Abstract
BACKGROUND:
Exposure to anesthetics is common in the majority of early survivors of life-threatening injuries. Whether and to what degree general anesthetics influence outcomes from major trauma is unknown. Potential confounding effects of general anesthetics on outcome measures are usually disregarded. We hypothesized that exposure to isoflurane or sevoflurane modulates the outcome from blunt trauma with brain injury (bTBI).
METHODS:
We tested the hypothesis in a novel model of bTBI implemented in Drosophila melanogaster. Fruit flies of the standard laboratory strain w1118 were cultured under standard conditions. We titrated the severity of bTBI to a mortality at 24 hrs (MI24) of approximately 20% under control conditions. We administered standard doses of isoflurane and sevoflurane before, before and during, or after bTBI and measured the resulting MI24. We report the MI24 as mean±SD.
RESULTS:
Isoflurane or sevoflurane administered for two hours before bTBI reduced the MI24 from 22.3±2.6 to 10.4±1.8 (p<10−9, n=12) and from 19.3±0.9 to 8.9±1.1 (p<0.0001, n=8), respectively. In contrast, administration of isoflurane after bTBI increased the MI24 from 18.5±4.3 to 25.3±9.1%, (p=0.0026, n=22) while sevoflurane had no effect (22.4±7.1 and 21.5± 5.8, n=22).
CONCLUSIONS:
In a whole animal model of bTBI, general anesthetics were not indifferent with respect to early mortality. Therefore, collateral effects of general anesthetics should be considered in the interpretation of results obtained in vertebrate trauma models. Invertebrate model organisms can serve as a productive platform to interrogate anesthetic targets that mediate collateral effects and to inform trauma research in higher organisms about the potential impact of anesthetics on outcomes.
INTRODUCTION
The response to life-threatening injury activates cellular signaling cascades that trigger organism-wide, tightly regulated immune and inflammatory responses to limit damage and initiate repair.1 Research aimed at predicting outcome from severe trauma strongly indicates a role for genetic background in molding the trauma-coping mechanisms.2 However, linking genomic patterns to outcome from trauma has proven difficult in mammalian models3 and in humans.4 Leveraging the experimental flexibility of invertebrate model organisms may be a useful strategy to achieve a better understanding of the genetic influence on trauma outcome.
Although humans and Drosophila melanogaster (fruit flies) do not look very similar, evolutionary conservation has allowed findings initially made in flies to lead to clinically important discoveries in humans. These include how Hox genes control development of the human body plan, how Toll pathways mediate the human innate immune response, how the period and clock genes coordinate circadian rhythms, how chromatin-based mechanisms regulate epigenetic inheritance in humans, and how Wnt, Notch, and Ras signal transduction pathways contribute to cancer in humans (for overview 5). Furthermore, results from flies translate to humans even when they were derived from studies of tissues that have no direct counterpart in humans.
We have shown that injuries induced by contact and inertial forces in fruit flies mimic characteristics of blunt trauma with associated traumatic brain injury (bTBI) in mammals.6 Furthermore, we have shown that naturally occurring genetic polymorphisms substantially modulate the resilience to bTBI.7 We and others have also demonstrated that cardinal pharmacodynamic and pharmacokinetic characteristics of the volatile general anesthetics (VGAs) isoflurane (ISO) and sevoflurane (SEVO) are conserved between flies and humans.8 Here we used flies to investigate the influence of exposure to VGAs on early mortality after bTBI because limited animal welfare concerns make it possible to isolate the effects of anesthesia in the context of bTBI. Future experiments will explore the genetic and genomic modifiers of VGA-bTBI interaction using the rich genetic toolbox available for fruit flies. The goal of this exploratory study was to test the hypothesis that VGAs modulate mortality from bTBI. We used the Mortality Index 24 hours after bTBI (MI24) as our primary endpoint and found differences between ISO and SEVO. These findings are relevant for the interpretation of experimental work in trauma models that include anesthesia and suggests the existence of collateral effects of anesthesia in the heretofore unexamined context of blunt trauma.
METHODS
This manuscript adheres to the applicable ARRIVE reporting guidelines (preclinical animal research).
Approval from Institutional Animal Care and Use Committee has been waived.
Fly Lines and Culturing
All experiments used 0–7 or 1–8 day old w1118 flies. All flies were maintained on molasses food at 25°C, as described in Katzenberger et al., 2016.6
Blunt Trauma With Associated Traumatic Brain Injury (bTBI)
bTBI was inflicted with a High-Impact Trauma (HIT) device operated following standardized protocols6,7,9 (for a visual demonstration see Katzenberger et al.7). Eight vials were used to simultaneously expose two experimental conditions (Fig. 1A), with each condition represented by four vials of 60 flies each. Results from two experimental or two control samples (e.g., vials 1 and 2 or 5 and 6, respectively) were averaged and considered a single replicate, so n=2 for the experiment illustrated in Figure 1A. The standard bTBI protocol took 20 min and consisted of four strikes from the HIT device with 5 min recovery between strikes and was administered either before, during or after exposure to anesthetics as illustrated in Fig. 1B (long bTBI rectangles). To maintain exposure to VGAs during the co-exposure condition, foam plugs were used to contain flies in vials during the bTBI protocol. In the pre-exposure condition, to passively eliminate VGAs, which diffuse freely, cotton balls were used to contain flies in vials during the bTBI protocol. To maintain exposure to VGAs when bTBI was being inflicted in the 15 min co-exposure condition, four strikes from the HIT device were administered in quick succession within less than 2.5 min (Fig. 1B, short bTBI rectangles). We have previously shown that the MI24 does not differ between the 20 min and 2.5 min bTBI protocols.6 Two HIT devices were used that produced bTBI of slightly different severities under the same bTBI protocol; one device was used for Figures 2–4 and another device was used for Figure 5.
Figure 1.
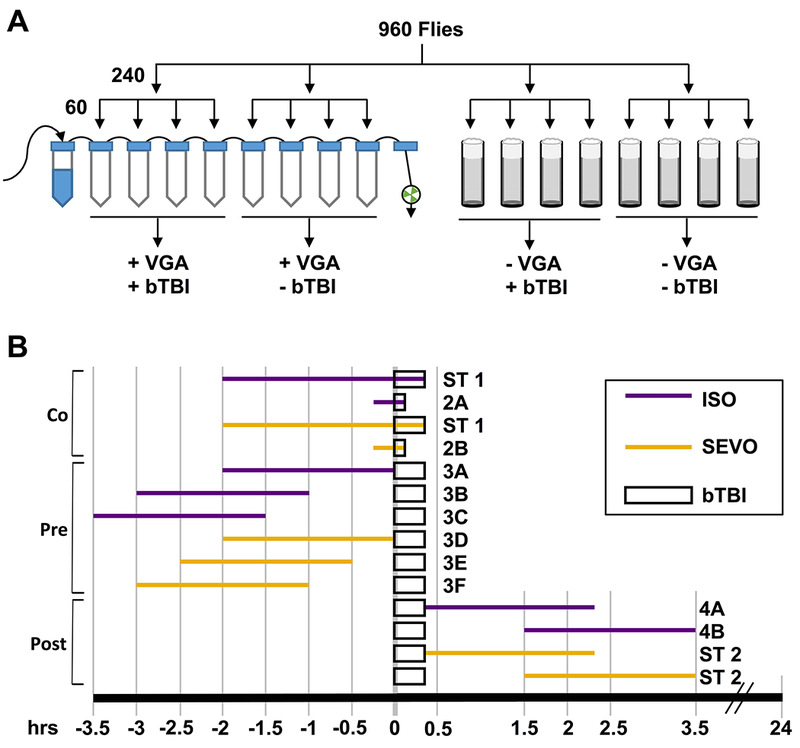
Diagrams that explain the workflow for experiments presented in Figures 2–4. (A) A group of mixed-sex, 1–8 day old w1118 flies was equally divided for examination under four conditions: anesthetic and trauma (+VGA +bTBI), anesthetic and no trauma (+VGA −bTBI), no trauma without anesthesia (−VGA +bTBI), and no anesthetic and no trauma (−VGA −bTBI). The +VGA −bTBI and −VGA −bTBI samples served to determine the percent mortality without trauma and were subtracted from the percent mortality of the +VGA +bTBI and −VGA +bTBI samples, respectively, to calculate the Mortality Index at 24hrs (MI24) values. (B) Timelines of the co- (Co), pre- (Pre) and post- (Post) exposures to anesthetics relative to the infliction of either the standard bTBI protocol or the rapid bTBI protocol (long and short rectangles, respectively). Purple and yellow lines indicate the duration (short: 15 min, long 2 hrs) and timing of exposure to 2% ISO or 3.5% SEVO, respectively. Lettering at the end of each line indicates the figure panel showing the results. ST indicates that results are presented only in the Supplemental Table. Timelines are drawn to scale in hrs and the MI24 was determined 24 hrs after initiation of the bTBI protocol.
Figure 2.
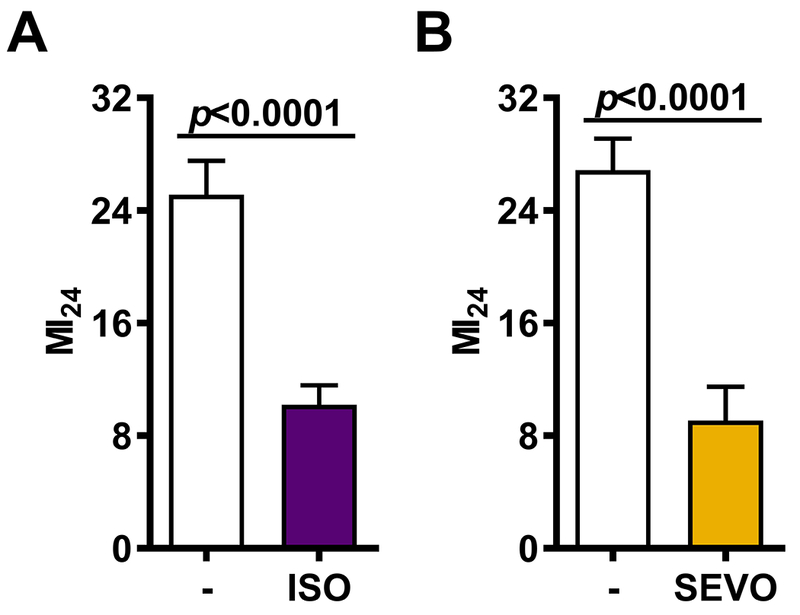
Co-exposure to volatile general anesthetics (VGAs) reduces the risk of mortality following blunt trauma with traumatic brain injury (bTBI). The MI24 was determined for mixed-sex, 0-7 or 1-8 day old w1118 flies either not exposed to anesthetic (−) or exposed to ISO (A) or SEVO (B) for 15 min prior to and during bTBI. Error bars represent the 95% Confidence Intervals.
Figure 4.
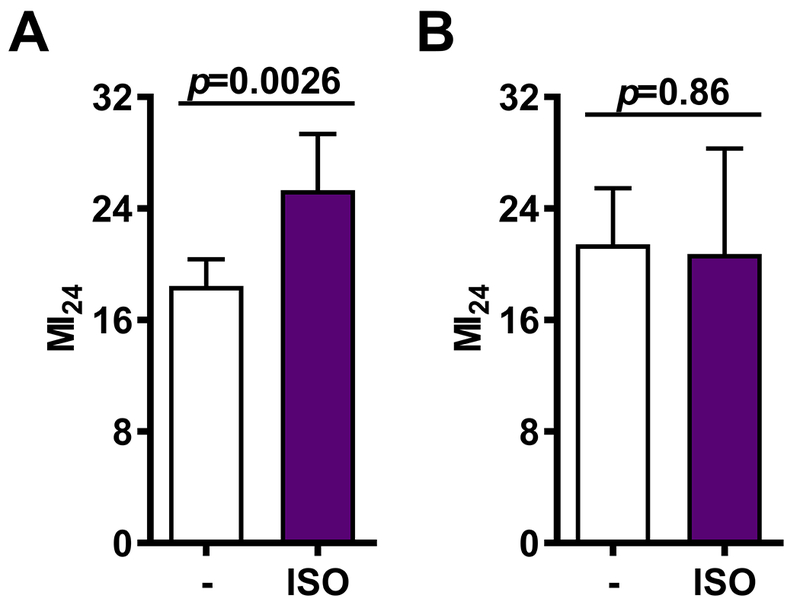
Exposure to ISO shortly after bTBI increases the risk of mortality due to bTBI. The MI24 was determined for mixed-sex, 0-7 or 1-8 day old w1118 flies that received the standard bTBI protocol and were either not exposed to anesthetic (−) or exposed to ISO for 2 hrs immediately afterward (A, P=0.003) or 1.5 hrs later (B, P=0.86). Error bars represent 95% Confidence intervals. SEVO had no effect (Supplementary Table 2)
Figure 5.
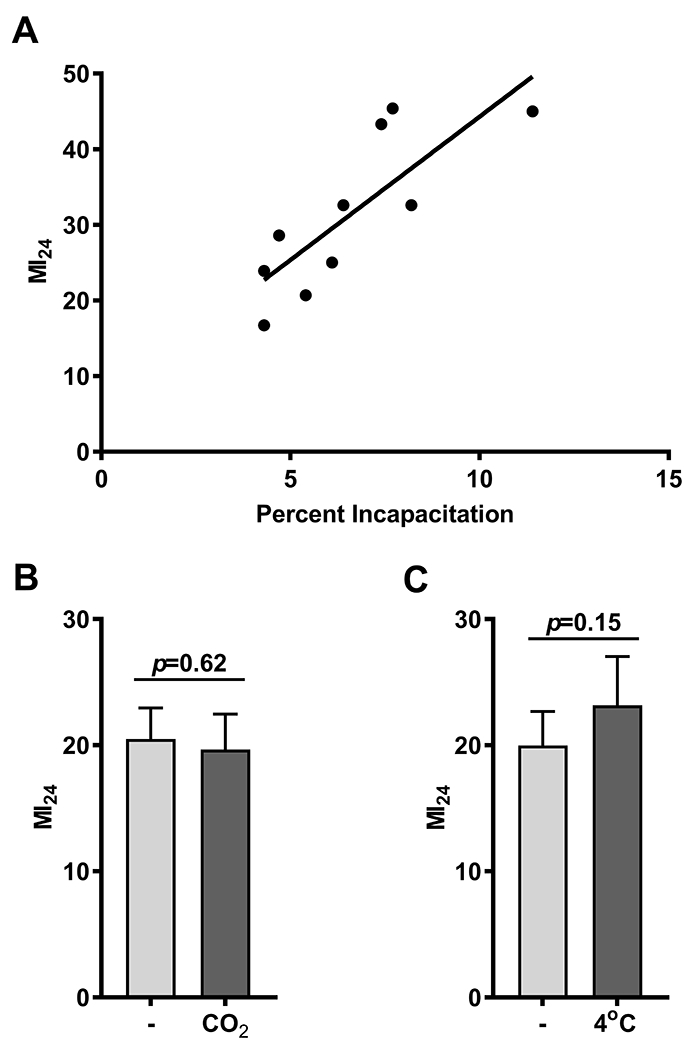
The risk of mortality following bTBI is correlated with the risk of incapacitation following bTBI and is not affected by mobility at the time of bTBI. (A) The MI24 following the standard bTBI protocol versus the percent incapacitation following one strike from the HIT device for 10 mixed-sex strains (w1118 and nine RAL lines) at 0-7 days old. Pearson correlation coefficient 0.80, 95% Confidence Interval 0.355 - 0.952, R2 = 0.6476. (B) The MI24 of mixed-sex, 0-7 day old w1118 flies either mobile (−) or immobile due to exposure to CO2 (CO2) at the time of bTBI. No difference between the MI24s of immobilized and mobile flies (20.5±3.4 and 19.7±3.9, mean± SD). (C) The MI24 of mixed-sex, 0–7 day old w1118 flies either mobile (−) or immobile due to exposure to low temperature (4°C) at the time of bTBI. The MI24s of immobilized and mobile flies are not different (20±3.8 and 23.2±5.4, mean± SD).
Experiments in Figure 5 used 0–7 day old mixed-sex fly lines from the Drosophila melanogaster Genetic Reference Panel (DGRP) (RAL lines 161, 352, 381, 409, 427, 439, 774, 892, and 897) and w1118, a standard laboratory strain of Drosophila melanogaster.10
For the primary outcome measure of mortality following bTBI, dead flies were counted 24 hrs after bTBI. We defined the Mortality Index at 24 hrs (MI24) as the percentage of flies that died within 24 hrs following bTBI minus the percentage of matching uninjured flies that died within the same 24 hr period. The overall average mortalities for uninjured flies were very low: 0.71±0.11% for flies not exposed to anesthetic, 0.84±0.13% for flies exposed to ISO, and 0.86±0.1% for flies exposed to SEVO.
Incapacitation
After a single strike from the HIT device, a fraction of flies were immobilized for varying periods of time. We defined those that remained immobile for a minimum of 1 min and regained mobility (most within 5 min) as ‘incapacitated’. (6 Supplemental Movie 1 and Fig. 5A).
VGA Administration
ISO and SEVO were delivered in air into the Serial Anesthesia Array (SAA), as described previously.11 In brief, commercial, agent-specific vaporizers and a custom-made SAA were used to ensure rapid administration of equal doses to all eight vials, resulting in equivalent exposure to anesthetics in all vials (Fig. 1A).
Analogous to the commonly used quantification of anesthetic exposure in MAC-hours, we define ‘dose’ as the product of agent concentration in air (in v/v%) and exposure duration (in hrs), i.e., %hr. We examined three anesthetic regimens: pre-exposure (VGA administration discontinued prior to bTBI), co-exposure (VGA present before and during bTBI), and post-exposure (VGA administered after bTBI).
Non-VGA Immobilization
Groups of 60, mixed-sex, 0–7 day old w1118 flies were immobilized with either CO2 or exposure to cold (i.e., a water-ice bath at 4°C) and subjected them to the standard bTBI protocol while they were immobile. Immobility was maintained throughout the experiment by re-exposure to CO2 or water-ice between each of four strikes from the HIT device.
Statistical Analysis
The principal outcome measure is the MI24. We tested the hypothesis that ISO and SEVO have an effect on MI24. We measured the MI24 under control conditions and after application of ISO or SEVO at different time points and for different durations. The principal null hypothesis is that ISO and SEVO have no effect on the MI24. Because control MI24s were normally distributed and we had no a priori assumptions about the effect of our intervention, we tested the hypothesis by comparing the control MI24 with the MI24 after drug exposure using two-tailed, unpaired t-tests with the significance level set at p = 0.05. We considered an effect size of 25%, in either direction, as biologically significant.
Sample size justification: in previously published work comparing the MI24 between different fly strains, we found that an n of 8 was sufficient to reject the null hypothesis (no difference between two strains) with a power of 95% with a population mean MI24 of 25±4% and an effect size (i.e. difference in MI24) of 20%. Therefore, we set the minimum number of replicates for rejecting the null hypothesis of no difference between anesthetic-exposed and -unexposed flies to eight. However, we used a higher number of replicates in experiments when the sample SD was higher to reduce the likelihood of falsely accepting the null hypothesis.
To test whether secondary events that lead to incapacitation also lead to mortality, we determined the incapacitation fraction and the MI24 after bTBI in separate experiments in 10 different strains. For incapacitation and the MI24, each data point in Figure 5A is the mean of eight replicates. The results for incapacitation are also based on eight independent experiments, each with three vials of 20 flies (480 flies total for each experiment). For the MI24, we report the mean values from eight independent experiments, each consisting of one vial with 60 flies. The lower number of flies per vial for incapacitation was necessary for accurate scoring. We used the Pearson correlation coefficient and report the 95% confidence interval.
Confounding effects (Figs. 5B and C): We tested the hypothesis that immobilization by CO2 or cold reduces the MI24.
We calculated the Risk Ratio for death comparing anesthetic-unexposed to exposed flies as described by Viera 12 and report it, together with detailed numerical results, in Supplementary Tables 1–3.
Unless otherwise stated, unpaired t-tests were used to test for differences between mean values. We set the statistical significance criterion at p < 0.05. Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for graphing.
RESULTS
ISO and SEVO Present During bTBI Reduce Mortality
In vivo trauma models typically include exposure to anesthetics to instrument the preparation prior to the administration of trauma and, in most vertebrate models, during trauma as well. In fact, even a brief exposure to ISO, e.g., for the purpose of euthanasia, influenced mRNA expression in both healthy mice and after TBI.13 We examined whether exposure to ISO or SEVO at various time points before, during, or after the time of bTBI influenced mortality in 1–8 day old w1118 flies (Fig. 1B) Co-exposure to ISO and SEVO for only 15 min reduced the MI24 by 59.4% from 25.3±2.9% to 10.2±1.6% (p<10−7) and by 68.5% from 26.9±3.1% to 9.1±3.4% (p<10−9), respectively (Figs. 2A and B and Supplementary Table 1). Longer exposures prior to bTBI to either 4 %hr ISO or 7 %hr SEVO did not further reduce the MI24: 53.5% and 53.9% reduction of MI24 (from 22.3±0.8 to 10.4±0.5; p < 10−10 and from 19.3% to 8.9% p < 10−5) for ISO and SEVO, respectively (Supplementary Table 1).
We conclude that both ISO and SEVO exert protective effects in the context of bTBI that saturate within a short exposure time.
Protection by ISO Outlasts SEVO
To test whether the protective effect of VGAs is contingent on the presence of high concentrations of the agents during trauma, we discontinued anesthesia for various time intervals prior to the administration of bTBI. We chose the time intervals based on our previous finding that 50 minutes after 4 %hr of ISO w1118 flies are behaviorally indistinguishable from non-anesthetized controls.8 Flies reach the same degree of recovery about twice as fast after SEVO anesthesia of equal depth and duration.11 Hence, bTBI was administered immediately after pre-exposures to both VGAs and at intervals of 60 and 90 minutes after 4 %hr of ISO as well as 30 and 60 min after 7 %hr of SEVO (Fig. 3, Supplementary Table 1).
Figure 3.
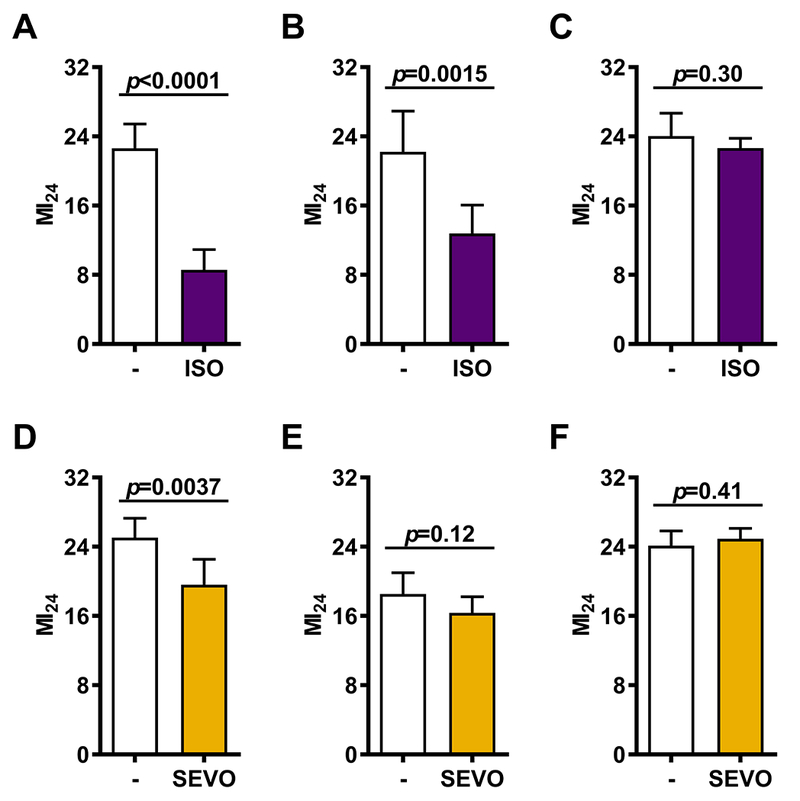
The time between pre-exposure to VGAs and infliction of bTBI differentially affects the ability of ISO and SEVO to reduce the risk of mortality following bTBI. The MI24 was determined for mixed-sex, 0-7 or 1-8 day old w1118 flies either not exposed to anesthetic (−) or exposed to ISO for 2 hrs prior to infliction of the standard bTBI protocol immediately (A), 1 hr later (B) or 1.5 hrs later (C). Alternatively, the MI24 was determined for mixed-sex, 0-7 or 1-8 day old w1118 flies either not exposed to anesthetic (−) or exposed to SEVO for 2 hrs prior to infliction of the standard bTBI protocol immediately (D), 0.5 hrs later (E), or 1 hr later (F). Error bars represent the 95% Confidence Intervals.
Initiation of the standard bTBI protocol within 5 min after terminating anesthesia resulted in a differential reduction of the MI24: 62.1% (from 22.6±3.9 to 8.6±3.3; Fig. 3A) for ISO and 21.7% (from 25.0±5.0 to 19.6±6.6, Fig. 3D) for SEVO. Increasing the time interval between anesthesia and bTBI widened the differential effects of ISO and SEVO. For ISO, a significant reduction of the MI24 was still detectable after an interval of 60 but not 90 min (Figs. 3B and C). By contrast, for SEVO, no reduction of the MI24 was detectable after intervals of either 30 or 60 min (Figs. 3E and F). We conclude that both ISO and SEVO reduce early mortality after bTBI, that this effect is time-limited and may be contingent on the persistence of low VGA concentrations in the fly body. However, the effect of ISO persisted for a longer duration than that of SEVO, even after accounting for its slower elimination.
ISO Administered Shortly After bTBI Increases Mortality
VGA administration after ischemia/reperfusion injury modulates the extent of tissue damage in mammalian models. This effect may be agent- and tissue-dependent,14–16 possibly less robust than pre-conditioning17 and has been studied in less detail than pre-conditioning. We examined whether exposure to VGAs after bTBI (post-exposure) might influence the MI24. We found that when the standard bTBI protocol was immediately followed (i.e., within 5 min) by 4 %hr ISO, the MI24 increased by 36.8% from 18.5±4.4 to 25.3±9.2 (CI 16.6 to 20.4 and 21.3 to 29.3; p=0.0026, Fig. 4A and Supplementary Table 2). However, when ISO anesthesia was delayed by 90 min, the MI24 did not differ between unexposed and exposed flies (22.4±7.1% and 21.5±5.8%, Fig. 4B and Supplementary Table 2). Neither immediate post-exposure to 7 %hr SEVO nor exposure delayed by 90 min affected the MI24 (Supplementary Table 2). We conclude that the effects of VGAs on the risk of mortality following bTBI are agent-specific and fade within a limited time.
Mortality May be Due to Brain Injury
To investigate the extent to which temporary incapacitation, a manifestation of brain injury, correlates with acute mortality after bTBI, we determined the percent incapacitation of 10 fly lines: w1118 (a standard laboratory strain) and nine wild-type, inbred strains (RAL lines) from the Drosophila melanogaster Genetic Reference Panel (DGRP) that had different MI24s ranging from 16.7 to 45.4 when injured at 0–7 days old. We found that the percent incapacitation was positively correlated with the MI24 (r=0.80, 95% CI 0.355 – 0.952) (Fig. 5A), suggesting that nervous system-based mechanisms leading to temporary concussion-like incapacitation also lead to mortality following bTBI.
Immobility and Intrinsic Toxicity do not Confound the VGA-bTBI Data
To investigate whether immobility at the time of bTBI affects mortality in the absence of anesthetics, we determined the MI24 of 0–7 day old w1118 flies immobilized with either CO2 or exposure to cold. We found that flies immobilized by CO2 or cold had the same MI24 as fully mobile flies (Figs. 5B and C, respectively, and Supplementary Table 2). Therefore, immobility by itself does not affect the risk of mortality following bTBI.
Flies are generally somewhat more sensitive to VGAs than mammals. Published anesthetic EC50s for ISO in different strains of Drosophila melanogaster range from 0.2118 to 1.3 v/v%,19 depending on the definition of anesthesia. Using a customized negative geotaxis-based assay, we determined that the EC50s of 1–8 day old w1118 flies were 0.41% and 0.68% for ISO and SEVO, respectively.8 Because VGAs have narrow safety margins in many animals and because it is unknown whether VGAs administered in air can be lethal in flies, we determined the effect of incremental doses of ISO and SEVO on the percent mortality 24 hrs after termination of exposure. We found that exposure of 1–8 day old w1118 flies up to 18 %hr with either agent did not cause mortality (Fig. 6A). The highest ISO dose tested (24 %hr, i.e., 4% ISO for 6 hrs) resulted in 8.6±1.3% mortality, and the highest SEVO dose tested (39 %hr, i.e., 6.5% SEVO for 6 hrs) resulted in 6.5±0.5% mortality. Normalization of the ISO and SEVO data to their respective EC50s that were determined in the same fly strain and at the same age, revealed that ISO and SEVO had equivalent toxicity profiles (Fig. 6B and Supplementary Table 3). We conclude that flies tolerate concentrations of ISO and SEVO in the range of those commonly administered for anesthetic purposes for long time periods without obvious harm. The doses we used to examine VGA effects in bTBI (4 and 7 %hr of ISO and SEVO, respectively) are well below the doses that increase mortality in the absence of trauma.
Figure 6.
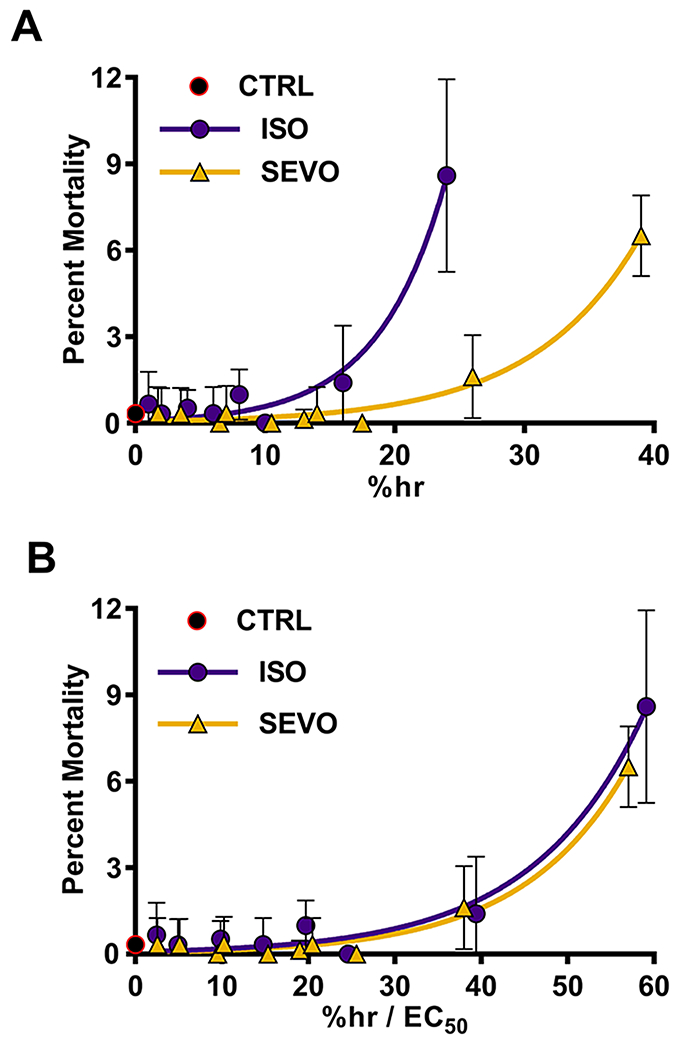
ISO and SEVO are well tolerated in flies. (A) Mixed-sex, 0-7 or 1-8 day old w1118 flies were exposed to different doses (%hr) of ISO or SEVO and the percent of flies that died within 24 hrs (percent mortality) was measured. Flies not exposed to anesthetic (CTRL) were examined to establish the baseline percent mortality. (B) %h values from panel A for ISO and SEVO were normalized to their respective EC50 values (ISO, 0.41±0.03 and SEVO, 0.68±0.05), as determined by Olufs et al.8
DISCUSSION
While virtually every vertebrate trauma model of severe injury involves the use of anesthetic/sedative drugs, potential interactions of anesthetics per se with the response to trauma are rarely the focus of analysis. In light of research conducted over the last two decades, the potential of VGAs to cause a plethora of effects beyond those resulting in the familiar clinical phenotype of anesthesia is undeniable.20–24 Therefore, we specifically investigated the effect of ISO and SEVO on the risk of mortality in a fly model of blunt trauma with concomitant brain injury (bTBI). Trauma in flies clearly does not equal trauma in humans. However, basic molecular and cellular processes triggered by life-threatening tissue destruction are likely to overlap to some degree between flies and mammals. When seen from this perspective, flies offer a model with ‘tractable complexity’ to examine, in an intact organism, the pathobiology of life-threatening injury. Our principal findings demonstrate that, at least in flies, VGAs modulate the outcome from bTBI.
VGAs Differentially Influence 24-hour Mortality
The presence of ISO or SEVO prior to and during bTBI significantly reduced early mortality (Figs. 2 and 3). When either VGA was present during bTBI the reduction in the MI24 was similar between agents for both 15 min and 2 h exposures (Fig. 2 and Supplemental Table 1). Interposition of a time interval between exposure to anesthetic and bTBI, revealed a subtle but gradually increasing difference between ISO and SEVO (Fig. 3). By the time the flies had recovered to pre-anesthetic levels of spontaneous activity (60 min for ISO and 30 min for SEVO),8 only ISO reduced the MI24 (Figs. 3B and E). Increasing the interval between anesthesia and bTBI to 90 min abolished the mortality-reducing effect of ISO (Fig. 3C).
We examined whether immobility during bTBI has a protective effect in the absence of VGAs. Our experiments indicate that immobility alone is neither sufficient (as demonstrated by immobilization using CO2 or exposure to cold, Figs. 5B and C) nor necessary (as demonstrated by the 60 min ISO pre-exposure experiment, Fig. 3B) to reduce mortality. We conclude that the reduction in mortality is due to a ‘pharmacological’ protective effect of ISO and, by extension, of SEVO as well.
What accounts for the longer duration of the protective effect of ISO relative to SEVO (Fig. 3)? The most straightforward explanation is pharmacokinetics and is supported by the equally protective effect of ISO and SEVO in the co-exposure conditions, i.e., when anesthetics were intentionally maintained during trauma administration (Fig. 2). As emergence is faster for SEVO than for ISO,8 a delay between anesthesia and bTBI will result in a higher residual concentration of ISO than of SEVO at the time of trauma and hence more protection (Figs. 3A and D). This purely pharmacokinetic explanation is however, weakened by other pre-exposure experiments: only ISO effectively protected flies that had recovered long enough to become behaviorally indistinguishable from unanesthetized animals, i.e., a recovery of 60 min for ISO and 30 min for SEVO (Figs. 3B and E). These experiments suggest that, whatever the mechanism, ISO may be a more efficient protective agent than SEVO, i.e., in addition to a pharmacokinetic difference there may also be a pharmacodynamic difference between the agents. A pharmacodynamic contribution is supported by our surprising discovery that ISO but not SEVO increased the MI24 when administered after bTBI (Fig. 4 and Supplementary Table 2), an experiment where pharmacokinetic differences should not be of concern. The clear difference between agents in this condition suggests that despite their chemical similarity and the indistinguishable ‘anesthetic’ phenotype, ISO and SEVO have overlapping but not identical cellular/molecular effect profiles. Importantly, this difference is revealed by a change in the physiological context of drug exposure: ‘anesthesia’ was indistinguishable in uninjured and bTBI flies for both ISO and SEVO, but the expression of collateral effects differed dramatically between contexts only for ISO. Therefore, concerns about the potential of VGAs to differentially confound outcome measures in trauma models are justified.
VGAs Differentially Modulate Molecular Effectors of Collateral Effects
The transient receptor potential (TRP) chemosensor family provides instructive examples of how differential modulation of molecular targets by chemically similar VGAs result in collateral effects with clearly different phenotypic presentations. ISO, but not SEVO, activates TRPA1, resulting in enhanced neurogenic inflammation in vivo.25 The TRP family also provides an example of the importance of the biological context for VGA activity. A number of modern VGAs fail to activate the TRPV1 channel directly; however, they sensitize the channel to capsaicin, protons and heat in vitro.26 Under these conditions, ISO has a stronger effect than SEVO. For example, ISO directly activates TRPV1 only after stimulation of protein kinase C and under concomitant application of bradykinin, a situation that might be encountered in the wake of tissue trauma, indicating the importance of the biochemical context for VGA activity.26 Analogous differential effects of ISO and SEVO have been reported for TRPA- and TRPV-mediated calcitonin gene-related peptide (CGRP) release from an ex vivo trachea model.27 A further example of differing results of exposure to VGAs is provided by the differential effect of ISO, SEVO and desflurane on cyclophilin-modulated mitochondrial H2O2 production 28 and the different phenotypes of ISO and desflurane on mitochondrial function and learning and memory.29
Anesthetic Modulation of Trauma
The most detailed experiments to date that are relevant to the present study were conducted in rodents using the controlled cortical impact (CCI) TBI model. Statler et al.30 compared seven anesthetic / sedative drugs (including ISO) administered for one hour after CCI to a control group without post-CCI anesthesia. None of the anesthetic regimens improved any outcome measure when compared to anesthesia-free recovery from CCI, but amongst the tested anesthetics, post-CCI exposure to ISO resulted in better neuronal survival in the hippocampus than exposure to ketamine. By 5 to 16 days after CCI, no differences in behavioral measures were detectable among the treatment groups. Notably, CCI was administered under ISO anesthesia in all groups. This early exposure to ISO before and during CCI may have confounded any intrinsic differences between the subsequently administered anesthetics. This possibility is supported by the work of Luh et al.,31 who exposed mice to ISO, SEVO or a combination of midazolam, even brief exposures to anesthetics modulate certain short-term outcome measures of CCI. Our model differs from those cited above in that the brain injury is but one component of the blunt polytrauma. What is the evidence that brain injury plays a decisive role fentanyl and medetomidine (and their antagonists to reverse anesthesia) during the time required to prepare for and inflict CCI (analogous to our co-exposure protocol). In contrast to Statler et al., only short-term outcomes were assessed (maximum 24 hours). At this point, the lesion volume was smaller and the neurological function was better in the ISO group, indicating that for mortality? We believe that the most parsimonious explanation of the pathogenesis of incapacitation following contact- and inertia-induced trauma is a concussion-like brain injury. The correlation between incapacitation and the MI24 across ten genotypes (Fig. 5A) as well as the findings of progressive neurodegeneration and shortened lifespan in the absence of overt injury to other organs6 suggest that brain injury not only occurs in our model but is also an important contributor to mortality.
Drosophila melanogaster as a Model for Discovery and Analysis of Collateral Anesthetic Effects in the Context of Trauma
Our results confirm that VGAs can profoundly affect the response of an organism to blunt trauma and that the effect of VGAs is context-dependent, i.e., it differs between naïve and traumatized organisms. Among the reasons contributing to the paucity of data in this area are, on one hand the public’s concerns about and regulatory agencies’ requirements for humane treatment of laboratory animals and, on the other hand, the lack of awareness among trauma researchers of the broad spectrum of biological activities of VGAs beyond ‘anesthesia’ per se. The delayed manifestation of such collateral effects (as suggested by findings in neurodevelopment and oncology) would require longer-term follow-up for either confirmation or refutation, further complicating the logistics and adding substantially to the cost of experiments.
Together with our previous demonstration of the reproducibility of key pharmacokinetic and pharmacodynamic properties of VGAs in flies,8 these data indicate that flies can be used as relevant model organisms in trauma research: collections of inbred, near isogenic fly lines 10,32 can be used for unbiased phenotype to genotype screens of wildtype genomes, while collections of deletions covering virtually the whole genome33 and a wide selection of mutants are available for genotype-to-phenotype screening. Future research may identify specific risk-conferring genetic variants, which then be translated for research in higher animals.
In summary, we found that VGAs differentially modulate early mortality in an invertebrate model of blunt trauma.
Supplementary Material
Supplementary movie 1. Recovery from Incapacitation. A fly lying motionless supine on the bottom of the ‘TBI’ vial (arrow) recovers mobility within a short period of time at the end of the observation period. Videographer Zach Olufs, participants: 40 w1118 flies, 15 MB, 40-fold speed, 9 seconds.
KEY POINTS.
Question: Do volatile general anesthetics modulate outcome from blunt trauma?
Finding: Isoflurane and sevoflurane differentially affected 24-hour-mortality in a Drosophila melanogaster model of blunt trauma.
Meaning: The use of general anesthetics may affect the consequences of blunt trauma in mammalian experimental models.
ACKOWLEDGEMENTS
We thank Mr. Russ Ward, Clinical Engineer, Department of Clinical Engineering University of Wisconsin Hospital and Clinics and Mark G. Perkins, BS, Department of Anesthesiology, Madison, Wisconsin for expert help with administration systems for volatile anesthetics.
Funding: This work was supported by the UW-Madison Department of Anesthesiology and the National Institutes of Health grant (R21 NS091893).
Footnotes
DISCLOSURES
Name: Julie A. Fischer, BS.
Contribution: This author helped with the acquisition and analysis of the data and drafting of the work.
Attestation: Julie A. Fischer approved this version of the manuscript and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest: The author declares no conflicts of interest.
Name: Zachariah P. G. Olufs, BS.
Contribution: This author helped with the acquisition and analysis of the data and drafting of the work.
Attestation: Zachariah P. G. Olufs approved this version of the manuscript and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest: The author declares no conflicts of interest.
Name: Rebeccah J. Katzenberger, MS.
Contribution: This author helped conduct the study and analyze the data.
Attestation: Rebeccah J. Katzenberger approved this version of the manuscript and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest: The author declares no conflicts of interest.
Name: David A. Wassarman, PhD.
Contribution: This author helped design the study, analyze and interpret the data, and prepare the manuscript.
Attestation: David A. Wassarman approved the final manuscript and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest: The author declares no conflicts of interest.
Name: Misha Perouansky, MD.
Contribution: This author helped design the study, analyze and interpret the data, and prepare the manuscript.
Attestation: Misha Perouansky approved this version of the manuscript and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and is the archival author and the corresponding author.
Conflicts of Interest: The author declares no conflicts of interest.
REFERENCES
- 1.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. [DOI] [PubMed] [Google Scholar]
- 2.De Maio A, Torres MB, Reeves RH. Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock. 2005;23(1):11–17. [DOI] [PubMed] [Google Scholar]
- 3.Al Nimer F, Lindblom R, Strom M, et al. Strain influences on inflammatory pathway activation, cell infiltration and complement cascade after traumatic brain injury in the rat. Brain Behav Immun. 2013;27(1):109–122. [DOI] [PubMed] [Google Scholar]
- 4.Tompkins RG. Genomics of injury: The Glue Grant experience. J Trauma Acute Care Surg. 2015;78(4):671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duronio RJ, O’Farrell PH, Sluder G, Su TT. Sophisticated lessons from simple organisms: appreciating the value of curiosity-driven research. Dis Model Mech. 2017;10(12):1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzenberger RJ, Loewen CA, Wassarman DR, Petersen AJ, Ganetzky B, Wassarman DA. A Drosophila model of closed head traumatic brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(44):E4152–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzenberger RJ, Loewen CA, Bockstruck RT, Woods MA, Ganetzky B, Wassarman DA. A Method to Inflict Closed Head Traumatic Brain Injury in Drosophila. Journal of visualized experiments : JoVE. 2015(100):e52905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olufs ZPG, Loewen C, Ganetzky B, Wassarman DA, Perouansky M. Genetic variability affects absolute and relative potencies and kinetics of the anesthetics isoflurane and sevoflurane in Drosophila melanogaster. Scientific Reports. 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katzenberger RJ, Ganetzky B, Wassarman DA. Age and Diet Affect Genetically Separable Secondary Injuries that Cause Acute Mortality Following Traumatic Brain Injury in Drosophila. G3 (Bethesda). 2016;6(12):4151–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay TF, Richards S, Stone EA, et al. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482(7384):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olufs ZPG, Loewen CA, Ganetzky B, Wassarman DA, Perouansky M. A new Drosophila melanogaster model reveals the influence of genetic variability on volatile anesthetic pharmacology. Scientific Reports. 2017;submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viera AJ. Odds ratios and risk ratios: what’s the difference and why does it matter? South Med J. 2008;101(7):730–734. [DOI] [PubMed] [Google Scholar]
- 13.Staib-Lasarzik I, Kriege O, Timaru-Kast R, et al. Anesthesia for euthanasia influences mRNA expression in healthy mice and after traumatic brain injury. J Neurotrauma. 2014;31(19):1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redel A, Stumpner J, Tischer-Zeitz T, et al. Comparison of isoflurane-, sevoflurane-, and desflurane-induced pre- and postconditioning against myocardial infarction in mice in vivo. Exp Biol Med (Maywood). 2009;234(10):1186–1191. [DOI] [PubMed] [Google Scholar]
- 15.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101(6):1313–1324. [DOI] [PubMed] [Google Scholar]
- 16.Li JT, Wang H, Li W, et al. Anesthetic isoflurane posttreatment attenuates experimental lung injury by inhibiting inflammation and apoptosis. Mediators Inflamm. 2013;2013:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurtrey RJ, Zuo Z. Isoflurane preconditioning and postconditioning in rat hippocampal neurons. Brain Res. 2010;1358:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kottler B, Bao H, Zalucki O, et al. A sleep/wake circuit controls isoflurane sensitivity in Drosophila. Curr Biol. 2013;23(7):594–598. [DOI] [PubMed] [Google Scholar]
- 19.Dawson AG, Heidari P, Gadagkar SR, Murray MJ, Call GB. An airtight approach to the inebriometer: from construction to application with volatile anesthetics. Fly. 2013;7(2):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007;27(6):1108–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jevtovic-Todorovic V, Absalom AR, Blomgren K, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stollings LM, Jia LJ, Tang P, Dou H, Lu B, Xu Y. Immune Modulation by Volatile Anesthetics. Anesthesiology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuki K, Astrof NS, Bracken C, et al. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 2008;22(12):4109–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–1250. [DOI] [PubMed] [Google Scholar]
- 25.Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 2008;105(25):8784–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornett PM, Matta JA, Ahern GP. General anesthetics sensitize the capsaicin receptor transient receptor potential V1. Mol Pharmacol. 2008;74(5):1261–1268. [DOI] [PubMed] [Google Scholar]
- 27.Kichko TI, Niedermirtl F, Leffler A, Reeh PW. Irritant Volatile Anesthetics Induce Neurogenic Inflammation Through TRPA1 and TRPV1 Channels in the Isolated Mouse Trachea. Anesthesia and analgesia. 2015;120(2):467–471. [DOI] [PubMed] [Google Scholar]
- 28.Harisseh R, Chiari P, Villedieu C, et al. Cyclophilin D modulates the cardiac mitochondrial target of isoflurane, sevoflurane and desflurane. J Cardiovasc Pharmacol. 2017. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Xu Z, Wang H, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Annals of neurology. 2012;71(5):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Statler KD, Alexander H, Vagni V, et al. Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. J Neurotrauma. 2006;23(1):97–108. [DOI] [PubMed] [Google Scholar]
- 31.Luh C, Gierth K, Timaru-Kast R, Engelhard K, Werner C, Thal SC. Influence of a brief episode of anesthesia during the induction of experimental brain trauma on secondary brain damage and inflammation. PLoS One. 2011;6(5):e19948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King EG, Macdonald SJ, Long AD. Properties and power of the Drosophila Synthetic Population Resource for the routine dissection of complex traits. Genetics. 2012;191(3):935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook RK, Christensen SJ, Deal JA, et al. The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome Biol. 2012;13(3):R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary movie 1. Recovery from Incapacitation. A fly lying motionless supine on the bottom of the ‘TBI’ vial (arrow) recovers mobility within a short period of time at the end of the observation period. Videographer Zach Olufs, participants: 40 w1118 flies, 15 MB, 40-fold speed, 9 seconds.


