Abstract
Gastrointestinal nematodes (GINs) of ruminants are prevalent and have major economic impacts worldwide. The insight studies of immune responses triggered against GINs are of great concern to understand interaction between host’s immune system and parasite. T-helper 2 cytokines drive the effector cell mechanisms which include eosinophils and mast cells. The immune responses are controlled by Th2 secreted interleukins (IL); IL3, IL-4, IL-5, IL-9, IL-10 and IL-13. B-Cell immune response is incorporated in defense mechanisms developed against GINs specially immunoglobulins (Ig); IgA, IgE and IgG. The immune resistance of the infected host is presented by failure of larval establishment or hypobiosis, low worm burden and decreased female fecundity. The host–parasite interaction is a complex series that affected by host’s genetic constitution, nutrition, age and physiological status. The GINs have different immune evasion mechanisms to improve their survival within the host. Also, management of the host influences GINs parasitism. Thus, the aim of this review is to highlight the hallmarks of immune responses that endorse GINs parasitism. The insights studies of the triggered immune responses developed against GINs will improve the appropriate protective immune strategy.
Keywords: GINs, Cytokines, MMC, Eosinophils, Ruminants, Evasion
Introduction
Gastrointestinal nematodes of the ruminants’ livestock are highly complex multicellular parasitic organisms that cause gastroenteric verminosis (McRae et al. 2015; Belina et al. 2017). The nematode infections usually elicit serious pathophysiological disorders causing significant economic losses in livestock. They cause severe anemia, loss of appetite and poor growths, consequently lead to losses in meat, milk and wool production and sometimes deaths occur (González et al. 2003; Toscan et al.2017). The life cycle of nematode parasites consists of two phases, pre-parasitic (free-living) and parasitic. The pre-parasitic phase takes place in the environmental surroundings, while the parasitic phase occurs inside the susceptible host. The vast majority of nematode’ life cycles consists of egg, five larval (L) stages; L1, L2, L3, L4 and L5 and adult stage. The third larval stage is the infective stage that penetrates the intestinal mucosa and matures to L4 then L5 (immature adult stage) and finally to adult stage. The successful infection is contributed to health status and immunity of the infected hosts (Angulo-Cubillán et al. 2007; Kandil et al. 2017).
Both the innate and adaptive immune responses are the classical pathways to kill and/or expel the parasitic nematode or to exacerbate a disease. Once the gastrointestinal mucosal tissues are penetrated by the larvae of nematodes, the interaction between epithelial cells, innate lymphoid cells (ILCs), antigen-presenting cells (APC) such as dendritic cells and intestinal macrophages are stimulated to generate an immune response in co-regulation with complement fixation and mucus secretions to resist the primary infection (Garza 2014; McRae et al. 2015). Subsequently, to control the further secondary infections the specific adaptive immune mechanisms are triggered.
The antigen specific immune mechanisms (processing and presentations) induce both humeral and cellular immunity. Th2 lymphocytes coordinate the humoral immune response which secrete the main cytokines IL3, IL4, IL5, IL9, IL10 and IL13 (Abo-Aziza et al. 2017; EL Namaky et al. 2017; Rodrigues et al. 2017). T-helper 2 cytokines drive the effector cell mechanisms which include eosinophils and mast cells. Additionally, the immunity against GINs is typically associated with different Ig isotypes (IgA, IgE and IgG) responses. IgA and IgE are the most characteristic features at the local mucosal sites of helminth infections, however IgG is predominantly detected in serum of infected animals (Angulo-Cubillán et al. 2007; McRae et al. 2015; Kandil et al. 2016).
This host-parasite relationship is manifested by different phenomena as hypobiosis of larval stages, expulsion of adult stages, decreasing adult female fecundity, changes in morphological characters of the adult. Several factors could affect GINs parasitism and influence the immune responses developed against them. These factors could be a host factor; related to its genetic constitution, age and/or physiological status, management factors such as nutrition and chemoprophylaxis therapy or parasite factors which contributed to nematode immunomodulation strategy (Balic et al. 2000; González et al. 2003; McRae et al. 2015; González-Garduño et al. 2017).
Hence, this review aimed to understand GINs-ruminants relationship and the effector immune strategies developed against them. This fundamental understanding highlights the right way to diagnose and control GINs infections in ruminants.
Gastrointestinal nematodes of ruminants
GINs are the major constraint to health, productivity and welfare of ruminants. Most species of these nematodes are related to order Strongylida, family Trichostrongyloidea. Most prevalent genera which infect small and large ruminants include Haemonchus, Cooperia, Ostertagia, Teladorsagia, Bunostomum, Trichostrongylus, Oesophagostomum, Chabertia, Nematodirus, Protostrongylus and Trichuris (Amarante and Amarante 2016; Belina et al. 2017). They usually habituate abomasum, small intestine and some lesser extent to large intestine. In general, the species of GINs are bounded to one host species. Nonetheless, particular exclusions are found among different ruminants such as Trichostrongylus axei (T. axei) and Haemonchus placei (H. placei) which is found in cattle and sheep. Haemonchus longistipes (H. longistipes) is a nematode of camel, but it can infect goats (Elshahawy et al. 2014).
In Egypt, most prevalent GINs in sheep and goat populations are H. contortus, T. axei, Bunostomum spp., Strongyloides papillosus (S. papillosus), Nematodirus spp., Ostertagia trifurcata, Chabertia spp. and Trichuris ovis (Khalafalla et al. 2011; Al-Gaabary et al. 2012; Elshahawy et al. 2014; Al-Aboody and Omar 2016). In cattle and buffaloes, Cooperia spp., Haemonchus spp., T. axei, Oesophagostomum spp., S. papillosus and Ostertagia spp. are usually found (Sobhy 2005; Al-Aboody and Omar 2016). In camels, nematode helminths are presented by H. longistipes, Trichostrongylus spp., Strongyloides spp., Trichuris spp., and Nematodirus spp. (Mahmoud et al. 2008; Abdel-Rady 2014). Although the nigh phylogenetic relationship between GINs species, there are some differences related to intestinal niches and/or period of larval developmental strategy that influence the immune responses induced against these nematodes. Life cycles of the diverse GINs are simple and direct. After ingestion of the infective L3, they lose their protective sheath and penetrate mucosa of gastro-intestinal tract depending on which nematode is involved (Balic 1999; Miller and Horohov 2006). They reach maturity in mucosal and submucosal membranes to L4 and L5 stages and then return to the lumen. The mature adult stages laid eggs which disseminated with the fecal matter of infected host. Several defense mechanisms are induced by the infectious nematode parasites and series of activities occurred and mediated by particular components such as cytokines, eosinophils, mast cells and antibodies. Most of prepatent periods of different GINs ranged from 18 to 21 days but it may be extended to a longer period of stopped larval developmental stages called hypobiosis which may be triggered by host’s immune response (Miller and Horohov 2006; Angulo-Cubillán et al. 2007).
The immune responses triggered against gastrointestinal nematodes
During gastrointestinal nematode infections, the host’s immune system and infectious nematodes orchestrate different series of defense mechanisms (Grencis et al. 2014). Humeral, cellular immune responses and immunomodulatory mechanisms are induced and subsequently resulted in resistance to host, resilience of negative effects developed and/or creation of the disease (Zvinorova et al. 2016).
Gastrointestinal mucosal immunity
Concerning the non-specific and specific arms of immunity, the gastrointestinal tract is covered with a mucus layer which is considered the first line of defense against GINs. This mucus layer is formed from high molecular weight glycoproteins called mucin which is normally secreted by goblet cells. Thus, nematodes have to pass through this physical barrier to reach epithelial tissue (Grencis et al. 2014).
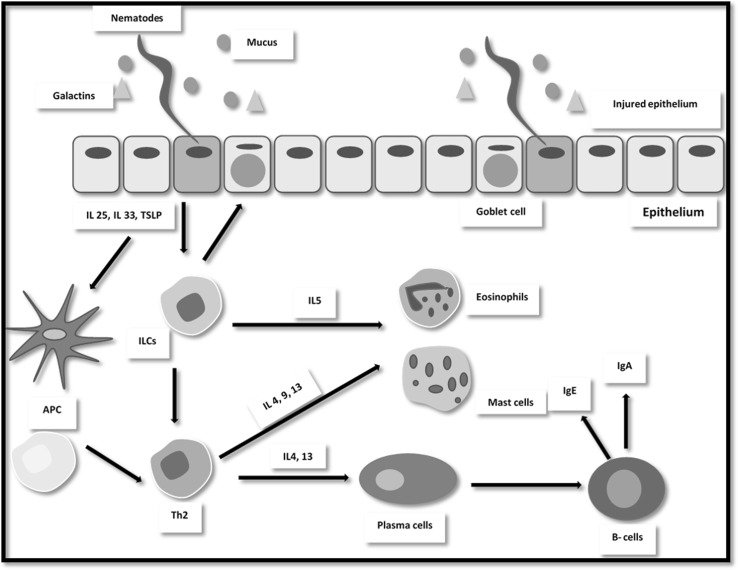
Once the infective L3 stages infect their susceptible hosts, they unsheathe their protective sheath and penetrate mucosa of the intestinal niche. After 1–2 days, they evolve into L4 stage which matures to L5 (immature adult stage) within 2–3 days and then evolve to adult stage in the lumen of gastrointestinal tract (Miller and Horohov 2006; McRae et al. 2015). As a consequence of injuring intestinal epithelial tissue, IL33, IL25 and thymic stromal lymphopoietin are released. These IL33, IL25 signals (i) initiate ILCs activation thus produce type 2 cytokines; IL5, IL9 and IL13. (ii) Induce MMC hyperplasia. (iii) Stimulate Th2 immune response to produce their cytokines mediators; IL4, IL5, IL9, IL10 and IL13 as well as induce IL-5 and IL-13 that released by B cells (Table 1, Fig. 1). Thus, these processes elicit type 1 hypersensitivity reaction and eosinophils and mucosal mast cell effector mechanisms (Meeusen et al. 2005; Komai-Koma et al. 2011; Allen and Sutherland 2014; Garza 2014).
Table 1.
Major cytokines mediated immune responses against gastrointestinal nematode infections
| Cytokines | Released by | Functions | References |
|---|---|---|---|
| IL33 | Injured epithelium | Induce MMC hyperplasia, initiate ILCs and stimulate Th2 immunity | Allen and Sutherland (2014), Garza (2014) |
| IL25 | Injured epithelium | Induce MMC hyperplasia, initiate ILCs, stimulate Th2 immunity and act as anti-inflammatory factor | Allen and Sutherland (2014), Garza (2014) |
| IFNγ | Th1 and damaged cells | Activate macrophages, induce proliferation of CD8 lymphocyte and Promote IgG2 switching | Estes and Brown (2002), McRae et al. (2015) |
| IL2 | Th1 | Activate macrophages and induce proliferation of CD8 lymphocyte, | McRae et al. (2015) |
| TNF | Th1 | Activate macrophages and induce proliferation of CD8 lymphocyte, | McRae et al. (2015) |
| IL3 | Th2 | Induce mastocytosis and eosinophils infiltration | Dawicki and Marshall (2007), Shin et al. (2009) |
| IL4 | Th2, MMC and eosinophils | Induce MMC and globule leucocyte hyperplasia, induce eosinophil infiltration and promote IgE and IgG1 production | Estes and Brown (2002), Lacroux et al. (2006), McRae et al. (2015) |
| IL5 | Th2, MMC and eosinophils | Induce infiltration of eosinophils and MMC hyperplasia and promote IgA, IgE production | Pernthaner et al. (2005), Shin et al. (2009) |
| IL6 | MMC and eosinophils | Induce infiltration of eosinophils, stimulate enteric nerves and IgE differentiation | Dawicki and Marshall (2007), McRae et al. (2015) |
| IL9 | Th2 | Promote MMC activity | McRae et al. (2015) |
| IL10 | Th2and eosinophils | IgE differentiation | Duque and Descoteaux (2014), McRae et al. (2015) |
| IL12 | APC | Stimulate Th1 and CD8 T cells | Duque and Descoteaux (2014) |
| IL13 | Th2and eosinophils | Induce infiltration of eosinophils and MMC hyperplasia, stimulate mucus production and promote IgG1 switching | Estes and Brown (2002), Pernthaner et al. (2005), Dawicki and Marshall (2007), McRae et al. (2015) |
| IL18 | Treg | Promote IgG2 switching | McRae et al. (2015) |
| IL17, IL21 | Treg | Control the induced Th2 | McRae et al. (2015) |
Fig. 1.
Immune response strategies triggered against gastrointestinal nematodes
Epithelial cells express and secrete galectins (Gal-15) which are carbohydrate binding molecules. These molecules firming cellular adhesion on nematode surface and interact with the mucin. This interaction results in increasing mucus viscosity thus impairing movement of the nematode parasite (Meeusen et al. 2005). Additionally, alternate complement pathway is activated by infective larval antigen that binds some opsonins on its surface. Thus, C3a and C5a chemotactic peptides complement are generated and promote the recruitment of eosinophils to site of infection which induce inflammatory responses (Angulo-Cubillán et al. 2007; Garza 2014).
Moreover, lymph nodes are incorporated in mucosal immunity where APC are activated and played a particular role in antigen recognition and presentation. Thus, a secondary infection could induce faster and potent local immune response (González et al. 2003).
Antigen recognition and presenting cells
Dendritic cells and macrophages are type of innate immunity cells which known as APC. They are the initiating and linking factor between innate and adaptive immune responses (McRae et al. 2015). Dendritic cells are found in most of tissue. As well as, macrophages are the differentiated monocytes in different tissues (Garza 2014). The phagocytosis process is initiated by activation of dendritic and macrophages via interferon-gamma (IFNγ). Simultaneously, APC express a toll-like receptor for recognition of pathogen associated molecular pattern of invading parasite and a major histocompatibility class II (MHC-II) receptor for antigen presentation (Werling et al. 2006; Duque and Descoteaux 2014).
There are two types of activated macrophages. (i) M1 “killer” macrophages are activated by IFNγ, induce inflammatory process and release tumor necrosis factor (TNF), IL1, IL6, IL8 and IL-12 plus certain chemotactic cytokine that attract eosinophils to site of infection. (ii) M2 ‘‘repair’’ macrophages which are activated by IL4 and IL13 and induce tissue repair via secretion of high level of IL10 (act as anti-inflammatory factor) (Mosser and Edwards 2008; Duque and Descoteaux 2014) (Table 1, Fig. 1).
Effector cell mechanisms
Effector immune paradigms involve GINs infection are usually expressed by eosinophil infiltration, MMC hyperplasia and Th2 immune strategies (Huang and Appleton 2016) (Fig. 1).
Role of eosinophils
Eosinophilia (increasing in number of eosinophils in peripheral blood circulation and tissue), is a typical sequence of parasitic infections (Shin et al. 2009).
The activated eosinophils release IL-4, IL-5, IL-6, IL-10, and IL-13 thus regulate B cell for IgE differentiation (Souza et al. 2015). Also, various chemokines are secreted by eosinophils as eotaxin-1 which strengths eosinophils infiltration, monocyte basic protein that regulate mast cells and galectins (Gal-14) which interact with Gal-15 to strength adhesion of cells on parasite surface (Meeusen et al. 2005; Souza et al. 2015).
Many authors investigated the immune response developed against GINs via estimation of the effector cells level in blood or tissue of the infected host during course of infection. The susceptible sheep group infected with H. contortus presented lower eosinophils count accompanied with higher worm burden than the resistant group (Shakya et al. 2009; Ortolani et al. 2013). The primary infections in immunized sheep induce more rapid tissue eosinophilia with closer association to tissue larvae when compared to repeated infection; the eosinophilia was restricted to presence of challenge larvae (Balic et al. 2002, 2006). As an effector cell, eosinophils play a crucial role in parasite killing. Induction of eosinophil degranulation in order to release their secondary granular proteins such as major basic protein, eosinophil cationic protein, eosinophil peroxidase, and eosinophil neurotoxin is dependent on IL5, C5a and IgA stimuli (Shin et al. 2009; Garza 2014) (Table 1). The larvae associated with eosinophil showed marked losses in internal integrity and distortion to circular sheath in transmission electron microscope sections (Balic et al. 2006). However, the effective mechanisms that expel worms from intestine differ among infectious nematode (Gebreselassie et al. 2012).
On the other hand, there is non-significant relation between tissue eosinophilia and worm burden of Teladorsagia circumcincta (T. circumcincta) infection in sheep and this result differs from that immune responses triggered against H. contortus (Henderson and Stear 2006). Consequently, the immune response could move forward other immunological pathways.
Role of mucosal mast cells and globule leucocytes
Mucosal mast cells and globule cells hyperplasia (increasing number of cells) occur at intestinal mucosal layer in particular to GINs infection (Balic et al. 2000). Mast cells are granular leucocytes that have a specific receptor (FcεR1) on its surface for IgE binding and granules contain specific mediators that induce type I hypersensitivity and stimulate parasitic expulsion such as histamine, serotonin, proteinases, prostaglandins, leukotrienes, 5-hydroxy tryptamine and bradykinin (Abraham and St. John 2010). As well as, they release cytokines; as IL4, IL5 and IL6 that act as pro-eosinophilic factor, stimulate enteric nerves, regulate IgA production and induce T helper immune response. Mastocytosis is triggered either by an antigenic parasite stimulus in particular at primary infection or by IL-3 and IL-9 Th2 cytokines as a secondary adaptive immune response (Dawicki and Marshall 2007; McRae et al. 2015). Degranulation of this activated MMC is dependent on IgE binding to its receptor. Subsequently, these released mediators induce immediate hypersensitivity thus increase permeability of the mucus membrane at site of infection, enabling transmission of complement and antibodies to niche lumen, activate mucus secretion, stimulate enteric nerves and smooth muscles contraction resulting in flushing and expulsion of intestinal nematodes (Dawicki and Marshall 2007; Abraham and St. John 2010; McRae et al. 2015) (Table 1).
Mast and globule cells particularly reflect the immune status of the host against GINs infections. In previous studies, authors recorded that globule cells and MMC are indicator of H. contortus infection in both breeds (Native and Suffolk) of sheep (Shakya et al. 2009; Ortolani et al. 2013). They are found in high level on day 7 post infection and associated with worm number reduction (Garza 2014). However, MMC and globule leucocytes activity may differ between ruminant’s species. Infected goats with T. circumcincta and Trichostrongylus vitrinus showed more globule leucocytes than MMC compared to sheep when were challenged with the same nematodes (Huntley et al. 1995). Unfortunately, degranulation of MMC couldn’t mimic its count, because they lose their granules after its activation, thus the reliable monitoring assay to estimate activity of MMC is measuring of the released chymotrypsin-like proteases in serum and intestinal mucus (Rothwell 1989; Miller 1996). On contrary, the increased globule and MMC numbers in abomasal mucosa are negatively correlated with recruitment of eosinophil numbers and larval stages of GINs particularly during primary infection at day 7, 15 and 28 post infection, but it is apparent at day 3 post infection in previously infected animals (Lacroux et al. 2006; Balic et al. 2006). Therefore, the secondary infection is almost orchestrated by Th2 lymphocyte and their released cytokines.
T-Helper 2 and their cytokine mediators
There are different types of T lymphocytes; CD4, CD8 and γ β subsets which played a vital role in host immune defense mechanisms. The γ β lymphocytes were previously known as non-B non-T lymphocytes. They are abundantly established in blood of ruminants and locate in intestinal epithelium, consequently have important role in immune response to GINs. The CD8 lymphocytes play main role as cytotoxic T cells against intracellular pathogens or damaged cells (McRae et al. 2015). CD4 T-Lymphocytes cells recognize specific antigens with MHC class II molecules. It is divided into 3 subpopulations; Th1, Th2 and Th17 or named regulatory T cell (Treg cells). Th1 releases IFN-γ, IL-2 and TNF-β and usually incorporated the defense mechanism against intracellular pathogens, induce propagation of CD8 lymphocytes and activate macrophages in particular during GINs infection (Sorci et al. 2013; McRae et al. 2015).
At early stage of helminth infection, Th1 lymphocytes stimulated by IL-12 which produced by dendritic cells and macrophages. These activated Th1 lymphocytes release TNF-α and coordinate the cellular immune response. However, with the progression of infection, the strong Th2 polarization induced by these helminths, and the consequent downregulation of the Th1 profile (Abo-Aziza et al. 2017; Rodrigues et al. 2017).
Th2 lymphocytes produce IL3, IL-4, IL-5, IL-9, IL-10 and IL-13 and coordinates immune response that developed against GINs (Meeusen et al. 2005; Angulo-Cubillán et al. 2007; McRae et al. 2015). The immune response regulation is dependent on type of the expressed cytokine profile in the course of infection (Ortolani et al. 2013). A vast majority of previous literatures investigated the role of different ILs that regulate immune responses to GINs in ruminants (Fig. 1).
For instance, primary and previously infected sheep with H. contortus showed high level of IL-4 mRNA that expressed in the lymph node and mucosa of their abomasa and gradually increased from 7 dpi and 3 dpi to 28 dpi, respectively, thus increase recruitment of sensitive effector cells (Lacroux et al. 2006). On the other hand, the intestinal lymph node cells of sheep infected with T. colubriformis showed IL5 and IL13 expression which have the same signaling to induce infiltration of eosinophils and MMC hyperplasia (Pernthaner et al. 2005). IL13 plays a role in mucus production and regeneration of intestinal epithelium during GINS infections and along with IL9 can promote activation of MMC. As well as, Th2 cytokines have pivotal role in differentiation of Ig produced during infection with nematodes. In sheep, IL5 induces eosinophilia and at the same time promotes IgA production, while IL4 induces switching of induced B cell to produce IgE subclass (Finkelman et al. 1988; Harriman et al. 1988; McRae et al. 2015). In cattle, IL4 and IL13 trigger IgG1 and IgE production, however IFN-γ and IL18 induce switching of IgG2 differentiation (Estes and Brown 2002).
On the other hand, the triggered Th2 immune response is regulated and controlled by Treg cell through secretion of IL17 and IL21 (McRae et al. 2015) (Table 1).
B-Cell (antibody) immune response
T helper 2 lymphocytes and their secreted cytokines profiles; IL4, IL5, IL10 and IL13 particularly induce appropriate Ig isotypes; IgA, IgE, and IgG production and switching during GINs infection (Estes and Brown 2002; Angulo-Cubillán et al. 2007; McRae et al. 2015). These antibodies subclasses are dependably produced by activated B cells either in primary or secondary infections. Each antibody isotype has a specific role in defense mechanism triggered against GINs infections and their increased logarithmic level occur within 7–14 days post infection (Lacroux et al. 2006).
IgA is usually secreted locally at intestinal mucosa and also, can be detected in serum of infected animal. The role of the local IgA immune response has been showed in T. circumcincta infected sheep, where the production of local IgA against somatic or L4 excretory secretory products resulted in reduced fecundity and length of worms (Halliday et al. 2007; McRae et al. 2014). In addition, H. contortus-specific IgA in association with other factors as Gal-11 and Gal-14 could increase eosinophils adherence and reduce nematode motility in response to infection (Lacroux et al. 2006). Ostertagia-specific IgA antibodies increase in the serum of calves either they are artificially or naturally infected with Ostertagia spp. (Balic et al. 2000). Also, IgA activity which developed against GINs (Mecistocirrus digitatus and Cooperia punctate) showed differences among different breeds of cattle. The Brangus and Brown Swiss breeds had higher IgA activity than Guzerat breed. The animals that showed high level of IgA levels in their sera indicate a high level of resistance against GINs infection (González-Garduño et al. 2017).
The immune responses triggered against GINs infections are predominantly associated with IgE-mediated immediate hypersensitivity response at intestinal mucosal niche. IgE plays an important role in inducing degranulation of MMC. In previous literatures, many authors stated that elevation of IgE serum antibodies has been observed during infection with H. contortus (Kooyman et al. 2000), T. colubriformis (Shaw et al. 1998) and T. circumcincta (Pettit et al. 2005). Moreover, IgE level in cattle during Ostertagia ostertagi (O. ostertagi) infections is elevated (Gasbarre et al. 2001).
The specific IgG response developed against GINs challenge is predominantly found in sera of infected animals. In case of Ostertagia and Cooperia-infected calves, IgG is the main subclass that characterizes these infections. Total IgG levels have been used successfully to estimate humeral adaptive immunity to O. ostertagi in cattle (Premier et al. 2004), as well as H. contortus in sheep (Kandil et al. 2015, 2016, 2017). In sheep that were genetically resistant to H. contortus, serum IgG levels were significantly higher than in random-bred sheep (Gill et al. 2000). As well as, high level of IgG was observed in T. colubriformis challenged sheep (Shaw et al. 1998). However, in different cattle breeds; Guzerat, Brangus, Charolais and Brown Swiss, IgG activity triggered against adult worm crude antigen of Cooperia punctata or Mecistocirrus digitatus showed little differences (González-Garduño et al. 2017).
Hence, the culmination of IL-4, IL5 and other interleukins mRNA expression (Th2 orientation), recruitment of eosinophils and MMC, IgA, IgE and IgG production result in an appropriate immune response which distort survival and establishment of GINs infection. This interference is manifested by various phenotypes of infected nematodes in resistant host.
Immune response manifestations
The immune responses against GINs resulting from exposure to L3, L4 and adult are manifested by the failure of larval establishment, the expulsion of adult worms, changes in the morphology of adult nematodes and the reduction in the fecundity of female nematodes, thus control the number of eggs excreted in the feces each day and contaminate the pasture. These manifestations are previously stated by many authors as parasitological evaluating parameters to host resistance (González et al. 2003; Meeusen and Piedrafita 2003; Miller and Horohov 2006; Prada et al. 2014).
Failure of larval establishment
The evidence for cell immunity in parasite rejection has been deduced from the attempts to block the final effector response and histopathology changes. Mechanism of rapid expulsion to infective larvae before they reach their intestinal niche, within few hours may occur. This rapid expulsion is a subsequent manifestation to MMC hyperplasia and globule leucocytes activation. Though, a negative correlation between the number of established larvae and MMC and globule cells is recorded (Meeusen and Piedrafita 2003), while delayed expulsion occurs if these larvae reach their intestinal niche. The eosinophils and lymphocytes infiltration are responsible for this delayed expulsion; thus, they could kill and damage these larvae (Meeusen and Balic 2000; Meeusen and Piedrafita 2003).
Reduction in the rate of development of larvae may also occur. This retarded larval growth needs multiple larval challenges and is stage-specific, to stimulate host immunity against this stage (Balic et al. 2000; González et al. 2003). Otherwise, hypobiosis phenomena (arrested larval development) could occur in association with host resistance to GINs infection; O. ostertagi, H. placei, H. contortus, T. circumcincta and T. colubriformis. As well as, it could be affected with seasonal variation, strain and density of nematodes (González et al. 2003).
Expulsion of adult nematodes
In ruminants, expulsion of adult GINs is uncommon after a primary infection. The developed resistance gradually increases in ruminants as a consequence of repeated exposure to infection, therefore acquired immunity specific to the adult induces an immediate hypersensitivity resulted in expulsion of such nematodes; O. ostertagi, H. placei, H. contortus, T. circumcincta and T. colubriformis (González et al. 2003). Sometimes, a self-cure phenomenon (spontaneous expulsion of adult) occurs when massive numbers of larvae invade the host over a very short exposure period. This larval invasion results in IgE mediated hypersensitivity and increases abomasal pH, thus nematode expulsion happens (Miller and Horohov 2006; Garza 2014).
Increased mortality and/or dampened establishment of nematode helminths result in low worm load. The recovered worm burden of Cooperia spp. was much lower than of Ostertagia spp. after mixed challenge infection and this diversity occurs due to earlier adaptive immunity developed against Cooperia spp. than Ostertagia spp. (Hilderson et al. 1995; Ploeger et al. 1995).
Changes in morphology
The changes in worm morphology are exhibited as the size reduction of the vulval flap and reduced length of adult O. ostertagi, T. circumcincta and H. contortus (Sutherland et al. 1999; Gasbarre et al. 2001; González et al. 2003). This reduced length may be due to inhibited growth, a selective expulsion of large worms or shrinkage of worms during the infection (Miller and Horohov 2006).
Decreasing in female fecundity
Decreasing in female fecundity and numbers of eggs excreted by an adult nematode is one of manifested measures that indicate immune resistance of the host against GINs. The female fecundity and parasite development were dampened in secondary infected lambs with H. contortus compared to primary infected animals (Lacroux et al. 2006). IgA production may be a major mechanism in controlling fecundity of GINs and this response is influenced by diet quality (Toscan et al. 2017). The number of eggs per female in Ostertagia spp., H. contortus and T. colubriformis in infected cattle, sheep and goats significantly reduced after previous exposure to infection (González et al. 2003).
Factors affecting immune response to gastrointestinal nematodes
Immune responses developed against GINs infections are persuaded by the culmination of numerous factors that encountering the host-parasite relationship. These factors could be related to involved host, infectious nematode and/or managing system that incorporated them (Angulo-Cubillán et al. 2007; Cooper and Eleftherianos 2016).
Host related factors
Resistance to GINs is differed either between breeds of small and large ruminants (Hou et al. 2012; Garza 2014; González-Garduño et al. 2017) or between species (Huntley et al. 1995). Genotypic selection of traits as a natural resistance to GINs recovers individuals which could increase activity of the expressed IL4, IL5, IL13 and increase production of IgE and IgA antibodies (Hou et al. 2012; Souza et al. 2015). Genetic selection of lines of sheep or goats within a breed should be based on main phenotypic criteria related to low nematode fecal egg count (to improve host resistance), as well as criteria measuring production of animals under parasitic challenge (to improve host resilience) (Zvinorova et al. 2016).
Arguably, Brangus and Brown Swiss breeds of cattle showed higher IgA levels in their sera and saliva than in Guzerat and Charolais breeds. However, Brangus and Guzerat breeds had higher eosinophils levels than that detected in Brown Swiss and Charolais breeds which developed against infection with GINs (González-Garduño et al. 2017). Additionally, prominent eosinophils infiltration is found in abomasal mucosa of Gulf Coast Native lambs when compared with Suffolk lambs nonetheless, both breeds displayed elevated MMC and IL13 levels when exposed to H. contortus infection (Garza 2014).
At the species level, sheep has ample MMC in their gastrointestinal tract mucosa compared with goats. Thus, goats are susceptible to infection with T. circumcincta and T. vitrinus than sheep and sequentially the mast cell proteinase is found in higher concentration in infected tissues of sheep (Huntley et al. 1995). In addition, infection of goats with H. contortus larvae unable to induce resistance against homologous challenge infection but recent reports have reported that some tropical goat breeds are genetically resistant to infection with H. contortus (Baker 1998; Zvinorova et al. 2016).
The efficacy of the immune system increases with age of the animal, thus young animals are more susceptible to infection compared to older one. Therefore, young hosts acquire heavier infection than older hosts, nonetheless the developed immunity is weaker.
As well as, the physiological status of the animal influence host immunity, thus at the peri-parturient and post-parturient periods, the immune response becomes weaker against GINs infection (Miller and Horohov 2006; Garza 2014).
Parasitic immune evasion strategies
Gastrointestinal nematodes evade the host’s effector immune mechanisms by different ways. One of them is evasion from immunological memory which developed before by the infected host. The host can build specific immune response against common variant of adult’s integument of infectious nematode, however one or more newly expressed variants can rise by this parasite to evade (Frank 2002; Kandil et al. 2016). Also, most of the early larval stages of GINs represent a speedy molting at their intestinal niche to evolve to mature stages (1–5 days). This rapid switching restricts the specialized strategies of the host’s immune system (Meeusen et al. 2005).
Cystatins are cysteine protease inhibitors (have immunomodulatory effect) that secreted by nematodes to inhibit cysteine proteases such as legumains or cathepsin L and S, and/or to enhance production of anti-inflammatory IL10. Legumains are important to antigen processing and presentation, thus it interfere generation of MHC class II molecules and subsequently impair Th2 adaptive immune response, while cathepsin is involved in polypeptide synthesis. Also, cystatins mount upregulation of IL10 those downregulate Th2 cytokines and inducing anti-inflammatory effects (Shin et al. 2009; Sorci et al. 2013; Cooper and Eleftherianos 2016). As well as, nematode helminths can produce specific component (ES-62) that interrupts the effector immune mechanisms, thus leading to dampen in eosinophils infiltration, MMC hyperplasia and impair B and T cell proliferation (Shin et al. 2009). Additionally, this parasite could release sperm-coating protein-like extracellular domain (SCP/TAPS) which plays a role in the inhibition of neutrophils and platelets activity. Recently, micro-RNAs containing vesicles are secreted by some nematode species. These vesicles are taken by macrophages and thus downregulate IL33 and impair Th2 immune response (Quintana et al. 2015).
Interestingly, specific immune responses could differ according to nematode species. For instance, immunity developed against O. ostertagi is weak and slowly, while Oesophagostomum radiatum triggers rapid effective immune response that may avoid reinfection (Gasbarre 1997). On the other hand, Cooperia spp. or H. placei, require a longer period of exposure before this level of protective immunity is seen (Balic et al. 2000).
Management factors
The manipulation of the host nutrition (increasing of energy and protein component in diet) could represent a choice to overcome pathophysiological consequences of parasites and improve immunological status of the host (Torres-Acosta 2003). The addition of food supplements to animal’s ration as copper oxide wire particles, cobalt and phosphorus has an effective role in reducing fecal nematode egg count (Sykes and Coop 2001; Burke et al. 2007; Soli et al. 2010). Also, vitamin E supplement causes negative relationship between eosinophilia and worm burden (Garza 2014).
Also, usage of bioactive forages containing tannins has direct effect on GINs infections, thus impair their larval establishment and decrease female fecundity. Moreover, these tannins affect development of free-living stages in dung (Martínez-Ortíz-de-Montellano et al. 2010; Shalaby 2013). Thus, well-fed animals reasonably respond better to nematode infections than those with low food intake (Bricarello et al. 2005; Toscan et al. 2017).
The use of short term grazing rotation system in order to affect survival of L3 stages on the pasture have been found effective in tropical and subtropical area compared to temperate area in particular H. contortus larvae (Barger et al. 1994; Garza 2014). Also, pasture contamination with infective larval stages could decrease if highly susceptible animals to GINs infection are culled (Pisseri et al. 2013).
The regular usage of chemo-prophylactic and chemo-therapeutic drugs schedule leads to evolve GINs resistance strains to commercial anti-helminthic drugs on some evaluated cattle farms (Ramos et al. 2016). Alternatively, usage of candidate vaccine to control GINs in particular H. contortus has a promising trend to control this infection. For instance, recombinant rHcp26/23 vaccine induces protective immune response against sheep haemonchosis (Kandil et al. 2017). Additionally, route of administration could affect induced immune response. The potent mucosal and IgA responses were induced by direct injection of antigen into intestinal and rectal mucosa than other route of administration (Premier et al. 2004).
Conclusion
This review highlights GINs-ruminants relationship and the effector immune strategies developed against GINs infections. The fundamental understanding to this relationship could emerge better diagnostics and control to GINs infections in ruminants.
To combat GINs infections, increased mucus, eosinophilia, MMC activation, Th2 immune response polarization and production of specific IgA, IgE and IgG antibodies are elicited as typical features which bound to developed immunity by the infected host.
Th2 cytokines coordinate the effector cell mechanisms and play pivotal role in immune strategies against GINs infection.
As clearly, identification of key molecules associated with the immune responses developed by the host could prompt improvement of an appropriate protective immune strategy.
Insights concerning host resistance, nematode immunomodulatory mechanisms and surrounding environments could deliver hallmarks to overcome anthelminthic resistance phenomenon and drug residues in animal products. As well as, these insights could improve genetic selection of resistant and resilient host.
Recommendations
Genetic selection of such ruminants within a breed could improve host resistance and resilience against GINs.
Improvement of host nutrition (increasing of energy and protein component in diet), besides the use of bioactive forages containing tannins and food supplements could improve host immune response that triggered against GINs.
The use of short term grazing rotation system and limitation of chemoprophylactic drugs could control GINs infections.
Routine fecal examination before treatment and culling of highly susceptible hosts to GINs could overcome anthelminthic drug resistance phenomenon and drug residues in animal products.
The use of candidate vaccine could maintain a protective immunity pathway to control GINs infections.
Conflict of interest
The author declares that there is no conflict of interest.
Ethical standards
This review article is prepared and presented by the author in accordance with the ethical standards.
References
- Abdel-Rady A. Epidemiological studies on parasitic infestations in camels (Camelus dromedarius) in Egypt. IJAVMS. 2014;8:142–149. [Google Scholar]
- Abo-Aziza FAM, Hendawy SHM, El Namaky AH, Ashry HM. Th1/Th2 balance and humoral immune response to potential antigens as early diagnostic method of equine Strongylus nematode infection. Vet World. 2017;10:679–687. doi: 10.14202/vetworld.2017.679-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham SN, St. John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aboody MS, Omar MA. Prevalence of gastrointestinal nematodes of farm animals by copro-culture. Russ J Parasitol. 2016;36:168–174. [Google Scholar]
- AL-Gaabary MH, Osman SA, Abo EL-Soud KM, Hassan AI. Studies on gastrointestinal nematodes infection in sheep with special reference to Haemonchus contortus. Assiut Vet Med J. 2012;58:31–40. [Google Scholar]
- Allen JE, Sutherland TE. Host protective roles of type2 immunity: parasite killing and tissue repair flip sides of the same coin. Semin Immunol. 2014;26:329–340. doi: 10.1016/j.smim.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarante AFT, Amarante MRV. Advances in the diagnosis of the gastrointestinal nematode infections in ruminants. Braz J Vet Res Anim Sci. 2016;53:127–137. doi: 10.11606/issn.1678-4456.v53i2p127-137. [DOI] [Google Scholar]
- Angulo-Cubillán FJ, García-Coiradas L, Cuquerella M, José CF, Alunda M. Haemonchus contortus-sheep relationship: a review. Rev Cient. 2007;17:577–587. [Google Scholar]
- Baker RL. A review of genetic resistance to gastrointestinal nematode parasites in sheep and goats in the tropics and evidence for resistance in some sheep and goat breeds in sub-humid coastal Kenya. AGRI. 1998;24:13–30. [Google Scholar]
- Balic A (1999) Immunobiology of Haemonchus contortus infections in sheep. Ph.D. thesis, University of Melbourne, Australia
- Balic A, Bowles VM, Meeusen EN. The immunobiology of gastrointestinal nematode infections in ruminants. Adv Parasitol. 2000;45:181–241. doi: 10.1016/S0065-308X(00)45005-0. [DOI] [PubMed] [Google Scholar]
- Balic A, Bowles VM, Meeusen EN. Mechanisms of immunity to Haemonchus contortus infection in sheep. Parasite Immunol. 2002;24:39–46. doi: 10.1046/j.0141-9838.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- Balic A, Cunningham CP, Meeusen EN. Eosinophil and nematode interactions with Haemonchus contortus larvae in the ovine gastrointestinal tract. Parasite Immunol. 2006;28:107–115. doi: 10.1111/j.1365-3024.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- Barger IA, Siale K, Banks DJD, Le Jambre LF. Rotational grazing for control of gastrointestinal nematodes of goats in a wet tropical environment. Vet Parasitol. 1994;53:109–116. doi: 10.1016/0304-4017(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Belina D, Abdurahman G, Mengistu S, Eshetu A. Gastrointestinal nematodes in ruminants: the parasite burden, associated risk factors and anthelmintic utilization practices in selected districts of east and western Hararghe, Ethiopia. J Vet Sci Technol. 2017;8:433–440. doi: 10.4172/2157-7579.1000433. [DOI] [Google Scholar]
- Bricarello PA, Amarante AFT, Rocha RA, Cabral Filho SL, Huntley JF, Houdijk JGM, Abdalla AL, Gennari SM. Influence of dietary protein supply on resistance to experimental infection with Haemonchus contortus in Il de France and Santa Ines lambs. Vet Parasitol. 2005;134:99–109. doi: 10.1016/j.vetpar.2005.05.068. [DOI] [PubMed] [Google Scholar]
- Burke JM, Morrical D, Miller JE. Control of gastrointestinal nematodes with copper oxide wire particles in a flock of lactating Polypay ewes and offspring in Iowa, USA. Vet Parasitol. 2007;146:372–375. doi: 10.1016/j.vetpar.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Cooper D, Eleftherianos I. Parasitic nematode immunomodulatory strategies: recent advances and perspectives. Pathogens. 2016;5:58–70. doi: 10.3390/pathogens5030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawicki W, Marshall JS. New and emerging roles for mast cells in host defense. Curr Opin Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Duque GA, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:1–12. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Namaky AH, Hendawy SH, Abo-Aziza FA, Ashry HM. Cytokines and immunoglobulin G response in donkeys with spontaneous Setaria equina infection. BJVM. 2017 doi: 10.15547/bjvm.2049. [DOI] [Google Scholar]
- Elshahawy IS, Metwally AM, Ibrahim DA. An abattoir-based study on helminthes of slaughtered goats (Capra hircus L., 1758) in Upper Egypt, Egypt. Helminthologia. 2014;51:67–72. doi: 10.2478/s11687-014-0210-2. [DOI] [Google Scholar]
- Estes DM, Brown WC. Type 1 and type 2 in regulation of Ig isotype expression in cattle. Vet Immunol Immunopathol. 2002;90:1–10. doi: 10.1016/S0165-2427(02)00201-5. [DOI] [PubMed] [Google Scholar]
- Finkelman FD, Katona IM, Urban JF. IL-4 is required to generate andsustain in vivo IgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- Frank SA. Immunology and evolution of infectious disease. Princeton: Princeton University Press; 2002. [PubMed] [Google Scholar]
- Garza JJ (2014) Comparison of immune responses during gastrointestinal helminth self-cure expulsion between resistant Gulf Coast Native and susceptible Suffolk sheep. LSU Doctoral Dissertations, 1019
- Gasbarre LC. Effects of gastrointestinal nematode infection on the ruminant immune system. Vet Parasitol. 1997;72:327–343. doi: 10.1016/S0304-4017(97)00104-0. [DOI] [PubMed] [Google Scholar]
- Gasbarre LC, Leighton EA, Sonstegard T. Role ofthe bovine immune system and genome in resistance to gastrointestinal nematodes. Vet Parasitol. 2001;98:51–64. doi: 10.1016/S0304-4017(01)00423-X. [DOI] [PubMed] [Google Scholar]
- Gebreselassie NG, Moorhead AR, Fabre V, Gagliardo LF, Lee NA, Lee JJ, Appleton JA. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol. 2012;188:17–425. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Altmann K, Cross ML, Husband AJ. Induction of T helper 1 and T helper 2 type immune responses during Haemonchus contortus infection in sheep. Immunology. 2000;99:458–463. doi: 10.1046/j.1365-2567.2000.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JF, Molina JM, Ruiz A, Conde de Felipe MM, Rodríguez- Ponee E. The immune response against gastrointestinal nematodes in ruminants: a review. Rev Iber Parasitol. 2003;63:97–115. [Google Scholar]
- González-Garduño R, Arellano MEL, Mendoza de Gives P, García JA, Magdeleine CM, Hernández GT, Hernández JO, Hinojosa-Cuéllar JA. Comparative response of IgA and IgG activity and hematological parameters among four main beef-cattle breeds infected with gastrointestinal nematodes in the warm humid tropic of Mexico. Ann Anim Sci. 2017;17:819–833. doi: 10.1515/aoas-2016-0089. [DOI] [Google Scholar]
- Grencis RK, Humphreys NE, Bancroft AL. Immunity to gastrointestinal nematodes: mechanisms and myths. Immunol Rev. 2014;260:183–205. doi: 10.1111/imr.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday AM, Routledge CM, Smith SK, Matthews JB, Smith WD. Parasite loss and inhibited development of Teladorsagia circumcincta in relation to the kinetics of the local IgA response in sheep. Parasite Immunol. 2007;29:425–434. doi: 10.1111/j.1365-3024.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- Harriman GR, Kunimoto DY, Elliott JF, Paetkau V, Strober W. The role of IL-5 in IgA B cell differentiation. J Immunol. 1988;140:3033–3039. [PubMed] [Google Scholar]
- Henderson NG, Stear MJ. Eosinophils and IgA responses in sheep infected with Teladorsagia circumcincta. Vet Immunol Immunopathol. 2006;112:62–66. doi: 10.1016/j.vetimm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Hilderson H, Vercruysse J, Clearebout E, De Graaf DC, Fransen J, Berghen B. Interactions between Ostertagia ostertagi and Cooperia oncophora in calves. Vet Parasitol. 1995;56:107–119. doi: 10.1016/0304-4017(94)00656-W. [DOI] [PubMed] [Google Scholar]
- Hou Y, Liu GE, Bickhart DM, Matukumalli LK, Li C, Song J, Gasbarre LC, Van Tasel CP, Sonstegard TS. Genomic regions showing copy number variations associate with resistance or susceptibility to gastrointestinal nematodes in Angus cattle. Funct Integr Genomics. 2012;12:81–92. doi: 10.1007/s10142-011-0252-1. [DOI] [PubMed] [Google Scholar]
- Huang L, Appleton JA. Eosinophil in helminth infection: defender and dupes. Trends Parasitol. 2016;32:798–807. doi: 10.1016/j.pt.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley JF, PattersonM MackellarA, JacksonF StevensonLM, Coop RL. A comparison of the mast cell and eosinophil responses of sheep and goats to gastrointestinal nematode infections. Res Vet Sci. 1995;58:5–10. doi: 10.1016/0034-5288(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Kandil OM, Eid NA, Elakabawy LM, Abdelrahman KA, Helal MA. Immunodiagnostic potency of different Haemonchus contortus antigens for diagnosis of experimentally and naturally haemonchosis in Egyptian sheep. APG. 2015;6:238–247. [Google Scholar]
- Kandil OM, Hendawy SHM, El Namaky AH, Gabrashanska MP, Nanev VN. Evaluation of different Haemonchus contortus antigens for diagnosis of sheep haemonchosis by ELISA and their cross reactivity with other helminthes. J Parasit Dis. 2016 doi: 10.1007/s12639-016-0865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil OM, Abdelrahman KA, Shalaby HA, Hendawy SHM, Abu El Ezz NMT, Nassar SA, Miller JE. Evaluation of crude larval protein and recombinant somatic protein 26/23 (rHcp26/23) immunization against Haemonchus contortus in sheep. Vet World. 2017;10:758–763. doi: 10.14202/vetworld.2017.758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafalla RE, Elseify MA, Elbahy NM. Seasonal prevalence of gastrointestinal nematode parasites of sheep in Northern region of Nile Delta, Egypt. Parasitol Res. 2011;108:337–340. doi: 10.1007/s00436-010-2066-9. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Ds Gilchrist, McKenzie ANJ, Goodyear CS, Xu D, Liew FY. IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol. 2011;186:2584–2591. doi: 10.4049/jimmunol.1002103. [DOI] [PubMed] [Google Scholar]
- Kooyman FN, Schallig HD, Van Leeuwen MA, MacKellar A, Huntley JF, Cornelissen AW, Vervelde L. Protection in lambs vaccinated with Haemonchus contortus antigens is age related and correlates with IgE rather than IgG1 antibody. Parasite Immunol. 2000;22:13–20. doi: 10.1046/j.1365-3024.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- Lacroux C, Nguyen THC, Andreoletti O, Prevot F, Grisez C, Bergeaud J, Gruner L, Brunel J, Francois D, Dorchies P, Jacquiet P. Haemonchus contortus (Nematoda: Trichostrongylidae) infection in lambs elicits an unequivocal Th2 immune response. Vet Res. 2006;37:607–622. doi: 10.1051/vetres:2006022. [DOI] [PubMed] [Google Scholar]
- Mahmoud MA, Amin MM, Youssef RR, El-Kattan A, Goda ASA, Abou El-Naga TR. Studies on some endoparasites of camels in the Southeastern area of Egypt. SCVMJ. 2008;13:81–92. [Google Scholar]
- Martínez-Ortíz-de-Montellano C, Vargas-Magaña JJ, Canul-Ku HL, Miranda-Soberanis R, Capetillo-Leal C, Sandoval-Castro CA, Hoste H, Torres-Acosta JFJ. Effect of a tropical tannin-rich plant Lysiloma latisiliquum on adult populations of Haemonchus contortus in sheep. Vet Parasitol. 2010;172:283–290. doi: 10.1016/j.vetpar.2010.04.040. [DOI] [PubMed] [Google Scholar]
- McRae KM, Good B, Hanrahan JP. Response to Teladorsagia circumcincta infection in Scottish Blackface lambs with divergent phenotypes for nematode resistance. Vet Parasitol. 2014;206:200–207. doi: 10.1016/j.vetpar.2014.10.023. [DOI] [PubMed] [Google Scholar]
- McRae KM, Stear MJ, Good B, Keane OM. The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunol. 2015;37:605–613. doi: 10.1111/pim.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen ENT, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16:95–101. doi: 10.1016/S0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- Meeusen ENT, Piedrafita D. Exploiting natural immunity to helminth parasites for the development of veterinary vaccines. Int J Parasitol. 2003;33:1285–1290. doi: 10.1016/S0020-7519(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Meeusen ENT, Balic A, Bowles V. Cells, cytokines and other molecules associated with rejection of gastrointestinal nematode parasites. Vet Immunol Immunopathol. 2005;108:121–125. doi: 10.1016/j.vetimm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Miller HRP. Mucosal mast cells and the allergic response against nematode parasites. Vet Immunol Immunopathol. 1996;54:331–336. doi: 10.1016/S0165-2427(96)05696-6. [DOI] [PubMed] [Google Scholar]
- Miller JE, Horohov DW. Immunological aspects of nematode parasite control in sheep. J Anim Sci. 2006;84:124–132. doi: 10.2527/2006.8413_supplE124x. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolani EL, Leal ML, Minervino AHH, Aires AR, Coop RL, Jackson F, Suttle NF. Effects of parasitism on cellular immune response in sheep experimentally infected with Haemonchus contortus. Vet Parasitol. 2013;196:230–234. doi: 10.1016/j.vetpar.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Pernthaner A, Cole SA, Morrison L, Hein WR. Increased expression of interleukin-5 (IL-5), IL-13, and tumor necrosis factor alpha genes in intestinal lymph cells of sheep selected for enhanced resistance to nematodes during infection with Trichostrongylus colubriformis. Infect Immun. 2005;73:2175–2183. doi: 10.1128/IAI.73.4.2175-2183.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit JJ, Jackson F, Rocchi M, Huntley JF. The relationship between responsiveness against gastrointestinal nematodes in lambs and the numbers of circulating IgE-bearing cells. Vet Parasitol. 2005;134:131–139. doi: 10.1016/j.vetpar.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Pisseri F, De Benedictis C, Roberti di Sarsina P, Azzarello B. Sustainable animal production, systemic prevention strategies in parasitic diseases of ruminants. Altern Integ Med. 2013;2:2–7. [Google Scholar]
- Ploeger HW, Kloosterman A, Rietveld FW. Acquired immunity against Cooperia spp. and Ostertagia spp. in calves: effect of level of exposure and timing of the midsummer increase. Vet Parasitol. 1995;58:61–74. doi: 10.1016/0304-4017(94)00711-K. [DOI] [PubMed] [Google Scholar]
- Prada JCJ, Stear MJ, Mair C, Singleton D, Stefan T, Stear A, Marion G, Matthews L. An explicit immunogenic model of gastrointestinal nematode infection in sheep. J R Soc Interface. 2014;11:20140416. doi: 10.1098/rsif.2014.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premier RR, Jacobs HJ, Lofthouse SA, Sedgmen BJ, Meeusen ENT. Antibody isotype profiles in serum and circulating antibody-secreting cells following mucosal and peripheral immunisations of sheep. Vet Immunol Immunopathol. 2004;98:77–84. doi: 10.1016/j.vetimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Quintana JF, Makepeace BL, Babayan SA, Ivens A, Pfarr KM, Blaxter M, Debrah A, Wanji S, Ngangyung HF, Bah GS. Extracellular Onchocerca-derived small RNAs in host nodules and blood. Parasit Vectors. 2015;8:58–69. doi: 10.1186/s13071-015-0656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F, Portella LP, Rodrigues FS, Reginato CZ, Potter L, Cezar AS, Sangioni LA, Vogel FSF. Anthelmintic resistance of gastrointestinal nematodes of beef cattle in the state of Rio Grande do Sul, Brazil. Int J Parasitol Drugs Drug Resist. 2016;6:93–101. doi: 10.1016/j.ijpddr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues GC, Vale VLC, Silva MC, Sales TS, Raynal JT, Pimentel ACM, Trindade SC, Meyer RJ. Immune response against Haemonchus contortus and the Th1-Th2 paradigm in helminth infection. EC Microbiol. 2017;9:152–159. [Google Scholar]
- Rothwell TLW. Immune expulsion of parasitic nematodes from the alimentary tract. Int J Parasitol. 1989;19:139–168. doi: 10.1016/0020-7519(89)90003-9. [DOI] [PubMed] [Google Scholar]
- Shakya KP, Miller JE, Horohov DW. A Th2 type of immune response is associated with increased resistance to Haemonchus contortus in naturally infected Gulf Coast Native lambs. Vet Parasitol. 2009;163:57–66. doi: 10.1016/j.vetpar.2009.03.052. [DOI] [PubMed] [Google Scholar]
- Shalaby HA. Anthelmintic resistance; how to overcome it? Iran J Parasitol. 2013;8:18–32. [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Gatehouse TK, McNeill MM. Serum IgE responses during primary and challenge infections of sheep with Trichostrongylus colubriformis. Int J Parasitol. 1998;28:293–302. doi: 10.1016/S0020-7519(97)00164-1. [DOI] [PubMed] [Google Scholar]
- Shin MH, Lee YA, Min DY. Eosinophil-mediated tissue inflammatory responses in helminth infection. Korean J Parasitol. 2009;47:125–131. doi: 10.3347/kjp.2009.47.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhy SG (2005) Some studies in helminth parasites of abomasum of cattle and buffaloes in Kafr-Elsheikh province. M.V.Sc. Thesis, Kafr-Elsheikh University
- Soli F, Terrill TH, Shaik SA, Getz WR, Miller JE, Vanguru M, Burke JM. Efficacy of copper oxide wire particles against gastrointestinal nematodes in sheep and goats. Vet Parasitol. 2010;168:93–96. doi: 10.1016/j.vetpar.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Sorci G, Cornet S, Faivre B. Immune evasion, immunopathology and the regulation of the immune system. Pathogens. 2013;2:71–91. doi: 10.3390/pathogens2010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza BMPS, Lambert SM, Nishi SM, Benavides MV, Berne MEA, Madruga CR, Almeida MAO. Galectins and collectinis expression are increased in Haemonchus contortus infected Corriedale sheep. Rev Bras Parasitol Vet. 2015;24:317–323. doi: 10.1590/S1984-29612015056. [DOI] [PubMed] [Google Scholar]
- Sutherland IA, Brown AE, Green RS, Miller CM, Leathwick DM. The immune response of sheep to larval challenge with Ostertagia circumcincta and Ostertagia ostertagi. Vet Parasitol. 1999;84:125–135. doi: 10.1016/S0304-4017(99)00079-5. [DOI] [PubMed] [Google Scholar]
- Sykes AR, Coop RL. Interaction between nutrition and gastrointestinal parasitism in sheep. N Z Vet J. 2001;49:222–226. doi: 10.1080/00480169.2001.36236. [DOI] [PubMed] [Google Scholar]
- Torres-Acosta FFJ (2003) The effect of supplementary feeding in browsing Criollo kids and Hair sheep naturally infected with gastrointestinal nematodes. In: 6th international symposium on the nutrition of herbivores, Mérida, Mexico, pp 18–24
- Toscan G, Cadore GC, Limana JFT, Weber A, Palma HH, Duarte MMF, Sangioni LA, Vogel FSF. Immune response of sheep naturally infected with Haemonchus spp. on pastures with two different nutritional conditions. Semin Cienc Agrar. 2017;38:809–819. doi: 10.5433/1679-0359.2017v38n2p809. [DOI] [Google Scholar]
- Werling D, Piercy J, Coffey TJ. Expression of toll-like receptors (TLR) by bovine antigen presenting cells—potential role in pathogen discrimination. Veterinary Immunol Immunopathol. 2006;112:2–11. doi: 10.1016/j.vetimm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Zvinorova PI, Halimani TE, Muchadeyi FC, Matika O, Riggio V, Dzama K. Breeding for resistance to gastrointestinal nematodes—the potential in low—input/output small ruminant production system. Vet Parasitol. 2016;225:19–28. doi: 10.1016/j.vetpar.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]