Abstract
Background
Insulin resistance (IR) is an important risk factor for subclinical atherosclerosis. This study evaluated the relationship between the triglyceride glucose (TyG) index, which is a simple and reliable surrogate marker for IR, and arterial stiffness.
Methods
This study included 2560 Korean subjects without a previous history of coronary artery disease, stroke, and malignancies who participated in a community-based cohort study. Arterial stiffness was measured using the brachial-ankle pulse wave velocity (baPWV).
Results
All participants were stratified into four groups based on the quartile of the TyG index. The prevalence of metabolic syndrome and diabetes significantly increased with increasing TyG index quartile. The mean baPWV was significantly different among all groups (group I [lowest]: 1421 ± 242 vs. group II: 1480 ± 244 vs. group III: 1534 ± 260 vs. group IV [highest]: 1575 ± 279 cm/s; p < 0.001). The TyG index values were correlated with baPWV (r = 0.224, p < 0.001). Multiple regression analysis showed that age (β = 0.410), male gender (β = 0.051), increased blood pressure (β = 0.266), and TyG index (β = 0.158) were associated with baPWV (p < 0.05, respectively). TyG index was independently related to baPWV in both non-diabetics and diabetics.
Conclusions
The TyG index is independently associated with arterial stiffness in a relatively healthy Korean population.
Keywords: Insulin resistance, Arterial stiffness
Introduction
Cardiovascular (CV) disease is a major cause of morbidity and mortality. It is well-established that insulin resistance (IR) is associated with an increased risk of metabolic abnormalities, including hyperglycemia, dyslipidemia, and hypertension [1, 2]. In addition, a number of studies have revealed that IR is one of the most important contributing factors to CV disease [3, 4].
Recently, the triglyceride glucose (TyG) index was used as a simple and reliable surrogate marker of IR. Several studies reported that the TyG index is closely correlated with the homeostatic model assessment of insulin resistance (HOMA-IR) index, which has been traditionally used to measure IR [5–7]. However, there is a paucity of data on the relationship between the TyG index and subclinical atherosclerosis, especially arterial stiffness which has independent prognostic value for the risk of CV events. Moreover, although previous studies suggested the possibility of a somewhat different atherosclerotic change in diabetics compared to non-diabetics, data on the usefulness of TyG index on subclinical atherosclerosis according to diabetic status is currently unavailable. In clinical practice, brachial-ankle pulse wave velocity (baPWV) is used as a simple and reliable tool for the measurement of arterial stiffness because of its high reproducibility. Therefore, the present study evaluated the association between TyG index and arterial stiffness measured using baPWV in a relatively healthy Korean population.
Methods
Participants
This is a cross-sectional investigation analyzing baseline data collected for a prospective cohort study. We used the data of 2560 subjects who participated in baseline health examinations for a community-based cohort study in the Seoul area between April 2010 and November 2012. Subjects with a clinical history of cerebrovascular hemorrhage or infarction, neurological abnormalities, or malignancy were excluded from this study. The study protocol was approved by the local ethics committee of our institution, and informed consent for the procedure was obtained from each individual.
All blood samples were obtained after 8 h of fasting and analyzed for triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and glucose. Waist circumference was measured at the midpoint between the lower border of the rib cage and the iliac crest. The TyG index was calculated as ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL) / 2]. Body mass index (BMI) was calculated as weight (kg) / height (m2). Metabolic syndrome was defined as when 3 or more of the following were present: (a) blood pressure ≥ 130 mmHg systolic or ≥ 85 mmHg diastolic or anti-hypertensive treatment; (b) HDL cholesterol < 40 mg/dL in males or < 50 mg/dL in females; (c) fasting triglycerides ≥150 mg/dL; (d) abdominal obesity based on waist circumference ≥ 90 cm in males or ≥ 80 cm in females; and (e) impaired fasting glucose, defined as fasting glucose ≥100 mg/dL or established diabetes based on the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) definition [8]. Diabetes was defined as either fasting glucose ≥126 mg/dL, a referral diagnosis of diabetes, or antidiabetic treatment.
Measurement of baPWV
All subjects abstained from beverages or caffeine-containing food for at least 45 min prior to baPWV measurement. After a subject had been resting in the supine position for at least 5 min in a quiet room, blood pressure and baPWV were measured using an automated waveform analyzer (Colin VP-2000, Colin Medical Instruments Corp., Komaki, Japan). Briefly, baPWV was measured in subjects’ bilateral upper and lower extremities using plethysmographic sensor that simultaneously recorded blood pressure, an electrocardiogram, and heart sounds. baPWV was calculated as the length between arterial sites divided by time interval and was measured in both brachial and posterior tibial arteries. The higher value of baPWV measured on either side of each patient was used for analysis.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation. Categorical variables are presented as absolute values and proportions. To compare the characteristics among the TyG index groups, one-way analysis of variance was used for continuous variables, and the χ2-test or Fisher’s exact test was used for categorical variables, as appropriate. Correlational analysis between the TyG index and baPWV was performed using Pearson’s correlation test. Univariate and multivariate linear regression analysis was performed to identify the association between independent variables and arterial stiffness. Variables with p < 0.05 in the univariate analysis were considered confounding variables and entered into multivariate linear regression analysis. All statistical analyses were performed using the Statistical Package for the Social Sciences version 19 (SPSS, Chicago, Illinois), and a p-value of < 0.05 was considered significant for all analyses.
Results
Baseline characteristics
Table 1 shows the clinical characteristics of the participants. All participants were stratified into four groups based on their TyG index levels. The mean levels of TyG index were 8.7 ± 0.2, 9.2 ± 0.1, 9.5 ± 0.1, and 10.0 ± 0.3 in groups I (lowest), II, III, and IV (highest), respectively. There were significant differences in anthropometric indices, including BMI, waist circumference, and systolic and diastolic blood pressure. The prevalence of metabolic syndrome was 10.0, 19.1, 40.2, and 79.2% and that of diabetes was 7.2, 9.1, 17.2, and 30.6% in groups I, II, III, and IV, respectively.
Table 1.
Baseline characteristics
| Quartile of the TyG index | p | ||||
|---|---|---|---|---|---|
| I (lowest) (n = 622) | II (n = 658) | III (n = 640) | IV (highest) (n = 640) | ||
| Age, years | 59 ± 8 | 60 ± 8 | 61 ± 8 | 60 ± 8 | < 0.001 |
| Male, n (%) | 147 (23.6) | 179 (27.2) | 224 (35.0) | 292 (45.6) | < 0.001 |
| Systolic blood pressure, mmHg | 119 ± 14 | 122 ± 15 | 124 ± 15 | 127 ± 15 | < 0.001 |
| Diastolic blood pressure, mmHg | 71 ± 10 | 73 ± 9 | 75 ± 9 | 77 ± 10 | < 0.001 |
| Heart rate, bpm | 65 ± 9 | 66 ± 8 | 68 ± 10 | 69 ± 10 | < 0.001 |
| Anti-hypertensive drugs, n (%) | 195 (31.4) | 278 (42.2) | 295 (46.1) | 326 (50.9) | < 0.001 |
| Smoking, n (%) | 114 (18.3) | 134 (20.4) | 188 (29.4) | 259 (40.5) | < 0.001 |
| BMI, kg/m2 | 23.8 ± 2.9 | 24.5 ± 2.9 | 25.3 ± 2.9 | 25.8 ± 2.9 | < 0.001 |
| Waist circumference, cm | 80 ± 8 | 83 ± 8 | 85 ± 8 | 87 ± 9 | < 0.001 |
| Laboratory | |||||
| Total cholesterol, mg/dL | 191 ± 33 | 198 ± 36 | 203 ± 37 | 205 ± 37 | < 0.001 |
| Triglyceride, mg/dL | 66 ± 13 | 99 ± 13 | 133 ± 21 | 217 ± 81 | < 0.001 |
| HDL cholesterol, mg/dL | 64 ± 15 | 57 ± 13 | 51 ± 13 | 45 ± 11 | < 0.001 |
| LDL cholesterol, mg/dL | 114 ± 29 | 122 ± 32 | 128 ± 34 | 122 ± 35 | < 0.001 |
| Fasting glucose, mg/dL | 93 ± 11 | 96 ± 11 | 101 ± 15 | 115 ± 31 | < 0.001 |
| Creatinine, mg/dL | 0.76 ± 0.19 | 0.77 ± 0.18 | 0.79 ± 0.18 | 0.82 ± 0.20 | < 0.001 |
| Metabolic syndrome, n (%) | 62 (10.0) | 126 (19.1) | 257 (40.2) | 507 (79.2) | < 0.001 |
| Diabetes mellitus, n (%) | 45 (7.2) | 60 (9.1) | 110 (17.2) | 196 (30.6) | < 0.001 |
| Anti-diabetic treatment, n (%) | 41 (6.6) | 56 (8.5) | 96 (15.0) | 154 (24.1) | < 0.001 |
| TyG index | 8.7 ± 0.2 | 9.2 ± 0.1 | 9.5 ± 0.1 | 10.0 ± 0.3 | < 0.001 |
Values are given as the mean ± standard deviation or number (%)
BMI body mass index, HDL high-density lipoprotein, LDL low-density lipoprotein, TyG triglyceride glucose
Relationship between the TyG index and baPWV
The mean baPWV significantly increased with increasing quartiles of the TyG index (group I [lowest]: 1421 ± 242 vs. group II: 1480 ± 244 vs. group III: 1534 ± 260 vs. group IV [highest]: 1575 ± 279 cm/s; p < 0.001) (Fig. 1). The levels of the TyG index were significantly correlated with baPWV (r = 0.224, p < 0.001) (Fig. 2).
Fig. 1.
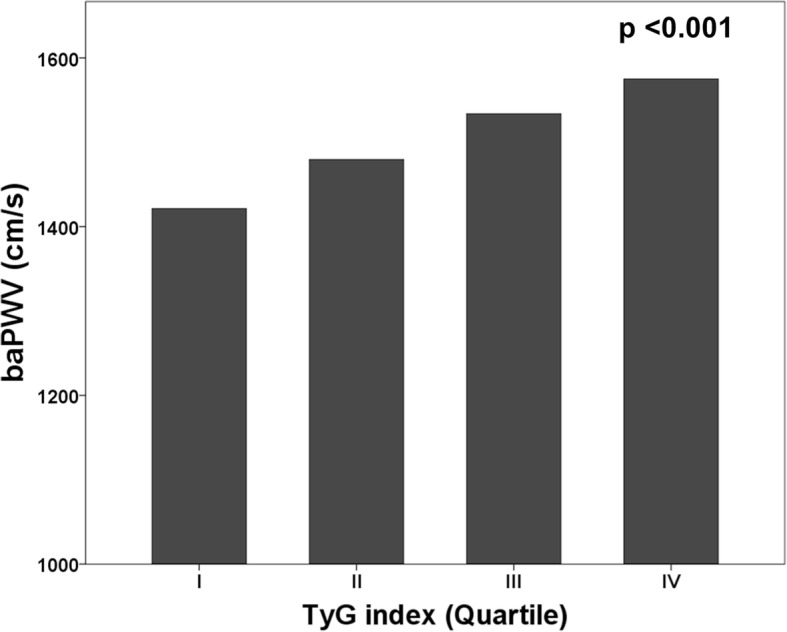
Comparison of baPWV according to TyG index group
Fig. 2.
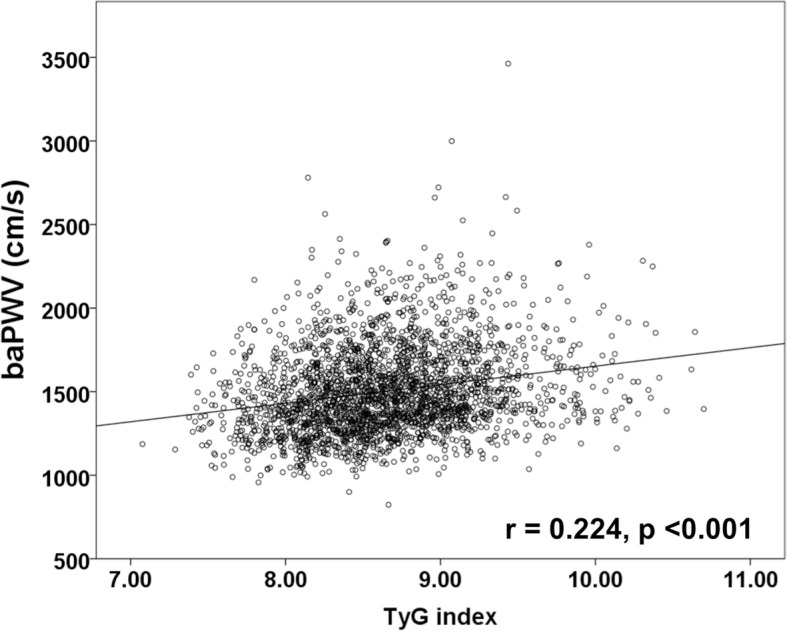
Correlation between TyG index and baPWV
Association between clinical variables and baPWV
Univariate linear regression analysis showed that age (β = 0.479, p < 0.001), male gender (β = 0.137, p < 0.001), abdominal obesity (β = 0.083, p < 0.001), increased blood pressure (β = 0.391, p < 0.001), decreased HDL (β = 0.057, p = 0.004), smoking (β = 0.114, p < 0.001), and TyG index (β = 0.224, p < 0.001) were significantly associated with baPWV. Multivariate linear regression analysis showed that age (β = 0.410, p < 0.001), male gender (β = 0.051, p < 0.043), increased blood pressure (β = 0.266, p < 0.001), and TyG index (β = 0.158, p < 0.001) were significantly associated with baPWV (Table 2).
Table 2.
Association between clinical variables and baPWV
| Univariate | Multivariate | |||
|---|---|---|---|---|
| β | p | β | p | |
| Age, years | 0.479 | < 0.001 | 0.410 | < 0.001 |
| Male | 0.137 | < 0.001 | 0.051 | 0.043 |
| Abdominal obesity | 0.083 | < 0.001 | −0.032 | 0.065 |
| Increased blood pressure | 0.391 | < 0.001 | 0.266 | < 0.001 |
| Decreased HDL | 0.057 | 0.004 | −0.026 | 0.140 |
| LDL > 130 mg/dL | 0.001 | 0.998 | ||
| Smoking | 0.114 | < 0.001 | −0.021 | 0.396 |
| TyG index | 0.224 | < 0.001 | 0.158 | < 0.001 |
HDL high-density lipoprotein, LDL low-density lipoprotein, TyG triglyceride glucose
Increased blood pressure was defined as blood pressure ≥ 130 mmHg systolic or ≥ 85 mmHg diastolic or anti-hypertensive treatment
Decreased HDL was defined as HDL cholesterol < 40 mg/dL in males or < 50 mg/dL in females
Relationship of TyG index to baPWV according to diabetic status
Multiple linear regression models were analyzed to identify the association between TyG index and baPWV according to the established diabetic status. TyG index was independently related to the baPWV after consecutive adjustment for confounding variables in both non-diabetics and diabetics (Table 3).
Table 3.
Impact of TyG index on baPWV according to diabetic status
| Non-diabetes | Diabetes | |||
|---|---|---|---|---|
| β | p | β | p | |
| Model 1 | 0.171 | < 0.001 | 0.131 | 0.004 |
| Model 2 | 0.161 | < 0.001 | 0.126 | 0.006 |
| Model 3 | 0.134 | < 0.001 | 0.125 | 0.009 |
| Model 4 | 0.137 | < 0.001 | 0.122 | 0.011 |
HDL high-density lipoprotein, TyG triglyceride glucose
Definitions of increased blood pressure and decreased HDL are present in Table 2
Model 1 Adjusted for age
Model 2 Adjusted for age and gender
Model 3 Adjusted for age, gender, abdominal obesity, increased blood pressure, and decreased HDL
Model 4 Adjusted for age, gender, abdominal obesity, increased blood pressure, decreased HDL, and smoking
Discussion
The main finding of present study was that the TyG index was significantly associated with arterial stiffness measured by baPWV after adjusting for other metabolic abnormalities. This result provides evidence that IR has a substantial role in subclinical atherosclerosis in a general population.
The homeostatic model assessment of insulin resistance (HOMA-IR) has been traditionally used to estimate IR [9, 10]. However, insulin levels must be determined to calculate the HOMA-IR index. In South Korea, insulin levels are usually measured for established diabetics. Thus, HOMA-IR is an inconvenient parameter to identify IR in the general population. Recently, several studies reported that the TyG index is closely correlated with HOMA-IR [11, 12]. In addition, some studies suggested that the TyG index had better predictive value for IR than HOMA-IR [6, 13]. Thus, the TyG index is being considered as a simple and useful surrogate marker of IR.
Early detection of atherosclerosis is important for preventing major CV events in the general population. In clinical practice, subclinical atherosclerosis is mostly evaluated at health check-ups with several tools, i.e., coronary artery calcium score (CACS), carotid intima-media thickness, plaque, and pulse wave velocity. Although IR might be a substantial risk factor for the development of CV disease, few studies evaluated the association between IR and subclinical atherosclerosis. In particular, data on the relationship between the TyG index and subclinical atherosclerosis have been limited. Irace et al. reported that the TyG index is strongly associated with carotid atherosclerosis, as assessed by Doppler ultrasonography, after adjusting for traditional CV risk factors [14].14 Importantly, they emphasized that the TyG Index is better related to carotid atherosclerosis than HOMA-IR. Kim et al. also reported similar findings that the TyG index is more independently associated with the presence of coronary artery atherosclerosis assessed using CACS than is HOMA-IR in 4319 healthy Korean subjects [15]. In the CRONOS-ADM (Coronary CT angiography evaluation for clinical outcomes in asymptomatic patients with type 2 diabetes mellitus) registry, a higher TyG index is associated with increased risk of coronary artery stenosis in asymptomatic subjects with type 2 diabetes [16]. However, data on the association between the TyG index and arterial stiffness has been limited. The present study investigated the relationship between the TyG index and the arterial stiffness assessed by baPWV in Korean adults without a previous history of major CV events or malignancies. We also found that the TyG index was significantly associated with arterial stiffness after adjusting for confounding factors.
It is well-known that IR is a major characteristic of metabolic syndrome [8, 17]. Additionally, despite the difference in the clinical features of diabetes according to ethnicity [18], IR has a pivotal role in the development of diabetes. Recently, a longitudinal study performed in 2900 non-diabetic adults indicated that the TyG index measured at a single point could be an indicator of the risk for incident diabetes [19]. Participants with TyG index ≥8.8 regardless of obesity had a significantly high risk for diabetes in this study. We confirmed that the prevalence of metabolic syndrome and diabetes significantly increased with increasing quartiles of the TyG index in the present study. Considering that IR has a substantial role in metabolic abnormalities, IR might be an important target to reduce the risk of CV disease. Although previous studies reported the different atherosclerotic change in diabetics compared to non-diabetics, we could identify that TyG index was useful IR parameter for predicting subclinical atherosclerosis in both non-diabetics and diabetics.
This study has some limitations. First, the present study includes only a Korean population. Second, we have not been able to eliminate the possible effects of underlying medications on subclinical atherosclerosis because of the observational design of this study. Third, the impact of IR on arterial stiffness may differ across different age groups. However, it was difficult to perform a sub-analysis of different age groups because none of the cohort study participants were very young. Fourth, we did not measure HOMA-IR because the examination of insulin levels is not usually included in general health check-ups at our institution. However, the close relationship between the TyG index and HOMA-IR was already well-established, as previous described. Fifth, we did not have information on the physical activity of participants. Finally, we did not evaluate the intra- and inter-observer correlation coefficient for the measurement of baPWV. However, it is well-known that the measurement of baPWV is simple and reliable for identifying arterial stiffness because of its high reproducibility [20, 21]. Despite these limitations, we could identify the independent impact of the IR estimated by the TyG index on arterial stiffness, which is an important marker of subclinical atherosclerosis.
In conclusion, the TyG index was independently associated with arterial stiffness measured by baPWV in a relatively healthy Korean population. This result suggested that that IR has a substantial role in subclinical atherosclerosis and might be an important target to prevent major CV disease.
Acknowledgements
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant no. HI13C0715).
Funding
Not applicable.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- baPWV
Brachial-ankle pulse wave velocity
- BMI
Body mass index
- CVD
Cardiovascular disease
- HDL
High-density lipoprotein
- HOMA-IR
Homeostatic model assessment of insulin resistance
- IR
Insulin resistance
- LDL
Low-density lipoprotein
- TyG
Triglyceride glucose
Authors’ contributions
All the authors listed in the manuscript participated in the design of the study and writing of the manuscript. KW and GP performed the statistical analysis. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol was approved by our institution’s ethics committee, and informed consent for the procedure was obtained from each individual.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ki-Bum Won, Email: kbwon99@naver.com.
Gyung-Min Park, Email: 0733719@uuh.ulsan.kr.
Sang-Eun Lee, Email: TKDDMSS@yuhs.ac.
In-Jeong Cho, Email: injeongcho@yuhs.ac.
Hyeon Chang Kim, Email: hckim@yuhs.ac.
Byoung Kwon Lee, Email: cardiobk@yuhs.ac.
Hyuk-Jae Chang, Email: hjchang@yuhs.ac.
References
- 1.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of insulin resistance in metabolic disorders: the Bruneck study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 2.Bonora E, Targher G, Alberiche M, Bonadonna RC, Zenere MB, Saggiani F, Muggeo M. Intracellular partition of plasma glucose disposal in hypertensive and normotensive subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2001;86:2073–2079. doi: 10.1210/jcem.86.5.7455. [DOI] [PubMed] [Google Scholar]
- 3.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio heart study. Diabetes Care. 2002;25:1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 4.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–324. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- 5.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 6.Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 9.Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–534. doi: 10.1046/j.1464-5491.2002.00745.x. [DOI] [PubMed] [Google Scholar]
- 10.Cutfield WS, Jefferies CA, Jackson WE, Robinson EM, Hofman PL. Evaluation of HOMA and QUICKI as measures of insulin sensitivity in prepubertal children. Pediatr Diabetes. 2003;4:119–125. doi: 10.1034/j.1399-5448.2003.t01-1-00022.x. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Han K, Yang HK, Kim MK, Yoon KH, Kwon HS, Park YM. Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and nutrition examination survey, 2008–2010. Clin Endocrinol. 2015;82:213–220. doi: 10.1111/cen.12502. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, Lee JH, Yim HW, Kang MI, Lee WC, Son HY, Yoon KH. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju metabolic disease cohort (CMC) study. PLoS One. 2014;9:e90430. doi: 10.1371/journal.pone.0090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, Gnasso A. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67:665–672. doi: 10.1111/ijcp.12124. [DOI] [PubMed] [Google Scholar]
- 15.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16:108. doi: 10.1186/s12933-017-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EY, Yang HK, Lee J, Kang B, Yang Y, Lee SH, Ko SH, Ahn YB, Cha BY, Yoon KH, Cho JH. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis. 2016;15:155. doi: 10.1186/s12944-016-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCEP Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 19.Lee DY, Lee ES, Kim JH, Park SE, Park CY, Oh KW, Park SW, Rhee EJ, Lee WY. Predictive value of triglyceride glucose index for the risk of incident diabetes: a 4-year retrospective longitudinal study. PLoS One. 2016;11:e0163465. doi: 10.1371/journal.pone.0163465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munakata M, Ito N, Nunokawa T, Yoshinaga K. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am J Hypertens. 2003;16:653–657. doi: 10.1016/S0895-7061(03)00918-X. [DOI] [PubMed] [Google Scholar]
- 21.Lee HS, Kim HL, Kim H, Hwang D, Choi HM, Oh SW, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Incremental prognostic value of brachial-ankle pulse wave velocity to single-photon emission computed tomography in patients with suspected coronary artery disease. J Atheroscler Thromb. 2015;22:1040–1050. doi: 10.5551/jat.29918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


