Abstract
Methemoglobinemia is a rare but clinically important condition that should be considered among differential diagnosis' in cyanosis. The conventional treatments of methemoglobinemia are high flow oxygen and methylene blue therapies.
We present a 52-year-old male patient who admitted to emergency room with severe cyanosis and dyspnea after he was exposed to paint thinner and zinc phosphate solution. The patient was diagnosed with methemoglobinemia with a MetHb level of 49.1mm/Hg in his arterial blood gas test. Patient's symptoms and increased MetHb levels were resistant to high flow oxygen and methylene blue therapies so hyperbaric oxygen therapy (HBO) as an alternative treatment was initiated and the patient was cured promptly.
In this case presentation, we aim to discuss the alternative treatment modalities in methemoglobinemia patients with persistent hypoxia and cyanosis, who are unresponsive to standard methylene blue treatment.
Keywords: Methemoglobinaemia, Methylene blue therapy, Hyperbaric oxygen therapy, Cyanosis
1. Introduction
Methemoglobinemia is a relatively rare but clinically important condition that should be considered among differential diagnosis' in cyanosis.1,2 This condition results from the oxidation of ferrous iron (Fe++) into ferric iron (Fe+3) in the hemoglobin (Hb) molecule. The amount of methemoglobin (MetHb) is less than 1% of the total Hb, under normal conditions. With the increased blood concentrations of MetHb; Hb oxygen dissociation curve shifts and the affinity of the Hb molecule to oxygen (O2) increases. This decreases oxygen delivery to peripheral tissues.3 Aromatic amines, nitrobenzene, methyl nitrite, sulphonamides, naphthalene, silver nitrate, copper sulfate, and zinc sulfate are potential toxic agents that may cause methemoglobinemia. Moreover; fire fume inhalation may also cause this condition.3,4
Our aim is to discuss therapeuticin patients who are unresponsive to standard methylene blue treatment with persistent hypoxia and cyanosis.
2. Case presentation
A 52-year-old male, working at a steel-wire factory was brought to our emergency department (ED) after a syncope attack. He declared that he passed out while he was cleaning the factory. He was conscious with a Glasgow Coma Scale (GCS) of 14 at his arrival to ED, which was 30 minutes after the incident. He had no known diseases except migraine in his medical history. His family and friends confirmed that he had no suicidal behavior.
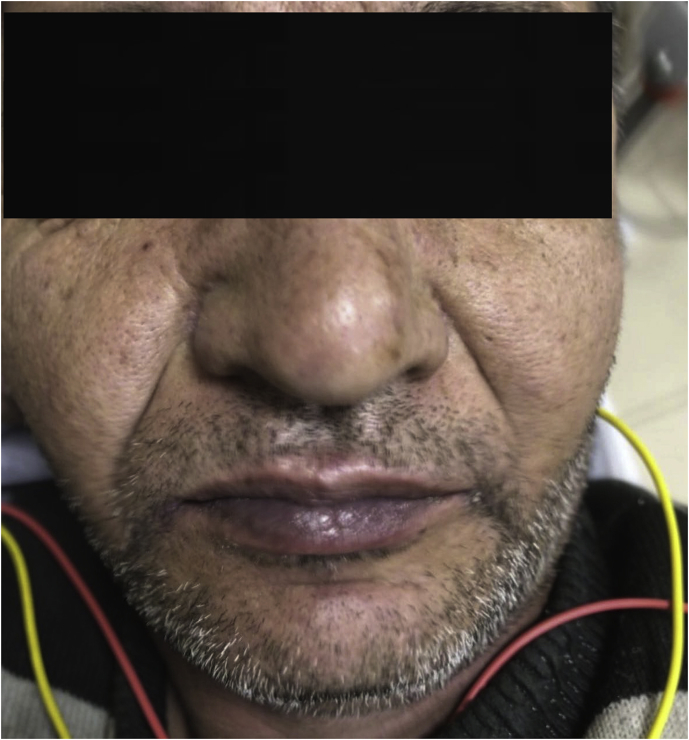
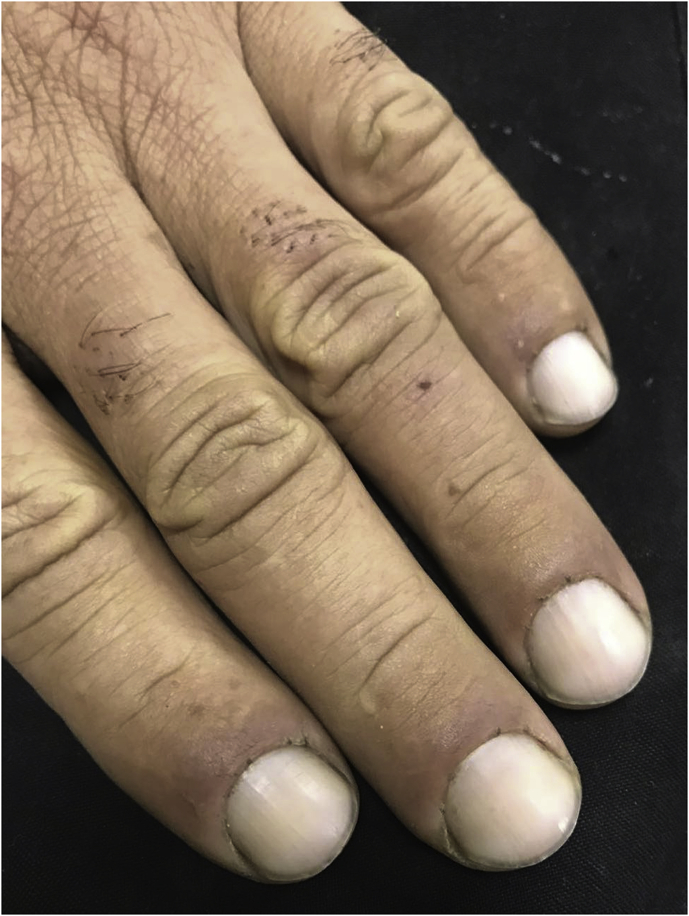
Patient's complaints were dizziness, nausea, and dyspnea. In his physical examination severe cyanosis and respiratory insufficiency were present. He had circumoral and facial cyanosis (Fig. 1). He also had cyanosis all around his fingers and fingernails (Fig. 2). There were no other pathological findings.
Fig. 1.
Circumoral and facial cyanosis.
Fig. 2.
Cyanosis around fingers and fingernails.
He was monitorised in the resuscitation room. Initial vital signs were as follows: BP: 110/80 mmHg, pulse rate: 105 bpm, respiratory rate: 22/min, SO2: 70%, temperature: 36 °C. Syncope work-up tests were initiated and computerized cranial tomography (CT) was ordered. The fingertip blood glucose was 128 mg/dL. The initial arterial blood gas was as follows: pH: 7.40, PO2: 43.8 mmHg, PCO2:41.9 mmHg, COHb: 1.1 mmHg, lactate: 1.2 mmol/L, MetHb: 49.1 mmHg. Intracranial pathologies were ruled-out by a normal cranial CT.
An IV line was established and high flow oxygen was initiated at a rate of 3 L/min through a face mask. Blood biochemistry revealed glucose 206 mg/dl; creatinine 0.6 mg/dL, BUN 13 mg/dL, CK-MB 28 mg/dL, CK 662 mg/dL and a negative Troponin T. All coagulation and CBC parameters were within normal limits. The second blood gas analysis (venous sample) was as follows: pH: 7.40, PO2: 31.5 mmHg, PCO2: 41.4 mmHg, COHb: 1.4 mmHg, lactate: 0.8 mmol/L, MetHb: 43.5 mmHg. He was consulted to the Nephrology and Hematology departments. However; neither hemodialysis nor apheresis was indicated. Methylene Blue, 1 mg/kg infusion in 5 min was initiated. After thirty minutes, MetHb was decreased to 18.1 mmHg. High flow oxygen therapy was continued. and consulted the patient for hyperbaric oxygen therapy (HBO) because there was no regression in the respiratory symptoms at the 8th hour of his admission to the ED. Hyperbaric oxygen therapy of 2.2 atm in a multi-place chamber (Baroks O2multi Series Mul32) unit was performed for 90 minutes, since respiratory systems did not regressed back at the 8th hour of ED admission.. MetHb level was decreased from 18.1 mmHg to 1.5 mmHg immediately after the HBO therapy. The patient was observed for 24 hours under oxygen until all symptoms were disappeared. The detailed patient history taken then revelaed that the factory was using Zinc-phosphate in liquid tanks for the production of steel lining, and thinner was widely used in the area. He was discharged from the hospital after the blood gases and vital signs recovered back to normal.
3. Discussion
Methemoglobinemia is a clinical condition in which ferrous iron (Fe++) in the hemoglobin molecule is oxidized to ferric iron (Fe+3) form. In methemoglobinemia, the delivery of the oxygen from hemoglobin to the tissue is diminished. Hypoxia and hypoperfusion at the cellular level is present. Although the patient has severe oxygen depletion, the blood gas analysis is usually within normal limits with normal oxygen saturation (SO2). There is a typical mismatch of oxygen saturation between the results of pulse oximetry and blood gas analysis.3,4 In concordance with the level of the methemoglobin, the tissue hypoxia results in significant cyanosis, cardiac ischemia, central nervous system symptoms such as stroke, seizure, lactic acidosis, and even coma leading to death. However, there may be individual differences in the metabolizing enzymes, and the concentration of toxin may not correlate to the symptoms.3
The most common form of methemoglobinemia is the acquired form, which is secondary to chemical agents used in industry. There is also a congenital type of methemoglobinemia seen during childhood in patients with nicotinamide adenine dinucleotide phosphate (NADPH) MetHb reductase enzyme deficiency. This enzyme is also useful in therapy because it converts methylene blue to leukomethylene blue, which in turn reduces MetHb to Hb. It must be noted that in congenital glucose-6-phosphate dehydrogenase (G6PD) deficiency, methylene blue is contraindicated since these patients cannot produce NADPH. The absence of NADPH may worsen symptoms due to the conversion of Hb into MetHb by methylene blue.4, 5, 6
In our case report, the patient was exposed to paint thinner besides Zinc phosphate solution. Paint thinner is a solvent used for cleaning oil-based paints. It is composed of highly toxic agents such as acetone, benzene, carbon tetrachloride, and toluene.6
The methemoglobinemia patients usually have the dominant symptoms of the respiratory system with typical cyanosis and dyspnea. Patients also experience tachycardia, headache, vertigo, and syncope. When MetHb is between 10% and 20% cyanosis is observed. Above 20% dyspnea, tachycardia and central nervous system signs are present. Severe lactic acidosis starts above 50%, and death is presumed above 70%.5
Although the diagnosis of methemoglobinemia is not difficult, the treatment success is variable. The mainstay of the therapy is high flow oxygen. Methylene blue is an oxidizing agent used at a dose of 1–2 mg/kg. On the other hand, methylene blue has a narrow therapeutic index. It is not a harmless drug and has many side effects. It inhibits nitric oxide synthase and guanylate cyclase enzymes. It also reacts with RBC to form leukomethylene blue, which reduces oxidized Hb and converts the ferric ion (Fe+3) to ferrous (Fe++) which can carry oxygen molecule. Methylene blue is reported to be highly cardiotoxic. It may cause arrhythmias, heart failure, and shock. It decreases renal and mesenteric blood flow. It also has several nephrotoxic and metabolic side effects and drug interactions.7 If the patient symptoms do not subside after the therapeutic 1–2 mg/kg dose range; the emergency physician should consider alternative treatment modalities, such as HBO. HBO was the second alternative therapy we used in our case, and it proved to be successful. Each patient has different time course for the therapy even after HBO because theoretically, each patient has a different enzymatic system.8 HBO therapy has been studied for the treatment of methemoglobinemia since the 1970s.8 Majority of these studies used HBO as an additional treatment to methylene blue and found that it is an effective treatment in methemoglobinemia patients.8, 9, 10, 11 HBO therapy inhibits the oxidation of hemoglobin by nitrite, and reduces MetHb levels at a rate of 8% per hour.8 In our case, MetHb reduction was even greater.
There are also other alternative treatment modalities: ascorbic acid and riboflavin have been used in selected patients, but they are not conventional.12 In the literature, extracorporeal methods such as plasmapheresis and hemodialysis have also been used for refractory methemoglobinemia patients. When oliguria is detected, hemodialysis should be started promptly. Neurological status is the critical point for deciding whether to give plasmapheresis or not. These two methods are effective in the elimination of the agent, and they also reduce the defeceted RBCs when the patient is transfused during the therapy.5 But in our case, we preferred HBO therapy over hemodialysis and plasmapheresis since HBO unit was closer in the distance and more reachable.
4. Conclusion
Methemoglobinemia diagnosis is a fast and relatively easy procedure. It should be considered among other differential diagnoses' in cyanosis. Alternative treatment regimens should be initiated when the patient is unresponsive to standard Methylene Blue treatment. Hyperbaric oxygen therapy is a promising alternative treatment in patients with persistent hypoxia and cyanosis.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
Contributor Information
Ismail Altintop, Email: draltintop1@hotmail.com.
Erkman Sanri, Email: erkmansanri@gmail.com.
Mehmet Tatli, Email: drmehmettatli@gmail.com.
Mehmet Emin Akcin, Email: meminakcin@hotmail.com.
Arzu Denizbasi, Email: denizbasi@gmail.com.
References
- 1.Al-Lawati A., Murch N. Acquired methemoglobinaemia. Sultan Qaboos Univ Med J. 2012;12(2):237–241. doi: 10.12816/0003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghotra S., Jangaard K., Pambrun C., Fernandez C.V. Congenital methemoglobinaemia due to Hb F-M-Fort Ripley in a preterm newborn. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-214381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright R.O., Lewander W.J., Woolf A.D. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34(5):646–656. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 4.Skold A., Cosco D.L., Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J. 2011;104(11):757–761. doi: 10.1097/SMJ.0b013e318232139f. [DOI] [PubMed] [Google Scholar]
- 5.Shatila W., Verma A., Adam S. Plasmapheresis in severe methemoglobinemia following occupational exposure. Transfus Apher Sci. 2017;56(3):341–344. doi: 10.1016/j.transci.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Ash-Bernal R., Wise R., Wright S.M. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltim) 2004;83(5):265–273. doi: 10.1097/01.md.0000141096.00377.3f. [DOI] [PubMed] [Google Scholar]
- 7.Umbreit J. Methemoglobin–it's not just blue: a concise review. Am J Hematol. 2007;82(2):134–144. doi: 10.1002/ajh.20738. [DOI] [PubMed] [Google Scholar]
- 8.Cho Y., Park S.W., Han S.K., Kim H.B., Yeom S.R. A case of methemoglobinemia successfully treated with hyperbaric oxygenation monotherapy. J Emerg Med. 2017;53(5):685–687. doi: 10.1016/j.jemermed.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 9.Lindenmann J., Fink-Neuboeck N., Schilcher G., Smolle-Juettner F.M. Severe methaemoglobinaemia treated with adjunctive hyperbaric oxygenation. Diving Hyperb Med. 2015;45(2):132–134. [PubMed] [Google Scholar]
- 10.Lindenmann J., Matzi V., Kaufmann P. Hyperbaric oxygenation in the treatment of life-threatening isobutyl nitrite-induced methemoglobinemia–a case report. Inhal Toxicol. 2006;18(13):1047–1049. doi: 10.1080/08958370600904629. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein G.M., Doull J. Treatment of nitrite-induced methemoglobinemia with hyperbaric oxygen. Proc Soc Exp Biol Med. 1971;138(1):137–139. doi: 10.3181/00379727-138-35846. [DOI] [PubMed] [Google Scholar]
- 12.Dhibar D.P., Sahu K.K., Jain S., Kumari S., Varma S.C. Methemoglobinemia in a case of paint thinner intoxication, treated successfully with vitamin C. J Emerg Med. 2017 doi: 10.1016/j.jemermed.2017.10.035. S0736-4679(17)31103-4. [DOI] [PubMed] [Google Scholar]