Synopsis
Eosinophilic esophagitis is a chronic disorder characterized by symptoms of esophageal dysfunction and esophageal inflammation with intraepithelial eosinophils. EoE represents an important cause of upper gastrointestinal morbidity. Primary care providers are pivotal for timely and accurate recognition of symptoms of eosinophilic esophagitis, for facilitating diagnoses through specialist referrals, and for understanding management strategies. This process begins with a thorough understanding of the clinical features of EoE, its associated atopic conditions, and its evolving epidemiology.
Keywords: eosinophilic esophagitis, dysphagia, food bolus impaction, heartburn
Introduction
Eosinophilic esophagitis (EoE) is a chronic disorder characterized clinically by symptoms of esophageal dysfunction and histologically by eosinophilic infiltration of the esophageal epithelium.1–3 The disease belongs to the spectrum of eosinophilic gastrointestinal disorders whereby eosinophilic inflammation of the gastrointestinal tract occurs in the absence of secondary causes. Prior to the 1990s when esophageal eosinophilia was thought to be solely due to reflux esophagitis,4 EoE was rarely recognized. However, by the mid-1990s, seminal papers described the condition,5 and the number of publications on EoE increased dramatically.6 EoE represents an important contributor to upper gastrointestinal morbidity throughout the world, a growing health problem, and a significant burden for healthcare systems.7,8
Important roles exist across medical specialties for the co-management of EoE. This includes primary care providers, allergists, gastroenterologists, pathologists, and nutritionists treating both adult and pediatric patients. For the primary care provider, the identification and referral of patients with suspected EoE is indispensible. Despite the increase in EoE-pertinent literature, patients endorse a protracted diagnostic delay following symptom initiation. Furthermore, this delay has yet to decrease and correlates with prognosis.1,9 Primary care providers are thus pivotal not only for timely and accurate diagnosis, but also to recognize the existence of co-morbid conditions and to initiate specialist referrals.2
This article aims to provide the critical information necessary to facilitate the incorporation of primary care providers into the co-management of EoE. To achieve this, we provide an overview of EoE clinical, endoscopic, and histologic features as well as treatment options and future directions in management.
Clinical presentation
Symptoms reported by eosinophilic esophagitis patients
The diagnosis of EoE starts with a thorough investigation of presenting symptoms. These vary by patient age (Table 1).
Table 1.
Common symptoms associated with eosinophilic esophagitis
| Adolescents and adults | Children |
|---|---|
|
| |
| Solid food dysphagia | Nausea and vomiting |
| Food bolus impaction | Regurgitation |
| Heartburn | Heartburn |
| Chest pain | Abdominal pain |
| Chest pain | |
| Anorexia/feeding refusal/failure to thrive | |
Practitioners should consider EoE in adolescents and adults when the predominant complaint is esophageal dysphagia,10 which is reported by 60–100% of patients,6,11,12 and food impaction can be seen in more than 25%.13,14 Heartburn (30–60%) and non-cardiac chest pain (8–44%) are commonly reported, 11,15 and EoE may be present in 1–8% of patients with proton pump inhibitor (PPI) refractory reflux symptoms.16,17 Abdominal pain, nausea, vomiting, diarrhea, GI bleeding, and weight loss are uncharacteristic in adults with EoE, and a different process or more diffuse eosinophilic gastrointestinal disorder (e.g. eosinophilic gastroenteritis, eosinophilic colitis) should be considered when these features predominate.
Children with EoE report non-specific complaints (Table 1).18 Difficulty feeding, choking, refusal of food, and vomiting are also found in infants and toddlers.19,20 When constitutional symptoms, such as fever and weight loss predominate, an alternative disease should be sought.
Elucidating symptoms of dysphagia can be a subtle task, as many patients have subconsciously developed compensatory eating behaviors over years to minimize symptoms. Specific behaviors to assess include:
Eating slowly
Excessive food chewing
Lubrication of food boluses or drinking a copious amount of liquid after each bite
Repeated swallows to facilitate food bolus passage
Avoidance of troublesome foods
Crushing or avoiding pills
Co-morbid conditions associated with eosinophilic esophagitis
Atopy is commonly encountered in EoE cohorts, and for adult EoE patients, the prevalence of any atopic condition is 20–80%.12 Children with EoE have a prevalence of 30–50% and 50–75% for asthma and allergic rhinitis, which compares to 10–30% for either condition in the general pediatric population. Furthermore, children with EoE are more likely to develop environmental allergies and IgE-mediated food allergy (e.g. urticaria, anaphylaxis).21,22 Moreover, a family history of an atopic disorder is found in more than 50% of EoE patients.23
EoE also develops in association with some genetic syndromes, including inherited connective tissue disorders that exhibit hypermobility.24,25 However, this is rare and only 1% of EoE patients present with this phenotype.
Familial susceptibility to eosinophilic esophagitis
While EoE does not demonstrate classic Mendelian inheritance, there is a genetic component26 and a familial history of EoE increases individual risk for the condition above the approximately 1/2000 seen in the general population (see epidemiology, below). The risk varies by the particular relationship:19
Any first degree relative: 1.8% individual risk (recurrence risk ratio: 33)
Father: 2.4% individual risk (recurrence risk ratio: 43)
Mother: 0.6% individual risk (recurrence risk ratio: 10)
Brother: 3.5% individual risk (recurrence risk ratio: 64)
Sister: 1.3% individual risk (recurrence risk ratio: 24)27
There is also significant concordance for EoE in both monozygotic (40%) and dizygotic twins (30%). The latter findings implicates the role of early-life exposures in EoE susceptibility.27
Endoscopic findings common to eosinophilic esophagitis
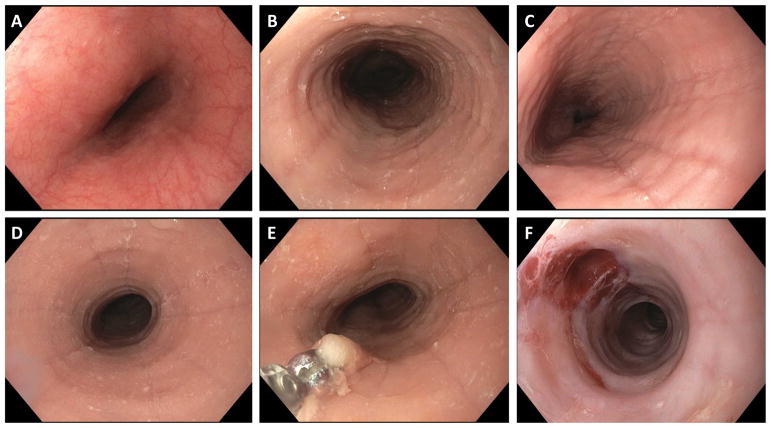
Multiple, though non-specific,28 structural changes of the esophagus are seen with EoE,2 and these can vary by patient age.11 Fibrostenotic findings such as esophageal rings, strictures, or narrowing are more common in adults, while inflammatory findings such as white plaques/exudates, linear furrows and edema/decreased vascularity are more common in children (Figure 1). While all findings can be seen across the age spectrum, the difference in findings by age is thought to reflect a fibrotic esophageal response to chronic eosinophilic inflammation.29–31 Other findings include a diffusely narrowed or small-caliber esophagus,32,33 and crepe-paper mucosa (e.g. tearing of the esophageal mucosa from passage of an endoscope). Endoscopic findings of EoE are frequently described using the EoE endoscopic reference score (EREFS), which stands for the five key findings of Edema, Rings, Exudates, Furrows, and Strictures.34 This system provides greater uniformity in the description of findings, identifies and discriminates between non-EoE and EoE patients, and correlates with treatment.35,36
Figure 1.
Endoscopic images of EoE. (A) The endoscopic appearance of the normal esophagus. Note the uniform and smooth appearance of the esophageal mucosa, with the fine vascular pattern clearly visible. (B) An EoE patient with evidence of esophageal rings, furrows, edema, and exudates. (C) An EoE patient with esophageal edema, deep furrows, and mild exudates. (D) An EoE patient with a focal stricture, in addition to mild rings, furrows, edema, and exudates. (E) Esophageal biopsy underway. (F) An EoE patient with a very narrow caliber esophagus and tight rings, as well as edema, after esophageal dilation. Good dilation effect (mucosal rent) is seen in the 11 o’clock position.
Histologic features of eosinophilic esophagitis
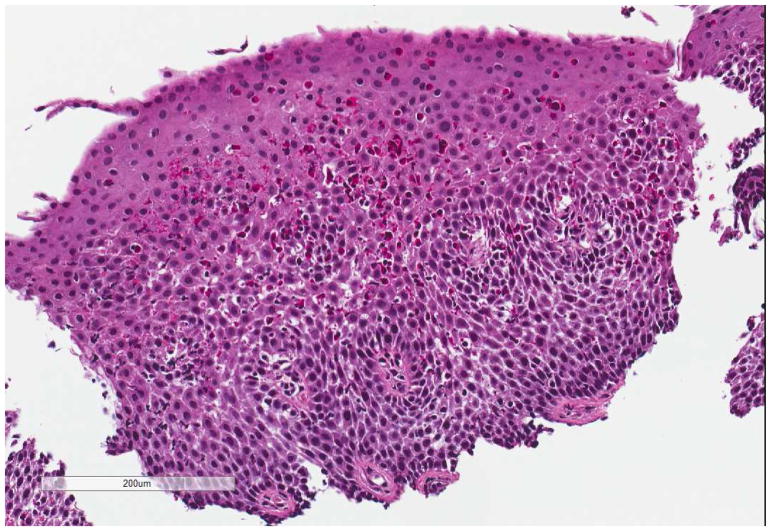
All patients exhibit increased intraepithelial eosinophils that may be found in all regions of the esophagus (Figure 2). Eosinophil surface layering and eosinophilic microabscesses, dilated intercellular spaces, a thickened mucosa with basal layer hyperplasia and papillary elongation, and extracellular deposition of eosinophil granule proteins such as eosinophil peroxidase are also found.37,38 Patients with fibrostenotic complications of EoE (e.g. rings, strictures) exhibit increased collagen deposition within the lamina propria.39
Figure 2.
Histologic image of an esophageal biopsy in EoE. In addition to the prominent eosinophilic infiltration (>15 eos/hpf), there is eosinophil degranulation, basal zone hyperplasia, and spongiosis.
Diagnostic criteria
Diagnostic criteria and consensus guidelines definition
EoE is a chronic immune and antigen-mediated clinicopathologic disease,1–3 and diagnostic criteria require both the appropriate clinical and histologic features:
Symptoms of esophageal dysfunction (Table 1)
Presence of esophageal eosinophilia with a peak of at least 15 eosinophils in a high-power microscopy field (eos/hpf)
Exclusion of alternative etiologies of esophageal eosinophilia1
Though initially selected by expert opinion, the threshold of 15 eos/hpf achieves a sensitivity of 100% and a specificity of 96% for establishing the diagnosis.40 It is worth noting that patients with lower levels of eosinophilia and phenotypic features have been reported, and they may have EoE in appropriate settings.41
In regards to the third criterion stipulated above, previous guidelines1 required non-response to a PPI trial to establish the diagnosis. Patients responding to PPI were labeled with PPI-responsive esophageal eosinophilia (PPI-REE), which became an area of substantial controversy.42 However, this distinction is no longer required, and PPIs have evolved from a diagnostic tool to a treatment option.3
Alternative etiologies of esophageal eosinophilia
Esophageal eosinophilia is not pathognomonic for EoE. Alternative etiologies should be sought and ruled out following a thorough history, physical examination, and select laboratory tests. The most prevalent competing or overlapping diagnosis is GERD. Less common competing diagnoses include:1–3
Achalasia
Infection
Connective tissue diseases
Crohn’s disease
Pill esophagitis
Hypereosinophilic syndrome
When more generalized eosinophilic infiltration of the gastrointestinal tract is noted, the findings may be consistent with eosinophilic gastroenteritis and/or colitis with esophageal involvement.
Since GERD can induce esophageal eosinophilia and produces similar symptoms to EoE, the differentiation of the two conditions proves challenging.43 Complicating this matter, the relationship between GERD and EoE remains controversial. For instance, GERD and EoE may simply overlap, EoE may induce GERD through impaired esophageal clearance of refluxate, or GERD may conceivably cause EoE by damaging the epithelial border and thus allowing for the presentation of antigens and a subsequent allergic response.44 Altogether, symptoms of reflux should be sought and treated, and pH monitoring has not successfully discriminated GERD from EoE.45
Epidemiology
EoE is found globally. Many cases have been reported in North America, South America, Europe, and Australia. Cases also exist from Asia and the Middle East. India and Sub-Saharan Africa are exceptional with no cases documented from these areas.46 EoE is more common in cold and arid climates, and in rural areas, 47 and most frequently affects those younger than 50,48 men, and caucasians.7
According to data derived largely from North American and European cohorts, the pooled incidence rate of EoE is 3.7/100,000 patient years (95% confidence interval: 1.7 – 6.5).49 Additionally, all studies examining EoE incidence have found an increasing incidence over time50 not explained by disease awareness or utilization of endoscopy.11 Similarly, prevalence data estimated an overall pooled prevalence of 22.7/100,000 (95% confidence interval: 12.4 – 36.0), 49 and this value has also increased over time.7
The aforementioned changes in EoE incidence and prevalence suggest that environmental factors, as opposed to genetic factors, drive the changing epidemiology,46 but the exact etiology is not known. Early life exposures including antibiotic use in infancy, cesarean delivery, preterm birth, and lack of breastfeeding have been implicated as disease risk factors.27,51 It has been hypothesized that these factors may affect the microbiome and the developing immune system. In addition, decreased Helicobacter pylori prevalence, increased proton pump inhibitor use, changes in food sources, and food packaging have also been implicated in the changing epidemiology of EoE.7
Pathogenesis
Animal models, genetic studies, co-morbid allergic disorders, and the efficacy of elimination diets suggest that EoE is an atopic condition.26 Most patients are sensitive to one or more foods52 and have aeroallergen hypersensitivity2 or respiratory allergy.53 Similarly, the role of antigen sensitization is supported by clinical and histologic improvements with elimination diets devoid of precipitating allergens.54 Mounting data show that EoE is not IgE-mediated55 and IgG4 may have a role in disease pathogenesis.55
EoE is also Th2 mediated. Th2 cells produce inflammatory cytokines including IL4, IL5, and IL13 that in turn increase eotaxin-3. The latter molecule is a potent chemokine inducing eosinophilic infiltration into and activation within the esophagus.56 Once activated, eosinophils produce additional factors such as TGF-beta. TGF-beta promotes tissue remodeling of the esophagus that contributes to the fibrostenotic complications of EoE.57,58
Treatments
Drugs, diet, and dilation encapsulate the treatment paradigms for EoE.59 Drugs and diet reduce EoE-associated inflammation while dilation targets esophageal strictures and narrowing. Treatment choice is predicated on patient preference, clinical features, and cost. Goals of therapy include clinical and histologic improvement and reduction in long-term complications. No Food and Drug Administration approved medication exists for EoE, and as such, all medications are used off label in the U.S. Many patients with EoE will continue to be followed by their primary care provider, so it is important to be familiar with EoE treatment options, even if these are initially directed by specialists.
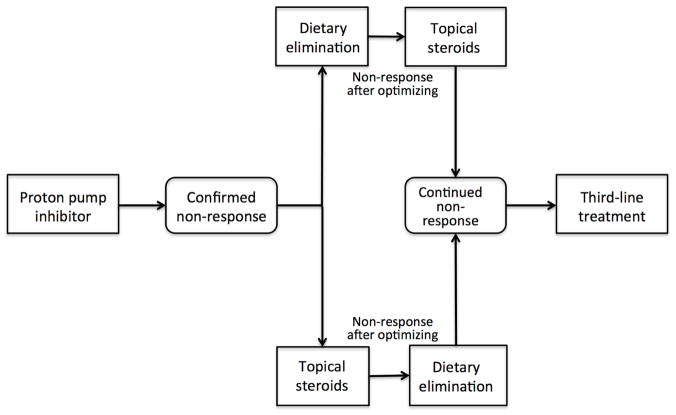
PPIs are an initial pharmacologic choice for EoE. If PPI non-response occurs (Figure 3), corticosteroids or elimination diet are utilized. Treatment should be optimized and factors associated with response (e.g. adherence, drug dose, inadvertent antigen exposure, esophageal infection, stricture) assessed at each follow-up visit.60
Figure 3.
Treatment algorithm for the primary and secondary treatment of eosinophilic esophagitis.
Corticosteroids, whether delivered topically or systemically, improve the clinical symptoms and histologic features of EoE. Systemic corticosteroids are now reserved for patients requiring a prompt therapeutic response, such as severe symptoms or growth failure, owing to their long-term adverse effects as well as the results of an RCT illustrating similar efficacy of topical and systemic corticosteroids.61 Methods for delivering topical corticosteroids include swallowing fluticasone (puffed into the mouth from an asthma multi-dose inhaler (MDI) and then swallowed) or budesonide (mixed in a viscous slurry from the aqueous asthma nebulizer formulation).
The decision to utilize an elimination diet depends upon multiple factors:
Acceptability of the diet by patient and family
Provider expertise
Availability of dieticians
Types of elimination diets include elemental liquid amino acid-based formulations,62 empiric elimination diets,54,63 and allergy test-directed diets. 64–66
Efficacy of pharmacologic and dietary treatment strategies
Studies analyzing adults and children illustrated that between 33–74% of patients with EoE respond to PPIs.45,67 Moreover, a meta-analysis documented a pooled PPI response rate of approximately 50%.68 The long-term efficacy of PPIs is less clear. A recent prospective study documented that most pediatric patients (78%) remain in clinical and histologic remission following one year of maintenance therapy,69 and similar data are available for adults.70 Additionally, temporary discontinuation of PPI therapy results in recurrence of symptoms and/or histologic relapse. However, PPI reintroduction recaptures response in most patients.
Topical corticosteroid efficacy has been well studied in multiple randomized controlled trials, and several meta-analyses summarize their data.71,72 They have consistently produced reductions in esophageal eosinophil counts versus comparator and are capable of maintaining remission in a proportion of patients. The typical doses utilized for oral viscous budesonide as well as fluticasone in clinical trials are summarized below (Table 2).
Table 2.
Typical doses of topical corticosteroids for eosinophilic esophagitis
| Topical corticosteroid | Age | Dose |
|---|---|---|
| Fluticasone via MDI | Children | 440 – 880 mcg/day |
| Adults and adolescents | 880 – 1760 mcg/day | |
| Oral viscous budesonide | Children | 1 mg/day |
| Adults and adolescents | 2–4 mg/day |
Correct techniques must be stressed to optimize esophageal deposition of topical corticosteroids. Topical corticosteroids should be taken following meals and patients should avoid eating or drinking for 30 to 60 minutes after swallowing the medication. Additionally, MDIs are ideally administered at end expiration following a breath hold.
A common complication of topical corticosteroid use is esophageal candidiasis, which is seen on follow-up endoscopy in 10–20% of EoE patients.73 A rare complication is herpes esophagitis.74 Additionally, a recent systematic review noted that adrenal suppression following topical corticosteroids is uncommon.75
The efficacy of food elimination diets varies according to the diet utilized. A meta-analysis of adult EoE patients reported that elemental diets were effective in 91%, empiric elimination diets in 72%, and allergy test-directed diets in 46%.64 Elemental diets produce histologic remission in most patients. However, practical limitations limit their use (e.g. cost, palatability, gastrostomy tube placement, quality of life).1 Conversely, allergy test-directed diets produce lower rates of remission as a consequence of their low predictive value for the identification of culprit foods.54,76 Empiric diets have thus become the elimination strategy of choice. The most common and best described is the ‘six-food elimination diet’ that removes dairy, wheat, egg, soy, peanut/tree nut, and fish/shellfish. For patients undergoing dietary elimination, working with a nutritionist or dietician is recommended to help increase compliance, decrease inadvertent contamination, and prevent nutritional deficiencies.77
The role of esophageal dilation in eosinophilic esophagitis
Esophageal stricture or narrowing is treated best by dilation, which is safe and effective when done cautiously. This is an important treatment to improve symptoms of dysphagia, but it does not impact the underlying eosinophilic inflammation.78 A meta-analysis determined a 0.3% risk for perforation, though at expert centers, and this value is comparable to the risk of perforation following dilation of non-EoE patients.79
Future directions
Future directions in the management of EoE pertain to diagnosis and follow up, topical corticosteroids formulations, and novel treatments. Researchers are actively seeking less invasive and more efficient diagnostic tools (e.g. tethered capsule endoscopy, un-sedated trans-nasal endoscopy, cytosponge-obtained esophageal tissue collection, or string-based analysis of esophageal inflammatory factors).80–83 Genetic features may also assist in the diagnosis of EoE: the EoE transcriptome may accurately identify the disease84 and assist in predicting clinical outcomes.85 Serum biomarkers are of intense interest, but to date none have been found to be ready for clinical use. 86,87
New formulations for topical corticosteroid delivery are under study. An effervescent budesonide tablet was highly effective as a means of delivery in an RCT,88 and has been recommended for approval for EoE in Europe. Another RCT found pre-prepared viscous budesonide to be both safe and effective and capable of inducing clinical, endoscopic, and histologic remission.89
Small molecules including angiotensin receptor blockers (e.g. losartan)(NCT1808196), JAK kinase inhibitors,90 and OCT00045991, an oral drug that blocks the effects of prostaglandin D2, are emerging as potential treatment options and have been studied to varying degrees. Biologic agents, including anti-IL-5 monoclonal antibodies (which are approved for eosinophilic asthma),92–94 anti-IL13 antibodies95,96, and the anti-IL-4r blocker dupilumab (which has recently been approved eczema)97, are also under study. The place of these biologics in the treatment algorithm for EoE has yet to be determined.
In addition to the aforementioned future directions, health care transition from pediatric-to adult-focused systems represents an important and under-studied topic.98 In one study, most patients and parents of children with EoE were found to be unfamiliar with health care transition. Readiness for transition was also low compared with other chronic diseases.99 As such, exploration of barriers limiting transition readiness should be a priority especially in light of the large cohort of EoE patients transitioning to adult care, and coordination of this care transition is another role for primary care providers.
Conclusion
Eosinophilic esophagitis is a chronic disorder characterized by symptoms of esophageal dysfunction and esophageal inflammation with intraepithelial eosinophils. EoE represents an important global contributor to gastrointestinal morbidity. Primary care providers are pivotal for timely and accurate detection of symptoms potentially related to EoE, referral to proper specialists for diagnosis, coordination of care between multiple providers as well as transition of care from pediatric to adult providers, all with the goal of improving patient quality of life and to reduce long-term EoE complications.
Key points.
Eosinophilic esophagitis has emerged as an important contributor to upper gastrointestinal morbidity.
Primary care providers are indispensible for the timely and accurate diagnosis of eosinophilic esophagitis.
A delay in diagnosis of eosinophilic esophagitis contributes to the risk of long-term complications.
Acknowledgments
Financial support: This research was funded by NIH Awards T32 DK007634 (CCR) and R01 DK101856 (ESD).
Footnotes
Potential competing interests: None of the authors report any potential conflicts of interest with this paper. Dr. Dellon is a consultant for Adare, Alivio, Banner, Enumeral, GSK, Receptos/Celegene, Regeneron, and Shire, receives research funding from Adare, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire, and has received educational grants from Banner and Holoclara.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108(5):679–92. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 2.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur Gastroenterol J. 2017;5(3):335–358. doi: 10.1177/2050640616689525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winter HS, Madara JL, Stafford RJ, et al. Intraepithelial eosinophils: a new diagnostic criterion for reflux esophagitis. Gastroenterology. 1982;83(4):818–823. [PubMed] [Google Scholar]
- 5.Attwood SEA, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia - A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38(1):109–116. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: A systematic review. Am J Gastroenterol. 2007;102(10):2300–2313. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2017;154(2):319–332. doi: 10.1053/j.gastro.2017.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol. 2015;110(5):626–632. doi: 10.1038/ajg.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed CC, Koutlas NT, Robey BS, et al. Prolonged Time to Diagnosis of Eosinophilic Esophagitis Despite Increasing Knowledge of the Disease. Clin Gastroenterol Hepatol. 2018 Mar; doi: 10.1016/j.cgh.2018.01.028. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellon ES, Kim HP, Sperry SLW, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc. 2014;79(4):577–85. doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, Endoscopic, and Histologic Findings Distinguish Eosinophilic Esophagitis From Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2009;7(12):1305–1313. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology. 2014;147(6):1238–1254. doi: 10.1053/j.gastro.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperry SLW, Crockett SD, Miller CB, et al. Esophageal foreign-body impactions: Epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc. 2011;74(5):985–991. doi: 10.1016/j.gie.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiremath GS, Hameed F, Pacheco A, et al. Esophageal Food Impaction and Eosinophilic Esophagitis: A Retrospective Study, Systematic Review, and Meta-Analysis. Dig Dis Sci. 2015;60(11):3181–3193. doi: 10.1007/s10620-015-3723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonsalves N, Policarpio-Nicolas M, Zhang Q, et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64(3):313–319. doi: 10.1016/j.gie.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 16.Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of Eosinophilic Esophagitis in an Adult Population Undergoing Upper Endoscopy: A Prospective Study. Clin Gastroenterol Hepatol. 2009;7(4) doi: 10.1016/j.cgh.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Dellon ES, Speck O, Woodward K, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013;108(12):1854–1860. doi: 10.1038/ajg.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: A 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3(12):1198–1206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 19.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351(9):940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 20.Liacouras Ca, Markowitz JE. Eosinophilic esophagitis: A subset of eosinophilic gastroenteritis. Curr Gastroenterol Rep. 1999;1:253–258. doi: 10.1007/s11894-999-0043-1. [DOI] [PubMed] [Google Scholar]
- 21.Simon D, Marti H, Heer P, et al. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. J Allergy Clin Immunol. 2005;115(5):1090–1092. doi: 10.1016/j.jaci.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Assa’ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119(3):731–738. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 23.Assa’ad A. Eosinophilic Esophagitis: Association with Allergic Disorders. Gastrointest Endosc Clin N Am. 2008;18(1):119–132. doi: 10.1016/j.giec.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, et al. Allergy: TGFβ receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5(195):195ra94. doi: 10.1126/scitranslmed.3006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abonia JP, Wen T, Stucke EM, et al. High prevalence of eosinophilic esophagitis in patients with inherited connective tissue disorders. J Allergy Clin Immunol. 2013;132(2):378–386. doi: 10.1016/j.jaci.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Shea K, Aceves S, Dellon E, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018;154(2):333–345. doi: 10.1053/j.gastro.2017.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander ES, Martin LJ, Collins MH, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. 2014;134(5):1084–1092. doi: 10.1016/j.jaci.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HP, Vance RB, Shaheen NJ, Dellon ES. The Prevalence and Diagnostic Utility of Endoscopic Features of Eosinophilic Esophagitis: A Meta-analysis. Clin Gastroenterol Hepatol. 2012;10(9):988–996. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy Eur J Allergy Clin Immunol. 2010;65(1):109–116. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–1236. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Koutlas NT, Dellon ES. Progression from an Inflammatory to a Fibrostenotic Phenotype in Eosinophilic Esophagitis. Case Rep Gastroenterol. 2017;11(2):382–388. doi: 10.1159/000477391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Huprich J, Kujath C, et al. Esophageal Diameter Is Decreased in Some Patients With Eosinophilic Esophagitis and Might Increase With Topical Corticosteroid Therapy. Clin Gastroenterol Hepatol. 2012;10(5):481–486. doi: 10.1016/j.cgh.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 33.Eluri S, Runge TM, Cotton CC, et al. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest Endosc. 2016;83(6):1142–1148. doi: 10.1016/j.gie.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–495. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 35.Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin Gastroenterol Hepatol. 2016;14(1):31–39. doi: 10.1016/j.cgh.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wechsler J, Bolton S, Amsden K, et al. Eosinophilic Esophagitis Reference Score Accurately Identifies Disease Activity and Treatment Effects in Children. Clin Gastroenterol Hepatol. 2017 Dec 15; doi: 10.1016/j.cgh.2017.12.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Protheroe C, Woodruff SA, de Petris G, et al. A Novel Histologic Scoring System to Evaluate Mucosal Biopsies From Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2009;7(7):749–755. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43(2):257–268. doi: 10.1016/j.gtc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Aceves SS. Tissue remodeling in patients with eosinophilic esophagitis: What lies beneath the surface? J Allergy Clin Immunol. 2011;128(5):1047–1049. doi: 10.1016/j.jaci.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 40.Dellon S, Speck O, Woodward K, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol. 2015;28(3):383–90. doi: 10.1038/modpathol.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravi K, Talley NJ, Smyrk TC, et al. Low grade esophageal eosinophilia in adults: An unrecognized part of the spectrum of eosinophilic esophagitis? Dig Dis Sci. 2011;56(7):1981–1986. doi: 10.1007/s10620-011-1594-1. [DOI] [PubMed] [Google Scholar]
- 42.Molina-Infante J, Bredenoord AJ, Cheng E, et al. Proton pump inhibitor-responsive oesophageal eosinophilia: An entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65(3):521–531. doi: 10.1136/gutjnl-2015-310991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigo S, Abboud G, Oh D, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103(2):435–442. doi: 10.1111/j.1572-0241.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 44.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102(6):1301–1306. doi: 10.1111/j.1572-0241.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- 45.Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther. 2012;35(2):300–307. doi: 10.1111/j.1365-2036.2011.04922.x. [DOI] [PubMed] [Google Scholar]
- 46.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):201–218. doi: 10.1016/j.gtc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen ET, Hoffman K, Shaheen NJ, et al. Esophageal eosinophilia is increased in rural areas with low population density: Results from a national pathology database. Am J Gastroenterol. 2014;109(5):668–675. doi: 10.1038/ajg.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dellon ES, Jensen ET, Martin CF, et al. Prevalence of Eosinophilic Esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12(4):589–96. doi: 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arias Á, Pérez-Martínez I, Tenías JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. 2016;43(1):3–15. doi: 10.1111/apt.13441. [DOI] [PubMed] [Google Scholar]
- 50.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of Eosinophilic Esophagitis Over Three Decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7(10):1055–1061. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen ET, Kappelman MD, Kim HP, et al. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2013;57(1):67–71. doi: 10.1097/MPG.0b013e318290d15a. [DOI] [PubMed] [Google Scholar]
- 52.Greenhawt M, Aceves SS, Spergel JM, Rothenberg ME. The management of eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2013;1(4):332–340. doi: 10.1016/j.jaip.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Aceves SS. Food Allergy Testing in Eosinophilic Esophagitis: What the Gastroenterologist Needs to Know. Clin Gastroenterol Hepatol. 2014;12(8):1216–1223. doi: 10.1016/j.cgh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonsalves N, Yang GY, Doerfler B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; Food reintroduction identifies causative factors. Gastroenterology. 2012;142(7):1451–1459. doi: 10.1053/j.gastro.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Clayton F, Fang JC, Gleich GJ, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147(3):602–609. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 56.Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology. 2015;148(6):1143–1157. doi: 10.1053/j.gastro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):297–316. doi: 10.1016/j.gtc.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(1):206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Straumann A. Treatment of eosinophilic esophagitis: Diet, drugs, or dilation? Gastroenterology. 2012;142(7):1409–1411. doi: 10.1053/j.gastro.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 60.Dellon E. Management of refractory eosinophilic oesophagitis. Nat Rev Gastroenterol Hepatol. 2017;14(8):479–490. doi: 10.1038/nrgastro.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of Oral Prednisone and Topical Fluticasone in the Treatment of Eosinophilic Esophagitis: A Randomized Trial in Children. Clin Gastroenterol Hepatol. 2008;6(2):165–173. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98(4):777–782. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 63.Wolf WA, Jerath MR, Sperry SLW, et al. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12(8):1272–1279. doi: 10.1016/j.cgh.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arias Á, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: A systematic review and meta-analysis. Gastroenterology. 2014;146(7):1639–1648. doi: 10.1053/j.gastro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Henderson CJ, Abonia JP, King EC, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129(6):1570–1578. doi: 10.1016/j.jaci.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130(2):461–7. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 67.Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal Eosinophilic Infiltration Responds to Proton Pump Inhibition in Most Adults. Clin Gastroenterol Hepatol. 2011;9(2):110–117. doi: 10.1016/j.cgh.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 68.Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2016;14(1):13–22. doi: 10.1016/j.cgh.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 69.Gutiérrez-Junquera C, Fernández-Fernández S, Cilleruelo ML, et al. High prevalence of response to proton-pump inhibitor treatment in children with esophageal eosinophilia. J Pediatr Gastroenterol Nutr. 2016;62(5):704–710. doi: 10.1097/MPG.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 70.Molina-Infante J, Rodriguez-Sanchez J, Martinek J, et al. Long-term loss of response in proton pump inhibitor-responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and rhinoconjunctivitis. Am J Gastroenterol. 2015;110(11):1567–1575. doi: 10.1038/ajg.2015.314. [DOI] [PubMed] [Google Scholar]
- 71.Chuang MY, Chinnaratha MA, Hancock DG, et al. Topical Steroid Therapy for the Treatment of Eosinophilic Esophagitis (EoE): A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol. 2015;6(e82) doi: 10.1038/ctg.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cotton CC, Eluri S, Wolf WA, Dellon ES. Six-Food Elimination Diet and Topical Steroids are Effective for Eosinophilic Esophagitis: A Meta-Regression. Dig Dis Sci. 2017;62(9):2408–2420. doi: 10.1007/s10620-017-4642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131(5):1381–1391. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 74.Lindberg GM, Van Eldik R, Saboorian MH. A case of herpes esophagitis after fluticasone propionate for eosinophilic esophagitis. Nat Clin Pract Gastroenterol Hepatol. 2008;5(9):527–530. doi: 10.1038/ncpgasthep1225. [DOI] [PubMed] [Google Scholar]
- 75.Philpott H, Dougherty M, Reed CC, et al. Systematic review: adrenal insufficiency secondary to swallowed topical corticosteroids in eosinophilic esophagitis. Aliment Pharmacol Ther. 2018;47(8) doi: 10.1111/apt.14573. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Philpott H, Nandurkar S, Royce SG, et al. Allergy tests do not predict food triggers in adult patients with eosinophilic oesophagitis. A comprehensive prospective study using five modalities. Aliment Pharmacol Ther. 2016;44(3):223–233. doi: 10.1111/apt.13676. [DOI] [PubMed] [Google Scholar]
- 77.Groetch M, Venter C, Skypala I, et al. Dietary Therapy and Nutrition Management of Eosinophilic Esophagitis: A Work Group Report of the American Academy of Allergy, Asthma, and Immunology. J Allergy Clin Immunol Pract. 2017;5(2):312–324. doi: 10.1016/j.jaip.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 78.Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal Dilation in Eosinophilic Esophagitis: Effectiveness, Safety, and Impact on the Underlying Inflammation. Am J Gastroenterol. 2010;105(5):1062–1070. doi: 10.1038/ajg.2009.657. [DOI] [PubMed] [Google Scholar]
- 79.Egan JV, Baron TH, Adler DG, et al. Esophageal dilation. Gastrointest Endosc. 2006;63(6):755–760. doi: 10.1016/j.gie.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 80.Tabatabaei N, Kang D, Wu T, et al. Tethered confocal endomicroscopy capsule for diagnosis and monitoring of eosinophilic esophagitis. Biomed Opt Express. 2014;5(1):197. doi: 10.1364/BOE.5.000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friedlander JA, Deboer EM, Soden JS, et al. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc. 2016;83(2):299–306. doi: 10.1016/j.gie.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Katzka DA, Smyrk TC, Alexander JA, et al. Accuracy and Safety of the Cytosponge for Assessing Histologic Activity in Eosinophilic Esophagitis: A Two-Center Study. Am J Gastroenterol. 2017;112(10):1538–1544. doi: 10.1038/ajg.2017.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Furuta GT, Kagalwalla AF, Lee JJ, et al. The oesophageal string test: A novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut. 2013;62(10):1395–1405. doi: 10.1136/gutjnl-2012-303171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wen T, Stucke EM, Grotjan TM, et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology. 2013;145(6):1289–1299. doi: 10.1053/j.gastro.2013.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Butz BK, Wen T, Gleich GJ, et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology. 2014;147(2):324–33. doi: 10.1053/j.gastro.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dellon ES, Rusin S, Gebhart J, et al. Utility of a Noninvasive Serum Biomarker Panel for Diagnosis and Monitoring of Eosinophilic Esophagitis: A Prospective Study. Am J Gastroenterol. 2016;2015(6):821–827. doi: 10.1038/ajg.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wright B, Ochkur S, Olson N, et al. Normalized serum eosinophil peroxidase levels are inversely correlated with esophageal eosinophilia in eosinophilic esophagitis. Dis Esophagus. 2018;31(2) doi: 10.1093/dote/dox139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miehlke S, Hruz P, Von Arnim U, et al. Two new budesonide formulations are highly efficient for treatment of active eosinophilic esophagitis: Results from a randomized, double-blind, double-dummy, placebo-controlled multicenter trial. Gastroenterology. 2014;146(5):S-16. [Google Scholar]
- 89.Dellon ES, Katzka DA, Collins MH, et al. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis. Gastroenterology. 2017;152(4):776–786. doi: 10.1053/j.gastro.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 90.Cheng E, Zhang X, Wilson KS, et al. JAK-STAT6 Pathway Inhibitors Block Eotaxin-3 Secretion by Epithelial Cells and Fibroblasts from Esophageal Eosinophilia Patients: Promising Agents to Improve Inflammation and Prevent Fibrosis in EoE. PLoS One. 2016;11(6):e0157376. doi: 10.1371/journal.pone.0157376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Straumann A, Bussmann C, Perkins M. Treatment of eosinophilic esophagitis with the CRTH2-antagonist OCT000459: a novel therapeutic principle (abstr 856) Gastroenterology. 212AD;142(suppl 1):s-147. [Google Scholar]
- 92.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–463e3. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 93.Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: A randomised, placebo-controlled, double-blind trial. Gut. 2010;59(1):21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 94.Assa’ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 95.Rothenberg M, Wen T, Greenberg A, et al. A randomized, double-blind, placebo controlled trial of a novel recombinant, humanized, anti-interleukin-13 monoclonal antibody (RPC4046) in patients with active eosinophilic esophagitis: Results of the HEROES study. United Eur Gastroenterol J. 2016;4(Suppl):OP325. [Google Scholar]
- 96.Rothenberg ME, Wen T, Greenberg A, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol. 2015;135(2):500–507. doi: 10.1016/j.jaci.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 97.Hirano I, Dellon E, Hamilton J, et al. Dupilumab Efficacy and Safety in Adult Patients with Active Eosinophilic Esophagitis: A Randomized Double-Blind Placebo-Controlled Phase 2 Trial. Am J Gastroenterol. 2017;112(Suppl1):AB20. ACG 2017. [Google Scholar]
- 98.Dellon ES, Jones PD, Martin NB, et al. Health-care transition from pediatric to adult-focused gastroenterology in patients with eosinophilic esophagitis. Dis Esophagus. 2013;26(1):7–13. doi: 10.1111/j.1442-2050.2011.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Eluri S, Book WM, Kodroff E, et al. Lack of Knowledge and Low Readiness for Health Care Transition in Eosinophilic Esophagitis and Eosinophilic Gastroenteritis. J Pediatr Gastroenterol Nutr. 2017;65(1):53–57. doi: 10.1097/MPG.0000000000001415. [DOI] [PMC free article] [PubMed] [Google Scholar]