Abstract
Purpose of review:
Atherosclerotic cardiovascular disease (CVD) and type II diabetes (T2D) share common etiologic pathways that may long precede the development of clinically-evident disease. Early identification of risk markers could support efforts to individualize risk prediction and improve the efficacy of primary prevention, as well as uncover novel therapeutic targets.
Recent findings:
Altered metabolism of branched-chain amino acids (BCAAs), and their subsequent accumulation in circulation, may precede the development of insulin resistance and clinically manifest cardiometabolic diseases. BCAAs – the essential amino acids leucine, isoleucine, and valine – likely promote insulin resistance through activation of mammalian target of rapamycin complex 1 (mTORC1). Epidemiologic studies demonstrate robust associations between BCAAs and incident T2D, and Mendelian randomization supports a potentially causal relationship. More recently, there is emerging evidence that BCAAs are also associated with incident atherosclerotic CVD, possibly mediated by the development of T2D.
Summary:
In this article, we review the biochemistry of BCAAs, their potential contribution to cardiometabolic risk, the available evidence from molecular epidemiologic studies to date, and, finally, consider future research and clinical directions. Overall, BCAAs represent a promising emerging target for risk stratification and possible intervention, to support efforts to mitigate the burden of cardiometabolic disease in the population.
Keywords: branched-chain amino acids, type 2 diabetes, cardiovascular disease, metabolomics, epidemiology
INTRODUCTION
The pathogenesis of cardiovascular disease (CVD) is preceded by a prolonged preclinical state that involves dysregulated metabolism, vascular dysfunction, and progressive atherosclerosis. Identifying individuals at elevated CVD risk in the earliest phases of disease pathogenesis could provide opportunities to maximize the effectiveness of primary prevention strategies and reduce the burden of disease in the population.
In search of early markers of CVD risk and potential disease mediators, metabolomics has emerged as a powerful tool to characterize complex states of metabolic dysfunction.(1) Metabolomics involves the integrated profiling of small molecules (<1.0 kDa) in populations, and has been highly effective in identifying novel biomarkers of cardiometabolic disease.(2, 3) Resulting in part from a recent increase in metabolomics research, circulating concentrations of branched-chain amino acids (BCAAs) have been consistently associated with incident T2D as well as other cardiovascular disease risk factors, including excess body weight and obesity, insulin resistance, and T2D.(3) Research to elucidate the role of BCAAs in the development of cardiometabolic disease is ongoing, including efforts to determine whether there is a causal relationship amenable to intervention. In this review, we consider the biochemistry of BCAAs, their potential contribution to cardiometabolic risk, review the available evidence from molecular epidemiologic studies to date, and, finally, consider future directions.
BRANCHED-CHAIN AMINO ACIDS
Leucine, isoleucine, and valine, collectively BCAAs, are essential amino acids that contribute to protein synthesis and perform a wide range of well-characterized metabolic and physiologic functions.(4) BCAAs are ubiquitous throughout the diet, available from a variety of both vegetable and animal protein sources; however, determinants of between- and within-person variability in circulating BCAA concentrations are largely unknown. Short-term feeding trials in patients with chronic illness or healthy athletes demonstrate that increases in BCAA intake, either from BCAA-enriched diets or supplements, results in modest, temporary rises in plasma BCAA concentrations.(5–8) However, the long-term effects of BCAA intake and supplementation on circulating BCAA concentrations among more generalizable populations are lacking.
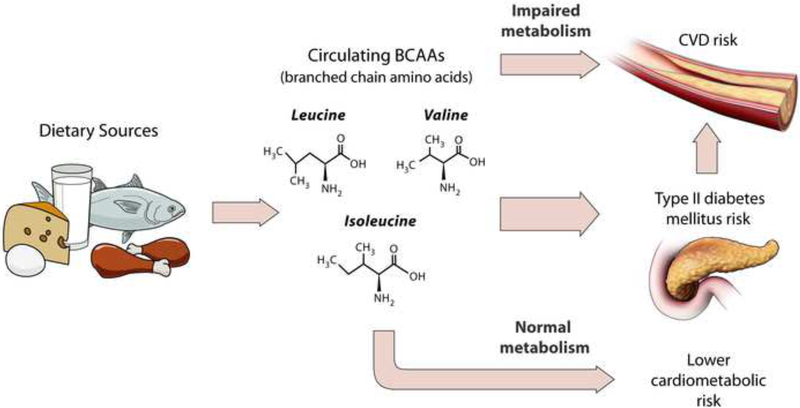
Although BCAAs are essential amino acids derived solely from the diet, correlations between diet and circulating BCAAs in the Nurses’ Health Study and Health Professionals Follow-up Study longitudinal cohorts appear at most modest (r~0.2), suggesting factors other than dietary intake may determine plasma concentrations of BCAAs.(9, 10) Further, circulating concentrations of BCAAs in plasma, serum, and urine, are strongly associated with poor cardiometabolic health, as reviewed below, while the magnitudes of the associations for dietary intakes of BCAAs with the same endpoints are lacking(11) or marginal(9, 12). Recently, we compared the dietary intakes of BCAAs and plasma concentrations of BCAAs in high risk women, and observed that women with elevated plasma levels, rather than dietary BCAAs alone, experienced a greater risk of incident T2D.(10) These findings suggest that underlying catabolic defects in BCAAs metabolism, resulting in elevated BCAA circulating concentrations, may be the relevant cardiometabolic risk factor, rather than the amount of dietary BCAA intake per se (Figure 1).
FIGURE 1.
Impaired metabolism of branched-chain amino acids, elevated circulating concentrations, and subsequent risks of type 2 diabetes and cardiovascular disease.
Identifying the determinants of impaired BCAA catabolism or elevated circulating BCAA concentrations are critical to understanding the role of BCAAs in cardiometabolic health and disease. Excess body weight and central obesity are positively correlated with circulating BCAAs across a number of disease-free study populations, implicating excess adiposity as an important determinant of circulating BCAA concentrations.(13–16) (17–19) Genetics may also influence individuals’ underlying BCAA metabolism, and, in turn, circulating BCAA levels, although likely to a small extent. A recent genome-wide association study (GWAS) identified 5 genetic regions significantly associated with higher plasma BCAAs, explaining 7.5%, 6.3%, and 5.3% of the heritability of isoleucine, leucine, and valine metabolites, respectively.(20) Decreased BCAA uptake by muscle and other tissue,(21) muscle breakdown or cachexia,(22) and lower branched-chain keto acid dehydrogenase (BCKD) enzyme activity(23) have also been identified as conditions resulting in elevated BCAA concentrations.(24) Other potential modifiable determinants of variability in concentrations of BCAAs, such as level of physical activity, overall dietary quality or other dietary factors, and the microbiome have not yet been fully elucidated, warranting further research.
ELEVATED BCAA CONCENTRATIONS AS A FEATURE OF POOR CARDIOMETABOLIC HEALTH
Accumulating evidence implicates dysregulated BCAA metabolism in the pathogenesis of T2D and CVD. Of the thousands of metabolites in circulation, BCAAs have been consistently identified across study populations, follow-up durations, and methods of metabolomic profiling, for their strong positive relationship with impaired glucose metabolism,(25) insulin resistance,(15, 26) and incident T2D,(3).These observations support the hypothesis that BCAA metabolism may be implicated early in the development of insulin resistance and eventual T2D.(27–29) Evidence from human and animal studies demonstrates that the controlled introduction of dietary amino acids, including BCAAs, has the ability to impair insulin action and signaling in skeletal muscle through upregulation of the mTOR pathway.(30–35) Further, small randomized trials of metformin, an insulin-sensitizing drug, did not demonstrate an effect on BCAA levels vs. placebo.(36, 37) These findings suggest that alterations in BCAAs metabolism may occur upstream of insulin resistance, rather than as a downstream consequence.
Importantly, recent evidence from Mendelian randomization analyses support a causal role for BCAA metabolism in T2D risk, wherein genetic determinants of elevated circulating BCAA levels were positively associated with risk of T2D.(20, 38) Further, these genetic predictors were related to impaired BCKD) activation, the rate-limiting step in BCAA break-down.
While the link between BCAAs with T2D is well-recognized, prospective analyses of circulating BCAA metabolites in relation to incident CVD events are sparse and/or inconsistent. Several prospective metabolome-wide studies of incident coronary heart disease(39, 40), coronary artery disease(41–43), and myocardial infarction (MI),(43) have failed to identify BCAAs as significantly associated with CVD, possibly owing to limited statistical power following adjustment for multiple hypothesis testing. However, in a candidate approach, the PREvención con DIeta MEDiterránea (PREDIMED) Mediterranean diet trial prospectively evaluated baseline plasma BCAA concentrations in relation to incident CVD among 970 men and women at high risk.(44) In BMI-adjusted models comparing the 4th and 1st quartiles, isoleucine, leucine, and valine baseline plasma concentrations were significantly associated with 2.9, 2.2, and 1.9-fold greater risks of incident cardiovascular events (primarily stroke), respectively.
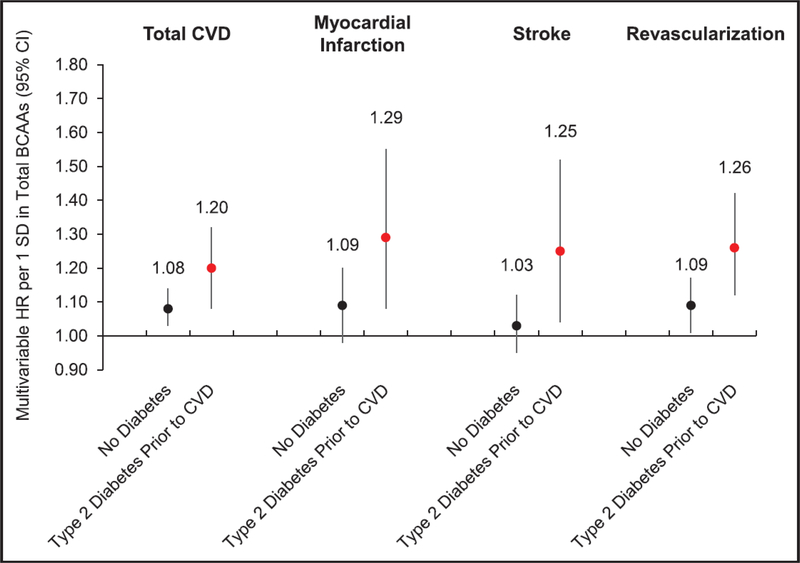
We recently analyzed BCAAs in relations to CVD risk among 27,041 women in the Women’s Health Study (WHS) prospective cohort who were free of CVD and cancer at the baseline blood collection.(45) We confirmed 2,207 first CVD events (MI, stroke, coronary revascularization) over an average 18.6 follow-up years. With multivariable models adjusting for age, BMI, and other established CVD risk factors, we observed that plasma concentrations of total BCAAs were positively associated with incident CVD, as shown in Figure 2.(adjusted hazard ratio [HR] per standard deviation [SD] increment=1.13; confidence interval (CI)=1.08 to 1.18). This magnitude of risk was comparable to the association between LDL cholesterol and CVD risk (per SD HR=1.12, CI=1.07 to 1.17). In WHS, BCAAs were associated with coronary events (MI: HR=1.16, CI=1.06 to 1.26; revascularization: HR=1.17, CI=1.11 to 1.25), but not significantly with total stroke (HR=1.07, CI=0.99 to 1.15). Notably, the relationship between BCAA concentrations and CVD risk was greater among women with T2D diagnosed prior to CVD (HR=1.20, CI=1.08 to 1.32), vs. women without T2D (HR=1.08, CI=1.03 to 1.14), and in those women the association with stroke as well as MI was also significant. Adjusting for LDL-C, an established CVD risk factor, did not attenuate these findings; however, adjusting for HbA1c and insulin resistance eliminated the associations of BCAAs with CVD. In a separate cross-sectional analysis, plasma BCAAs were positively correlated with carotid intima-media thickness (cIMT) among adult subjects with HbA1c values ≥5.6%, while no correlation between BCAAs and cIMT persisted among the subgroup of individuals with Hba1c<5.6%.(46) Thus, BCAAs may confer an elevated risk of CVD through insulin resistance- and T2D-related atherosclerosis risk.
FIGURE 2. Prospective association between baseline circulating total branched-chain amino acids (BCAAs) in relation to incident total cardiovascular disease (CVD), myocardial infarction (Ml), stroke, and revascularization risk in 27401 US women, according to type 2 diabetes mellitus diagnosis before CVD end point.
Multivariable-adjusted model includes the following: age (continuous), randomized treatment assignments (aspirin, beta- carotene, and vitamin E), fasting status at blood draw, menopausal status (pre, post, uncertain, and missing), current hormone therapy use, family history of Ml, White race/ethnicity, smoking status (never, past, current <15 c/d, current 15+ c/d). Alternative Healthy Eating Index (AHEl) diet quality score (quintiles), alcohol intake (4 categories), total physical activity metabolic equivalent of tasks (MET)-h/wk (quintiles), history of high cholesterol, history of hypertension, and body mass index (10 categories). A values for interaction: total CVD, P=0.036; Ml, P=0.059; stroke, P=0.066; and revascularization, P=0.019. Cl indicates confidence interval; and HR, hazard ratio.
BCAAs AND HEART FAILURE
Metabolic reprogramming is one of the hallmarks of heart failure. Alternations in myocardial substrate utilization plays can influence overall myocardial ATP generation, and affect cardiac function.(47) Over the past few years, accumulating evidence suggests that the suppression of BCAA catabolic gene expression along with concomitant tissue accumulation of branched-chain α-keto acids occurs in experimental and clinical cardiac failure, and that this may be mediated upstream through aberrant changes in Krüppel-like factor 15.(48) The accumulation of these branched-chain α-keto acids have been linked to heart failure through a potential direct effect to inhibit respiration and promote an increase in the release of potent reactive oxygen species, such as, superoxide, within the mitochondria. Intriguingly, the heart failure benefits of some of the newer antihyperglycemic agents, such as empagliflozin, has been suggested to be mediated through improving BCAA catabolism.(49) More recently, research in mice have demonstrated that impaired BCAA catabolism resulting in a buildup of BCAA’s served to impair glucose metabolism and increased the susceptibility of the heart to ischemia. Strikingly, approaches that augmented BCAA catabolism had beneficial effects on the myocardium.(50)
BCAAS AS MODIFIABLE TARGETS FOR PRIMARY PREVENTION
BMI, diet, and physical activity contribute substantially to the development of poor cardiometabolic health, and play pivotal roles in primary prevention strategies for T2Dand CVD.(51–53) A recent rat model demonstrated that up-regulating branched-chain ketoacid dehydrogenase (BCKDH), a critical step in BCAA catabolism, lowered circulating BCAA concentrations and led to improvements in glucose tolerance, independent of weight loss.(54) However, the identification of modifiable risk factors capable of modifying rates of BCAA catabolism and subsequent circulating BCAA concentrations in humans is sparse. Identifying improved BCAA metabolism, as reflected by lowered BCAA concentrations, as a relevant mediator underlying the impact of lifestyle on cardiometabolic health would allow for more efficient therapeutic strategies and the identification of high risk subgroups years prior to disease onset.
Two weight loss trials, POUNDS LOST and DIRECT, observed significant correlations between BCAA metabolite levels and weight loss between baseline and 2 years.(55) Further, small prospective studies of subjects undergoing bariatric weight-loss surgery found correlations between post-operative weight loss and BCAA levels, with one study reporting a significant 20% reduction in plasma BCAAs concurrent with 20% body weight loss.(56, 57) Collectively, these evidence support the potential modifiability of BCAA metabolism with weight loss and their potential as efficient targets for intervention and primary prevention. Another diet-induced weight loss trial with 7.4% average percent body weight loss did not observe significant reductions in individual or total BCAA concentrations. Lower valine concentrations, however, were independently correlated with improved HOMA-IR, a metric of insulin resistance, at 6 months follow-up.(58)
Evidence for effects of diet and physical activity, independent of weight loss, on circulating BCAA concentrations is mixed. Although BCAAs are essential amino acids derived solely from diet, short-term manipulations of dietary BCAA intake have resulted in only modest changes in circulating BCAA concentrations.(8, 59) One study in diet-induced obese mice reported that dramatically reducing dietary BCAAs by 66% led to reductions in weight and restored measures of glucose homeostasis, although results were not independent of unintended increases in the animals’ energy expenditure.(60) The PREDIEMD Mediterranean diet trial reported a significant reduction in plasma BCAAs between baseline and 1-year follow-up with the healthful Mediterranean dietary intervention, and no change in the low-fat control group.(61) Further, increases in isoleucine between baseline and 1 year, but not valine or leucine, was associated with a significant nearly 2-fold greater T2D risk compared with no change in isoleucine. Baseline BCAA concentrations were also associated with a significantly higher risk of incident CVD in the control group, but this association was mitigated in the Mediterranean diet intervention groups.(44) In contrast, the Diabetes Prevention Program, which observed significant effects of an intensive diet and lifestyle intervention on reductions in T2D incidence, did not observe significant changes in BCAAs between baseline and 2 years follow-up.(62)
A small cross-sectional analysis of physical activity in Chinese adults (N=277) found that BCAAs had the strongest association with physical activity among the ~300 metabolites evaluated, with lower BCAA levels associated with greater usual physical activity.(63) An analysis of twin studies compared a number of cardiometabolic markers, including lipids and BCAAs for active vs. inactive twins, observing significantly lower isoleucine levels among the regularly active individuals.(64) Finally, an aerobic exercise training intervention induced greater plasma BCAA turnover and increased insulin sensitivity among overweight trained subjects vs. overweight untrained subjects over 6 months.(65) Physical activity may increase BCAA degradation through increased BCAA metabolism-related gene expression in muscle and adipose tissue.(66) Overall, some lifestyle factors, including a Mediterranean-style dietary pattern and physical activity, may confer health benefits through improved BCAA metabolism. Although promising, limitations of the sparse literature include small sample size, cross-sectional study design, and poor control for potential confounders. Additional research is needed to identify and confirm which lifestyle and therapeutic interventions would effectively increase BCAA catabolism and reduce circulating BCAAs, and whether such improvements would appreciably modify risks of subsequent cardiometabolic disease.
CONCLUSIONS: FUTURE DIRECTIONS FOR BCAAS IN CARDIOVASCULAR DISEASE RESEARCH AND PREVENTION
BCAAs have been consistently observed as strongly associated with elevated T2D risk, and compelling experimental evidence and genetic epidemiologic studies suggest a potentially causal role of impaired BCAA metabolism in the development of insulin resistance and T2D.(20, 38, 54) However, their relationship with CVD is less consistent, but emerges particularly in those with intermediate T2D. Thus, impaired BCAA metabolism may represent a pathway consistent with the “common soil hypothesis”; a shared pathology predisposing to both cardiometabolic conditions.(67) Understanding where these potential common risk pathways converge and diverge along the road to CVD and T2D risk has important implications for how risk is identified and mitigated in vulnerable populations.
To date, little research has been undertaken to identify upstream determinants of dysregulated BCAA metabolism or elevated circulating BCAA levels. Future efforts to identify modifiable contributors to circulating BCAA concentrations, beyond dietary BCAAs or supplements, may optimize T2D prevention strategies, generate effective interventions for individuals at particularly high T2D risk, and lead to novel targeted therapeutics.
KEY POINTS:
Altered metabolism of branched-chain amino acids (BCAAs), and their subsequent accumulation in circulation, may precede the development of insulin resistance and cardiovascular disease.
While the link between BCAAs with T2D is well-recognized, prospective analyses of circulating BCAA metabolites in relation to incident CVD events are sparse and/or inconsistent.
BCAAs may confer an elevated risk of CVD through insulin resistance and T2D-related atherosclerosis.
Identifying the determinants of impaired BCAA catabolism or elevated circulating BCAA concentrations are critical to understanding the role of BCAAs in cardiometabolic health and disease.
ACKNOWLEDGEMENTS
Dr. Mora has received institutional research grant support from the National Heart, Lung, and Blood Institute (R01HL134811 and K24 HL136852), and the National Institute of Diabetes and Digestive and Kidney Diseases (DK112940). The BCAA NMR measurements in the Women’s Health Study were provided at no additional charge by LabCorp, Inc.
P. R. Lawler receives research funding from the Peter Munk Cardiac Centre, the Ted Rogers Foundation for Heart Research, and the Heart and Stroke/Richard Lewar Centre of Excellence in Cardiovascular Research at the University of Toronto. The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, and final approval of manuscript.
Footnotes
Potential conflicts of interest:
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Cheng S, Shah SH, Corwin EJ, Fiehn O, Fitzgerald RL, Gerszten RE, et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circulation Cardiovascular genetics. 2017. April;10(2). PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts LD, Koulman A, Griffin JL. Towards metabolic biomarkers of insulin resistance and type 2 diabetes: progress from the metabolome. The lancet Diabetes & endocrinology. 2014. January;2(1):65–75. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 3.Guasch-Ferre M, Hruby A, Toledo E, Clish CB, Martinez-Gonzalez MA, Salas-Salvado J, et al. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes care. 2016. May;39(5):833–46. PubMed PMID: Pubmed Central PMCID: 4839172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu G Functional amino acids in nutrition and health. Amino acids. 2013. September;45(3):407–11. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Vuzelov E, Krivoshiev S, Ribarova F, Boyadjiev N. Plasma levels of branched chain amino acids in patients on regular hemodialysis before and after including a high-protein supplement in their diet. Folia medica. 1999;41(4):19–22. PubMed PMID: . [PubMed] [Google Scholar]
- 6.Hiroshige K, Sonta T, Suda T, Kanegae K, Ohtani A. Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001. September;16(9):1856–62. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2013. October;28(5):580–8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Nelson AR, Phillips SM, Stellingwerff T, Rezzi S, Bruce SJ, Breton I, et al. A protein-leucine supplement increases branched-chain amino acid and nitrogen turnover but not performance. Medicine and science in sports and exercise. 2012. January;44(1):57–68. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. International journal of epidemiology. 2016. October;45(5):1482–92. PubMed PMID: Pubmed Central PMCID: 5100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobias DK, Clish C, Mora S, Li J, Hu FB, Manson JE, et al. Dietary Intakes and Circulating Concentrations of Branched-Chain Amino Acids in Relation to Incident Type 2 Diabetes Risk among High-Risk Women with a History of Gestational Diabetes Mellitus. Clinical chemistry. 2018;Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata C, Nakamura K, Wada K, Tsuji M, Tamai Y, Kawachi T. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: the Takayama study. American journal of epidemiology. 2013. October 15;178(8):1226–32. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 12.Isanejad M, LaCroix AZ, Thomson CA, Tinker L, Larson JC, Qi Q, et al. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women’s Health Initiative. The British journal of nutrition. 2017. June;117(11):1523–30. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higashi T, Hayashi H, Kaida T, Arima K, Takeyama H, Taki K, et al. Prognostic Impact of Visceral Fat Amount and Branched-Chain Amino Acids (BCAA) in Hepatocellular Carcinoma. Annals of surgical oncology. 2015. December;22 Suppl 3:S1041–7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi N, Mahbub MH, Takahashi H, Hase R, Ishimaru Y, Sunagawa H, et al. Plasma free amino acid profiles evaluate risk of metabolic syndrome, diabetes, dyslipidemia, and hypertension in a large Asian population. Environmental health and preventive medicine. 2017. April 7;22(1):35 PubMed PMID: Pubmed Central PMCID: 5664911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatric obesity. 2013. February;8(1):52–61. PubMed PMID: Pubmed Central PMCID: 3519972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rietman A, Stanley TL, Clish C, Mootha V, Mensink M, Grinspoon SK, et al. Associations between plasma branched-chain amino acids, beta-aminoisobutyric acid and body composition. Journal of nutritional science. 2016;5:e6 PubMed PMID: Pubmed Central PMCID: 4791517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nature medicine. 2017. July;23(7):859–68. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 18.Goffredo M, Santoro N, Trico D, Giannini C, D’Adamo E, Zhao H, et al. A Branched-Chain Amino Acid-Related Metabolic Signature Characterizes Obese Adolescents with Non-Alcoholic Fatty Liver Disease. Nutrients. 2017. June 22;9(7). PubMed PMID: Pubmed Central PMCID: 5537762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy RA, Moore SC, Playdon M, Meirelles O, Newman AB, Milijkovic I, et al. Metabolites Associated With Lean Mass and Adiposity in Older Black Men. The journals of gerontology Series A, Biological sciences and medical sciences. 2017. October 01;72(10):1352–9. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, et al. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS medicine. 2016. November;13(11):e1002179 PubMed PMID: Pubmed Central PMCID: 5127513 serves on the journal’s editorial board. MIM is a member of the Editorial Board of PLOS Medicine. MIM is a member of advisory boards for NovoNordisk and Pfizer. MIM received honoraria for speaking engagements: NovoNordisk, Pfizer, Eli Lilly. MIM receives research funding from: NovoNordisk, Pfizer, Eli Lilly, Takeda, Servier, Sanofi-Aventis, Boehringer Ingelheim, Janssen, Merck, Roche, Astra-Zeneca. EDK is an employee of Metabolon Inc., a fee-for-service metabolomics provider and received salary and stock options as compensation. IB and her spouse own stock in GlaxoSmithKline and Incyte Corporation. SB acts as an occasional paid statistical referee for PLOS Medicine, however had no reviewer role in this paper. The other authors report no conflict of interest relative to this study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatazawa Y, Tadaishi M, Nagaike Y, Morita A, Ogawa Y, Ezaki O, et al. PGC-1alphamediated branched-chain amino acid metabolism in the skeletal muscle. PloS one. 2014;9(3):e91006 PubMed PMID: Pubmed Central PMCID: 3956461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nature medicine. 2014. October;20(10):1193–8. PubMed PMID: Pubmed Central PMCID: 4191991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burrage LC, Nagamani SC, Campeau PM, Lee BH. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Human molecular genetics. 2014. September 15;23(R1):R1–8. PubMed PMID: Pubmed Central PMCID: 4170715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holecek M Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutrition & metabolism. 2018;15:33 PubMed PMID: Pubmed Central PMCID: 5934885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trico D, Prinsen H, Giannini C, de Graaf R, Juchem C, Li F, et al. Elevated alpha-Hydroxybutyrate and Branched-Chain Amino Acid Levels Predict Deterioration of Glycemic Control in Adolescents. The Journal of clinical endocrinology and metabolism. 2017. July 1;102(7):2473–81. PubMed PMID: Pubmed Central PMCID: 5505187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CC, Watkins SM, Lorenzo C, Wagenknecht LE, Il’yasova D, Chen YD, et al. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes care. 2016. April;39(4):582–8. PubMed PMID: Pubmed Central PMCID: 4806771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon MS. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients. 2016. July 01;8(7). PubMed PMID: Pubmed Central PMCID: 4963881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature reviews Endocrinology. 2014. December;10(12):723–36. PubMed PMID: Pubmed Central PMCID: 4424797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell metabolism. 2009. April;9(4):311–26. PubMed PMID: Pubmed Central PMCID: 3640280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saha AK, Xu XJ, Balon TW, Brandon A, Kraegen EW, Ruderman NB. Insulin resistance due to nutrient excess: is it a consequence of AMPK downregulation? Cell cycle. 2011. October 15;10(20):3447–51. PubMed PMID: Pubmed Central PMCID: 3356833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, et al. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010. October;59(10):2426–34. PubMed PMID: Pubmed Central PMCID: 3279521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. The Journal of biological chemistry. 2001. October 12;276(41):38052–60. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 33.Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007. June;56(6):1600–7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 34.Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002. March;51(3):599–605. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 35.Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005. September;54(9):2674–84. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 36.Preiss D, Rankin N, Welsh P, Holman RR, Kangas AJ, Soininen P, et al. Effect of metformin therapy on circulating amino acids in a randomized trial: the CAMERA study. Diabetic medicine : a journal of the British Diabetic Association. 2016. November;33(11):1569–74. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 37.Irving BA, Carter RE, Soop M, Weymiller A, Syed H, Karakelides H, et al. Effect of insulin sensitizer therapy on amino acids and their metabolites. Metabolism: clinical and experimental. 2015. June;64(6):720–8. PubMed PMID: Pubmed Central PMCID: 4525767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xuan L, Hou Y, Wang T, Li M, Zhao Z, Lu J, et al. Association of branched chain amino acids related variant rs1440581 with risk of incident diabetes and longitudinal changes in insulin resistance in Chinese. Acta diabetologica. 2018. May 31 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 39.Ganna A, Salihovic S, Sundstrom J, Broeckling CD, Hedman AK, Magnusson PK, et al. Large-scale metabolomic profiling identifies novel biomarkers for incident coronary heart disease. PLoS genetics. 2014. December;10(12):e1004801 PubMed PMID: Pubmed Central PMCID: 4263376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation. 2018. February 20;137(8):841–53. PubMed PMID: Pubmed Central PMCID: 5854187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang XZ, Zheng SX, Hou YM. A Non-Targeted Liquid Chromatographic-Mass Spectrometric Metabolomics Approach for Association with Coronary Artery Disease: An Identification of Biomarkers for Depiction of Underlying Biological Mechanisms. Medical science monitor : international medical journal of experimental and clinical research. 2017. February 2;23:613–22. PubMed PMID: Pubmed Central PMCID: 5301958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basak T, Varshney S, Hamid Z, Ghosh S, Seth S, Sengupta S. Identification of metabolic markers in coronary artery disease using an untargeted LC-MS based metabolomic approach. Journal of proteomics. 2015. September 8;127(Pt A):169–77. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 43.Fan Y, Li Y, Chen Y, Zhao YJ, Liu LW, Li J, et al. Comprehensive Metabolomic Characterization of Coronary Artery Diseases. Journal of the American College of Cardiology. 2016. September 20;68(12):1281–93. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvado J, et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clinical chemistry. 2016. April;62(4):582–92. PubMed PMID: Pubmed Central PMCID: 4896732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobias DK, Lawler PR, Harada PH, Demler OV, Ridker PM, Manson JE, et al. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circulation Genomic and precision medicine. 2018. April;11(4):e002157 PubMed PMID: Pubmed Central PMCID: 5880282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mels CM, Schutte AE, Schutte R, Huisman HW, Smith W, Fourie CM, et al. The link between vascular deterioration and branched chain amino acids in a population with high glycated haemoglobin: the SABPA study. Amino acids. 2013. December;45(6):1405–13. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 47.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiological reviews. 2010. January;90(1):207–58. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 48.Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation. 2016. May 24;133(21):2038–49.PubMed PMID: Pubmed Central PMCID: 4879058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kappel BA, Lehrke M, Schutt K, Artati A, Adamski J, Lebherz C, et al. Effect of Empagliflozin on the Metabolic Signature of Patients With Type 2 Diabetes Mellitus and Cardiovascular Disease. Circulation. 2017. September 5;136(10):969–72. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 50.Li T, Zhang Z, Kolwicz SC Jr., Abell L, Roe ND, Kim M, et al. Defective Branched-Chain Amino Acid Catabolism Disrupts Glucose Metabolism and Sensitizes the Heart to Ischemia-Reperfusion Injury. Cell metabolism. 2017. February 7;25(2):374–85. PubMed PMID: Pubmed Central PMCID: 5301464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001. September 13;345(11):790–7. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 52.Hu FB. Diet and cardiovascular disease prevention the need for a paradigm shift. Journal of the American College of Cardiology. 2007. July 3;50(1):22–4. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 53.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. The New England journal of medicine. 2000. July 6;343(1):16–22. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 54.White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman JM, et al. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell metabolism. 2018. May 11 PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Y, Ceglarek U, Huang T, Li L, Rood J, Ryan DH, et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. The American journal of clinical nutrition. 2016. February;103(2):505–11. PubMed PMID: Pubmed Central PMCID: 4733257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, et al. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes. 2013. August;62(8):2757–61. PubMed PMID: Pubmed Central PMCID: 3717831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanvold SE, Vinknes KJ, Bastani NE, Turner C, Loken EB, Mala T, et al. Plasma amino acids, adiposity, and weight change after gastric bypass surgery: are amino acids associated with weight regain? European journal of nutrition. 2017. August 30 PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 58.Haufe S, Engeli S, Kaminski J, Witt H, Rein D, Kamlage B, et al. Branched-chain amino acid catabolism rather than amino acids plasma concentrations is associated with diet-induced changes in insulin resistance in overweight to obese individuals. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2017. October;27(10):858–64. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 59.Maida A, Chan JSK, Sjoberg KA, Zota A, Schmoll D, Kiens B, et al. Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Molecular metabolism. 2017. August;6(8):873–81. PubMed PMID: Pubmed Central PMCID: 5518726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. The Journal of physiology. 2018. February 15;596(4):623–45. PubMed PMID: Pubmed Central PMCID: 5813603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruiz-Canela M, Guasch-Ferre M, Toledo E, Clish CB, Razquin C, Liang L, et al. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: case-cohort study within the PREDIMED Trial. Diabetologia. 2018. July;61(7):1560–71. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walford GA, Ma Y, Clish C, Florez JC, Wang TJ, Gerszten RE, et al. Metabolite Profiles of Diabetes Incidence and Intervention Response in the Diabetes Prevention Program. Diabetes. 2016. May;65(5):1424–33. PubMed PMID: Pubmed Central PMCID: 4839205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao Q, Moore SC, Keadle SK, Xiang YB, Zheng W, Peters TM, et al. Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. International journal of epidemiology. 2016. October;45(5):1433–44. PubMed PMID: Pubmed Central PMCID: 5100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kujala UM, Peltonen M, Laine MK, Kaprio J, Heinonen OJ, Sundvall J, et al. Branched-Chain Amino Acid Levels Are Related with Surrogates of Disturbed Lipid Metabolism among Older Men. Frontiers in medicine. 2016;3:57 PubMed PMID: Pubmed Central PMCID: 5122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glynn EL, Piner LW, Huffman KM, Slentz CA, Elliot-Penry L, AbouAssi H, et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia. 2015. October;58(10):2324–35. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kivela R, Silvennoinen M, Lehti M, Rinnankoski-Tuikka R, Purhonen T, Ketola T, et al. Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010. November;24(11):4565–74. PubMed PMID: Pubmed Central PMCID: 2974413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stern MP. Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes. 1995. April;44(4):369–74. PubMed PMID: . [DOI] [PubMed] [Google Scholar]