Abstract
Background:
Burn patients suffer muscle mass loss associated with hyperinflammation and hypercatabolism. The mitochondria are affected by this metabolic alteration. Mitochondrial fission activates a caspase cascade that ultimately leads to cell death. We postulate that burn-induced muscle loss is associated with increased mitochondrial fission and subsequent functional impairment. Further, we investigated whether the cytokine IL-6 plays a major role in mitochondrial fission-associated cell death after burn.
Methods:
Murine myoblast C2C12 cells were treated with 10% serum isolated either from control rats or 40% total body surface area burned rats. Mitochondria were labeled with MitoTracker Green for live cell images. Mitochondrial function was assessed with an Enzo Mito-ID membrane potential cytotoxicity kit. Protein signals were detected by Western blot analysis. Moreover, recombinant IL-6 was applied to stimulate C2C12 to differentiate the role of cytokine IL-6; lastly, we treated burn serum-stimulated cells with IL-6 antibodies.
Results:
Caspase 3 activity increased in C2C12 cells with burn serum stimulation, suggesting increased cell death in skeletal muscle after burn. Mitochondrial morphology shortened and mitochondrial membrane potential decreased in cells treated with burn serum. Western blot data showed that mitofusion-1 expression significantly decreased in burn serum-treated cells, supporting the morpho-logic observation of mitochondrial fission. Mitochondrial fragmentation increased with IL-6 stimulation, and IL-6 antibody decreased caspase 3 activity and mitochondrial membrane potential improved in burn serum-stimulated cells.
Conclusion:
Burn serum caused muscle cell death associated with increased mitochondrial fission and functional impairment. This alteration was alleviated with IL-6 antibody treatment, suggesting the cytokine plays a role in mitochondrial changes in muscle after systemic injury.
Keywords: Burn serum, caspase 3, IL-6, mitochondrial fission, muscle cell
INTRODUCTION
Severe burn causes hyperinflammation and systemic increase of cytokines with other stress hormones inducing an extended hypermetabolic response (1). In this event, hypercatabolism of the muscle persists with increased protein breakdown (2). Muscle mass loss and muscle atrophy is correlated with the severity of the injury (3). At the cellular level, muscle cell death overwhelms myogenic activation, leading to a disturbance in muscle tissue homeostasis. Over the long term, muscle atrophy or muscle cachexia worsen a burn patient’s progress, interfere with muscle rehabilitation, and even increase overall mortality (4).
Mitochondria are abundant in skeletal muscle, and mitochondrial function impairment has been observed after thermal injury (5, 6). Cree et al. (7) reported that mitochondrial oxidative capacity decreased as much as 50% in pediatric patients with over 40% total body surface area (TBSA) burn. Mitochondrial functional impairment serves as a major mechanism activating the cell death pathway followed by oxidative stress (ROS) and a high level of intracellular calcium (8). However, little is known mitochondrial dynamics in response to burn injury.
Mitochondrial function is highly correlated with its structure, and is maintained dynamically in response to external or internal stimulation (9). Mitochondrial dynamics include mitochondrial fission and fusion cycle. A known group of proteins regulates mitochondrial morphological plasticity. The main fusion proteins are mitofusin 1 and 2 (Mfn1 and 2) and Opa1. As GTPase proteins, Mfn1 and Mfn2 fuse the outer mitochondrial membrane while Opa1 fuses the inner mitochondrial membrane. Dynamin-related protein (Drp1) is involved in regulating the mitochondrial fission process and its associated cell apoptosis regulators Bak and Bax (10). Under starvation or other nutritionally adverse conditions, mitochondrial fusion occurs to maintain mitochondrial function (11); when a mitochondrion is damaged, the adaptive fission process is initiated to preserve the remaining mitochondria. During the fission step, cytochrome C is released from mitochondria, triggering the caspase cascade, which ultimately leads to cell death (12).
Severe burn causes hyperinflammation and has been observed in both animal and human studies. In human studies, patients with over 40% TBSA burn express high levels of IL-6 (13). In a previous burn animal study, an increased cytokine profile, which included IL-1β and IL-6, was observed in rat serum (14). IL-6 is a major pro-inflammatory cytokine identified in burn patients (15). Also IL-6 contributes to cardiac inflammation and dysfunction (16). The role of IL-6 in muscle wasting and cachexia is reported in cancer (17), which could be due to mitochondrial remodeling. White et al. reported that IL-6 increased oxidative stress and fission protein FIS1 in vitro, and the reduction of fusion protein expression and mitochondrial content was alleviated in ApcMin/+ cachexic mice with IL-6 receptor antibody treatment (18). In the current study, we hypothesized that IL-6 has a key role in regulating muscle cell death through mitochondrial fission related to muscle cachexia after burn. The aims of this study were to investigate the role of mitochondrial dynamics in burn serum-stimulated muscle cells; second, to investigate the specific role of IL-6 in the regulation of mitochondrial-mediated cell death after burn.
MATERIALS AND METHODS
Cell culture
C2C12 mouse myoblasts (ATCC, Manassas, Va) were grown in Dulbecco’s Modified Eagle Medium (DMEM) culture containing 1% penicillin/streptomycin, 1% glutamate, and 10% fetal bovine serum (FBS). Culture media and reagents were purchased from ATCC. Cells were incubated in a humidified 37°C incubator with 5% CO2 during the experiment. Then 0.1 × 106cells/well or 8000cells/well were plated into a 12-well or 96-well culture plate, and 80,000 cells into a 35mm glass bottom culture dish (Thermo Scientific, Waltham, Mass) for 24h with culture media prior to experiments. Each experiment was repeated in triplicate.
Burn rat serum collection
Adult male Sprague Dawley rats (276–300 g; Charles River Laboratories, Wilmington, Mass) received a 40% TBSA scald burn. The burn procedure was approved by the University of Texas Southwestern institutional animal care and use committee, as previously described (19). Animals were euthanized at 6h (n=3) and 48h (n=3) after burn. Whole blood was collected via cardiac puncture and placed in a serum separation tube (BD, Franklin Lakes, NJ). The serum was separated at 2,500rpm centrifugation for 30 min at 4°C and aliquots were stored at −80°C. Serum was also collected from 3 non-burn normal rats to serve as a control.
Rat serum stimulation
Cell culture media was replaced with DMEM media containing either 10% normal rat serum or 10% burn rat serum for C2C12 cells.
IL-6 stimulation
C2C12 cells were replaced with fresh DMEM media with 10% FBS. Recombinant rat IL-6 protein (R&D System, Minneapolis, Minn) was added into culture media for final concentration from 0.01, 0.1, 1, 10, and 100ng/mL.
IL-6 antibody treatment
C2C12 cells were incubated with DMEM containing: 10% normal rat serum, 10% burn rat serum, or 10% burn rat serum with 0.5μg/mL of rat IL-6 antibody (R&D System).
Fluorescent image of live cells
C2C12 cells were labeled with 3nM MitoTracker Green (Life Technologies, Waltham, Mass) in a 35mm glass bottom culture dish, and live cell images were taken with a ×60 oil objective lens by a Nikon Eclipse TI fluorescent microscope (Nikon, Tokyo, Japan). MitoTracker Green is a fluorescent dye applied in live cells that bond to mitochondrial membrane lipids regardless of membrane potential (20). The settings of the microscope were consistent during the experiment: offset=−2, objective=×60, filter=PGFP, 0.448=1.08, HV(G)=50, and pinhole=1.2. Cell images were randomly taken from five scope views per dish with 0.2 to 0.5 μm per slice and 15–25 slices in total for Z-stack of pictures. The images were processed with NIS element AR4.200 software to quantify mitochondrial volume, fluorescent intensity, and elongation index. The software processed the entire Z-stack of images in a cell to calculate a final volume. The middle slice of the Z-stack was selected to analyze both intensity and elongation of mitochondria.
Western blot analysis
C2C12 cells were lysed with M-PER mammalian lysis buffer with a protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, Mass). The cell lysate was vortexed and incubated on ice for 30 min. Supernatant of the lysate was isolated for 15 min at 15,000rpm 4°C centrifugation. Twenty micrograms of lysate was subsequently analyzed by SDS-PAGE and Western blot following the published procedure (21). Band intensities were quantified with the GeneSnap/ GeneTools software (Syngene, Frederick, Md). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was utilized as a loading control. All antibodies, including Mfn1 and 2, and Drp1, were purchased from Cell Signaling Technology (Danvers, Mass). SuperSignal West Pico Chemiluminescent Substrate was purchased from Thermo Fisher Scientific.
Caspase-3 activity measurement
Twenty micrograms of cell lysate was measured for caspase 3 activity using EnzChek Caspase-3 Assay Kit #2 (Thermo Fisher).
MTT cell proliferation assay
Cell proliferation in burn serum-stimulated C2C12 cells was detected by an MTT cell proliferation assay kit from ATCC. The measurement procedure was followed per the manufacturer’s protocol.
Mitochondrial function assessment
Mitochondrial function was estimated with mitochondrial potential membrane integrity. The measurement procedure was followed per the manual instruction of the Mito-ID Membrane potential cytotoxicity kit (Enzo Life Sciences, Farmingdale, NY).
Statistical analysis
Results are presented as means±standard error of the mean (SEM). The data were analyzed using paired Student t tests, and one-way ANOVA where appropriate. Differences were considered significant at P<0.05.
RESULTS
C2C12 cells with burn serum stimulation
C2C12 myoblasts were stimulated with 10% rat serum collected at 6h after burn. No change of caspase 3 activity was found in cells treated with 10% FBS or 10% rat normal serum. Caspase 3 activity was significantly increased in C2C12 cells with burn rat serum for 24h compared with normal rat serum-treated cells (P<0.05). MTT assay showed no change in cell proliferation observed among three groups (Fig. 1).
Fig. 1. Burn serum increased cell death but not cell proliferation in C2C12 mouse myoblasts.
(A) Caspase 3 activities and (B) MTT assay of cell proliferation in C2C12 cells incubated with DMEM culture for 48 h, respectively, labeled as DMEM (with 10% FBS), Norm (with 10% normal rat serum), and Burn (with 10% 6-h post-burn rat serum). An asterisk (*) indicates P< 0.05, paired t test, Norm versus Burn. DMEM indicates Dulbecco Modified Eagle Medium.
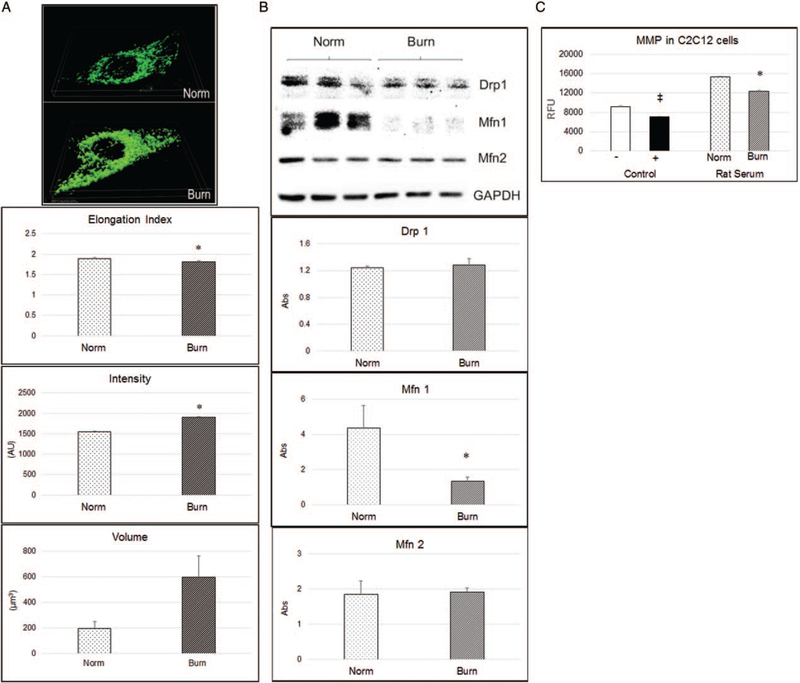
3D cell images demonstrated mitochondrial morphologic changes in burn serum-stimulated C2C12 cells at 48h after stimulation. Fluorescent staining displayed mitochondria with rod shapes in cells treated with 10% normal rat serum, while becoming brighter and circular shaped in cells treated with 10% burn rat serum. Statistical results showed that the mitochondrial elongation index significantly decreased, with an increased intensity signal (P<0.05), indicating that mitochondrial fragmentation increased in response to burn serum stimulation (Fig. 2A).
Fig. 2. Mitochondrial structure and function disturbance in burn serum-stimulated C2C12 cells.
Burn serum was collected from rats 6 h after burn. A, 3D volume of live cell image with DMEM culture containing 10% normal rat serum (Norm) and 6-h burn rat serum (Burn) at 48 h. Cells were labeled with 3 nM of MitoTracker Green 15 min prior to experiment. Statistical quantification of mitochondrial elongation index, intensity, and volume. B, Western blot showing mitochondrial marker changes with burn serum stimulation. C, Statistics analysis of MMP in C2C12 cell with burn serum for 48-h stimulation; to the left are control groups for assay validation with 3 μM of CCCP (+). A double cross symbol (‡) indicates P< 0.05, paired t test, versus positive control; to the right are bars showing MMP in Norm and Burn rat serum stimulation. An asterisk (*) indicates P< 0.05, paired t test, Norm versus Burn. CCCP indicates carbonyl cyanide m-chlorophenyl hydrazone; DMEM, Dulbecco Modified Eagle Medium; MMP, mitochondrial membrane potential.
Western blot data showed that the absorbance ratio of Mfn1 normalized to GAPDH significantly decreased in cells with burn serum stimulation. Mitochondrial fusion protein Mfn1 decreased further supporting mitochondrial fragmentation in response to burn serum stimulation (Fig. 2B).
Data from the mitochondrial membrane potential (MMP) assay showed that membrane potential decreased in response to burn serum stimulation. Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) is a chemical inhibitor of oxidative phosphorylation which served as a positive control to decrease mitochondrial membrane potential. Adding 3μM of CCCP into C2C12 cells, fluorescent intensity (RFU) decreased about 23%. We found a significant 20% decrease of RFU in cells with burn rat serum stimulation compared with normal serum stimulation (P<0.05; Fig. 2C).
Comparing the effects of rat serum collected at 6 and 48h after burn, we found a less intense signal and higher elongation index in C2C12 cells with 48-h burn serum stimulation versus 6-h burn serum stimulation, indicating less mitochondrial fragmentation in cells with 48-h burn serum stimulation. We further found MMP level significantly increased in cells with 48-h burn serum (Fig. 3).
Fig. 3. Comparison of mitochondrial response to rat serum collected at 6 and 48h after burn.
A, MitoTrack Green-labeled cell mitochondrial morphology changes in response to burn serum for 48-h treatment. The top image is with normal rat serum stimulation, the middle is 6-h burn serum (6HPB) and the bottom is 48-h burn (48HPB) serum stimulation. B, Statistical figure of mitochondrial morphology quantification. C, MMP level in cells stimulated with rat serum collected from 6 and 48 h after burn. Rat serum were from nine rats in total and three animals in each group. An asterisk (*) indicates P< 0.05, paired t test, versus Norm group.
IL-6 in regulating mitochondrial dynamics in C2C12 cells
C2C12 cells were treated with increasing doses of rat recombinant IL-6 protein (r-IL-6) from 0, 0.01, 0.1, 10, and 100ng/mL. We found significantly increased intensity signals and an increased elongation index in all IL-6-stimulated cells at 48h (P<0.05). Treatment with 0.01ng/mL of r-IL6 showed a four-fold significant increase of mitochondrial volume in C2C12 cells (P<0.05; Fig. 4).
Fig. 4. IL-6 disturbed mitochondrial morphology in C2C12 cells.
The (A) elongation index, (B) intensity, and (C) mitochondrial volume altered with r-IL-6 stimulation for 48 h. An asterisk (*) indicates P< 0.05, one way ANOVA, versus baseline.
Last, rat IL-6 antibody significantly decreased caspase 3 activity in burn serum-treated cells at 48h (P<0.05). In cells cultured with 10% burn rat serum (collected 6h after burn), 0.5μg/mL of IL-6 antibody treatment reversed mitochondrial fragmentation, demonstrated by the recovered elongation index and decreased mitochondrial intensity (P<0.05; Fig. 5).
Fig. 5. IL-6 antibody decreased mitochondrial fission and caspase 3 activities in burn serum-stimulated C2C12 cells.
A, The elongation index change. B, The mitochondrial intensity in burn serum-stimulated cells with IL-6 antibody treatment. C, Caspase 3 activity in response to conditions respectively labeled as Norm (with 10% normal rat serum) and Burn (with 10% burn rat serum) and Burn +IL-6 Ab. An asterisk (*) indicates P< 0.05, paired t test, Norm versus Burn. A plus sign (þ) indicates P< 0.05, paired t test, Burn versus Burn+IL-6 Ab.
DISCUSSION
In the current study, we labeled C2C12 myoblasts with mitochondrial fluorescent dye and took live cell images by confocal laser microscopy to observe mitochondrial morphology in response to burn serum stimulation. This study revealed mitochondrial fragmentation with functional impairment in muscle cells following burn serum stimulation, and increased mitochondrial fragmentation activated a caspase cascade leading to cell death. Furthermore, IL-6 caused mitochondrial fragmentation in C2C12 cells, while neutralizing IL-6 in burn rat serum reduced the caspase 3 activity, demonstrated by decreased mitochondrial fragmentation in murine myoblasts. This suggests the role of IL-6 in regulating cell death through adjusting mitochondrial dynamics.
In the study, we endeavored to quantify mitochondrial morphology with parameters including elongation index, intensity, and mitochondrial volume. In estimating the three parameters, we found that mitochondrial fragmentation increased in burn serum-stimulated muscle cells. The mitochondrial elongation index is determined by a Ferret-specific formula from Nikon. The higher the elongation index, the more rod-like the object. Normal mitochondria have a rod-like structure, and a decrease in elongation would suggest a loss of this structure (22). The decrease of the elongation index suggests a loss of normal mitochondrial morphology in cells with the post 6-h burn serum stimulation.
In response to burn serum stimulation, mitochondrial volume increased, which is either due to increased mitochondrial fragmentation, mitochondrial swelling, or both. The morphologic change of mitochondrial swelling was reported in burn rats’ liver by electronic microscopic images (23), and also displayed indirectly in isolated fresh mitochondria from animal heart tissue at 6 and 24h (24). Swelling of mitochondrial cristae promotes ROS production and even leads to cell apoptosis (25). In the current study, we speculated that both mitochondrial fission and swelling occurred associated with increased cell death in muscle cells.
De Vos et al. (26) pointed out that mitochondrial-shape changes are caused by both Drp-1-dependent fission and swelling. Drp1 is the mitochondrial fission protein that regulates cell death by releasing cytochrome C (27). In the current study, we found that the expression of mitochondrial fusion protein Mfn1 was decreased in myoblasts stimulated with burn serum. Santel et al. (28) revealed that the completion of the Mfn1 GTPase cycle is required for mitochondrial network formation. Though Drp 1 was recognized to mediate mitochondrial fission leading to cell apoptosis (29), the ablation of Mfn1 with its homologue Mfn2 leads to mitochondrial fragmentation with apoptotic death (30). The current study further supports the role of Mfn1 in regulating mitochondrial fission related to cell death.
Cytokine IL-6 has a broad biological response presented in both pro-and anti-inflammatory properties (31). IL-6 directly interferes with lipolysis and mitochondrial dysfunction in vitro (32). Our question is whether IL-6 is the major contributor to morphologic changes in burn-stimulated muscle cells. In this study, we treated mouse myoblasts with rat recombinant IL-6protein to induce the similar mitochondrial fragmentation with decreased MMP in myoblasts. Furthermore, we attempted to ablate the effect of burn serum stimulation through co-incubation with an antibody against rat IL-6. The ablation of IL-6 was able to preserve mitochondrial morphology and decreased caspase 3 activity levels. These data indicate the importance of IL-6 in regulating mitochondrial-dependent muscle cell death.
In addition, a previous study showed inflammatory cytokines are overexpressed in rats with 60% TBSA burn. IL-6 levels were raised for 3h and reached a spike at 6h, and then gradually dropped with a higher level remaining compared with normal rats (14).
The elevated IL-6 level in 6-h burn serum relative to the 48-h burn serum may explain the difference of mitochondrial elongation in burn serum-stimulated cells between the two experiments.
Though we observed IL-6 disturbing mitochondrial homeostasis in burn serum-stimulated myoblasts, we agree other cytokines such as TNF might play important roles as well. We previously observed that TNF plays a role in insufficient myogenic activation, contributing to muscle mass loss after burn (33). A recent study from Ueki’s report showed elevated fibrinogen levels in plasma-related muscle wasting in burn mice. They confirmed that fibrinogen increased cytokine TNF and chemokine MCP-1 to further damage mitochondria in myotubes (34). Without mentioning the effect of IL-6, the authors conveyed the complexity of cytokine in triggering mitochondrial disturbances and its effect on muscle homeostasis after burn.
Without testing in our study, we also noticed the potential effect of one culture media condition in mitochondrial plasticity. The cell culture in DMEM has a high glucose level (4.5g/L d-glucose). Down-regulated Mfn1 with increased mitochondrial fission was reported recently in vitro with high glucose-induced kidney cell death with decreased autophagy (35). The possible pathway involved an miRNA 140-negative interaction with Mfn1 in cardiomyocyte apoptosis (36). Our previously published study showed that miR-140–3p related to mitochondrial dynamics regulation increased 1.17 fold in plantaris muscle from 40% TBSA burn rats 14 days later (37).
Burn trauma patients with muscle cachexia are associated with a poor prognosis. The current treatments such as growth hormone and insulin in burn patients showed benefits based on mitochondrial function improvement (38, 39). In a human study, growth hormone infusion for 14h (150ug/h) increased the muscle mitochondrial ATP production rate and citrate synthase activity in healthy subjects (40). Insulin has been shown to increase mitochondrial fusion in myocardiocytes (41). The fact that those anabolic agents improve muscle cachexia seen in postburn trauma patients suggests that metabolic control postburn may be an effective treatment route.
The current mitochondrial dynamics study might illuminate a new pathway to perhaps slow down the rate of catabolism and allow for patients to have a better prognosis. More precise treatments for specifically targeting mitochondrial protection may be one avenue to improve patient outcomes. Along these lines, mitochondrial-targeted vitamin E was previously shown to exhibit a protective effect on myocardiocyte damage in septic rats through mitochondrial function maintenance (42). In conclusion, we found that burn serum disrupted mitochondrial structure and function, increased mitochondrial fragmentation, and increased cell apoptosis. Mfn1 plays a role in negatively regulating mitochondrial fragmentation. IL-6 increased mitochondrial fragmentation with function impairment in muscle cells. IL-6 antibody diminished burn serum-induced mitochondrial fragmentation and reduced cell death in myoblasts.
ACKNOWLEDGMENTS
Our lab technicians Ming Mei and Kevin DeSpain and medical students Rohan Kulangara and Christian Maxwell collected data for the study, and staff medical editor Dave Primm also supported this work.
This work was supported by funds from the Golden Charity Guild Charles R. Baxter, MD, Chair Department funding and the UT Southwestern medical student summer research program. The authors report no conflicts of interest.
REFERENCES
- 1.Herndon DN, Tompkins RG: Support of the metabolic response to burn injury.Lancet 363(9424):1895–1902, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA,Wolfe RR, Herndon DN: Persistence of muscle catabolism after severe burn. Surgery 128(2):312–319, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Newsome TW, Mason AD Jr, Pruitt BA Jr: Weight loss following thermal injury. Ann Surg 178(2):215–217, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R,Suman OE, Mlcak RP, Herndon DN: Long-term persistence of the pathophysiologic response to severe burn injury. PLoS One 6(7):e21245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padfield KE, Astrakas LG, Zhang Q, Gopalan S, Dai G, Mindrinos MN, Tompkins RG, Rahme LG, Tzika AA: Burn injury causes mitochondrial dysfunction in skeletal muscle. Proc Natl Acad Sci U S A 102(15): 5368–5373, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter C, Herndon DN, Bhattarai N, Ogunbileje JO, Szczesny B, Szabo C, Toliver-Kinsky T, Sidossis LS: Differential acute and chronic effects of burn trauma on murine skeletal muscle bioenergetics. Burns 42(1):112–122, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cree MG, Fram RY, Herndon DN, Qian T, Angel C, Green JM, Mlcak R, Aarsland A, Wolfe RR: Human mitochondrial oxidative capacity is acutely impaired after burn trauma. Am J Surg 196(2):234–239, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan H, Chai J, Sheng Z, Yao Y, Yin H, Liang L, Shen C, Lin J: Effect of burn injury on apoptosis and expression of apoptosis-related genes/proteins in skeletal muscles of rats. Apoptosis 14(1):52–65, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Anesti V, Scorrano L: The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta 1757(5–6):692–699, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Gao AW, Canto C, Houtkooper RH: Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol Med 6(5):580–589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Chan DC: Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18(R2):R169–R176, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babbar M, Sheikh MS: Metabolic stress and disorders related to alterations in mitochondrial fission or fusion. Mol Cell Pharmacol 5(3):109–133, 2013. [PMC free article] [PubMed] [Google Scholar]
- 13.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, Branski LK, Gauglitz GG, Mlcak RP, Herndon DN: Pathophysiologic response to severe burn injury. Ann Surg 248(3):387–401, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, Jeschke MG: Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock 30(5):503–507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh FL, Lin WL, Shen HD, Fang RH: Changes in circulating levels ofinterleukin 6 in burned patients. Burns 25(2):131–136, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Wang HY, Bassel-Duby R, Maass DL, Johnston WE, Horton JW, Tao W: Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am J Physiol Heart Circ Physiol 292(5):H2408–H2416, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Martignoni ME, Kunze P, Hildebrandt W, Kunzli B, Berberat P, Giese T, Kloters O, Hammer J, Buchler MW, Giese NA, et al. : Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin Cancer Res 11(16):5802–5808, 2005. [DOI] [PubMed] [Google Scholar]
- 18.White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, Kostek MC, Matesic LE, Carson JA: IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/þ mouse. Skelet Muscle 2:14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson DL, Maass DL, White J, Sikes P, Horton JW: Caspase inhibition reduces cardiac myocyte dyshomeostasis and improves cardiac contractile function after major burn injury. J Appl Physiol (1985) 103(1):323–330, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Pendergrass W, Wolf N, Poot M: Efficacy of MitoTracker Green and CMXros-amine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry A 61(2):162–169, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Song J, Zhang XJ, Boehning D, Brooks NC, Herndon DN, Jeschke MG:Measurement of hepatic protein fractional synthetic rate with stable isotope labeling technique in thapsigargin stressed HepG2 cells. Int J Biol Sci 8(2): 265–271, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mai S, Klinkenberg M, Auburger G, Bereiter-Hahn J, Jendrach M: Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J Cell Sci 123(Pt 6):917–926, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Jeschke MG, Gauglitz GG, Song J, Kulp GA, Finnerty CC, Cox RA, Barral JM, Herndon DN, Boehning D: Calcium and ER stress mediate hepatic apoptosis after burn injury. J Cell Mol Med 13(8B):1857–1865, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X, Costantini T, Lopez NE, Wolf PL, Hageny AM, Putnam J, Eliceiri B, Coimbra R: Vagal nerve stimulation protects cardiac injury by attenuating mitochondrial dysfunction in a murine burn injury model. J Cell Mol Med 17(5):664–671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Moraes R, Gioseffi G, Nobrega AC, Tibirica E: Effects of exercise training on the vascular reactivity of the whole kidney circulation in rabbits. J Appl Physiol (1985) 97(2):683–688, 2004. [DOI] [PubMed] [Google Scholar]
- 26.De Vos KJ, Allan VJ, Grierson AJ, Sheetz MP: Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol 15(7):678–683, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Suen DF, Norris KL, Youle RJ: Mitochondrial dynamics and apoptosis. Genes Dev 22(12):1577–1590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT: Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci 116(Pt 13):2763–2774, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ: The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1(4):515–525, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Sugioka R, Shimizu S, Tsujimoto Y: Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem 279(50):52726–52734, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Hunter CA, Jones SA: IL-6 as a keystone cytokine in health and disease. Nat Immunol 16(5):448–457, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Ji C, Chen X, Gao C, Jiao L, Wang J, Xu G, Fu H, Guo X, Zhao Y: IL-6 induces lipolysis and mitochondrial dysfunction, but does not affect insulin-mediated glucose transport in 3T3-L1 adipocytes. J Bioenerg Biomembr 43(4):367–375, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Saeman MR, De Libero J, Wolf SE: Skeletal muscle loss is associated with TNF mediated insufficient skeletal myogenic activation after burn. Shock 44(5):479–486, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueki R, Liu L, Kashiwagi S, Kaneki M, Khan MA, Hirose M, Tompkins RG, Martyn JA, Yasuhara S: Role of elevated fibrinogen in burn-induced mitochondrial dysfunction: protective effects of glycyrrhizin. Shock 46(4):382–389, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee WC, Chiu CH, Chen JB, Chen CH, Chang HW: Mitochondrial fission increases apoptosis and decreases autophagy in renal proximal tubular epithelial cells treated with high glucose. DNA Cell Biol 35(11):657–665, 2016. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Li Y, Jiao J, Wang J, Li Y, Qin D, Li P: Mitofusin 1 is negatively regulated by microRNA 140 in cardiomyocyte apoptosis. Mol Cell Biol 34(10):1788–1799, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song J, Saeman MR, Baer LA, Cai AR, Wade CE, Wolf SE: Exercise altered the skeletal muscle microRNAs and gene expression profiles in burn rats with hindlimb unloading. J Burn Care Res 38(1):11–19, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramirez RJ, Wolf SE, Barrow RE, Herndon DN: Growth hormone treatment in pediatric burns: a safe therapeutic approach. Ann Surg 228(4):439–448, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cree MG, Zwetsloot JJ, Herndon DN, Qian T, Morio B, Fram R, Sanford AP, Aarsland A, Wolfe RR: Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg 245(2):214–221, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Short KR, Moller N, Bigelow ML, Coenen-Schimke J, Nair KS: Enhancement of muscle mitochondrial function by growth hormone. J Clin Endocrinol Metab 93(2):597–604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parra V, Verdejo HE, Iglewski M, Del Campo A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez-Crisosto C, et al. : Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFkappaBOpa-1 signaling pathway. Diabetes 63(1):75–88, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao X, Carlson D, Sun Y, Ma L, Wolf SE, Minei JP, Zang QS: Mitochondrial ROS induces cardiac inflammation via a pathway through mtDNA damage in a pneumonia-related sepsis model. PLoS One 10(10):e0139416, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]