Abstract
The structural diversity and localization of cell surface glycosphingolipids (GSLs), including gangliosides, in glycolipid-enriched microdomains (GEMs, also known as lipid rafts) render them ideally suited to play important roles in mediating intercellular recognition, interactions, adhesion, receptor function, and signaling. Gangliosides, sialic acid-containing GSLs, are most abundant in the nerve tissues. The quantity and expression pattern of gangliosides in brain change drastically throughout development and these changes are mainly regulated through stage-specific expression of glycosyltransferase genes. We previously demonstrated for the first time that efficient histone acetylation of the glycosyltransferase genes in mouse brain contributes to the developmental alteration of ganglioside expression. We further demonstrated that acetylation of histones H3 and H4 on the N-acetylgalactosaminyltransferase I (GalNAcT, GA2/GM2/GD2/GT2-synthase; B4galnt1) gene promoter resulted in recruitment of trans-activation factors. In addition, we showed that epigenetic activation of the GalNAcT gene was detected and accompanied by an apparent induction of neuronal differentiation of neural stem cells (NSCs) responding to an exogenous supplement of ganglioside GM1. Most recently, we found that nuclear GM1 binds with acetylated histones on the promoters of the GalNAcT as well as on the NeuroD1 genes in differentiated neurons. Here, we will introduce epigenetic regulation of ganglioside synthase genes in neural development and neuronal differentiation of NSCs.
Keywords: Brain development, DNA methylation, Epigenetics, Glycosyltransferase, Ganglioside, Histone acetylation, Neural stem cell, Neuronal progenitor cell, Neuronal differentiation
Introduction
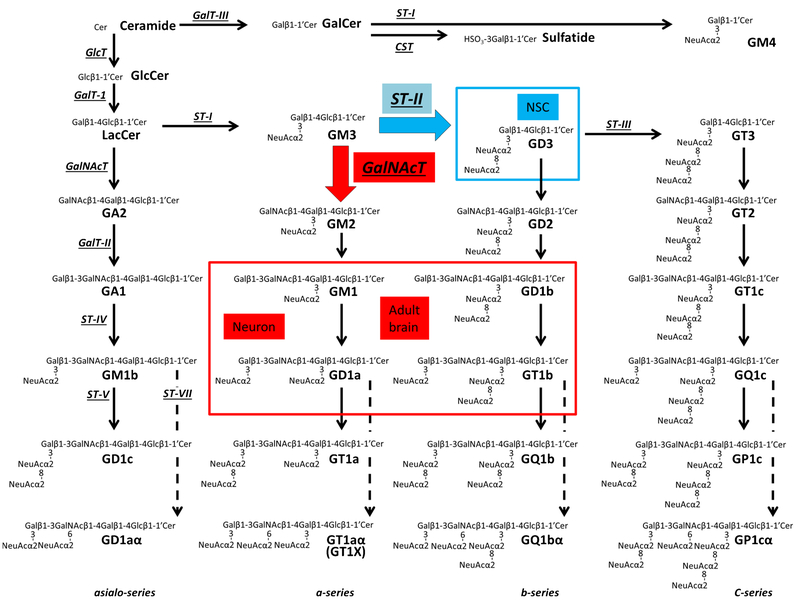
Gangliosides, sialic acid–containing glycosphingolipids (GSLs), are composed of a ceramide moiety linked to an oligosaccharide chain (Fig. 1). Gangliosides are expressed primarily, but not exclusively, on the outer leaflet of the plasma membrane of cells in all vertebrates. They are particularly abundant in nervous tissues. In early embryonic rodent brains, the pattern of ganglioside expression is characterized by the expression of a large amount of simple gangliosides, such as GM3 and GD3 [1]. In later developmental stages, more complex gangliosides prevail, particularly GM1, GD1a, GD1b, and GT1b, which account for more than 80 % of the total gangliosides. Thus, the expression of neural gangliosides changes dramatically during cellular differentiation and brain development, indicating that ganglioside expression and neurodevelopmental events are closely correlated. This unique expression pattern of specific gangliosides can be used for specific cell lineage markers and may reflect the functional roles they play in a specific developmental stage. Abundant evidence supports the notion that GSLs, including ganglio-sides, serve regulatory roles in cellular events, including proliferation and neural differentiation, as exemplified by neuritogenesis, axonogenesis, and synaptogenesis [2, 3]
Fig. 1.
Structures and biosynthetic pathways of glycosphingolipids (GSLs). Cer, ceramide; CST, cerebroside sulfotransferase (Gal3st1, sulfatide synthase); GalNAc-T, N-acetylgalactosaminyltransferase I (B4galnt1, GA2/GM2/GD2/GT2 - synthase); G a l T- I, galactosyltransferase I (B4galt6, lactosylceramide synthase); GalT-II, galactosyltransferase II (B3galt4, GA1/GM1/GD1b/GT1c-synthase); GalT-III, galactosyltransferase III (Ugt8a, galactosylceramide synthase); GlcT, glucosyltransferase (Ugcg, glucosylceramide synthase); ST-I, sialyltransferase I (St 3 gal5, GM3-synthase); ST-II, sialyltransferase II (St8Sia1, GD3-synthase); ST-III, sialyltransferase III (St8Sia3, GT3-synthase); ST-IV, sialyltransferase IV (St 3 gal2, GM1b/GD1a/GT1b/GQ1c-synthase); ST-V, sialyltransferase V (St8sia5, GD1c/GT1a/GQ1b/GP1c-synthase); ST-VII, sialyltransferase VII (St6galnac6, GD1aα/GT1aα/GQ1bα/GP1cα-synthase). Official symbols of genes are represented in italics in this figure legend
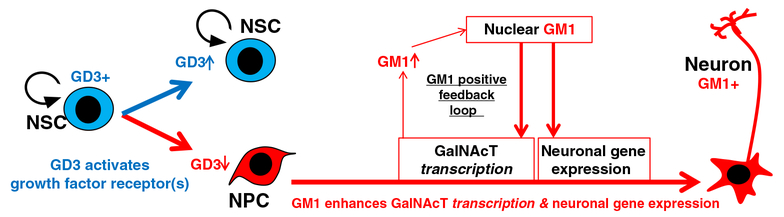
The brain tissue is composed of a variety of neural cells, including neurons and glia; those cells are generated from progenitor cells that are recognized as neural stem cells (NSCs). NSCs are undifferentiated neural cells characterized by their high proliferative potential and the capacity for self-renewal with retention of multipotency to differentiate into neurons and glia. Specific gangliosides are considered to have critical roles in the regulation of NSC cell-fate determination (Fig. 2). We have shown that GD3 is the predominant ganglioside in NSCs [4]. The interaction of GD3 with epidermal growth factor receptor (EGFR) plays a crucial role in maintaining the self-renewal capacity of NSCs by directing the EGFR through the recycling pathway rather than through the degradative pathway after endocytosis [5]. Although Sialyltransferase II (ST-II, GD3-synthase; St8sia1) knockout (KO) mice and N-acetylgalactosaminyltransferase I (GalNAcT, GA2/GM2/GD2/GT2-synthase; B4galnt1) KO mice do not have explicit phenotypes in early development [6], it has been revealed that GD3 is required for long-term maintenance of NSCs populations in postnatal mouse brain [5, 7]. Deficiency in GD3 leads to developmental and behavior deficits, such as depression [7]. GalNAcT-KO mice exhibit impaired movement and have virtually all the neuropathological symptoms of Parkinson disease [8]. For specialization of neuronal cells, GM1 potentiates nerve growth factor and regulates neurite outgrowth to contribute to neuronal differentiation. Intriguingly, our recent studies revealed that epigenetic activation of the GalNAcT gene, but not the ST-II gene, occurred and was accompanied by an apparent induction of neuronal differentiation in NSCs responding to an exogenous supplement of ganglioside GM1 [9]. Supplemental GM1 enhanced GalNAcT expression of mRNA, while exogenous GD3 had no significant effect in this expression. The binding of acetylated histone H4 (AcH4) on the GalNAcT promoter region was elevated by GM1 addition, but neither by GD3 nor GD1b supplementations. Unlike GD3 and GD1b, GM1 has epigenetic effect for neuronal differentiation. This report apparently showed that the epigenetic regulation in neuronal cells by GM1 did not influence on the genome at random, but at specifically controlled pinpoint loci [9].
Fig. 2.
Specific gangliosides modulate NSC function and neuronal differentiation. Our published data suggest that GD3 maintains NSC’s characteristics and GM1 epigenetically promotes neuronal differentiation of NSCs. ST-II (GD3S) and GalNAcT (GM2/GD2S) are located at a key branching point of a- and b-series ganglioside biosynthetic pathways (Fig. 1). GD3 modulates NSC proliferation by interacting with growth factor receptors and regulating growth factor-induced signaling. The synthesis of GD3 is switched into the synthesis of complex gangliosides (GM1, GD1a, GD1b, and GT1b), resulting in terminal differentiation and loss of the “stemness” of NSCs. During neuronal differentiation, the expression of complex a-series gangliosides, especially GM1, is augmented by a GM1-modulated epigenetic gene regulation mechanism of GalNAcT. Nuclear GM1 modulates transcriptional activity of neuronal genes, such as Neuro D1. NSC, neural stem cell; NPC, neuronal progenitor cell
Epigenetic regulation of gene expression involving DNA methylation, histone modifications, chromatin remodeling, and non-coding RNAs, is an important mechanism governing stage-specific gene expression in developing mammalian brains as well as other tissues [10, 11]. The most common post-translational histone modification is acetylation, which occurs by addition of acetyl groups to the lysine residues of the amino-terminal tails of core histones. Acetylation of his-tone H3 or H4 can alter the interaction between histones and DNA to allow for relaxation of chromatin. Thus, changes in histone acetylation status are often associated with transcriptional activation and repression [12, 13]. In NSCs, induction of histone acetylation promotes neuronal differentiation and inhibits glial differentiation through up-regulation of neurogenic transcription factors [14]. Histone acetylation is controlled by histone acetyl transferases (HATs) and histone deacetylases (HDACs) [15]. Intriguingly, recent studies have suggested that glycans can contribute to epigenetic gene regulation as modulators. For example, a Bcarbohydrate-like^ molecule, inositol tetraphosphate (IP4; inositol 1, 3, 4, 5– tetrakisphosphate) acts as a stimulator to the activity of class I HDACs (HDAC1/2/3) [16–18]. Additionally, OGlcNAcylation has been reported to regulate localization, activity, and stability of many transcription factors, as well as their interactions with other proteins and DNA [19, 20]. As such, glycosyl modification, such as O-GlcNAcylation, is now considered as an important modulatory mechanism to epigenetic control of gene expression [21].
We have previously reported that efficient histone acetylation of glycosyltransferase (GT) genes in mouse brain contributes to the developmental alteration of ganglioside expression [22]. Further, we have demonstrated that acetylation of histones H3 and H4 on the GalNAcT gene promoter leads to recruitment of trans-activation factors SP1 and AP-2 [9]. More recently, we found that nuclear GM1 binds with acetylated histones on the promoters of the GalNAcT as well as on the neurogenic transcription factor, NeuroD1 gene, in differentiated neurons [23]. Here, we will further elaborate the importance of epigenetic regulation of ganglioside synthase genes in neural development and neuronal differentiation of NSCs.
Histone acetylation and gene activation of the 5′ region of GalNAcT and ST-II genes during brain development
The quantity and expression pattern of gangliosides in brain change drastically throughout development and are mainly regulated through stage-specific expression of GT genes [1, 24, 25]. A diagram of the biosynthetic pathways of gangliosides is shown in Fig. 1. Of particular importance is the regulation of GTs located at the branching points that govern pathway shifts for ganglioside biosynthesis. For example, regulation of the two key GTs at the branching point leading to GD3 and GM2 synthesis constitutes one of the critical steps for the expression of GD3 and N-acetylgalactosaminyl-containing “brain-type” gangliosides in the a- and b- series. Our analyses of the whole brain have shown that the GalNAcT gene encounters drastic activation since the late embryonic stage of mice, and its expression level is further elevated from birth through adulthood; transcription of the sialyltransferase II (ST-II, GD3-synthase; St8sia1) gene is also increased during brain development, albeit less pronounced in comparison [22, 25]. We have demonstrated that acetylated histone H3 (AcH3) of the chromatin in the 5′ region of the mouse GalNAcT and ST-II genes is highly correlated to the developmental changes of their mRNA levels in the mouse brain. In that study, the 5′ DNA regions of the GalNAcT and ST-II loci appeared to be unmethylated in either the embryonic or postnatal mouse brain. These observations suggest that transcriptional activation of GalNAcT and ST-II genes in developing mouse brains is not regulated by DNA methylation, at least in the 5′ DNA regions [22]. Subsequently, Taniguchi’s group suggested that for the brain-specific synthesis of branched O-mannose glycan that was catalyzed by N-acetyl-glucosaminyltransferase-IX (GnT-IX), the mRNA expression of GnT-IX could be triggered by the neurogenic transcription factor NeuroD1 as well as an insulator protein CTCF as a consequence of active histone marks deposited around GnT-IX gene’s transcription start site [26, 27].
Histone acetylation recruits transcription factors onto the promoters of glycosyltransferase genes in NSCs
We have further elucidated the differential regulatory mechanisms underlying epigenetic activation for the GalNAcT and ST-II genes during neuronal differentiation in a NSC culture system. This study has showed that during neuronal differentiation of NSCs, not only AcH3, but also AcH4, exhibited a pattern of increment similar to that of enhanced mRNA expression of GalNAcT and ST-II. It has been reported that GT promoters are often controlled by transcription factors Sp1 and AP-2 [28–30]. The promoter activity of the human GalNAcT gene is known to be activated by Sp1 and AP-2, and that for the ST-II gene by Sp1 by way of promoter association [30]. A putative binding site for the transcription factor AP-2 was found within the 5′ untranslated region (5′ UTR) of the GalNAcT gene and in the first exon of the ST-II gene, while a binding site for the transcription factor Sp1 was present only in the 5′ UTR of the ST-II gene [31]. When the cellular HDAC activity was globally inhibited by valproic acid (VPA), more GalNAcT or ST-II mRNA was detected, which could be triggered due to a loading boost of the transcription factors AP-2 and Sp1 on the promoter region [9]. Individually knocking down HDAC1 and HDAC2 gene expression increased the levels of AcH3 and AcH4. However, GalNAcT and ST-II genes did not show any corresponding change in the histone acetylation status, promoter binding abilities for the transcription factors, or mRNA expression level. Intriguingly, when both HDAC1 and HDAC2 were knocked down, the expression of GalNAcT mRNA was upregulated and AP-2- or Sp1-loading was significantly increased, reflecting the elevated level of histone acetylation on the GalNAcT gene. This was not the case for the ST-II gene, however, which remained unchanged with respect to the levels of mRNA, the binding of transcription factors, or the AcH status. Our results indicated that transcription of GalNAcT and ST-II could be regulated by different HDAC isoforms, since double-knockdown of HDAC1 and HDAC2 led to GalNAcT gene trans-activation, but not ST-II.
Nuclear GM1 at the nuclear periphery is associated with acetylated histones in neurons
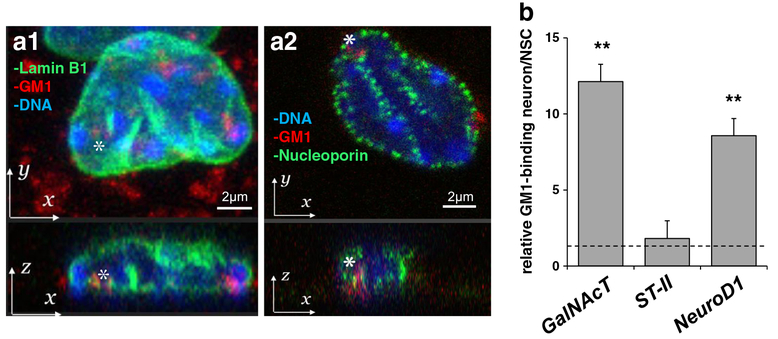
Glycolipid-enriched microdomains (GEMs) or lipid rafts on the cell plasma membrane surface are increasingly recognized as an important site for signaling transduction. Nuclear lipid domains on the nuclear envelope have also recently been suggested to play a similar role [32]. Both GM1 and GD1a have been detected in the inner and outer nuclear membranes [33]. The nuclear distribution of gangliosides in the developing brain reflects their composition in the total brain [2, 34], i.e., GD3 is abundant in the nuclear membranes of the embryonic brain and adult brain type gangliosides (GM1, GD1a, GD1b, GT1b) become more abundant in the nuclei of postnatal brain cells [34]. With regard to gangliosides associated with chromatins, GD3 is reported to interact with histone H1 in the nucleus [35]. It has also been shown that nuclear sphingolipids participate in epigenetic regulation of gene expression by controlling histone acetylation [36]. For these reasons, it is reasonable to assume that membrane lipids, including GSLs, may contribute, in a stage- and cell-specific manner, to modulate gene expression as happening on the nuclear membrane. The nuclear envelope, including the nuclear lamina and nuclear pore complexes, is a key structure to maintain chromatin architecture and cell-specific gene expression [37]. We have confirmed that GM1 is indeed present and colocalized with lamin B1 and nucleoporin at the nuclear periphery of neuronal cells derived from NSCs (Fig. 3a). Our co-immunoprecipitation experiments have clearly revealed that nuclear GM1 genuinely interacts with AcH3 and AcH4, which are both active epigenetic marks, but not with H3K27me3, an inactive epigenetic mark. This result strongly suggests that GM1-engaged chromatins are transcriptionally active. We have further investigated the interaction between GM1 and putative genetic loci, including the GalNAcT and ST-II genes. ChIP assay was performed using anti-GM1 antibody, and revealed that GM1 was increased at the promoter region of the GalNAcT gene in neurons (Fig. 3b). Such a tendency did not appear for the ST-II gene loci. Interestingly, after neuronal differentiation, more GM1 was accumulated on the promoter of the neurogenic transcription factor NeuroD1 gene, which commits the transition of NSCs to neuronal progenitor cells (NPCs). These results are consistent with the notion that GM1 is involved in the chromatin complex that promotes neuronal differentiation.
Fig. 3.
Nuclear GM1 in at the nuclear periphery and is accumulated in the activated GalNAcT and NeuroD1 genes in neurons. (a) Localization of GM1 at the nuclear periphery in neurons. Differentiated neurons were fixed, stained with fluorescent cholera toxin B subunit (CtxB, red fluorescent) to determine the localization of GM1. Cells were co-stained with lamin B1 (green fluorescence in a1) or nucleoporin (green fluorescence in a2). Nuclear DNA was counterstained with Hoechst 33,258. Z-projection: bottom panel is x-z plane. (b) GM1 is accumulated in the activated GalNAcT and NeuroD1 genes. The nuclear fraction of differentiated neurons with prior formaldehyde cross-linking was used for anti-GM1 immunoprecipitation. The amount of specific DNA fragments co-precipitated with GM1 was analyzed by quantitative real-time PCR. The data indicate the relative GM1 binding ability in neurons. The value of NSC samples is defined as 1.0 and represented with a dashed line. Each bar represents mean ± SD of 3 to 4 independent experiments (n = 3–4). ** (p < 0.01) indicates the level of significance in two-tailed t-tests of differences between differentiated neurons versus NSCs
GM1 promotes neuronal differentiation with enhanced histone acetylation
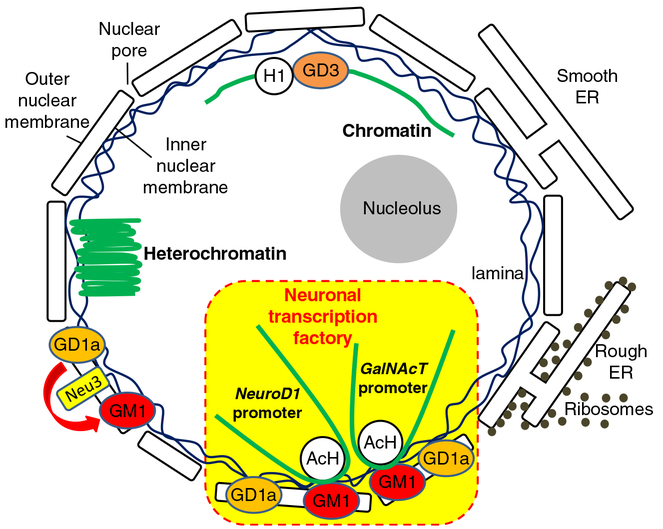
It is known that GM1 enhances neurite outgrowth, and up-regulation of GM1 in the nuclear membrane accompanies the process of neurite outgrowth [38, 39]. Ledeen et al. have proposed that the elevated level of GM1 has a modulatory effect on Ca2+ homeostasis in the nucleus, which is mediated by a tight association of GM1 with the Na+/Ca2+ exchanger [8, 40]. The supplement of GM1 in our NSC culture led to neuronal differentiation as well. A notable observation from our own study was that GM1-induced NSCs produced more GalNAcT transcripts as well as a higher level of acetylated histones on the gene promoter where more transcription factors are recruited. On the other hand, the ST-II gene did not show any significant change. Given that the global acetylation state of histones was not changed, the GM1-triggered epigenetic activation appears to be specific for certain targeted genes, including GalNAcT, in this enhanced neurogenic process. This result might represent a potential mechanism accounting for the correlations between the ganglioside pattern shift and epigenetic modifications of ganglioside synthase expression during neuronal differentiation and neural development. In this regard, GM1 might play a crucial role in modulating the “pathway switch” in ganglioside expression in the developing brain. We propose that GM1 may generate a positive feedback loop (Fig. 2) for NSCs to enhance neuronal differentiation and thereby to produce more GM1 and other “brain-type” gangliosides, such as GD1a, GD1b and GT1b by increasing the GalNAcT message level. Since the content of GM1 in the nuclear membrane is increased during neuronal differentiation [39], it is possible that the nuclear GM1-lipid domains may serve as a docking site at the nuclear periphery specifically for active chromatins. Our working model on the role of nuclear gangliosides, which are to epigenetically modulate the gene expression in neuronal cells is shown schematically in Fig. 4. In the nuclear envelope, GM1 and GD1a are present [8, 41] and sialidase activity has also been identified [42, 43]. Further, it has been reported that neuraminidases (sialidases) Neu3 and Neu1 are present in the inner and outer nuclear membranes, respectively [33]. Since these neuraminidases can convert GD1a to GM1, nuclear GD1a may serve as a precursor of GM1 on site. Alternatively, GM1 is found to be associated with lamin B1, nucleoporin, and chromosomes during a breakdown of the nuclear envelope at mitosis of NSCs. Thus, GM1 may hitchhike into the reforming nucleus with nuclear envelope vesicles or with chromosomes as a passenger. In either case, GM1 can gain access to the chromosomes for modulating transcriptional activity of neurogenic genes, such as GalNAcT and NeuroD1.
Fig. 4.
A model depicting the regulation of glycosyltransferase GalNAcT gene by gangliosides for neuronal differentiation. Nuclear GM1 interacts with acetylated histones (AcH) which are active epigenetic marks. GM1 binds with the GalNAcT and the NeuroD1genes, and GM1 enhances histone acetylation on the promoters of the GalNAcT as well as on the NeuroD1 genes in differentiated neurons. These associations of GM1 and these genes were found in differentiated neurons, but not in undifferentiated NSCs. The interaction of GM1 and the GalNAcT gene promoter occurs in a differentiation stage-specific manner. At a later differentiation stage, the nuclear GM1-lipid domains may serve as a docking site at the nuclear periphery for specific active chromatins for neuronal differentiation
Conclusion and future studies
Although recent evidence of GSLs, including gangliosides, has shed light on their roles in modulating signaling pathways during cellular differentiation and reprograming, mice deficient in some of these molecules show only subtle phenotypic abnormalities compared with the wild-type animals in early development. Clearly, the biological function of one glycoconjugate can be substituted by another, albeit with less efficiency. However, the aberrant ganglioside expression becomes progressively more serious in adult stage and pathogenic conditions. The “biological redundancy” can be considered for more important functions of these molecules. Epigenetic regulation is an important regulatory mechanism for controlling the expression pattern of gangliosides during cellular differentiation and brain development. Most interestingly, our data suggest that nuclear gangliosides themselves can modulate epigenetic gene expression, presumably by a feed-back mechanism. It would be interesting if different gangliosides can modulate a certain gene expression during different stages of development. Ganglioside expression profiles and glycogene expression patterns are associated not only with CNS development but also with pathogenic mechanisms of neurodegenerative diseases in CNS, such as Alzheimer’s disease [44–46], Parkinson disease [47], Huntington’s disease [48], amyotrophic lateral sclerosis [49, 50], and multiple sclerosis [51, 52]. In addition, the aberrant and elevated expression of gangliosides has been also observed in many types of cancer cells, thereby promoting tumor survival. Epigenetic regulations for the ganglioside expression, and vice versa, should provide clues underlying the pathogenic mechanisms, which are useful in developing novel strategies for disease treatment and tissue damage repair. Futures studies will prove fruitful in this regard.
Acknowledgments
This work was supported in part by a VA Merit Review Award (1 IO1BX001388 to RKY), NIH grants (RO1 NS26994 and RO1 NS11853 to RKY) and Mizutani Foundation for Glycoscience (150026 to YI).
Footnotes
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Yu RK, Macala LJ, Taki T, Weinfield HM, Yu FS: Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J. Neurochem 50(6), 1825–1829 (1988) [DOI] [PubMed] [Google Scholar]
- 2.Yu RK, Itokazu Y: Glycolipid and glycoprotein expression during neural development. Advances in neurobiology. 9, 185–222 (2014). doi:10.1007/978-1-4939-1154-7_9 [DOI] [PubMed] [Google Scholar]
- 3.Yu RK, Tsai YT, Ariga T, Yanagisawa M: Structures, biosyn-thesis, and functions of gangliosides–an overview. Journal of oleo science. 60(10), 537–544 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakatani Y, Yanagisawa M, Suzuki Y, Yu RK: Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology. 20(1), 78–86 (2010). doi:10.1093/glycob/cwp149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Yu RK: Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc. Natl. Acad. Sci. U. S. A 110(47), 19137–19142 (2013). doi:10.1073/pnas.1307224110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa K, Ohmi Y, Ohkawa Y, Tajima O, Furukawa K: Glycosphingolipids in the regulation of the nervous system. Advances in neurobiology. 9, 307–320 (2014). doi:10.1007/978-1-4939-1154-7_14 [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Cheng A, Wakade C, Yu RK: Ganglioside GD3 is required for neurogenesis and long-term maintenance of neural stem cells in the postnatal mouse brain. The journal of neuroscience: the official journal of the society for. Neuroscience. 34(41), 13790–13800 (2014). doi:10.1523/JNEUROSCI.2275-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ledeen RW, Wu G: The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci 40(7), 407–418 (2015). doi:10.1016/j.tibs.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 9.Tsai YT, Yu RK: Epigenetic activation of mouse ganglioside synthase genes: implications for neurogenesis. J. Neurochem 128(1), 101–110 (2014). doi:10.1111/jnc.12456 [DOI] [PubMed] [Google Scholar]
- 10.Hirabayashi Y, Gotoh Y: Epigenetic control of neural precursor cell fate during development. Nat Rev Neurosci 11(6), 377–388 (2010). doi:10.1038/nrn2810 [DOI] [PubMed] [Google Scholar]
- 11.Jobe EM, McQuate AL, Zhao X: Crosstalk among Epigenetic Pathways Regulates Neurogenesis. Frontiers in neuroscience. 6, 59 (2012). doi:10.3389/fnins.2012.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh J, Gage FH: Epigenetic control of neural stem cell fate. Curr Opin Genet Dev 14(5), 461–469 (2004). doi:10.1016/j.gde.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 13.Mehler MF: Epigenetics and the nervous system. Ann. Neurol 64(6), 602–617 (2008). doi:10.1002/ana.21595 [DOI] [PubMed] [Google Scholar]
- 14.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH: Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. U. S.A 101(47), 16659–16664 (2004). doi:10.1073/pnas.0407643101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M: Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 325(5942), 834–840 (2009). doi:10.1126/science.1175371 [DOI] [PubMed] [Google Scholar]
- 16.Jamaladdin S, Kelly RD, O’Regan L, Dovey OM, Hodson GE, Millard CJ, Portolano N, Fry AM, Schwabe JW, Cowley SM: Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A 111(27), 9840–9845 (2014). doi:10.1073/pnas.1321330111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson PJ, Fairall L, Santos GM, Schwabe JW: Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature. 481(7381), 335–340 (2012). doi:10.1038/nature10728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millard CJ, Watson PJ, Celardo I, Gordiyenko Y, Cowley SM, Robinson CV, Fairall L, Schwabe JW: Class I HDACs share a common mechanism of regulation by inositol phosphates. Mol. Cell 51(1), 57–67 (2013). doi:10.1016/j.molcel.2013.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozcan S, Andrali SS, Cantrell JE: Modulation of transcription factor function by O-GlcNAc modification. Biochim. Biophys. Acta 1799(5–6), 353–364 (2010). doi:10.1016/j.bbagrm.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardiville S, Hart GW: Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab 20(2), 208–213 (2014). doi:10.1016/j.cmet.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis BA, Hanover JA: O-GlcNAc and the epigenetic regulation of gene expression. The Journal of biological chemistry. 289(50), 34440–34448 (2014). doi:10.1074/jbc.R114.595439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki Y, Yanagisawa M, Ariga T, Yu RK: Histone acetylation-mediated glycosyltransferase gene regulation in mouse brain during development. J. Neurochem 116(5), 874–880 (2011). doi:10.1111/j.1471-4159.2010.07042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai YT, Itokazu Y, Yu RK: GM1 Ganglioside is Involved in Epigenetic Activation Loci of Neuronal Cells. Neurochemical research. 41(1–2), 107–115 (2016). doi:10.1007/s11064-015-1742-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouvier JD, Seyfried TN: Ganglioside composition of normal and mutant mouse embryos. J. Neurochem 52(2), 460–466 (1989) [DOI] [PubMed] [Google Scholar]
- 25.Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK: Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem 103(6), 2327–2341 (2007). doi:10.1111/j.1471-4159.2007.04910.x [DOI] [PubMed] [Google Scholar]
- 26.Kizuka Y, Kitazume S, Okahara K, Villagra A, Sotomayor EM, Taniguchi N: Epigenetic regulation of a brain-specific glycosyltransferase N-acetylglucosaminyltransferase-IX (GnT-IX) by specific chromatin modifiers. The Journal of biological chemistry. 289(16), 11253–11261 (2014). doi:10.1074/jbc.M114.554311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kizuka Y, Kitazume S, Yoshida M, Taniguchi N: Brain-specific expression of N-acetylglucosaminyltransferase IX (GnT-IX) is regulated by epigenetic histone modifications. The Journal of biological chemistry. 286(36), 31875–31884 (2011). doi:10.1074/jbc.M111.251173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia T, Gao L, Yu RK, Zeng G: Characterization of the promoter and the transcription factors for the mouse UDP-gal:betaGlcNAc beta1,3-galactosyltransferase gene. Gene. 309(2), 117–123 (2003) [DOI] [PubMed] [Google Scholar]
- 29.Xia T, Zeng G, Gao L, Yu RK: Sp1 and AP2 enhance promoter activity of the mouse GM3-synthase gene. Gene. 351, 109–118 (2005). doi:10.1016/j.gene.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 30.Yu RK, Bieberich E, Xia T, Zeng G: Regulation of ganglioside biosynthesis in the nervous system. Journal of lipid research. 45(5), 783–793 (2004). doi:10.1194/jlr.R300020-JLR200 [DOI] [PubMed] [Google Scholar]
- 31.Takashima S, Kono M, Kurosawa N, Yoshida Y, Tachida Y, Inoue M, Kanematsu T, Tsuji S: Genomic organization and transcriptional regulation of the mouse GD3 synthase gene (ST8Sia I): comparison of genomic organization of the mouse sialyltransferase genes. Journal of biochemistry. 128(6), 1033–1043 (2000) [DOI] [PubMed] [Google Scholar]
- 32.Lucki NC, Sewer MB: Nuclear sphingolipid metabolism. Annu Rev Physiol. 74, 131–151 (2012). doi:10.1146/annurev-physiol-020911-153321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Wu G, Miyagi T, Lu ZH, Ledeen RW: Sialidase occurs in both membranes of the nuclear envelope and hydrolyzes endogenous GD1a. J. Neurochem 111(2), 547–554 (2009). doi:10.1111/j.1471-4159.2009.06339.x [DOI] [PubMed] [Google Scholar]
- 34.Saito M, Sugiyama K: Characterization of nuclear gangliosides in rat brain: concentration, composition, and developmental changes. Arch. Biochem. Biophys 398(2), 153–159 (2002). doi:10.1006/abbi.2001.2725 [DOI] [PubMed] [Google Scholar]
- 35.Tempera I, Buchetti B, Lococo E, Gradini R, Mastronardi A, Mascellino MT, Sale P, Mosca L, d’Erme M, Lenti L: GD3 nuclear localization after apoptosis induction in HUT-78 cells. Biochem. Biophys. Res. Commun 368(3), 495–500 (2008). doi:10.1016/j.bbrc.2007.12.196 [DOI] [PubMed] [Google Scholar]
- 36.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S: Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 325(5945), 1254–1257 (2009). doi:10.1126/science.1176709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talamas JA, Capelson M: Nuclear envelope and genome interactions in cell fate. Front. Genet 6, 95 (2015). doi:10.3389/fgene.2015.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu G, Lu ZH, Ledeen RW: GM1 ganglioside in the nuclear membrane modulates nuclear calcium homeostasis during neurite outgrowth. J. Neurochem 65(3), 1419–1422 (1995) [DOI] [PubMed] [Google Scholar]
- 39.Wu G, Lu ZH, Ledeen RW: Induced and spontaneous neuritogenesis are associated with enhanced expression of ganglioside GM1 in the nuclear membrane. The Journal of neuroscience: the official journal of the Society for Neuroscience. 15(5 Pt 2), 3739–3746 (1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie X, Wu G, Lu ZH, Ledeen RW: Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J. Neurochem 81(6), 1185–1195 (2002) [DOI] [PubMed] [Google Scholar]
- 41.Kotzerke J, Stibane C, Dralle H, Wiese H, Burchert W: Screening for pheochromocytoma in the MEN 2 syndrome. Henry Ford Hosp Med J 37(3–4), 129–131 (1989) [PubMed] [Google Scholar]
- 42.Saito M, Hagita H, Ito M, Ando S, Yu RK: Age-dependent reduction in sialidase activity of nuclear membranes from mouse brain. Exp. Gerontol 37(7), 937–941 (2002) [DOI] [PubMed] [Google Scholar]
- 43.Saito M, Fronda CL, Yu RK: Sialidase activity in nuclear membranes of rat brain. J. Neurochem 66(5), 2205–2208 (1996) [DOI] [PubMed] [Google Scholar]
- 44.Ariga T, Itokazu Y, McDonald MP, Hirabayashi Y, Ando S, Yu RK: Brain gangliosides of a transgenic mouse model of Alzheimer’s disease with deficiency in GD3-synthase: expression of elevated levels of a cholinergic-specific ganglioside, GT1aalpha. ASN Neuro 5(2), 141–148 (2013). doi:10.1042/AN20130006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ariga T, Wakade C, Yu RK: The pathological roles of ganglioside metabolism in Alzheimer’s disease: effects of gangliosides on neurogenesis. Int. J. Alzheimers Dis 2011, 193618 (2011). doi:10.4061/2011/193618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itokazu Y, Yu RK: Amyloid beta-peptide 1–42 modulates the proliferation of mouse neural stem cells: Upregulation of Fucosyltransferase IX and notch signaling. Mol. Neurobiol (2014). doi:10.1007/s12035-014-8634-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu G, Lu ZH, Kulkarni N, Ledeen RW: Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans.J. Neurosci. Res 90(10), 1997–2008 (2012). doi:10.1002/jnr.23090 [DOI] [PubMed] [Google Scholar]
- 48.Maglione V, Marchi P, Di Pardo A, Lingrell S, Horkey M, Tidmarsh E, Sipione S: Impaired ganglioside metabolism in Huntington’s disease and neuroprotective role of GM1. The journal of neuroscience: the official journal of the society for. Neuroscience. 30(11), 4072–4080 (2010). doi:10.1523/JNEUROSCI.6348-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rapport MM: Implications of altered brain ganglioside profiles in amyotrophic lateral sclerosis (ALS. Acta Neurobiol. Exp. (Wars). 50(4–5), 505–513 (1990) [PubMed] [Google Scholar]
- 50.Rapport MM, Donnenfeld H, Brunner W, Hungund B, BartfeldH.: Ganglioside patterns in amyotrophic lateral sclerosis brain regions. Ann. Neurol 18(1), 60–67 (1985). doi:10.1002/ana.410180111 [DOI] [PubMed] [Google Scholar]
- 51.Yu RK, Ledeen RW, Eng LF: Ganglioside abnormalities in multiple sclerosis. J. Neurochem 23(1), 169–174 (1974) [DOI] [PubMed] [Google Scholar]
- 52.Yu RK, Ueno K, Glaser GH, Tourtellotte WW: Lipid and protein alterations of spinal cord and cord myelin of multiple sclerosis.J. Neurochem 39(2), 464–477 (1982) [DOI] [PubMed] [Google Scholar]