Abstract
Cellular mechanical properties play an integral role in bacterial survival and adaptation. Historically, the bacterial cell wall and, in particular, the layer of polymeric material called the peptidoglycan were the elements to which cell mechanics could be primarily attributed. Disrupting the biochemical machinery that assembles the peptidoglycan (e.g., using the β-lactam family of antibiotics) alters the structure of this material, leads to mechanical defects, and results in cell lysis. Decades after the discovery of peptidoglycan-synthesizing enzymes, the mechanisms that underlie their positioning and regulation are still not entirely understood. In addition, recent evidence suggests a diverse group of other biochemical elements influence bacterial cell mechanics, may be regulated by new cellular mechanisms, and may be triggered in different environmental contexts to enable cell adaptation and survival. This review summarizes the contributions that different biomolecular components of the cell wall (e.g., lipopolysaccharides, wall and lipoteichoic acids, lipid bilayers, peptidoglycan, and proteins) make to Gram-negative and Gram-positive bacterial cell mechanics. We discuss the contribution of individual proteins and macromolecular complexes in cell mechanics and the tools that make it possible to quantitatively decipher the biochemical machinery that contributes to bacterial cell mechanics. Advances in this area may provide insight into new biology and influence the development of antibacterial chemotherapies.
Graphical Abstract

Bacteria inhabit a wide range of different environments in which they experience fluctuating physical and chemical stresses. For example, osmotic pressure across the bacterial cell wall arises from a mismatch in the intracellular and extracellular concentration of solutes. Sudden changes in the extracellular concentration of solutes create an osmotic pressure in bacteria that may reach ~20 atm.1 To survive, bacteria have evolved cell walls to mechanically resist osmotic pressure and osmoregulatory machinery that senses pressure and transports solutes into and out of cells to reduce pressure. The current model of bacterial mechanics is one in which the polymeric meshwork surrounding cells, termed the peptidoglycan, provides significant mechanical properties. The peptidoglycan is a macro-molecular cellular “exoskeleton” that stabilizes the cell wall and provides structural integrity to the cell. Additional structural elements have been uncovered recently, indicating that the peptidoglycan is one element of a larger set of macromolecular materials that influence cell mechanics.2,3 Several new tools enable studies of bacterial mechanics at the single-cell level2,4,5 and provide a proteome/genome-wide view of mechanomicrobiology.6
In contrast to those of microbes, eukaryotic cell mechanics are much better understood. Eukaryotic studies provided insight into the progression of human diseases7–10 in which changes in cellular mechanics are important.9,10 For example, the infection of red blood cells by the parasite Plasmodium falciparum, which is primarily responsible for the mortality caused by malaria,11 causes a 10-fold increase in the stiffness of infected red blood cells. Changes in red blood cell mechanics arise from increased membrane stiffness and alterations in the spectrin cytoskeletal protein network that reduce the flow of blood and eventually lead to a loss of microcirculation.7,12 Changes in cell mechanics are also linked to a wide range of human health conditions and diseases, including asthma, osteoporosis, cancer, glaucoma, and osteoarthritis.10 Finally, mechanical stress applied to eukaryotic cells, through substrate elasticity, can alter cell physiology and control development; e.g., altering matrix elasticity steers the mesenchymal stem down different lineages.13
The study of eukaryotic cell mechanics has provided insight into the importance of control over cell mechanics in normal cellular function and in different states of disease.14 Likewise, the study of bacteria may uncover roles for cell mechanics linked to their cellular function and applications in the infection of eukaryotic hosts. In addition, the problem of widespread drug resistance of bacteria to antibiotics may benefit from studies in this area, in which a more detailed understanding of bacterial mechanics can uncover the physical effects of current antibiotics, uncover new therapeutic targets, and provide insight into the mechanisms of resistance of clinical antibiotics.
MECHANICAL CHARACTERISTICS OF BACTERIAL CELLS
The mechanical properties of cells are most frequently described by the Young’s modulus and bending rigidity.2–4, 15–19 Below we provide a brief definition and overview of these terms.
Young’s Modulus.
The stiffness of a material can be defined by its Young’s modulus (or tensile elasticity), which is characterized by the relationship between the applied stress on the material (force per unit area) and the resulting strain (fractional change in length). The Young’s modulus is defined by the slope of the stress/strain curve in the linear region and is measured in units of pascals (newtons per square meter). If a physical load is applied to material in the linear region, the material will deform, and removing the load will return the material to its preload state. Stress applied to a material outside of the linear regime results in the permanent and irreversible deformation of a material.
Bending Rigidity or Flexural Rigidity.
Bending rigidity (units of newtons per square meter) is the resistance of a material to bending under a load and represents the product of the Young’s modulus and the second moment of inertia. In rod-shaped bacteria, the second moment of inertia is equivalent to πr3h, where r is the radius of a bacterial cell and h is the thickness of the mechanically relevant material being studied. Previous studies of whole cell mechanics have focused on the peptidoglycan layer of the bacterial cell wall, which is found in Gram-positive and Gram-negative bacteria. Importantly, the bending rigidity can provide insight into the orientation of structural elements within cells, e.g., biomolecular elements that play a mechanical role, such as peptide bonds within the peptidoglycan, that are oriented perpendicular to the long axis of bacterial cells3,20 and may be difficult to interrogate using other measurements.2 The bending rigidity can also be used to determine the Young’s modulus through its inherent relation to bending rigidity.
COMPONENTS OF THE BACTERIAL CELL WALL CONTRIBUTE TO CELL MECHANICS
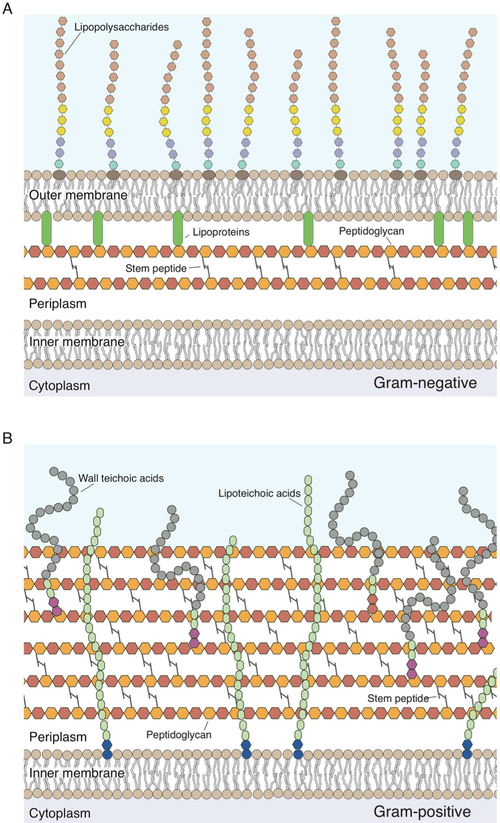
Bacteria can be broadly classified into Gram-negative (Figure 1A) and Gram-positive cells (Figure 1B) based on the presence of an outer membrane and the thickness of the peptidoglycan layer. Gram-negative bacteria contain both a cytoplasmic and outer membrane; in addition to phospholipids, the outer membrane contains lipopolysaccharides (LPS) (Figure 1A). Gram-positive bacteria do not have an outer membrane or LPS; however, they contain wall teichoic acids (WTA) and lipoteichoic acids (LTA) that are polysaccharides covalently attached to the peptidoglycan and inserted into the cytoplasmic membrane, respectively (Figure 1B). The peptidoglycan layer is thinner in Gram-negative cells and thicker in Gram-positive bacteria and is described in more detail in Peptidoglycan. We summarize the structure and mechanical function of these classes of materials below.
Figure 1.
Structure of the bacterial cell walls. (A) Cartoon depicting the structure of the Gram-negative cell wall. The peptidoglycan thickness is ~4 nm; monosaccharides in the peptidoglycan are represented as hexagons, and the colors demonstrate that this material consists of repeating disaccharide building blocks. Peptide cross-links in the peptidoglycan are depicted as gray lines. Monosaccharides in lipopolysaccharides are depicted as hexagons. Aqua and purple denote the inner polysaccharide core; yellow denotes the outer polysaccharide core, and brown denotes the O-antigen. Lipoproteins (green) connect the outer membrane to the peptidoglycan. (B) Cartoon depicting the Gram-positive bacterial cell wall. The peptidoglycan thickness is ~19–33 nm. Lipoteichoic acid is inserted into the membrane and consists of a glycolipid anchor (blue) and poly(glycerol phosphate) (green). The wall teichoic acid is directly cross-linked to the peptidoglycan through a linkage unit (red) and consists of glycerol phosphate (green) and poly(alditol phosphate).
Not all bacteria fit neatly into the Gram-negative and Gram-positive categories. Corynebacteria spp., Mycobacteria spp., and Nocardia spp. have a unique cell wall terminated with an outer membrane that is adjacent to a layer of mycolic acids, which makes them structurally, and possibly mechanically, unique. Corynebacteria spp. are considered to be Gram-positive bacteria; however, Mycobacteria spp. and Nocardia spp. are impermeable to many membrane dyes and only very weakly stain using the Gram-positive dyes. Not true Gram-positive bacteria, these organisms are classified as “acid-fast bacteria” because of their insensitivity to the acid treatment in the Gram-positive staining method. These organisms have unique cell walls,21,22 yet very little is known about their cell mechanics compared to those of Gram-negative and Gram-positive bacteria.
Gram-Negative Lipopolysaccharides (LPS).
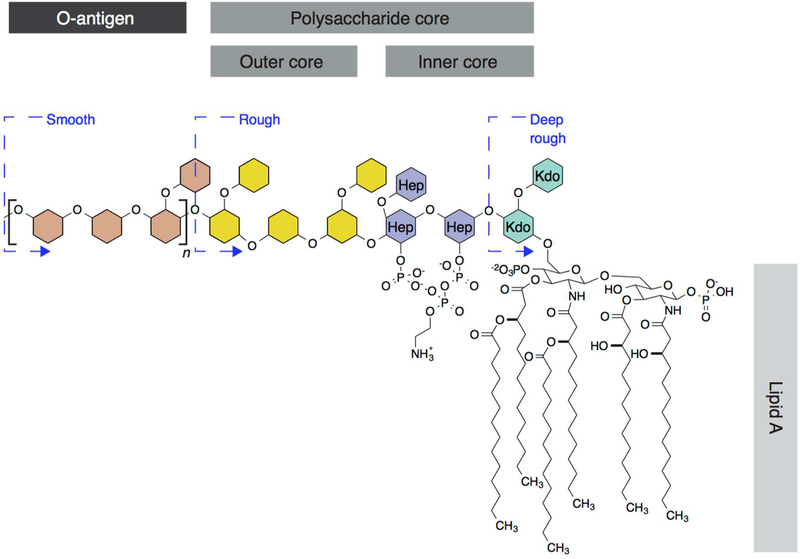
LPS is expressed by most Gram-negative bacteria, plays an important role in the function and structural integrity of the outer lipid membrane, and is linked to the pathology of certain bacteria in humans.23 LPS make up a family of large molecules (>100 kDa) containing a lipid moiety attached to a long-chain polysaccharide and are located in the outer leaflet of the outer membrane (Figure 1A).24 LPS molecules consist of three distinct structural regions (Figure 2): (1) lipid A, which is the physical anchor between the LPS and the outer lipid membrane; (2) the inner and outer polysaccharide core; and (3) a hydrophilic O-antigen. Lipid A contains six saturated fatty acyl chains (Figure 2), rather than the two to four fatty acyl chains characteristic of most prokaryotic membrane lipids,25 and is found only in the LPS of Gram-negative bacteria. The tight packing of the hydrophobic acyl chains in lipid A plays a role in stabilizing the outer membrane.26 The inner core of LPS is highly conserved among bacterial species23 and typically consists of 3-deoxy-d-manno-octulosonic acid (Kdo) and heptose sugars (Figure 2). With the exception of Neisseria meningitides,27 lipid A and at least one Kdo (from the inner LPS core) are required for cell viability in LPS-producing, Gram-negative bacteria. The other two sections of the molecule are not essential for cell viability and display a low degree of conservation among bacterial species (Figure 2).23 The O-antigen consists of polysaccharides with a range of lengths (Figure 2)23,25 and is implicated in the potential virulence of pathogenic strains.28 LPS truncations produce physical aberrations in the structure of cells and the morphology of bacterial colonies. Wild-type bacterial cells containing intact LPS have an outer cell morphology that is continuous and defect-free and are termed “smooth”. Bacterial cells that have lost the O-antigen are classified as “rough” mutants; cells that have lost both the outer core and the O-antigen are classified as “deep-rough” mutants (Figure 2), and electron microscopy reveals that they have a rough, uneven membrane morphology.29 LPS mutant cells are more permeable to small molecules than wild-type cells are30 and more susceptible to environmental stress.31 These changes in morphology indicate LPS may play a structural and mechanical role in cells; however, this hypothesis remains untested.
Figure 2.
Structure of LPS in Gram-negative bacteria. LPS consists of three primary regions: lipid A, the polysaccharide core (composed of an inner and outer core), and the O-antigen. The monosaccharides of LPS, polysaccharide core, and O-antigen are represented schematically as hexagons to simplify the structure of the molecule. The inner core is highly conserved among species and is composed of 3-deoxy-d-manno-octulosonic acid (Kdo) (aqua) and heptose (Hep) (purple). The outer core (yellow) and O-antigen (brown) are variable among bacteria. n represents the number of O-antigen repeats, which vary in length depending on the species and can be as large as 40 repeating units. Alterations in the length of the LPS (depicted by the blue dashed lines) result in physical alterations in colony and cell morphology that ranges from smooth to deep rough.
LPS are negatively charged molecules that electrostatically repel other LPS molecules,25 leading to physical separation between the molecules and a subsequent increase in membrane permeability. To overcome electrostatic repulsion and increase stability, LPS typically bind tightly to divalent cations such as Ca2+ or Mg2+. Atomic force microscopy (AFM) has been used to monitor the effect of divalent cations on LPS and membrane architecture; treating cells with EDTA removes divalent cations and causes a loss of ~40–50% of the LPS from the outer membrane.32 Removing divalent cations from an asymmetric membrane bilayer consisting of phosphatidylcholine in the inner leaflet and deep-rough mutant LPS in the outer leaflet caused the LPS to flip between the outer and inner leaflets.33 Flipping LPS between the leaflets minimizes the repulsive electrostatic forces that arise between adjacent, charged LPS molecules33 and may be responsible for the release of LPS from the outer membrane of intact cells into the extracellular environment.32
The relationship between the stability of the LPS layer and its contribution to membrane permeability has been well established; however, there is a surprisingly small number of studies linking LPS to the mechanical properties of Gram-negative bacteria. Experiments pointing to a mechanical role for the LPS layer are based on in vitro measurements of membrane fluidity and the viscoelastic properties of LPS in lipid vesicles and in model membrane bilayers.
Bacterial membrane lipids form a stable lamellar phase bilayer in which the hydrophilic portion of the molecules is aligned at the water interface and the hydrophobic portion is sequestered away from water and reduces the free energy of the structure. Similar to membrane lipids, isolated LPS or lipid A forms stable lamellar phases consisting of bilayers and multilayers in aqueous solution.34,35 LPS-containing membranes are stabilized by the presence of divalent cations, which increase the level of order and rigidity of multilamellar LPS layers.35,36 Addition of Ca2+ to a “deep-rough” LPS monolayer containing only lipid A and two Kdo sugars (Figure 2) produces a cross-linked elastic gel.37 Furthermore, increasing the polysaccharide length of the LPS enabled the formation of a similar gel in the absence of Ca2+, possibly because of an increased extent of hydrogen bonding, and resulted in additional lateral compression of the LPS monolayer.37,38 These in vitro studies support LPS stabilization by divalent cations and by the length of the polysaccharide chain. Additional in vivo studies may illuminate the magnitude of the mechanical contribution between divalent cations and LPS, the mechanical response of the LPS to changing environmental conditions,39 and the role of LPS in outer membrane homeostasis.29,40 The techniques for studying the mechanical properties of bacterial cells highlighted in the section below may be helpful for exploring the contribution of LPS to cell mechanics.
Gram-Positive Wall Teichoic Acids (WTAs).
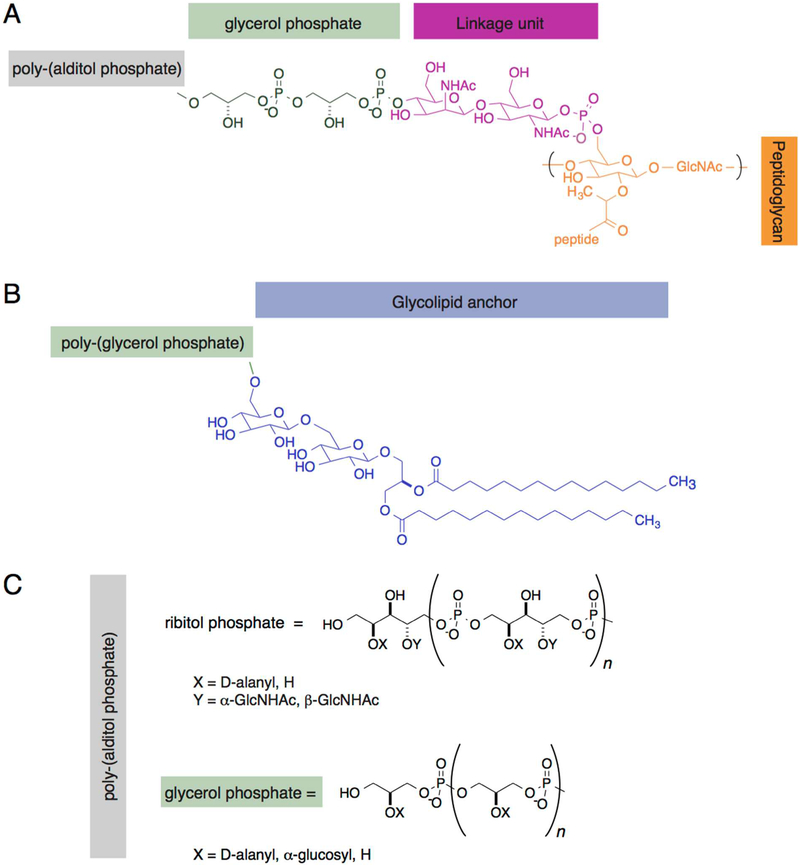
WTAs are abundant, nonessential glycopolymers attached to the peptidoglycan layer in Gram-positive bacteria (Figure 1B), account for ~50% of the weight of the cell wall,41 and play a role in membrane integrity.42 WTA consists of two primary structural features (Figure 3A): (1) a disaccharide linkage connecting WTAs to the peptidoglycan and (2) a primary polymeric chain that typically consists of alditol phosphate (glycerol phosphate or ribitol phosphate) repeats that are 20–40 units in length (Figure 3C).43 The disaccharide linkage unit is highly conserved among bacterial species44 and consists of N-acetylmannosamine-N-acetylglucosamine-1-phosphate covalently attached to the peptidoglycan through phosphodiester bonds to the N-acetylmuramyl saccharide (Figure 3A).45 The polymeric backbone of the WTA main chain is negatively charged because of the presence of phosphate; however, WTA is generally zwitterionic because of the positively charged d-alanine esters that decorate the polyol phosphate backbone (Figure 3A,C).46 By altering the groups attached to the WTA backbone, cells modulate their antibiotic susceptibility, increase virulence, and improve their survival.43 In Clostridium difficile, the addition of d-alanine to teichoic (both wall teichoic and lipoteichoic) acids is directly related to exposure of the bacteria to the host innate immune factor, cationic antimicrobial peptides (CAMPs), and provides a mechanism for resisting this family of antimicrobial agents.47 Additionally, the presence of WTAs is crucial for maintaining cell wall structure and cell shape. For example, deletion of WTAs in Bacillus subtilis changes cell morphology42 and peptidoglycan thickness.48 B. subtilis WTAs are also important for the correct function of proteins involved in cell elongation;42,49 in spherical Staphylococcus aureus cells, WTAs appear to be promiscuous and play roles in both cell elongation and cell division.48
Figure 3.
Glycopolymers of Gram-positive bacteria. (A) Wall teichoic acids (WTAs) are cross-linked to the peptidoglycan (orange) through a linkage unit (red), which is followed by two glycerol phosphate units (green) and repeating poly(alditol phosphate) units depicted in panel C. Dashed lines indicate the connection to the cross-linked peptidoglycan. (B) Lipoteichoic acids (LTAs) consist of a glycolipid anchor (blue) and repeating poly(glycerol phosphate) units. (C) The structure of poly(alditol phosphate) in WTAs and LTAs consists of glycerol phosphate or ribitol phosphate polymers that range in length from 20 to 40 repeat units (n = 20–40). X and Y indicate the location of chemical modifications to the polysaccharide chain of WTAs and LTAs.
Although little is presently known about the mechanical contribution of WTAs in Gram-positive bacteria, the influence of this family of molecules on cell morphology and peptidoglycan thickness may affect the mechanical properties of the cell. In support of this hypothesis, the absence of WTAs in S. aureus cells is hypothesized to remove the electrostatic repulsion that occurs between neighboring WTA molecules and results in a cell with a more compact layer of peptidoglycan.50
Gram-Positive Lipoteichoic Acids (LTAs).
In addition to WTAs, Gram-positive bacteria contain a second family of glycopolymers termed lipoteichoic acids (LTAs), which extend from the cytoplasmic membrane to the extracellular space immediately surrounding cells (Figure 1B). LTA molecules consist of two distinct structural components (Figure 3B): (1) a glycolipid anchor that attaches LTA to the inner membrane of all Gram-positive bacteria and (2) the main polymeric chain consisting of glycerol phosphate (Figure 3B,C).51 LTA is generally considered essential for cell viability and plays a role in the construction and placement of the peptidoglycan layer and in cellular integrity.48 For example, LTAs in the rod-shaped bacterium B. subtilis are involved in cell division.49 A suppressor mutation in GdpP, a cyclic di-AMP phosphodiesterase in S. aureus, enables the deletion of LTA and causes an increase in the extent of peptidoglycan cross-linking.52 Although there are no direct measurements of cell mechanics in this mutant, this result may indicate that LTA plays a functional role in cell mechanics and can be compensated by increasing the stiffness of the peptidoglycan layer.
Similar to WTA, LTA is negatively charged and contains a main polymeric chain decorated with d-Ala esters that creates a zwitterionic layer (Figure 3B,C). Incorporation of d-Ala into LTA is regulated through the dlt operon (consisting of four genes, dltA, dltB, dltC, and dltD) and provides cells with several adaptive advantages, including improved adhesion to host cell surfaces and cell invasion and resistance to cationic antimicrobial peptides.51 For example, LTA in S. aureus cells is modified with d-Ala esters.53 A loss of d-Ala esters caused no visible change in the S. aureus cell shape; however, transmission electron microscopy demonstrates that these cells experience an increase in peptidoglycan thickness, a concave membrane topology, and an elevated frequency of cell lysis. d-Alanylation of S. aureus LTA is essential for cell viability in the absence of WTA.48 In contrast, the loss of d-alanylation of LTA in Streptococcus agalactiae does not alter the cell morphology or peptidoglycan thickness; instead, the Young’s modulus of the cell wall was reduced from 173.3 to 7.9 MPa, which represents a 21-fold change in mechanical properties and indicates that LTA is a significant mechanical element in Gram-positive bacteria.54 The importance of d-alanylated LTA for these phenotypes and its regulation are not understood presently; however, it is clearly connected to cell wall architecture and cell mechanics, and future studies will aid in characterizing these roles.
Isolated LTA forms unstable monolayers in the absence of membrane phospholipids.55 Mixing LTAs with dipalmitoyldi-phosphatidylglycerol, a phospholipid found in the membranes of Gram-positive bacteria, increases the stability and rigidity of the monolayer membrane.55 LTA is also stabilized in phospholipid vesicles containing diacylglycerol and phosphatidylglycerol.56 These studies illustrate that the membrane acts as a scaffold to stabilize the LTA layer in Gram-positive bacteria and suggests a role for LTA and WTA in cell mechanics.57
Peptidoglycan.
The peptidoglycan is the cross-linked polymeric meshwork that encapsulates bacterial cells (Figure 1A,B), with the notable exceptions of Mycoplasma and Ureaplasma,58 and has historically been considered to be the canonical material in bacteria that imparts cell mechanical properties. The peptidoglycan thickness varies in Gram-negative (2.5–6.5 nm thick when fully hydrated) and Gram-positive bacteria (19–33 nm thick when fully hydrated) (Figure 1A,B).59–63 Although varying in thickness, the peptidoglycan of Gram-negative and Gram-positive bacteria shares a similar structure consisting of polysaccharide chains cross-linked with peptides (Figure 4A–C).
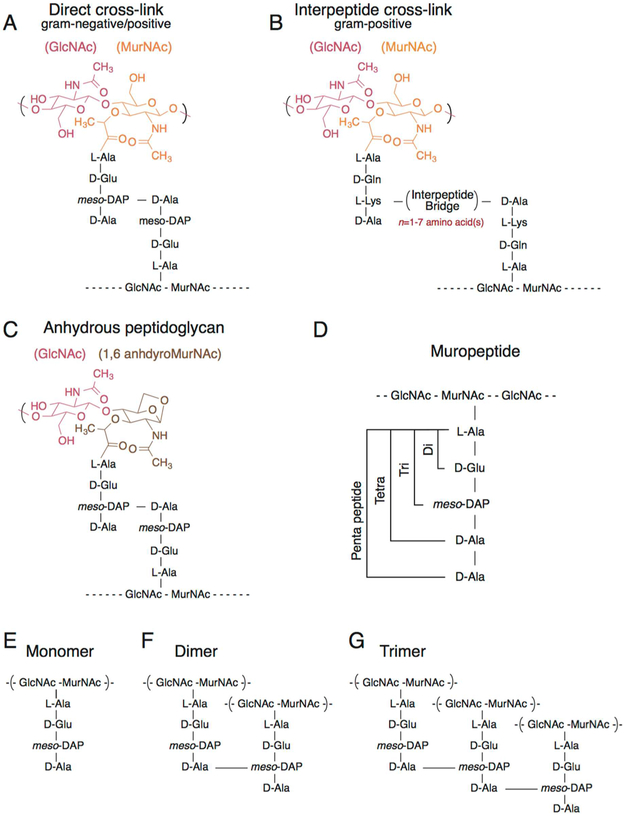
Figure 4.
Structure of the peptidoglycan. (A) Structure of cross-linked meso-DAP containing peptidoglycan found in both Gram-negative and Gram-positive bacteria. A 3–4 cross-link is depicted between meso-DAP in position 3 and d-Ala in position 4. (B) Structure of l-Lys peptidoglycan cross-linked through an interpeptide bridge ranging from one to seven amino acids that is found only in Gram-positive bacteria. A 3–4 cross-link is shown between l-Lys in position 3 and d-Ala in position 4. (C) Structure of anhydrous-terminated peptidoglycan containing 1,6-anhydroMurNAc. (D) Cartoon depicting the length of the stem peptides ranging from di (two amino acids) to tri (three amino acids) to tetra (four amino acids) to penta (five amino acids). (E) Structure of monomeric peptidoglycan containing a meso-DAP tetrapeptide. (F) Structure of dimeric peptidoglycan containing a meso-DAP tetrapeptide cross-linked at position 4–3. (G) Structure of trimeric peptidoglycan containing a meso-DAP tetrapeptide cross-linked at position 4–3.
The glycan backbone of peptidoglycan is highly conserved across bacteria and consists of alternating N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) monomers linked by a β-1,4 glycosidic bond.59 A “stem peptide” is attached to the C-3 hydroxyl group of each MurNAc monomer. In Gram-negative bacteria, the most common stem peptide consists of the five-amino acid sequence l-Ala-d-Glu-meso-diaminopimelic acid (meso-DAP)-d-Ala-d-Ala (Figure 4A). This primary five-unit peptide structure is shared by some Gram-positive bacteria (Figure 4B); meso-DAP at position 3 in the peptide can be replaced by l-Lys.64 Cross-linking the stem peptides tethers the polysaccharide chains (Figure 4). In Gram-negative bacteria, adjacent peptides are cross-linked to produce an abundant 3–4 linkage between position 3 (meso-DAP) and position 4 (d-Ala)65 or a less common 3–3 linkage (Figure 4A).66,67 In Gram-positive bacteria, adjacent peptides are cross-linked68,69 or may be tethered through an interpeptide bridge69 to produce 2–4 or 3–4 linkages (Figure 4A).59 The interpeptide bridge may be one to seven peptides in length and consist of various amino acids (Figure 4B).59
Peptidoglycan contains >50 individual types70 of peptidoglycan subunits [also termed muropeptides (Figure 4D)] that are characterized as monomeric (Figure 4E) (not containing cross-links), dimeric (Figure 4F) (containing one cross-link), or trimeric (Figure 4G) (containing two cross-links) depending on the number of cross-links formed to a single peptide stem.71 A high degree of cross-linking is indicative of a stiff peptidoglycan layer, and the relative abundance of peptide cross-links in peptidoglycan is an indicator of the stiffness of the material.71,72 Peptidoglycan is a porous material and lacks an ordered macromolecular structure; the pore size of Gram-negative peptidoglycan ranges from 4 to 25 nm in diameter.50,73–75 Electron cryotomography studies of Gram-negative bacteria reveal that the stem peptides are aligned along the long axis of the cell and glycans are wrapped circumferentially around the cell,60 which is hypothesized to impart directional (e.g., anisotropic) mechanical properties on cells. This organization enables the peptide bonds to swell and shrink with changes in turgor pressure, while the more rigid glycan chains remain relatively unchanged.76 In contrast to structural features that have been revealed in Gram-negative bacteria, the thickness of the peptidoglycan layer in Gram-positive bacteria (Figure 1B), the orientation of the stem peptides, and the position of the glycan strands relative to the axis of the cell are generally unknown. Solid-state nuclear magnetic resonance (NMR) studies of isolated peptidoglycan layers are starting to provide insight into its structure and the orientation of its components.77 There have been several proposed models of peptidoglycan orientation and growth in Gram-positive bacteria,78–80 including a model similar to the structure in Gram-negative bacteria.
The Young’s modulus of peptidoglycan has been measured in wild-type Gram-negative and Gram-positive bacteria.17,18,81–83 A recent understanding of the roles of the different penicillin-binding proteins (PBPs) in peptidoglycan synthesis84 and new analytical tools for rapidly determining peptidoglycan structure (e.g., cross-linking and glycan length), such as UPLC-MS,85 has enabled studies to quantitatively investigate how changing peptidoglycan structure affects its stiffness. A recent screen of all nonessential genes in Escherichia coli confirmed that cell wall and membrane biogenesis genes represent the largest functional family of genes that are connected to cell mechanics.6 The study demonstrated that removing the major PBP, PBP1b, decreased E. coli cell stiffness by ~50% (to 12 MPa), which may be approaching a lower limit required to maintain cell viability in E. coli. Combining the use of antibiotics to reduce PG biosynthesis, methods for measuring cell mechanics (as described in a later section), and imaging to determine when cells have lysed may enable the determination of a lower limit of PG mechanics required to maintain viability. Determining an upper and lower limit for bacterial cell stiffness can help frame these and other values measured and provide a sense of scale for understanding their relevance. A lower-limit measurement may entail measuring the stiffness of l-forms or bacteria that lack a peptidoglycan layer of the cell wall (e.g., Mycoplasma).
Increasing glycan strand length and cross-linking level enhances the mechanical properties of bacterial cells.86–88 For example, four enzymes with essential glucosaminidase activity in S. aureus are responsible for hydrolysis of the bonds connecting GlcNAc and MurNAc.88 Loss of the glucosaminidase activity of these enzymes increases glycan strand chain length, which has been hypothesized to increase the number of cross-links per strand and increase cell wall stiffness.88 Altering the glycan chain length has been proposed as a mechanism for increasing the stiffness of peptidoglycan when the level of cross-linking is reduced.89 S. aureus enzyme penicillin-binding protein 4 (PBP4) is a nonessential enzyme that provides further cross-linking of peptides in the peptidoglycan, and its deletion reduces the Young’s modulus of cells by 2–4-fold.87 The small magnitude of the stiffness changes upon PBP4 deletion is consistent with its role as a nonessential enzyme in PG cross-linking.
The antibiotic lysostaphin decreases the level of peptide cross-linking in S. aureus and reduces the Young’s modulus of the cell by ~10-fold and may alter cell stiffness by decreasing the level of cross-linking or targeting multiple cross-linking enzymes.86 Classes of biomolecules (e.g., membranes) in the cell envelope that interact with the peptidoglycan through proteins, such as the lipoprotein Lpp (see below), are also candidates for influencing cell stiffness.
Lipid Bilayers.
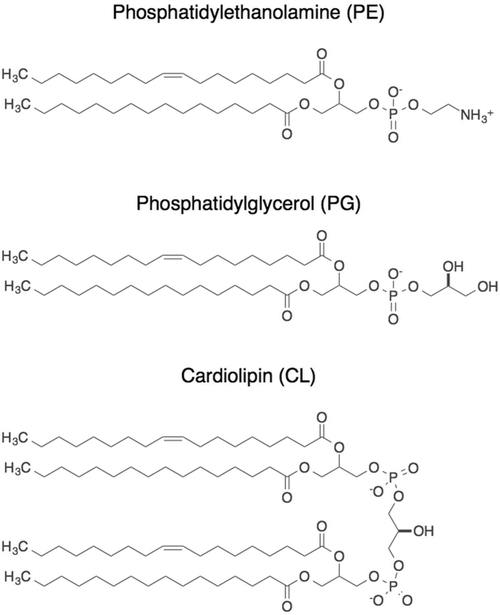
Phospholipid membranes are essential liquid crystalline structures that encapsulate the cytoplasm in all bacteria. Gram-positive bacteria contain a single inner phospholipid bilayer, and Gram-negative bacteria have two lipid bilayers (Figure 1A). These membranes consist of three primary families of phospholipids: phosphatidylethanolamine (70–80% of total lipids), phosphatidylglycerol (20–25% of total lipids), and cardiolipin (5–10% of total lipids) (Figure 5). Bacterial membranes can contain a mixture of phospholipids, proteins, lipopolysaccharides, and lipoteichoic acids, and two primary forces hold membranes together: (1) electrostatic interactions between charged polar lipid head groups and their association with divalent cations and (2) van der Waals forces between adjacent fatty acyl chains.
Figure 5.
Major phospholipids of Gram-negative and Gram-positive bacteria. In E. coli membranes, phosphatidylethanolamine (PE) represents 70–80% of total lipids, phosphatidlyglycerol (PG) represents 20–25% of total lipids, and cardiolipin (CL) represents 5–10% of total lipids. Phospholipids are shown with alkyl tails representing the most common degree of unsaturation found in E. coli membranes. Double bonds can be located in different positions and have different geometries (cis as shown, or trans), and alkyl tails can have multiple unsaturated bonds.
Similar to the case for the peptidoglycan, changes in membrane thickness can affect the bending rigidity of the lipid membrane.90 Bending rigidity increases with the square of the bilayer thickness, and membranes have an approximate bending energy of ~20 kBT.91,92 E. coli membranes consist of three classes of phospholipids that contain acyl groups with the following ratio of total acyl chain length per lipid to total number of unsaturations present: phosphatidylglycerol (32:1), phosphatidylethanolamine (32:1), cardiolipin (66:2); phosphatidylglycerol and phosphatidylethanolamine contain two acyl groups, and cardiolipin contains four acyl groups. Figure 5 highlights these most abundant E. coli lipids and the single unsaturated acyl chain.93 The introduction of unsaturated lipids alters the geometry of acyl chains, making them shorter and wider, reduces their packing order in the membrane, and alters lipid bilayer rigidity.94 For example, the presence of two or more cis double bonds in a fatty acyl chain alters packing and results in a membrane with a 2-fold decrease in rupture tension and an ~2–5-fold increase in water permeability.95 Not surprisingly, the composition of lipid mixtures can alter the mechanical properties of membranes. Vesicles consisting of an E. coli lipid extract are ~50% less stiff than those containing only phosphatidylglycerol (in this case, in which each chain is unsaturated).96 Combining techniques for measuring cell mechanics with the large number of available mutants in which lipid composition has been altered will make it possible to quantitatively frame the magnitude of membrane contributions on cell mechanics.
BACTERIAL PROTEINS THAT HYPOTHETICALLY CONTRIBUTE TO CELL STIFFNESS
The penicillin-binding proteins (PBPs) make up a class of proteins that affect the mechanical properties of the cell by directly altering the cross-linking and glycan strand length of the peptidoglycan and were described previously in Peptidoglycan. Additionally, other membrane proteins, including OmpA97 and the Tol–Pal complex,97 interact with multiple layers of the cell wall and may influence cell mechanics.98,99 Proteins in this category modulate the physical properties of cells through interactions with components of the cell wall. Of the ~4300 genes present in E. coli, only a few genes encode proteins that are known to alter the mechanical properties of bacterial cells without affecting characteristics of the peptidoglycan layer (beyond the PBPs), which we summarize below.
MreB.
In many rod-shaped Gram-negative and Gram-positive bacteria, insertion of new peptidoglycan is coordinated by the bacterial actin cytoskeleton homologue, MreB.100 MreB monomers polymerize into filaments that rotate circumferentially around the long axis of cells.101–104 E. coli MreB is positioned in contact with the cytoplasmic membrane through an N-terminal amphipathic helix105 and has been hypothesized to localize a protein complex containing proteins that perform peptidoglycan synthesis and degradation to regions of the cell wall with negative curvature.106,107 The directed motion of MreB in these regions of the cell is correlated with peptidoglycan assembly and enables cells to maintain a rod shape, 107 as inhibiting MreB function causes cells to gradually change shape from a rod to a sphere.108 Despite the attention that MreB has received and the large number of studies to date, a clear picture of its role in cell physiology has yet to emerge. Although the direct connection of MreB to the assembly of the load-bearing material, peptidoglycan, remains controversial, MreB is a candidate for studies to test whether alterations in its structure and function change cell mechanics.
Several measurements have been performed to determine the contribution of MreB to bacterial cell mechanics. Depolymerization of MreB in E. coli cells followed by applying a bending stress demonstrated that these cells had an ~30% decrease in bending rigidity compared to that of wild-type cells.3 Using a compressive force to measure the longitudinal stiffness of cells, no change in stiffness was observed after depolymerizing MreB using the small molecule antagonist of polymerization, A22;2 these results were attributed to the time scale of MreB inhibition and its residence time on the cell membrane. Specifically, to affect the longitudinal stiffness of cells, MreB would need to remain attached to the cell membrane for a time scale that is incompatible with the rapid turnover of MreB filaments, their detachment from the cell wall, their diffusion, and their reattachment to the cell wall to coordinate peptidoglycan growth.109
Lpp.
Lpp (also termed Braun’s lipoprotein) is the most abundant lipoprotein in E. coli and is located in the inner leaflet of the outer membrane (Figure 1A).110 Lpp is also present in other Gram-negative bacteria; however, it is typically found only in bacteria that are enteric and endosymbionts in which it enables them to adapt to high-osmolarity environments.98 The Lpp is covalently attached to the peptidoglycan layer, and it physically tethers the outer membrane and the peptidoglycan together; ~30% of the 5 × 105 copies of Lpp in each cell are covalently attached to the meso–DAP residue in the peptide stem of the peptidoglycan.110 Loss of Lpp from cells increases the level of formation of membrane vesicles and decreases membrane integrity.111 In E. coli, loss of Lpp reduces the effective rigidity of cells by 42% and decreases the viscosity of the membrane bilayer, which may make cells more deformable in response to external forces.112
Newly Discovered Proteins.
With the aim of developing an understanding of how bacteria control their mechanical properties, we recently developed a high-throughput measurement technology discussed in the next section below to identify nonessential genes in E. coli that modulate bacterial stiffness. We identified 41 candidate genes (of ~4000 studied) that decreased bacterial stiffness when they were deleted and five genes that increased bacterial stiffness upon their deletion. We found that bacterial cell stiffness is modulated by a diverse set of functional gene categories: the largest category of genes represented cell wall and membrane biogenesis (e.g., mrcB, lpoB, and pal), followed by energy production and conversion (e.g., iscA, iscU, and gor), DNA replication/recombination and repair (e.g., holC, dnaT, and recA), and amino acid transport and metabolism (e.g., glnA, trpB, and gmhB).6 We determined that the deletion of the proteins encoded by these genes altered cell stiffness without changing the chemical composition of peptidoglycan (using liquid chromatography to analyze its composition). These results led us to conclude that these proteins encode mechanical elements or are connected to control over the peptidoglycan or other mechanically relevant material in the cell wall. The identification of these diverse proteins indicates that cell stiffness is dependent on the function and coordination of numerous intracellular pathways and suggests a rich area for biochemical, biophysical, and cell biological studies.
MEASURING THE MECHANICAL PROPERTIES OF BACTERIAL CELLS
The small physical dimensions of bacteria (having a length scale that is typically several micrometers) presents a challenge for quantitatively measuring their mechanical properties. Early studies of the mechanical properties of bacteria took advantage of tensile testing (a bulk material technique) to measure the properties of aggregates of bacterial cells encapsulated in secreted polymers and yielded “composite” Young’s modulus measurements of 10 MPa.18 Several other techniques have been developed to measure the mechanical properties of bacterial cells, including microfluidic-based assays, optical trapping, growth measurements in encapsulated polymers, and AFM, which yield Young’s modulus values of 0.05–769 MPa for different bacteria2 and are described below. Measurements of mechanical properties are sensitive to experimental conditions, and significant differences appear in values measured by different techniques.2 Consequently, it is best to compare values between different bacteria, mutants, and conditions measured by a single technique and important to compare samples that have been prepared similarly;2 for example, dehydrating a sample can lead to a drastic increase in cell stiffness,19 treatment with a chelating agent decreases cell stiffness,16 and the ionic strength of the media used in experiments can drastically alter the measured stiffness.113
Measurements of Osmotic Sensitivity.
Osmotic pressure is the force applied per unit area on a semipermeable membrane to prevent the movement of water across it because of a mismatch in the concentration of solutes on either side of the membrane. Changes in the osmotic environment cause rapid changes in the osmotic pressure of bacteria and can lead to cell lysis through the movement of water into the cell or dehydration of the cytoplasm through the movement of water out of the cell.114 Osmotic pressure is countered by the mechanical properties of the cell wall and other associated structures, and it can be probed experimentally by microscopy or growth-based assays, making it a potentially convenient way to measure the connection between biochemical changes and mechanical alterations in cells.
Two types of methodologies, bulk measurements and singlecell measurements, have been used to determine the sensitivity of bacteria to changes in osmotic pressure. Bulk measurements of osmotic sensitivity entail diluting cells into a hypoosmotic medium in which cells swell or diluting in a hyperosmotic medium in which cells shrink or undergo plasmolysis.115 The surviving fraction of cells after osmotic shock is determined by plating the cells on nutrient agar, incubating to grow surviving cells, and counting the resulting colony-forming units (or surviving cells).116 This is one of the most accessible measurements available; however, it does not produce quantitative values of direct cell properties. Instead, it yields indirect information about the mechanical status of cells. More recent methodologies make it possible to directly monitor the response of single bacterial cells to osmotic shock using microfluidic-based systems in conjunction with microscopy to apply an osmotic shock and monitor single-cell response and survival.117–119 These studies indicate that the rate of applying an osmotic shock is an important variable in cell survival; a slower change in the local osmotic environment is correlated with a higher number of surviving cells.117 By monitoring the response of single cells to osmotic shock, we can quantify the change in cell length and width,119 which provides an indirect measurement of the extendibility of the peptides and polysaccharides within the peptidoglycan, respectively.120 When it is desirable to quickly determine whether the mechanical properties of a cell may have changed, osmotic shift experiments are an ideal first step.
Atomic Force Microscopy.
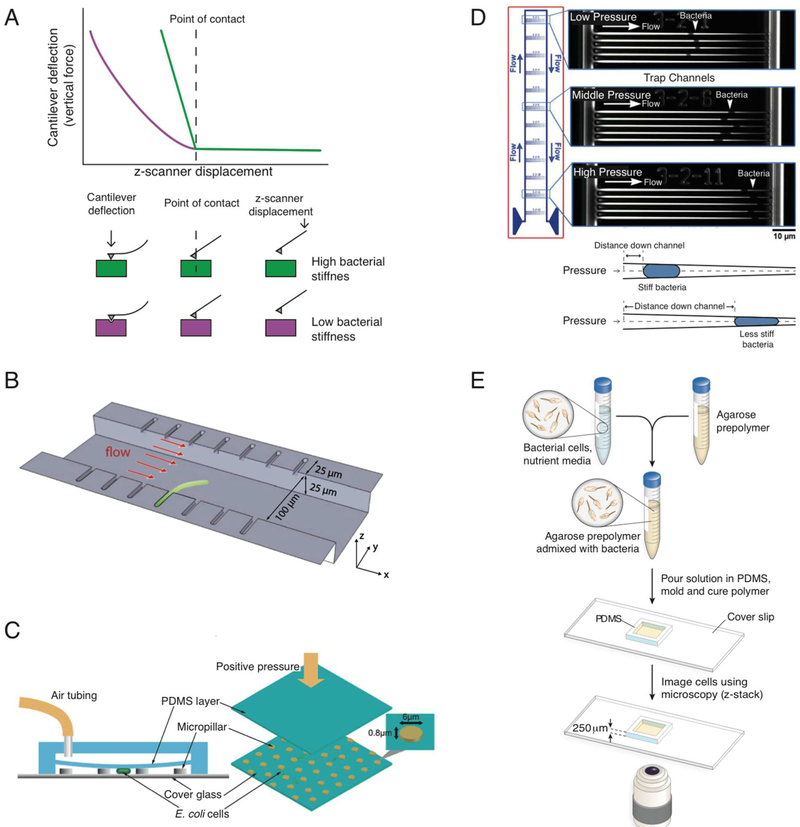
AFM is a technique that has been used to measure the mechanical properties and surface topography of bacteria at nanometer resolution (Figure 6A).121 The Young’s modulus of a bacterial cell or a sample of an isolated, intact cell wall can be determined from force–distance curves by measuring the deflection of a cantilever containing an applied load122 and fitting the data with a Hertz model that assumes a bacterial cell is an isotropic and linear elastic solid.17 However, not all samples exhibit these idealized properties, and several different models may be required to extract salient mechanical data.17 Peak force tapping AFM is an imaging mode that is often used to quantify mechanical properties across an entire cell surface, as it accounts for heterogeneity in the properties of bacterial cells.73,123 AFM has provided important insight into the mechanical properties of cells, many of which have been highlighted in previous sections of this review, including measurements of the Young’s modulus for whole cells17,81,86,87 and isolated peptidoglycan.19 AFM is a powerful technique with several caveats that should be considered when making stiffness measurements of bacteria or isolated peptidoglycan sacculi. Samples should be kept fully hydrated throughout the experiment, as imaging in air has been shown to increase measured values.19 The response of the AFM probe is sensitive to its orientation with respect to the sample surface, i.e., perpendicular or at an arbitrary angle, and measurements of cells should be kept to the center of bacterial cells to obtain the most accurate measurements.124 If the mechanical force applied to the bacterial cell by the AFM probe is excessive, the tip can pierce the cell wall and cause a sudden drop in the measured stiffness.125 AFM is a powerful technique, yet nuanced and best performed by experts.
Figure 6.
Techniques for measuring bacterial cell stiffness. (A) Atomic force microscopy. The top panel shows a force–distance curve of a bacterium with high bacterial stiffness (green) and low bacterial stiffness (purple). The bottom panel is an illustration of an AFM probe contacting the surface of a cell with high or low stiffness. (B) Microchannel measurements of cell bending rigidity. Bacteria are loaded and filamented in microfluidic channels, and fluid flow through the central channel applies a force on cells; cells bend when the magnitude of force is sufficient. Cartoon reproduced with permission from ref 4. Copyright 2014 National Academy of Sciences. (C) Transverse compression microfluidic device. The left panel is a side view of the poly(dimethylsiloxane) (PMDS) microfluidic device. The right panel is a three-dimensional view of the device. Cells are placed within a microfluidic device consisting of a glass coverslip patterned with 0.8–0.9 μm tall micropillars positioned below a polymer layer with a height that can be controlled by air pressure. Cartoon reproduced with permission from ref 122. Copyright 2002 American Society for Microbiology. (D) Extrusion loading microfluidic device. In the top panel, 12 parallel channels have diameters that taper between the entrance and exit; fluid flow pushes cells into channels and applies loads on them ranging from 0.0037 to 0.045 MPa. In the bottom panel, at equivalent pressures, cells that are more deformable are forced further down tapered channels than stiff cells. Cartoon reproduced with permission from ref 19. Copyright 1999 American Society for Microbiology. (E) Bacterial growth encapsulated in agarose. Cells are mixed with a solution of warm agarose, poured into a PDMS mold, and gelled. The cells are imaged at 1 min intervals using phase contrast microscopy to monitor cell growth. Cartoon reproduced with permission from ref 2. Copyright 2012 John Wiley & Sons, Inc.
Microfluidic-Based Assays.
Microfluidic structures have physical dimensions that can match individual or small groups of cells and provide unique capabilities for measuring cell mechanics, including Young’s modulus, bending rigidity, and osmotic pressure. A microfluidic system for measuring cell bending rigidity was reported in which bacteria are filamented inside of channels positioned so that the majority of the volume of the cell is oriented within a main flow channel (Figure 6B).4 The flow of fluid through the central channel applies a force to cells and, when the magnitude is sufficient, causes cells to bend; measuring cell deflection and fitting to a mechanical model enable the determination of bending rigidity. This approach was used to measure the bending rigidity of Gram-negative (5 × 10−20 N m2) and Gram-positive bacteria (2.4 × 10−19 N m2) and extract values of Young’s modulus of 30 and 20 MPa, respectively.4 There are several challenges with this technique. For example, measurements are very sensitive to the relative diameter of the cell and the neck of the channel in which the cell is positioned; a mismatch in these dimensions produces large deviations in the pressure measured for deforming cells under a fluid flow. Another challenge is that all of the experimental steps, including cell growth, need to be performed in the device. Measurements with this device require cells that are ~50 μm long, which is approximately 1 order of magnitude longer than most bacteria and limits the technique to rod-shaped bacteria. To accommodate the length requirement, cells can be elongated (i.e., “filamented”) using an antibiotic that inhibits cell division (e.g., cephalexin or aztreonam) or engineered to overexpress the cell division inhibitor, SulA. The effect of cephalexin or aztreonam on cell mechanics, potentially at the site of blocked division, is not known. However, it has been shown that overexpression of SulA has the potential to mask changes in cell stiffness.6 This technique provides quantitative data about bending rigidity; however, it requires fabrication of channels (the channel systems are not commercially available) and is limited to providing values of bending mechanics, making it less approachable than the techniques presented that do not require special materials or instruments (e.g., osmotic shifts).
Another approach to measuring cell mechanical properties is based on transverse compression. Measuring the rate of change in the radius of curvature of E. coli cells under compression was used to extract a Young’s modulus value for cells.126 Briefly, cells are placed within a microfluidic device consisting of a glass coverslip patterned with 0.8–0.9 μm tall micropillars that are positioned below a PDMS layer with a height that can be controlled by air pressure. Applying a pressure compresses the polymer ceiling and applies a force on cells, while the micropillars provide a “stop” to ensure that the deformation of cells (diameter, ~1 μm) does not extend beyond 10–20% of their initial dimensions (Figure 6C). This approach produced Young’s modulus measurements of 22 MPa and a turgor pressure of 140 kPa (~1.4 atm) for E. coli cells. Under compression, mechanical stress was concentrated at the periphery of cells and caused membrane blebbing.
Extrusion loading microfluidic techniques are based on previously developed micropipette-based methods, which have been used to study the mechanical properties of neutrophils.127 An extrusion loading microfluidic system has been reported in which the channel width at the entrance is 1.4 μm and tapers to a width of 250 nm at the exit;20 12 tapered, parallel channels apply a load on bacterial cells ranging from 0.0037 to 0.045 MPa. Cells are loaded in microfluidic channels with very stiff walls, and fluid flow pushes them deeper into the channel; their deformability controls the distance they travel within the channel before stopping (Figure 6D). Extrusion loading has not been used to quantitatively measure specific numerical values for the Young’s modulus, bending rigidity, or turgor pressure of bacterial cells. However, this technique makes it possible to monitor qualitative changes in bacterial stiffness based on the distance a cell is forced into the tapered channel by the applied load. This microfluidic system qualitatively demonstrated that Gram-negative cells (e.g., E. coli) were less stiff than Gram-positive cells (e.g., B. subtilis),20 an observation that has been demonstrated previously.2,4 Loading samples and operation of these microfluidic devices are challenging; the authors indicate that ~30% of the devices produced unacceptable variation in either channel occupancy or pressure. An additional caveat of using this technique is the comparison of cells of different sizes, as it would require the use of a scaling factor to relate the cell size to the dimensions of the channels. Although some techniques2,4 can be used only to measure rod-shaped bacteria, this device makes it possible to compare cells with variable shapes. The low fabrication yield and requirements for microfabrication provide limitations on this technique.
Cell Growth Encapsulated in Polymers.
A recently described method, known as cell length analysis of mechanical properties (CLAMP), demonstrates that measuring the rate of growth of individual cells in polymer environments can estimate values of the Young’s modulus of Gram-negative (E. coli, ~100 MPa; Pseudomonas aeruginosa, ~150 MPa) and Gram-positive bacteria (B. subtilis, ~150 MPa).2 Microscopy was used to measure the growth of single cells embedded in agarose hydrogels of tunable mechanical stiffness, and a decrease in relative cell elongation over time was observed in agarose gels with an increased stiffness (Figure 6E). A finite-element model of the growth of a thin, elastic shell was used to fit the experimental data and extract values of the composite Young’s modulus. One underlying deficiency of this technique is the necessity to increase the number of gel percentages used to embed bacterial cells to decrease the error in fitting data. The current theory and modeling is also limited to rod-shaped bacteria.
A recent modification of this method, known as general regulators affecting bacterial stiffness (GRABS), enables the use of a plate reader to automate cell growth measurements in agarose gels and was used to assay a genome-wide collection of nonessential gene mutants (knockouts) in E. coli to identify 46 modulators of cell stiffness.6 Briefly, we measured the optical density (OD) of mutant cells embedded both in 1% agarose and in liquid growth medium. By calculating the percent change in the OD of each mutant compared to that of wild-type cells, we generated a metric known as the GRABS score in which a negative score indicated a decrease in bacterial cell stiffness and a positive score indicated an increase in cell stiffness. Although the GRABS technique determines a qualitative score, we used a microfluidic system for measuring cell bending rigidity to confirm a correlation between the GRABS score and bacterial cell stiffness. This method provides a new capability for rapidly assessing the mechanical contributions of a large collection of mutants and assessing the mechanical genomics of the cell.6 It also brings to light the complementary use of multiple techniques to first rapidly but qualitatively identify genes of interest and then to quantitatively probe these genes to determine their effect on the stiffness of bacterial cells.
The repertoire of tools that have emerged for measuring bacterial mechanics provides exciting new capabilities that will accelerate the development of this field. These techniques can be separated into qualitative and quantitative techniques: qualitative techniques (e.g., osmotic shifts and GRABS) provide two of the most experimentally approachable techniques for querying changes in cell mechanics and may be an excellent starting point for further experiments. Of these techniques, GRABS provides capabilities to assay large numbers of different strains and mutants in parallel. Methods for determining quantitative changes in mechanics require expensive instrumentation (e.g., AFM) or microfabricated materials, which are not commercially available. Of the microchannel techniques, the most compelling may be the method for measuring bending moduli as hundreds of cells can be assessed in parallel,4 the systems can be reused, they are made in materials that are easy to prototype, and the theory has been developed for fitting data and extracting stiffness values.
CONCLUSIONS
The study of bacterial cell mechanics is an area of microbiological and biophysical research that is gaining inertia. The introduction of new tools for measuring bacterial cell mechanics, particularly methods that are accessible to scientists in areas outside of mechanics and physics, enables experiments that impact an understanding of the biology, biophysical, and genetics underlying this property of bacteria and its conservation across the bacterial kingdom. These methods for directly measuring cell mechanics can be combined with other techniques that provide insight into biochemical or structural changes in cells, including (1) ultraperformance liquid chromatography–mass spectrometry (UPLC-MS) to rapidly determine the structure of the peptidoglycan (e.g., cross-linking and glycan length),85 (2) AFM to map the macroscale features of the peptidoglycan (e.g., pores, holes, defects, and glycan strand orientation),73,74 (3) electron cryotomography to visualize changes in the dimension of the cell envelope (e.g., peptidoglycan, periplasm, and lipid membrane thickness),128 and (4) genetic screens to identify genes and proteins that are connected to changes in bacterial cell mechanics.6
This area of research has an opportunity to illuminate both fundamental and applied science. For example, bacteria undergo changes in phenotype when occupying new niches. Uropathogenic E. coli cells change shape during urinary tract infections, and their new morphology is hypothesized to enable them to avoid predation and engulfment by macrophages.129 Genetic drift can lead to changes in bacterial cell shape linked to known mechanical components of the cell, including the peptidoglycan130 and MreB.131 Some bacteria that have adapted to live within a host environment exhibit a loss of peptidoglycan or a decrease in the number of genes that encode proteins that construct this material.132 It is plausible that some of these adaptive changes are accompanied by alterations in bacterial cell mechanics. Studying these processes may shed light on how bacteria adapt to their environment and provide insight into mechanisms for controlling their growth in specific niches that is relevant to antimicrobial chemotherapies. Identifying and characterizing proteins connected to changes in cell mechanics may also yield new targets for designing antibiotics that can be used to make cells more prone to osmotic pressure, environmental perturbations, or small molecule inhibitors that target other biochemical machinery.
A current challenge in this field is that there are not yet enough quantitative data with which to compare measurements and interpret their physical meaning. Additional studies will create a baseline for bacterial cell mechanics, enable an understanding of minimum and maximum values, and define mechanical values in terms of the structure and organization of cells. This field is at an early and exciting stage in its development and may uncover new biology related to bacterial adaptation and evasion by predators and insight into antimicrobial technologies.
ACKNOWLEDGMENTS
We thank Manohary Rajendram, Hannah Tuson, and Joseph Dillard for reading the review and providing feedback.
Funding
The National Institutes of Health (1DP2OD008735-01) and National Science Foundation (DMR-1121288) provide support for the research in this area within our lab.
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Reuter M, Hayward NJ, Black SS, Miller S, Dryden DT, and Booth IR (2014) Mechanosensitive channels and bacterial cell wall integrity: does life end with a bang or a whimper? J. R. Soc., Interface 11, 20130850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Tuson HH, Auer GK, Renner LD, Hasebe M, Tropini C, Salick M, Crone WC, Gopinathan A, Huang KC, and Weibel DB (2012) Measuring the stiffness of bacterial cells from growth rates in hydrogels of tunable elasticity. Mol. Microbiol 84, 874–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wang S, Arellano-Santoyo H, Combs PA, and Shaevitz JW (2010) Actin-like cytoskeleton filaments contribute to cell mechanics in bacteria. Proc. Natl. Acad. Sci U. S. A 107, 9182–9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Amir A, Babaeipour F, McIntosh DB, Nelson DR, and Jun S (2014) Bending forces plastically deform growing bacterial cell walls. Proc. Natl. Acad. Sci. U. S. A 111, 5778–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Louise Meyer R, Zhou X, Tang L, Arpanaei A, Kingshott P, and Besenbacher F (2010) Immobilisation of living bacteria for AFM imaging under physiological conditions. Ultramicroscopy 110, 1349–1357. [DOI] [PubMed] [Google Scholar]
- (6).Auer GK, Lee TK, Rajendram M, Cesar S, Miguel A, Huang KC, and Weibel DB (2016) Mechanical Genomics Identifies Diverse Modulators of Bacterial Cell Stiffness. Cell Syst 2, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, and Seufferlein T (2005) Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater. 1, 15–30. [DOI] [PubMed] [Google Scholar]
- (8).Suwanarusk R, Cooke BM, Dondorp AM, Silamut K, Sattabongkot J, White NJ, and Udomsangpetch R (2004) The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J. Infect. Dis 189, 190–194. [DOI] [PubMed] [Google Scholar]
- (9).Boal DH (2002) Mechanics of the cell, Cambridge University Press, Cambridge, U.K. [Google Scholar]
- (10).Rodriguez ML, McGarry PJ, and Sniadecki NJ (2013) Review on cell mechanics: experimental and modeling approaches. Appl Mech. Rev. 65, 060801. [Google Scholar]
- (11).Olliaro P (2008) Editorial commentary: mortality associated with severe Plasmodium falciparum malaria increases with age. Clin. Infect. Dis 47, 158–160. [DOI] [PubMed] [Google Scholar]
- (12).Zhang Y, Huang C, Kim S, Golkaram M, Dixon MW, Tilley L, Li J, Zhang S, and Suresh S (2015) Multiple stiffening effects of nanoscale knobs on human red blood cells infected with Plasmodium falciparum malaria parasite. Proc. Natl. Acad. Sci. U. S. A 112, 6068–6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Engler AJ, Sen S, Sweeney HL, and Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. [DOI] [PubMed] [Google Scholar]
- (14).Moeendarbary E, and Harris AR (2014) Cell mechanics: principles, practices, and prospects. Wiley Interdiscip Rev. Syst. Biol. Med 6, 371–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cerf A, Cau JC, Vieu C, and Dague E (2009) Nanomechanical properties of dead or alive single-patterned bacteria. Langmuir 25, 5731–5736. [DOI] [PubMed] [Google Scholar]
- (16).Chen YY, Wu CC, Hsu JL, Peng HL, Chang HY, and Yew TR (2009) Surface rigidity change of Escherichia coli after filamentous bacteriophage infection. Langmuir 25, 4607–4614. [DOI] [PubMed] [Google Scholar]
- (17).Gaboriaud F, Bailet S, Dague E, and Jorand F (2005) Surface structure and nanomechanical properties of Shewanella putrefaciens bacteria at two pH values (4 and 10) determined by atomic force microscopy. J. Bacteriol 187, 3864–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Thwaites JJ, and Mendelson NH (1985) Biomechanics of bacterial walls: studies of bacterial thread made from Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A 82, 2163–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Yao X, Jericho M, Pink D, and Beveridge T (1999) Thickness and elasticity of gram-negative murein sacculi measured by atomic force microscopy. J. Bacteriol 181, 6865–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sun X, Weinlandt WD, Patel H, Wu M, and Hernandez CJ (2014) A microfluidic platform for profiling biomechanical properties of bacteria. Lab Chip 14, 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hoffmann C, Leis A, Niederweis M, Plitzko JM, and Engelhardt H (2008) Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. U. S. A 105, 3963–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Dover LG, Cerdeno-Tarraga AM, Pallen MJ, Parkhill J, and Besra GS (2004) Comparative cell wall core biosynthesis in the mycolated pathogens, Mycobacterium tuberculosis and Corynebacterium diphtheriae. Fems Microbiol Rev. 28, 225–250. [DOI] [PubMed] [Google Scholar]
- (23).Erridge C, Bennett-Guerrero E, and Poxton IR (2002) Structure and function of lipopolysaccharides. Microbes Infect. 4, 837–851. [DOI] [PubMed] [Google Scholar]
- (24).Lameire N, and Mehta RL (2000) Complications of dialysis, Marcel Dekker, New York. [Google Scholar]
- (25).Delcour AH (2009) Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta, Proteins Proteomics 1794, 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Yu Z, Qin W, Lin J, Fang S, and Qiu J (2015) Antibacterial mechanisms of polymyxin and bacterial resistance. BioMed Res. Int 2015, 679109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Steeghs L, den Hartog R, den Boer A, Zomer B, Roholl P, and van der Ley P (1998) Meningitis bacterium is viable without endotoxin. Nature 392, 449–450. [DOI] [PubMed] [Google Scholar]
- (28).Lerouge I, and Vanderleyden J (2002) O-antigen structural variation: mechanisms and possible roles in animal/plant-microbe interactions. Fems Microbiol Rev. 26, 17–47. [DOI] [PubMed] [Google Scholar]
- (29).Smit J, Kamio Y, and Nikaido H (1975) Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J. Bacteriol 124, 942–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yethon JA, Heinrichs DE, Monteiro MA, Perry MB, and Whitfield C (1998) Involvement of waaY, waaQ, and waaP in the modification of Escherichia coli lipopolysaccharide and their role in the formation of a stable outer membrane. J. Biol. Chem 273, 26310–26316. [DOI] [PubMed] [Google Scholar]
- (31).Linkevicius M, Anderssen JM, Sandegren L, and Andersson DI (2016) Fitness of Escherichia coli mutants with reduced susceptibility to tigecycline. J. Antimicrob. Chemother. 71, 1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Amro NA, Kotra LP, Wadu-Mesthrige K, Bulychev A, Mobashery S, and Liu GY (2000) High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: Structural basis for permeability. Langmuir 16, 2789–2796. [Google Scholar]
- (33).Clifton LA, Skoda MW, Le Brun AP, Ciesielski F, Kuzmenko I, Holt SA, and Lakey JH (2015) Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 31, 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Labischinski H, Barnickel G, Bradaczek H, Naumann D, Rietschel ET, and Giesbrecht P (1985) High state of order of isolated bacterial lipopolysaccharide and its possible contribution to the permeation barrier property of the outer membrane. J. Bacteriol 162, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Naumann D, Schultz C, Sabisch A, Kastowsky M, and Labischinski H (1989) New insights into the phase behavior of a complex anionic amphiphile: architecture and dynamics of bacterial deep rough lipopolysaccharide membranes as seen by FTIR, X-Ray, and molecular modeling techniques. J. Mol. Struct 214, 213–246. [Google Scholar]
- (36).Le Brun AP, Clifton LA, Halbert CE, Lin BH, Meron M, Holden PJ, Lakey JH, and Holt SA (2013) Structural Characterization of a Model Gram-Negative Bacterial Surface Using Lipopolysaccharides from Rough Strains of Escherichia coli. Biomacromolecules 14, 2014–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Herrmann M, Schneck E, Gutsmann T, Brandenburg K, and Tanaka M (2015) Bacterial lipopolysaccharides form physically cross-linked, two-dimensional gels in the presence of divalent cations. Soft Matter 11, 6037–6044. [DOI] [PubMed] [Google Scholar]
- (38).Ivanov IE, Kintz EN, Porter LA, Goldberg JB, Burnham NA, and Camesano TA (2011) Relating the physical properties of Pseudomonas aeruginosa lipopolysaccharides to virulence by atomic force microscopy. J. Bacteriol 193, 1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Gaboriaud F, Dague E, Bailet S, Jorand F, Duval J, and Thomas F (2006) Multiscale dynamics of the cell envelope of Shewanella putrefaciens as a response to pH change. Colloids Surf., B 52, 108–116. [DOI] [PubMed] [Google Scholar]
- (40).Kamio Y, and Nikaido H (1976) Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry 15, 2561–2570. [DOI] [PubMed] [Google Scholar]
- (41).Brown S, Santa Maria JP Jr., and Walker S (2013) Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol 67, 313–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).D’Elia MA, Millar KE, Beveridge TJ, and Brown ED (2006) Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J. Bacteriol 188, 8313–8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Swoboda JG, Campbell J, Meredith TC, and Walker S (2010) Wall teichoic acid function, biosynthesis, and inhibition. ChemBioChem 11, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Araki Y, and Ito E (1989) Linkage units in cell walls of gram-positive bacteria. Crit. Rev. Microbiol 17, 121–135. [DOI] [PubMed] [Google Scholar]
- (45).Hancock IC (1997) Bacterial cell surface carbohydrates: structure and assembly. Biochem. Soc. Trans. 25, 183–187. [DOI] [PubMed] [Google Scholar]
- (46).Weidenmaier C, and Peschel A (2008) Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol 6, 276–287. [DOI] [PubMed] [Google Scholar]
- (47).McBride SM, and Sonenshein AL (2011) The dlt operon confers resistance to cationic antimicrobial peptides in Clostridium difficile. Microbiology 157, 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Santa Maria JP Jr., Sadaka A, Moussa SH, Brown S, Zhang YJ, Rubin EJ, Gilmore MS, and Walker S (2014) Compound-gene interaction mapping reveals distinct roles for Staphylococcus aureus teichoic acids. Proc. Natl. Acad. Sci. U. S. A 111, 12510–12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Schirner K, Marles-Wright J, Lewis RJ, and Errington J (2009) Distinct and essential morphogenic functions for wall- and lipo-teichoic acids in Bacillus subtilis. EMBO J. 28, 830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Marquis RE (1973) Immersion refractometry of isolated bacterial cell walls. J. Bacteriol 116, 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Percy MG, and Grundling A (2014) Lipoteichoic acid synthesis and function in gram-positive bacteria. Annu. Rev. Microbiol 68, 81–100. [DOI] [PubMed] [Google Scholar]
- (52).Corrigan RM, Abbott JC, Burhenne H, Kaever V, and Grundling A (2011) c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 7, e1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Oku Y, Kurokawa K, Matsuo M, Yamada S, Lee BL, and Sekimizu K (2009) Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J. Bacteriol 191, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, Shimoni E, Trieu-Cuot P, and Shai Y (2012) D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B streptococcus by increasing the cell wall density. PLoS Pathog. 8, e1002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Gutberlet T, Markwitz S, Labischinski H, and Bradaczek H (1991) Monolayer investigations on the bacterial amphiphile lipoteichoic acid and on lipoteichoic acid dipalmitoyl-phosphatidylglycerol mixtures. Makromol Chem., Macromol Symp. 46, 283–287. [Google Scholar]
- (56).Gutberlet T, Frank J, Bradaczek H, and Fischer W (1997) Effect of lipoteichoic acid on thermotropic membrane properties. J. Bacteriol 179, 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Labischinski H, Naumann D, and Fischer W (1991) Small and medium-angle X-ray analysis of bacterial lipoteichoic acid phase structure. Eur. J. Biochem 202, 1269–1274. [DOI] [PubMed] [Google Scholar]
- (58).Razin S, Yogev D, and Naot Y (1998) Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev 62, 1094–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Vollmer W (2007) Structure and biosynthesis of the murein (peptidoglycan) sacculus. Periplasm, 198–213. [Google Scholar]
- (60).Gan L, Chen S, and Jensen GJ (2008) Molecular organization of Gram-negative peptidoglycan. Proc. Natl. Acad. Sci. U. S.A 105, 18953–18957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Beeby M, Gumbart JC, Roux B, and Jensen GJ (2013) Architecture and assembly of the Gram-positive cell wall. Mol. Microbiol 88, 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Matias VR, and Beveridge TJ (2005) Cryo-electron microscopy reveals native polymeric cell wall structure in Bacillus subtilis 168 and the existence of a periplasmic space. Mol. Microbiol 56, 240–251. [DOI] [PubMed] [Google Scholar]
- (63).Matias VR, and Beveridge TJ (2006) Native cell wall organization shown by cryo-electron microscopy confirms the existence of a periplasmic space in Staphylococcus aureus. J. Bacteriol 188, 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Vollmer W, Blanot D, and de Pedro MA (2008) Peptidoglycan structure and architecture. Fems Microbiol Rev. 32, 149–167. [DOI] [PubMed] [Google Scholar]
- (65).Ghosh AS, Chowdhury C, and Nelson DE (2008) Physiological functions of D-alanine carboxypeptidases in Escherichia coli. Trends Microbiol. 16, 309–317. [DOI] [PubMed] [Google Scholar]
- (66).Lavollay M, Arthur M, Fourgeaud M, Dubost L, Marie A, Veziris N, Blanot D, Gutmann L, and Mainardi JL (2008) The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by L,D-transpeptidation. J. Bacteriol 190, 4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Peltier J, Courtin P, El Meouche I, Lemee L, Chapot-Chartier MP, and Pons JL (2011) Clostridium difficile has an original peptidoglycan structure with a high level of N-acetylglucosamine deacetylation and mainly 3–3 cross-links. J. Biol. Chem 286, 29053–29062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Atrih A, Bacher G, Allmaier G, Williamson MP, and Foster SJ (1999) Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J. Bacteriol 181, 3956–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Navarre WW, and Schneewind O (1999) Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev 63, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Vollmer W, and Bertsche U (2008) Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim. Biophys. Acta, Biomembr 1778, 1714–1734. [DOI] [PubMed] [Google Scholar]
- (71).Desmarais SM, De Pedro MA, Cava F, and Huang KC (2013) Peptidoglycan at its peaks: how chromatographic analyses can reveal bacterial cell wall structure and assembly. Mol. Microbiol 89, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Glauner B (1988) Separation and quantification of muropeptides with high-performance liquid chromatography. Anal. Biochem 172, 451–464. [DOI] [PubMed] [Google Scholar]
- (73).Saar Dover R, Bitler A, Shimoni E, Trieu-Cuot P, and Shai Y (2015) Multiparametric AFM reveals turgor-responsive net-like peptidoglycan architecture in live streptococci. Nat. Commun 6, 7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Turner RD, Hurd AF, Cadby A, Hobbs JK, and Foster SJ (2013) Cell wall elongation mode in Gram-negative bacteria is determined by peptidoglycan architecture. Nat. Commun 4, 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Gumbart JC, Beeby M, Jensen GJ, and Roux B (2014) Escherichia coli peptidoglycan structure and mechanics as predicted by atomic-scale simulations. PLoS Comput. Biol 10, e1003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).van den Bogaart G, Hermans N, Krasnikov V, and Poolman B (2007) Protein mobility and diffusive barriers in Escherichia coli: consequences of osmotic stress. Mol. Microbiol 64, 858–871. [DOI] [PubMed] [Google Scholar]
- (77).Kim SJ, Chang J, and Singh M (2015) Peptidoglycan architecture of Gram-positive bacteria by solid-state NMR. Biochim. Biophys. Acta, Biomembr 1848, 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Ghuysen JM (1989) The rigid matrix of bacterial-cell walls - a citation classic commentary on the use of bacteriolytic enzymes in determination of wall structure and their role in cell-metabolism. Current Contents. Agriculture Biology & Environmental Sciences, 20. [Google Scholar]
- (79).Hayhurst EJ, Kailas L, Hobbs JK, and Foster SJ (2008) Cell wall peptidoglycan architecture in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A 105, 14603–14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Vollmer W, and Holtje JV (2001) Morphogenesis of Escherichia coli. Curr. Opin. Microbiol 4, 625–633. [DOI] [PubMed] [Google Scholar]
- (81).Pelling AE, Li Y, Shi W, and Gimzewski JK (2005) Nanoscale visualization and characterization of Myxococcus xanthus cells with atomic force microscopy. Proc. Natl. Acad. Sci. U. S. A 102, 6484–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Schar-Zammaretti P, and Ubbink J (2003) The cell wall of lactic acid bacteria: surface constituents and macromolecular conformations. Biophys. J 85, 4076–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Thwaites JJ, Surana UC, and Jones AM (1991) Mechanical properties of Bacillus subtilis cell walls: effects of ions and lysozyme. J. Bacteriol 173, 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Sauvage E, Kerff F, Terrak M, Ayala JA, and Charlier P (2008) The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. Fems Microbiol Rev. 32, 234–258. [DOI] [PubMed] [Google Scholar]
- (85).Kuhner D, Stahl M, Demircioglu DD, and Bertsche U (2015) From cells to muropeptide structures in 24 h: peptidoglycan mapping by UPLC-MS. Sci. Rep 4, 7494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Francius G, Domenech O, Mingeot-Leclercq MP, and Dufrene YF (2008) Direct observation of Staphylococcus aureus cell wall digestion by lysostaphin. J. Bacteriol 190, 7904–7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Loskill P, Pereira PM, Jung P, Bischoff M, Herrmann M, Pinho MG, and Jacobs K (2014) Reduction of the peptidoglycan crosslinking causes a decrease in stiffness of the Staphylococcus aureus cell envelope. Biophys. J 107, 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Wheeler R, Turner RD, Bailey RG, Salamaga B, Mesnage S, Mohamad SA, Hayhurst EJ, Horsburgh M, Hobbs JK, and Foster SJ (2015) Bacterial cell enlargement requires control of cell wall stiffness mediated by peptidoglycan hydrolases. mBio 6, e00660–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Vollmer W, and Holtje JV (2004) The architecture of the murein (peptidoglycan) in gram-negative bacteria: vertical scaffold or horizontal layer(s)? J. Bacteriol 186, 5978–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Purushothaman S, Cicuta P, Ces O, and Brooks NJ (2015) Influence of High Pressure on the Bending Rigidity of Model Membranes. J. Phys. Chem. B 119, 9805–9810. [DOI] [PubMed] [Google Scholar]
- (91).Bermudez H, Hammer DA, and Discher DE (2004) Effect of bilayer thickness on membrane bending rigidity. Langmuir 20, 540–543. [DOI] [PubMed] [Google Scholar]
- (92).Phillips R, Ursell T, Wiggins P, and Sens P (2009) Emerging roles for lipids in shaping membrane-protein function. Nature 459, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Oliver PM, Crooks JA, Leidl M, Yoon EJ, Saghatelian A, and Weibel DB (2014) Localization of anionic phospholipids in Escherichia coli cells. J. Bacteriol 196, 3386–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Russell NJ, and Nichols DS (1999) Polyunsaturated fatty acids in marine bacteria–a dogma rewritten. Microbiology 145 (4), 767–779. [DOI] [PubMed] [Google Scholar]
- (95).Olbrich K, Rawicz W, Needham D, and Evans E (2000) Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J 79, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).White GF, Racher KI, Lipski A, Hallett FR, and Wood JM (2000) Physical properties of liposomes and proteoliposomes prepared from Escherichia coli polar lipids. Biochim. Biophys. Acta, Biomembr 1468, 175–186. [DOI] [PubMed] [Google Scholar]
- (97).Schwechheimer C, and Kuehn MJ (2015) Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol 13, 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Yeh YC, Comolli LR, Downing KH, Shapiro L, and McAdams HH (2010) The caulobacter Tol-Pal complex is essential for outer membrane integrity and the positioning of a polar localization factor. J. Bacteriol 192, 4847–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Clavel T, Germon P, Vianney A, Portalier R, and Lazzaroni JC (1998) TolB protein of Escherichia coli K-12 interacts with the outer membrane peptidoglycan-associated proteins Pal, Lpp and OmpA. Mol. Microbiol 29, 359–367. [DOI] [PubMed] [Google Scholar]
- (100).Jones LJ, Carballido-Lopez R, and Errington J (2001) Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104, 913–922. [DOI] [PubMed] [Google Scholar]
- (101).Garner EC, Bernard R, Wang W, Zhuang X, Rudner DZ, and Mitchison T (2011) Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science 333, 222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Dominguez-Escobar J, Chastanet A, Crevenna AH, Fromion V, Wedlich-Soldner R, and Carballido-Lopez R (2011) Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science 333, 225–228. [DOI] [PubMed] [Google Scholar]
- (103).van Teeffelen S, Wang S, Furchtgott L, Huang KC, Wingreen NS, Shaevitz JW, and Gitai Z (2011) The bacterial actin MreB rotates, and rotation depends on cell-wall assembly. Proc. Natl. Acad. Sci. U. S. A 108, 15822–15827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Ouzounov N, Nguyen JP, Bratton BP, Jacobowitz D, Gitai Z, and Shaevitz JW (2016) MreB Orientation Correlates with Cell Diameter in Escherichia coli. Biophys. J 111, 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Salje J, van den Ent F, de Boer P, and Lowe J (2011) Direct membrane binding by bacterial actin MreB. Mol. Cell 43, 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Renner LD, Eswaramoorthy P, Ramamurthi KS, and Weibel DB (2013) Studying biomolecule localization by engineering bacterial cell wall curvature. PLoS One 8, e84143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Ursell TS, Nguyen J, Monds RD, Colavin A, Billings G, Ouzounov N, Gitai Z, Shaevitz JW, and Huang KC (2014) Rod-like bacterial shape is maintained by feedback between cell curvature and cytoskeletal localization. Proc. Natl. Acad. Sci. U. S. A 111, E1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Bean GJ, Flickinger ST, Westler WM, McCully ME, Sept D, Weibel DB, and Amann KJ (2009) A22 disrupts the bacterial actin cytoskeleton by directly binding and inducing a low-affinity state in MreB. Biochemistry 48, 4852–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Defeu Soufo HJ, Reimold C, Linne U, Knust T, Gescher J, and Graumann PL (2010) Bacterial translation elongation factor EF-Tu interacts and colocalizes with actin-like MreB protein. Proc. Natl. Acad. Sci. U. S. A 107, 3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Cowles CE, Li Y, Semmelhack MF, Cristea IM, and Silhavy TJ (2011) The free and bound forms of Lpp occupy distinct subcellular locations in Escherichia coli. Mol. Microbiol 79, 1168–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Cascales E, Bernadac A, Gavioli M, Lazzaroni JC, and Lloubes R (2002) Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J. Bacteriol 184, 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Vadillo-Rodriguez V, Schooling SR, and Dutcher JR (2009) In situ characterization of differences in the viscoelastic response of individual gram-negative and gram-positive bacterial cells. J. Bacteriol 191, 5518–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Volle CB, Ferguson MA, Aidala KE, Spain EM, and Nunez ME (2008) Quantitative changes in the elasticity and adhesive properties of Escherichia coli ZK1056 prey cells during predation by bdellovibrio bacteriovorus 109J. Langmuir 24, 8102–8110. [DOI] [PubMed] [Google Scholar]
- (114).Wood JM (2015) Bacterial responses to osmotic challenges. J. Gen. Physiol 145, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Schwarz H, and Koch AL (1995) Phase and electron microscopic observations of osmotically induced wrinkling and the role of endocytotic vesicles in the plasmolysis of the Gram-negative cell wall. Microbiology 141 (12), 3161–3170. [DOI] [PubMed] [Google Scholar]
- (116).Anraku Y, and Heppel LA (1967) On the nature of the changes induced in Escherichia coli by osmotic shock. J. Biol. Chem 242, 2561–2569. [PubMed] [Google Scholar]
- (117).Bialecka-Fornal M, Lee HJ, and Phillips R (2015) The rate of osmotic downshock determines the survival probability of bacterial mechanosensitive channel mutants. J. Bacteriol 197, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Rojas E, Theriot JA, and Huang KC (2014) Response of Escherichia coli growth rate to osmotic shock. Proc. Natl. Acad. Sci. U. S. A 111, 7807–7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Pilizota T, and Shaevitz JW (2013) Plasmolysis and cell shape depend on solute outer-membrane permeability during hyperosmotic shock in E. coli. Biophys. J 104, 2733–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Vollmer W, and Seligman SJ (2010) Architecture of peptidoglycan: more data and more models. Trends Microbiol. 18, 59–66. [DOI] [PubMed] [Google Scholar]
- (121).Binnig G, Quate CF, and Gerber C (1986) Atomic force microscope. Phys. Rev. Lett 56, 930–933. [DOI] [PubMed] [Google Scholar]
- (122).Dufrene YF (2002) Atomic force microscopy, a powerful tool in microbiology. J. Bacteriol 184, 5205–5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Deng Y, Sun M, and Shaevitz JW (2011) Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys. Rev. Lett 107, 158101. [DOI] [PubMed] [Google Scholar]
- (124).Wright CJ, and Armstrong I (2006) The application of atomic force microscopy force measurements to the characterisation of microbial surfaces. Surf. Interface Anal 38, 1419–1428. [Google Scholar]
- (125).Suo Z, Avci R, Deliorman M, Yang X, and Pascual DW (2009) Bacteria survive multiple puncturings of their cell walls. Langmuir 25, 4588–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Si F, Li B, Margolin W, and Sun SX (2015) Bacterial growth and form under mechanical compression. Sci. Rep 5, 11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Needham D, and Hochmuth RM (1992) A sensitive measure of surface stress in the resting neutrophil. Biophys. J 61, 1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Milne JL, and Subramaniam S (2009) Cryo-electron tomography of bacteria: progress, challenges and future prospects. Nat. Rev. Microbiol 7, 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Horvath DJ Jr., Li B, Casper T, Partida-Sanchez S, Hunstad DA, Hultgren SJ, and Justice SS (2011) Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect. 13, 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Philippe N, Pelosi L, Lenski RE, and Schneider D (2009) Evolution of penicillin-binding protein 2 concentration and cell shape during a long-term experiment with Escherichia coli. J. Bacteriol 191, 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Monds RD, Lee TK, Colavin A, Ursell T, Quan S, Cooper TF, and Huang KC (2014) Systematic perturbation of cytoskeletal function reveals a linear scaling relationship between cell geometry and fitness. Cell Rep. 9, 1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).McCutcheon JP, and Moran NA (2012) Extreme genome reduction in symbiotic bacteria. Nat. Rev. Microbiol 10, 13–26. [DOI] [PubMed] [Google Scholar]