To the Editor:
Iron deficiency anemia (IDA) is the most prevalent extraintestinal laboratory manifestation observed in patients with inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn disease (CD).1 IDA is more prevalent in children and adolescents with IBD compared to adults, and it often goes unrecognized or under treated.1, 2 Persistent anemia in pediatric patients with IBD may lead to reduced growth and development, impaired cognition, and a decrease in quality of life.3 Patients with IBD are at increased risk for the development of IDA, especially during intervals of acute or chronic gastrointestinal blood loss or poor enteral nutrition. As such, the application of safe and cost-effective therapies is important in addressing this modifiable aspect of pediatric IBD treatment. Parenteral iron therapies are receiving increasing attention with respect to their effectiveness in addressing IDA, while the use of oral iron supplementation for treatment of patients with IBD and IDA has become less prevalent. This trend is rooted in beliefs that enteral iron formulations may be poorly absorbed, clinically intolerable, or display decreased bioavailability in patients with IBD.4 However, there is relatively little controlled data to support these perceptions. Lack of research studying oral iron effectiveness, particularly in the context of increased disease activity, impedes progress in the development of pediatric IBD care guidelines regarding rational iron therapies. We conducted a retrospective chart review of our center’s 11-year experience using oral iron to study its clinical effectiveness in treatment of anemia associated with pediatric UC flare in real world practice.
We performed a retrospective cohort study of pediatric patients (≤ 21 years of age) with biopsy-confirmed UC admitted to Boston Children’s Hospital (BCH) for management of worsening disease activity between 2003 and 2014. We identified patients in the electronic medical record with an International Classification of Disease-9-Clinical Modification (ICD-9-CM) (556.xx) or ICD-10-CM (K51.xx) listing for ulcerative colitis. We excluded patients with potentially confounding comorbidities, including hematologic malignancy, underlying hematological diagnosis, or history of renal disease / treatment with dialysis. We also excluded patients transferred to the General Surgery service to undergo colectomy.
We defined anemia using hemoglobin nomograms developed from the National Health and Nutrition Examination Survey (NHANES) to classify each patient dichotomously as “anemic” or “not anemic” at the time of hospital discharge. We defined anemia as ≤ 2.5% hemoglobin values using age and gender-based nomograms.5 Data were summarized using descriptive statistics while the effectiveness of oral iron supplementation from discharge to follow-up clinic visit was studied using repeated-measures mixed model regression. We controlled for inflammation using platelet value, an acute phase reactant,6 due to the high-frequency (>50%) of missing data for more traditional biochemical markers of inflammation, including erythrocyte sedimentation rate (ESR) and C-reactive protein (C-RP), at the time of discharge. White blood cell count (WBC) can be elevated in the context of oral and parenteral steroid use, and for this reason excluded as a surrogate marker for inflammation. Models also adjusted for hemoglobin values at discharge. All comparisons were two-sided with statistical significance level set at P<0.05. All analyses were performed with SAS v.9.4 statistical software (Cary, NC).
A total of 174 patients met criteria for study inclusion. The mean age of the cohort was 13.8 ± 4.2 years, and 59% were female. The majority (90%) of the cohort received IV steroid treatment while admitted, with the remaining patients treated with anti-TNF agents. A standardized outpatient tapering protocol was used for patients treated with IV steroids following discharge. One hundred (56%) patients were anemic by our definition at the time of discharge. The mean hemoglobin value in the anemic group was 9.8 ± 1.2 g/dL. Five patients (2%) in the entire cohort underwent iron studies at discharge limiting analyses at outpatient follow-up. Thirty-two of the 100 (32%) anemic patients were prescribed standard oral iron supplementation therapy at time of their discharge. No patients received IV iron therapy during their admission.
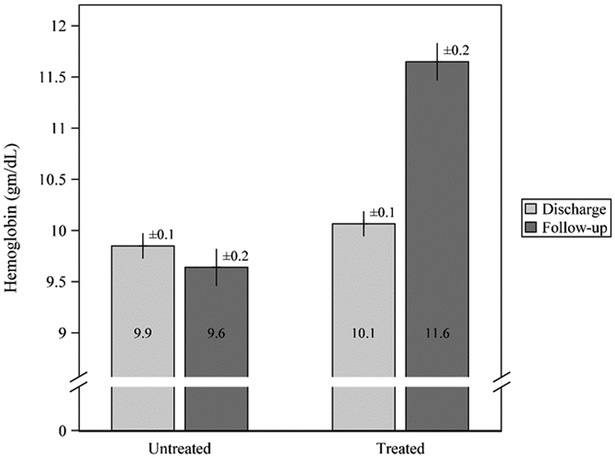
The median duration from hospital discharge to ambulatory follow-up appointment was 28 (IQR 19 – 42) days for UC patients with anemia. Figure 1 depicts the mean hemoglobin values from discharge to the follow-up clinic visit for the anemic cohort, stratified by oral iron supplementation status. Hemoglobin values at the time of discharge did not differ statistically between the treated and untreated groups (P=0.35). In those prescribed oral iron supplementation at the time of their discharge, hemoglobin values increased approximately 2.0 (95% CI 1.5, 2.5) g/dL by the time of their outpatient follow-up visit. In contrast, patients not prescribed oral iron supplementation experienced a much smaller change in average hemoglobin values (0.2 g/dL; 95% CI −0.1, 0.6). The mean difference in hemoglobin values between iron supplemented and untreated patients (1.8 g/dL (95% CI 1.2, 2.4) at the time of their outpatient follow-up visits was highly different statistically (P<0.0001).
Figure 1.
Mean hemoglobin (Hgb) among n=100 pediatric UC patients admitted to BCH between 2003 and 2014 for management of moderate to severe UC disease activity who were anemic at discharge. Results are from a repeated-measures mixed model with adjustment for Hgb at discharge. Shown are mean ± standard error. There was no difference in mean Hgb at discharge (P=0.35). Subjects with iron supplementation experienced a statistically significant increase in mean Hgb by the follow-up clinic visit, while those without iron supplementation did not (mean increase 2.0±0.3 vs. 0.2±0.2 gm/dL, respectively; P<0.0001). F
Our findings demonstrate a high prevalence of anemia in this single center, retrospective cohort study of pediatric UC patients discharged from the hospital following admission for disease flare management. Prescription of oral iron supplementation to anemic patients at the time of discharge was uncommon, occurring in only 32% of patients. Finally, our data demonstrate a significantly larger increase in average hemoglobin value at initial outpatient follow-up in those anemic UC patients that were prescribed oral iron supplementation at the time of their discharge, as compared to anemic UC patients not supplemented, who experienced no significant change in hemoglobin value.
The 56% prevalence of anemia estimated from our data is consistent with previously published studies of pediatric IBD-associated anemia.1 In our cohort of patients with UC being discharged following admission for disease management, over half were anemic at discharge. Our cohort of hospitalized patients with active disease, therefore, represents a group at high-risk for anemia and one that could benefit from intervention. Our prevalence findings emphasize the importance of screening to identify patients with anemia immediately following or during periods of active disease, given the high prevalence of treatable sequelae.
Our data further demonstrate that oral iron supplementation in patients with active UC can result in a significant increase in hemoglobin values by the time of their initial outpatient follow-up ambulatory clinic visits. In this subset of anemic patients, after controlling for hemoglobin at discharge and inflammation, the average hemoglobin level among subjects receiving oral iron supplementation increased by 2 g/dL (95% CI 1.5–2.5) by the time of first outpatient follow-up appointment, which occurred at a median duration of 30 days following discharge. Equally as important, our findings suggest that anemic patients not receiving oral iron supplementation did not experience a marked change in mean hemoglobin value at first outpatient follow-up despite receipt of standard UC inpatient therapies. As such, treating the mucosal and systemic inflammatory state that characterize a flare in UC disease, in the absence of targeted iron supplementation, does not appear to be sufficient to reverse anemia in the first 30 days following hospital discharge. This further underscores the need for recognition and active management of iron deficiency in this anemia-vulnerable pediatric patient population. One of our study strengths is a longitudinal cohort design taking into account repeated measures of hemoglobin and accounting for between-subject variation as well as within-subject variation.
In summary, we demonstrate a high prevalence of anemia in a population of pediatric UC patients admitted to our center for treatment of disease flare. We demonstrate that treatment of UC disease activity alone, without targeted management of highly prevalent anemia, does not result in improved hemoglobin trajectory. Lastly, our data suggest that oral iron supplementation is clinically effective for treatment of iron-deficiency anemia associated with recent UC flare in pediatric patients discharged from the hospital.
Acknowledgments
Funding source: Supported by the National Institutes of Health (P30-DK034987 [to Joseph A. Galanko])
Footnotes
Scholarship without named authorship: Tim Yang, Sara Rogerson, Sarah Manely
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Resources
- 1.Goodhand JR, Kamperidis N, Rao A, et al. Prevalence and management of anemia in children, adolescents, and adults with inflammatory bowel disease. Inflamm Bowel Dis 2012;18:513–9. [DOI] [PubMed] [Google Scholar]
- 2.Sjoberg D, Holmstrom T, Larsson M, et al. Anemia in a population-based IBD cohort (ICURE): still high prevalence after 1 year, especially among pediatric patients. Inflamm Bowel Dis 2014;20:2266–70. [DOI] [PubMed] [Google Scholar]
- 3.Niepel D, Klag T, Malek NP, et al. Practical guidance for the management of iron deficiency in patients with inflammatory bowel disease. Therap Adv Gastroenterol 2018;11:1756284818769074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemeth E TM, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004;306:2090–2093. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population. Available at: https://www.cdc.gov/nutritionreport/99-02/part_3.html. Accessed October 1, 2016.
- 6.Parry MFJB, Scully B, Neu HC Thrombocytosis: an acute-phase reactant, not an adverse reaction to the new beta-lactam antibiotics. Diagn Microbiol Infect Dis 1984;2:229–231. [DOI] [PubMed] [Google Scholar]