Abstract
Background:
Multiple case definitions for post-diarrheal hemolytic uremic syndrome (D+ HUS) associated with Shiga toxin-producing Escherichia coli (STEC) are used across public health, research, and clinical practice.
Methods:
To identify a single definition of D+ HUS for standardized use, we evaluated the comparability and validity of four common, heterogeneous definitions: the Council of State and Territorial Epidemiologists (CSTE) definition, hematology-focused and age-focused definitions from the literature, and hospital diagnosis. We reviewed medical records from 471 hospitalized E. coli O157:H7 cases reported in Washington State, 2005–2014. We assessed 1) reliability across definitions, 2) comparability of temporal trends, and 3) sensitivity and specificity using an omnibus reference standard, developed using a combination of definition agreement and clinical outcomes. With the standard, we classified cases as definite, borderline, or unlikely/not post- diarrheal D+ HUS.
Results:
Reliability was highest between the age-focused definition and hospital diagnosis (к=0.84), and temporal trends were largely comparable across definitions. For definite D+ HUS cases, the age-focused definition had the highest overall validity [100% sensitivity, 95% confidence interval (CI): 94%, 100%; 96% specificity, 95% CI: 94%, 98%]. The CSTE definition had low specificity (75%, 95% CI: 70%, 79%).
Conclusions:
In this review, the CSTE definition overestimated the burden of D+ HUS, and the age-focused definition provided the best overall reliability and validity to define post-diarrheal D+ HUS. Disease monitoring and research activities should consider using the age-focused D+ HUS definition.
Keywords: E. coli O157:H7, hemolytic uremic syndrome, Shiga toxin-producing Escherichia coli, surveillance
INTRODUCTION
Post-diarrheal hemolytic uremic syndrome (D+ HUS1) is characterized by hemolytic anemia, thrombocytopenia, and renal injury, often necessitates renal replacement therapy (Garg et al., 2003; Tarr et al., 2005), and has a case fatality of 3–5% (Gould et al., 2009; Mody et al., 2015; Scheiring et al., 2008). Incidence is highest among children <5 years-old (Centers for Disease Control and Prevention, 2016; Crim et al., 2015; Crim et al., 2014; Marder et al., 2017). D+ HUS surveillance is a surrogate for the incidence of Shiga toxin-producing Escherichia coli (STEC), including E. coli O157, its most common cause (Centers for Disease and Prevention, 1995; De Schrijver et al., 2008; Schimmer et al., 2006; Werber et al., 2008). Such data can indicate the underlying E. coli O157:H7 burden, assist in outbreak recognition, and detect trends that signal expanding etiologic roles for non-O157 STEC serogroups (Mahon et al., 1997). D+ HUS enumeration might also be more sensitive to underlying STEC incidence, because most HUS cases are easily diagnosed and are concentrated in relatively few centers, usually pediatric tertiary care institutions. However, not all jurisdictions systematically track and/or report D+ HUS incidence (Centers for Disease Control and Prevention, 2016), so much of our knowledge on D+ HUS trends arises from U.S. FoodNet reports (e.g. Centers for Disease Control, 2017).
One potential barrier to meaningful D+ HUS surveillance at any level is uncertainty as to the most appropriate case definition. The Council of State and Territorial Epidemiologists (CSTE) provides a case definition for reporting D+ HUS to CDC in the U.S., without regard to etiologic agent (Council of State and Territorial Epidemiologists, 2009). However, that case definition differs in important ways from case definitions used in the HUS and STEC literature, of which there are many.
The ideal case definition maximizes the inclusion of bona fide D+ HUS while minimizing inclusion of individuals without D+ HUS. Because D+ HUS is easily diagnosed by widely available laboratory tests, this entity should be amenable to a standardized case definition. However, the current spectrum of case definitions includes imprecise measurements, employing semi-quantitative criteria such as thrombocytopenia or anemia without provision of cut-points, and some using a qualitatively “abnormal” urinalysis.
Given the heterogeneity and potential limitations of extant definitions, we analyzed over 400 hospitalized cases of E. coli O157:H7 infection in Washington State to compare case definitions and identify one suitable for standardized use among the patients who developed possible or definite D+ HUS following STEC infection. To this end, we assessed the reliability, temporal trends, and validity of four of the most common D+ HUS case definitions.
METHODS
Record abstraction
We conducted a retrospective review of all hospitalized, culture-confirmed E. coli O157:H7 cases reported to Washington State Department of Health between 2005 and 2014 through passive surveillance. We obtained records from each hospital listed on Department of Health case report forms and abstracted the data in Appendix Table 1. We piloted the data abstraction form on 12 cases (5 pediatric, 7 adult; 1 with diagnosed D+ HUS) and revised the instrument before starting the full review. Cases who spent at least one midnight in an inpatient medical facility were considered hospitalized.
This review was conducted to enhance surveillance activities and therefore deemed exempt by the Washington State Institutional Review Board.
D+ HUS definitions
We considered four primary definitions of D+ HUS (Appendix Table 2). Cases are reported to CDC using the 1996 CSTE confirmed and probable criteria for postdiarrheal D+ HUS (reaffirmed 2009), which require anemia: (Harriet Lane Service (Johns Hopkins Hospital), 2009); hematuria, proteinuria, serum creatinine concentration ≥1.0 mg/dL for children <13 years- old and ≥1.5 mg/dL for ≥13-year-olds, or 50% increase in serum creatinine concentration from baseline; D+ HUS following diarrhea; and evidence of microangiopathic changes or D+ HUS onset within 21 days of diarrhea onset (https://wwwn.cdc.gov/nndss/conditions/hemolytic-uremic-syndrome-post-diarrheal/case-definition/1996/;Council of State and Territorial Epidemiologists, 2009). Two definitions from the literature, referred to here as the hematology- focused definition and age-focused definition, were also included. The hematology-focused definition has been employed in studies using FoodNet data (Mody et al., 2015; Mody et al., 2012; Ong et al., 2012). It resembles the CSTE definition but includes thrombocytopenia, does not accept hematuria or proteinuria as sufficient evidence of renal injury, and requires microangiopathic changes. The age-focused definition has been used in publications of D+ HUS case series from the Pacific Northwest (Ake et al., 2005; Klein et al., 2002; Wong et al., 2000; Wong et al., 2012), elsewhere in North America (Freedman et al., 2017), and Europe (Bielaszewska et al., 2006; Bielaszewska et al., 2007). It requires, all on the same day, hematocrit <30%, platelet count <150,000/mm3, and serum creatinine concentration above the upper limit of normal for age (Meites, 1989). A diagnosis of D+ HUS in the discharge note or charge codes was included as the fourth definition.
Comparability
We assessed comparability of the definitions in two ways. First, we estimated the reliability of the four definitions to identify the same population of D+ HUS cases by calculating the kappa statistic (к), with 95% confidence intervals (CIs), for each combination of definitions. Second, we calculated and graphed the annual incidence rate of D+ HUS across all age groups (U. S. Census Bureau, 2012).
Validity
To assess validity, we developed an omnibus reference standard based on definition agreement and common complications. We classified cases as “definite”, “borderline”, or “unlikely/not” D+ HUS (Appendix Table 3). Definite cases were those that: 1) all four definitions agreed were D+ HUS, 2) three definitions agreed were D+ HUS and were anuric, or 3) at least two definitions agreed were D+ HUS and received dialysis. Of the remaining cases, borderline cases were those that: 1) three definitions agreed were D+ HUS or 2) two definitions agreed were D+ HUS and had urine output <0.5ml/kg/hr. All other cases were considered unlikely/not D+ HUS.
Using the omnibus reference standard, we calculated the sensitivity and specificity of each definition, with exact binomial 95% confidence intervals, to accurately distinguish 1) definite D+ HUS cases and 2) the combination of definite and borderline D+ HUS cases, from non-HUS cases. We stratified sensitivity and specificity by age [<10 vs. >10 years, chosen because of a steep drop-off in incidence at 10 years old (Tarr et al., 2018)] to determine if definition validity differed by age group. To identify causes of imperfect sensitivity or specificity, we examined cases that differed from the omnibus reference standard. In sensitivity analysis, we examined validity for common modifications of definitions with objective criteria.
R (R Core Team, 2017) was used for all analyses.
RESULTS
Of 1160 culture-confirmed E. coli O157:H7 cases in Washington State during the study period, 471 (41%) were hospitalized (Appendix Figure 1), and we obtained and abstracted hospital records for 433 (92%) of these cases. No hospital was listed on the case report form for 18 cases, and records for 20 additional cases could not be located at the hospital listed (Appendix). Of the 433 reviewed cases, 161 (37%) met one or more D+ HUS definition. The CSTE definition classified 154 as D+ HUS; the hematology-focused definition, 58; the age- focused definition, 76; and hospital diagnosis, 92 (Table 1). In comparison, the omnibus reference standard classified 75 as D+ HUS (62 definite, 13 borderline). The average annual incidence of D+ HUS for all ages varied between 0.09 (hematology-focused) and 0.23 (CSTE) per 100,000 people (all ages). For individuals <18 years-old, the average annual incidence of D+ HUS varied by definition between 0.31 (hematology-focused) and 0.59 (CSTE) per 100,000 (Table 1).
Table 1. Clinical outcomes by D+ HUS definition among hospitalized E. coli O157:H7 patients, Washington State, 2005–2014.
| Variable | CSTE* | Hematology- focused |
Age- focused |
Hospital Diagnosis |
Full Cohort |
|---|---|---|---|---|---|
| Number of cases | 154 | 58 | 76 | 92 | 433 |
| Incidence per 100,000 | |||||
| <18 years-old | 0.59 | 0.31 | 0.41 | 0.51 | − |
| All ages | 0.23 | 0.09 | 0.11 | 0.14 | − |
| Bloody diarrhea (%) | 149 (98) | 57 (100) | 74 (99) | 91 (99) | 407 (96) |
| Missing | 2 | 1 | 1 | 0 | 11 |
| Vomiting (%) | 114 (77) | 49 (88) | 65 (89) | 75 (85) | 259 (65) |
| Missing | 5 | 2 | 3 | 4 | 37 |
| Days hospitalized, median (IQR) | 7 (4, 14) | 13.5 (10, 21) | 13 (10, 19) | 12 (7, 17) | 3 (2, 6) |
| Missing | 1 | 0 | 0 | 0 | 7 |
| Urine output | |||||
| Anuria (%) | 30 (28) | 26 (49) | 29 (44) | 28 (36) | 34 (17) |
| <0.5 ml/kg/hr (%) | 50 (46) | 35 (66) | 42 (64) | 42 (54) | 69 (35) |
| Missing | 46 | 5 | 10 | 14 | 233 |
| Underwent dialysis (%) | 41 (28) | 33 (58) | 39 (52) | 39 (42) | 41(10) |
| Missing | 8 | 1 | 1 | 0 | 37 |
| Died (%) | 4 (2.7) | 2 (3.4) | 3 (3.9) | 2 (2.2) | 5(1.2) |
| Missing | 7 | 0 | 0 | 0 | 32 |
Abbreviations: CSTE, Council for State and Territorial Epidemiologists; D+ HUS, post-diarrheal hemolytic uremic syndrome; IQR, interquartile range
Includes confirmed and probable definitions.
The frequencies of bloody diarrhea and vomiting were similar across definitions. Other clinical outcomes did vary across definitions, with the CSTE definition generally identifying a D+ HUS case pool with lower average severity than the other definitions (Table 1).
Five cases died during the study period (Appendix Table 4). Case fatality was 1.2% among all hospitalized E. coli O157:H7 cases and, among D+ HUS cases, varied by definition between 2.2% (hospital diagnosis) and 3.9% (age-focused). One fatal case was not defined as D+ HUS by any definition, and two were D+ HUS cases by all definitions.
Comparability
Reliability of the CSTE definition to identify the same pool of D+ HUS cases as any of the other definitions was fair (Table 2), using the classification of Byrt (poor: <0.20, fair: 0.21–0.40, moderate: 0.41–0.60, good: 0.61–0.80, very good: 0.81–1.00) (Byrt, 1996). Agreement between the hematology-focused definition and hospital diagnosis was also good (к=0.72; 95% CI: 0.62, 0.81). Agreements between the age-focused definition and 1) the hematology-focused definition (к=0.81; 95% CI: 0.71, 0.90) and 2) hospital diagnosis (к=0.84; 95% CI: 0.75, 0.94) were very good.
Table 2. Reliability (к) of different definitions to identify the same pool of D+ HUS cases.
| D+ HUS |
Not D+ HUS |
CSTE* | Hematology- focused |
Age-focused | ||||
|---|---|---|---|---|---|---|---|---|
| к | 95% CI | к | 95% CI | к 95% CI | ||||
| CSTE* | 154 | 274 | ||||||
| Hematology-focused | 58 | 375 | 0.44 | 0.36, 0.51 | ||||
| Age-focused | 76 | 353 | 0.52 | 0.44, 0.61 | 0.81 | 0.71, 0.90 | ||
| Hospital Diagnosis | 92 | 320 | 0.59 | 0.50, 0.68 | 0.72 | 0.62, 0.81 | 0.84 0.75, 0.94 | |
Abbreviations: CI, confidence interval; CSTE, Council for State and Territorial Epidemiologists
D+ HUS, post-diarrheal hemolytic uremic syndrome
Includes confirmed and probable definitions.
Incidence trends were similar across three of the definitions (Figure 1). The CSTE definition estimated substantially higher incidence in all years. Most large trends, such as the surge of cases in 2013, were detected by all four definitions. However, the definitions were not always consistent. In 2009, the CSTE and hospital diagnosis classifications indicated an increase in incidence while the age-focused definition indicated a decline and the hematology-focused definition detected no change.
Figure 1. Temporal patterns of D+ HUS among E. coli O157:H7 patients, by definition.
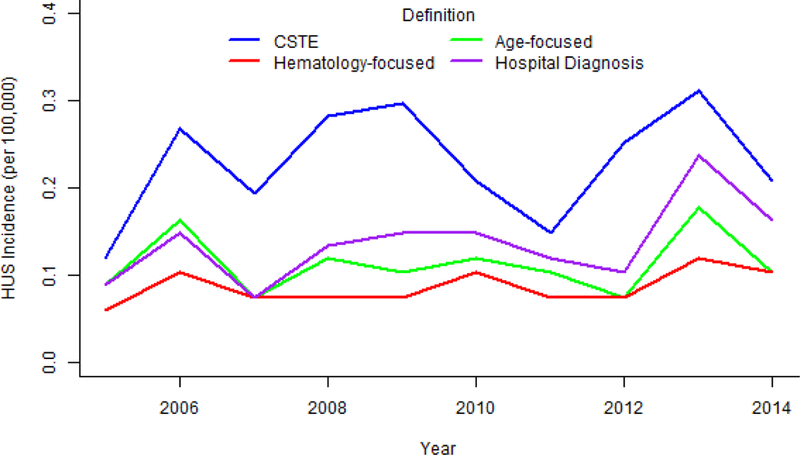
The CSTE definition estimated substantially higher incidence of D+ HUS than other definitions in all years. The hematology-focused definition obscured some of the variation in incidence over time (e.g. 2007–2009). CSTE contains confirmed and probable cases. Abbreviations: CSTE, Council of State and Territorial Epidemiologists; D+ HUS, post-diarrheal hemolytic uremic syndrome
Definition validity
The omnibus reference standard classified 62 cases as definite, and an additional 13 cases as borderline, D+ HUS, leaving 358 hospitalized E. coli O157:H7 cases unlikely to have had D+ HUS (Appendix Figure 1). Of the 62 definite cases, 55 cases met all four definitions, 1 case had anuria and met three definitions, and 6 cases received dialysis and met at least two definitions (Appendix Table 3). CSTE, age-focused, and hospital diagnosis each identified 100% of definite D+ HUS cases (Figure 2, Appendix Table 5). The hematology-focused and age-focused definitions were highly specific, but the CSTE definition had considerably lower specificity to identify definite D+ HUS cases (75%, 95% CI: 70%, 79%). For identifying definite D+ HUS, the age-focused definition had the strongest overall validity (Figure 2). The CSTE definition and hospital diagnosis both identified 100% of definite and borderline D+ HUS cases. Sensitivity to identify the combined definite-borderline D+ HUS case pool was notably low for the hematology-focused definition at 77% (95% CI: 66%, 86%). Specificity of all definitions increased when including borderline D+ HUS cases in the standard. For the combination of definite and borderline D+ HUS, the age-focused definition and hospital diagnosis were both strong, with higher specificity in the former and higher sensitivity in the latter.
Figure 2. Sensitivity and specificity for D+ HUS definitions among all hospitalized E. coli O157:H7 cases (A) and stratified by age (B), Washington State, 2005–2014.
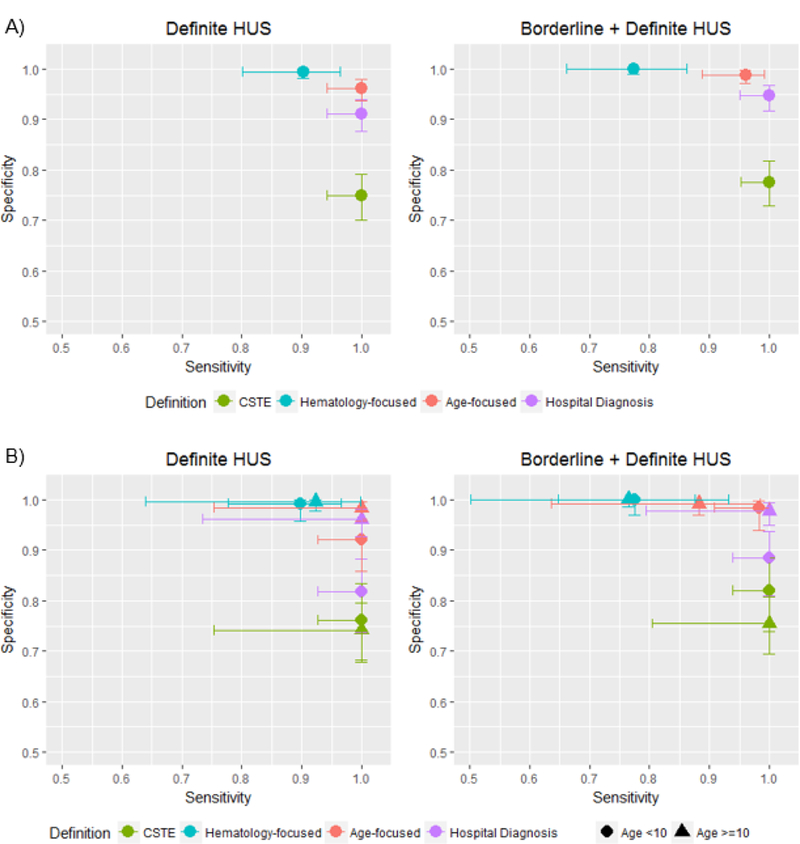
Sensitivity and specificity were calculated among all hospitalized E. coli O157:H7 cases (A) and stratified by age (B). Definitions were compared to the omnibus reference standard classifying cases as definite, borderline, or unlikely/not D+ HUS. Exact binomial 95% confidence intervals were used. Only cases with data for the omnibus reference standard and the respective definition are included in each calculation. CSTE contains confirmed and probable cases. Abbreviation: CSTE, Council of State and Territorial Epidemiologists; D+ HUS, post-diarrheal hemolytic uremic syndrome
Using the omnibus reference standard, approximately four times more children <10 years-old were classified as having had D+ HUS compared to older children and adults (Appendix Table 5). Most definitions were similar across age groups, with the largest difference in hospital diagnosis specificity. Among children <10, specificity was 82% (95% CI: 74%, 88%) for definite D+ HUS (Figure 2). The comparable value among cases ≥10 was 96% (95% CI: 93%, 98%). The sensitivity of the age-focused definition also differed somewhat for the combination of definite and borderline cases, identifying only 88% (95% CI: 64%, 99%) of D+ HUS cases ≥10 years-old vs. 98% (91% CI 95%, 100%) of D+ HUS cases <10 years-old.
The low specificity of the CSTE definition appeared to be driven by lack of criteria for thrombocytopenia and inclusion of hematuria or proteinuria as evidence of kidney injury (Appendix Table 6). Low sensitivity of the hematology-focused definition appeared to be driven by the requirement for evidence of microangiopathic changes and crude criteria for serum creatinine concentration. Moderate specificity of the age-focused definition for definite cases appeared to be driven by creatinine values that met age-standard cutoffs but not CSTE and hematology-focused creatinine cutoffs. Factors affecting differences between the omnibus reference standard and hospital diagnosis could not be evaluated, because diagnosis criteria are not standardized.
Sensitivity analysis
Using normal values for serum creatinine concentrations from the Harriet Lane Handbook (2009) instead of Meites (Meites, 1989) changed the age-focused classification of three cases, two from D+ HUS to non-D+ HUS and one from non-D+ HUS to D+ HUS (Appendix). This did not appreciably alter the D+ HUS case group or validity of the age-focused definition (Appendix Table 7). When excluding cases without thrombocytopenia, defined as platelet concentration <150,000/mm3, the modified CSTE definition classified 102 E. coli O157:H7 cases as D+ HUS (Appendix Table 7). This increased the specificity of the CSTE definition, though not to levels comparable with the other definitions. Without the requirement for microangiopathic changes, the modified hematology-focused definition classified 69 cases as D+ HUS. This increased the sensitivity of the hematology-focused definition. However, multiple cases were still missed, most of whom were classified as borderline D+ HUS by the omnibus reference standard.
DISCUSSION
We found substantial variation in the hospitalized E. coli O157:H7 cases identified as having D+ HUS using four common definitions, with an almost-threefold difference in the number of cases identified by the most stringent and the most liberal definitions. Agreement, as calculated by kappa, was good or very good for all definition combinations except those involving the CSTE definition. This suggests that cases ascertained using the CSTE definition should not be directly compared to D+ HUS cases defined using the other definitions, because the CSTE case pool includes many additional cases. This lack of comparability is reinforced when examining D+ HUS trends by definition over the study period.
Regarding validity, the age-focused definition identified all definite D+ HUS cases, with 96% specificity. Hospital diagnosis also identified all definite D+ HUS cases and was somewhat less specific at 91%. These were the most balanced definitions we evaluated for combined definite and borderline cases, as well. The CSTE definition erred on the side of over-inclusion, inflating the number of cases identified by the omnibus reference standard by a factor of two. The hematology-focused definition performed in the opposite direction, with an overly- restrictive definition that excluded 10% of definite D+ HUS cases and 85% of borderline cases. Using modified forms of the CSTE and hematology-focused definitions improved their performance, but neither rose to the level of the age-focused definition or hospital diagnosis.
We identified four domains in which the objective definitions diverged from the omnibus reference standard: thrombocytopenia, microangiopathic changes, serum creatinine concentrations, and use of urinalysis values. Other authors have raised concerns about reliance on anemia as a criterion with which to define D+ HUS, because patients can be hemoconcentrated at a point in illness at which other criteria are met (Ardissino et al., 2014; Balestracci et al., 2015). Though this is an important consideration for clinical practice, anemia did not emerge as a problematic criterion in our analysis, likely because the hemoconcentration is temporary and brisk hemolysis soon follows.
The CSTE statement on postdiarrheal D+ HUS (Council of State and Territorial Epidemiologists, 2009) notes, “If a platelet count obtained within 7 days after onset of the acute gastrointestinal illness is not less than 150,000/mm3, other diagnoses should be considered.” However, thrombocytopenia is not included in the matrix of criteria for D+ HUS. As evidenced by a recent review that catalogued D+ HUS definitions as part of its methods (Freedman et al., 2016), thrombocytopenia is common. Our results demonstrate that thrombocytopenia is, indeed, critical to the differentiation of D+ HUS and non-D+ HUS cases, and sensitivity analysis demonstrated an increase in the specificity of the CSTE definition when we added thrombocytopenia as a criterion.
Criteria requiring microangiopathic changes on smear examination may be overly restrictive. In our review, peripheral blood smears were not documented as having been conducted for 44 of the 106 discrepant cases (Appendix Table 6). Even when done, some smears were performed early in illness before evidence of injury to erythrocytes could appear and not performed again with subsequent complete blood counts when hemolysis is well underway, which may explain why two definite/borderline D+ HUS cases had no smear evidence of schistocytes. Evidence of intravascular hemolysis is ideal, but, in reality, case management may not require this information if other clinical and laboratory elements are consistent with STEC- associated D+ HUS. In our analysis, sensitivity of the hematology-focused definition increased when it was modified to not require microangiopathic changes.
The CSTE and hematology-focused definitions relied on insensitive creatinine criteria, grouping cases into only two age groups, relative to the five age groups used by the age-focused definition, based on Meites (Meites, 1989). This was a common cause of discordance between the hematology-focused definition and the omnibus reference standard. While our findings support the use of age-specific serum creatinine concentrations as an important component of the D+ HUS case definition, we acknowledge problems with this determination. First, the degree of renal injury in E. coli O157:H7 infections likely lies on a spectrum, and employment of a rigid cut-point for categorical definition purposes is somewhat arbitrary. For example, there is evidence of renal tubular injury in infected children who do not develop D+ HUS (Chandler et al., 2002). Also, as a consequence of illness, many infected children have had poor protein intake for several days, and creatinine values might be misleadingly low. Under such a scenario, a normal value might actually reflect some degree of renal dysfunction.
Though the CSTE definition uses the same insensitive serum creatinine concentrations as the hematology-focused definition, it did not demonstrate low sensitivity because it accepts hematuria or proteinuria in lieu of elevated creatinine. However, these alternative criteria appear responsible for some of the CSTE definition’s low specificity. Urines may be contaminated in patients with diarrhea, and protein and hemoglobin detected in urinalyses might not accurately reflect kidney injury (Ahn et al., 2009; Davis et al., 2013; Holtz et al., 2009). Moreover, hematuria, based on dip-criteria, could reflect filtered serum free hemoglobin secondary to intravascular erythrocyte destruction, and not red cells of kidney origin, and D+ HUS not be useful markers of kidney injury. Given that D+ HUS definitions based on urinalysis-dependent criteria offer no value in acute illness management in the absence of azotemia, we believe that age-specific serum creatinine criteria provide a sensitive and specific means of resolving these limitations.
There is no true reference standard for D+ HUS, so no test can be conducted to definitively determine its presence or absence. We attempted to take an agnostic approach to developing a standard against which we could evaluate definitions. As such, we avoided including laboratory criteria that are often used in definitions and instead relied upon definition agreement and clinical outcomes of interest. However, a different reference standard might certainly yield different results. We believe that our approach reflects the expertise of clinicians and researchers in the D+ HUS field, because similar definitions and/or hospital diagnosis reinforce one another through the agreement criteria in the omnibus reference standard, as well as clinical relevance, because more serious outcomes are given more weight in the standard.
Though this study offers evidence-based analysis of the consequences of definitions using a large dataset, we acknowledge several limitations. First, we noted secular trends in D+ HUS incidence, but these observations were based on a small annual number of D+ HUS cases. However, we observed a similar magnitude of difference between incidence estimates for the hematology-focused definition and hospital diagnosis as observed in a study by Ong et al. that compared similar definitions applied to FoodNet cases (Ong et al., 2012). Second, this analysis was retrospective and relied on a statewide, passive STEC surveillance system, with hospitalizations spread unevenly across 71 facilities. Therefore, the care administered and the medical records from which we abstracted data were heterogeneous. The variation observed in the performance and/or documentation of peripheral blood smears is an example of this variability. Repeat serum creatinine testing also varied, with some facilities only having documented that the level was checked once, which may have resulted in missed D+ HUS cases. This may explain why a one-year-old child with 3+ schistocytes and serum creatinine of 0.9mg/dL (Appendix Table 6, case 72) was missed by the hematology-focused definition. Additionally, testing standards for serum creatinine changed during the study period with the recommendation that isotope dilution mass spectrometry (IDMS) methods be used to standardize laboratory serum creatinine assays (Myers et al., 2006). We do not have specific information on the impact of this change at individual facilities, but it is possible that it resulted in lower measurements of serum creatinine in the latter years of the study (Delanaye et al., 2017).
Our analysis was limited to measures of acute kidney injury common in STEC-associated D+ HUS surveillance and literature, focusing on serum creatinine concentration at the time of illness. Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease (RIFLE) and Acute Kidney Injury Network (AKIN) classifications provide more rigorous criteria for diagnosing acute kidney injury, including change in serum creatinine or estimated glomerular filtration rate (Lopes and Jorge, 2013). Consistent with these guidelines, some definitions (e.g. the CSTE definition) incorporate criteria for a 50% increase in serum creatinine over baseline. Baseline data prior to illness were not available for any patients in this series, in view of the scope of the review. While early serum creatinine data were available for some cases, these were necessarily those who sought care early in illness, which in itself may alter HUS risk, and we could not be certain they constituted baseline levels, which, in any event, are rarely known in previously healthy children. To avoid unpredictably biasing the analysis, we opted not to consider early-illness creatinine level for those cases in which it was available.
In some cases, clinical circumstances precluded documentation of urine output, because urine was mixed with stool and could not be distinguished. However, in the majority of cases missing urine output, this information was not documented in the chart. If cases with missing urine output were anuric or had low output, they may have been falsely excluded from the definite or borderline D+ HUS classifications in our omnibus reference standard. At most, three cases classified as borderline D+ HUS would have been definite cases, and five cases classified as unlikely D+ HUS would have been borderline. Specificity for CSTE, age-focused, and hospital diagnosis would have increased slightly, and sensitivity for the hematology-focused definition would have decreased. Nonetheless, the heterogeneity in clinical practice and gaps in documentation reflect the real-world conditions faced by surveillance programs, which may learn about a case only after the peak of illness or recovery. A definition that remains valid even when applied to real-world data is critical to accurate surveillance.
Although HUS may arise for several reasons, including “atypical” HUS associated with a complement disorder, pneumococcal-associated HUS, O157 and non-O157 STEC-associated HUS, we have used only E. coli O157 cases in this analysis and the definitions used are specific to D+ HUS (CSTE definition) and STEC-associated HUS (hematology-focused and age-focused definitions). Our results cannot be generalized to atypical or pneumococcal-associated HUS, neither of which is commonly preceded by diarrhea. Because development of D+ HUS in E. coli O157 cases is linked to the pathogen’s Shiga toxin, however, our findings likely generalize to D+ HUS associated with other Shiga toxin-producing bacteria, including other STEC serogroups and Shigella dysenteriae serotype 1.
The choice of a D+ HUS definition has pragmatic value for public health, research, and patients, and the desired characteristics of a definition may depend on its use. In general, inaccurate classifications can obscure true fluctuations in disease, as suggested by the observed variation across definitions of D+ HUS incidence over time. Low-specificity definitions inflate the burden of D+ HUS, while low-sensitivity definitions underestimate the burden of D+ HUS. Either situation may result in the misallocation of public health resources, but high sensitivity may be prioritized during the initial phases of an outbreak when case ascertainment is key. Lack of reliability between definitions, such as that observed between the CSTE definition and others, implies that incidences or burdens of D+ HUS ascertained using different definitions are not always directly comparable.
For D+ HUS research, using a definition with substantial misclassification, particularly low specificity, likely produces biased estimates, and heterogeneity of definitions across studies limits their ability to be compared and combined, e.g., as part of a meta-analysis. Additionally, D+ HUS over-diagnosis may have adverse future consequences for patients who had no azotemia, because a diagnosis of D+ HUS confers lifelong risk for chronic renal disease, most particularly if the D+ HUS was accompanied by anuria (Garg et al., 2003), and could engender unwarranted concerns, as well as life and health insurance risk.
CONCLUSIONS
The STEC landscape has continually shifted over the past decades, with advances in diagnostics, improved clinical management, protection of the food supply, and evolving serogroups. Our review revealed likely over- and underestimation of D+ HUS burden by the CSTE and hematology-focused definitions, respectively, even if modified forms of these definitions are used. Hospital diagnosis is inherently subjective and could vary with the experience of the provider, and our analysis showed a concerning amount of over-diagnosis among children <10, the age group with the greatest D+ HUS burden. The age-focused definition appears to provide the best ascertainment of severe cases while minimizing inflation of D+ HUS burden and should be considered for surveillance and research purposes. D+ HUS surveillance remains an important component of the public health strategy for STEC, just as when it was initiated 20 years ago (Mahon et al., 1997). This review was undertaken to inform D+ HUS surveillance in Washington State, which has not yet been implemented, in part because of the lack of clarity surrounding an optimal D+ HUS definition. Our analysis identifies a valid definition, and given the proven benefit of D+ HUS surveillance in detecting outbreaks (Werber et al., 2008), we encourage the broader adoption of routine D+ HUS surveillance using this standardized definition.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the work and participation of hospital personnel at the 71 facilities involved in this review, as well as the guidance and support of the Communicable Disease Epidemiology team at Washington State Department of Health. We thank Dr. Stephen Freedman for helpful comments on the manuscript.
FUNDING
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health [F31AI126834]; the National Institute of Environmental Health Sciences, National Institutes of Health [T32ES015459]; and the Washington University Digestive Diseases Research Core Center [5P30 DK052574]. The funding bodies had no role in the design, data collection, analysis, or interpretation of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1 Abbreviations:
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CSTE
Council of State and Territorial Epidemiologists
- FoodNet
Foodborne Diseases Active Surveillance Network
- D+ HUS
post-diarrheal hemolytic uremic syndrome
- OR
odds ratio
- STEC
Shiga toxin-producing Escherichia coli
- U.S.
United States of America
Footnotes
CONFLICT OF INTEREST
Declarations of interest: P.I.T. is a consultant to, holder of an equity interest in, and a member of the Scientific Advisory Board of, MediBeacon Inc, which is developing a technique to measure glomerular filtration rates in humans.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn CK, Holt NJ, Tarr PI, 2009. Shiga-Toxin Producing Escherichia coli and the Hemolytic Uremic Syndrome: What Have We Learned in the Past 25 Years? 634, 1–17. [DOI] [PubMed] [Google Scholar]
- Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, Klein EJ, Tarr PI, 2005. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 115, e673–680. [DOI] [PubMed] [Google Scholar]
- Ardissino G, Possenti I, Tel F, Testa S, Paglialonga F, 2014. Time to change the definition of hemolytic uremic syndrome. Eur. J. Intern. Med. 25, e29. [DOI] [PubMed] [Google Scholar]
- Balestracci A, Martin SM, Toledo I, 2015. Hemoconcentration in hemolytic uremic syndrome: time to review the standard case definition? Pediatr. Nephrol. 30, 361. [DOI] [PubMed] [Google Scholar]
- Bielaszewska M, Friedrich AW, Aldick T, Schürk-Bulgrin R, Karch H, 2006. Shiga Toxin Activatable by Intestinal Mucus in Escherichia coli Isolated from Humans: Predictor for a Severe Clinical Outcome. Clin. Infect. Dis. 43, 1160–1167. [DOI] [PubMed] [Google Scholar]
- Bielaszewska M, Kock R, Friedrich AW, von Eiff C, Zimmerhackl LB, Karch H, Mellmann A, 2007. Shiga toxin-mediated hemolytic uremic syndrome: time to change the diagnostic paradigm? PLoS One 2, e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt T, 1996. How good is that agreement? Epidemiology 7, 561. [DOI] [PubMed] [Google Scholar]
- Centers for Disease, C., Prevention, 1995. Community outbreak of hemolytic uremic syndrome attributable to Escherichia coli O111:NM--South Australia 1995. MMWR Morb. Mortal. Wkly. Rep. 44, 550–551, 557–558. [PubMed] [Google Scholar]
- Centers for Disease Control, 2017. Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet 2015 Surveillance Report (Final Data), in: U.S. Department of Health and Human Services, C. (Ed.), Atlanta, Georgia. [Google Scholar]
- Centers for Disease Control and Prevention, 2016. Summary of Notifiable Diseases, 2014. MMWR Morb. Mortal. Wkly. Rep. 63, 1–154. [Google Scholar]
- Chandler WL, Jelacic S, Boster DR, Ciol MA, Williams GD, Watkins SL, Igarashi T, Tarr PI, 2002. Prothrombotic coagulation abnormalities preceding the hemolytic-uremic syndrome. N. Engl. J. Med. 346, 23–32. [DOI] [PubMed] [Google Scholar]
- Council of State and Territorial Epidemiologists, 2009. Public Health Reporting and National Notification for Hemolytic Uremic Syndrome (post-diarrheal), Atlanta, Georgia.
- Crim SM, Griffin PM, Tauxe R, Marder EP, Gilliss D, Cronquist AB, Cartter M, Tobin D’Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Wolpert B, Henao OL, 2015. Preliminary Incidence and Trends of Infection with Pathogens Transmitted Commonly Through Food — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2006–2014. MMWR Morb. Mortal. Wkly. Rep. 64, 495–498. [PMC free article] [PubMed] [Google Scholar]
- Crim SM, Iwamoto M, Huang JY, Griffin PM, Gilliss D, Cronquist AB, Cartter M, Tobin-D’Angelo M, Blythe D, Smith K, Lathrop S, Zansky S, Cieslak PR, Dunn J, Holt KG, Lance S, Tauxe R, Henao OL, Centers for Disease Control and Prevention, 2014. Incidence and trends of infection with pathogens transmitted commonly through food-- Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2006–2013. MMWR Morb. Mortal. Wkly. Rep. 63, 328–332. [PMC free article] [PubMed] [Google Scholar]
- Davis TK, McKee R, Schnadower D, Tarr PI, 2013. Treatment of Shiga toxin-producing Escherichia coli infections. Infect. Dis. Clin. North Am. 27, 577–597. [DOI] [PubMed] [Google Scholar]
- De Schrijver K, Buvens G, Posse B, Van den Branden D, Oosterlynck O, De Zutter L, Eilers K, Pierard D, Dierick K, Van Damme-Lombaerts R, Lauwers C, Jacobs R, 2008. Outbreak of verocytotoxin-producing E. coli O145 and O26 infections associated with the consumption of ice cream produced at a farm, Belgium, 2007. Euro Surveill. 13. [DOI] [PubMed] [Google Scholar]
- Delanaye P, Cavalier E, Pottel H, 2017. Serum Creatinine: Not So Simple! Nephron 136, 302–308. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Eltorki M, Chui L, Xie J, Feng S, MacDonald J, Dixon A, Ali S, Louie M, Lee BE, Osterreicher L, Thull-Freedman J, 2017. Province-Wide Review of Pediatric Shiga Toxin-Producing Escherichia coli Case Management. J. Pediatr. 180, 184–190 e181. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Xie J, Neufeld MS, Hamilton WL, Hartling L, Tarr PI, Alberta Provincial Pediatric Enteric Infection, T., Nettel-Aguirre A, Chuck A, Lee B, Johnson D, Currie G, Talbot J, Jiang J, Dickinson J, Kellner J, MacDonald J, Svenson L, Chui L, Louie M, Lavoie M, Eltorki M, Vanderkooi O, Tellier R, Ali S, Drews S, Graham T, Pang XL, 2016. Shiga Toxin-Producing Escherichia coli Infection, Antibiotics, and Risk of Developing Hemolytic Uremic Syndrome: A Meta-analysis. Clin. Infect. Dis. 62, 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, Salvadori M, Haynes RB, Clark WF, 2003. Long-term Renal Prognosis of Diarrhea- Associated Hemolytic Uremic Syndrome: A Systematic Review, Meta-analysis, and Meta-regression. JAMA 290, 1360–1370. [DOI] [PubMed] [Google Scholar]
- Gould LH, Demma L, Jones TF, Hurd S, Vugia DJ, Smith K, Shiferaw B, Segler S, Palmer A, Zansky S, Griffin PM, 2009. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000–2006. Clin. Infect. Dis. 49, 1480–1485. [DOI] [PubMed] [Google Scholar]
- Harriet Lane Service (Johns Hopkins Hospital), 2009. The Harriet Lane Handbook: A Manual for Pediatric House Officers, 18th ed. Elsevier Mosby, Philadelphia, PA. [Google Scholar]
- Holtz LR, Neill MA, Tarr PI, 2009. Acute Bloody Diarrhea: A Medical Emergency for Patients of All Ages. Gastroenterology 136, 1887–1898. [DOI] [PubMed] [Google Scholar]
- Klein EJ, Stapp JR, Clausen CR, Boster DR, Wells JG, Qin X, Swerdlow DL, Tarr PI, 2002. Shiga toxin-producing Escherichia coli in children with diarrhea: a prospective point- of-care study. J. Pediatr. 141, 172–177. [DOI] [PubMed] [Google Scholar]
- Lopes JA, Jorge S, 2013. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 6, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BE, Griffin PM, Mead PS, Tauxe RV, 1997. Hemolytic uremic syndrome surveillance to monitor trends in infection with Escherichia coli O157:H7 and other shiga toxin- producing E. coli. Emerg. Infect. Dis. 3, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder EP, Cieslak PR, Cronquist AB, Dunn J, Lathrop S, Rabatsky-Ehr T, Ryan P, Smith K, Tobin D’Angelo M, Vugia DJ, Zansky S, Holt KG, Wolpert BJ, Lynch M, Tauxe R, Geissler AL, 2017. Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food and the Effect of Increasing Use of Culture-Independent Diagnostic Tests on Surveillance — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013–2016. MMWR Morb. Mortal. Wkly. Rep. 66, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites S, 1989. Pediatric Clinical Chemistry: Reference (Normal) Values, 3rd ed. AACC Press, Washington, D.C. [Google Scholar]
- Mody RK, Gu W, Griffin PM, Jones TF, Rounds J, Shiferaw B, Tobin-D’Angelo M, Smith G, Spina N, Hurd S, Lathrop S, Palmer A, Boothe E, Luna-Gierke RE, Hoekstra RM, 2015. Postdiarrheal hemolytic uremic syndrome in United States children: clinical spectrum and predictors of in-hospital death. J. Pediatr. 166, 1022–1029. [DOI] [PubMed] [Google Scholar]
- Mody RK, Luna-Gierke RE, Jones TF, Comstock N, Hurd S, Scheftel J, Lathrop S, Smith G, Palmer A, Strockbine N, Talkington D, Mahon BE, Hoekstra RM, Griffin PM, 2012. Infections in pediatric postdiarrheal hemolytic uremic syndrome: factors associated with identifying shiga toxin-producing Escherichia coli. Arch. Pediatr. Adolesc. Med. 166, 902–909. [DOI] [PubMed] [Google Scholar]
- Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH, National Kidney Disease Education Program Laboratory Working, G., 2006. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin. Chem. 52, 5–18. [DOI] [PubMed] [Google Scholar]
- Ong KL, Apostal M, Comstock N, Hurd S, Webb TH, Mickelson S, Scheftel J, Smith G, Shiferaw B, Boothe E, Gould LH, 2012. Strategies for surveillance of pediatric hemolytic uremic syndrome: Foodborne Diseases Active Surveillance Network (FoodNet), 2000–2007. Clin. Infect. Dis. 54 Suppl 5, S424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Scheiring J, Andreoli SP, Zimmerhackl LB, 2008. Treatment and outcome of Shiga-toxin- associated hemolytic uremic syndrome (HUS). Pediatr. Nephrol. 23, 1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer B, Eriksen HM, Nygard K, Grahek-Ogden D, Madssen T, Hajdu A, Lovoll O, Stavnes TL, Lassen J, Kapperud G, Aavitsland P, 2006. An outbreak of haemolytic uraemic syndrome associated with minced beef, Norway, January-February 2006: preliminary report. Euro Surveill. 11, E060302–060301. [DOI] [PubMed] [Google Scholar]
- Tarr GAM, Oltean HN, Phipps AI, Rabinowitz P, Tarr PI, 2018. Strength of the association between antibiotic use and hemolytic uremic syndrome following Escherichia coli O157:H7 infection varies with case definition. Int. J. Med. Microbiol. 308, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr PI, Gordon CA, Chandler WL, 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365, 1073–1086. [DOI] [PubMed] [Google Scholar]
- U. S. Census Bureau, 2012. 2012 TIGER/Line Shapefiles (machine-readable data files). U.S. Census Bureau. [Google Scholar]
- Werber D, Frank C, Wadl M, Karch H, Fruth A, Stark K, 2008. Looking for tips to find icebergs--surveillance of haemolytic uraemic syndrome to detect outbreaks of Shiga toxin- producing E. coli infection. Euro Surveill. 13. [PubMed] [Google Scholar]
- Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI, 2000. The risk of the hemolytic- uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342, 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CS, Mooney JC, Brandt JR, Staples AO, Jelacic S, Boster DR, Watkins SL, Tarr PI, 2012. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin. Infect. Dis. 55, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


