Abstract
The immune response to heparin is one of the most common drug-induced allergies, and yet, atypical for a drug hypersensitivity reaction. Whereas most drug-induced allergies are rare, idiosyncratic and life-long, the allergic response to heparin is common, predictable in certain clinical settings and transient. Advances in the last decade with regards to structural characterization of the PF4/heparin antigenic complex, contributions of innate immunity and development of animal models have provided insights into the distinctive features of the HIT immune response. Recent descriptions of the crystal structure of the PF4/heparin complex, alongside other biophysical studies, have clarified the structural requirements for immunogenicity and heparin-dependency of antibody formation. Studies of interactions of PF4 with bacterial cell walls as well as epidemiologic associations of anti-PF4/heparin antibody formation and infection suggest a role for immune priming and explain the rapid evolution of an isotype-switched immune response in sensitized patients. Murine models have greatly facilitated investigations of cellular basis of the HIT response and identified a major role for T-cells and marginal zone B-cells, but key findings have yet to be validated in human disease. This chapter will summarize recent investigations of the HIT immune response in the context of major pathways of immune activation and identify areas of uncertainty.
Introduction
The immune response to heparin, as signified by development of anti-platelet factor 4 (PF4)/heparin antibodies, is one of the leading causes of drug-induced allergic reactions among hospitalized patients. Yet, much remains unknown about its pathogenesis. Specifically, little is known about the reasons why so many patients react to heparin, why heparin and PF4, which, individually, are considered “self” antigens, become recognized as “non-self” when combined together and why the immune system limits its recognition of the antigenic complex, leading to transient antibody responses in the majority of sensitized patients. Recent investigations utilizing cellular studies and animal models have shed light on each of these biological questions. The following chapter will first summarize the distinctive features of the HIT immune response, followed by a review of recent structural studies of the PF4/heparin antigen and cellular studies of the HIT immune response in vitro and in vivo. These findings will be discussed in the context of our current understanding of innate and adaptive immune mechanisms.
The humoral response to PF4/heparin
Naturally occurring anti-PF4/heparin antibodies are rare in healthy individuals. In a study of ~3800 blood bank donors, anti- PF4/heparin antibodies were detected in ~3.1%, using a low cut-off for antibody positivity (OD >0.4), and in 0.3% using a higher cut-off (OD>1) in a commercial enzyme-linked immuonosorbent assay (ELISA) (1). Similar findings were noted in another study of 4029 healthy subjects, wherein ~4.4% of patients had low levels of PF4/heparin- IgG (OD>0.5–1.0) and <0.5% were noted to have high-level IgG (OD >1.0) (2).
While antibody formation is unusual in healthy subjects, anti-PF4/heparin autoantibodies have been reported in the context of inflammation and/or orthopedic surgery. To date, 12 cases of “spontaneous HIT” have been described in the literature (3–8). All of the reported cases were associated with high-titer platelet activating anti-PF4/heparin antibodies without prior heparin exposure and were associated with clinical complications of thrombocytopenia and/or thrombosis. Of the 12 patients, 7 patients had recent orthopedic surgery and 3 had recent infection. These findings suggest that infection, post-operative inflammation and/or mechanical compression (9) facilitates antigen exposure through heightened platelet and/or endothelial activation (10).
The preponderance of PF4/heparin seroconversions, however, occurs in the wake of unfractionated heparin (UFH) or low molecular weight heparin (LMWH) therapy. Anti-PF4/heparin antibodies occur in ~27–61% of patients after cardiac surgery (11–14) and in 8–17% medical and surgical patients treated with UFH or LMWH therapy for treatment or prophylaxis of venous thromboembolic (VTE) disease (15, 16). Seroconversion rates appear to be lower in certain clinical populations, including pediatric (17, 18) and obstetric (19) patients.
An unusual feature of the HIT immune response is the timing and pattern of seroconversions. In heparin naïve individuals, PF4/heparin seroconversions occur within 4–14 days of drug exposure (20). Importantly, isotype-switched IgG antibodies to PF4/heparin can be detected as early as 4 days after drug exposure, generally without antecedent IgM antibodies (20, 21). In a subset of patients, antibody formation is followed 5–14 days later by clinical complications of thrombocytopenia and/or thrombosis (21). As well, a clinical variant known as delayed-onset HIT, can result in disease manifestations up to 30 days after heparin exposure.
Once antibodies form, titers of anti-PF4/heparin wane over time, becoming undetectable over three to four months (22, 23). Patients who are re-exposed to the drug months to years after antibody disappearance often do not have recrudescent disease, and rarely seroconvert to antiPF4/heparin positivity (23–25), perhaps, with the exception of patients who are re-exposed for cardiac surgery, who have higher rates of seroconversions (26).
The structural basis of PF4/heparin immunogenicity
Significant advances have been made in recent years in understanding the structural basis for immunogenicity of PF4/heparin complexes. These studies, largely stemming from observations of PF4/heparin binding sites recognized by HIT antibodies, suggest that that PF4 conformational changes caused by heparin are likely relevant for antibody formation.
The structural basis of HIT antibody binding to antigen was first documented by Greinacher and colleagues, who in their seminal studies in the 1990’s showed that HIT antibodies recognized multimolecular complexes of PF4 and heparin formed at distinct stoichiometric ratios (27). Building on these observations, Rauova and colleagues in 2005 demonstrated that antigenic multimolecular complexes form over a narrow range of PF4 and heparin molar ratios (PHRs) leading to the generation of sizeable ultra-large complexes (>670kDa) (28). Additional biophysical studies by Greinacher and colleagues in 2006 (29) and Suvarna and colleagues in 2007 (29) showed that formation of PF4/heparin ULCs occurred through charge-neutralization. Additoinal structural studies by Brandt et. al. and Kreimann et.al have shown that conformational changes leading to expression of neoepitopes are not merely due to formation of ULCs but need to meet an energy threshold (>−4000 calories/molPF4) for effective binding of antibodies(30, 31).
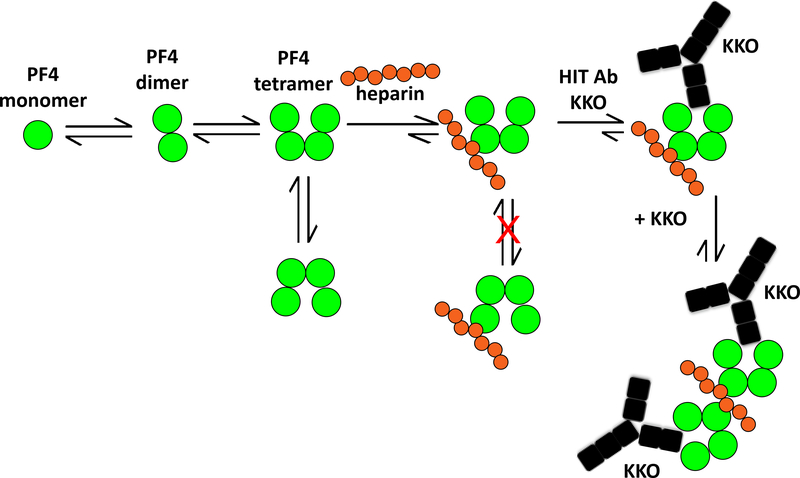
The structural basis of ULC formation was further clarified by Cai and colleagues, who, in 2015, crystallized the PF4/fondaparinux complex (Figure 1) (32) as well as the crystal structure of the immune complex using the monoclonal antibody, KKO, which recognizes complexes of human PF4 and heparin (33). The initial characterization of the crystal structure of PF4 in 1994 revealed that the tetramer displays a pseudosymmetry with respect to its dimers. The two dimers involved in tetramer formation show an “open” and “closed” conformation, as defined by intra-chain distances of surface exposed amino acids (Figure 1) (34). Cai et. al., demonstrated that binding of fondaparinux (and presumably, heparin) occurs assymetrically on the PF4 tetramer to the “closed” site of the PF4 tetramer. This asymmetric binding of heparin has important consequences for ULC formation and expression of neoeopitopes for antibody binding. These studies showed that heparin binds to three monomers of one PF4 tetramer forming a groove on the “closed” end. Heparin also simultaneously binds to the C-terminal of another tetramer to promote bridging of two tetramers. In so doing, heparin assumes a linear configuration, which results in binding of additional tetramers (Figure 1). Another important effect of heparin binding is the further stabilization of the asymmetric conformation of the PF4 tetramer, resulting in exposure of neo-epitopes on the “open” side to which HIT antibodies bind, which are also immunogenic (32).
Figure 1. A cartoon summarizing a model of PF4/heparin ULC formation {adapted from Cai et. al (32)}.
PF4 molecules (dark grey circles) exist in an equilibrium among monomers, dimers and tetramers. Upon binding to heparin (light grey circles), the configuration of the tetramer is stabilized. Binding of heparin to PF4 also stabilizes the heparin in a linear configuration allowing binding of additional PF4. The net result is the generation of stable ultralarge immune complexes. As a result, the open end of the PF4 tetramer is oriented, revealing neo-epitopes that are recognized by HIT-antibody KKO (black).
Figure reproduced with permission from the authors.
In vivo studies corroborate heparin’s effect on the immunogenicity of PF4. Like many carbohydrate compounds, heparins are poorly immunogenic (35). While the drug is minimally sensitizing on its own (36), heparin acquires potent immunogenicity when combined with PF4 or other positively charged proteins. Mice injected with mouse (m)PF4/heparin ULCs develop antibodies to mPF4/heparin complexes in 7–14 days (37, 38) in a heparin dependent manner. Studies have shown that anti-mPF4/heparin antibodies are serologically similar to anti-human (h)PF4/heparin antibodies with respect to heparin-dependent binding and ability to activate platelets in a FcγRIIA dependent manner (38). Similar to antibody binding requirements, the immunogenicity of mPF4/heparin complexes in vivo is highly correlated with the stoichiometry of PF4 and heparin (PF4:heparin molar ratios, PHRs). Higher rates of seroconversions occur with greater PF4 content (PHRs >10:1), whereas low PF4 and high heparin concentrations are associated with marked diminution of the immune response (low PHRs <1:5). Similar findings have been noted in studies utilizing heparin and other positively charged proteins (protamine/lysozyme) (39).
The innate immune response in HIT
While it is generally accepted that PF4/heparin complexes are immunogenic in vivo, the extent to which this antigen behaves as a conventional antigen has been the subject of debate. PF4/heparin ULCs consist of repetitive patterned structures, and in many ways, resemble the repetitive charged carbohydrate structures found on microbial pathogens. Because the innate immune response is designed to recognize patterned structures on microbial surfaces, several studies have focused on the role of innate immune mechanisms in HIT.
The innate immune system carries out its sentinel functions through a network of humoral-based proteins (pattern recognition molecules or PRMs) and/or cell-surface receptors (pattern recognition receptors or PRRs). Binding of PRMs and PRRs to conserved patterns on the surface of microorganisms unleashes a cascade of inflammatory responses, characterized by complement activation, cytokine release, migration of phagocytic cells, and activation of professional antigen-presenting cells (APCs). These responses, while rapid and generalized for the purpose of containing micro-organisms, are also essential for programming subsequent adaptive immune responses. In this latter context, APCs activated in the course of an innate immune response, provide important co-stimulatory signals for T-cells. To date, investigations of the innate immune system in HIT have examined the role of bacterial infection and PRRs in the development of anti-PF4/heparin antibodies.
Bacterial infections as sensitizing events in HIT
As noted above, an unusual feature of the HIT immune response is the development of isotype-switched antibodies within 4–5 days of heparin exposure. To explain the early IgG response, Greinacher and colleagues postulated that HIT may arise from prior antigen exposure. Data in support of this hypothesis derive from population studies and cellular investigations of bacterial interactions with PF4. To examine the role of bacterial infection as a primary sensitizing event, Krauel and colleagues were first to demonstrate interactions of bacteria with PF4. Using biotinylated PF4, these investigators showed that PF4 binds to both gram-positive and gram-negative bacteria and that binding was charge dependent, as heparin could compete with bacteria for binding PF4 (2). These investigators also showed that antibodies from patients with HIT recognize PF4 bound to bacteria and that mice suffering from polymicrobial sepsis develop an expected sequence of IgM and IgG PF4/heparin seroconversions (2). Subsequent studies by this group have shown that PF4 not only binds to phosphate residues on lipid A moieties of bacterial lipopolysaccharide (40), but also binds to short chain polyphosphates found in platelets (41). The clinical significance of these findings have been demonstrated in several studies of patients with and without infection. In a case-control designed study of 40 subjects with and without gum infection (periodontitis), subjects with severe periodontal disease, as indicated by increased probing depth, had a 7-fold higher risk for anti-PF4/heparin antibody positivity than control subjects (42). In a population study of 3500 subjects assessed for periodontal disease, periodontal disease significantly correlated with anti-PF4/heparin antibody reactivity, irrespective of immunoglobulin isotype (42). Finally, in a smaller study of 32 bacteremic patients, increased anti-PF4/heparin antibody levels were demonstrated in patients with gram-negative bacteria as compared to healthy controls (43). In these patients, subsequent heparin exposures would elicit anti-PF4/heparin antibody formation only if the optimal stoichiometric requirements of antigen formation were met (44).
Toll-like receptors (TLRs)
TLRs are a family of pattern recognition receptors (~10 described in humans) present on antigen presenting cells and B-cells that recognize patterned ligands on microbes (45). With the exception of TLR-3, all TLRs signal through the intracellular myeloid differentiation 88 (MyD88) adaptor protein. TLR-4, on the other hand, has both MyD88 dependent and independent modes of activation (46). Using a murine immunization model, our laboratory showed that MyD88 null mice respond similarly to immunization with PF4/heparin, suggesting that the MyD88 dependent TLRs are not critical to the HIT immune response (47). However, one recent study implies that TLR-4, a receptor that can function independently of MyD88, may be involved in immune activation. Using whole blood from healthy human subjects, Prechel and Walenga showed minimal IL-8 release when blood was incubated with PF4 alone or heparin alone. However, when whole blood was incubated with PF4/heparin complexes at varying stoichiometric ratios, they noted significant IL-8 release, with maximal IL-8 release occurring at PHR ratios of 12.5:1. Moreover, IL-8 release was highly variable among donors and dependent on TLR-4 signaling, as an anti-TLR-4 antibody abrogated IL-8 secretion (48). While additional studies are needed to show the cell-types and/or pathways activated by TLR-4, these findings provide an important first link between pattern recognition molecules and HIT.
The adaptive immune response in HIT
The adaptive immune response, like the innate immune system, has both humoral and cellular based effector mechanisms. Unlike responses of the innate immune system, which are immediate and non-specific, responses of the adaptive immune system occur days after antigen exposure and are highly antigen-specific. The hallmark of an adaptive immune response is involvement of T-cells and the generation of long-lived protective immunity.
To what extent T-cells are involved in HIT is currently unresolved. Clinically and scientifically, there is evidence to suggest that HIT may be T-cell dependent or T-cell independent (Table 1). T-cell dependent features of the immune response come from clinical observations of isotype switched IgG, which generally require T-cell help, evidence of restricted T-cell receptor usage in patients with HIT and murine studies showing requirements for T-cell help. However, there is also evidence to support T-cell independent pathways, including the serologic transience of anti-PF4/heparin antibodies, lack of immune recall (24, 49) and cellular studies involving marginal zone B-cells. The following sections summarize investigations of adaptive immune mechanisms in HIT.
Table 1:
Evidence for and against the role of T-cells in the HIT immune response
| Data supporting a role for T-cells in the PF4/heparin immune response | References |
|---|---|
| • Human studies showing T-cell skewing in two patients with HIT | (50) |
| • Murine studies: Lack of T-cells or T-cell depletion abrogates HIT antibody formation in mice | (38, 52) |
| Data against a role for T-cells in HIT in the PF4/heparin immune response | |
| • Lack of immune recall in HIT patients | (24, 49) |
| • Lack of memory B-cells in HIT | (61) |
| • Evidence of anti-PF4/heparin antibody formation in patients on T-cell immunosuppressive therapy | (51) |
| • Requirements for marginal zone B-cells in murine studies; not T-cell dependent | (57) |
Role of T-cells and Regulatory T-cells in HIT
To date, few studies have examined the contribution of T-cells in human HIT (50, 51). Studies by Bacsi and colleagues showed that T-cells isolated from HIT patients after an acute episode proliferate in response to PF4/heparin, but not to PF4 or heparin alone. Moreover, responding T-cells showed highly restricted T-cell receptor (TCR) usage, with skewing of TCRβ chains involving the BV 5.1 family (50). Another study examining the role of T-cells in immunosuppressed patients suggests that T-cells may not play a vital role in PF4/heparin seroconversions (51). In this study, 38 patients undergoing intensive T-cell immunosuppression for liver transplantation were monitored for seroconversions before and after liver transplantation with triple immunosuppressive therapy (calcineurin inhibitors, mycophenollic acid and corticosteroids). Despite effective immunosuppression, new seroconversions occurred in 5/33 patients (15%), of whom only two developed platelet activating antibodies. No patients in this study developed HIT.
Murine studies have shown a more consistent requirement for T-cells in the development of anti-PF4/heparin antibodies. In the first application of a murine immunization model, our laboratory showed that athymic mice (lacking T-cells) do not exhibit an anti-PF4/heparin immune response, unlike wild-type (WT) animals, which generate antibodies to mPF4/heparin (38). These findings were extended by Zheng and colleagues, who using the same murine immunization model, showed that depletion of T-helper cells (Th) with a polyclonal antibody abrogates the PF4/heparin immune response as compared to mice injected with control antibody (52). Using bone marrow transplant experiments involving WT and CD40 −/− B-cells, these investigators additionally showed requirements for B-cell CD40, a co-receptor for T-cell help via CD40 ligand.
Because T-cell involvement generally implies development of protective memory, it has been challenging to reconcile murine studies, showing T-cell involvement, with clinical observations of human HIT, wherein immune recall is generally lacking. Peripheral tolerance, however, is one mechanism that reconciles these seemingly discrepant observations. Regulatory T-cells, or Tregs, are a subset of circulating CD4+ T cells (CD4+CD25+) that maintain peripheral tolerance by suppressing the activation and expansion of antigen-specific lymphocytes (53). Tregs are particularly important for regulating autoreactive B- and T-cells. Studies by Fleischer and colleagues showed that PF4 inhibits T cell proliferation induced by activating antibodies (anti-CD3 and anti-CD28). These effects were due to decreased synthesis and secretion of IL-2 (54) and were mediated through low-affinity interactions with GAGs on the T cell surface (54). Liu and colleagues expanded on these observations by showing that these inhibitory effects of PF4 primarily occurred with non-regulatory T cells (CD4+CD25-). However, PF4 enhances the proliferation of Treg cells, but these PF4-stimulated Tregs lost their normal suppressive function on non-regulatory T-cells (55). Thus, if circulating PF4/heparin complexes inhibited Treg function, this would allow for transient expression of anti-PF4/heparin (self-reactive) antibodies by non-regulatory T-cells; as PF4/heparin complexes are cleared from circulation, there would restoration of Treg function and resumption of peripheral tolerance mechanisms. Studies have yet to characterize the contribution of Tregs in murine models or to human disease through ex-vivo studies.
Role of B-cells and B-cell subsets in HIT
The presence of T-cell independent features in the HIT immune response have prompted increased scrutiny of B-cell subsets that work independently of T-cell help. Three lineages of B-cells are recognized in mice and humans based on their cellular ontogeny and anatomic location: 1) B1 B-cells, which are found at birth and are primarily involved in T-cell independent responses, 2) marginal zone (MZ) B-cells, primarily found in the splenic marginal zone, have the capacity to participate in both T-cell dependent and independent responses and 3) follicular B-cells, the most abundant B-cell subsets, which are conventional B-lymphocytes that circulate in the spleen and lymph nodes and generate high-affinity, isotype-switched antibodies in response to T-cell help (56). Because the splenic MZ traps large multivalent antigens, and because MZ B-cells contribute to T-cell independent immune responses, Zheng and colleagues examined the contribution of MZ B-cells to the development of anti-PF4/heparin antibodies. In these studies, B-cell specific Notch2-deficient mouse strain (CD19CreNotch2fl/fl), had marked impairment in anti-mPF4/heparin antibody production as compared to WT mice injected with mPF4/heparin. They additionally showed that adoptive transfer of MZ B-cells into mice lacking mature B-cells (μMT mice) facilitated development of anti-mPF4/heparin antibodies, but did not occur in μMT mice receiving only follicular B-cells (57). These findings were comparable to impaired antibody responses in the B-cell/Notch2-deficient mice to trinitrophenyl-ficoll, a T-cell independent antigen, suggesting that anti-PF4/heparin antibody production only required MZ B-cells and not T-cells. However, these findings are distinctly at odds with earlier studies using athymic mice (38) and studies involving T-helper cells (52), highlighting the challenges of murine studies, especially those involving genetically modified strains.
Other investigators have examined the contribution of B1 cells, another B-cell subset involved in T-cell independent immunity. Krauel and colleagues investigated pediatric patients and murine splenectomy/sepsis models (58) on the basis that MZ B-cells are under-developed in early infancy and are anatomically restricted to the spleen in mice. In these studies, the authors found evidence of anti-PF4/heparin IgM in infants aged 1–6 months after cardiac surgery, anti-PF4/heparin IgM antibody secreting cells in the cord blood of healthy volunteers and evidence of antibody production in splenectomized mice with polymicrobial sepsis. While these studies show that B-cell subsets, other than MZ B-cells, contribute to IgM antibody production, they only provide indirect evidence of B1 cell involvement.
Finally, two groups have examined the contribution of peripheral B-cell tolerance as a mechanism for transient antibody production in HIT. Using peripheral blood mononuclear cells from healthy subjects and splenocytes from WT mice, Zheng and colleagues demonstrated production of anti-PF4/heparin antibodies (IgM isotype) from B-cells after stimulation with the inflammatory oligodeoxynucleotides, deoxycytosine-deoxyguanosine (CpG) (59). In other studies, they showed that WT mice injected only with CpG also produced both IgG and IgM anti-PF4/heparin and that PKCδ deficient mice, mice lacking signaling molecule critical for B-cell anergy and tolerance maintenance, developed spontaneous IgG and IgM anti-PF4/heparin antibodies. Similar findings were recently noted by Krauel and colleagues, who demonstrated the presence of antibody-secreting B-cells in human cord blood and peripheral blood of healthy donors after stimulation with CpG (58). These findings implicate B-cell tolerance as another potential mechanism for regulating anti-PF4/heparin antibody production.
Antigen presenting cells
To date, little attention has been given to the role of antigen presentation in HIT. With conventional immune responses, APCs initiate adaptive immune response by taking up antigen, degrading the antigen in lysosomes and packaging degraded peptides alongside MHC class II proteins to T-cells. In recent studies, we examined the uptake and processing of PF4/heparin antigen by monocytes and dendritic cells. Using confocal microscopy and flow cytometry, we demonstrated that monocytes preferentially take up PF4/heparin complexes in a heparin-dependent manner and that uptake was non-specific, inhibitable by cytochalasin D (an inhibitor of cytoskeletal reorganization) and amiloride (an inhibitor of macropinocytosis). In other studies we observed that uptake was associated with endosomal processing and accompanied by cellular activation as indicated by CD83 and MHCII expression (60).
Summary
Despite increased scientific attention and development of murine models in recent years, a number of fundamental questions remain unanswered about the immune pathogenesis of HIT. To what extent the PF4/heparin serves as a conventional antigen, whether T-cells are essential to the immune response, and what regulatory mechanisms keep the immune response in check remain at the forefront of scientific investigation. These issues are of both clinical and scientific significance. Clinical benefits of understanding the immunologic basis of HIT can lead to identification of risk factors for seroconversion and determination of which patients can be safely re-exposed to drug. Understanding the scientific principles governing HIT can lead to discovery of unique pathways that can be disrupted and help to identify regulatory mechanisms that can be manipulated for control of autoimmune or allergic disease.
Acknowledgments
Supported by the National Institutes of Health P01 HL110860 (GMA)
References
- 1.Hursting MJ, Pai P, McCracken JE, et al. Platelet Factor 4/Heparin Antibodies in Blood Bank Donors. Am J Clin Pathol 2010; 134: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krauel K, Potschke C, Weber C, et al. Platelet factor 4 binds to bacteria, inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood 2011; 117: 1370–1378. [DOI] [PubMed] [Google Scholar]
- 3.Warkentin TE, Makris M, Jay RM, et al. A Spontaneous Prothrombotic Disorder Resembling Heparin-induced Thrombocytopenia. Amer J Med 2008; 121: 632–636. [DOI] [PubMed] [Google Scholar]
- 4.Jay RM, Warkentin TE. Fatal heparin-induced thrombocytopenia (HIT) during warfarin thromboprophylaxis following orthopedic surgery: another example of ‘spontaneous’ HIT? J Thromb Haemost 2008; 6: 1598–1600. [DOI] [PubMed] [Google Scholar]
- 5.Warkentin TE, Basciano PA, Knopman J, et al. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood 2014; 123: 3651–3654. [DOI] [PubMed] [Google Scholar]
- 6.Pruthi RK, Daniels PR, Nambudiri GS, et al. Heparin-induced thrombocytopenia (HIT) during postoperative warfarin thromboprophylaxis: a second example of postorthopedic surgery ‘spontaneous’ HIT. J Thromb Haemost 2009; 7: 499–501. [DOI] [PubMed] [Google Scholar]
- 7.Ketha S, Smithedajkul P, Vella A, et al. Adrenal haemorrhage due to heparin-induced thrombocytopenia. Thromb Haemost 2013; 109: 669–675. [DOI] [PubMed] [Google Scholar]
- 8.Okata T, Miyata S, Miyashita F, et al. Spontaneous heparin-induced thrombocytopenia syndrome without any proximate heparin exposure, infection, or inflammatory condition: Atypical clinical features with heparin-dependent platelet activating antibodies. Platelets 2015; 26: 602–607. [DOI] [PubMed] [Google Scholar]
- 9.Bito S, Miyata S, Migita K, et al. Mechanical prophylaxis is a heparin-independent risk for anti–platelet factor 4/heparin antibody formation after orthopedic surgery. Blood 2016; 127: 1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warkentin TE. Knee replacement and HIT without heparin. Blood 2016; 127: 961–962. [DOI] [PubMed] [Google Scholar]
- 11.Bauer TL, Arepally G, Konkle BA, et al. Prevalence of heparin-associated antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation 1997; 95: 1242–1246. [DOI] [PubMed] [Google Scholar]
- 12.Visentin GP, Malik M, Cyganiak KA, et al. Patients treated with unfractionated heparin during open heart surgery are at high risk to form antibodies reactive with heparin:platelet factor 4 complexes. J Lab Clin Med 1996; 128: 376–383. [DOI] [PubMed] [Google Scholar]
- 13.Pouplard C, May MA, Iochmann S, et al. Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular-weight heparin : clinical implications for heparin-induced thrombocytopenia. Circulation 1999; 99: 2530–2536. [DOI] [PubMed] [Google Scholar]
- 14.Trossaert M, Gaillard A, Commin PL, et al. High incidence of anti-heparin/platelet factor 4 antibodies after cardiopulmonary bypass surgery. Br J Haematol 1998; 101: 653–655. [DOI] [PubMed] [Google Scholar]
- 15.Amiral J, Peynaud-Debayle E, Wolf M, et al. Generation of antibodies to heparin-PF4 complexes without thrombocytopenia in patients treated with unfractionated or low-molecular-weight heparin. Am J Hematol 1996; 52: 90–95. [DOI] [PubMed] [Google Scholar]
- 16.Girolami B, Prandoni P, Stefani PM, et al. The incidence of heparin-induced thrombocytopenia in hospitalized medical patients treated with subcutaneous unfractionated heparin: a prospective cohort study. Blood 2003; 101: 2955–2959. [DOI] [PubMed] [Google Scholar]
- 17.Obeng EA, Harney KM, Moniz T, et al. Pediatric heparin-induced thrombocytopenia: prevalence, thrombotic risk, and application of the 4Ts scoring system. J Pediatr 2015; 166: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakil NH, Kanaan AO, Donovan JL. Heparin-induced thrombocytopenia in the pediatric population: a review of current literature. J Pediatr Pharmacol Ther: 2012; 17: 12–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fausett MB, Vogtlander M, Lee RM, et al. Heparin-induced thrombocytopenia is rare in pregnancy. Am J Obstet Gynecol 2001; 185: 148–152. [DOI] [PubMed] [Google Scholar]
- 20.Greinacher A, Kohlmann T, Strobel U, et al. The temporal profile of the anti-PF4/heparin immune response. Blood 2009; 113: 4970–4976. [DOI] [PubMed] [Google Scholar]
- 21.Warkentin TE, Sheppard JA, Moore JC, et al. Studies of the immune response in heparin-induced thrombocytopenia. Blood 2009; 113: 4963–4969. [DOI] [PubMed] [Google Scholar]
- 22.Cines DB, Kaywin P, Bina M, et al. Heparin-associated thrombocytopenia. N Engl J Med 1980; 303: 788–795. [DOI] [PubMed] [Google Scholar]
- 23.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med 2001; 344: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 24.Potzsch B, Klovekorn WP, Madlener K. Use of heparin during cardiopulmonary bypass in patients with a history of heparin-induced thrombocytopenia. N Engl J Med 2000; 343: 515. [DOI] [PubMed] [Google Scholar]
- 25.Lubenow N, Kempf R, Eichner A, et al. Heparin-induced thrombocytopenia: temporal pattern of thrombocytopenia in relation to initial use or reexposure to heparin. Chest 2002; 122: 37–42. [DOI] [PubMed] [Google Scholar]
- 26.Warkentin TE, Sheppard J-AI. Serological investigation of patients with a previous history of heparin-induced thrombocytopenia who are re-exposed to heparin. Blood 2014; 123: 2485–2493. [DOI] [PubMed] [Google Scholar]
- 27.Greinacher A, Potzsch B, Amiral J, et al. Heparin-associated thrombocytopenia: isolation of the antibody and characterization of a multimolecular PF4-heparin complex as the major antigen. Thromb Haemost 1994; 71: 247–251. [PubMed] [Google Scholar]
- 28.Rauova L, Poncz M, McKenzie SE, et al. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood 2005; 105: 131–138. [DOI] [PubMed] [Google Scholar]
- 29.Greinacher A, Gopinadhan M, Gunther J-U, et al. Close Approximation of Two Platelet Factor 4 Tetramers by Charge Neutralization Forms the Antigens Recognized by HIT Antibodies. Arterioscler Thromb Vasc Biol 2006; 26: 2386–2393. [DOI] [PubMed] [Google Scholar]
- 30.Brandt S, Krauel K, Gottschalk KE, et al. Characterisation of the conformational changes in platelet factor 4 induced by polyanions: towards in vitro prediction of antigenicity. Thromb Haemost 2014; 112: 53–64. [DOI] [PubMed] [Google Scholar]
- 31.Kreimann M, Brandt S, Krauel K, et al. Binding of anti–platelet factor 4/heparin antibodies depends on the thermodynamics of conformational changes in platelet factor 4. Blood 2014; 124: 2442–2449. [DOI] [PubMed] [Google Scholar]
- 32.Cai Z, Yarovoi SV, Zhu Z, et al. Atomic description of the immune complex involved in heparin-induced thrombocytopenia. Nat Commun 2015; 6: 8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arepally GM, Kamei S, Park KS, et al. Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood 2000; 95: 1533–1540. [PubMed] [Google Scholar]
- 34.Zhang X, Chen L, Bancroft DP, et al. Crystal structure of recombinant human platelet factor 4. Biochemistry 1994; 33: 8361–8366. [DOI] [PubMed] [Google Scholar]
- 35.Huhle G, Harenberg J, Malsch R, et al. Monoclonal antibodies against heparin and heparinoids. Semin Thromb Hemost 1997; 23: 17–22. [DOI] [PubMed] [Google Scholar]
- 36.Gitel S, Medina V, Wessler S. Preparation and identification of a population of antibodies that recognize carbodiimide-modified heparin. Blood 1985; 65: 902–911. [PubMed] [Google Scholar]
- 37.Suvarna S, Espinasse B, Qi R, et al. Determinants of PF4/heparin immunogeneicity Blood 2007; 110: 4253–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suvarna S, Rauova L, McCracken EK, et al. PF4/heparin complexes are T cell-dependent antigens. Blood 2005; 106: 929–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chudasama SL, Espinasse B, Hwang F, et al. Heparin modifies the immunogenicity of positively-charged proteins. Blood 2010; 116 : 6046–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krauel K, Weber C, Brandt S, et al. Platelet factor 4 binding to lipid A of Gram-negative bacteria exposes PF4/heparin-like epitopes. Blood 2012; 120: 3345–3352. [DOI] [PubMed] [Google Scholar]
- 41.Brandt S, Krauel K, Jaax M, et al. Polyphosphates form antigenic complexes with platelet factor 4 (PF4) and enhance PF4-binding to bacteria. Thromb Haemost 2015; 114: 1189–1198. [DOI] [PubMed] [Google Scholar]
- 42.Greinacher A, Holtfreter B, Krauel K, et al. Association of natural anti-platelet factor 4/heparin antibodies with periodontal disease. Blood 2011; 118: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 43.Pongas G, Dasgupta SK, Thiagarajan P. Antiplatelet factor 4/heparin antibodies in patients with gram negative bacteremia. Thromb Res 2013; 132: 217–220. [DOI] [PubMed] [Google Scholar]
- 44.Warkentin TE, Cook RJ, Marder VJ, et al. Anti-PF4/heparin antibody formation postorthopedic surgery thromboprophylaxis: the role of non-drug risk factors and evidence for a stoichiometry-based model of immunization. J Thromb Haemost 2010; 8: 504–512. [DOI] [PubMed] [Google Scholar]
- 45.Pandey S, Kawai T, Akira S. Microbial Sensing by Toll-Like Receptors and Intracellular Nucleic Acid Sensors. Cold Spring Harb Perspect Biol 2015; 7:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 2007; 7: 353–364. [DOI] [PubMed] [Google Scholar]
- 47.Suvarna S, Qi R, Hollingsworth JW, et al. Platelet factor 4-heparin complexes trigger immune responses independently of the MyD88 pathway. Br J Haematol 2008; 142: 671–673. [DOI] [PubMed] [Google Scholar]
- 48.Prechel MM, Walenga JM. Complexes of platelet factor 4 and heparin activate Toll-like receptor 4. J Thromb Haemost 2015; 13: 665–670. [DOI] [PubMed] [Google Scholar]
- 49.Potschke C, Selleng S, Broker BM, et al. Heparin-induced thrombocytopenia: further evidence for a unique immune response. Blood 2012; 120: 4238–4245. [DOI] [PubMed] [Google Scholar]
- 50.Bacsi S, De Palma R, Visentin GP, et al. Complexes of heparin and platelet factor 4 specifically stimulate T cells from patients with heparin-induced thrombocytopenia/thrombosis. Blood 1999; 94: 208–215. [PubMed] [Google Scholar]
- 51.Bakchoul T, Assfalg V, Zöllner H, et al. Anti-platelet factor 4/heparin antibodies in patients with impaired graft function after liver transplantation. J Thromb Haemost 2014; 12: 871–878. [DOI] [PubMed] [Google Scholar]
- 52.Zheng Y, Yu M, Padmanabhan A, et al. Critical role of CD4 T cells in PF4/heparin antibody production in mice. Blood 2015; 125: 1826–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 2001; 182: 18–32. [DOI] [PubMed] [Google Scholar]
- 54.Fleischer J, Grage-Griebenow E, Kasper B, et al. Platelet Factor 4 Inhibits Proliferation and Cytokine Release of Activated Human T Cells. J Immunol 2002; 169: 770–777. [DOI] [PubMed] [Google Scholar]
- 55.Liu CY, Battaglia M, Lee SH, et al. Platelet factor 4 differentially modulates CD4+CD25+ (regulatory) versus CD4+CD25- (nonregulatory) T cells. J Immunol 2005; 174: 2680–2686. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman W, Lakkis FG, Chalasani G. B Cells, Antibodies, and More. Clin J Am Soc Nephrol 2016; 11: 137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Y, Yu M, Podd A, et al. Critical role for mouse marginal zone B cells in PF4/heparin antibody production. Blood 2013; 121: 3484–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krauel K, Schulze A, Jouni R, et al. Further insights into the anti-PF4/heparin IgM immune response. Thromb Haemost 2016; 115: 752–761.. [DOI] [PubMed] [Google Scholar]
- 59.Zheng Y, Wang AW, Yu M, et al. B-cell tolerance regulates production of antibodies causing heparin-induced thrombocytopenia. Blood 2014; 123: 931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joglekar M, Khandelwal S, Cines DB, et al. Heparin enhances uptake of platelet factor 4/heparin complexes by monocytes and macrophages. J Thromb Haemost 2015; 13: 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selleng K, Schutt A, Selleng S, et al. Studies of the anti-platelet factor 4/heparin immune response: adapting the enzyme-linked immunosorbent spot assay for detection of memory B cells against complex antigens. Transfusion 2010; 50: 32–39. [DOI] [PubMed] [Google Scholar]