Abstract
Background
Adolescents with type 1 diabetes (T1D) commonly have poor glycemic control. We aimed to test the efficacy of the Flexible Lifestyles Empowering Change (FLEX) adaptive behavioral intervention on primary (HbA1c) and secondary (psychosocial and metabolic) outcomes at 18 months.
Methods
Youth (13–16 years, T1D duration > 1 yr, and HbA1c of 64–119 mmol/mol [8∙0–13∙0%]) from two clinical sites were eligible. FLEX used motivational interviewing and problem solving skills training to enhance self-management. Intervention fidelity was assessed via audiotaped sessions. Intent-to-treat analyses used mixed effects models, with fixed effects including site, timepoint, intervention group, intervention by timepoint, and baseline level of primary (HbA1c) or secondary outcomes (alpha=0∙05).
Findings
Youth recruited from 05/01/2014 to 04/04/2016 were randomized to FLEX (n=130) or usual care control (n=128). Mean (SD) diabetes duration was 6∙4 (3∙8) years, and 71% used insulin pump therapy. Retention was 93∙4%. Intervention fidelity score was 4∙4/5 for motivational interviewing and 97∙4% for session content. At 18 months, HbA1c was not statistically significantly different between intervention [baseline: 82 (17) mmol/mol; follow-up: 83 (18)] and control [baseline: 80 (14); follow-up: 82 (17)]; change in intervention vs. control = −0∙7, 95% CI (−4∙7, 3∙4), p=0∙75. The intervention was associated with improved scores for motivation (p=0∙009), problem solving (p=0∙04), diabetes self-management profile (p=0∙01), youth report of overall quality of life (p=0∙01), selected domains related to fear of hypoglycemia (p<0∙05), parent report of diabetes family conflict (p<0∙0001), total cholesterol (p=0∙04), and diastolic blood pressure (p=0∙01).
Interpretation
The FLEX intervention did not significantly change HbA1c among these adolescents with elevated HbA1c, but did positively impact several psychosocial outcomes over 18 months. Further analyses will reveal information regarding drivers of positive response to intervention and will point to future directions for improvement in the approach.
Funding
NIH/NIDDK UC4DK101132 (MPIs: Mayer-Davis, Maahs, Seid) and the Helmsley Charitable Trust (PI Mayer-Davis). FLEX is registered on clinicaltrials.gov, NCT01286350.
Introduction
It is well established that adolescence is a time of significant vulnerability for youth with type 1 diabetes (T1D). From the population-based SEARCH for Diabetes in Youth Study, 17% had poor glycemic control (HbA1c ≥ 9∙5%),1 with similar findings reported by the Type 1 Diabetes Exchange 2 in the US and Europe,1,3 who reported mean HbA1c consistently above clinical targets. Moreover, adolescents with T1D have high levels of general and diabetes-related stress 4 and declining quality of life during the first six years after T1D diagnosis, correlated with worse glycemic control.5–7 Finally, an adverse cardiovascular risk profile is common among adolescents with T1D.8,9 The prevalence of chronic vascular complications of diabetes ranged from 5∙8% to 14∙4% among adolescents and young adults with T1D, including diabetic kidney disease, retinopathy, peripheral neuropathy, cardiovascular autonomic neuropathy, arterial stiffness, and hypertension.10
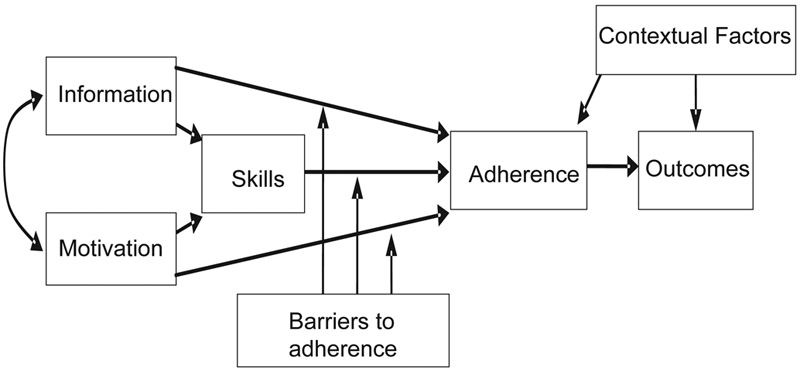
To address these compelling clinical concerns in adolescents with elevated HbA1c, we developed a behavioral intervention for adolescents with T1D called “Flexible Lifestyles Empowering Change (FLEX)”. The conceptual framework for the FLEX intervention employed established theories of health behavior, such as the Health Belief Model, Transtheoretical Model, and Theory of Reasoned Action,11 and analysis and integration of theory in social and health psychology,12 which together posit that knowledge, motivation, and skills are necessary for behavior change (Figure 1). Our novel application of these theories integrated motivational interviewing (MI)13 and problem solving skills training (PSST)14 approaches into a coherent intervention, supplementing with tools and skill building tailored to overcome specific barriers to adherence for individual patients. FLEX focused on the participant primarily, as opposed to the family as in other interventions, primarily to facilitate the adolescent’s growing independence and separation from their families.15 A randomized pilot study of this intervention (n=61 youth with T1D, age 12–16 years with HbA1c from 64–119 mmol/mol (8∙0–13∙0%) at baseline) yielded strong retention of study participants (95% during the three-month trial), high acceptability of the program, and evidence of improvement in HbA1c.16 We then further developed the intervention into an 18-month program and initiated a fully powered, randomized controlled trial of the FLEX intervention. Here we report findings for the primary and secondary outcomes of this trial.
Figure 1.
FLEX intervention conceptual framework
Methods
Study design
We conducted a randomized controlled trial comparing the FLEX intervention to usual care control, over 18 months of intervention delivery and follow-up. We describe elsewhere the FLEX trial design and measurement methods, details of the intervention, and baseline sample characteristics.17 The study was conducted at two pediatric endocrinology diabetes clinics in Colorado and Ohio, USA, with institutional review board approval for ethical conduct of human subjects research at each institution and at the coordinating center located in North Carolina.
Participants
Youth with a physician diagnosis of T1D, age 13–16 years at baseline, with T1D duration > 1 year and HbA1c between 64–119 mmol/mol (8∙0–13∙0%), without other serious medical conditions or pregnancy, were invited to participate. One caregiver was required to participate actively in the study. Described in detail elsewhere,17 we used a two-step recruitment process, incorporating MI strategies to provide substantial opportunity for parents and youth to discuss their potential participation in the trial. Written informed consent was provided by a parent, and written assent was provided by the adolescent.
Randomization and masking
After completion of baseline measures, adolescents and their participating caregiver were randomized to intervention or usual care control via an automated computer program to intervention or control (1:1) using a block size of 4, stratified by site and, within site, by HbA1c (< 9% or ≥ 9%). Participants were informed verbally of their randomization assignment. Clinical staff were not informed of the assignment. As with most behavioral trials, although assessment staff and coaches were different individuals, assessment staff blinding could not be guaranteed. The planned number of participants to recruit was 250, conservatively allowing for loss to follow-up of up to 20%. Additionally, the study protocol was to increase this enrollment target by the number of participants who dropped out before the three-month visit.
Procedures: Intervention
In FLEX, a patient-centered MI approach to health behavior change was used to help patients resolve ambivalence about change and to enhance intrinsic motivation by creating a motivational frame for change. Once this motivation for change was achieved, PSST, a systematic approach to problem solving, was presented as a way for patients to make the changes they desired. Because individual youth with T1D may have specific barriers to adherence and because these might change over time, we supplemented the MI/PSST approach with a flexible array of tools designed to address these barriers. The FLEX “toolbox” included elements of Behavioral Family Systems Therapy – Diabetes [BFST-D;18] as well as materials to support T1D education (e.g., guidance for diet and physical activity relative to insulin dosing), social support, and the use of communication technology (e.g., text messaging, alarms, or calendar appointments) for reminders or motivational boosters. These tools were used as necessary, as defined by the participants’ goals, problem solving strategies, and specific circumstances. Materials were consistent with the American Diabetes Association (ADA) 19 and International Society of Pediatric and Adolescent Diabetes (ISPAD) 20 clinical practices. The intervention was standardized in a detailed intervention manual that included session goals, materials, and sample scripts for each session.
The coaches were professionals who are typically members of a T1D care team (e.g., dietitian, nurse educator). The intervention was delivered individually to the adolescent at the participant’s usual diabetes care clinic location. Parents were included during some portions of FLEX-Basic session (see below) and as needed in subsequent intervention sessions. Parents were instructed that their role was to support the participant’s attempts at self-management. During the first 12 weeks of intervention, the core of the FLEX intervention (FLEX-Basic) was delivered, which entailed four sessions (40–60 min) that systematically established the MI and PSST processes, introduced concepts of family communication and teamwork, and introduced participants to available toolbox resources. Thereafter, the coach met with each participant three to four times in each of the subsequent six-month periods. The intervention period was a total of 18 months.
Following FLEX-Basic, the intervention was adaptive in that the intensity of sessions was determined by an a priori decision rule that assigned intervention participants to FLEX Regular or FLEX Check-In according to change in HbA1c since the last standardized measurement visit. The participant was assigned to FLEX Check-In if s/he met one of the following criteria: 1) HbA1c reached target (≤ 58 mmol/mol; or 7∙5%), or 2) previous HbA1c was no more than 75 mmol/mol (9∙0%) and decreased by 6 mmol/mol or more since the previous visit, or 3) previous HbA1c was > 75 mmol/mol (9∙0%) and HbA1c decreased by 11 mmol/mol or more (1∙0%). Otherwise, the participant proceeded to FLEX Regular. This decision rule, which was applied anew following each standardized measurement visit, is consistent with ISPAD and ADA clinical practice guidelines 19,20 and with other clinical trials that use HbA1c change of ≥ 0∙5% in youth with T1D as a marker of success.21 FLEX Regular entailed three to four 40–60 minute coaching sessions over six months, whereas FLEX Check-In entailed one 10–15 min phone call per month. In FLEX Regular, sessions included review of goal attainment, discussion of barriers and use of problem solving skills to address those barriers, and establishing new goals as appropriate. FLEX Check-In included brief support for ongoing success in goal setting and problem solving.
To ensure intervention fidelity, meaning that the intervention would be delivered as designed, coaches received training in MI and PSST and in the specifics of intervention delivery. Coaches were “certified” for each of the FLEX Basic sessions and for FLEX Regular and FLEX Check-In based on review of audiotaped practice sessions. Throughout the trial, study investigator psychologists (MS, JK) provided regular supervision conference calls for coaches. All in-person FLEX sessions were audio-taped. Fidelity of intervention delivery was assessed via review of a 10% random selection of the audio-taped sessions for assessment of adherence to MI principles using the Motivational Interviewing Treatment Integrity (MITI) system, 22 which incorporates five domains of evocation, collaboration, autonomy, direction, and empathy, and by review of content fidelity using a content checklist.
Procedures: Control Condition
Participants in the usual care control condition were not matched to intervention for attention. Both intervention and control participants were mailed a brief report of key clinical measures, including body mass index, laboratory values (HbA1c and lipids), blood pressure, and select behavioral self-reported results (physical activity/inactivity, dietary intake, self-monitoring of blood glucose, missed insulin). All participants also received a copy of Pink Panther™ Understanding Diabetes, a T1D educational book for patients and families.
Procedures: Standardized Measurements
Standardized data collection was implemented by FLEX assessment staff trained and certified as competent to perform all study procedures. Questionnaires, for the adolescent and participating caregiver, were available for completion online through the secure FLEX study website, or if participants preferred, questionnaires could be completed during the in-person study measurement visits. The full set of study measurements were obtained at baseline and 6 and 18 months post-randomization; a limited set of measurements were obtained at 3 post-randomization and 12 months post-baseline17.
Laboratory data
A central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, Seattle, WA, USA) provided oversight and conducted all assays. At all timepoints, HbA1c was measured in whole blood by using an automated nonporous ion exchange HPLC system (model G-7; Tosoh Bioscience). At the full measurement visits and drawn after at least an eight-hour fast, measurements of plasma cholesterol, triglycerides, and HDL cholesterol concentrations were performed on a Hitachi 917 autoanalyser (Boehringer Mannheim Diagnostics). LDL cholesterol was calculated by the Friedewald equation for those with triglycerides < 4·52 mmol/l and by the beta-quantification procedure for those with triglycerides ≥ 4·52 mmol/l.
Clinical measures
At baseline and at 6 and 18 months post-randomization, a blinded continuous glucose monitor (CGM; iPro®2 Professional CGM; Medtronic Diabetes, Northridge, CA) was worn for a seven-day period to measure interstitial glucose levels in real time throughout the day and night. Cutpoints for glucose used to describe hypoglycemia were established according to recommended values.23 Height was measured using a stadiometer, and weight was measured to the nearest 0∙1 kg using an electronic scale. Body mass index (BMI, weight (kg) / height (m)2) was calculated and then converted to an age- and sex-specific BMI z-score according to the Centers for Disease Control and Prevention growth charts. Blood pressure was measured after five minutes of rest using an aneroid manometer. The second and third of three measures were averaged for systolic and diastolic pressures.
Questionnaires
Baseline internal consistency and comparison to similar samples indicated that all questionnaires had adequate psychometric properties.17 The Motivation and Intention questionnaire was initially developed for an adolescent asthma population.14 For motivation, the questionnaire was modified for relevance to T1D self-management, including checking blood glucose, taking insulin, eating a healthy diet, and getting enough exercise. Intention was measured with respect to “managing my diabetes” based on the Intention Measurement for Adherence Studies, which has been shown to respond to interventions.24 The Social Problem Solving Inventory – Revised: Short (SPSI-R:S) was used to assess adolescents’ cognitive, affective, and behavioral abilities to resolve problems in everyday living.25 The Diabetes Self-Management Profile – Self Report (DSMP-SR) 26 was used to assess usual practices of diabetes management during the preceding three months, across five domains: exercise, management of hypoglycemia, diet, blood glucose testing, and insulin administration and dose adjustment; with higher scores indicating more diabetes self-management behaviors. The DSMP-SR was modified for the present study with updated language and to allow for a single questionnaire to be administered regardless of insulin regimen. Symptoms of depression were assessed using the Centers for Epidemiologic Study – Depression Scale (CES-D), with higher scores reflecting more depressive symptoms.27 The Pediatric Quality of Life Inventory™ – Generic Core Scales (PedsQL™ Generic) measures health-related quality of life (HRQOL) in four domains (physical, emotional, social, and school functioning) during the previous month, with higher scores reflecting better HRQOL.28 The PedsQL was completed by both the adolescent (self-report) and the participating caregiver (proxy-report of adolescent HRQOL). Fear of hypoglycemia was completed by both the adolescent and parents and measured in three domains:29 maintaining high blood sugar, helplessness/worry about low blood sugar, and worry about negative social consequences. The Diabetes Family Conflict Scale (DFCS) 30 was used to assess both adolescent- and parent-reported diabetes-related family conflict, with higher scores indicating more conflict about direct and indirect diabetes management tasks.
Procedures: Participant Incentives
All participants received incentives for completing the measurement visits ($120 for baseline, $200 for 6-month, $250 for 18-month; and $50 for each of the abbreviated brief visits at three and 12 months). Participants received an additional incentive for completion of CGM data collection at baseline, six months, and 18 months ($50 for wearing the CGM device for seven days and $25 for returning the device). Parents received a $20 incentive for participating in each of the five standardized measurement visits.
Outcomes
The primary outcome was HbA1c, which captures average glycemia during the prior 6–8 weeks.31 Secondary outcomes included motivation and intention, problem solving skills, self-management behaviors, symptoms of depression, HRQOL, fear of hypoglycemia, diabetes family conflict, risk factors for T1D complications (BMI, blood pressure, and plasma lipids), and hypoglycemia derived from CGM (percent time below 3∙0 and 3∙9 mmol/l [54 and 70 mg/dl]). We collected all measures at all timepoints, except for fear of hypoglycemia, risk factors for T1D complications, and hypoglycemia derived from CGM, which were only collected at baseline, six months and 18 months.
Statistical Analysis
Using the pilot study as a source of data for sample size calculations,16 the sample size of 200 was planned to allow 80% power for detection of change in HbA1c. In the FLEX pilot, we observed an intervention effect of 4∙1 mmol/mol, corresponding to an effect size (standardized mean difference) of 0∙58 in the high-fidelity intervention group. The 200 participants in FLEX allows 80% power for detection of a smaller effect size of 0∙39 at an alpha of 0∙05. Based on pilot data, this effect size was a reasonable target for secondary outcomes as well. Analyses were intent-to-treat. At baseline, we compared the intervention and control groups with respect to participant characteristics and study outcomes using chi-square and t-tests (Wilcoxon-Mann-Whitney where appropriate). To test the effect of the FLEX intervention on each of the planned primary and secondary outcomes, we use a mixed effects model, incorporating all available data. Linear mixed models were used for all outcomes except number of minutes per day spent in hypoglycemic ranges, which were dichotomized into some/none due to the large number of zeroes. Fixed effects included site, timepoint (categorical), intervention group, intervention x timepoint, and the baseline level of the outcome. A random intercept was included to account for within-participant correlation. For the primary outcome, modeled at four follow-up visits, the within-subject covariance matrix was given an unstructured format based on having the lowest AIC and BIC among unstructured, compound symmetric, and first-order autoregressive.32 The secondary outcomes were modeled at only six- and 18-month follow-ups. A test of the appropriate interaction parameter at a significance level of 0∙05 was used to test the intervention’s effect on each primary or secondary outcome. The impact of baseline HbA1c on the intervention effect was assessed by adding a time x treatment x stratum interaction to the mixed model for the primary outcome. All mixed models included clinical site as a fixed effect, and a time x treatment x site interaction was used to explore treatment effect by site. Analyses were conducted using SAS, version 9∙3 PROC MIXED and PROC GLIMMIX. A data and safety monitoring committee, convened by the National Institutes of Health, National Institute of Diabetes Digestive Diseases and Kidney, oversaw the study.
Trial Registration
The trial is registered on clinicaltrials.gov, NCT01286350.Role of the Funding Source The sponsor of this study was represented on the steering committee (CH) and, as part of this committee, contributed to the collaborative development of the study design, oversight of its execution, interpretation of the data, and the review and editing of this paper (CH). All authors in the writing group had access to all data. JC had direct access to raw data for statistical analyses. The corresponding author (EJMD) had full access to all of the data and the final responsibility to submit for publication.
Results
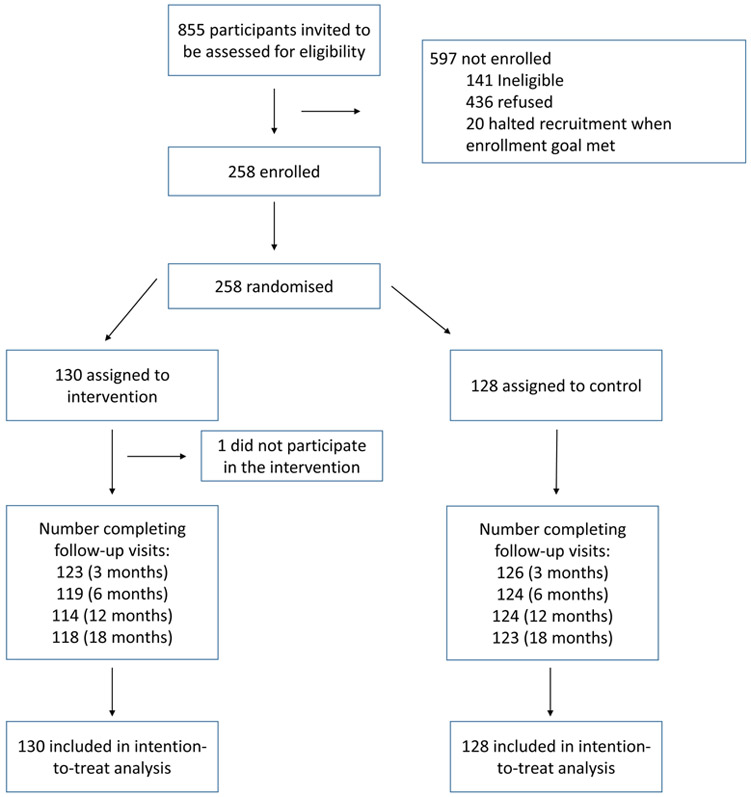
Participants were recruited from 05/01/2014 to 04/04/2016. Shown in Figure 2, of 855 participants invited to be assessed for eligibility, 141 (16∙5%) were found to be ineligible, 436 (51∙0%) refused, and 258 (30∙2%) enrolled and completed a baseline visit along with a participating caregiver. This included the planned 250 and 8 more to account for the 8 participants (6 intervention and 2 control) who dropped out prior to the three-month visit. Adolescents who enrolled and completed a baseline visit were more likely to be Caucasian (p=0 ∙02) and have private insurance (p=0∙001) compared to those who did not; however, there were no differences in HbA1c from the medical record, sex, age, or disease duration (all p>0∙05). Of those who refused participation, attempts to contact were unsuccessful for n=115; n=114 were considered “passive refusals” due to unsuccessful contact attempts following a first contact, and n=207 actively refused participation. Those who refused to participate most commonly cited lack of interest, lack of time, or travel distance.
Figure 2:
Consort Diagram: FLEX Intervention Randomized Control Trial
The majority of caregivers (n=217, 84%) were mothers or stepmothers of the participant. At baseline, mean (SD) age was 14∙9 (1∙1) years, diabetes duration was 6∙4 (3∙8) years, 71% used insulin pump therapy, and HbA1c was 81 (9∙6%) (1∙2). Across all visits, hypoglycemia was experienced in 37–48% (< 3∙9 mmol/L) and 15–23% (< 3∙0 mmol/L) of participants, with a median time spent in hypoglycemia per 24-hour period ranging from 1–7 minutes (< 3∙0 mmol/L) and 17–30 minutes (< 3∙9 mmol/L).
Details of participant characteristics by group assignment are shown in Table 1; no statistically significant differences between intervention and control groups were identified for any of these characteristics. Retention, defined as study participants continuing to attend study visits, exceeded the study goal, with 93∙4% completing the 18-month follow-up visit. Fidelity of intervention delivery based on the MITI scoring of 220 sessions averaged across the five MI domains for all coaches was 4∙60 (0∙43) out of 5, compared to a fidelity goal of 4∙0. All four coaches exceeded 4∙0 in all five domains, with the exception of one coach who averaged 3∙96 in one domain (empathy). Average content completeness was 97∙4% across all sessions evaluated, compared to a fidelity goal of > 90% for each session. Of the 130 individuals randomized to intervention, 91% attended all four FLEX Basic sessions by month six. Between six and 12 months of the intervention, 70% of participants met the intervention attendance goal; this remained stable, with 70% meeting the goal between 12 and 18 months. Based on the a priori decision rules that relied on change in HbA1c, the percent of intervention participants who participated in the “check-in” rather than the “regular” sessions was 17% for the 3–6 month time period, and 16% and 19% for the 6–12 month period and 12–18 month period, respectively.
Table 1.
Baseline Characteristics of FLEX Participants (n=258)
| Control (N=128) | FLEX Intervention (N=130) |
|
|---|---|---|
|
Demographic and Clinical
Characteristics |
||
| Age (years) | 14.9 (1.1) | 14.8 (1.1) |
| Female sex | 53.9 | 45.4 |
| Race and Ethnicity | ||
| Non-Hispanic white | 100 (78.1%) | 100 (76.9%) |
| Black | 4 (3.1%) | 7 (5.4%) |
| Hispanic | 17 (13.3%) | 16 (12.3%) |
| Other | 7 (5.5%) | 7 (5.4%) |
| Public Health Insurance | 20 (15.8%) | 27 (20.8%) |
| Single adult home | 19 (15.0%) | 15 (11.9%) |
| Duration of diabetes (years) | 6.39 (3.71) | 6.48 (3.76) |
| HbA1c above 75 mmol/mol (9.0%) (%) | 79 (61.7%) | 91 (70.0%) |
| Average number of hypoglycemic (<3.88 mmol/L) episodes lasting 15 or more minutes per 24-hr period† |
0.47 (0.86) | 0.45 (0.8) |
| Weight Status | ||
| Under- or normal weight | 78 (60.9%) | 86 (66.2%) |
| Overweight | 33 (25.8%) | 26 (20.0%) |
| Obese | 17 (13.3%) | 18 (13.9%) |
| Insulin Regimen | ||
| Multiple daily injection | 34 (26.8%) | 41 (31.8%) |
| Pump | 93 (73.2%) | 88 (68.2%) |
| Used CGM in past month | 29 (22.7%) | 24 (18.5%) |
| Primary Outcome | ||
| HbA1c (mmol/mol) | 80 (4) | 83 (13) |
| Secondary Outcomes | ||
| Motivation | 7.7 (1.6) | 7.5 (1.6) |
| Intention | 9.1 (1.0) | 9.1 (1.0) |
| Problem solving (SPSI) | 106.3 (12.3) | 105.1 (13.3) |
| Diabetes self-management (DSMP) - Youth |
55.5 (11.4) | 54.6 (11.7) |
| Diabetes self-management (DSMP) - Parent |
52.7 (11.6) | 50.6 (12.3) |
| Depression symptoms (CES-D) | 9.16 (7.73) | 9.25 (8.91) |
| Generic QOL (PedsQOL) - Youth | 81.1 (11.7) | 80.7 (13.1) |
| Generic QOL (Peds QOL) - Parent | 78 (14) | 77.1 (14.8) |
| Fear of hypoglycemia, Maintain High BG - Youth |
1.25 (0.88) | 1.17 (0.91) |
| Fear of hypoglycemia, Helplesseness/Worry - Youth |
1.11 (0.57) | 1.13 (0.57) |
| Fear of hypoglycemia, Worry about negative social consequences - Youth |
1.04 (0.76) | 1.12 (0.7) |
| Fear of hypoglycemia, Maintain High BG – Parent | 1.11 (0.79) | 1.11 (0.74) |
| Fear of hypoglycemia, Helplesseness/Worry - Parent | 1.45 (0.74) | 1.49 (0.73) |
| Fear of hypoglycemia, Worry about negative social consequences - Parent | 0.52 (0.58) | 0.59 (0.61) |
| Diabetes Family Conflict - Youth | 1.36 (0.36) | 1.35 (0.3) |
| Diabetes Family Conflict - Parent | 1.41 (0.28) | 1.45 (0.33) |
| BMI z-score | 0.71 (0.89) | 0.60 (0.98) |
| Total Cholesterol (mmol/L) | 4.47 (0.84) | 4.40 (0.87) |
| LDL Cholesterol (mmol/L) | 2.59 (0.77) | 2.46 (0.7) |
| HDL Cholesterol (mmol/L) | 1.43 (0.32) | 1.43 (0.35) |
| Triglycerides (mmol/L) | 0.99 (0.54) | 1.26 (2.31) |
| Systolic blood pressure (mmHg) | 101 (9) | 102 (9) |
| Diastolic blood pressure (mmHg) | 66 (7) | 67 (9) |
| CGM - Time < 3.0 mmol/L (min/day) | 4.5 (0–31.5) | 4.3 (0–31.5) |
| CGM - Time <3.9 mmol/L (min/day) | 28.8 (5.2–80.7) | 31.4 (5.0–72.1) |
| CGM - Time >10.0 mmol/L (min/day) | 846 (733–1082) | 906 (764–1055) |
| CGM - Time >13.9 mmol/L (min/day) | 486 (327–706) | 535 (336–649) |
Data are mean (SD), n (%) or median (Q1-Q3).
Missing data: Fear of hypoglycemia (participants) is missing 4 responses in the control group and 1 in the intervention group due to an administration error; the remaining psychosocial scales are missing up to 2 participants per intervention group due to missing responses. Fasting lipids are available in 126 control and 129 intervention. 115 control and 119 intervention participants provided CGM data.
CGM – continuous glucose monitoring; SPSI – Social Problem Solving Inventory; DSMP – Diabetes Self Management Profile; QOL – quality of life; CES-D – Centers for Epidemiologic Studies – Depression scale.
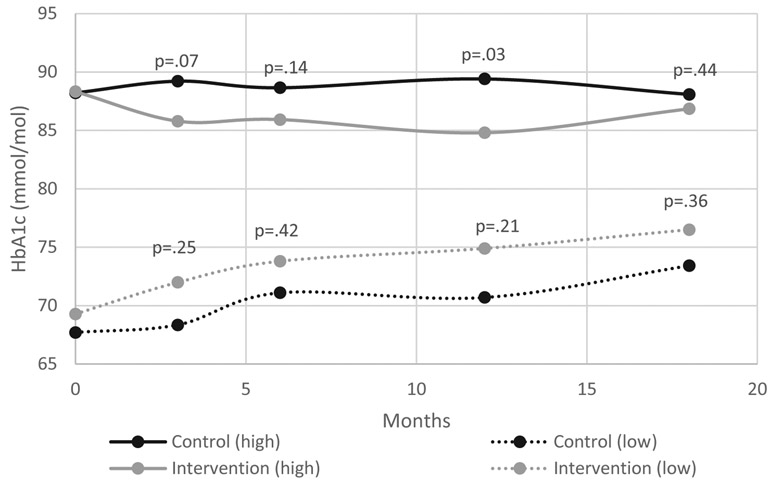
A statistically significant intervention effect on the primary outcome (HbA1c) was not observed at 18 months (primary endpoint) or at any of the other follow-up visits (Table 2). One coach at one of the clinical sites had existing patients who were subsequently enrolled in the study (two intervention patients with a total of 10 visits and two control patients with a total of eight visits over the course of the 18 months study time). This coach was instructed to treat control patients in the usual manner. When data from these individuals was excluded from the analysis, results were highly similar (Table 2 footnote). Figure 3 shows intervention effects according to baseline HbA1c. In stratified models, the model-estimated intervention effect was statistically significant for the high HbA1c subgroup at 12 months (−4∙76 mmol/mol, 95% CI −8∙92 to −0∙60, p=0∙03) but not at 18 months. The stratum x treatment interaction was not significant at any timepoint. Additionally, the site x treatment interaction was not significant.
Table 2:
Intervention effects on HbA1c (mmol/mol)
| Control mean (SD) |
Intervention mean (SD) |
Model-estimated* intervention effect on Hba1c (95%CI, p) |
|
|---|---|---|---|
| Baseline | 80 (14) | 83 (13) | |
| 3mo | 81 (15) | 81 (13) | −1.4 (−3.9–1.1, 0.28) |
| 6mo | 82 (16) | 82 (15) | −1.5 (−4.5–1.5, 0.34) |
| 12mo | 82 (16) | 82 (16) | −2.1 (−5.4–1.2, 0.21) |
| 18mo | 82 (17) | 84 (19) | −0.7 (−4.7–3.4, 0.75) |
from a mixed model for HbA1c at four follow-up visits, controlling for baseline HbA1c and clinical site. Models were repeated after exclusion of two control participants who were seen by one coach as part of their usual diabetes care, raising concern for contamination. For 3, 6, 12 and 18 months respectively, model estimated intervention effects on HbA1c (95% CI) were: −1.3 (−3.9–1.2, 0.31); −1.4 (−4.4–1.7, 0.38); −2.1 (−5.4–1.3, 0.22); and −0.7 (−4.7–3.4, 0.75).
Figure 3.
Model estimated intervention effect on HbA1c according to baseline HbA1c [64–75 mmol/mol (low) vs > 75 mmol/mol (high)]
Table 3 summarizes the intervention’s effect on the secondary outcomes at 18 months. A beneficial intervention effect was observed for motivation (p=0∙01) and problem solving skills (p=0∙024) but not for intention. Self-reported scores for diabetes self-management were improved among intervention compared to control as reported by adolescents (p=0∙01) but not for caregiver proxy-report of adolescent self-management. Though CES-D scores favored the intervention, the effect was not statistically significant at 18 months (p=0∙053). Self-reported HRQOL scores improved to a greater extent among intervention compared to control participants (p=0∙009) but not for caregiver proxy-reports. Although all three domains of fear of hypoglycemia decreased more in the intervention group than the control for both adolescent and parent report, the differences were only significant in behaviors to maintain high blood sugar as reported by parents (p=0∙005) and worry/helplessness as reported by the adolescents (p=0∙04). Parental, but not adolescent, report of diabetes family conflict was improved among intervention compared to control (p<0∙0001).
Table 3:
Intervention effects on secondary outcomes
| Baseline | 18 months | ||||
|---|---|---|---|---|---|
| Control Mean (SD) or percent (N=128) |
Intervention Mean (SD) or percent, (N=130) |
Control Mean (SD) or percent, (N=123) |
Intervention Mean (SD) or percent, (N=118) |
Model- estimated† intervention effect (95% CI; p) |
|
|
Motivation and Problem Solving |
|||||
| Total Motivation | 7.7 (1.6) | 7.5 (1.5) | 7.6 (1.6) | 8 (1.7) | 0.45 (0.10, 0.80; 0.011) |
| Total Intention | 9.1 (1) | 9.1 (1) | 9.1 (1.2) | 9.2 (1) | 0.11 (−0.14, 0.36; 0.38) |
| SPSI Total Score | 106.3 (12.3) | 105.1 (13.3) | 108.6 (13.1) | 110.9 (13.8) | 2.86 (0.37, 5.34; 0.024) |
|
Diabetes Self- Management Assessment Profile (DSMP-SR) |
|||||
| DSMP - Youth | 55.5 (11.4) | 54.6 (11.7) | 54.6 (10.2) | 56.7 (11.3) | 2.61 (0.56, 4.67; 0.013) |
| DSMP - Parent | 52.7 (11.6) | 50.6 (12.3) | 50.7 (13) | 50.2 (13.4) | 1.58 (−0.64, 3.80; 0.16) |
|
Depressive symptoms (CES-D) |
9.16 (7.73) | 9.25 (8.91) | 8.46 (7.08) | 6.63 (7.12) | −1.64 (−3.31, 0.02; 0.05) |
| Quality of Life | |||||
| PedsQL - Youth | 81.1 (11.7) | 80.7 (13.1) | 82.2 (12.6) | 85.2 (11.4) | 3.18 (0.80, 5.56; 0.0089) |
| PedsQL - Parent | 78 (14) | 77.1 (14.8) | 81 (14.4) | 82.5 (12.8) | 1.96 (−0.70, 4.61; 0.15) |
|
Fear of Hypoglycemia |
|||||
| Maintain High BG- Youth |
1.25 (0.88) | 1.17 (0.91) | 1.28 (0.88) | 1.07 (0.95) | −0.18 (−0.38, 0.02; 0.084) |
| Helplessness / Worry - Youth |
1.11 (0.57) | 1.13 (0.57) | 1.09 (0.61) | 0.93 (0.62) | −0.16 (−0.32, −0.01; 0.036) |
| Worry about negative social consequences - Youth |
1.04 (0.76) | 1.12 (0.7) | 0.91 (0.72) | 0.80 (0.69) | −0.16 (−0.33, 0.01; 0.070) |
| Maintain High BG- Parent |
1.11 (0.79) | 1.11 (0.74) | 1.11 (0.72) | 0.88 (0.64) | −0.21 (−0.35, −0.06; 0.0051) |
| Helplessness / Worry - Parent |
1.45 (0.74) | 1.49 (0.73) | 1.49 (0.8) | 1.38 (0.81) | −0.13 (−0.31, 0.04; 0.13) |
| Worry about negative social consequences - Parent |
0.52 (0.58) | 0.59 (0.61) | 0.58 (0.65) | 0.51 (0.62) | −0.12 (−0.26, 0.02; 0.088) |
|
Diabetes Family Conflict |
|||||
| Family Conflict - Youth |
1.36 (0.36) | 1.35 (0.3) | 1.32 (0.43) | 1.26 (0.38) | −0.06 (−0.15, 0.03; 0.20) |
| Family Conflict - Parent |
1.41 (0.28) | 1.45 (0.33) | 1.41 (0.31) | 1.28 (0.29) | −0.14 (−0.21, −0.07; 0.0001) |
|
Cardiovascular Disease Risk Factors |
|||||
| BMIz | 0.71 (0.89) | 0.6 (0.98) | 0.8 (0.84) | 0.7 (0.93) | −0.03 (−0.11, 0.06; 0.55) |
| Total cholesterol (mmol/l) | 4.47 (0.84) | 4.4 (0.87) | 4.62 (1) | 4.42 (0.8) | −0.17 (−0.33, −0.01; 0.038) |
| LDL cholesterol (mmol/l) | 2.59 (0.77) | 2.46 (0.7) | 2.69 (0.78) | 2.53 (0.69) | −0.08 (−0.2, 0.03; 0.16) |
| HDL cholesterol (mmol/l) | 1.43 (0.32) | 1.43 (0.35) | 1.39 (0.32) | 1.38 (0.4) | 0 (−0.06, 0.06; 0.97) |
| Triglycerides (mmol/l) | 0.99 (0.54) | 1.26 (2.31) | 1.18 (0.94) | 1.16 (1.26) | −0.16 (−0.34, 0.02; 0.079) |
| Systolic blood pressure (mmHg) | 101 (9) | 102 (9) | 105 (9) | 105 (9) | −1.54 (−3.41, 0.32; 0.10) |
| Diastolic blood pressure (mmHg) | 66 (7) | 67 (9) | 70 (7) | 68 (8) | −2.14 (−3.86, −0.43; 0.015) |
|
Hypoglycemia (CGM data) †† |
|||||
| Any time under 3.0 mmol/l | 70 (61%) | 74 (62%) | 58 (55%) | 46 (49%) | 1.4 (0.8, 2.6; 0.27) |
| Any time under 3.9 mmol/l | 97 (84%) | 102 (86%) | 81 (77%) | 71 (76%) | 1.2 (0.6, 2.3; 0.60) |
Data are mean (SD) or n (%).
Note: Baseline missing data are described in Table 1. At 18 months, some psychosocial variables had a small amount of missing data, up to 2 per group, and sample sizes for fasting lipids and CGM for control/intervention are 119/114 and 105/93 respectively.
from a mixed model for all timepoints, controlling for the baseline level of the outcome and clinical site.
Dichotomized as any vs. none for a logistic mixed effects model. The table reports percent experiencing any hypoglycemia; effects are odds ratios.
CES-D – Centers for Epidemiologic Studies – Depression scale
CGM – continuous glucose monitoring
A treatment effect was observed for diastolic blood pressure (−2∙14 mmHg, 95% CI −3∙86 to −0∙43) and for total cholesterol (- 0∙17 mmol/l, 95% CI −0∙33 to −0∙01). No intervention effect was observed for BMIz, systolic blood pressure, or other lipid parameters (LDL and HDL cholesterol, triglycerides). Intervention participants were no more or less likely to spend time in hypoglycemia than control participants; this was corroborated by looking at other CGM measures of hypoglycemia (proportion of participants spending no more than 15 minutes in hypoglycemia, number of hypoglycemic episodes lasting 15 minutes or longer; data not shown). A total of 54 serious adverse events were identified; 34 of these were diabetes-related, including low blood glucose requiring assistance (n=3) and high blood glucose with diabetic ketoacidosis and emergency response (n=25). The non-diabetes-related serious adverse events were hospitalizations typically related to acute trauma from accidents or mental health-related admissions. None of the events were study-related, and the rate of serious adverse events was similar in the intervention and control groups.
Discussion
The central premise of FLEX (Figure 1) was that an intervention to improve information (targeted diabetes education), motivation (creating a motivational framework for change), and skills (problem solving skills), would reduce barriers to adherence (e.g. diabetes specific family conflict, fear of hypoglycemia) and improve diabetes self-management, leading to better glycemic control, psychosocial outcomes, and CVD risk for adolescents with T1D. The FLEX study, executed with high study retention and intervention fidelity, did not show efficacy with respect to the primary outcome of HbA1c at 18 months post-randomization. Although statistically significant, effect estimates for diastolic blood pressure and total cholesterol were small from a clinical perspective and might be the result of a type 1 error. However, efficacy was supported for several secondary outcomes, including motivation, problem solving skills, diabetes self-management as self-reported by adolescents, adolescent-reported HRQOL, and parameters related to fear of hypoglycemia and diabetes family conflict. From the perspective of clinical importance, the difference in health-related QOL is similar to the minimal clinically important difference for youth with T1D 33 even though scores in both groups were within normal limits. Other secondary outcome variables in psychosocial domains have less information regarding minimal clinically important differences. Nevertheless, the overall pattern of secondary outcomes suggests broad improvement in psychosocial well-being.
Evidence for behavioral interventions that reliably improve long-term HbA1c for adolescents with T1D is equivocal. The initial FLEX pilot yielded preliminary evidence of benefit,16 and Wysocki et al. showed that a T1D-specific behavioral family systems therapy approach to enhance family communication and problem solving improved HbA1c.18 Channon et al. showed that MI in adolescents with T1D reduced HbA1c,34 but this was not replicated by Wang et al.35 In that trial, MI was not effective despite confirmation of the fidelity of MI counseling; instead, the diabetes education arm showed improvement in HbA1c. Overall, a mixed literature now exists, with inconsistent evidence of benefit from various behavioral strategies implemented in a variety of settings. Thus, there remains a general lack of coherence regarding the optimal combination of intervention components that result in consistent benefit for glycemic control, psychosocial well-being, and other outcomes for adolescents with T1D.
Our findings of efficacy with regard to psychosocial outcomes, such as HRQOL and symptoms of depression, is consistent with previous literature. Problem solving based interventions in T1D have improved self-efficacy,36 increased frequency of checking blood glucose,37 and improved anxiety, stress, and coping,38 and QOL.39,40 Wysocki et al.’s trial improved diabetes management adherence as well as HbA1c.18 Channon et al. showed that MI in adolescents with T1D improved QOL.34
The FLEX trial improved motivation, problem solving skills, barriers to adherence, and self-management, despite having no impact on HbA1c. This suggests the need for further investigation into the connection between these constructs and glycemic control. One potential target is insulin dosing behavior, as insufficient insulin is the root cause of persistent hyperglycemia in T1D. The FLEX intervention was designed to be responsive to the high degree of treatment burden in T1D, which includes detailed attention to glucose values, food choices, exercise, and other variables that often are not a priority to adolescents. As such, a range of behaviors were considered appropriate targets for problem solving. Ensuring adequate insulin, while avoiding hypoglycemia, likely requires a more targeted approach to glucose monitoring and insulin delivery than was provided in the FLEX intervention as delivered. This more specific focus should be included in future applications of the FLEX methodology.
Emerging technologies, such as CGM and hybrid closed loop insulin pumps, also have potential to improve glucose control and reduce the burden of disease as part of a targeted strategy, but have not been investigated in detail in adolescents with elevated HbA1c such as participated in FLEX (mean HbA1c=81 mmol/mol (9.6%) with 66% >75 mmol/mol (9.0%) at baseline).41 Fear of hypoglycemia is a specific barrier to tighter glucose control that may merit more focused attention.42 Use of new technologies to improve insulin dosing and increased attention to fear of hypoglycemia in the context of a comprehensive behavior change and motivational strategy represent evolution of the field and future directions of study.
The FLEX trial had several limitations. First, the inclusion criteria for age (13–16 years at baseline) limits generalizability, although it accomplished the design intent to account for developmental readiness for the intervention and avoided the challenges in follow-up for adolescents who, when older, would be likely to move away from home to attend college or begin work. Second, two of the measures were altered for the purposes of the present study; and, although the baseline data were similar to published norms for these two measures, the psychometric properties of these updated measures need to be analyzed with a wider sample in the future. Third, although the intervention was carefully documented in a manual of procedure, clear training procedures were established and followed, fidelity of intervention delivery was monitored throughout the trial, and fidelity goals were exceeded, the number of coaches was relatively small (total of four across two sites), limiting our ability to generalize. Finally, although the trial was embedded in active pediatric endocrinology clinical practices, by design, it was implemented as an efficacy trial with rigorous monitoring of all study procedures and was not integrated into clinical practice, being coordinated with the participant’s usual diabetes care provider only sporadically if a specific need arose (e.g., need for adjustment in insulin regimen). Given the findings of the FLEX trial and the current literature, it appears that the intervention holds promise for improvements in psychosocial outcomes, including HRQOL, but that changes in the intervention approach are needed to achieve the goal of improved glycemic control. Moreover, an upward tendency in HbA1c was noted in both the low Control and Intervention groups during the course of the study. This is consistent with an increased HbA1c reported in cross-sectional and longitudinal data in the T1DX 43 in which HbA1c peaked at 17 years of age, further emphasizing the need for effective interventions for this patient population. Our working hypothesis related to future work is twofold. First, a more directed approach toward achievement of appropriate insulin dosing, and greater integration with ongoing clinical care, is likely needed. Second, heterogeneity in participant profile may drive variations in response to behavioral interventions, including the FLEX intervention. Further, understanding this heterogeneity may inform how to better match existing intervention components, or identify new components, to optimize outcomes for participant subgroups or individuals accordingly. Next steps in the research process will be to utilize state-of-the-art statistical approaches, such as residual-weighted learning methods,44 to identify whether responder and non-responder subgroups exist in the FLEX study population with respect to observed effects on primary and secondary outcomes, and then to identify patient characteristics that align with intervention components to drive response. Included in this effort is the requirement for a deeper, systematic understanding of the intervention delivery. To this end, application of the recent Behavior Change Technique (BCT) Taxonomy, developed by Michie and colleagues 45 is underway to identify BCTs related to six behaviors presumed relevant to improving HbA1c (blood glucose checking, use of CGM, insulin dosing, diet, physical activity, and parental support). Together, this is the information required to move forward with plans to build on the FLEX intervention to optimize metabolic, behavioral, and psychosocial outcomes for youth with T1D.
In conclusion, the FLEX behavioral intervention did not significantly change the primary outcome, but did positively impact several psychosocial outcomes over the 18 months of the trial. Further analyses will reveal information regarding drivers of positive response to intervention and will point to directions for improvement in interventions designed to optimize health and well-being for adolescents living with T1D.
Evidence before this study
We searched PubMed for English-language clinical trials from database inception until March 28, 2018, with the terms “behavioral intervention” AND “type 1 diabetes”, (“motivational interviewing” OR “problem solving skills training”) AND “type 1 diabetes”, (“carbohydrate counting” OR “insulin dosing”) AND “type 1 diabetes”, (“diabetes education” OR “telemedicine” OR “telehealth” OR “m-health”) AND “type 1 diabetes”, (“diabetes self-management” OR “adherence” OR “social support”) AND “type 1 diabetes”, (“family conflict” OR “family communication”) AND “type 1 diabetes”, (“quality of life” OR “depression”) AND “type 1 diabetes”. The search yielded 51 trials examining the effect of a behavioral intervention on the clinical outcome of hemoglobin A1c (HbA1c). Of these, 12 trials showed modest improvements in glycemic control (generally < 0∙50% in HbA1c). Six trials centered on family-based interventions such as family communication or relationships and family diabetes management practices, one of which used a family-based motivational interviewing technique. One trial focused on motivational interviewing alone. The remaining five incorporated carbohydrate counting, assisted insulin adjustments, online social support, problem solving, behavioral pairing, and auditory guided imagery. We found no trials that investigated the use of a behavioral intervention that integrates aspects of motivational interviewing with problem solving and family communication to improve glycemic control among adolescents with type 1 diabetes.
Added value of this study
The FLEX intervention is, to our knowledge, the first randomized controlled trial to test the efficacy of an adaptive behavioral intervention that integrates motivational interviewing and problem solving skills training tailored to patients and their families to promote self-management and improve measures of blood glucose control in youth with type 1 diabetes. Despite high retention and fidelity, the intervention did not provide the hypothesized benefit on glycemic control at 18-month post-randomization, as measured by HbA1c. However, there were statistically significant benefits on secondary outcomes, including motivation and problem solving, which were central to the intervention approach, and other markers of psychosocial functioning.
Implications of all of the available evidence
There remains a general lack of coherence in the literature regarding the optimal combination of intervention components to benefit glycemic control and other outcomes among adolescents with type 1 diabetes. The impact of FLEX on secondary psychosocial outcomes but not HbA1c suggests that a more directed approach to achieve appropriate insulin dosing may be more effective towards achieving change in this clinical endpoint. Current technologies, including continuous glucose monitoring, may be useful in this regard. Further, additional research is needed to identify potential drivers of response or non-response to any intervention strategy.
Acknowledgements
The FLEX Study is indebted to the many youth and their families whose participation made this study possible.
The writing group for this manuscript wishes to acknowledge the contributions of the following individuals to the FLEX Study: Nancy Morwessel, MSN, APRN, CNP, CDE; Emily Smith, BS; Michelle Hull; and Daniel H. Grossoehme, DMin, MS; for Cincinnati Children’s Hospital and Medical Center. Alexis Bouffard, RN, MA, CDE; Tonya Jenkins, RD, CDE; Emily E. Simmons, BA; and Mariana Villarreal, BS; for the University of Colorado Barbara Davis Center. Thomas Songer, PhD, for University of Pittsburgh. Timothy Wysocki, PhD, for Nemours Children’s Health System. DMM has research support from grant P30DK116074.
DMM has research support from the NIH, JDRF, NSF, and the Helmsley Charitable Trust, and his institution has research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, and Roche. DMM has consulted for Abbott, the Helmsley Charitable Trust, Sanofi, and Eli Lilly and has served on an advisory board for Insulet. EJMD has consulted for Helmsley Charitable Trust. There are no other interests to declare.
Footnotes
Contributors EJMD had primary responsibility to draft and finalize the manuscript. JC conducted statistical analyses. CH represented NIH/NIDDK. All authors provided substantial input to the manuscript, including review for accuracy and contribution to interpretation of findings.
Declaration of Interests
The views expressed in this paper are those of the authors and do not necessarily represent the positions of the NIH, the DHHS, or the Federal Government.
Full Professors: Mayer-Davis, Maahs and Seid.
References
- 1.Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. JPediatr. 2009;155(5):668–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes. 2015. [DOI] [PubMed] [Google Scholar]
- 3.Charalampopoulos D, Hermann JM, Svensson J, et al. Exploring Variation in Glycemic Control Across and Within Eight High-Income Countries: A Cross-sectional Analysis of 64,666 Children and Adolescents With Type 1 Diabetes. Diabetes care. 2018;41(6):1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rechenberg K, Whittemore R, Holland M, Grey M. General and diabetes-specific stress in adolescents with type 1 diabetes. Diabetes research and clinical practice. 2017;130:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood KK, Beavers DP, Yi-Frazier J, et al. Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. Journal of Adolescent Health. 2014;55(4):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naughton MJ, Joyce P, Morgan TM, et al. Longitudinal associations between sex, diabetes self-care, and health-related quality of life among youth with type 1 or type 2 diabetes mellitus. The Journal of pediatrics. 2014;164(6):1376–1383. e1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence JM, Joyce P, Black MH, et al. Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. The Journal of pediatrics. 2012;161(2):201–207. e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez BL, Fujimoto W, Mayer-Davis E, et al. Prevalence of Cardiovascular Disease Risk Factors in U.S. Children and Adolescents with Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care. 2006;29(8):1891–1896. [DOI] [PubMed] [Google Scholar]
- 9.Dabelea D, Talton JW, D’Agostino R, et al. Cardiovascular risk factors are associated with increased arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. Diabetes care. 2013;36(12):3938–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. Jama. 2017;317(8):825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein ND, Rothman AJ, Sutton SR. Stage theories of health behavior: conceptual and methodological issues. Health Psychol. 1998;17(3):290–299. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–473. [DOI] [PubMed] [Google Scholar]
- 13.Miller W, Rollnick S. Motivational interviewing: Preparing people for change. Journal for Healthcare Quality. 2003;25(3):46.12691106 [Google Scholar]
- 14.Seid M, D’Amico EJ, Varni JW, et al. The In Vivo Adherence Intervention For at Risk Adolescents With Asthma: Report of a Randomized Pilot Trial. JPediatrPsychol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nansel TR, Iannotti RJ, Liu A. Clinic-integrated behavioral intervention for families of youth with type 1 diabetes: randomized clinical trial. Pediatrics. 2012;129(4):e866–e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer-Davis EJ, Seid M, Crandell J, et al. Flexible Lifestyles for Youth (FL3X) behavioural intervention for at-risk adolescents with Type 1 diabetes: a randomized pilot and feasibility trial. Diabetic medicine : a journal of the British Diabetic Association. 2015;32(6):829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kichler JC, Seid M, Crandell J, et al. The Flexible Lifestyle Empowering Change (FLEX) intervention for self-management in adolescents with type 1 diabetes: Trial design and baseline characteristics. Contemporary clinical trials. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wysocki T, Harris MA, Buckloh LM, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. JPediatrPsychol. 2006;31(9):928–938. [DOI] [PubMed] [Google Scholar]
- 19.Association AD. 4. Foundations of care: education, nutrition, physical activity, smoking cessation, psychosocial care, and immunization. Diabetes care. 2015;38(Supplement 1):S20–S30. [DOI] [PubMed] [Google Scholar]
- 20.Rewers M, Pillay K, de Beaufort C, et al. International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014;15(Suppl 20):102–114. [DOI] [PubMed] [Google Scholar]
- 21.Mauras N, Beck R, Xing D, et al. A Randomized Clinical Trial to Assess the Efficacy and Safety of Real-Time Continuous Glucose Monitoring in the Management of Type 1 Diabetes in Young Children Aged 4 to <10 Years. Diabetes Care. 2012;35(2):204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsberg L, Kallmen H, Hermansson U, Berman AH, Helgason AR. Coding counsellor behaviour in motivational interviewing sessions: inter-rater reliability for the Swedish Motivational Interviewing Treatment Integrity Code (MITI). Cogn BehavTher. 2007;36(3):162–169. [DOI] [PubMed] [Google Scholar]
- 23.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes care. 2017;40(12):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller WR, Johnson WR. A natural language screening measure for motivation to change. AddictBehav. 2008;33(9):1177–1182. [DOI] [PubMed] [Google Scholar]
- 25.D’Zurilla TJ, Nezu AM, Maydeu-Olivares A. Social problem-solving inventory--revised (SPSI-R). Multi-Health Systems; 2002. [Google Scholar]
- 26.Wysocki T, Buckloh LM, Antal H, Lochrie A, Taylor A. Validation of a self-report version of the diabetes self-management profile. PediatrDiabetes. 2012;13(5):438–443. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LD. The CES-D scale: a self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 28.Varni J, Seid M, Kurtin P. The PedsQL’4.0: Reliability and validity of the Pediatric Quality of Life Inventory 4.0 version. Med Care. 2001. [DOI] [PubMed] [Google Scholar]
- 29.Shepard JA, Vajda K, Nyer M, Clarke W, Gonder-Frederick L. Understanding the construct of fear of hypoglycemia in pediatric type 1 diabetes. Journal of pediatric psychology. 2014;39(10):1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised Diabetes Family Conflict Scale. Diabetes Care. 2007;30(7):1764–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. ClinChemLab Med. 2002;40(1):78–89. [DOI] [PubMed] [Google Scholar]
- 32.Vallejo G, Ato M, Valdés T. Consequences of misspecifying the error covariance structure in linear mixed models for longitudinal data. Methodology. 2008;4(1):10–21. [Google Scholar]
- 33.Hilliard ME, Lawrence JM, Modi AC, et al. Identification of minimal clinically important difference scores of the PedsQL in children, adolescents, and young adults with type 1 and type 2 diabetes. Diabetes care. 2013;36(7):1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Channon SJ, Huws-Thomas MV, Rollnick S, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care. 2007;30(6):1390–1395. [DOI] [PubMed] [Google Scholar]
- 35.Wang YC, Stewart SM, Mackenzie M, Nakonezny PA, Edwards D, White PC. A randomized controlled trial comparing motivational interviewing in education to structured diabetes education in teens with type 1 diabetes. Diabetes Care. 2010;33(8):1741–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howells L, Wilson AC, Skinner TC, Newton R, Morris AD, Greene SA. A randomized control trial of the effect of negotiated telephone support on glycaemic control in young people with Type 1 diabetes. DiabetMed. 2002;19(8):643–648. [DOI] [PubMed] [Google Scholar]
- 37.Cook S, Herold K, Edidin DV, Briars R. Increasing problem solving in adolescents with type 1 diabetes: the choices diabetes program. Diabetes Educ. 2002;28(1):115–124. [DOI] [PubMed] [Google Scholar]
- 38.Hains AA, Davies WH, Parton E, Totka J, moroso-Camarata J. A stress management intervention for adolescents with type 1 diabetes. Diabetes Educ. 2000;26(3):417–424. [DOI] [PubMed] [Google Scholar]
- 39.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. JPediatr. 2000;137(1):107–113. [DOI] [PubMed] [Google Scholar]
- 40.Grey M, Boland EA, Davidson M, Yu C, Tamborlane WV. Coping skills training for youths with diabetes on intensive therapy. ApplNursRes. 1999;12(1):3–12. [DOI] [PubMed] [Google Scholar]
- 41.Sherr J, Tauschmann M, Battelino T, et al. ISPAD guidelines-Diabetes Technologies Chapter. Pediatric Diabetes. In press 2018. [DOI] [PubMed] [Google Scholar]
- 42.Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Current diabetes reports. 2016;16(8):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatric Diabetes. 2016;17(5):327–336. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Mayer-Hamblett N, Khan U, Kosorok MR. Residual weighted learning for estimating individualized treatment rules. Journal of the American Statistical Association. 2017;112(517):169–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of behavioral medicine. 2013;46(1):81–95. [DOI] [PubMed] [Google Scholar]