Abstract
Three diterpenoid alkaloids, including an unreported compound, were isolated from the roots of Aconitum kusnezoffii Reichb. On the basis of spectral analysis, these three compounds were determined to be 1,15-dimethoxy-3-hydroxy-14-benzoyl-16-ketoneoline, benzoylaconine and aconitine.
Keywords: roots of Aconitum kusnezoffii Reichb., diterpenoid alkaloid, aconitum
1. Introduction
Aconitum kusnezoffii Reichb., belonging to the genus Aconitum L., is distributed in the Xinjiang, Sichuan and Jilin provinces of China. Although recently there were some reports on flowers [1] and leaves [2] of this plant, roots of Aconitum kusnezoffii Reichb. had been long used in Traditional Chinese Medicine as an analgesic and cardiotonic herbal medicine. Diterpenoid alkaloids [3,4] and polysaccharide [5] have been isolated from its roots to date. To our knowledge, diterpenoid alkaloids extracted from the genus Aconitum L. have various pharmacological properties [6,7,8,9,10]. In the search for biologically active alkaloids from the roots of Aconitum kusnezoffii Reichb., a detailed study was carried out. This led to the isolation of the new diterpenoid alkaloid 1,15-dimethoxy-3-hydroxy-14-benzoyl-16-ketoneoline, along with two known diterpenoid alkaloids, benzoylaconine and aconitine. Herein, the structure of the new compound was determined based on MS, IR, and NMR spectral data, and the known ones were identified by comparing their NMR data with those in the literature [3].
2. Results and Discussion
Compound 1 was obtained as a white solid. Its molecular formula was determined to be C32H43O9N by ESI-MS (M/Z 586. 861 [M+H]+). The 1H-NMR spectroscopic data of compound 1 showed three groups of absorptions for a benzoyl moity at δH 7.47 (2H, t, H-4ʺ), 7.61 (1H, t, H-5ʺ), and 7.96 (2H, d, H-3ʺ); N-ethyl protons at δH 2.72 (1H, m, H-1ʹα), 2.74 (1H, m, H-1ʹβ), and 1.14 (3H, t, H-2ʹ); and four O-methyl protons at δH 3.28 (3H, s, CH3O-1) 3.29 (3H, s, CH3O-6), 3.30 (3H, s ,CH3O-18), and 3.82 (3H, s, CH3O-15). The 13C-NMR and DEPT spectra of 1 revealed the presence of 32 carbons, which were assignable to a tertiary methyl group at δC 12.2 (C-2), an oxygenated methylene group at δC 76.3 (C-18), an oxygenated quaternary carbon at δC 77.5 (C-8), two quaternary carbons at δC 43.6 (C-4) and 51.2 (C-11), four methylene groups at δC 31.8 (C-12), 32.6 (C-2), 48.5 (C-1ʹ) and 49.3 (C-19), two carbonyl groups at δC 166.0 (C-1ʺ) and 211.8 (C-16) and aromatic hydrocarbon signals at δC 128.7 (C-4ʺ), 129.1 (C-2ʺ), 129.7 (C-3ʺ) and 133.8 (C-5ʺ), respectively. After further analysis of the spectroscopic data, compound 1 was identified as a neoline-type diterpenoid alkaloid [11].
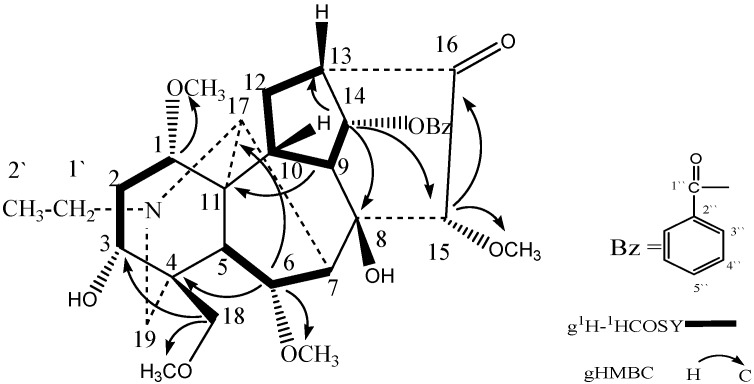
The g1H–1H COSY, gHSQC and gHMBC spectra allowed the complete assignment of the chemical shifts of compound 1 (Table 1) [12,13,14,15]. The 1H-NMR and 13C-NMR resonances of compound 1 were similar to those of chasmanine [3], which was also isolated from roots of Aconitum kusnezoffii Reichb., except for the different configurations of C-3, C-15, C-16 and C-14. The main differences are that the chemical shifts of C-3, C-15, C-16 and H-14 are δC 71.0, 85.8, 211.8 and δH 5.44 (d, J = 5.0 Hz) respectively in compound 1, and δC 35.0, 38.7, 81.9 and δH 4.12 (t, J = 4.5 Hz) respectively in chasmanine, which suggested that C-3, C-15 and C-16 in compound 1 belong to an oxygen-containing group. In the gHMBC spectrum, the correlations between H-18 and C-3, and between H-15 and CH3O-15 were important to confirm the presence of oxygen groups at C-3 and C-15. Further analysis of the 13C NMR data suggested that C-16 in compound 1 could be a carbonyl group, and not methoxyl group. The gHMBC correlations from C-1ʺ to H-14 and H-3ʺ confirmed the position of a benzoyl group. In addition, it is noteworthy that the chemical shift of H-14 in compound 1 was more deshielded than the same proton in chasmanine as the result of the steric effect of the carbonyl group at C-16. On the basis of the above spectral data, compound 1 was determined to be 1,15-dimethoxy-3-hydroxy-14-benzoyl-16-ketoneoline (Figure 1).
Table 1.
1D and 2D NMR Data for Compound 1 in CDCl3.
| δH | δC | g1H_1HCOSY | gHSQC | |
|---|---|---|---|---|
| 1 | 3.20 m | 82.5 | H-2α, H-2β | + |
| 2α | 1.97 m | 32.6 | H-1, H-2β, H-3 | + |
| 2β | 2.38 m | H-1, H-2α, H-3 | + | |
| 3 | 3.88 d J = 4.9 Hz | 71.0 | H-2α, H-2β | + |
| 4 | - | 43.6 | - | - |
| 5 | 2.23 d J = 6.4 Hz | 46.5 | H-6, H-7, H-17 | + |
| 6 | 3.95 d J = 6.6 Hz | 83.7 | H-5, H-7, H-17 | + |
| 7 | 2.79 m | 42.3 | H-6 | + |
| 8 | - | 77.5 | - | - |
| 9 | 2.84 m | 38.0 | H-10, H-14 | + |
| 10 | 2.33 m | 44.2 | H-9, H-12α, H-12β | + |
| 11 | - | 51.2 | - | - |
| 12α | 1.92 m | 31.8 | H-10, H-12β, H-13 | + |
| 12β | 2.91 dd J = 15.3 Hz J = 6.9 Hz | H-10, H-12α | + | |
| 13 | 2.67 m | 49.4 | H-12α | + |
| 14 | 5.44 d J = 5.0 Hz | 78.3 | H-9 | + |
| 15 | 3.86 s | 85.8 | - | + |
| 16 | - | 211.8 | - | - |
| 17 | 3.15 s | 62.1 | H-5, H-6 | + |
| 18α | 3.59 d J = 8.7 Hz | 76.3 | H-18β | + |
| 18β | 3.70 d J = 8.7 Hz | H-18α | + | |
| 19α | 2.65 d J = 11.9 Hz | 49.3 | H-19β | + |
| 19β | 3.25 d J = 11.9 Hz | H-19α | + | |
| CH3O-1 | 3.28 s | 55.9 | - | + |
| CH3O-6 | 3.29 s | 58.3 | - | + |
| CH3O-15 | 3.82 s | 62.4 | - | + |
| CH3O-18 | 3.30 s | 59.2 | - | + |
| N-CH2 1ʹα | 2.72 m | 48.5 | H-2ʹ | + |
| 1ʹβ | 2.74 m | H-1α, H-2ʹ | + | |
| -CH3 2ʹ | 1.14 t J = 7.0 Hz | 12.2 | H-1ʹα, H-1ʹβ | + |
| Bz | ||||
| 1ʺ | - | 166.0 | - | - |
| 2ʺ | - | 129.1 | - | - |
| 3ʺ | 7.96 d J = 7.6 Hz | 129.7 | H-4ʺ | + |
| 4ʺ | 7.47 t J = 7.5 Hz | 128.7 | H-3ʺ, H-5ʺ | + |
| 5ʺ | 7.61 t J = 7.1 Hz | 133.8 | H-4ʺ | + |
Figure 1.
Structure and Key Correlations of Compound 1.
3. Experimental
3.1. General
IR spectra were recorded using a Thermo Nicolet Nexus spectrophotometer. 1D and 2D NMR spectra were obtained on Bruker AV400; δH values are expressed in parts per million relative to the solvent (CDCl3) signal. DEPT, g1H-1H COSY, gHSQC, and gHMBC experiments were carried out with the pulse sequences given by Bruker. LC-MS were performed on a UPLC instrument (Waters, USA) coupled with a ZQ 2000 mass spectrometer (Waters, USA) using an XTerra MS C18 2.1 × 150 mm column (Waters, USA) and a C18HCE 4.6 × 150 mm column (self-made). A Waters Auto-Purification Factory was used in this study and consisted of a sample inJector (Waters 2777 sample manager), a passive splitter, a compensation pump (515 HPLC pump), an eight-channel UV detector (MUX-UV 2488), a four-channel MS detector (Micromass ZQ2000), four Waters 2525 binary gradient modules, and four Waters 2757 sample managers using XTerra MS C18 19 × 150 mm (Waters, USA) and C18HCE 10 × 150 mm (self-made) columns. Data were collected using a MassLynx workstation.
3.2. Plant Material
Roots of Aconitum kusnezoffii Reichb. were collected in YiLi, Xinjiang Province, China, in July 2008. The herb was authenticated by Xi Rong He, Institute of Medication, Xiyuan Hospital of China Academy of Traditional Chinese Medicine. A voucher specimen (CAOWU 200807) was deposited at the School of Chemical Engineering, Dalian University of Technology.
3.3. Extraction and Isolation
Dried powdered roots of the plant (50.0 kg) were defatted with EtOH (500.0 L) over 12 h. After removing the solvent under vacuum at 45 ºC, the residue was applied to X-4 macroporous absorption resin and eluted with H2O followed in sequence by 30%, 50% and 100% EtOH (v/v, aqueous-EtOH). The EtOH fractions were separated by column chromatography on silica gel eluted with petroleum, petroleum/EtOAc (v/v, 5:1), EtOAc and EtOH to obtain four fractions. Fraction 4 (15.0 g) was treated with a Waters Auto-Purification Factory using an XTerra 19 × 150 mm column. The injection volume was 1.2 mL. The flow rate was 17 mL/min. The mobile phases were 30 mM ammonium acetate buffer (pH = 8.0) and acetonitrile. Linear gradient elution was adopted starting from 10% Bp to 90% Bp: 0–40 min, 10–50% Bp; 40–45 min, 50–90% Bp and 45–60 min, 90–90% Bp. Fraction 4 was separated to afford 40 fractions of 1.5 min each. Fractions 16-17 were separated by Pre-HPLC with formic acid/water (0.2:100, v/v) and acetonitrile on C18HCE 10 × 150 mm. The injection volume was 1.0 mL. The flow rate was 3 mL/min. Linear gradient elution was as follows: 0–10 min, 5–20% Bp; 10–30 min, 20–25% Bp and 30–50 min, 25–50% Bp. Fractions of 1 min each were collected. This separation yielded 1,15-dimethoxy-3-hydroxy-14-benzoyl-16-ketoneoline (4.1 mg), benzoylaconine (5.2 mg) and aconitine (3.2 mg).
3.4. Spectral Data
1,15-Dimethoxy-3-hydroxy-14-benzoyl-16-ketoneoline: amorphous solid; IR (KBr) vmax 3480 (OH), 2935 (CH), 1780 (C=O, ester), 1451, 1382, 1320, 1282, 1180, 1,097, 983, 711 cm-1; UV λmax (CAN/H2O) 232 nm; 1H- and 13C-NMR data, Table 1; ESI-MS M/Z 586.861 [M+H]+ (calcd for C32H43O9N, 585. 041).
4. Conclusions
Based on the results of our present study, roots of Aconitum kusnezoffii Reichb. contain diterpenoid alkaloids, which possess diverse biological properties, as the principal secondary metabolites. A new diterpenoid alkaloid, 1,15-dimethoxy-3-hydroxy-14-benzoyl-16-ketoneoline, together with two known alkaloids, benzoylaconine and aconitine, were separated from roots of Aconitum kusnezoffii Reichb., and their structures identified by spectral analysis.
Acknowledgements
We thank the Key lab of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences for support.
Supplementary Materials
Supplementary materials can be accessed at http://www.mdpi.com/1420-3049/16/4/3345/s1.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Wang Y., Song F.R., Xu Q.X., Liu Z.Q., Liu S.Y. Characterization of aconitine-type alkaloids in the flowers of Aconitum kusnezoffii by slectrospray ionization tandem mass spectrometry. Acta Pharm. Sin. 2003;38:962–970. doi: 10.1002/jms.510. [DOI] [PubMed] [Google Scholar]
- 2.Yu H.L., Jia S.S. A norterpenoids alkaloid, Beiwucine from the leaves of Aconitum kusnezoffii Reichb as Mongolia Medicine. Acta Pharm. Sin. 2000;35:232–234. [Google Scholar]
- 3.Li Z.B., Lu G.H., Chen D.L., Wang F.P. Chemical study on the alkaloids of "CAO WU". Nat. Prod. Res. Dev. 1997;9:9–14. [Google Scholar]
- 4.Zinurova E.G., Khakimova T.V., Spirikhin L.V., Yunusov M.S., Gorovoi P.G., Tolstikov G.A. A new norditerpenoid alkaloid acsonine from the roots of Aconitum Kusnezoffii Reichb. Russ. Chem. Bull. 2001;50:311–312. doi: 10.1023/A:1009550922824. [DOI] [Google Scholar]
- 5.Sun Y.J., Chen Y., Wu J.J., Wang B.S., Guo Z.R. Studies on the isolation, purification and composition of Acontium kusnezoffii polysaccharide. Chin. Pharm. J. 2000;35:731–733. [Google Scholar]
- 6.Heubach J., Schüle A. Cardiac effects of Lappaconitine and N-Deacetyltappaconitine, Two diterpenoid alkaloids from plants of the Aconitum and Delphinium species. Planta Med. 1998;64:22–26. doi: 10.1055/s-2006-957359. [DOI] [PubMed] [Google Scholar]
- 7.Saito H., Ueyama T., NaKa N., Yagi J., Okamoto T. Pharmacological studies of Lgnavine, an aconitum alkaloid. Chem. Pharm. Bull. 1982;30:1844–1850. doi: 10.1248/cpb.30.1844. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Heiden R., Spijksma G., Reijmers T., Wang M., Xu G., Hankemeier T., Greef J., Murayama M., Mori T., Bando H., Amiya T. Studies on the constituents of aconitum species. IX. The pharmacological properties of pyro-type Aconitine alkaloids, components of processed aconite powder 'kako-bushi-matsu': analgesic, antiinflammatory and acute toxic activities. J. Ethnopharmacol. 1991;35:159–164. doi: 10.1016/0378-8741(91)90068-O. [DOI] [PubMed] [Google Scholar]
- 9.Ameri A. The effects of Aconitum alkaoids on the central nervous system. Prog. Neurobiol. 1998;56:211–235. doi: 10.1016/S0301-0082(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X.Y., Wang Y., Li Y., Chen X.Q., Yang H.H., Yue J.M., Hu G.Y. Songorin, a diterpenoid alkaloid of the genus Aconitum, is a novel GABAA receptor antagonist in rat brain. Neurosci. Lett. 2003;337:33–36. doi: 10.1016/S0304-3940(02)01299-5. [DOI] [PubMed] [Google Scholar]
- 11.Hanuma J.B., Katz A. 1H-NMR spectra of norditerpenoid Alkaloids. A Review. J. Nat. Prod. 1994;57:1473–1483. doi: 10.1021/np50113a001. [DOI] [Google Scholar]
- 12.Pelletier S.W., Chokshi H.P., Desai H.K. Separation of diterpenoid alkaloid mixtures using vacuum liquid chromatography. J. Nat. Prod. 1986;49:892–900. doi: 10.1021/np50047a021. [DOI] [Google Scholar]
- 13.Liu H.M., Katz A. Diterpenoid alkaloids from Aphids Brachycaudus aconiti and Brachycaudus napelli feeding on Aconitum napellus. J. Nat. Prod. 1996;59:135–138. doi: 10.1021/np960047r. [DOI] [Google Scholar]
- 14.Arlandini E., Ballabio M., Gioia B., Colombo M.L., Tomé F. N-Deethylaconitine from Aconitum napellus ssp. Vulgare. J. Nat. Prod. 1987;50:937–939. doi: 10.1021/np50053a030. [DOI] [Google Scholar]
- 15.Joshi B.S., Srivastava S.K., Barber A.D., Desai H.K., Pelletier S.W. Selective demethylation of some Aconitine-Type norditerpenoid alkaloids. J. Nat. Prod. 1997;60:439–443. doi: 10.1021/np9606862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.