Abstract
Significance:
This is a report on the use of peripheral prisms (p-prisms) for patients with left neglect and homonymous visual field defects (HVFD).
Purpose:
To investigate if patients with left hemispatial neglect and HVFDs benefit from p-prisms to expand the visual field and improve obstacle detection.
Methods:
Patients (24 with HVFDs; 10 of whom had left-neglect) viewed an animated, virtual, shopping-mall corridor and reported if they would have collided with a human obstacle which appeared at various offsets up to 13.5° from their simulated walking path. There were 40 obstacle presentations on each side, with and without p-prisms. No training with p-prisms was provided and gaze was fixed at the center of expansion.
Results:
Detection on the side of the HVFD improved significantly with p-prisms in both groups from 26% to 92% in the left-neglect group and 43% to 98% in the non-neglect group (both p < 0.001). There was a tendency for greater improvement in the neglect patients with p-prisms. For collision judgments, both groups exhibited a large increase in perceived collisions on the side of the HVFD with the prisms (p < 0.001), with no difference between the groups (p = 0.93). Increased perceived collisions represent a wider perceived safety margin on the side of the HVFD.
Conclusions:
Within the controlled conditions of this simulated, collision-judgment task, patients with left neglect responded well to initial application of p-prisms exhibiting improved detection and wider safety margins on the side of the HVFD that did not differ from non-neglect patients. Further study of p-prisms for neglect patients in free-gaze conditions after extended wear and in real-world mobility tasks is clearly warranted.
Keywords: peripheral prisms, peli prisms, spatial neglect, unilateral spatial neglect, visual neglect, hemispatial neglect, mobility, stroke, homonymous, hemianopia
Homonymous visual field defects are loss of vision on the same side in each eye due to post-chiasmal visual pathway damage. Homonymous visual field defects are common after stroke occurring in 29%1 to 50%2 of cases, but also frequently occur after trauma, aneurysm rupture and with brain tumors.3–5 Homonymous visual field defects impair detection of hazards on the side of the visual field loss resulting in functional limitations.6–9 Peripheral prisms10 (p-prisms; also known as “EP prisms” or “Peli lens”; Chadwick Optical, Souderton, PA) (Figure 1) expand the binocular visual field of patients with homonymous visual field defects by shifting two sectors of the peripheral visual field from the affected hemifield of one eye into the intact hemifield.10, 11 The visual field expansion, which has also been referred to as field-of-view expansion,12, 13 can be up to 30° in the oblique 57Δ design and up to 40° in the horizontal 57Δ design.14 P-prisms have shown positive results in multiple open-label clinical studies10, 11, 15–17 and a randomized controlled clinical trial;18 however, patients with hemispatial neglect in addition to homonymous visual field defects were excluded from those studies. Our study begins to address this gap in the literature.
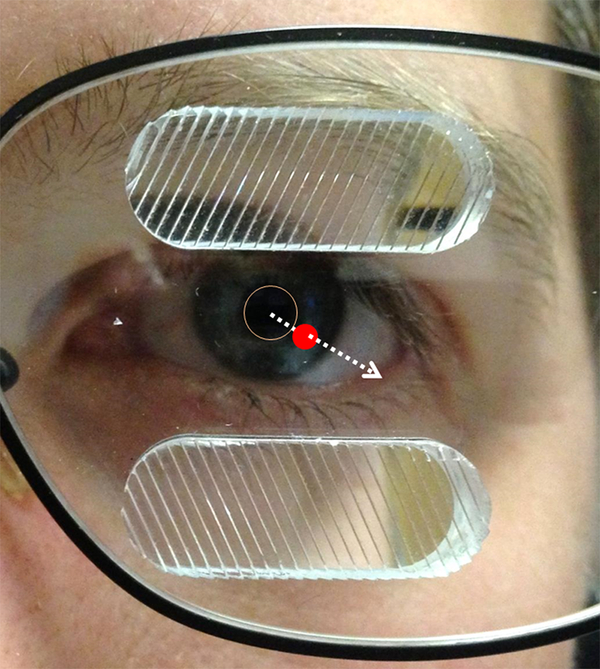
Figure 1.
Standard unilateral fitting of oblique 57Δ peripheral prisms (p-prisms) on the left eye for left homonymous visual field defect (HVFD) with the segments laterally centered on the visual axis in primary gaze (indicated by the dashed arrow). The red dot is the position of the visual axis at the spectacle plane (picture was taken from slightly off to the left and up from the photographer’s perspective). The p-prisms straddle the border of the HVFD such that half of the prism is within the intact (right) visual field when in primary position of gaze.
Hemispatial neglect is often defined as a failure to respond, report, or orient to sensory stimuli located on the side opposite the brain lesion, which causes functional disability and cannot be attributed to primary sensory or motor impairments.19 Left neglect is far more common than right neglect due to right cerebral hemisphere specialization for spatial functions20; therefore, we limited investigations to left neglect. Multiple studies have found that left neglect is associated with worse functional disability after stroke with longer hospitalizations, less independence, and more functional decline chronically post stroke.21–26 In terms of mobility, left neglect is associated with increased falls27 and further decline in mobility chronically after stroke.28
Left neglect and homonymous visual field defects, which often occur together, are likely to have a negative synergistic effect on detection of left side obstacles during mobility.29,30 Scanning to the left side is a compensation for an homonymous visual field defect that can be used to reduce the impact,9, 31 but leftward scanning is reduced in left neglect with longer and more frequent fixations to the right,32 which may limit the ability to compensate for a homonymous visual field defect. Consistent with this, patients with left neglect and homonymous visual field defects were found to have worse detection of left-sided obstacles in a walking-simulation collision judgment task when compared to patients with homonymous visual field defects but without neglect.30 Leftward cueing (frequently reminding the patient to look and move to the left) is the leading rehabilitation strategy for left neglect used by therapists on stroke and rehabilitation units,33 but it relies on the development of left-sided awareness and intact memory formation. P-prisms might be a helpful addition to standard approaches because they have the potential to utilize intact involuntary visual attentional systems in the right visual field (where p-prism images of left-sided obstacles appear).34, 35 Thus, patients with homonymous visual field defects plus left neglect may have a greater need for and benefit from a mobility aid such as peripheral prisms than patients with homonymous visual field defects without neglect. On the other hand, while patients with a homonymous visual field defect and neglect may benefit from peripheral prisms, it is also possible they will have a reduced ability to detect obstacles visible through the peripheral prisms because of problems with vigilance (ability to sustain arousal), and visual attention.34–38 Obstacles appearing in the peripheral prisms from the left visual field are multiplexed over the right visual field with reduced contrast and clarity. Those obstacles may be less salient and thus less likely to be detected by patients with left neglect.
This study was designed to determine if patients with left neglect and a homonymous visual field defect have the capacity to detect and make a judgment about the multiplexed images from the peripheral prisms, and whether that differs from patients with a homonymous visual field defect without neglect. Our primary hypothesis was that patients with homonymous visual field defects and left neglect using peripheral prisms would show improved detection but due to problems with vigilance and visual attention might show less improvement than patients with homonymous visual field defects without neglect. This hypothesis was tested in a realistic virtual shopping mall environment, using a paradigm similar to a prior study without peripheral prisms.30 We evaluated the ability of patients with left homonymous visual field defects and neglect to detect a life-size human obstacle with and without peripheral prisms immediately after prisms were first fitted compared to patients with homonymous visual field defects without neglect. By fixing gaze at a central cross in the direction of virtual walking, the confounding variable of compensatory scanning for improved detection was minimized.
P-prisms may also affect the perceived safety margin in collision judgments after obstacle detection. This safety margin is a “buffer” added to the internal representation of space that one occupies, and has been measured by asking study participants to judge whether a human obstacle would have caused a collision.30,39 This safety margin was found to be smaller on the neglected left side than the right in patients with left neglect and homonymous visual field defects.30 With peripheral prisms, the safety margin might be larger because of improved detection and due to the prismatic shift causing images of obstacles in the affected field to appear closer to the walking path. Based on pilot data (Houston KE, et al. OVS 2015;92:E-abstract 155256), we hypothesized that there would be more perceived collisions for prism-side obstacles in both HFVD groups due to improved detection and because of the apparent displacement of the obstacle toward the walking path as seen through the peripheral prisms.
METHODS
The study was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from the participants after explanation of the nature and possible consequences of the study. The protocol was approved by the institutional review board of Massachusetts Eye and Ear.
Participants
Patients with complete homonymous visual field defects were targeted for recruitment; however, incomplete homonymous visual field defects and quadranopia were allowed so long as there were visual field defects in the hemi-central 30° which may have obscured detection of obstacles when walking, and which would have been accessible via the peripheral prisms. Other inclusion criteria were age greater than 14 (youngest age approved by our IRB) and best-corrected visual acuity of 20/50 or better in either eye. Patients were not excluded based on time since onset.
Twenty four patients with homonymous visual field defects were screened, enrolled, and completed the study (Table 1). Patients were referred from other studies or through a database search of prior research participants at the Schepens Eye Research Institute Mobility Enhancement and Vision Rehabilitation Center of Excellence, or from the authors’ clinics. As such, recruitment was well targeted and all patients screened were eligible. The study required at minimum, one visit, and none of the patients who enrolled dropped out or were unable to complete the study visit. Most patients (n = 21) attended 2 visits related to this study: A screening and intake visit which included ordering of permanent p-prism glasses, and a second visit consisting of data collection in the virtual shopping mall. Three patients were only able or willing to attend 1 visit, and data collection was done on the same day as the intake using temporary press-on peripheral prisms applied at that visit.
Table 1.
Patient Characteristics.
| HVFD (non-neglect) | Left Neglect (and Left HVFD) | Tests for between group differences† | |
|---|---|---|---|
| Total, n | 14 | 10 | |
| Right HVFD | 9 | 0 | |
| Incomplete HVFD | 6 | 0 | |
| Female, n (%) | 50% | 30% | p = 0.330 |
| Age, years, median (range) | 53 (22 to 86) | 64 (24 to 84) | p = 0.62 |
| Years since onset, median (range) | 5 (0.1 to 17) | 5 (0.1 to 22) | p = 0.38 |
| MMSE‡, median (range) | 29 (24 to 30) | 28 (13 to 30) | p = 0.59 |
HVFD = homonymous visual field defect
Wilcoxon Rank-Sum Test except gender (Fisher exact test)
Mini-Mental State Exam (maximum score = 30); non-neglect (n = 13), left neglect (n = 10); data missing for one participant.
Left neglect was diagnosed when at least one of the following criteria were met (see Table 2): (1) Positive Schenkenberg Line Bisection Test defined as rightward mean deviation > 10%40: (2) Positive Bells Cancellation Test41 defined as ≥ 4 omissions more on the left compared to the right side of the test sheet; (3) Positive report of symptoms of left neglect in both of two questions from the Catherine Bergego Scale42 (“Experience difficulty adjusting left sleeve or slipper?” and “Experience difficulty in finding your way toward the left?”). These questions captured neglect behaviors in personal and extra-personal space not evaluated by paper and pencil tests (Line Bisection and Bells); (4) History of left neglect diagnosis either reported in the study intake history or found on a review of the patient’s medical record. Patients with history of left neglect, even without other signs of neglect in our examinations, were included in the neglect group because a prior study found they behaved like the neglect patients on the collision judgment task used in this study.30 The author who supervised study intake (KH) provided a clinical impression of neglect severity based on the diagnostic tests and other behaviors observed during testing. This type of severity grading is commonly performed in clinical practice related to documentation guidelines set forth by the U.S. Center for Medicare Services.43 The clinical impression is reported in Table 2. The 14 patients classified as non-neglect did not meet any of the neglect criteria. All 10 patients classified as having left neglect met at least one of the four criteria and most met multiple criteria (see Table 2).
Table 2.
Diagnostic Testing and Other Characteristics of the Left Neglect Group.
| Patient | Time Since Onset (yrs) | Bells Test Omissions (R – L) | Line Bisection Error Mean % Deviation‡ | CBS† Trouble Adjust Left Sleeve | CBS† Trouble Finding Way to Left | History of Neglect | Number of Diagnostic Criteria Met | Clinical Impression of Neglect Severity |
|---|---|---|---|---|---|---|---|---|
| HSN1 | 8 | 5 | −12.0 | Yes | Yes | Yes | 3 | moderate |
| HSN2 | 6 | 0 | −9.0 | No | Yes | Yes | 2 | moderate |
| HSN3 | 5 | 0 | −8.8 | No | No | Yes | 1 | mild |
| HSN4 | 17 | −4 | 0.5 | No | No | Yes | 2 | moderate |
| HSN5 | 0.3 | −11 | 50.2 | No | No | Yes | 3 | severe |
| HSN6 | 7 | −2 | −21 | No | No | Yes | 2 | mild |
| HSN7 | 5 | 3 | −9.5 | Yes | Yes | Yes | 3 | mild |
| HSN8 | 2 | −16 | 62.5 | Yes | Yes | Yes | 5 | severe |
| HSN9 | 1 | −10 | 15.4 | Yes | Yes | Yes | 5 | severe |
| HSN10 | 0.08 | −7 | 26 | No | Yes | No | 3 | severe |
CBS = Catherine Bergego Scale (neglect symptom scale)
positive values = rightward line bisection error, negative values = leftward line bisection error.
Bold values are positive for left neglect. Patients in the non-neglect group did not meet any criteria.
Fitting of peripheral prisms was done by study staff using standard methods recommended by the manufacturer (Chadwick Optical, Souderton, PA). None of the patients reported using peripheral prisms previously. One patient without neglect had superior right quadranopia and was tested with only the upper p-prism. The fixation cross was adjusted downwards (approximately 15°) until the human obstacle in the virtual mall was not visible to the patient (being in the area of field loss) for all trials, with and without the peripheral prisms. As we had previously found no difference among patients without neglect in the obstacle detection and collision judgment tasks in the virtual mall between right homonymous visual field defects and left homonymous visual field defects,30 we included patients with either side of hemianopia if they had no neglect (9 of 14 had right homonymous visual field defects). However, patients with neglect all had left sided neglect and homonymous visual field defects. Cognitive status was evaluated using the Mini-Mental State Exam.44 At the study intake visit the patients had permanent 57Δ oblique p-prism glasses ordered (Chadwick Optical, Souderton PA) and performed the virtual mall task once the glasses arrived a few weeks later. The 3 patients without neglect who could only attend for 1 visit were fitted with 40Δ press on peripheral prisms (Chadwick Optical, Souderton PA), as no press-on version of a 57Δ prism exists. Two of the patients were tested with oblique 40Δ press-on peripheral prisms, and one was tested with horizontal 40Δ press-on peripheral prisms. They performed the virtual mall task on the same day as the study intake.
Visual Field Expansion
The visual field expansion with peripheral prisms on binocular Goldmann perimetry was expected to be ~30° when tested with 57Δ oblique and 18° and 15° when tested with 40Δ horizontal and oblique peripheral prisms, respectively.14 Visual field expansion was consistent with the prism diopter value for all but two patients: One each with and without neglect (both were tested with the 57Δ oblique). Instead of the expected 30°, the patient with left neglect (HSN 8) only achieved 18° and the patient without neglect achieved 22°. Visual field expansion for all patients was also confirmed in the virtual-mall environment prior to data collection.
Collision Judgment Task
The virtual shopping mall collision judgment task has been described in detail previously.30, 39 To summarize, patients sat with their head in a chin-and-head rest 100 cm from a wide (170 cm by 120 cm) rear-projection screen (visual angle 81° by 62°), displaying a virtual model of a shopping-mall hallway (Figure 2). The program simulated walking motion along a straight path at 1.5 m/s generating optic flow similar to that experienced during actual walking. During each trial the patient maintained fixation on a target (a cross) positioned in the hallway at the center of expansion in the optic flow field. By fixing gaze at a central cross in the direction of virtual walking, the potentially confounding variable of increased scanning, that may improve detection, was minimized. Fixation was monitored by a second experimenter standing near the display who provided frequent reminders to continue looking at the fixation cross. If a fixation loss occurred during the obstacle appearance, the trial was repeated. The walking path was always straight but the angle relative to the mall corridor center varied such that the patient could be walking down the center of the mall corridor or angled toward stores on the left or right. While “walking”, a single realistically-sized human obstacle (0.7 m (8°) wide x 0.7 m deep x 2 m tall (23°)) suddenly appeared at offsets from the walking path/fixation point ranging from 0° (directly in the walking path) up to 13.5° of visual angle to the left and right, measured from the inside edge of the obstacle. Offsets were balanced on each side and stimulus locations were presented in a pre-determined, random order. The obstacle was stationary in the optic flow field (and so moved on the screen) appearing at a virtual distance of 5 m remaining visible for 1 s, and disappearing 3.5 m from the patient. The simulated motion (“walking”) then stopped and the trial ended. Patients were instructed to imagine walking down the mall corridor and to indicate, after the trial had ended, if they would have made any contact with the human obstacle if the walking had continued. Response options were “collision”, “no collision”, or “nothing” if no obstacle was present/seen. Catch trials where no obstacle appeared were randomly inserted at a rate of 9% (8 catch trials/per 88 trial session).
Figure 2.
Virtual shopping mall experimental set-up photographed from above. Patients were seated with head fixed in a head-chin rest and eyes fixed on a central cross. Optic flow was generated to simulate walking and then a single realistically-sized human obstacle (0.7 m (8°) wide x 0.7 m deep x 2 m tall (23°) (as shown here on the left) would suddenly appear and then disappear after 1s, at which time the simulated walking would stop and the patient would be asked for a response of “collision”, “no collision”, or “no obstacle” if no human obstacle was seen. The human obstacle appeared at offsets from the walking path/fixation point ranging from 0° (directly in the walking path) up to 13.5° of visual angle to the left and right, measured from the inside edge of the obstacle. There were 88 trials (40 on each side and 8 catch trials where no obstacle appeared).
A 20 trial practice session was conducted to familiarize the patient with the task and lens condition (without or with p-prism), followed by the data collection session which consisted of a total of 88 trials: 8 catch plus 80 with the obstacle presentation, 40 obstacles per side. The experiment consisted of a session without peripheral prisms and a session with peripheral prisms on the same day, with the order being balanced such that half the patients in each group performed the p-prism condition first and half performed the no p-prism condition first. No explanation of the peripheral prisms was given other than the knowledge that they were designed to improve the visual field and instructions to always look between the prism segments at the fixation cross throughout the experiment. The experiment was performed before the patients had any experience of wearing the glasses in their habitual environments. An experimenter positioned the patient in the chin-and-head rest so that gaze was between the prism segments when fixating on the target, and then confirmed field expansion using a perimetry test similar to a tangent screen,45 with a stationary version of the shopping mall used as the background. Most patients were tested with permanent 57Δ oblique peripheral prisms which provided approximately 30° (W) x 20° (H) of field expansion. Three had lower power (40Δ) peripheral prisms (15 to 18°) and two had unexplained lower than expected visual field expansion with the 57Δ, as described above. All p-prism designs used would be expected to expand the field sufficiently to detect even the most eccentric obstacle in the mall, which appeared at 13.5° eccentricity. Therefore at least part of the obstacle would have been visible in the peripheral prisms for even the most eccentric obstacles. We expected that detection of obstacles on the side of the homonymous visual field defect when not wearing prisms would be consistent with the Goldmann visual field perimetry (e.g. patients would show detection up to 5° eccentricity if the patient had 5° of macular sparing on perimetry), whereas with peripheral prisms detection would improve to 100%, regardless of eccentricity. For collision judgments we expected most obstacles detected in the peripheral prisms would be perceived as being on a collision course, since they were not allowed to look over and so could only see the shifted prism image which appeared nearer to the walking path.
Patients exhibiting greater than 50% detection on the side of the visual field defect without peripheral prisms in the virtual mall were classified as having incomplete homonymous visual field defects. Some of these patients measured as having a complete homonymous visual field defect on Goldmann Perimetry (V4e) but retained some perception of form in the impaired field, enough to detect and make collision judgments in the mall. Others had areas of intact field where part of the obstacle was visible on some trials. The analyses were performed both with and without the incomplete homonymous visual field defect group, which was comprised of 6 patients, all in the non-neglect group.
Statistical Analyses
Mixed-effects logistic regression analyses were performed for detection and collision responses using trials that included a stimulus. For detection, only the defect-side trials were analyzed because seeing-side performance was at ceiling (almost 100%). For the collision responses, both seeing and homonymous visual field defect side trials were included and detection failures were coded as “no collision”, with the rationale that a failed detection was equivalent to the perception of “no collision” in the participant’s information for collision avoidance. Responses of patients with right and left homonymous visual field defects without neglect (non-neglect) were not statistically different for detection (z = 1.22, p = 0.22) or collisions (z = 1.02, p = 0.31), and so their data were combined and this non-neglect homonymous visual field defect group was then compared to the left-neglect group. Variables included p-prism (binary), neglect status (binary), side of the obstacle (homonymous visual field defect or seeing side; collision responses only), an interaction term between p-prism and neglect status, and covariates age, MMSE score,44 gender, and duration of vision loss. Subject was included as a random factor. Offset of the obstacle from the virtual walking path was also included as a categorical random factor to control for its expected negative but non-linear relationship with detection and perceived collision response. Age was included as a covariate because it had previously been shown to be a negative predictor for the success of rehabilitation.46 Duration was included because it has been shown to be associated with changes in performance on spatial tasks in patients with homonymous visual field defect and neglect.47 MMSE score was included because those with a lower score may have more difficulty understanding the concept of the p-prism glasses and the shifted image or the task itself, and so may have worse responses. Gender may have an effect on collision judgments. The impact of neglect severity on detection, measured using the line bisection and Bells test scores were included as covariates because they have been previously related to detection failures for objects on the left side during mobility.48,49
All statistical analyses were performed with STATA/IC 14.2 (College Station, TX). As multiple analyses were conducted, α ≤ 0.01 was taken to indicate statistical significance. Since the sample sizes were small, we also note marginal significances (tendency), where 0.01 < α ≤ 0.10 to mitigate the risk of rejecting a true effect. When reporting proportions of detections or collision responses, we provide the average proportion for the group or condition.
RESULTS
Obstacle Detections
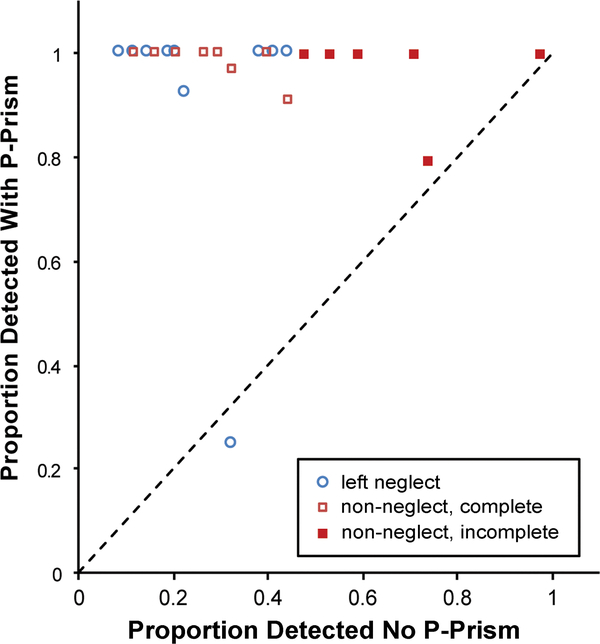
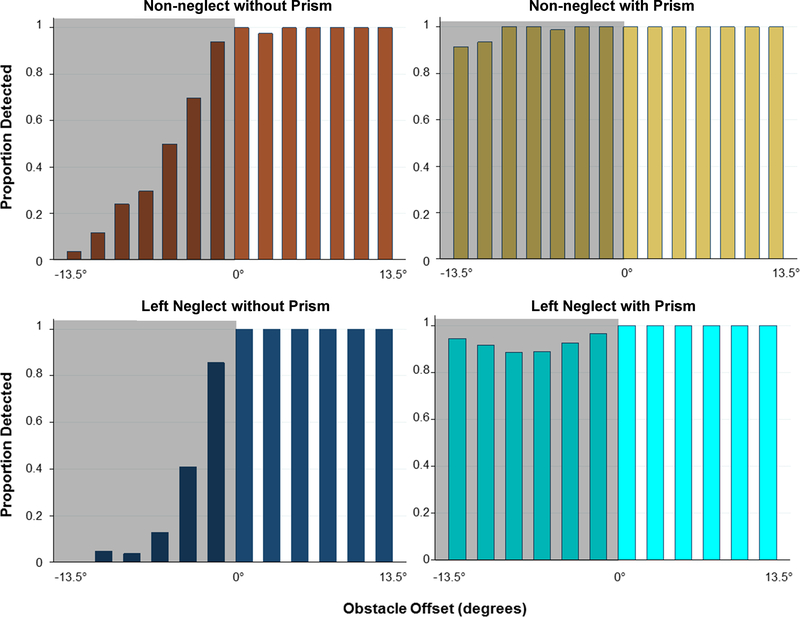
There were only 0.2% false positive (detections reported in catch trials) and 0.1% false negative (detection failures on seeing side) responses. As expected, seeing-side detection was near perfect at 100% in the non-neglect and 99.8% in the left-neglect group. Individual side obstacle detection rates with and without peripheral prisms are shown by scatter plot in Figure 3 and group data are presented in Figure 4. Without peripheral prisms there was some detection of stimuli on the homonymous visual field defect side in both groups, which either resulted from incomplete vision loss or failure to hold fixation steady. Data for all 24 patients were entered into a mixed-effects logistic regression for analyses of detection. Without peripheral prisms, there was no difference in homonymous visual field defect side detection between the left-neglect and the non-neglect groups (z = 0.29, p = 0.77), as there were large between-patient differences in response rates and substantial overlap of the distributions of the two groups (Figure 3; x-axis). With peripheral prisms, homonymous visual field defect side detection improved significantly in both groups, with the left-neglect group improving from 26% (90/352) to 92% (317/344; z = 13.2, p < 0.001) and the non-neglect group improving from 43% (229/535) to 98% (524/535; z = 12.2, p < 0.001). There was a tendency for improvement with peripheral prisms to be higher in the neglect group (z = 1.77, p = 0.08). There were no effects of the covariates gender, age, or Line Bisection or Bells test performance on detection rates (z < 0.73, p > 0.47). When considering all participants both with and without peripheral prisms, there was a tendency for higher MMSE scores (z = 1.93, p = 0.05) and a longer duration of homonymous visual field defect (z = 2.14, p=0.03) to be associated with better detection. A post-hoc analysis that included magnitude of visual-field expansion (degrees on Goldmann perimetry) as a covariate confirmed that magnitude of expansion, which was less for some patients for reasons described in the methods section, was not related to detections (z = 1.21, p = 0.23). When patients with incomplete hemianopia (n = 6, all in non-neglect group) were excluded from the analysis, the homonymous visual field defect-side detection of the non-neglect group without peripheral prisms was 26% and with prisms was 99%. The effect of the peripheral prisms on detection was statistically greater in the non-neglect group (z = 2.91, p = 0.004), but other results were comparable. However, the association with duration of homonymous visual field defect was not present when only considering the participants with complete hemianopia (n = 18; z = 1.21, p = 0.23).
Figure 3.
Scatter plot of detection for each patient on the side with homonymous visual field defect (HVFD) without (x-axis) and with (y-axis) peripheral prisms (p-prisms). “Non” refers to the non-neglect group for those with complete HVFD and incomplete HVFD. One patient with left neglect and one without neglect did not improve substantively (data points close to the unity line).
Figure 4.
Average proportion detected by obstacle offset without and with peripheral prisms (p-prisms) for the non-neglect (n=14) and left-neglect (n = 10) groups. The shaded area with negative x-axis values represents the homonymous visual field defect (HVFD) side. Gaze was fixed throughout the testing. Without p-prisms there was equally poor HVFD side detection in the non-neglect and left neglect groups (p = 0.77) and both groups improved significantly with p-prisms (p < 0.001). There was a tendency for the improvement in HVFD side detection with the p-prisms to be higher in the neglect group (p = 0.08).
Collision Safety Margin Judgment
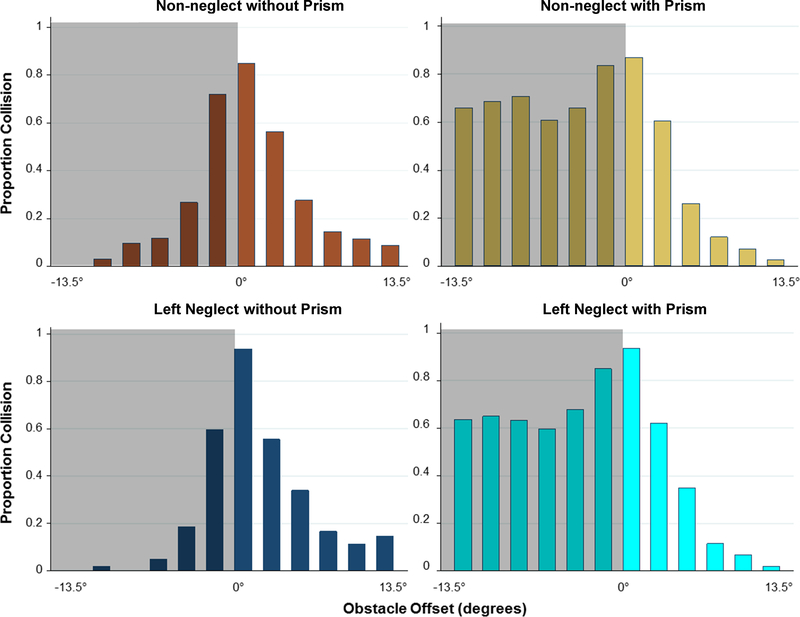
Data for collision judgments are shown in Figure 5. Data for all 24 patients were entered into a new mixed-effects logistic regression for analyses of collision responses. The seeing side response for both groups shows expected normal behavior (decreasing collision responses with increasing obstacle eccentricity) to which the homonymous visual field defect side was compared. The image shift from the peripheral prisms increased the number of perceived collisions on the homonymous visual field defect side in both groups (non-neglect from 22% (119/535) to 70% (373/535), z = 12.16, p < 0.001; left-neglect from 15% (53/352) to 68% (233/344), z = 11.04, p < 0.001) and was not different between the groups (z = 0.13, p = 0.90). There was an increased likelihood of reporting a collision with increasing time since onset of homonymous visual field defect (z = 3.67, p < 0.001) and a tendency if female (z = 2.01, p = 0.05). There were no effects of the other covariates age, Line Bisection, or Bells Test performance on perceived collisions (all z < 1.39, p > 0.16). There was no effect of peripheral prisms on the seeing side collision judgments of the non-neglect group (34% (184/535) without peripheral prisms, 33% (178/536), z = 0.75, p = 0.45) or the neglect group (38% (132/352) without, 36% (123/343), z = 0.12, p = 0.91). A post-hoc analysis adding magnitude of visual field expansion (extent of field in degrees on Goldmann perimetry) as a covariate found that the expansion was not related to collision responses (z = 1.28, p = 0.20).
Figure 5.
Average collision judgments by offset of the obstacle from the walking path without and with peripheral prisms (p-prisms) for the non-neglect and left-neglect groups. On the affected (homonymous visual field defect (HVFD)) side (negative x-axis values), p-prisms improved (p < 0.001) but exaggerated the safety margin an equal amount in left-neglect and non-neglect groups, p = 0.90 (i.e. patients report most obstacles detected via the p-prisms as an impending collision).
DISCUSSION
Our primary hypothesis, that patients with left neglect would show improved detection with peripheral prisms, was supported by our findings, and they did show slightly less improvement than patients without neglect when excluding non-neglect patients with incomplete homonymous visual field defect. This difference was mainly due to one patient with left neglect who demonstrated little to no improvement with the peripheral prisms, but all others improved at or very near to 100% detection (Figure 3). Improvements in detection did not require acclimation or training– patients in this study were tested upon first application of the peripheral prisms after only a few minutes of wear without any explanation other than that they would expand the view on the affected side. These findings of essentially normalized detection in 9 of 10 patients with left homonymous visual field defect and left neglect without any training are encouraging first findings for treatment with peripheral prisms. The ability to benefit from peripheral prisms, which shift the image of left side obstacles to the right side, is consistent with the previous reports of relatively intact involuntary (exogenous) orienting of attention to stimuli in the right visual field - where the images of left side obstacles appear when wearing the peripheral prisms.34,35 P-prisms therefore appear to be able to take advantage of the intact right field attentional systems for automatic detection of left side obstacles, at least in the relatively simple mobility situation used. Interestingly, prior studies in patients with left neglect but without homonymous visual field defect found the left field, when intact, still had severely impaired involuntary orienting;34, 35 therefore, it is possible peripheral prisms could be useful for left neglect even if the left visual field is intact or only partially affected (all but one patient with neglect in this study had complete homonymous visual field defect). This might be considered for future research.
The second hypothesis, that peripheral prisms would increase perceived collisions on the homonymous visual field defect-side and be similar in patients with and without left neglect, was also supported by our findings. An increased collision response was expected for both groups because the prism image appeared as though closer to the walking path than it actually was and patients did not see the true location of the obstacle on most trials (eyes were fixed straight ahead and obstacles were in the impaired field). A prior study found that patients with complete homonymous visual field defects without neglect who wore peripheral prisms for nine weeks continued to show a similar misinterpretation of visual direction for stimuli only visible in the peripheral prisms, yet, despite the misinterpretation, they reported improved mobility on a survey.15 This may have been because in their natural environments these patients were making appropriate gaze shifts to ascertain the actual location of obstacles after detection via the peripheral prisms,15 as instructed at the time of fitting. While detection of the prism image relies on involuntary mechanisms, this gaze shift after detection is likely an endogenous (voluntary, goal-directed) orienting of attention (i.e. the p-prism image is a cue to the patient to look left). Endogenous orienting was found to be partially impaired in patients with left neglect in a prior study not involving peripheral prisms when a visual cue to look to the left was provided in the central visual field.34, 35 Failure to shift gaze to the veridical location of the obstacle after detection in the peripheral prisms (as we instruct p-prism wearers) may lead to unnecessary or over-exaggerated avoidance maneuvers, and warrants investigation.
While improvements with peripheral prisms in our study paradigm are encouraging, there are limitations to acknowledge. The present study did not include extended wearing periods and mobility behaviors were not measured during actual walking so it remains unknown how avoidance maneuvers by neglect patients in response to the information from the peripheral prisms may differ from those without neglect. Another limitation are unavoidable effects of the study task on vigilance (ability to sustain arousal), which is frequently reduced in patients with left neglect.37,50 Vigilance was likely artificially elevated due to the frequent appearance of obstacles and regular reminders of the task by the experimenters. Thus, our experiment may not represent the detection or collision-decision performance of people with left neglect in real-world settings when obstacles are usually encountered infrequently and less regularly, and when they are free to look around naturally. The head was stabilized and gaze was fixed because the purpose of this study was to determine whether neglect patients can detect and make a collision judgment using multiplexed images from the peripheral prisms. This is not a natural situation but it did control for changes in scanning behavior and potentially confounding postural abnormalities common in left neglect which may reduce the efficacy of the peripheral prisms. For example, Prévost’s sign, a rightward eye and head deviation after stroke in patients with left neglect, is often severe initially, largely recovers over the course of a year,51,52 and would result in the peripheral prisms being decentered from the visual axis toward the blind field lessening the field expansion.
The sample size of patients with left neglect was only ten, and while sufficiently powered to detect changes in our primary outcomes, any covariate analysis should be cautiously considered. The main purpose of including covariates (age, duration, MMSE, gender, Bell’s and Line Bisection scores) was to reduce the effect of uncontrolled (known) variables in the analyses and was not to determine their significance as predictors of treatment success/failure. The covariate outcomes could be considered preliminary for planning future studies. Another limitation is that patients and experimenters were not masked to intervention – they knew they were wearing a device to expand the field and no sham device was used to control for placebo effect. However, changes in collision judgments were consistent with the prism image displacement suggesting detection was indeed from the peripheral prisms and not the result of increased effort to utilize residual field, as might be caused by placebo effect or bias. We also acknowledge the heterogeneity of our neglect sample. Neglect is a combination of symptoms representing biased spatial functions such that any population studied has some heterogeneity.53 For example, in the present study neglect patients 4 & 6 exhibited left side omissions on the Bells test but had normal line bisection (Table 2) and patient 10 had severe deficits in peripersonal space (paper and pencil tests) and was symptomatic with mobility (finding way to the left), but did not have symptoms in personal space (adjusting left sleeve or slipper). Symptom dissociation is well known in left neglect and is thought to represent different subtypes of the syndrome (e.g. personal, peripersonal, allocentric vs. egocentric, motor-intentional vs. representational neglect).54 Our sample was not large enough to evaluate responses by neglect subtype or severity, but is a potential consideration for future research.
CONCLUSIONS
In patients with homonymous visual field defects and left neglect, initial response to peripheral prisms was excellent in the controlled conditions of the virtual mall collision judgment task under steady central fixation. Patients could detect via the peripheral prisms an obstacle posing a collision threat in a relatively simple mobility situation. Our results suggest that peripheral prisms may be helpful in clinical care of patients with left hemianopia plus left neglect. However, further study is needed to evaluate what happens with free gaze and after detection, and the potential benefits in everyday life.
ACKNOWLEDGMENTS
We would like to thank Robert Goldstein for assistance with data consolidation and design and maintenance of the virtual mall simulator, Qu Tang for making modifications to the data collection software, Sarah Sheldon for data processing software, Jeffrey Churchill, Jean-Paul Wiegand, Azma Rehman, and Rui Liu for their assistance with data collection and processing, and Doris Apfelbaum and Amy Doherty for their assistance with scheduling and coordination.
Funded in part by the National Institutes of Health grants K12EY016335 (KH) and P30EY003790.
REFERENCES
- 1.Townend BS, Sturm JW, Petsoglou C, et al. Perimetric Homonymous Visual Field Loss Post-Stroke. J Clin Neurosci 2007;14:754–6. [DOI] [PubMed] [Google Scholar]
- 2.Rowe F, Brand D, Jackson CA, et al. Visual Impairment Following Stroke: Do Stroke Patients Require Vision Assessment? Age Ageing 2009;38:188–93. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Kedar S, Lynn MJ, et al. Homonymous Hemianopias: Clinical-Anatomic Correlations in 904 Cases. Neurology 2006;66:906–10. [DOI] [PubMed] [Google Scholar]
- 4.Schueller P, Micke O, Palkovic S, et al. 12 Years’ Experience with Intraoperative Radiotherapy (IORT) of Malignant Gliomas. Strahlenther Onkol 2005;181:500–6. [DOI] [PubMed] [Google Scholar]
- 5.Kupersmith MJ, Vargas ME, Yashar A, et al. Occipital Arteriovenous Malformations: Visual Disturbances and Presentation. Neurology 1996;46:953–7. [DOI] [PubMed] [Google Scholar]
- 6.Bowers AR, Mandel AJ, Goldstein RB, Peli E. Driving with Hemianopia: I. Detection Performance in a Driving Simulator. Invest Ophthalmol Vis Sci 2009;50:5137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberti CF, Peli E, Bowers A. Driving with Hemianopia: Iii. Detection of Stationary and Approaching Pedestrians in a Simulator Invest Ophthalmol Vis Sci 2014;55:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papageorgiou E, Hardiess G, Ackermann H, et al. Collision Avoidance in Persons with Homonymous Visual Field Defects under Virtual Reality Conditions. Vision Res 2012;52:20–30. [DOI] [PubMed] [Google Scholar]
- 9.Bahnemann M, Hamel J, De Beukelaer S, et al. Compensatory Eye and Head Movements of Patients with Homonymous Hemianopia in the Naturalistic Setting of a Driving Simulation. J Neurol 2015;262:316–25. [DOI] [PubMed] [Google Scholar]
- 10.Peli E Field Expansion for Homonymous Hemianopia by Optically-Induced Peripheral Exotropia. Optom Vis Sci 2000;77:453–64. [DOI] [PubMed] [Google Scholar]
- 11.Bowers AR, Keeney K, Peli E. Community-Based Trial of a Peripheral Prism Visual Field Expansion Device for Hemianopia. Arch Ophthalmol 2008;126:657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apfelbaum H, Peli E. Tunnel Vision Prismatic Field Expansion: Challenges and Requirements. Transl Vis Sci Technol 2015;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peli E, Jung JH. Multiplexing Prisms for Field Expansion. Optom Vis Sci 2017;94:817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung J-H, Peli E. Impact of High Power and Angle of Incidence on Prism Corrections for Visual Field Loss. Opt Eng 2014;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giorgi RG, Woods RL, Peli E. Clinical and Laboratory Evaluation of Peripheral Prism Glasses for Hemianopia. Optom Vis Sci 2009;86:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill EC, Connell PP, O’Connor JC, et al. Prism Therapy and Visual Rehabilitation in Homonymous Visual Field Loss. Optom Vis Sci 2011;88:263–8. [DOI] [PubMed] [Google Scholar]
- 17.Houston KE, Peli E, Goldstein RB, Bowers AR. Driving with Hemianopia Vi: Peripheral Prisms and Perceptual-Motor Training Improve Detection in a Driving Simulator. Transl Vis Sci Technol 2018;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowers AR, Keeney K, Peli E. Randomized Crossover Clinical Trial of Real and Sham Peripheral Prism Glasses for Hemianopia. JAMA Ophthalmol 2014;132:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heilman KM, Watson RT, Valenstein E. Neglect and Related Disorders In: Heilman K, Valenstein E, Eds. Clinical Neuropsychology, 3rd ed. New York: Oxford University Press; 1993. [Google Scholar]
- 20.Pedersen PM, Jorgensen HS, Nakayama H, et al. Hemineglect in Acute Stroke--Incidence and Prognostic Implications. The Copenhagen Stroke Study. Am J Phys Med Rehabil 1997;76:122–7. [DOI] [PubMed] [Google Scholar]
- 21.Paolucci S, Antonucci G, Gialloreti LE, et al. Predicting Stroke Inpatient Rehabilitation Outcome: The Prominent Role of Neuropsychological Disorders. Eur Neurol 1996;36:385–90. [DOI] [PubMed] [Google Scholar]
- 22.Paolucci S, Antonucci G, Grasso MG, Pizzamiglio L. The Role of Unilateral Spatial Neglect in Rehabilitation of Right Brain-Damaged Ischemic Stroke Patients: A Matched Comparison. Arch Phys Med Rehabil 2001;82:743–9. [DOI] [PubMed] [Google Scholar]
- 23.Kalra L, Perez I, Gupta S, Wittink M. The Influence of Visual Neglect on Stroke Rehabilitation. Stroke 1997;28:1386–91. [DOI] [PubMed] [Google Scholar]
- 24.Katz N, Hartman-Maeir A, Ring H, Soroker N. Functional Disability and Rehabilitation Outcome in Right Hemisphere Damaged Patients with and without Unilateral Spatial Neglect. Arch Phys Med Rehabil 1999;80:379–84. [DOI] [PubMed] [Google Scholar]
- 25.Paolucci S, Grasso MG, Antonucci G, et al. O ne-Year Follow-up in Stroke Patients Discharged from Rehabilitation Hospital. Cerebrovasc Dis 2000;10:25–32. [DOI] [PubMed] [Google Scholar]
- 26.Gillen R, Tennen H, McKee T. Unilateral Spatial Neglect: Relation to Rehabilitation Outcomes in Patients with Right Hemisphere Stroke. Arch Phys Med Rehabil 2005;86:763–7. [DOI] [PubMed] [Google Scholar]
- 27.Webster JS, Roades LA, Morrill B, et al. Rightward Orienting Bias, Wheelchair Maneuvering, and Fall Risk. Arch Phys Med Rehabil 1995;76:924–8. [DOI] [PubMed] [Google Scholar]
- 28.Paolucci S, Grasso MG, Antonucci G, et al. Mobility Status after Inpatient Stroke Rehabilitation: 1-Year Follow-up and Prognostic Factors. Arch Phys Med Rehabil 2001;82:2–8. [DOI] [PubMed] [Google Scholar]
- 29.Saj A, Honore J, Richard C, et al. Hemianopia and Neglect Influence on Straight-Ahead Perception. Eur Neurol 2010;64:297–303. [DOI] [PubMed] [Google Scholar]
- 30.Houston KE, Woods RL, Goldstein RB, et al. Asymmetry in the Collision Judgments of People with Homonymous Field Defects and Left Hemispatial Neglect. Invest Ophthalmol Vis Sci 2015;56:4135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Haan GA, Melis-Dankers BJ, Brouwer WH, et al. The Effects of Compensatory Scanning Training on Mobility in Patients with Homonymous Visual Field Defects: A Randomized Controlled Trial. PloS One 2015;10:e0134459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behrmann M, Watt S, Black SE, Barton JJ. Impaired Visual Search in Patients with Unilateral Neglect: An Oculographic Analysis. Neuropsychologia 1997;35:1445–58. [DOI] [PubMed] [Google Scholar]
- 33.Singh-Curry V, Husain M. Rehabilitation in Practice: Hemispatial Neglect: Approaches to Rehabilitation. Clin Rehabil 2010;24:675–84. [DOI] [PubMed] [Google Scholar]
- 34.Smania N, Martini MC, Gambina G, et al. The Spatial Distribution of Visual Attention in Hemineglect and Extinction Patients. Brain 1998;121(Pt. 9):1759–70. [DOI] [PubMed] [Google Scholar]
- 35.Ladavas E, Carletti M, Gori G. Automatic and Voluntary Orienting of Attention in Patients with Visual Neglect: Horizontal and Vertical Dimensions. Neuropsychologia 1994;32:1195–208. [DOI] [PubMed] [Google Scholar]
- 36.Bartolomeo P, Chokron S. Orienting of Attention in Left Unilateral Neglect. Neurosci Biobehav Rev 2002;26:217–34. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta M, Shulman GL. Spatial Neglect and Attention Networks. Annu Rev Neurosci 2011;34:569–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posner MI. Orienting of Attention: Then and Now. Q J Exp Psychol 2016;69:1864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo G, Woods RL, Peli E. Collision Judgment When Using an Augmented-Vision Head-Mounted Display Device. Invest Ophthalmol Vis Sci 2009;50:4509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenkenberg T, Bradforn DC, Ajax ET. Line Bisection and Unilateral Visual Neglect in Patients with Neurologic Impairment. Neurology 1980;30:509–17. [DOI] [PubMed] [Google Scholar]
- 41.Gauthier L, Dehaut F, Joannette Y. The Bells Test: A Quantitative and Qualitative Test for Visual Neglect. J Clin Exp Neuropsychol 1989;11:49–54. [Google Scholar]
- 42.Azouvi P, Olivier S, de Montety G, et al. Behavioral Assessment of Unilateral Neglect: Study of the Psychometric Properties of the Catherine Bergego Scale. Arch Phys Med Rehabil 2003;84:51–7. [DOI] [PubMed] [Google Scholar]
- 43.United States Center for Medicare Services (CMS). Reference II: 1997 Documentation Guidelines for Evaluation and Management Services. United States Center for Medicare Services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/referenceii.pdf. Accessed: April 3, 2018.
- 44.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A Practical Method for Grading the Cognitive Status of Patients for the Clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 45.Carlson NB, Kurtz D, Heath DA, Hines C. Clinical Procedures for Ocular Examinations. Norwalk, CT: Appleton & Lange; 1990. [Google Scholar]
- 46.Bagg S, Pombo AP, Hopman W. Effect of Age on Functional Outcomes after Stroke Rehabilitation. Stroke 2002;33:179–85. [DOI] [PubMed] [Google Scholar]
- 47.Saj A, Honore J, Braem B, et al. Time since Stroke Influences the Impact of Hemianopia and Spatial Neglect on Visual-Spatial Tasks. Neuropsychology 2012;26:37–44. [DOI] [PubMed] [Google Scholar]
- 48.Buxbaum LJ, Palermo MA, Mastrogiovanni D, et al. Assessment of Spatial Attention and Neglect with a Virtual Wheelchair Navigation Task. J Clin Exp Neuropsychol 2008;30:650–60. [DOI] [PubMed] [Google Scholar]
- 49.Ten Brink AF, Visser-Meily JMA, Nijboer TCW. Dynamic Assessment of Visual Neglect: The Mobility Assessment Course as a Diagnostic Tool. J Clin Exp Neuropsychol 2018;40:161–72. [DOI] [PubMed] [Google Scholar]
- 50.Finke K, Matthias E, Keller I, et al. How Does Phasic Alerting Improve Performance in Patients with Unilateral Neglect? A Systematic Analysis of Attentional Processing Capacity and Spatial Weighting Mechanisms. Neuropsychologia 2012;50:1178–89. [DOI] [PubMed] [Google Scholar]
- 51.Berger MF, Johannsen L, Karnath HO. Time Course of Eye and Head Deviation in Spatial Neglect. Neuropsychology 2008;22:697–702. [DOI] [PubMed] [Google Scholar]
- 52.Fruhmann Berger M, Pross RD, Ilg U, Karnath HO. Deviation of Eyes and Head in Acute Cerebral Stroke. BMC Neurol 2006;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buxbaum LJ, Ferraro MK, Veramonti T, et al. Hemispatial Neglect: Subtypes, Neuroanatomy, and Disability. Neurology 2004;62:749–56. [DOI] [PubMed] [Google Scholar]
- 54.Adair JC, Barrett AM. Spatial Neglect: Clinical and Neuroscience Review: A Wealth of Information on the Poverty of Spatial Attention. Ann N Y Acad Sci 2008;1142:21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]