Abstract
Altered consciousness and accompanying neurological symptoms are both complex and challenging cases for emergency physicians. These are not specific and may be a sign of a variety of medical conditions including stroke and delayed neurological sequelae (DNS) is a recurrent transient neuropsychiatric consequence of CO intoxication. DNS produces a spectrum of varying symptoms and the diagnosis is primarily made on the basis of clinical features and radiological findings from CT and conventional MRI. In clinical practice, serious CO intoxication is treated only with oxygen therapy although no effective treatment exists. Emergency physicians play a major role in managing patients presenting with CO intoxication and preventing DNS.
Keywords: Intoxication, Carbon monoxide, Emergency department, Altered consciousness
1. Introduction
Delayed neurologic sequelae (DNS) are recurrent–transient neuropsychiatric consequences of carbon monoxide (CO) intoxication. They manifest with alternating periods of exacerbation and remission that cause diffuse white matter or gray matter injury.1 The differential diagnosis includes similar neurological signs and symptoms, thus resulting in great uncertainty when evaluating patients presenting at emergency departments (EDs), particularly when CO intoxication is not suspected. We report a case of CO encephalopathy that manifested after a silent interval following index CO intoxication.
2. Case presentations
A 48-year-old man presented in the ED with loss of balance, gait disturbance, and urinary incontinence of one-week duration. Given that the patient's consciousness level did not permit obtaining a reliable history, relevant clinical and demographic information was obtained from his medical records and relatives. The patient's history was unremarkable with regard to co-morbidities or medication use other than admission to the ED at another hospital one month before for a syncope attack associated with CO exposure. Patient's relatives stated that magnetic resonance imaging (MRI) and electroencephalography did not indicate any pathology at that time. He was admitted to the intensive care unit of that hospital for CO toxicity, had three sessions of hyperbaric oxygen treatment (HBOT), and discharged without sequelae. Upon admission to our ED, physical examination findings were normal other than limited cooperation with the medical team. His vital signs were as follows: temperature 37 °C, pulse rate 104 bpm, blood pressure 114/70 mmHg, and oxygen saturation 96%. All other system examinations were normal. Significant laboratory findings included an elevated WBC count of 11.9 cells/mm³ and a creatine kinase level of 401 I/U. His electrocardiogram showed normal sinus rhythm.
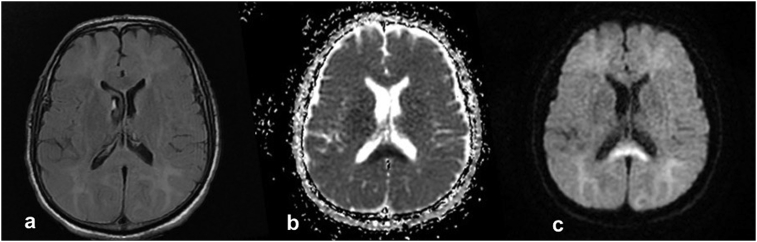
Cranial computed tomography (CT) was performed to investigate the cause of limited cooperation but revealed no acute pathological conditions. A neurology consult was scheduled to rule out acute neurological conditions. The lack of findings on cranial CT, the limited cooperation of the patient, and the changes in his consciousness resulted in a provisional diagnosis of DNS following CO exposure. Cranial MRI showed diffuse, symmetrical areas of pathological signal alterations in the deep white matter areas of both cerebral hemispheres. They were more prominent at the level of the centrum semiovale and extended to subcortical white matter areas. Those areas appeared hyperintense in T2A sequences but showed no uptake after intravenous contrast injection one-month after CO intoxication. Cranial MRI also showed areas of altered signals at the level of the body and splenium of the corpus callosum, which appeared hyperintense on diffusion-weighted imaging, and hypointense on apparent diffusion coefficient (ADC) maps. Initial imaging showed localized diffusion limitation (Fig. 1). After consultations with department of neurology, he was diagnosed with delayed central nervous system signs and symptoms secondary to CO intoxication, primarily on the basis of the patient's clinical and physical examination findings and cranial imaging signs. Hyperbaric oxygen therapy was planned and the patient was referred to a relevant medical facility.
Fig. 1.
Axial DWI MR (c) in the patient shows restricted diffusion. Correlative ADC (b) hypointensity was also demonstrated within the same geographic area (splenium of corpus callosum). Axial FLAIR MRI (a) for this patient in the ER is normal.
3. Discussion
The management of patients who present to the ED with altered consciousness and accompanying neurological symptoms is challenging. The presentation is generally not disease specific and may include signs of diverse medical conditions, including stroke and DNS.2 A final diagnosis must be made in the ED not only for appropriate treatment but also to avoid incorrect diagnoses and overly aggressive treatment which ha the potential to lead to complications.2,3 CO intoxication is a frequent diagnosis in EDs worldwide, which has a high morbidity and mortality.4 CO intoxication resolves in most patients following normobaric or hyperbaric oxygen therapy, but a minority of patients experience persistent neuropsychiatric abnormalities or delayed encephalopathy. The carboxyhemoglobin (COHb) level on admission after CO inhalation does not always correlate with clinical findings or prognosis. Chronic CO exposure may present itself with loss of dentition, gradual-onset neuropsychiatric symptoms, or recent impairment of cognitive ability inconsistent with DNS.1,5,6 Increasing evidence indicates that the brain damage caused by CO intoxication results from mitochondrial oxidative stress in the central nervous system, white matter demyelination resulting from the immune response, abnormal inflammatory responses, and apoptosis. Free radicals activate a cascade responsible for the appearance of the delayed effects seen in an estimated 1%–47% of patients with CO intoxication.5,7
DNS includes a broad spectrum of symptoms.5 The sequelae may vary from mild to severe headache, seizures, alteration in consciousness, lethargy, concentration problems, cognitive disturbances, emotional liability, personality changes, amnestic syndromes, dementia, psychosis, gait disturbances, movement disorders (e.g., parkinsonism), chorea, apraxia, agnosia, inaction, peripheral neuropathy, urinary incontinence, and even vegetative state.5,7, 8, 9 Delayed CO intoxication is diagnosed by the presence of a clinically silent period or lucid interval lasting for 2–40 days after acute intoxication followed by recurrent neuropsychiatric symptoms.7 The diagnosis of DNS is primarily made on the basis of the clinical features and radiological findings of CT and conventional MRI.8 MRI reveals abnormalities in the basal ganglia and/or white matter, as well as in the globus pallidus. These include newly formed hyperintense white matter lesions on T2-weighted images and fluid-attenuated inversion recovery (FLAIR), but they may be absent even in cases with chronic neurological symptoms.10,11 Cerebrospinal fluid (CSF) analysis is a valuable diagnostic tool, but it is highly invasive and time-consuming.1 Proton magnetic resonance spectroscopy is a noninvasive imaging modality that can detect neurochemical impairment associated with brain injury in the region of interest. Fractional anisotropy in diffusion tensor imaging is a recently introduced technique that is useful for CO diagnosis.7,11 However, the latter are even less accessible than MRI in the ED setting.
There is no effective treatment for delayed posthypoxic demyelination resulting from CO intoxication.12 Serious CO intoxication is treated only with oxygen therapy. HBOT acts to increase oxygen partial pressure in the blood, decrease COHb tension, and improve tissue oxygenation. It also reduces CO-induced mortality, limits cognitive impairment, and reduces the incidence of DNS. If the emergent HBOT starts within 24 hours after CO inhalation, it is more effective in reducing the incidence of neuropsychiatric sequelae than normobaric oxygen therapy. Its effectiveness for preventing DNS is not clear, and there are uncertainties surrounding the optimum HBOT protocol.5,9,13,14 Studies reporting increased fractional anisotropy after HBOT are consistent with the reversal of myelin damage by HBOT.15 Other experimental treatments have been evaluated in clinical trials, including anti-inflammatory and immunomodulatory agents such as immuneglobulin, interferon, glatiramer acetate, and steroids, but with limited success.12 Hydrogen-rich saline has a lower hydroxyl radical concentration than water, has low toxicity, and is thought to be neuroprotective in ischemia-reperfusion injury.14 Allopurinol reportedly protects against brain ischemia and oxidative-stress-induced reperfusion injury; it also decreases the severity of DNS by inhibiting xanthine oxidoreductase, which promotes free radical production during cerebral ischemia/reperfusion.5 CO toxicity stimulates oxidative stress in the brain, with protective responses including the accumulation of superoxide dismutase (SOD-1, SOD-2), HO-1, nitric oxide synthase, and nitrotyrosine. Thus, it is possible that vitamin C (ascorbic acid) and Mn (III) tetrakis (4-benzoic acid) porphyrin, which is a superoxide dismutase mimetic, might be effective for treating CO toxicity.16 The mechanism of CO toxicity is not clear; however, if it results in oxidative stress, neuronal toxicity, and post ischemic reperfusion injury responsible for delayed symptoms, antioxidants may prove to be a novel treatment approach.17, 18, 19
4. Conclusion
Neurological emergencies are complex and challenging diagnoses because of the limited time allotted in taking patient histories and performing physical examinations. The availability of medical resources for making accurate diagnoses may also be limited. Patients with CO intoxication usually present in the ED. Most are encountered in the acute phase, but some occasionally present with delayed symptoms. Awareness of the DNS of CO intoxication and its signs and symptoms is of utmost importance to ensure the effective management of neurological emergencies.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
Contributor Information
Bedriye Müge Sönmez, Email: mugesonmez06@yahoo.com.
Murat Doğan İşcanlı, Email: mdiscanli@gmail.com.
Selçuk Parlak, Email: selcukparlakdr@gmail.com.
Yasin Doğan, Email: dr.dogan89@hotmail.com.
Hilmi Gökhan Ulubay, Email: gokhanulubay@yahoo.com.
Emirhan Temel, Email: emirhantemel@gmail.com.
References
- 1.Beppu T., Nishimoto H., Fujiwara S. 1H-magnetic resonance spectroscopy indicates damage to cerebral white matter in the subacute phase after CO poisoning. J Neurol Neurosurg Psychiatry. 2011;82:869–875. doi: 10.1136/jnnp.2010.222422. [DOI] [PubMed] [Google Scholar]
- 2.Kuroda H., Fujihara K., Kushimoto S., Aoki M. Novel clinical grading of delayed neurologic sequelae after carbon monoxide poisoning and factors associated with outcome. Neurotoxicology. 2015;48:35–43. doi: 10.1016/j.neuro.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Dong G., Ren M., Wanga X. Allopurinol reduces severity of delayed neurologic sequelae in experimental carbon monoxide toxicity in rats. Neurotoxicology. 2015;48:171–179. doi: 10.1016/j.neuro.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Beppu T., Nishimoto H., Ishigaki D. Assessment of damage to cerebral white matter fiber in the subacute phase after carbonmonoxide poisoning using fractional anisotropy in diffusion tensor imaging. Neuroradiology. 2010;52:735–743. doi: 10.1007/s00234-009-0649-x. [DOI] [PubMed] [Google Scholar]
- 5.Thom S.R., Taber R.L., Mendiguren, Clark J.M., Hardy K.R., Fisher A.B. Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen. Ann Emerg Med. 1995;25:474–480. doi: 10.1016/s0196-0644(95)70261-x. [DOI] [PubMed] [Google Scholar]
- 6.Rose J.J., Wang L., Xu Q. Carbon monoxide poisoning: pathogenesis, management, and future directions of therapy. Am J Respir Crit Care Med. 2017;195:596–606. doi: 10.1164/rccm.201606-1275CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khot S., Walker M., Lacy J.M., Oakes P., Longstreth W.T., Jr. An unsuccessful trial of immunomodulatory therapy in delayed posthypoxic demyelination. Neurocritical Care. 2007;7:253–256. doi: 10.1007/s12028-007-0044-6. [DOI] [PubMed] [Google Scholar]
- 8.Gilmer B., Kilkenny J., Tomaszewski C., Watts J.A. Hyperbaric oxygen does not prevent neurologic sequelae after carbon monoxide poisoning. Acad Emerg Med. 2002;9:1–8. doi: 10.1111/j.1553-2712.2002.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 9.Sun Q., Cai J., Zhou J. Hydrogen-rich saline reduces delayed neurologic sequelae in experimental carbon monoxidetoxicity. Crit Care Med. 2011;39:765–769. doi: 10.1097/CCM.0b013e318206bf44. [DOI] [PubMed] [Google Scholar]
- 10.Prockop L.D. Carbon monoxide brain toxicity: clinical, magnetic resonance imaging, magnetic resonance spectroscopy, and neuropsychological effects in 9 people. J Neuroimaging. 2005;15:144–149. doi: 10.1177/1051228404273819. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda H., Fujihara K., Mugikura S., Takahashi S., Kushimoto S., Aoki M. Altered white matter metabolism in delayed neurologic sequelae after carbon monoxi poisoning: a proton magnetic resonance spectroscopic study. J Neurol Sci. 2016;360:161–169. doi: 10.1016/j.jns.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Rico M., Martinez-Rodriguez L., Larrosa-Campo D., Calleja S. Dilemma in the emergency setting: hypomagnesemia mimicking acute stroke. Int Med Case Rep J. 2016;9:145–148. doi: 10.2147/IMCRJ.S101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aslaner M.A., Boz M., Çelik A. Etiologies and delirium rates of elderly ED patients with acutely altered mental status: a multicenter prospective study. Am J Emerg Med. 2017;35:71–76. doi: 10.1016/j.ajem.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Hosseininejad S.M., Aminiahidashti H., Goli Khatir I., Ghasempouri S.K., Jabbari A., Khandashpour M. Carbon monoxide poisoning in Iran during 1999-2016: a systematic review and meta-analysis. J Forensic Leg Med. 2018;53:87–96. doi: 10.1016/j.jflm.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y.T., Chen S.Y., Lo C.P. Utilizing cerebral perfusion scan and diffusion-tensor MR imaging to evaluate the effect of hyperbaric oxygen therapy in carbon monoxide-induced delayed neuropsychiatric seqeulae- a case report and literature review. Acta Neurol Taiwan. 2015;24:57–62. [PubMed] [Google Scholar]
- 16.Akyol S., Erdogan S., Idiz N. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: an in-depth analysis. Redox Rep. 2014;19:180–189. doi: 10.1179/1351000214Y.0000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akyol S., Gulec M.A., Erdemli H.K., Akyol O. A new therapeutic approach for carbon monoxide poisoning: antioxidants. Toxicology. 2015;36:34–35. doi: 10.1016/j.tox.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Gulec M.A., Akyol O., Akyol S. A commentary on “The effectiveness of oxygen therapy in carbon monoxide poisoning is pressure- and time-dependent: a study on cultured astrocytes.”. Toxicol Lett. 2015;238:83. doi: 10.1016/j.toxlet.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Akyol S., Yuksel S., Pehlivan S. Possible role of antioxidants and nitric oxide inhibitors against carbon monoxide poisoning: having a clear conscience because of their potential benefits. Toxicol Lett. 2016;92:3–6. doi: 10.1016/j.mehy.2016.04.015. [DOI] [PubMed] [Google Scholar]