Abstract
Cardiac fibrosis is a common pathologic consequence of stress insult to the heart and is characterized by abnormal deposition of fibrotic extracellular matrix that compromises cardiac function. Cardiac fibroblasts are key mediators of fibrotic remodeling and are regulated by secreted stress-response proteins. The matricellular protein connective tissue growth factor (CTGF), or CCN2, is strongly produced by injured cardiomyocytes and although it is considered a pro-fibrotic factor in many organ systems, its role in cardiac fibrosis is controversial. Here we adopted a cell-specific genetic approach to conditionally delete CCN2 in either cardiomyocytes or activated fibroblasts. Fibrosis was induced by angiotensin II-based neurohumoral stimulation, an insult that strongly induces CCN2 expression from cardiomyocytes and to a lesser extent in fibroblasts. Remarkably, only CCN2 deletion from activated fibroblasts inhibited the fibrotic remodeling while deletion from cardiomyocytes (the main source of CCN2 in the heart) had no effects. In vitro experiments revealed that although efficiently secreted by both fibroblasts and cardiomyocytes, only fibroblast-derived CCN2 is proficient in its ability to fully activate fibroblasts. These results overall indicate that although secreted into the extracellular matrix, CCN2 acts in an autocrine fashion. Secretion of CCN2 by cardiomyocytes is not pro-fibrotic, while fibroblast-derived CCN2 can modulate fibrosis in the heart. In conclusion we found that cardiomyocyte-derived CCN2 is dispensable for cardiac fibrosis, while inhibiting CCN2 induction in activated fibroblasts is sufficient to abrogate the cardiac fibrotic response to angiotensin II. Hence, CCN2 is an autocrine factor in the heart.
Keywords: cardiac fibrosis, CTGF, CCN2, cardiomyocytes, myofibroblasts
Introduction
Heart failure is a complex clinical syndrome that originates from pathologic remodeling of the myocardium.1 In the dysfunctional heart, fibrosis is often observed as a consequence of abnormal deposition of extracellular matrix (ECM).2 Cardiac fibrosis disrupts myocardial architecture and interferes with normal electrical and mechanical properties of the heart, further driving and exacerbating the existing pathology.2,3 Although a variety of insults can lead to fibrotic cardiac remodeling, fibrosis originates from fibroblasts which have become activated and transformed into a pro-fibrotic cell type, the myofibroblast.4 Since fibroblasts are the main producer of connective tissue in the heart, understanding the molecular mechanisms regulating their activation into pro-fibrotic cells is critical to develop strategies that counteract cardiac fibrosis.
Secreted factors and cytokines are key regulators of fibroblast function.3 As an example, members of the TGFβ family of growth factors have been extensively characterized for their ability to stimulate myofibroblast (pro-fibrotic) phenotypes both in vitro and in vivo.5 In the heart, we have found that upregulation of TGFβ1 is sufficient to drive fibrosis, cardiac dysfunction, and mortality in a murine transgenic model.6,7 However, because of the pleiotropic effects of TGFβ1 and its critical functions not only in stress-responses but also in the maintenance of homeostasis, therapeutically targeting TGFβ1 is complicated and carries the risk for important adverse effects.
Connective tissue growth factor (CTGF), or CCN2, is a matricellular protein that is induced in the heart following cardiac injury.6,8,9 CCN2 is consistently associated with fibrotic remodeling in various organ systems and has been widely used as marker of fibrosis.10 In addition to its ability to mark fibrotic lesions, CCN2 has been implicated in the onset and progression of tissue fibrosis in skeletal muscle, skin, lung, and many other organs.11 In addition, TGFβ1 and angiotensin II are key upstream regulators of CCN2 expression.8,12 Our laboratory has recently analyzed the role of CCN2 as a downstream modulator of TGFβ1-dependent cardiac remodeling and, more generally, as an effector of stress-induced cardiac dysfunction.6 To our surprise, and as subsequently confirmed by independent investigators, decreased levels of CCN2 did not alter TGFβ1-driven cardiomyopathy or cardiac dysfunction upon chronic pressure-overload stimulation.6,13 Mechanisms behind these findings are as yet unknown, as is the precise role of CCN2 in the heart.
Using newly generated genetically engineered mice to specifically address CCN2 function in activated fibroblasts and cardiomyocytes, we show that although cardiomyocytes are the main source of CCN2 in the injured heart, cardiomyocyte-derived CCN2 is dispensable for fibrotic heart remodeling. In contrast, fibroblast-derived CCN2 drives myofibroblast function and cardiac fibrosis following neurohumoral stimulation. We have thereby revealed the autocrine action of CCN2 in the heart, and clarified the contribution of CCN2 to cardiac fibrosis.
Results
Analysis of cardiomyocyte-specific CCN2 deletion mice
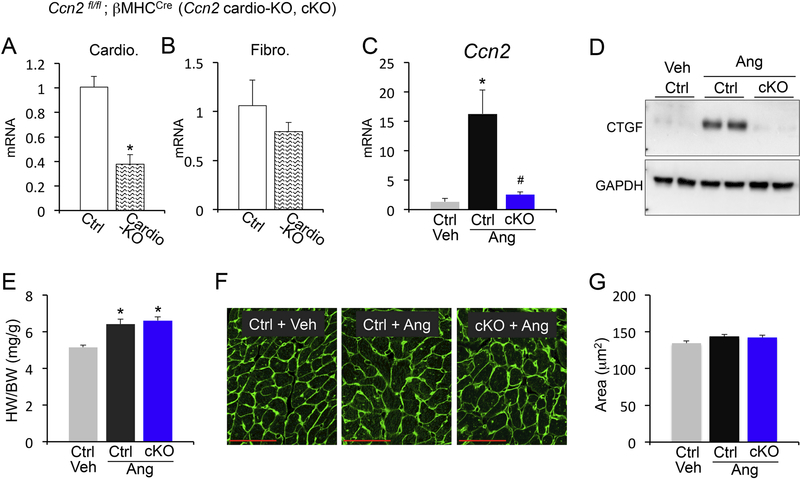
In the heart, CCN2 is strongly induced following injury, and is secreted primarily by cardiomyocytes.6 To delete CCN2 specifically in cardiomyocytes, we crossed Ccn2fl/fl mice with mice expressing cre recombinase under the control of the cardiomyocyte-restricted β-MHC promoter (Ccn2 cardio-KO; cKO). We confirmed a significant reduction of CCN2 expression from isolated cardiomyocytes, while expression of CCN2 from fibroblasts was unaffected (Figure 1A and B). Purity of cardiomyocyte and fibroblast preparations was confirmed using cell-specific markers (Supplemental Figure 1). Previous works have utilized complex systems such as chronic pressure overload and long-term infusion of combined angiotensin and phenylephrine to study CCN2, but have failed in revealing any effects in the heart.6,13 We therefore chose a simplified method where we induced cardiac fibrosis using a high dose of the pro-fibrotic agonist angiotensin II (ang) for one week. As expected, angiotensin II strongly induced CCN2 expression in the heart, which was remarkably abrogated in cardiomyocyte-specific CCN2 knockout mice (Figure 1C and D). This supported our previous findings whereby using different injury models we showed that cardiomyocytes are the main source of CCN2 in the stressed myocardium.6 Additionally, angiotensin II stimulation of cardiac growth was unaffected by cardiomyocyte-specific deletion of CCN2 (Figure 1E), which was confirmed at the cellular level by cross-sectional area quantifications (Figure 1F and G). Thus, cardiomyocytes are the main source of CCN2 when the heart is challenged by the neurohumoral agent angiotensin II, but CCN2 is dispensable for cardiomyocyte hypertrophy in this system.
Figure 1 – Analysis of cardiomyocyte-specific CCN2 deletion mice.
(A, B) qPCR analysis for Ccn2 mRNA expression normalized to Rpl7 housekeeping gene in isolated cardiomyocytes (A) or isolated cardiac fibroblasts (B) from the indicated genotypes. (C) qPCR analysis for Ccn2 mRNA expression normalized to Rpl7 housekeeping gene in hearts from vehicle (veh) treated control (ctrl) mice or angiotensin II (Ang) stimulated control (ctrl) or Ccn2 cardiac KO mice (cKO). n≥ 3 biological replicates / condition. (D) Western blot for CCN2 and GAPDH loading control from cardiac protein extracts from the indicated genotypes and treatments. (E) Heart weight to body weight ratio (HW/BW) from the indicated groups of mice. n≥ 5 biological replicates / condition. (F) Representative Wheat Germ Agglutinin (WGA) staining outlining cell borders (green) in cardiac cross-sections from vehicle and angiotensin II stimulated control and Ccn2 cKO mice. Scale bar is 50μm. (G) Quantification of cardiomyocyte cross-sectional areas from WGA stained sections using ImageJ NIH software. n≥3 hearts / condition, n=100 cells / heart. *p<0.05 vs. ctrl (A) or veh ctrl (C and D); #p<0.05 vs. ctrl ang treatment
Cardiomyocyte-derived CCN2 is dispensable for fibrotic remodeling
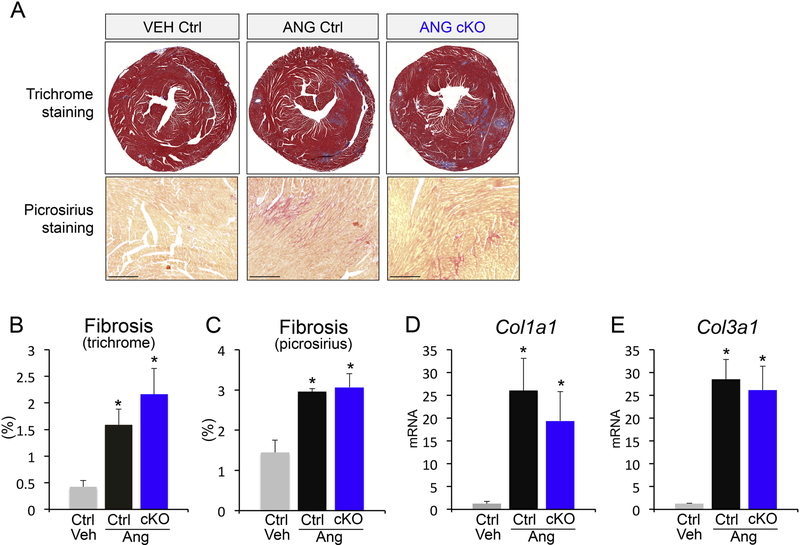
To assess the role of CCN2 in angiotensin-driven cardiac fibrosis, we analyzed collagen deposition in our cardiomyocyte-specific genetic Ccn2 deletion model. Histological assessment of fibrosis by Masson’s trichrome and picrosirius red staining revealed significant induction of cardiac fibrosis with angiotensin II, the levels of which were unchanged with cardiomyocyte-specific CCN2 deletion (Figure 2A-C). Finally, expression analysis of collagens I (Col1a1) and III (Col3a1), the main collagens that contribute to cardiac fibrosis, confirmed equal induction of collagen production in control and cardiomyocyte Ccn2 knockout (cKO) mice with angiotensin II (Figure 2D and E). These results indicate that cardiomyocyte-derived CCN2 is dispensable for both cardiomyocyte hypertrophy and angiotensin-induced fibrosis in the heart.
Figure 2 – Cardiomyocyte-derived CCN2 is dispensable for fibrotic remodeling.
(A) Representative Masson’s trichrome-stained histological sections for fibrosis (upper panels; blue) and picrosirius staining (bottom panels; red) in control and Ccn2 cKO mouse hearts with or without angiotensin II infusion. (B, C) Quantification of fibrosis from Masson’s trichromestained and picrosirius-stained histological sections of the indicated genotypes and treatments using ImageJ NIH software. Scale bar is 20μm. n≥5 animals / condition (D, E) qPCR analysis for collagen 1a1 (Col1a1) and collagen 3a1 (Col3a1) mRNA expression normalized to Rpl7 housekeeping gene in hearts from the indicated groups. n≥5 biological replicates / condition. *p<0.05 vs. veh ctrl
Myofibroblast-derived CCN2 is essential for cardiac fibrosis
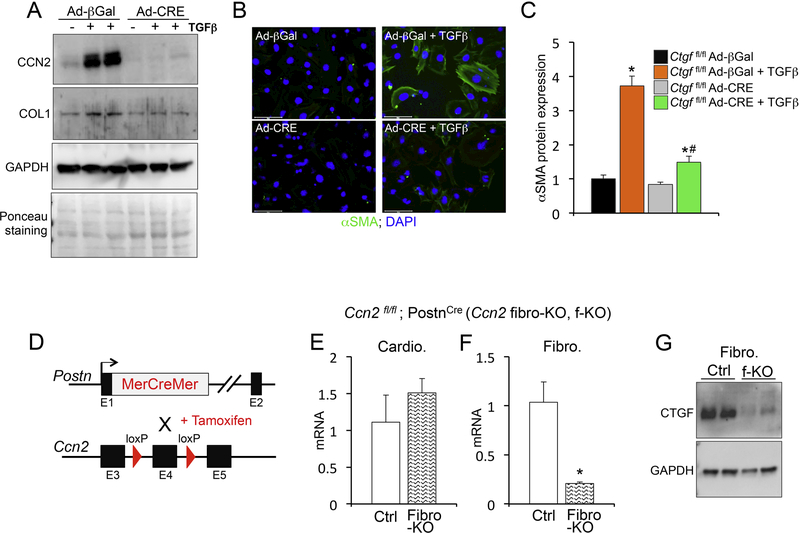
In cell culture systems CCN2 modulates fibroblast activity and differentiation into myofibroblasts, which are characterized by high level of alpha smooth muscle actin (α-Sma) and periostin (Postn).4,14 To test if CCN2 deletion in cultured fibroblasts affects myofibroblast activation, we generated Ccn2fl/fl mouse embryonic fibroblasts (MEFs) and utilized adenoviral-mediated cre expression to knock down CCN2. Using this isolated-cell system we induced myofibroblast activation with TGFβ1 treatment (one of the most potent in vitro fibroblast activators). CCN2 induction by TGFβ1 was abrogated in MEFs infected with adenoviral vectors encoding for cre recombinase [lacking CCN2] (Figure 3A). Concomitantly, we found that CCN2 deletion effectively inhibited TGFβ-induced collagen production (Figure 3A). In addition, activation of myofibroblasts by TGFβ, as measured by alpha smooth muscle (αSMA) expression and stress fiber formation, was significantly inhibited in the absence of CCN2 (Figure 3B and C). These results suggest that when CCN2 is deleted specifically from fibroblasts, the cells show a defective ability to become pro-fibrotic. Hence, we show that CCN2 is necessary for fibroblast activation and collagen production in vitro.
Figure 3 – Generation of myofibroblast-specific CCN2 loss of function mice.
(A) Western blot analysis for CCN2, collagen 1(COL1) and GAPDH loading control from protein extracts of mouse embryonic fibroblasts (MEFs) from Ccn2fl/fl mice infected with adenoviruses (Ad) expressing either beta-galactosidase (βGal) control or cre-recombinase (CRE) and treated with TGFβ for 24 hours. (B) Representative immunofluorescence images for alpha smooth muscle actin (αSMA) (green) and nuclei (DAPI; blue). Scale bar is 100μm. (C) Quantification of αSMA protein expression using ImageJ NIH software and represented relative to βGal baseline control. n≥ 400 cells / condition. (D) Schematic of mouse strategy to generate tamoxifen-inducible deletion of Ccn2 under the control of the periostin (Postn) genetic locus. (E, F) qPCR analysis for Ccn2 mRNA expression normalized to Rpl7 housekeeping gene in isolated cardiomyocytes (E) or isolated cardiac fibroblasts (F) from the indicated genotypes. n≥ 3 biological replicates / condition. (G) Western blot analysis for CCN2 and GAPDH loading control in isolated fibroblasts of the indicated genotypes. *p<0.05 vs. ctrl; #p<0.05 vs. Ad-βGal same treatment.
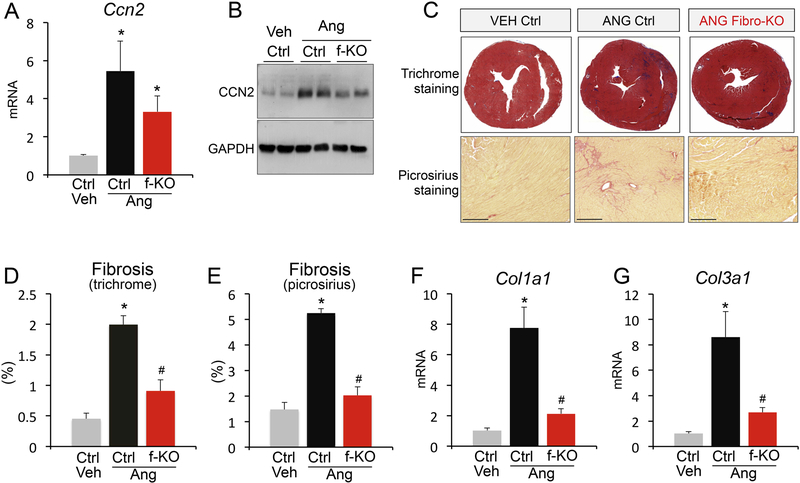
To test if this is true in vivo, we generated a myofibroblast-specific Ccn2 deletion mouse model, where Ccn2fl/fl mice were crossed to mice expressing tamoxifen-inducible cre (MerCreMer) from the genetic locus of periostin (Postncre) (Figure 3D). We call this mouse CCN2 fibroblast-KO or fibro-KO. Periostin is expressed in activated fibroblasts, i.e. myofibroblasts, in the injured heart and is therefore an effective marker to target pro-fibrotic fibroblasts.4,15 After isolating cardiomyocytes and primary cardiac fibroblasts treated with tamoxifen to induce cre expression, we confirmed fibroblast-specific deletion of CCN2 (Figure 3E-G). To analyze the contribution of fibroblast-derived CCN2 (Ccn2 fibro-KO; f-KO) to the development of cardiac fibrosis, we subjected Ccn2 fibroblast-KO and control mice to angiotensin II stimulation. We confirmed that cardiomyocytes are the main CCN2-expressing cell in injured hearts, as fibroblast-specific CCN2 deletion had only minor effects on CCN2 levels when total heart homogenates were used (Figure 4A and B). However, we found a remarkable reduction in cardiac fibrosis in Ccn2 fibroblast-KO mice by Masson’s trichrome and picrosirius analysis of histological heart sections (Figure 4C-E). In addition to histologically assessed fibrosis, expression of collagens I and III was dramatically reduced with angiotensin II stimulation in CCN2-null myofibroblasts, confirming an inhibition of fibrotic remodeling (Figure 4F and G). These data demonstrate that CCN2 is an autocrine factor in the heart and its function is dictated by its origin, whether cardiomyocyte or fibroblast. Our results highlight a previously unrecognized aspect of CCN2 biology and reveal cell-specific roles for this matricellular protein in the injured heart.
Figure 4 – Myofibroblast-derived CCN2 is essential for cardiac fibrosis.
(A) qPCR analysis for Ccn2 mRNA expression normalized to Rpl7 housekeeping gene in hearts from vehicle (veh) treated control (ctrl) mice or angiotensin II (Ang) stimulated control (ctrl) or Ccn2 fibroblast KO mice (f-KO). n≥ 3 biological replicates / condition. (B) Western blot for CCN2 and GAPDH loading control from cardiac protein extracts from the indicated genotypes and treatments. (C) Representative Masson’s trichrome-stained histological sections for fibrosis (upper panels; blue) and picrosirius staining (bottom panels; red) in control and Ccn2 fibroblast KO (fibro-KO) mouse hearts with or without angiotensin II infusion. Scale bar is 200μm. (D, E) Quantification of fibrosis from Masson’s trichrome-stained and picrosirius-stained histological sections of the indicated genotypes and treatments using ImageJ NIH software. n≥5 biological replicates / condition (F, G) qPCR analysis for collagen 1a1 (Col1a1) and collagen 3a1 (Col3a1) mRNA expression normalized to Rpl7 housekeeping gene in hearts from the indicated groups. n≥5 biological replicates / condition. *p<0.05 vs. veh ctrl; #p<0.05 vs. ctrl same treatment.
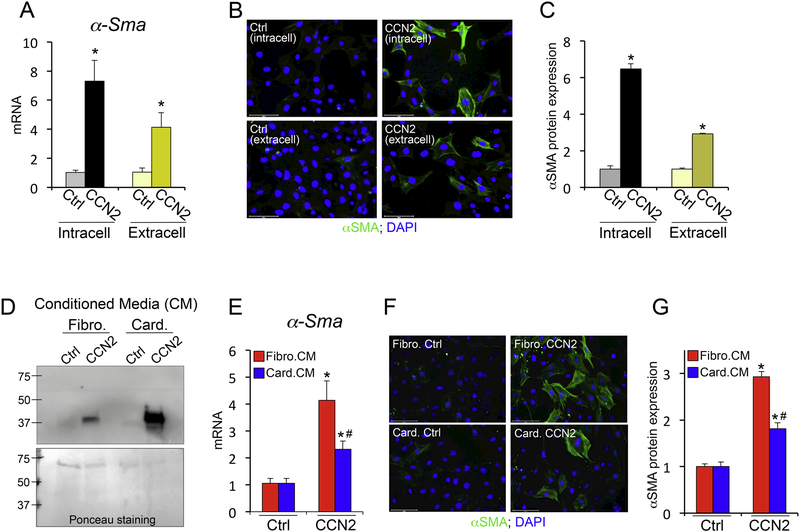
We reasoned that one possible explanation for our in vivo results showing that cardiomyocyte-derived CCN2 has no effect on modulating fibrosis could derive from the fact that CCN2 might exert intracellular pro-fibrotic roles when expressed by fibroblasts in addition to acting as extracellular factor. To answer this question we compared fibroblast activation following adenoviral-mediated overexpression of CCN2 (intracellular) and following extracellular stimulation with CCN2 as provided in the media. We found that both intracellularly-expressed and extracellularly-provided CCN2 were able to stimulate alpha smooth muscle expression in fibroblasts (Figure 5A-C). This result excluded the possibility of intracellular-derived pro-fibrotic effects that could justify a lack of effect from cardiomyocyte-derived CCN2. We then tested the hypothesis that cardiomyocyte-provided CCN2 is less potent in stimulating fibroblasts than fibroblast-provided CCN2. To do this we used adenoviruses encoding for CCN2 or beta galactosidase control and overexpressed these proteins in cultured cardiomyocytes and fibroblasts in serum free media. We then collected the media from these conditions and used secreted CCN2 to stimulate fibroblast activation. We found that both fibroblasts and cardiomyocytes efficiently secrete CCN2, but fibroblast-derived CCN2 more potently induced alpha smooth muscle expression in fibroblasts (Figure 5D-G). These results suggest that CCN2 reaches its full pro-fibrotic potential in an autocrine manner and corroborate our in vivo data demonstrating the cell-specificity of CCN2 effects.
Figure 5 – Differential potency of fibroblast- and cardiomyocyte-derived CCN2.
(A) qPCR analysis for α-Sma mRNA expression following intracellular CCN2 overexpression (intracell) or treatment with CCN2 exogenously provided in the media (extracell) on mouse embryonic fibroblasts (MEFs). Control (ctrl) is βGal overexpression or media from βGal overexpressing MEFs. n≥ 3 biological replicates / condition. (B) Representative immunofluorescence images for alpha smooth muscle actin (αSMA) (green) and nuclei (DAPI; blue). Scale bar is 100μm. (C) Quantification of αSMA protein expression using ImageJ NIH software and represented relative to control. n≥ 400 cells / condition. (D) Western blot for CCN2 presence in media derived from fibroblasts (fibro.) or cardiomyocytes (card.) treated with Ad-βGal (ctrl) or Ad-CCN2. Ponceau staining (bottom panel) indicates loading control. (E) qPCR analysis for α-Sma mRNA expression following stimulation of MEFs with conditioned media (CM) from fibroblasts (fibro.CM) or cardiomyocytes (card.CM). n≥ 3 biological replicates / condition. (F) Representative immunofluorescence images for alpha smooth muscle actin (αSMA) (green) and nuclei (DAPI; blue). Scale bar is 100μm. (G) Quantification of αSMA protein expression using ImageJ NIH software and represented relative to control. n≥400 cells / condition. *p<0.05 vs. ctrl; #p<0.05 vs. fibro.CM.
Materials and Methods
Mice generation and treatments.
The generation of Ccn2 loxP-targeted (fl) mice (Ccn2fl/fl) was previously described.16 Ccn2fl/fl mice were crossed with mice expressing cre recombinase under the control of the cardiac-specific β-MHC promoter to obtain heart-restricted deletion of Ccn2 (cardio-KO; cKO). Control mice for this group are Ccn2 +/+ β−MHCcre. Fibroblast-specific deletion of Ccn2 was obtained by crossing Ccn2fl/fl mice with periostin-dependent and tamoxifen-inducible cre expressing mice (Postncre)15 (fibro-KO; f-KO). Control mice used for this group are Ccn2 +/+ Postncre mice. For this latter model, tamoxifen was administered by intraperitoneal injection in control and experimental mice with pharmaceutical-grade tamoxifen dissolved in peanut oil for 5 consecutive days (100 mg/kg body weight), followed by maintenance on tamoxifen-citrate chow (400 mg/kg body weight, Harlan Laboratories) until the experiment was terminated. Infusion of angiotensin II (1.5 μg/kg/min) or vehicle control was performed by subcutaneous implantation of Alzet minipumps for 1 week (Durect Inc). 10–12 weeks-old males and females mice were used in this study. All experiments involving animals were approved by the Institutional Animal Care and Use Committee at The Ohio State University.
Cardiomyocyte and fibroblast isolation and treatments.
Adult cardiomyocytes were isolated in a modified manner as has been previously published.17 Briefly, whole hearts were excised in 37°C EDTA-containing buffers and flushed of blood. The aorta was clamped shut, and 4 mL of 37°C 4 mg/mL Worthington Collagenase dissolved in Tyrodes solution was injected directly into the apex of the heart. Perfusion in this manner was continued for 10 minutes. Following this, the ventricles were pulled into ~1mm3 pieces and cardiomyocytes were dissociated from the tissue for an additional 10 minutes. Following filtration, quenching of the reaction with Tyrodes+5%BGS, and cardiomyocyte sedimentation, the remaining suspension containing non-myocyte cells was plated in DMEM medium enriched with 10% bovine serum to obtain adherence and growth of cardiac fibroblasts. Mouse embryonic fibroblasts (MEFs) were isolated from day 12.5 to 13.5 embryos from Ccn2fl/fl mice. Adenoviral infections were achieved with 72 hours treatment with vectors encoding beta-galactosidase control (Ad-βGal) and 72 hours treatment with vectors encoding cre-recombinase (Ad-Cre). Treatments also included incubation with recombinant TGFβ1 (10ng/ml; R&D systems) or vehicle control, following Ad-βGal or Ad-Cre infection, for 24 hours.
Conditioned media (CM) experiments were performed as follows: Isolated neonatal rat cardiomyocytes (NRCMs) and MEFs were cultured in 12-well dishes. Cells were exposed to AdβGal or adenoviral CCN2 (Ad-CCN2) for 4 hours in serum-free media, after which time the media was changed to serum-free media for 48 hours. After 48 hours, the conditioned media was collected and transferred to MEFs plated in 12-well dishes for 48 hours. Following treatment, the cells were harvested for RNA extraction using Trizol or used for immunostaining (both detailed below).
Histological, mRNA and protein expression analysis.
Masson’s trichrome staining for fibrosis (blue) and picrosirius red staining were performed from histological sections generated from paraffin-embedded hearts. FITC-conjugated wheat germ agglutinin (Sigma-Aldrich; L-5266) was used to outline cardiomyocytes. RNA was extracted from ventricles or isolated cell populations using Trizol according to manufacturer’s instructions (Life Technologies). Reverse transcription was performed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Selected gene expression differences were analyzed by real-time qPCR using SYBR green (Biorad). Quantified mRNA expression was normalized to Rpl7 and expressed relative to controls. Primers used were: Ccn2 (5’-GGAAAACATTAAGAAGGG-3’; 5’-TGCACCATCTTTGGCAGTG-3’), Col1a1 (5’-GCTCCTCTTAGGGGCCACT-3’; 5’-ATTGGGGACCCTTAGGCCAT-3’), Col3a1 (5-AAATTCTGCCACCCCGAACT-3’; 5’-GCACCAGAATCTGTCCACCA-3’), α-Sma (5’-GGCATCCACGAAACCACCTA-3’; 5’-GTACTTGCGTTCTGGAGGGG-3’) and Rpl7 (5’-TGGAACCATGGAGGCTGT-3’; 5’-CACAGCGGGAACCTTTTTC-3’). Western blotting in conditioned media (CM) samples was performed by first concentrating the media by filter centrifugation and loading equal amounts of media from both cultured fibroblasts and cardiomyocytes. Membranes were transferred using the Bio-Rad Semi-Dry Transfer System, then probed overnight using Santa Cruz anti-CCN2 (1:250). Western blotting on cell and tissue lysates was performed using 20 μg of protein lysate and probed overnight using Santa Cruz anti-CCN2 (1:250), Fitzgerald anti-GAPDH (1:5000), and Rockland collagen type I antibodies (1:500). Immunofluorescence analysis of cultured fibroblasts was carried out as follows: cells were fixed for 20 minutes in 2% paraformaldehyde and stained using Sigma-Aldrich mouse anti-alpha smooth muscle actin, clone 1A4 (1:1000) overnight. Cells were then exposed to fluorescent secondary antibodies Molecular Probes Alexa Fluor donkey anti-mouse 488 (1:500) and DAPI (1:1000), and imaged on an Evos FL Auto 2 microscope (Thermo Fischer). For α-Sma quantifications, multiple 20X images of n≥3 wells / condition were taken and n≥400 cells / condition were quantified.
Statistics.
All results are presented as mean ± SEM. Statistical analysis was performed with unpaired 2-tailed t-test (for 2 groups) and 1-way ANOVA with Bonferroni correction (for groups of 3 or more). P values less than 0.05 were considered significant. Each experimental/control animal group have n≥5 mice. Each cell culture experiment has been performed in biological triplicates. For cell size measurements n=100 cell / heart were analyzed.
Discussion
CTGF, or CCN2, has been widely studied as a stress-response factor implicated in the fibrotic pathophysiology of a variety of organs.18 Most available reports have associated CCN2 expression with the development of tissue fibrosis, and the importance of this secreted factor for fibrotic remodeling has become more clear in recent years.11 In the heart, however, the fibrotic role of CCN2 is highly controversial. Work from our laboratory has shown that cardiomyocyte-restricted deletion of CCN2 has no effects on total cardiac remodeling, and an independent group has recently confirmed that when hearts lacking CCN2 are subjected to chronic pressure-overload stimulation, their cardiac function and levels of fibrosis do not differ from controls.6,13 These results are quite striking and unexpected, and have raised a number of questions focused on defining the role of CCN2 in the heart. CCN2 is strongly induced during cardiac injury and roles of CCN2 in cardiomyocyte hypertrophy and fibroblast activation have been reported.19–24 The confusion in the field, however, arises because the pro-fibrotic properties of CCN2 are complex and appear to be tissue- and insult- specific.
Taking advantage of a newly generated mouse line that allows for in vivo genetic manipulation specifically in activated fibroblasts, we now demonstrate that CCN2 is pro-fibrotic when expressed by fibroblasts, but not by cardiomyocytes. This provides evidence for an autocrine function of this secreted matricellular protein and could finally explain why transgenic overexpression of CCN2 from cardiomyocytes failed to induce cardiac fibrosis.6,19–22 CCN2 is a secreted factor that accumulates in the extracellular matrix (ECM) post-injury. In the heart, we have found that cardiomyocytes, not fibroblasts, are the main source of CCN2. This result makes our study even more striking, as although our genetic approach to delete CCN2 specifically in cardiomyocytes was more effective in reducing the overall CCN2 content in the injured heart, this model shows that cardiomyocyte-derived CCN2 does not modulate cardiac hypertrophy and fibrosis following angiotensin II treatment. This is in agreement with work published by us and others where overexpression of CCN2 in cardiomyocytes neither drives spontaneous fibrotic remodeling, nor influences the cardiac fibrotic response following injury.6,19–22 In contrast, in fibroblast cultures we were able to replicate the importance of CCN2 for fibroblast activation, myofibroblast transformation, and collagen deposition. Altogether, our results presented here indicate that CCN2 is an autocrine factor in the heart and affects fibroblast activation and fibrosis induction only when secreted by fibroblasts themselves.
Fibroblasts are the key cell type regulating ECM deposition and composition.4 Following cardiac injury, TGFβ1 activation or an increase in circulating angiotensin II levels are strong stimuli that perturb fibroblast quiescence and transform them into myofibroblasts.4 Myofibroblasts constitute the pro-fibrotic, periostin-positive, collagen-producing cells largely responsible for tissue fibrosis.25 In this study, we have utilized an injury model whereby infusing high doses of angiotensin II for one week allowed us to focus on the development of fibrosis without inducing cardiomyocyte dysfunction (as occurs during complex injury models such as chronic pressure overload or combination of multiple neurohumoral effectors). In addition, our use of a genetic approach where deletion of CCN2 is driven by the periostin locus provided us with a system where only stress-activated fibroblasts are depleted of CCN2. This system precludes the possibility of adaptations that can occur with global targeting approaches that affect cell homeostasis. Indeed, periostin is a secreted protein that marks activated fibroblasts and is not expressed at baseline in the heart.15
Because CCN2 is a secreted protein, the concept of cell-specific effects has been so far ignored. However, recent evidence suggests that even if they are secreted and active as extracellular factors, matricellular proteins can also exert intracellular functions.26 This is for example the case for thrombospodin proteins, which activate ER stress responses while they go through the secretory pathway.26,27 Although intracellular function of CCN2 could plausibly explain where the cell-specificity comes from, the experimental evidence provided here suggests that CCN2 can activate fibroblasts even if exogenously provided. We have also excluded the possibility that cardiomyocytes might not be as proficient in secreting CCN2, while also demonstrating that cardiomyocyte-secreted CCN2 is less potent in activating fibroblasts. These intriguing results might suggest cell-specific post-translational modifications in CCN2 that could alter its function. Although future work will be needed to address this point, our study highlights interesting new aspects of CCN2 biology in the heart.
Altogether, our in vivo genetic approach identifies the cell-specificity of CCN2 function in the heart and returns the attention to this protein for the treatment of cardiac fibrosis.
Supplementary Material
Highlights.
Cell-specific genetic manipulation of CCN2 in the heart revealed that cardiomyocyte-derived
CCN2 is dispensable for fibrotic remodeling
Fibroblast-restricted deletion of CCN2 demonstrated that fibroblast-derived CCN2 is essential
for cardiac fibrosis
CCN2 is required for fibroblast activation and functions as an autocrine factor
Acknowledgments
Sources of funding:
This work was supported by grants from the NIH R00HL 121284 and R01HL 136951 (to F.A.)
Non-standard abbreviations:
- CTGF
connective tissue growth factor
- POSTN
periostin
- MHC
myosin heavy chain
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kehat I & Molkentin JD Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 122, 2727–2735 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabbah HN, Sharov VG, Lesch M & Goldstein S Progression of heart failure: a role for interstitial fibrosis. Mol Cell Biochem 147, 29–34 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Manabe I, Shindo T & Nagai R Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res 91, 1103–1113 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Davis J & Molkentin JD Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70, 9–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leask A Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106, 1675–1680 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Accornero F, et al. Genetic Analysis of Connective Tissue Growth Factor as an Effector of Transforming Growth Factor beta Signaling and Cardiac Remodeling. Mol Cell Biol 35, 2154–2164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann MA, et al. TGF-beta1 affects cell-cell adhesion in the heart in an NCAM1-dependent mechanism. J Mol Cell Cardiol 112, 49–57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed MS, et al. Connective tissue growth factor--a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J Mol Cell Cardiol 36, 393–404 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Chen MM, Lam A, Abraham JA, Schreiner GF & Joly AH CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 32, 1805–1819 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Leask A & Abraham DJ All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 119, 4803–4810 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Ramazani Y, et al. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol (2018). [DOI] [PubMed] [Google Scholar]
- 12.Shi-wen X, et al. CCN2 is necessary for adhesive responses to transforming growth factor-beta1 in embryonic fibroblasts. J Biol Chem 281, 10715–10726 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Fontes MS, et al. CTGF knockout does not affect cardiac hypertrophy and fibrosis formation upon chronic pressure overload. J Mol Cell Cardiol 88, 82–90 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Kennedy L, et al. CCN2 is necessary for the function of mouse embryonic fibroblasts. Exp Cell Res 313, 952–964 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Kanisicak O, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 7, 12260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Shi-wen X, Abraham DJ & Leask A CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum 63, 239–246 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Ackers-Johnson M, et al. A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart. Circ Res 119, 909–920 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perbal B CCN proteins: multifunctional signalling regulators. Lancet 363, 62–64 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Ahmed MS, et al. Mechanisms of novel cardioprotective functions of CCN2/CTGF in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 300, H1291–1302 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Panek AN, et al. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS One 4, e6743 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravning J, et al. Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction. PLoS One 7, e52120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravning J, Ahmed MS, von Lueder TG, Edvardsen T & Attramadal H CCN2/CTGF attenuates myocardial hypertrophy and cardiac dysfunction upon chronic pressure-overload. Int J Cardiol 168, 2049–2056 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Wang X, et al. Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. Am J Physiol Cell Physiol 297, C1490–1500 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Yoon PO, et al. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol 49, 294–303 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Stempien-Otero A, Kim DH & Davis J Molecular networks underlying myofibroblast fate and fibrosis. J Mol Cell Cardiol 97, 153–161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch JM, et al. A thrombospondin-dependent pathway for a protective ER stress response. Cell 149, 1257–1268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanhoutte D, et al. Thrombospondin expression in myofibers stabilizes muscle membranes. Elife 5(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.