Abstract
Objective
We aimed to determine a comparison between the Quick Sequential Organ Failure Assessment (qSOFA) score and existing Sequential Organ Failure Assessment (SOFA) score when applied to severe sepsis & septic shock patients in the Emergency Department (ED) for prediction of in-hospital mortality in the setting of a tertiary care hospital ED in a low-middle income country.
Method
We conducted a prospective observational cohort study on 760 subjects. The qSOFA, SOFA score and in-hospital mortality were assessed by area under the receiver operating curve (AUROC). We calculated sensitivity and specificity for each score for outcomes at cut-offs of 0.92 and 0.63 for qSOFA and SOFA in Severe Sepsis respectively and 0.89 and 0.63 for qSOFA and SOFA in Septic shock respectively.
Results
In patients with severe sepsis, the AUROC of qSOFA for predicting mortality in subjects was 0.92 (95% CI; 0.89–0.94) with 96% sensitivity and 87% specificity in comparison to the AUROC of SOFA score which was 0.63 (95% CI; 0.55–0.70 with 71% sensitivity and 57% specificity. In patients with septic shock, the AUROC of qSOFA for predicting mortality in subjects was 0.89 (95% CI; 0.85–0.92) with 92% sensitivity and 85% specificity in comparison to the AUROC of SOFA score which was 0.63 (95% CI; 0.55–0.70 with 70% sensitivity and 59% specificity.
Conclusion
Our study concludes that qSOFA score is an effective tool at predicting in hospital mortality in comparison to SOFA score when applied to severe sepsis and septic shock patients in the setting of a tertiary care hospital ED of a low-middle income country however, further studies are needed before application for this purpose.
Keywords: qSOFA, SOFA, Sepsis
1. Introduction
Sepsis is a fatal syndrome with dire consequences.1, 2, 3 It progresses rapidly and delays in its identification and treatment can cause a higher mortality.4,5 Presently, there are many clinical scoring systems that measure the disease severity in septic population.6, 7, 8, 9, 10, 11 Many of these scores are time consuming and require information that is not readily available.
With the introduction of the Severe Inflammatory Response Syndrome (SIRS) criteria in 1991 for rapid bedside identification of sepsis6 to current era where various complex clinical outcome prediction model snow exist, a few of which that are notable to mention such as the Acute Physiology and Chronic Health Evaluation Score,7 the Simplified Acute Physiology Score III,8 the Logistic Organ Dysfunction Score,9 and the Mortality Probability Model III,10 were actually derived and validated in the intensive care unit (ICU) setting. Previous investigations have demonstrated these scores to be inadequate when applied to ED patients.11 The one ED-based scoring system, the Mortality in Emergency Department Sepsis score (MEDS), was designed for ED septic patients12,13 however, it is said to be inaccurate in severely ill patients.14 Previous investigators have determined an association between the organ dysfunction and mortality in ED septic patients.15 The Sequential Organ Failure Assessment (SOFA) score calculates the number and severity of dysfunction in six organ systems (Pulmonary, coagulation, hepatobiliary, cardiovascular, renal, and neurologic).16The Sepsis III definitions have introduced a new diagnostic tool termed the Quick Sequential Organ Failure Assessment (qSOFA) which enables rapid risk stratification of septic patients requiring prolonged ICU stay alongwith in hospital death. Patients having high qSOFA scores need further assessment by the SOFA score.17, 18, 19 The surviving sepsis campaign has suggested qSOFA to be used for prognostication only. Further implementation of this within existing guidelines for sepsis is yet to be seen.20
Our study aims to compare the qSOFA score and existing SOFA score when applied to severe sepsis & septic shock patients in the ED for prediction of in-hospital mortality in the setting of a tertiary care hospital ED in a low-middle income country.
2. Methods
We conducted a prospective observational cohort study in the ED from October to March 2017. The study was approved by the Ethical Review Committee (ERC) of (4328-EM-ERC-16) and informed consent was exempted. We recruited adult patients presenting to the ED, equal to or above 18 years of age and examined by an ED physician for assessment & fulfillment of the clinical criteria of severe sepsis or septic shock as per the guidelines of the Surviving Sepsis Campaign and were subsequently admitted to the hospital. Patients were considered as having severe sepsis when they fulfilled criteria for sepsis along with signs of acute organ dysfunction or hypoperfusion as defined either by sepsis-induced hypotension (systolic blood pressure (SBP) < 90 mm Hg or mean arterial pressure (MAP) < 70 mm Hg or a SBP decrease > 40 mm Hg or less than two standard deviations below normal for age in the absence of other causes of hypotension), serum lactate above upper limits normal, urine output < 0.5 mL/kg/h for more than 2 h despite adequate fluid resuscitation, acute lung injury (ALI) with PaO2/FIO2 < 250 in the absence of pneumonia as infection source, ALI with PaO2/FIO2 < 200 in the presence of pneumonia as infection source, serum creatinine> 2.0 mg/dL (176.8 μmol/L), total bilirubin >2 mg/dL (34.2 μmol/L), platelet count < 100,000 μL or coagulopathy (international normalized ratio > 1.5). Patients were considered having septic shock when they fulfilled criteria for severe sepsis with the presence of hypotension (systolic blood pressure <90 mm Hg) despite adequate fluid resuscitation.21
Patients who were below 18 years of age, pregnant, dead on arrival to the ED, suffered multiple trauma injuries, underwent major surgery in previous 30 days before ED arrival or had preexisting do-not- resuscitate orders were excluded. A sample size of 1267 subjects was calculated after achieving 80% power to detect a difference of −0.130 between two diagnostic tests whose sensitivities are 0.550 and 0.680. This procedure used a two-sided McNemar test with a significance level of 0.05. The prevalence of disease in the population is 0.090. The proportion of discordant pairs is 0.230. Eligible patients were be identified by daily review of ED census sheets and data collection was performed by trained research assistants. We recorded the date of visit, demographic data, vital sign parameters, severity of sepsis, diagnosis and focus of infection, comorbidity, lactate results, items of the qSOFA and SOFA score. The diagnosis of severe sepsis and septic shock was made by the treating ED physician when the patient was seen in the ED. Investigators calculated the qSOFA and the SOFA score of patients on arrival in ED. The patients were subsequently followed for their in hospital stay for all-cause mortality. Collected data was analyzed in SPSS version 19. Descriptive data was reported as mean and median for quantitative and proportions for qualitative data. The qSOFA, SOFA score and in-hospital mortality was assessed by area under the receiver operating curve (AUROC).
3. Results
We were able to achieve a calculated sample size of 760 patients due to limitation of resources therefore we decided to proceed with data analysis.
Table 1 shows that the mean age of participants was 59.6 + 17.2 years among the severe sepsis group and was 60.2 + 17.9 years among the septic shock group. Urinary tract infections were reported in majority septic shock patients compared to gastrointestinal infections reported in severely septic patients. The majority of septic shock patients (60%) were admitted to the Intensive care unit while 88.8% of severe sepsis patients were admitted to Intermediate care units. The mean lactate value among the severe sepsis group was 2.9 + 2.79 mmol/L and 4.2 + 3.7 mmol/L among the septic shock group. The proportion of death among participants with severe sepsis was 33.3% and it was observed to be even higher among subjects with septic shock i.e. 61.2%.
Table 1.
Baseline characteristics.
| Variables | Severe sepsis n = 421 (53.9%) | Septic Shock n = 339 (46.1%) |
|---|---|---|
| Socio-demographics: | ||
| Age (Mean ± SD in years) | 59.6 ± 17.2 | 60.2 ± 17.9 |
| Gender [N(%)] | ||
| Male | 242 (57.5) | 196 (57.7) |
| Female | 179 (42.4) | 143 (42.2) |
| Comorbids: | ||
| Malignancy [N(%)] | ||
| No | 386 (91.7) | 296 (87.4) |
| Yes | 35 (8.2) | 43 (12.6) |
| Cardiovascular [N(%)] | ||
| No | 202 (48.7) | 173 (51.1) |
| Yes | 219 (51.2) | 166 (48.9) |
| Diabetes [N(%)] | ||
| No | 185 (44.0) | 172 (50.7) |
| Yes | 236 (56.0) | 167 (49.2) |
| Neurological [N(%)] | ||
| No | 366 (87.0) | 298 (88) |
| Yes | 55 (12.2) | 41 (12.0) |
| Congestive heart failure [N(%)] | ||
| No | 17 (3.9) 404 (96.1) | 20 (5.8) |
| Yes | 404 (96.1) | 319 (94.2) |
| Psychiatric illness [N(%)] | ||
| No | 419 (99.5) | 337 (99.4) |
| Yes | 2 (0.5) | 2 (0.6) |
| Others comorbidities [N(%)] | ||
| No | 396 (94) | 292 (86) |
| Yes | 25 (5.9) | 47 (13.8) |
| Lower Respiratory tract infection [N (%)] | ||
| No | 234 (56) | 128 (37.7) |
| Yes | 187 (44) | 211 (62.2) |
| Urinary tract infection [N(%)] | ||
| No | 158 (77.0) | 271 (80.0) |
| Yes | 47 (22.9) | 68 (20.0) |
| Gastrointestinal infection [N(%)] | ||
| No | 320 (76.1) | 283 (83.4) |
| Yes | 101 (23.9) | 56 (16.5) |
| Skin/Joint infection [N(%)] | ||
| No | 365 (86.9) | 315 (93.1) |
| Yes | 56 (13.1) | 24 (7.0) |
| Hepatobiliary infection [N(%)] | ||
| No | 412 (97.9) | 329 (97.1) |
| Yes | 9 (2.11) | 10 (2.86) |
| Other sources [N(%)] | ||
| No | 382 (90.7) | 325 (95.8) |
| Yes | 39 (9.27) | 14 (4.0) |
| Unit of admission [N(%)] | ||
| Special care unit | 370 (88.8) | 136 (40.0) |
| Intensive care unit | 51 (12.1) | 203 (60.0) |
| SOFAparameters: | ||
| Lactate (Mean±SD in mmol/L) | 2.9 ± 2.79 | 4.2 ± 3.7 |
| PaO2/FiO2 ratio in mmHg [N(%)] | ||
| 0 | 49 (11.6) | 20 (5.8) |
| <400 = +1 | 215 (51.0) | 114 (33.6) |
| <300 = +2 | 104 (24.7) | 84 (24.7) |
| <200 & mechanically ventilated = +3 | 49 (11.6) | 90 (26.6) |
| <100 & mechanically ventilated = +4 | 4 (0.95) | 31 (9.2) |
| Platelets (×103/μL)[N(%)] | ||
| 0 | 310 (73.6) | 190 (56.0) |
| <150 = +1 | 55 (13.0) | 81 (24.0) |
| <100 = +2 | 33 (7.8) | 31 (9.1) |
| <50 = +3 | 18 (4.39) | 27 (8.0) |
| <20 = +4 | 5 (1.18) | 10 (2.9) |
| GCS[N(%)] | ||
| 0 | 111 (26.3) | 42 (12.3) |
| +1 | 238 (56.5) | 193 (57.1) |
| +2 | 47 (11.2) | 66 (19.4) |
| +3 | 20 (4.8) | 29 (8.5) |
| +4 | 4 (0.98) | 9 (2.8) |
| Total bilirubin in mg/dl [N(%)] | ||
| 0 | 304 (72.2) | 201 (59.4) |
| 1.2–1.9 = +1 | 55 (13.0) | 66 (19.4) |
| 2–5.9 = +2 | 31 (7.3) | 42 (12.5) |
| 6–11.9 = +3 | 18 (4.2) | 12 (3.5) |
| >12 = +4 | 13 (3.0) | 18 (5.3) |
| MAP or administration of vasopressin mics/kg/min [N(%)] | ||
| No hypotension = 0 | 170 (40.4) | 33 (9.7) |
| MAP < 70 mmHg = +1 | 127 (30.2) | 58 (17.1) |
| Dopamine ≤5 or dobutamine (any dose) = +2 | 16 (3.9) | 7 (2.2) |
| Dopamine >5 OR epinephrine ≤0.1 OR norepinephrine ≤ 0.1 = +3 | 99 (23.5) | 163 (48) |
| Dopamine >15 OR epinephrine >0.1 OR norepinephrine >0.1 = +4 | 9 (1.9) | 78 (22) |
| Creatinine in mg/dl [N(%)] | ||
| <1.2 = 0 | 100 (23.9) | 66 (19.4) |
| 1.2–1.9 = +1 | 85 (20.9) | 81 (24.0) |
| 2.0–3.4 = +2 | 57 (29.2) | 101 (29.7) |
| 3.5–4.9 = +3 | 124 (12.2) | 46 (13.7) |
| >5.0 = +4 | 55 (13.6) | 45 (13.1) |
| SOFA score [N(%)] | ||
| 0 to 6 = < 10% mortality | 240 (57.0) | 70 (20.5) |
| 7 to 9 = 15–20% mortality | 125 (29.7) | 130 (38.2) |
| 10 to 12 = 40–50% mortality | 46 (10.7) | 83 (24.5) |
| 13 to 14 = 50–60% mortality | 6 (1.46) | 34 (10.2) |
| 15 = >80% mortality | 2 (0.5) | 2 (0.57) |
| 15 to 24 = >90% mortality | 2 (0.5) | 20 (5.7) |
| qSOFA parameters: | ||
| New/worsened altered mentation [N(%)] | ||
| No | 199 (47.3) | 99 (29.1) |
| Yes (+1) | 222 (52.6) | 240 (70.8) |
| RR ≥ 22breaths/min [N(%)] | ||
| No | 137 (32.6) | 64 (18.8) |
| Yes (+1) | 284 (67.4) | 142 (81.1) |
| SBP ≤ 100 mmHg [N(%)] | ||
| No | 220 (52.2) | 64 (18.8) |
| Yes (+1) | 201 (47.8) | 275 (81.1) |
| qSOFA risk/score [N(%)] | ||
| Low (1) | 183 (43.4) | 52 (15.4) |
| High (>1) | 238 (56.5) | 287 (84.6) |
| Mortality [N(%)] | ||
| No | 280 (66.6) | 131 (38.8) |
| Yes | 141 (33.3) | 208 (61.2) |
Overall the SOFA score was highest among subjects with septic shock. However, a higher proportion of subjects (84.5%) with septic shock scored as high risk on qSOFA when compared to subjects with severe sepsis.
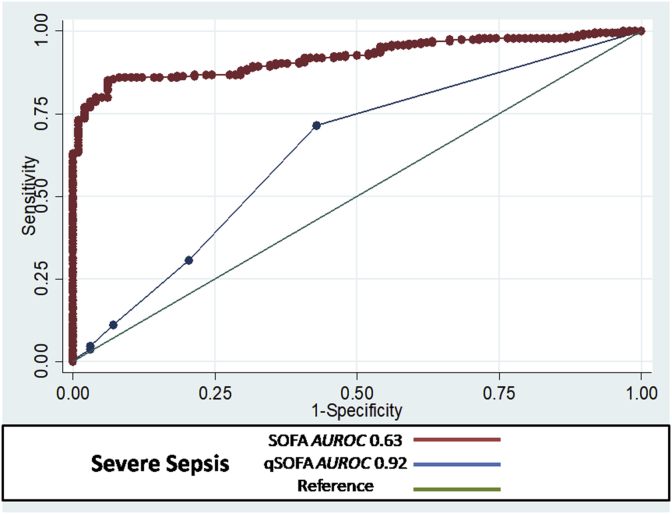
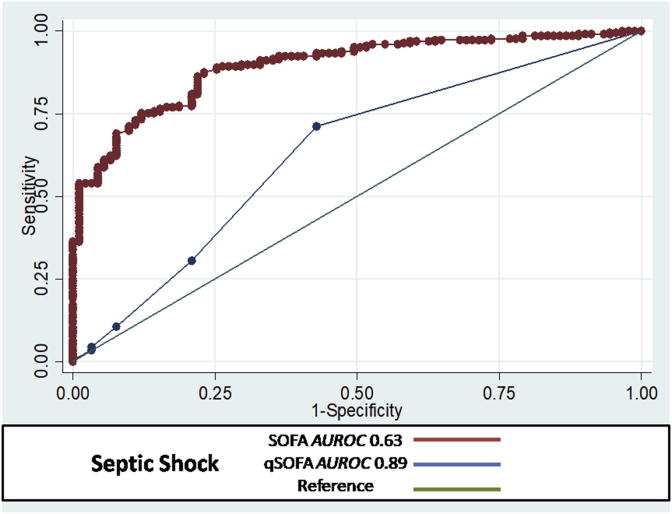
In patients with severe sepsis, the AUROC cutoff of qSOFA for predicting mortality in subjects was 0.92 (95% CI; 0.89–0.94) with 96% sensitivity and 87% specificity in comparison to the AUROC cutoff of SOFA score which was 0.63 (95% CI; 0.55–0.70 with 71% sensitivity and 57% specificity (Fig. 1). In patients with septic shock, the AUROC cutoff of qSOFA for predicting mortality in subjects was 0.89 (95% CI; 0.85–0.92) with 92% sensitivity and 85% specificity in comparison to the AUROC cutoff of SOFA score which was 0.63 (95% CI; 0.55–0.70 with 70% sensitivity and 59% specificity (Fig. 2).
Fig. 1.
QSOFA score in severe sepsis AUROC = 0.92 with 95% CI; 0.89–0.94, sensitivity = 96% and specificity = 87%. And SOFA score in severe sepsis AUROC = 0.63 with 95% CI; 0.55–0.70, Sensitivity = 71%, Specificity = 57%.
Fig. 2.
QSOFAscore in septic shock AUROC = 0.89 with 95% CI; 0.85–0.92, sensitivity = 92% and specificity = 85%. and SOFA score in septic shock AUROC = 0.63 with 95% CI; 0.55–0.70, Sensitivity = 70%, Specificity = 59%.
The results confirm that the model for qSOFA appears well calibrated and has adequate discriminative ability indicating its clinical applicability.
4. Discussion
Our study evaluated and compared performance of the qSOFA score and SOFA in septic ED patients from a low to middle income country with a high reported severity of illness and mortality than quoted locally3,22 as well as those from high income nations.17
The utility of qSOFA has been established in numerous instances within and outside the intensive care unit setting.17,23 Through our study, we established that qSOFA was reported high (>1 parameters which are Altered mentation, Systolic Blood Pressure and Respiratory rate) in accordance with the severity of sepsis with cumulative values of 56.5% in severe sepsis and 84.6% in septic shock patients. This is in contrast to prior literature, examples include one study that validated the qSOFA outside the ICU setting concluded with a low sensitivity identified in septic patients in pre-hospital setting.24Churpek et al. found that only 9% of the 30,667 patients admitted to an ED or a ward with defined infection suspicion had a qSOFA ≥2 at time of suspicion of infection25 and the qSOFA only had 29.9% sensitivity for detecting organ dysfunction according to the sepsis-3 definition in an Australian ED.26
Although, it has been reported previously that the discriminative ability of qSOFA is better than SIRS (qSOFA AUROC of 0.81 compared to SIRS AUROC of 0.76),23a recent retrospective study conducted in multicenter ICUs showed that the predictive ability for determining mortality of the qSOFA score is inferior to SOFA score with AUROC of 0.75 and 0.60 respectively.27 We were able to demonstrate that qSOFA score has better discriminative ability than SOFA score in assessing mortality in our ED septic patients. In patients with severe sepsis, the AUROC for predicting mortality was higher for qSOFA score (AUROC cutoff = 0.92 with 95% CI; 0.89–0.94, sensitivity = 96% and specificity = 87%) when compared to SOFA score (AUROC cutoff = 0.63 with 95% CI; 0.55–0.70, Sensitivity = 71%, Specificity = 57%). Similarly, in patients with septic shock, the AUROC for predicting mortality was greater for qSOFA score (AUROC cutoff = 0.89 with 95% CI; 0.85–0.92, sensitivity = 92% and specificity = 85%) when compared to SOFA score (AUROC cutoff = 0.63 with 95% CI; 0.55–0.70, Sensitivity = 70%, Specificity = 59%).
4.1. Limitations
Prospective larger multicenter studies in LMIC settings are needed to validate our results. We were not able to achieve our desired sample size therefore further studies are required. Secondly, our study included more critically-ill septic patients therefore our results may be limited in the application to all septic patients in EDs. The consequences of high predictive performance of qSOFA than SOFA are useful in our setting as this tool allows for rapid bedside analysis with indication for immediate therapy. However, we believe that there is a significant delay in our septic patients for receiving appropriate medical attention and it may be because of this lead time bias that we may be dealing with a sicker cohort of patients that demonstrated higher scoring values.
5. Conclusion
From our study, qSOFA score appears to be an effective tool at predicting in hospital mortality in comparison to SOFA score when applied to severe sepsis and septic shock patients in the setting of a tertiary care hospital ED of a low-middle income country. However, it is still necessary to rigorously evaluate its applicability in settings outside the ICU environment before concluding its utility beyond what it was designed for.
Fundings
N/A.
Acknowledgement
N/A.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
Contributor Information
Muhammad Akbar Baig, Email: dr_akbar2007@hotmail.com.
Sadaf Sheikh, Email: sadaf.sheikh@aku.edu.
Erfaan Hussain, Email: erfan.hussain@aku.edu.
Samina Bakhtawar, Email: saminaboghani786@gmail.com.
Muhammad Subhan Khan, Email: dr.msubhan@gmail.com.
Syed Mujtaba, Email: syed.mujtaba@aku.edu.
Shahan Waheed, Email: shahan.waheed@aku.edu.
References
- 1.Russell J.A., Walley K.R., Singer J. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008 Feb 28;358(9):877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 2.Sprung C.L., Annane D., Keh D. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 3.Khan N.U., Razzak J.A., Alam S.M. Emergency department deaths despite active management: experience from a tertiary care centre in a low‐income country. Emerg Med Australasia (EMA) 2007;19:213–217. doi: 10.1111/j.1742-6723.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 4.Vincent J.L., Abraham E., Annane D. Reducing mortality in sepsis: new directions. Crit Care. 2002;6(Suppl 3):S1–S18. doi: 10.1186/cc1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivers E.P., Coba V., Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anaesthesiol. 2008 Apr;21(2):128–140. doi: 10.1097/ACO.0b013e3282f4db7a. [DOI] [PubMed] [Google Scholar]
- 6.Bone R.C., Balk R.A., Cerra F.B. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman J.E., Kramer A.A., McNair D.S. Acute Physiology and Chronic Health Evaluation (Apache) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 8.Metnitz P.G.H., Moreno R.P., Almeida E. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods, and cohort description. Intensive Care Med. 2005;31:1336–1344. doi: 10.1007/s00134-005-2762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Gall J.R., Klar J., Lemeshow S. The Logistic Organ Dysfunction system.A new way to assess organ dysfunction in the intensive care unit.ICU Scoring Group. J Am Med Assoc. 1996;276:802–810. doi: 10.1001/jama.276.10.802. [DOI] [PubMed] [Google Scholar]
- 10.Higgins T.L., Teres D., Copes W.S. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III) Crit Care Med. 2007;35:827–835. doi: 10.1097/01.CCM.0000257337.63529.9F. [DOI] [PubMed] [Google Scholar]
- 11.Jones A.E., Fitch M.T., Kline J.A. Operational performance of validated physiologic scoring systems for predicting in-hospital mortality among critically ill emergency department patients. Crit Care Med. 2005;33:974–978. doi: 10.1097/01.ccm.0000162495.03291.c2. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro N.I., Wolfe R.E., Moore R.B. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31:670–675. doi: 10.1097/01.CCM.0000054867.01688.D1. [DOI] [PubMed] [Google Scholar]
- 13.Sankoff J.D., Goyal M., Gaieski D.F. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS) Crit Care Med. 2008;36:421–426. doi: 10.1097/01.CCM.0B013E3181611F6A0. [DOI] [PubMed] [Google Scholar]
- 14.Jones A.E., Saak K., Kline J.A. Performance of the mortality in ED sepsis score for predicting hospital mortality among patients with severe sepsis and septic shock. Am J Emerg Med. 2008;26:689–692. doi: 10.1016/j.ajem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro N., Howell M.D., Bates D.W. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006;48:583–590. doi: 10.1016/j.annemergmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Singer M., Deutschman C.S., Seymour C.W. The third international consensus definitions for sepsis and septic shock (Sepsis-3) J Am Med Assoc. 2016 Feb 23;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour C.W., Liu V.X., Iwashyna T.J. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) J Am Med Assoc. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seymour C.W., Coopersmith C.M., Deutschman C.S. Application of a framework to assess the usefulness of alternative sepsis criteria. Crit Care Med. 2016 Mar;44(3):e122–e130. doi: 10.1097/CCM.0000000000001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonelli M., DeBacker D., Dorman T. Surviving sepsis Campaign; 2016. Surviving Sepsis Campaign Responds to Sepsis-3.http://www.survivingsepsis.org/SiteCollectionDocuments/SSC-Statements-Sepsis-Definitions-3-2016.pdf Website. Available at: [Google Scholar]
- 21.Dellinger R.P., Levy M.M., Rhodes A. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013 Feb;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullah A.R., Hussain A., Ali I. A prospective observational study assessing the outcome of Sepsis in intensive care unit of a tertiary care hospital, Peshawar. Pak J Med Sci. 2016;32(3):688–693. doi: 10.12669/pjms.323.9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adhikari N.K.J., Rubenfeld G.D. qSOFA score for patients with sepsis in low- and middle-income countries. J Am Med Assoc. 2018 Jun 5;319(21):2175–2177. doi: 10.1001/jama.2018.6413. [DOI] [PubMed] [Google Scholar]
- 24.Dorsett M., Kroll M., Smith C.S. qSOFA has poor sensitivity for prehospital identification of severe sepsis and septic shock. Prehosp Emerg Care. 2017:1–9. doi: 10.1080/10903127.2016.1274348. [DOI] [PubMed] [Google Scholar]
- 25.Churpek M.M., Snyder A., Han X. qSOFA, SIRS, and early warning scores for detecting clinical deterioration in infected patients outside the ICU. Am J Respir Crit Care Med. 2016;195(7):906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams J.M., Greenslade J.H., McKenzie J.V. SIRS, qSOFA and organ dysfunction: insights from a prospective database of emergency department patients with infection. Chest. 2017;151(3):586–596. doi: 10.1016/j.chest.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 27.Raith E.P., Udy A.A., Bailey M. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. J Am Med Assoc. 2017;317:290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]