Abstract
In this survey, rodents and their endoparasites were investigated in the Jaz Murian depression and adjacent areas, southeast Iran. In total, 146 specimens of rodents belong to 13 species were trapped. In general, 10 different genera of endoparasites including 11 species were collected. The endoparasites were identified as follows: (1) Nematodes: Trichuris muris, Syphacia obvelata, Labiostomum sp., Labiostomum naimi, Mastrophorus muris, Aspicularis tetraptera and Heligmosomoides skrjabini, Physaloptera sp. (2) Cestodes: Choanotaenia sp., Raillietina sp., and Hymenolepis diminuta. Of 146 captured rodents, Tatera indica was found with high parasitic infestation (with 93% infested) comparing to Acomys dimidiatus (66%), Rattus rattus (50%), Meriones libycus (15%), Jaculus blanfordi (14%) and Mus musculus (8%) whereas, seven rodent species, Nesokia indica, Gerbillus nanus, Golunda ellioti, Calomyscus hotsoni, Apodemus witherbyi, Cricetulus migratorius and Microtus mystacinus were free from any parasitic infestation. Those six infested rodent species were collected from the center of the Jaz Murian depression, whereas seven non-infested rodents’ species except N. indica and G. nanus live in the marginal ranges of the Jaz Murian depression, therefore, these species inhabiting the central parts were supposed to be more important from the health aspect. The species, Labiostomum naimi collected from A. dimidiatus is the first report of this species in rodents from Iran.
Keywords: The Jaz Murian depression, Endoparasites, Rodents, Labiostomum naimi
Introduction
Rodents especially those living close to human habitats, play an important role in public health and economy (Nateghpour et al. 2015). Different disease agents such as, plague (Azizi and Azizi 2010), cutaneous leishmaniasis (Asgari et al. 2007; Kassiri et al. 2013), murine typhus and Lassa fever (Kia et al. 2010) can be transmitted by rodents to human (Khajeh et al. 2017).
Although, some investigations have been done on rodents’ endoparasites in Iran (Kia et al. 2010; Yousefi et al. 2014; Pakdel et al. 2013; Garedaghi and Khaki 2014; Meshkekar et al. 2014), there are few investigations concentrating to wild rodents and their endoparasites in the Jaz Murian depression (Nateghpour et al. 2015; Kassiri et al. 2013). Nateghpour et al. (2015) carried out a study on four species of rodents’ endoparasites (Tatera indica, Gerbillus nanus, Meriones hurrianea, Meriones libycus) in Iranshahr and Nikshahr, the easternmost corner of the Jaz Murian depression and reported Spirurida sp., Hymenolepis diminuta, Hymenolepis nana feraterna, Trichuris trichiura, Skerjabino taenia, Trichostrongylus spp. Entamoeba muris, Chilomastix mesnili and Leishmania spp.
Fasihi Harandi et al. (2016) studied endoparasites of small mammals (Tatera indica, Meriones libycus, Meriones persicus, Dryomys nitedula, Mus musculus, Paraechinus hypomelas, Lepus europaeus) in Kerman province, parts of the Jaz Murian depression. They isolated five different species of parasites including Trichuris muris, Moniliformis moniliformis, Hymenolepis diminuta, Hymenolepis nana, and Mastrophorus muris.
Parasites of the genus Trichuris have been reported from some rodents of the other parts of Iran such as Tatera indica (Rahdar et al. 2016), Meriones persicus and Microtus socialis (Kia et al. 2010), Hystrix indica (Yousefi et al. 2014), Mus musculus and Rattus norvegicus (Pakdel et al. 2013). Also, parasites of the genus Hymenolepis, Physaloptera, Heligmosomum, Aspicularis and Syphacia have been collected from rodents in Iran (Kia et al. 2010; Yousefi et al. 2014; Allymehr et al. 2012 and Garedaghi and Khaki 2014).
Akhtar (1947) described Labiostomum naimi from a Lagomorph Ochotona sp. in a village of Kabul, Afghanistan for the first time, then Babaev (1968) and Babaev and Sapargel’dyev (1970) recorded this parasite from O. rufescens in Turkmenia, USSR. It was also isolated from O. alpina in Mongolia (Tinnin et al. 2011) but it has not been reported in rodents.
In this survey, we tried to provide comprehensive sampling from different habitats and diverse climatic conditions of the Jaz Murian depression, to provide new insight into the fauna of wild rodents and their relative endoparasites. The information provided in this study can be applied to decrease human health challenges in the region.
Materials and methods
Study area
The Jaz Murian depression is located in the southeast Iran, in a tropical realm and considered as an endemic area for some zoonotic diseases such as malaria and cutaneous leishmaniasis (Kassiri et al. 2013; Fekri et al. 2013; Salmanzadeh et al. 2015; Vatandoost et al. 2011; Salahi-Moghadam et al. 2014; Salehi et al. 2010). The dry Jaz Murian depression (27°29′21″N, 58°32′46″E) covering about 25,000–30,000 square miles entirely surrounded by some mountain chains (Khajeh et al. 2015; Khajeh 2017). Locating near the border of Afghanistan and Pakistan, the Jaz Murian depression in the southeast Iran exposed to communication of refugees and nomads and also transportation of livestock which can increase the potential risk of the public health. Additionally, the health condition of the region is severely affected by low supply of public services and health care in adjacent areas of the neighboring countries. Therefore, discovering the potential risks to the public health in the region can help us to prevent spreading and enhance controlling.
Sampling
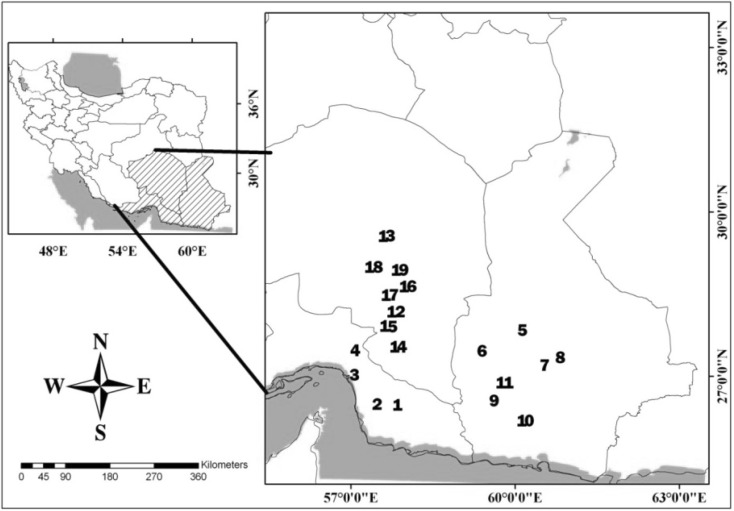
Sampling was conducted from December 2015 to June 2016 in 31 various regions of the Jaz Murian depression as shown in Fig. 1. We used 100 Sherman live traps which are appropriate for capturing rodents with date as bait (Khajeh et al. 2015). The traps were deployed for 2 consecutive nights at each study site. The traps were checked in the morning for the presence of rodents and the specimens were transferred to the Parasitology laboratory of the Department of Veterinary Clinical Sciences, Ferdowsi University of Mashhad. Since Jerboas cannot be trapped by live traps, we caught them with a hand net, using a searchlight and motorcycle at night.
Fig. 1.
Map showing the collecting sites of rodents used for this study
In the laboratory, specimens were subjected and prepared based on mammalogical procedure established by the American Society of mammalogists Animal Care and Use Committee (1998). The entire gastrointestinal tract was removed and placed in a Falcon tube containing 70% alcohol. The entire gastrointestinal tract was placed on the plates containing physiological saline. The stomach, small intestine, large intestine, cecum, liver and lung were studied using stereomicroscope and parasites were removed. In this study, wet-mount (with Lactophenol) for staining of nematodes and Eshnaider-acetocarmine method for staining of cestodes were used (Garedaghi and Hashemzade 2011).
Identification
The rodents were identified based on identification keys including (Corbet 1978) with consideration to new revisions on rodent’s species of Iran (Darvish et al. 2014, 2015; Ghorbani et al. 2015) and endoparasites with valid systematic helminthological keys (Khalil et al. 1994; Yamaguti 1962).
Statistical analysis
To elucidate the potential risk of each rodent species in prevalence of different parasites, the rodent’s species with the highest and the lowest infestation were determined. We also determined the parasites with the highest and the lowest prevalence in the rodents from Jaz Murian depression. Prevalence of the infected rodents, abundance of the endoparasites and mean intensity of parasites related to each hosts were calculated following (Margolis et al. 1982) as below:
Statistical analysis was estimated using SPSS 16 (SPSS Inc., Chicago, IL, USA).
Results
During the period of study, a total of 146 specimens of rodents belong to 13 different species were captured from the Jaz Murian depression and adjacent areas. The rodents included 36 specimens of House Mouse (Mus musculus) (24.56% of total rodents captured), 30 specimens of Indian Gerbil (Tatera indica) (20.54%), 21 specimens of Spiny Mouse (Acomys dimidiatus) (14.38%), 13 specimens of Baluchistan Gerbil (Gerbillus nanus) (8.9%), 13 specimens of Libyan Jird (Meriones libycus) (8.9%), eight specimens of Short-tailed Bandicoot (Nesokia indica) (5.47%), seven specimens of Jerboa (Jaculus blanfordi) (4.79%), six specimens of Hotson’s brush-tailed Mouse (Calomyscus hotsoni) (4.10%), six specimens of Steppe field Mouse (Apodemus witherbyi) (4.10%), two specimens of Black Rat (Rattus rattus) (1.36%), two specimens of Indian Bush Rat (Golunda ellioti) (1.36%), one specimen of the Eastern European Vole (Microtus mystacinus) (0.68%), and one specimen of Migrant Hamster (Cricetulus migratorius) (0.68%).
Amongst 146 examined specimens, 49 individuals (33.5% of total rodents captured) found to be infested with 10 different genera of endoparasites. The identified endoparasites were: nematodes including Trichuris muris, Syphacia obvelata, Labiostomum sp., Labiostomum naimi, Mastrophorus muris, Aspicularis tetraptera, Heligmosomoides skrjabini, Physaloptera sp. and cesteodes including Choanotaenia sp., Raillietina sp. and Hymenolepis diminuta (Table 1).
Table 1.
Diversity of wild rodents and their endoparasites in the Jaz Murian depression
| Parasites | Nematodes | Cestodes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rodents | Mastrophorus muris | Labiostomum sp. | L. naimi | Aspicularis tetraptera | Trichuris muris | Syphacia obvelata | Heligmosomoides skrjabini | Physaloptera sp. | Choanotaenia sp. | Hymenolepis diminuta | Raillieitina sp. |
| T. indica | 3, 4, 6, 15, 17 | – | – | 4 | 4, 6, 8, 10, 14, 16, 26, 27 | – | – | – | 10 | 4 | 10, 17, 26 |
| G. nanus | – | – | – | – | – | – | – | – | – | – | – |
| M. libycus | – | – | – | – | – | – | – | 16 | 19 | – | 22 |
| G. ellioti | – | – | – | – | – | – | – | – | – | – | – |
| A. witherbyi | – | – | – | – | – | – | – | – | – | – | – |
| M. musculus | 18 | – | – | – | – | 5 | 16 | – | – | 18 | – |
| N. indica | – | – | – | – | – | – | – | – | – | – | – |
| R. rattus | 3 | – | – | – | – | – | – | – | – | – | – |
| A. dimidiatus | 1, 2, 5 | 20 | 1, 2 | – | – | – | – | – | 2 | – | – |
| J. blanfordi | – | 11 | – | – | – | – | – | – | – | – | – |
| C. hotsoni | – | – | – | – | – | – | – | – | – | – | – |
| C. migratorius | – | – | – | – | – | – | – | – | – | – | – |
| M. mystacinus | – | – | – | – | – | – | – | – | – | – | – |
Numbers show the sampling localities in the Table 3 and figure
Indian Gerbil (T. indica) (93% infested) was found with the highest parasitic infestation comparing to Spiny Mouse (A. dimidiatus) (66%), Black Rat (R. rattus) (50%), Libyan Jird (M. libycus) (15%), Jerboa (J. blanfordi) (14%) and House Mouse (M. musculus) (8%). Seven species of rodents, Short-tailed Bandicoot (N. indica), Baluchistan Gerbil (G. nanus), Indian Bush Rat (G. ellioti), Hotson’s brush-tailed Mouse (C. hotsoni), Steppe field Mouse (A. witherbyi), Migrant Hamster (C. migratorius) and the Eastern European Vole (M. mystacinus) were free from any parasitic infestation (Table 2).
Table 2.
Prevalence (%) of infected rodents, abundance (%) and mean intensity (%) of endoparasites from the Jaz Murian depression
| Parasites | M. muris | Labiostomum sp. | L. naimi | A. tetraptera | T. muris | S. obvelata | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rodent species | Prevalence (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) |
| T. indica | 93.3 | 83.3 | 104.2 | 0 | 0 | 0 | 0 | 63.3 | 380 | 150 | 409.1 | 0 | 0 |
| G. nanus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M. libycus | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| G. ellioti | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. witherbyi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M. musculus | 8 | 2.8 | 1.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 44.4 | 533.3 |
| N. indica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| R. rattus | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. dimidiatus | 66 | 61.9 | 100 | 4.8 | 100 | 9.5 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| J. blanfordi | 14 | 0 | 0 | 14.3 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. hotsoni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. migratorius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M. mystacinus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parasites | H. skrjabini | Physaloptera sp. | Choanotaenia sp. | H. diminuta | Raillieitina sp. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rodent species | Prevalence (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) | Abundance (%) | Mean intensity (%) |
| T. indica | 93.3 | 0 | 0 | 0 | 0 | 3.3 | 100 | 16.7 | 250 | 10 | 300 |
| G. nanus | 0 | 0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 |
| M. libycus | 15 | 0 | 0 | 130.8 | 850 | 7.7 | 100 | 0 | 0 | 7.7 | 100 |
| G. ellioti | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. witherbyi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M. musculus | 8 | 2.8 | 100 | 0 | 0 | 0 | 0.0 | 8.3 | 150 | 0 | 0 |
| N. indica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| R. rattus | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. dimidiatus | 66 | 0 | 0 | 0 | 0 | 14.3 | 150 | 0 | 0 | 0 | 0 |
| J. blanfordi | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. hotsoni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. migratorius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M. mystacinus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Endoparasites composing Mastrophorus muris, Aspicularis tetraptera, Trichuris muris, Hymenolepis diminuta, Choanotaenia sp., Raillietina sp. were collected from Indian Gerbil (T. indica). For House Mouse (M. musculus), we collected endoparasites including Mastrophorus muris, Syphacia obvelata, Heligmosomoides skrjabini and Hymenolepis diminuta. Parasites of the genus Physaloptera sp. was only found in Libyan Jird (M. libycus). Also, we isolated cestodes of Choanotaenia sp. and Raillieitina sp. from this species. Spiny Mouse (A. dimidiatus) was infested with Mastrophorus muris, Labiostomum sp., Labiostomum naimi and Choanotaenia. Black Rat (R. rattus) was only infested with Mastrophorus muris. Finally, we collected Labiostomum sp. from Jerboa (J. blanfordi) and Spiny Mouse (A. dimidiatus) (Table 1). The highest abundance of the endoparasites was related to Trichuris muris in T. indica and the highest mean intensity of endoparasites attributed to Physaloptera sp. in M. libycus hosts (Table 2).
Discussion
Control of zoonotic parasites depends on reliable knowledge of their distribution in each region. Some zoonotic diseases such as Crimean-Congo hemorrhagic fever (CCHF) and zoonotic cutaneous leishmaniasis (ZCL) are endemic in southeast of Iran (Ansari et al. 2014; Kassiri et al. 2013). Rodents and their endoparasites are important not only from the point that they directly affected human health but also can play as a role of intermediate host to infect poultries and husbandries (Rafique et al. 2009; Meerburg et al. 2004; Meerburg and Kijlstra 2006), hence, controlling programs are necessary to reduce rodent’s impact.
Among rodents captured in the present study, House Mouse (M. musculus; 24.56% of total specimens captured) represented the most frequency and Migrant Hamster, (C. migratorius) and the eastern European Vole (M. mystacinus) showed the least frequency.
High diversity of endoparasites (11 different species) was demonstrated in the Jaz Murian depression. Parasites of the species Mastrophorus muris were the most common in the captured rodents and found in four species (T. indica, M. musculus, R. rattus and A. dimidiatus), which were scattered in all parts of the Jaz Murian dep. The species Trichuris muris had the highest frequency of infestation in Indian Gerbil (T. indica), which was distributed in eight localities of the Jaz Murian depression. Additionally, parasites of Aspicularis tetraptera were only found in the Indian Gerbil (T. indica) in Minab. Since, Indian Gerbils can be found in low altitudes (Khajeh et al. 2015) thus; existence of the species at the central parts of the Jaz Murian depression was expected. We collected Syphacia obvelata and Heligmosomoides skrjabini from House Mouse (M. musculus) in the village of Fariab and Houdian, southwest and north of the Jaz Murian depression respectively. Also, Physaloptera sp. was identified at Libyan Jird (M. libycus) in the village of Houdian.
The most infested species was Indian Gerbil (T. indica) in which 28 of 30 captured specimens (93%) were infested with six genera of endoparasites. Moreover, high abundance of some parasite species observed in this rodent may be related to their lifestyle (living in colonies) which provides appropriate condition for transmission of high amount of parasites. These six infested rodents species (T. indica, M. musculus, M. libycus, J. blanfordi, A. dimidiatus, R. rattus) were collected from residential or adjacent to residential area which increase the risk of pathogenesis (Tables 1, 3 and Fig. 1). Seven non-infested rodent’s species (G. nanus, G. ellioti, M. mystacinus, N. indica, A. witherbyi, C. migratorius and C. hotsoni) except N. indica and G. nanus which were free from any parasitic infestation live far from the human habitats, however, the results may be probably due to a low sample size. They were collected from Amjaz and Sartagheen (Anbarabad, Kerman province) in the top of the Jebal Bariz and Bashagard Mountain chains which presence of human associations were very low, therefor, this species were less important from the health aspect.
Table 3.
Sampling localities of the specimens of rodents in the Jaz Murian depression
| No. | No. on the map | Collecting station | GPS information | No. | No. on the map | Collecting station | GPS information | ||
|---|---|---|---|---|---|---|---|---|---|
| Locality | Latitude | Longitude | Locality | Latitude | Longitude | ||||
| 1 | 1 | Sardasht, Biskove | 26°29′ | 57°51′ | 17 | 7 | Bampour, Jafarabad | 27°12′ | 60°32′ |
| 2 | 2 | Sardasht, Koh-e-Heidar | 26°30′ | 57°29′ | 18 | 8 | Bampour, Aliabad | 27°11′ | 60°34′ |
| 3 | 3 | Minab,Tarom. | 27°01′ | 57°04′ | 19 | 9 | Iranshahr, Tighabad | 27°20′ | 60°45′ |
| 4 | 3 | Minab | 27°03′ | 57°06′ | 20 | 10 | Fannoj | 26°33′ | 59°38′ |
| 5 | 4 | Fariab | 27°28′ | 57°05′ | 21 | 11 | Nikshahr | 26°12′ | 60°13′ |
| 6 | 4 | Roudan | 27°27′ | 57°09′ | 22 | 6 | Fannoj, Maskoutan | 26°52′ | 59°49′ |
| 7 | 5 | Bazman, Kalgande | 27°51′ | 60°10′ | 23 | 12 | Dalgan, Bagh-e-Ebrahim | 27°29′ | 59°27′ |
| 8 | 5 | Bazman | 27°51′ | 60°10′ | 24 | 13 | Fariab, Sardak sargorij | 28°11′ | 57°51′ |
| 9 | 5 | Bazman, Kargokan | 27°50′ | 60°10′ | 25 | 14 | Kahnoj, Avazabad | 27°57′ | 57°40′ |
| 10 | 5 | Bazman | 27°51′ | 60°10′ | 26 | 15 | Roodbar | 27°31′ | 57°54′ |
| 11 | 5 | Bazman, Shandak | 27°50′ | 60°10′ | 27 | 16 | Kahnoj | 27°56′ | 57°41′ |
| 12 | 5 | Bazman, Sefidabad | 27°51′ | 60°11′ | 28 | 17 | Anbarabad, Amjaz | 28°36′ | 58°02′ |
| 13 | 5 | Bazman, Shandak | 27°46′ | 60°07′ | 29 | 19 | Anbarabad, | 28°28′ | 57°51′ |
| 14 | 6 | Chah-e-hashem | 27°28′ | 59°24′ | 30 | 18 | Jiroft, ebal-e- Barez | 28°54′ | 57°54′ |
| 15 | 6 | Dalgan | 27°29′ | 59°27′ | 31 | 19 | Anbarabad, Sartagheen | 28°36′ | 58°02′ |
| 16 | 6 | Houdian | 27°29′ | 59°27′ | |||||
Comparing to the similar study in NE Iran carried out by Arzamani et al. (2017) Mastrophorus muris, Labiostomum spp., Heligmosomoides skrjabini, Physaloptera sp., Choanotaenia sp., Raillieitina sp. which were not observed in their study were isolated in present investigation while Cysticercus fasciolaris was the most common parasite in the rodents from NE Iran. The reason may be related to the more humid climate in the northeast Iran which provide suitable habitats for larval stage of Taenia taeniaeformis and transmitting to new hosts, whereas the dry hot climate in Jaz Murian impose constrain to this tapeworm. In concordant to our results, Gerbils (M. persicus in Arzamani et al. (2017) and T. indica in the present study) show the highest infections with endoparasites. Hymenolepis nana which was previously recorded in rodents from Jaz Murian depression (Nateghpour et al. 2015; Fasihi Harandi et al. 2016) was not observed in present study. Generally, the most infested rodents in our study were also reported to be as a reservoir host for cutaneous leishmaniasis in Iran (Rassi et al. 2011; Vazirianzadeh et al. 2013; Davami et al. 2014; Wernery 2014) and provoke more attention for Gerbils population controlling. As reported in Rafique et al. (2009) and Majeed (2016) Hymenolepis nana and H. diminuta have prevalence in R. rattus in neighboring countries while we isolated Mastrophorus muris from the Black Rat in the present study. House mouse has been also reported to be infested by H. diminuta, H. nana, T. taeniaeformis, Vampirolepis sp., Protospirura sp., and Trichuris sp. in Pakistan while we recorded H. diminuta, H. skrjabini, S. obvelata, and M. muris from Mus musculus.
Although, there is no organized databank about endoparasites in Iran, based on available data, we reported L. naimi for the first time in rodents from Iran. This species (L. naimi) was collected from Spiny Mouse (A. dimidiatus) in Bashagard Mountain chain in south of the Jaz Murian depression.
Conclusion
The multiple impacts of rodents on human public health highlight the essential role of controlling programs and services by the governments in developing countries. Taking to account the human commensalism of the rodents with the highest infestation (Indian Gerbil, Spiny Mouse, Black Rat, Libyan Jird, Jerboa and House Mouse) and the overlap between their habitats with farms and villages in the southeast Iran, the necessity for monitoring and controlling of the rodent’s populations around villages and farms would be expected.
Acknowledgements
This research was supported by grants from the Vice President Research and Technology of Ferdowsi University of Mashhad, Iran under Project No. 3/32150. Permission number to collect specimens was authorized by The Iranian Department of Environment (Permission Number: 93/45436; 2014, 18th Nov.). This paper is dedicated to the memory of my supervisor, Prof. Jamshid Darvish, who passed away during this project. We would like also to thank anonymous reviewers for their instructive comments to the primary version of the manuscript.
Author contributions
AK, ARS and GRR shared in the study design, research topics and providing the funds. AK and ZM collected rodent samples and AK, ZM, and FG identified the rodent specimens and wrote the manuscript. AK and ARS collected the endoparasites and performed the laboratory work on endoparasites. AK, ZM, FG, AM, IM, GRR identified the endoparasites. All authors shared in interpretation of the results, and reviewed the manuscript.
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
Animals were captured, handled and euthanized while observing the regulations on animal welfare (28/1998).
Informed consent
For this type of study informed consent is not required.
References
- Akhtar SA. A new genus of nematodes, parasitic in the pika. Parasitology. 1947;38:104–106. doi: 10.1017/S0031182000016474. [DOI] [PubMed] [Google Scholar]
- Allymehr M, Tavassoli M, Manoochehri MH, Ardavan D. Ectoparasites and gastrointestinal helminths of house mice (Mus musculus) from poultry houses in northwest Iran. Comp Parasitol. 2012;79(2):283–287. doi: 10.1654/4534.1. [DOI] [Google Scholar]
- Animal Care and Use Committee Guidelines for the capture, handling, and care of mammals as approved by the American Society of Mammalogists. J Mammal. 1998;79:1416–1431. doi: 10.2307/1383033. [DOI] [Google Scholar]
- Ansari H, Shahbaz B, Izadi S, Zeinali M, Tabatabaee SM, Mahmoodi M, Holakouie-Naieni K, Mansournia MA. Crimean-Congo hemorrhagic fever and its relationship with factors in southeast Iran. J Infect Dev Ctries. 2014;8:749–757. doi: 10.3855/jidc.4020. [DOI] [PubMed] [Google Scholar]
- Arzamani K, Salehi M, Mobedi I, Adinezade A, Hasanpour H, Alavinia M, Darvish J, Shirzadi MR, Mohammadi Z. Intestinal helminths in different species of rodents in north Khorasan province, northeast of Iran. Iran J Parasitol. 2017;12(2):267–273. [PMC free article] [PubMed] [Google Scholar]
- Asgari Q, Motazedian MH, Mehrabani D, Oryan A, Hatam GR, Owji SM, Paykari H. Zoonotic cutaneous leishmaniasis in Shiraz, southern Iran: a molecular, isoenzyme and morphologic approach. J Res Med Sci. 2007;12:7–15. [Google Scholar]
- Azizi MH, Azizi F. A history of the human plague in Iran. Arch Iran Med. 2010;13(6):563–569. [PubMed] [Google Scholar]
- Babaev Y. Study of helminth fauna of Ochotona rufescens in Turkmenia. Arazity zhivotnykh i rastenii v Turkmenii. 1968;8:5–12. [Google Scholar]
- Babaev Y, Sapargel’dyev M. Helminth fauna of Ochotona rufescens (Lagomyidae) and some of its characteristics in different areas of Kopetdag (Turkmenian SSR). Izvestiya Akademii Nauk Turkmenskoi (Turkmenistan SSR Ylymlar Akademijasynyn Habarlary) Biol Nauki. 1970;1:58–65. [Google Scholar]
- Darvish J, Mohammadi Z, Mahmoudi A, Siahsarvie R. Faunistic and taxonomic study of rodents from northwestern Iran. IJAB. 2014;10:119–136. [Google Scholar]
- Darvish J, Mohammadi Z, Ghorbani F, Mahmoudi A, Dubey S. Phylogenetic relationships of Apodemus Kaup, 1829 (Rodentia: Muridae) species in the eastern Mediterranean inferred from mitochondrial DNA, with emphasis on Iranian species. J Mamm Evol. 2015;22:119–136. doi: 10.1007/s10914-015-9294-9. [DOI] [Google Scholar]
- Davami MH, Motazedian MH, Kalantari M, Asgari Q, Mohammadpour I, Sotoodeh-Jahromi A, Solhjoo K, Pourahmad M. Molecular survey on detection of Leishmani infestation in rodent reservoirs in Jahrom district, Southern Iran. J Arthropod Borne Dis. 2014;8:139–146. [PMC free article] [PubMed] [Google Scholar]
- Fekri S, Vatandoost H, Daryanavard A, Shahi M, Safari R, Raeisi A, Omar AS, Sharifi M, Azizi A, Ali AA, Nasser A, Hasaballah I, Hanafi-Bojd AA. Malaria situation in an endemic area, Southeastern Iran. J Arthropod Borne Dis. 2013;8:82–90. [PMC free article] [PubMed] [Google Scholar]
- Garedaghi Y, Hashemzade FH. Prevalence rate of endoparasites in wild rabbits of east-Azerbaijan province, Iran. Scholars research library. Ann Biol Res. 2011;2(6):31–35. [Google Scholar]
- Garedaghi Y, Khaki AA. Prevalence of gastrointestinal and blood parasites of rodents in Tabriz, Iran, with emphasis on parasitic zoonoses. Crescent J Med Biol Sci. 2014;1(1):9–12. [Google Scholar]
- Corbet GB. The mammals of the Palaearctic region: a taxonomic review. London: British Museum (Natural History); 1978. [Google Scholar]
- Ghorbani F, Mohammadi Z, Darvish J, Kami HG, Siahsarvie R. Morphological and morphometric characterization of the new records of the east European vole (Microtus Levis Miller, 1908) from northeast Iran. J Asia Pac Biodivers. 2015;8(3):233–237. doi: 10.1016/j.japb.2015.07.002. [DOI] [Google Scholar]
- Harandi MF, Madjdzadeh SM, Ahmadinejad M. Helminth parasites of small mammals in Kerman province, southeastern Iran. J Parasit Dis. 2016;40:106–109. doi: 10.1007/s12639-014-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiri H, Naddaf SR, Javadian EA, Mohebali M. First report on isolation and characterization of Leishamnia major from Meriones hurrianae (Rodentia: Gerbillinae) of a rural cutaneous leishmaniasis focus in south-eastern Iran. Iran Red Crescent Med J. 2013;15:789–793. doi: 10.5812/ircmj.6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajeh A (2017) Taxonomic revision of rodents in the Jaz Murian depression and identification of their parasites. Ph.D thesis, Biology Group, Faculty of Science, Ferdowsi University of Mashahd, Iran (in Persian)
- Khajeh A, Darvish J, Razmi G. A contribution on rodents fauna of Jaz Murian depression, southeast Iran. Biodivers J. 2015;7(2):203–214. [Google Scholar]
- Khajeh A, Razmi G, Darvish J. A study of ectoparasites in wild rodents in the Jaz Murian area in the southeast of Iran. Asian Pac J Trop Dis. 2017;7(7):418–421. doi: 10.12980/apjtd.7.2017D7-54. [DOI] [Google Scholar]
- Khalil LF, Jones A, Bray RA. Key to the cestodes parasites of vertebrates. Wallingford: CABI; 1994. [Google Scholar]
- Kia EB, Shahryary-Rad E, Mohebali M, Mahmoudi M, Mobedi I, Zahabium F, Zarei Z, Miahipoor A, Mowlavi GH, Akhavan AA, Vatandoost H. Endoparasites of rodents and their zoonotic importance in Germi, Dashte–Mogan, Ardabil province, Iran. Iran J Parasitol. 2010;3:44–49. [PMC free article] [PubMed] [Google Scholar]
- Majeed ShA. Prevalence of intestinal parasites in Rattus rattus in some districts in Baghdad/Iraq. Al-Anbar J Vet Sci. 2016;9(1):43–48. [Google Scholar]
- Margolis L, Esch GW, Holmes JC, Kuris AM, Shad GA. The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists) J Parasitol. 1982;68:131–133. doi: 10.2307/3281335. [DOI] [Google Scholar]
- Meerburg BG, Kijlstra A. Zoonotic risk of rodents in the cattle farming. Tijdschr Diergeneeskd. 2006;131(12):445–447. [PubMed] [Google Scholar]
- Meerburg BG, Bonde M, Brom FWA, Endepols S, Jensen AN, Leirs H, Lodal J, Singleton GR, Pelz HJ, Rodenburg TB, Kijlstra A. A towards sustainable management of rodents in organic animal husbandry. J Life Sci. 2004;52(2):195–205. [Google Scholar]
- Meshkekar M, Sadraei J, Mahmoodzadeh A, Mobedi I. Helminth Infestations in Rattus ratus and Rattus norvigicus in Tehran, Iran. Iran J Parasitol. 2014;9(4):548–552. [PMC free article] [PubMed] [Google Scholar]
- Nateghpour M, Motevalli-Haghi A, Akbarzadeh K, Akhavan AA, Mohebali M, Mobedi I, Farivar L. Endoparasites of wild rodents in southeastern Iran. J Arthropod Borne Dis. 2015;9(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Pakdel N, Naemm S, Rezaei F, Chalehchaleh AA. A survey on helminthic infestation in mice (Mus musculus) and rats (Rattus norvegicus and Rattus rattus) in Kermanshah, Iran. VRF. 2013;4(2):105–109. [PMC free article] [PubMed] [Google Scholar]
- Rafique A, Rana SA, Khan HA, Sohail A. Prevalence of some helminths in rodents captured from different city structures including poultry farms and human population of Faisalabad, Pakistan. Pak Vet J. 2009;29(3):141–144. [Google Scholar]
- Rahdar M, Vazirianzadeh B, Rointan ES, Amraei K. Identification of collected ectoparasites of rodents in the west of Khuzestan province (Ahvaz and Hovizeh), southwest of Iran. Asian Pac J Trop Dis. 2016;5:627–631. doi: 10.1016/S2222-1808(15)60902-1. [DOI] [Google Scholar]
- Rassi Y, Saghafipour A, Abai M, Oshaghi MA, Rafizadeh S, Mohe-bail M, Yaaghobi-Ershadi MR, Mohtarami F, Farzinnia B. Phlebotomus papatasi and Meriones libycus as the vector and reservoir host of cutaneous leishmaniasis in Qom-rood district, Qom province, central Iran. Asian Pac J Trop Med. 2011;4:97–100. doi: 10.1016/S1995-7645(11)60045-X. [DOI] [PubMed] [Google Scholar]
- Salahi-Moghadam A, Khoshdel AR, Barati M, Sedaghat MM. An overview and mapping of malaria and its vectors in Iran. HMJ. 2014;18:473–484. [Google Scholar]
- Salehi M, Mokhtari-Amirmajdi M, Eftekharzadeh-Mashhadi I, Hakemi Y, Eftekharzadeh-Mashhadi A, Mirinezhad A. Analysis of malaria epidemic features in Sistan and Baluchistan province, southeast of Iran. Iran Red Crescent Med J. 2010;12:247–255. [Google Scholar]
- Salmanzadeh S, Foroutan-Rad M, Khademvatan S, Moogahi S, Bigdeli S. Significant decline of malaria incidence in southwest of Iran (2001–2014) J Trop Med. 2015 doi: 10.1155/2015/523767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinnin DS, Ganzorig S, Gardner SL. Helminths of small mammals (Erinaceomorpha, Soricimorpha, Chiroptera, Rodentia, and Lagomorpha) of Mongolia. Austin: Natural Science Research Laboratory, Texas University; 2011. [Google Scholar]
- Vatandoost H, Emami SN, Oshaghi MA, Abai MR, Raeisi A, Piazzak N, Mahmoodi M, Akbarzadeh K, Sartipi M. Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan province, south-east Islamic Republic of Iran. East Mediterr Health J. 2011;17:439–445. doi: 10.26719/2011.17.5.439. [DOI] [PubMed] [Google Scholar]
- Vazirianzadeh B, Saki J, Jahanifard E, Zarean M, Amraee K, Navid Pour S. Isolation and identification of Leishmania species from sandflies and rodents collected from Roffaye district, Khuzestan province, southwest of Iran. Jundishapur J Microbiol. 2013;6:e10025. [Google Scholar]
- Wernery U. Zoonoses in the Arabian Peninsula. Saudi Med J. 2014;35:1455–1462. [PMC free article] [PubMed] [Google Scholar]
- Yamaguti S. Systema Helminthu. The nematodes of vertebrates. New York: Wiley; 1962. [Google Scholar]
- Yousefi A, Eslami A, Mobedi I, Rahbari S, Ronaghi H. Helminth infestations of house mouse (Mus musulus) and wood mouse (Apodemus sylvaticus) from the suburban areas of Hamadan city, Western Iran. Iran J Parasitol. 2014;9(4):511–518. [PMC free article] [PubMed] [Google Scholar]