Abstract
A study was undertaken to investigate the prevalence rate, site preference and extent of damage caused by myxosporidian parasites in Punjab, India. During the 6 months study, 600 carp fingerlings belonging to 4 genera i.e. Labeo rohita Hamilton, Cirrhinus mrigala Hamilton, Catla catla Thail and Carassius carassius were randomly collected from the polyculture nursery ponds located in different villages District Fatehgarh Sahib, Punjab. Organs such as fins, scales, gills, intestine, kidney, and eye-ball were examined for the presence of myxozoan parasites. In this study, gills of 321 (53.50%) were found to be infected with as many as 10 species of myxosporean parasites belonging to the genus Myxobolus. Gills were examined for the presence of plasmodia and infected organs were processed for histology. The gill plasmodial index (GPI) was counted for all the 10 species and this revealed a mild to severe infection was recorded. M. knobii, M. majraiensis, M. markiwi, M. naini, M. rocatlae, M. vascularis and M. venkateshi formed intralamellar vascular type of plasmodia (LV1), M. nanokiensis formed (LV3) and M. potularis and M. slendrii formed intrafilamental type of plasmodium (FV2). Out of 231 infected fishes, mixed infection was recorded in 44 fishes (13.70%), which exhibited biparasitism and polyparasitism. No infection was recorded in exotic carp i.e. C. carassius. Physicochemical parameters of water were also recorded for the period of 6 months comprising 4 months of winter and 2 months of spring. The present study indicated that the prevalence of myxozoan parasite was 43% in November (24.0 °C) and increased to 54% with the decrease in temperature (22.65 °C).
Keywords: Fingerlings, Myxobolus, Gills, Water parameters, GPI, Histology, Punjab
Introduction
Parasitic diseases are the major limiting factors in aquaculture in India because fish are usually polycultured in high density in a restricted water body, where fish pathogens can easily be transmitted amongst fish. Due to rich blood supply and a site of gaseous exchange, gills are prone to be more infected (Martins et al. 1997). Myxosporeans are common parasites of fish in aquaculture and include an extraordinary large number of species (Kaur and Singh 2012b). Myxozoan parasites can be located in almost every organ of the fish host, however, the gills are most commonly infested where they may cause mild or severe serious structural changes depending on the intensity of infection. The genus Myxobolus, which is the best studied genus of Myxosporea, contains more than 2700 species (Lom and Dyková 2006). Myxozoans are present in both natural and fish farming environments and may have obvious clinical signs when there is a host, parasite and environmental imbalance (Lom and Noble 1984). These parasites are common in juvenile carps in nursery ponds and the high mortality rates caused by their infections in the gills have raised serious concern among fish farmers. There are alarming economical losses due to Myxobolus spp. infestation of the major carp in the nursery ponds as reported by Sanaullah and Ahmed (1980), Kaur and Katoch (2016), Kaur and Ahmad (2016) and Ahmad and Kaur (2017). The present study was therefore undertaken to investigate the prevalence rate, site preference and extent of damage caused by myxosporidian parasites in Punjab, India. In addition, the physiochemical parameters of water were also recorded during the study period i.e. temperature, pH, DO, TDS and conductivity, during the winter and spring periods.
Materials and methods
The present study was conducted during 6 months (November 2014–April 2015), during which different species of fingerlings, such as Labeo rohita (Ham.) vern. rohu, Catla catla (Ham.) vern. thail, Cirrhinus mrigala (Ham.) vern. mrigal and Carassius carassius Linneaus vern. crucian carp were randomly collected fortnightly from polycultured nursery ponds located in the village Fagan Majra, District Fatehgarh Sahib, Punjab. 50 specimens of fingerlings were collected randomly during each sampling and out of four species, the specimens of C. mrigala and L. rohita were more in number (285) and (195) respectively and were available all the time, however other two species, C. catla and C. carassius were less available and only 60 number of specimens each were collected. Various organs such as gills, fin, scales, gall-bladder, gut, skin, kidney etc. were examined for the presence of myxozoan parasites. The infection was recorded in the form of minute to large-sized plasmodia located on or within the gills. Each plasmodia was measured and carefully picked with fine forceps under stereozoom binocular microscope and were teased on a clean slide, following this the smear was observed under the compound microscope for the presence of myxospores. Myxospores were treated with 8% KOH to evert the polar filaments. Permanent slides were made by fixing in Bouin’s fixative and stained with Ziehl–Neelsen and Iron haematoxylin. Fresh preparation myxospores were photographed under phase contrast microscope (Image Processing Unit Magnus MLX Model No. 12G961) and stained preparations with Leica photographic unit at Sophisticated Instrumentation Center, Punjabi University (Patiala). The myxosporean parasites were identified up to species level with the help of the genus Myxobolus Butschli (1882) provided by Eiras et al. (2005, 2014). Another key to the species of myxosporeans infecting freshwater fishes in Africa was proposed by Fomena and Bouix (1997) and a handbook on the myxosporeans of Indian fishes given by Kalavati and Nandi (2007) was followed for identification at the species level and also with the help of research papers reporting species regionally and globally. A Russian compilation by Bauer (1984) was also referred for earlier descriptions. At generic level, identification of the myxospores was done with the help of the key given by Lom and Dyková (1991) and revised key given by Kaur and Singh (2012a, b). For histopathological studies infected organs were fixed in Bouin’s fixative, dehydrated in ascending grades of ethanol, cleared in xylene, embedded in paraffin wax, sectioned at 8–10 µm and stained with Luna’s method (Luna 1968). The gill plasmodial index (GPI) was calculated on the basis of number of plasmodia present per gill (one side) visible under the stereozoom binocular microscope as per Kaur and Attri (2015). 0–0 (no infection); 1–5 (light infection-1); 5–10 (moderate infection-2); 10–20 (heavy infection-3); 20–50 (severe infection-4). For calculations of prevalence the following formula was applied.
The location of myxosporean plasmodia in various tissues of the gills was determined with the help of histological sections stained with Luna’s method and were categorized into types according to the guidelines of Molnár (2002a). According to tissue location 2 types of intralamellar-vascular type (LV: LV1 and LV3) and one intrafilamental-vascular (FV: FV2) plasmodia were recorded.
Intralamellar-vascular type (LV)
LV1 Plasmodium located centrally in the gill lamella
LV3 Large plasmodium deforming several gill lamellae
Intrafilamental-vascular type (FV2) Large plasmodia formed by the fusion of several plasmodia near the end of the gill filament (FV2)
Plasmodia according to size Type of Plasmodia were categorized into three types
Type A Plasmodia visible under binocular microscope (size range = 40–200 µm)
Type B Plasmodia visible under stereozoom microscope (size range = 0.2–0.9 mm)
Type C Plasmodia visible with naked eye (size range = 0.9–3.0 mm)
Type D Plasmodia of very large size (range = 3.0–10 mm)
To study the water quality parameters, sampling was done for 6 months from November 2014 to March 2015 at fortnightly interval from nursery ponds located at Fagan Majra, District Fatehgarh Sahib, Punjab. Water samples were collected from the surface up to the depth of 15 cm. Various water parameters were analyzed such as water temperature, pH, conductivity, TDS and dissolved oxygen (DO). All the parameters were recorded on the spot with the help of portable water testing kit.
Results
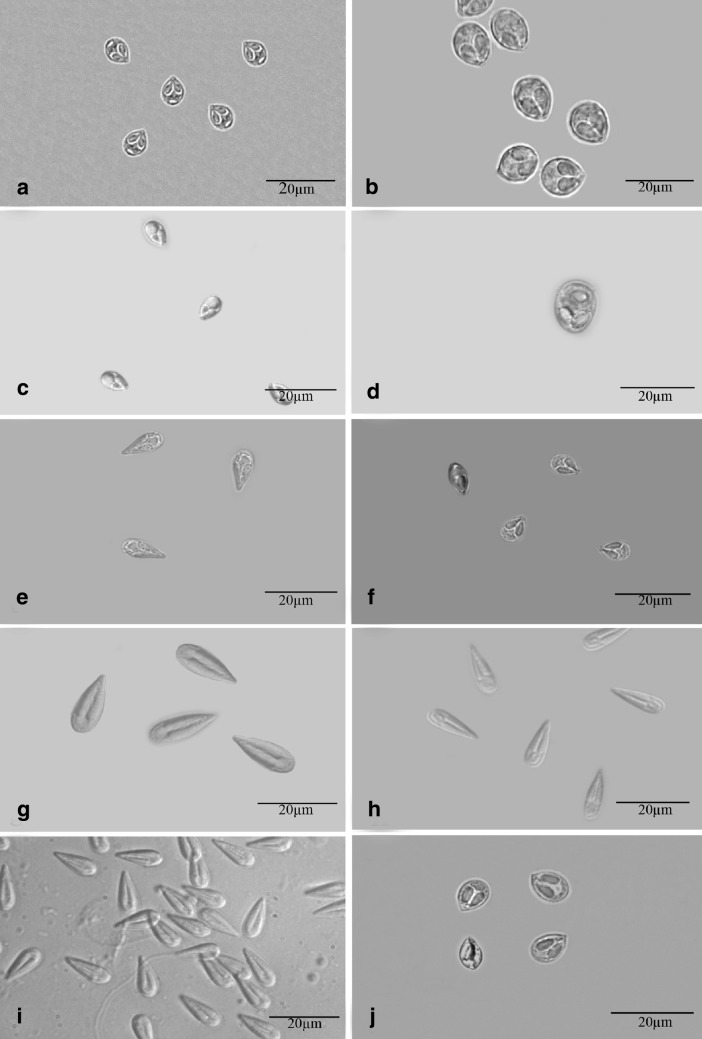
During this study, as many as 12 collection tours were made to the collection sites. A total of 600 carp fingerlings belonging to 4 genera i.e. L. rohita Hamilton (285), C. mrigala Hamilton (195), C. catla Thail (60) and C. carassius Linnaeus (60) were collected and examined. Although all the organs were thoroughly examined however, only gills were found infected and no infection was detected in fins, skin, scales, kidney, gall-bladder, gut etc. The total infection rate was 53.50% with highest in L. rohita (63.58%) followed by C. mrigala (58.94%), C. catla (48.33%) and exotic carp C. carassius free from any infection. In total, 10 species of myxosporean parasites belonging to the genus Myxobolus i.e. M. knobii Kaur and Ahmad (2017); M. majraiensis Kaur and Ahmad (2017); M. markiwi Kaur and Ahmad (2016); M. naini Kaur and Singh (2008); M. nanokiensis Kaur et al. (2015); M. potularis Madhavan et al. (2013); M. rocatlae Basu and Haldar (2002); M. slendrii Kaur and Singh (2010); M. vascularis Ahmad and Kaur (2017) and M. venkateshi Seenappa and Manohar (1981) have been collected and identified on the basis of myxospore morphology and morphometrics (Table 1; Fig. 3). The mean age of the fingerlings ranged from 2 to 3 months and mean length ranged from 2 to 4.5 cm (Table 2). C. mrigala showed susceptibility with as many as 5 species (M. knobii, M. nanokiensis, M. rocatlae, M. vascularis and M. venkateshi), L. rohita with 4 species (M. markiwi, M. naini, M. potularis and M. slendrii) and only one species (M. majraiensis) was recorded in C. catla (Table 3).
Table 1.
Different morphological characteristics of all the 10 species of Myxobolus
| Species | Host | Infected site | LS | WS | LPC | WPC | No. of filament coils |
|---|---|---|---|---|---|---|---|
| Myxobolus knobii (Kaur and Ahmad, 2017) | Cirrhinus mrigala | Gill lamellae | 5.83 | 4.29 | 1.95 | 1.70 | 3–4 |
| M. majraiensis (Kaur and Ahmad, 2017) | Catla catla | Gill lamellae | 8.58 | 5.27 | 3.47 | 1.80 | 5–6 |
| M. markiwi (Kaur and Ahmad, 2016) | Labeo rohita | Gill lamellae | 6.54 | 5.35 | 1.87 | 0.86 | 4–5 |
| M. naini (Kaur and Singh, 2008) | Labeo rohita | Gill lamellae | 13.73 | 9.05 | 5.66 | 3.36 | 6–7 |
| M. nanokiensis (Kaur et al. 2015) | Cirrhinus mrigala | Gill lamellae | 9.91 | 4.61 | 5.79 | 1.76 | 6–7 |
| M. potularis (Madhavan et al. 2013) | Labeo rohita | Gill filament | 7.83 | 4.79 | 4.00 | 1.23 | 7–9 |
| M. rocatlae (Basu and Haldar, 2002) | Cirrhinus mrigala | Gill lamellae | 15.62 | 5.10 | 10.00 | 1.30 | 9–10 |
| M. slendrii (Kaur and Singh, 2010) | Labeo rohita | Gill filament | 12.02 | 3.43 | 6.59 | 0.97 | 8–9 |
| M. vascularis (Ahmad and Kaur, 2017) | Cirrhinus mrigala | Gill lamellae | 10.73 | 4.88 | 6.51 | 1.80 | 8–9 |
| M. venkateshi (Seenappa and Manohar, 1981) | Cirrhinus mrigala | Gill lamellae | 8.41 | 5.59 | 4.77 | 2.04 | 5–6 |
Fig. 3.
Fresh myxospores of all the 10 species (Scale bar = 20 µm). a: Myxobolus knobii b: M. majraiensis c: M. markiwi d: M. naini e: M. nanokiensis. f: M. potularis g: M. rocatlae h: M. slendrii i: M. vascularis j: M. venkateshi
Table 2.
Myxozoan parasitism in the gills of aquacultured fingerlings from nursery ponds in Punjab, India
| Host examined | Fish age (mo) | Fish length (cm) | No. of fish | Prevalence | |
|---|---|---|---|---|---|
| Examined | Infected (%) | ||||
|
Cirrhinus mrigala
vern. mrigal |
2–2.5 | 2–3.5 | 285 | 168 | 58.94 |
|
Labeo rohita
vern. rohu |
2–3 | 2–4 | 195 | 124 | 63.58 |
|
Catla catla
vern. thail |
2–3 | 2–4.5 | 60 | 29 | 48.33 |
|
Carassius carassius
vern. crucian carp |
2–2.8 | 3 | 60 | 0 | 0 |
| Total | 600 | 321 | 53.50 | ||
Table 3.
Prevalence, nature of parasitism and gill plasmodial index (GPI) in cultured fingerlings in District Fatehgarh Sahib, Punjab
| Parasite species | Host | Locality | Location | Examined fishes | Infected fishes | Prevalence (%) | GPI |
|---|---|---|---|---|---|---|---|
| M. knobii n. sp. (Ahmad and Kaur, 2017) | Cirrhinus mrigala | Fagan Majra Pond, Punjab | Gill lamellae | 60 | 32 | 53.33 | 3 |
| M. majraiensis (Kaur and Ahmad, 2017) | Catla catla | Fagan Majra Pond, Punjab | Gill lamellae | 60 | 29 | 48.33 | 3 |
| M. markiwi (Kaur and Ahmad, 2016) | Labeo rohita | Fagan Majra Pond, Punjab | Gill lamellae | 52 | 29 | 57.76 | 1 |
| M. naini (Kaur and Singh, 2008) | L. rohita | Fagan Majra Pond, Punjab | Gill lamellae | 45 | 23 | 51.11 | 1 |
| M. nanokiensis (Kaur et al., 2015) | C. mrigala | Fagan Majra Pond, Punjab | Gill lamellae | 46 | 28 | 60.86 | 1 |
| M. potularis (Madhavan et al., 2013) | L. rohita | Fagan Majra Pond, Punjab | Gill filament | 44 | 40 | 90.90 | 4 |
| M. rocatlae (Basu and Haldar, 2002) | C. mrigala | Fagan Majra Pond, Punjab | Gill lamellae | 62 | 33 | 54.83 | 1 |
| M. slendrii (Kaur and Singh, 2010) | L. rohita | Fagan Majra Pond, Punjab | Gill filament | 54 | 32 | 59.25 | 1 |
| M. vascularis (Ahmad and Kaur, 2017) | C. mrigala | Fagan Majra Pond, Punjab | Gill lamellae | 59 | 37 | 62.71 | 3 |
| M. venkateshi (Seenappa and Manohar, 1981) | C. mrigala | Fagan Majra Pond, Punjab | Gill lamellae | 58 | 37 | 63.79 | 2 |
| _ | Carassius carassius | Fagan Majra Pond, Punjab | _ | 60 | 0 | 0 | _ |
| Total | 600 | 321 | 53.50 |
The size, shape and color of plasmodia varied from species to species. The plasmodia of M. potularis ranged from 3 to 10 mm in diameter attached to the gill filament, creamish, elongated in shape and were extraordinarily large and gave abscessed appearance to the gills of the fingerlings (Type D) (Fig. 1a) while as plasmodia of M. rocatle ranged from 0.1 to 0.2 mm in diameter attached to the gill lamellae, microscopic, round to oval, white, visible under binocular microscope (Type A) (Fig. 1b).
Fig. 1.
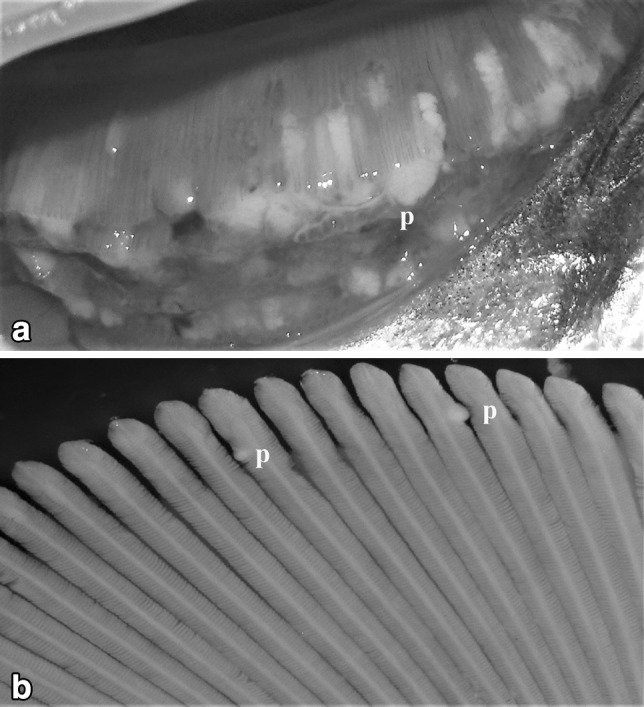
a Zoomed view of fresh gills of Labeo rohita heavily infected with myxozoan plasmodia Myxobolus potularis. b Gills of Cirrhinus mrigala infected small sized plasmodia of M. rocatlae
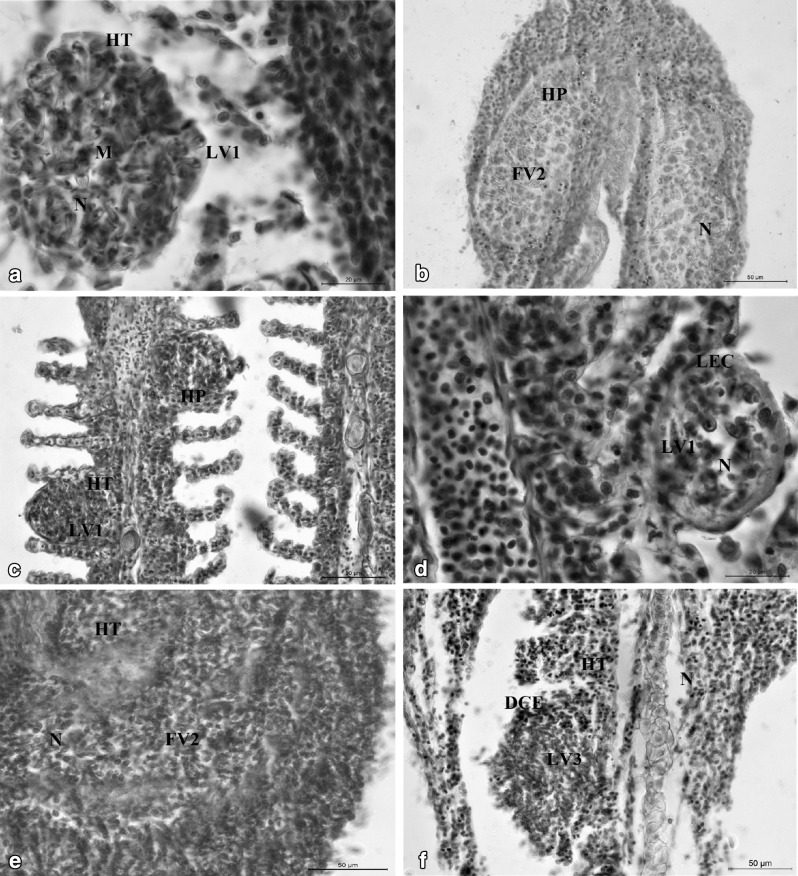
In the present study, myxozoan infection were recorded mostly in the gill lamellae located centrally within single gill lamella (LV1) or by the fusion of several gill lamella (LV3), causing hypertrophy and hyperplasia of lamellar cells. M. nanokiensis formed intralamellar vascular type of plasmodia (LV3) resulting in hyperplasia, hypertrophy, necrosis and destruction of cellular elements; M. rocatlae and M. vascularis formed intralamellar vascular type of plasmodia (LV1) results in hypertrophy, hyperplasia, lifting of epithelial cells and necrosis. The plasmodia of M. potularis and M. slendrii forming intrafilamental vascular type (FV2) type of plasmodia resulted in extensive hyperplasia, necrosis, and the total destruction of epithelial and vascular supply which may result in the suffocation of the fingerlings due to lack of oxygen and can ultimately lead to the death of the fish. Generally, both LV and FV type of plasmodia caused severe damage leads to vacuolization of the stratified epithelium and vascular endothelium of the filament as well as lamellae (Table 4; Fig. 2a–f).
Table 4.
Parasite, host, clinical signs, visibility categories (vis. cat.), location, type of plasmodium and lesions caused by myxozoan parasites in the gills of aquaculture fingerlings from Punjab. Visibility category A: not visible under stereozoom B: visible under stereozoom C: visible with the naked eye. D: Very large type of plasmodia visible with the naked eye. Plasmodium type (per tissue location): LV1: intralamellar vascular plasmodium located centrally in the gill lamella; LV3: Large plasmodium by the fusion of several gill lamellae; FV2: large plasmodia formed by the fusion of several plasmodia near the end of the gill filament (Vis. Cat. See Materials and Methods)
| Parasite species | Host (s) | Clinical signs | Vis. Cat. | Location | Type | Lesions |
|---|---|---|---|---|---|---|
| Myxobolus knobii (Kaur and Ahmad, 2017) | Cirrhinus mrigala | Mucous laden gills | A | Gill lamellae | LV1 | Hypertrophy of lamellar cells |
| M. majraiensis (Kaur and Ahmad, 2017) | Catla catla | Mucous laden gills, pale | A | Gill lamellae | LV1 | Hypertrophy and fusion of adjacent lamellae |
| M. markiwi (Kaur and Ahmad, 2016) | Labeo rohita | Pale gills | A | Gill lamellae | LV1 | Hypertrophy of lamellar cells |
| M. naini (Kaur and Singh, 2008) | L. rohita | Mucous laden gills, pale | A | Gill lamellae | LV1 | Hypertrophy of lamellar cells |
| M. nanokiensis (Kaur et al., 2015) | C. mrigala | Mucous laden gills | A | Gill lamellae | LV3 | Hypertrophy, hyperplasia and distortion of adjacent lamellae |
| M. potularis (Madhavan et al., 2013) | L. rohita | Gills full of abcesses, mucous laden | D | Gill filament | FV2 | Necrosis and hypertrophy of the gills |
| M. rocatlae (Basu and Haldar, 2002) | C. mrigala | Mucous laden and pale hemorrhagic gills | A | Gill lamellae | LV1 | Hypertrophy and hyperplasia of cellular elements |
| M. slendrii (Kaur and Singh, 2010) | L. rohita | Mucous laden gills | A | Gill filament | FV2 | Vascular hypertrophy |
| M. vascularis (Ahmad and Kaur, 2017) | C. mrigala | Mucous laden gills | C | Gill lamellae | LV1 | Hypertrophy of gill lamellae |
| M. venkateshi (Seenappa and Manohar, 1981) | C. mrigala | Mucous laden and pale hemorrhagic gills | A | Gill lamellae | LV1 | Hypertrophy of cellular elements |
Fig. 2.
a, b Sagittal sections of infected gills of M. rocatlae and M. slendrii showing LV1 and FV2 type of plasmodia and histopathological effects. LV1: Intralamellar vascular; N: necrosis; HT: hypertrophy; FV2: Intrafilamental vascular; HP: hyperplasia. c, d Sagittal sections of infected gills of M. vascularis n. sp. and M. rocatlae showing both LV1 type of plasmodia and histopathological effects: LV1: Intralamellar vascular; HT: hypertrophy; HP: hyperplasia; N: necrosis; LEC: lifting of epithelial cells. e, f Sagittal sections of infected gills of M. potularis and M. nanokiensis showing FV2 and LV3 type of plasmodia respectively and histopathological effects: FV2: Intrafilamental vascular; LV3: Intralamellar vascular; HT: hypertrophy; N: necrosis; DCE; degeneration of cellular elements
The present study indicated high prevalence of myxozoan parasites during the study period of winter months. M. potularis was found most prevalent with (90.90%) followed by M. venkateshi (63.79%), M. vascularis (62.71%), M. nanokiensis (60.86%), M. slendrii (59.25%), M. markiwi (55.76%), and low prevalence was recorded for M. majraiensis (48.33%), M. naini (51.11%), M. knobii (53.33%) and M. rocatlae (54.83%) (Fig. 3a–j; Table 3).
In this study, out of 321 infected fishes examined, mixed infection was recorded in 46 fishes (14.33%). The most common combination is M. naini + M. slendrii (63.04%) in L. rohita followed by M. naini + M. slendrii + M. knobii + M. venkateshi (39.95%) in C. mrigala (Table 5).
Table 5.
Occurrence of mixed infections of myxozoan parasites in the gills of cultured fingerlings in Fagan Majra, Punjab
| No. of mixed infected fishes | Percentage of mixed infection (%) | Parasites involved in mixed infection | Percentage of parasites involved in mixed infection (%) |
|---|---|---|---|
| 321 | 46 (14.33%) |
Myxobolus naini + M. slendrii Myxobolus naini + M. slendrii + M. knobii + M. venkateshi |
29 (63.04%) 17 (36.95%) |
During the entire sampling period, age and length of the fingerlings was recorded. The age of the fingerlings was recorded as 2–3 months and length ranged from 2 to 4.5 cm. The study clearly indicated moderate to severe infection in fingerlings as indicated by the gill plasmodial index (GPI). Gill plasmodial index (GPI) was recorded for all the 10 myxobolid species and ranged from 0 to 4. Maximum number of plasmodia were recorded in L. rohita infected with M. potularis with GPI of 4 indicating severe infection followed by M. majraiensis infecting Catla catla, M. knobii infecting C. mrigala and M. vascularis infecting C. mrigala with GPI of 3 indicating heavy infection. GPI 2 was recorded in M. venkateshi indicated moderate infection and for M. slendrii, M. rocatlae, M. nanokiensis, M. naini, and M. markiwi indicated light infection i.e. (GPI 1) (Table 3).
In addition, at the time of collection, the physico-chemical parameters of water were also recorded for the period of 6 months (Nov. 2014–Apr. 2015), comprising of 4 months of winter and 2 months of spring seasons. The present study indicated that the prevalence of myxozoan parasite was 43% in November (24.0 °C) and increased to 54% with the decrease in temperature (22.65 °C). Further, with decrease in temperature in January (14.35 °C) and February (18.1 °C), the infection rate was observed as 51% to 56% respectively, and in March the infection rate decreases (36%) through April (30%), with the increase in temperature (24.6 °C)–(24.5%). The pH level showed slight variation during the entire sampling period and ranged 7.71–8.46. Alkaline pH 8.46 was recorded in the month of January with maximum rate of infection (56%). In January, the minimum value of DO 8.3 was recorded with highest rate of myxozoan infection. An increase in the rate of DO from 9.05, 10.8 and 11.2 was recorded in February, March and April months respectively, indicating thereby that with the increase in DO, there was decrease in the percentage of myxozoan infection in aquaculture fingerlings of the nursery pond under study. The conductivity (1.47 Ms) and TDS (915 mg/l) was recorded in the month of January exhibiting highest rate of myxozoan infection (Table 6).
Table 6.
Physico-chemical analysis of water in nursery ponds located in the Village Fagan Majra, Punjab
| Month | Water temp. | pH | Dissolved oxygen (DO) mg/dm3 | Conductivity | TDS | Myxozoan prevalence (%) |
|---|---|---|---|---|---|---|
| November | 23.4 °C 24.6 °C (24.0) |
7.44 7.98 (7.71) |
8.2 9.2 (8.7) |
1.28 1.26 (1.27) |
910 880 (895) |
43 |
| December | 26.1 °C 19.2 °C (22.65) |
7.24 8.58 (7.91) |
8.4 9.3 (8.85) |
1.35 1.29 (1.32) |
870 840 (855) |
54 |
| January | 12.4 °C 16.3 °C (14.35) |
8.29 8.64 (8.46) |
5.7 10.9 (8.3) |
1.46 1.48 (1.47) |
910 920 (915) |
56 |
| February | 17.4 °C 18.8 °C (18.1) |
7.74 8.82 (8.28) |
9.5 8.6 (9.05) |
1.37 1.41 (1.30) |
950 830 (890) |
51 |
| March | 25.8 °C 23.4 °C (24.6) |
8.11 8.33 (8.22) |
10.02 11.4 (10.8) |
1.28 1.39 (1.33) |
910 890 (900) |
36 |
| April | 24.0 °C 25.0 °C (24.5) |
8.18 8.21 (8.19) |
10.8 11.6 (11.2) |
1.34 1.36 (1.35) |
900 870 (885) |
30 |
Discussion
In the present study, prevalence rate of myxozoans was recorded to be 53.50% in the cultured fingerlings of Indian major carps in Punjab. Recently, Kaur and Katoch (2016) reported 28.6% of myxozoan infection in fully growing cultured carps. In wild carp species, 36% prevalence rate was recorded by Kaur and Singh (2012a, b). The infection rate was highest in Labeo rohita (63.58%) followed by C. mrigala (58.94%) and C. catla (48.33%). Kalavati and Nandi (2007) also recorded L. rohita as most susceptible to the myxozoan parasites followed by C. mrigala and C. catla. They also found a high parasite virulence in C. catla, with mortality up-to 80–90%. Kaur and Singh (2012a, b) also recorded the C. catla as the most susceptible to myxozoans followed by C. mrigala and L. rohita in wetlands of Punjab. Kaur and Katoch (2016) also recorded a highest parasitic infection in L. rohita (40.48%) than other carp fishes. During the study, M. potularis was found most prevalent with (90.90%) followed by M. venkateshi (63.79%), M. vascularis (62.71%), M. nanokiensis (60.86%), M. slendrii (59.25%), M. markiwi (55.76%), M. rocatlae (54.83%), M. knobii (53.33%), M. naini (51.11%) and M. majraiensis (48.33%). Kaur and Singh (2012a, b) also recorded 64% prevalence for M. naini, 63% for M. patialensis and 23% for M. slendrii. Kaur and Katoch (2016) also described highest prevalence in M. potularis (62%) followed by M. nanokiensis (43.75%), M. longisporous (40%), Thelohanellus bifurcata (40%), T. dykovae (38.57%) and M. venkateshi (31.25%). In the present study, only gills were found infected. According to Chandra (1987), the gills of fish have rich blood supply and an important media for infectious agents, hence they serve as rich site of disease production. Longshaw et al. (2005) and Kaur and Singh (2012a, b) also recorded that the majority of infection with Myxobolus species was in the gills. In many states of India, Kalavati and Nandi (2007) observed gill myxoboliasis as the most widely spread disease among aquaculture carps and reported severe mortality due to myxozoan parasites during November and December 2000 in Andhra Pradesh. According to Molnár (2002b) and Eszterbauer et al. (2013) most of myxozoan species infected gills and this probably was because of the easy release of the spores for transmission of the parasite to the new host.
In the present study, a mixed infection was recorded only in L. rohita and C. mrigala, with the combination of M. naini + M. slendrii (63.04%) followed by M. naini + M. slendrii + M. knobii + M. venkateshi (36.95%) respectively. Holzer et al. (2005) also detected 3 myxozoan species i.e. Sphaerospora truttae, Chloromyxon schorovi and Tetracapsuloids bryosalmonae in the renal tubules as mixed infection. Recently, Kaur and Katoch (2016) observed mixed infections of myxozoan parasites in the gills of aquacultured cyprinids from Punjab as biparasitism, triparasitism and polyparasitism. In this study, the gill plasmodial index (GPI) was higher in M. potularis (4) indicating a severe infection. Fish fingerlings become more susceptible to myxozoan infection because of their immature immune system as discussed by Anderson (1974). According to Schmidt-Posthaus et al. (2013) brown trout fish infected with T. bryosalmonae older than 1 year have been characterized to have low prevalence of infection and less pathology in comparison to young brown trout. Sitja-Bobadilla and Alvarez-Pellitero (1993) have recorded host age-dependent differences in myxozoan prevalence and linked it to the host maturation. Haaparanta et al. (1994) found that gills of younger perch (Perca fluviatilis) were more heavily infected by Henneguya creplini in comparison to adults. Kaur and Attri (2015) recorded GPI index 2 indicating moderate infection for Henneguya bicaudi infecting C. mrigala in natural habitat. Kaur and Katoch (2016) recorded variable myxozoan infections in cultured fishes depending upon the species involved from light to severe as indicated by the GPI and also detected highest GPI score (4) in M. nanokiensis, M. longisporus, M. potularis and M. slendrii. Physico-chemical parameters of pond water were recorded for the period of 6 months, comprising 4 months of winter and 2 months of spring seasons. Nearly all the parameters were within the range of optimal values for carp production as suggested by Alabaster and Lloyd (1982), Boyd (1982), Piper et al. (1982) and Svobodova et al. (1993) (Table 7). During the study, the water parameters were conducive to gill myxoboliasis and water temperature, pH, DO, conductivity and TDS were the important criteria associated with disease outbreak. Hossain et al. (2008) also reported that the parasitic community of fish showed considerable variation with the environmental conditions. Also Banerjee and Bandopadhyay (2010) observed that the water temperature, pH, and DO are important water parameters that are related to disease infestation as they fluctuated more rapidly. Awal et al. (2001) and Saha et al. (2012) also affirmed the role of water temperature and DO in inducing myxozoan infection in carps.
Table 7.
Comparison of mean values of physicochemical water parameters with the optimal range for aquaculture ponds given by Alabaster and Lloyd (1982), Boyd (1982), Piper et al. (1982) and Svobodova et al. (1993)
| Parameter | Unit | Optimal value | Mean value (Present study) |
|---|---|---|---|
| Temperature | °C | 22–26 (28) | 21.36 |
| pH | (H +)mol/L | 6.5–8.5 | 8.12 |
| Dissolved oxygen | mg/l | > 5 | 9.48 |
| Conductivity | Ms | – | 1.34 |
| TDS | mg/l | < 1000 | 890 |
The present study indicated a high prevalence of myxozoan parasites during winter months. Banerjee and Bandopadhyay (2010) also reported highest parasitic infection during winter season i.e. December–February when the water quality degrades due to decrease in the dissolved oxygen level and temperature. Ahmad et al. (1991) also recorded more infection in the winter season than the other months of the year.
Plasmodia of LV1 and LV3 types were recorded in the gill lamellae and FV2 in the gill filaments. Recently, Kaur and Katoch (2016) observed that majority of plasmodia were recorded in gill lamellae. LV1 type of plasmodium cause dilation of the infected gill lamella at the base leaving normal structure at its middle and tip initially and when fully mature occupy whole of the gill lamella. In LV3 type of plasmodia, the gill lamellae adjoining the infected lamella bend towards the plasmodium in such a way that it tends to cover it on the sides. The adjoining gill lamellae seem to bear young plasmodia developing at their base. The mature plasmodium ruptures at the tip releasing myxospores which are seen to attach to the fresh gill lamellae. Complete necrosis of cellular elements and degeneration of gill lamellae is also observed due to the large sized plasmodium. Kaur et al. (2014) also reported that large-sized plasmodia damaged more than 50% of the gills causing respiratory distress and suffocation. (Here some lines were deleted from similar studies up to Kaur and Katoch 2014). FV2 type of plasmodia showed severe infection damaging almost 95% of the gill filament and also overlying gill lamellae due to the hypertrophy, hyperplasia, vacuolization of the stratified epithelium and vascular endothelium of the filament. Kalavati and Narasimhamurti (1985) and also Kaur and Katoch (2014) observed that rupturing of cysts can also lead to hemorrhages, and may result in considerable loss of respiratory surface.
The present study is the first record of the species diversity and prevalence of myxozoan parasites infecting fingerlings in nursery ponds in Punjab, India. The infection rate was recorded to be higher in fingerlings as compared to fish of 2–3 years of age. Therefore, it is emphasized that the management strategies should to be focused on the fingerling stage in hatchery ponds before their further distribution to the farmers in the rest of the Punjab state.
Acknowledgements
The authors acknowledge the facilities provided by Instrumentation lab Punjabi University, Patiala. Financial support for this study was provided by UGC-CAS grant Department of Zoology, Panjab University, Chandigarh.
Conflict of interest
There is no conflict of interest to disclose.
Ethical approval
Not required as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), because we are dealing with fish samples and the chemicals we are using are not harmful to the environment.
References
- Ahmad I, Kaur H. Myxobolus vascularis n. sp. (Cnidaria: Myxozoa: Myxosporea), a new parasite infecting fingerlings of Indian major carps in aquaculture in Punjab, India. Bull Pure Appl Sci Zool. 2017;36:57–70. doi: 10.5958/2320-3188.2017.00009.2. [DOI] [Google Scholar]
- Ahmad A, Ali SMK, Samad A. Probable cause of fish ulcer in Bangladesh. Nutr News. 1991;14(1):3. [Google Scholar]
- Alabaster JS, Lloyd R. Water quality criteria for fresh water fish. London: Butterworths; 1982. p. 315. [Google Scholar]
- Anderson DP. Fish immunology. In: Sneiszko SF, Axelrod HR, editors. Diseases of fishes. New Jersey: FH Publication; 1974. [Google Scholar]
- Awal MA, Begum AA, Chandra KJ, Ahmed GU, Kurohmaru M. Myxosporidian infection of gills and skin among carp from nursery ponds in Bangladesh: histopathology. Vet Archiv. 2001;71(5):265–276. [Google Scholar]
- Banerjee S, Bandopadhyay PK. Observation on prevalence of ectoparasites in carp fingerlings in two districts of West Bengal. J Parasit Dis. 2010;34(1):44–47. doi: 10.1007/s12639-010-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Haldar DP. Observations on three new species of Myxobolus Butschli, 1882 from hybrid carps of West Bengal, India. Indian J Environ Ecoplan. 2002;6(3):629–640. [Google Scholar]
- Bauer ON. Parasites of the Lena River. Izv Vsesoyuz Nauchno-Issled Inst Ozer Irechn ryb Khozyaistva. 1984;27:157–175. [Google Scholar]
- Boyd CE. Water quality management of pond fish culture. Dev Aquacult Fish Sci 9. Amsterdam: Elsevier; 1982. p. 318. [Google Scholar]
- Butschli O. Myxosporidia. In: Winter CF, editor. Bronn’s Klassens und Ordnungen des Tierreichs, Vol. I Protozoa. 2. Leipzig: C.F. Winter; 1882. pp. 590–603. [Google Scholar]
- Chandra KJ (1987) Fish health monitoring and control of disease. In: Training manual of training on integrated farming to the Upazila fisheries officer. DoF Bang, p 155
- Eiras JC, Malta JCO, Varela AMB, Pavanelli GC. Myxobolus insignis sp. n. (Myxozoa, Myxosporea, Myxobolidae), a parasite of the Amazonian teleost fish, Semaprochilodus insiginis (Osteichthyes: Prochilodontidae) Mem Inst Oswaldo Cruz. 2005;100:245–247. doi: 10.1590/S0074-02762005000300005. [DOI] [PubMed] [Google Scholar]
- Eiras JC, Zhang J, Molnár K. Synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea, Myxobolidae) described between 2005 and 2013. Syst Parasitol. 2014;88(1):11–36. doi: 10.1007/s11230-014-9484-5. [DOI] [PubMed] [Google Scholar]
- Eszterbauer E, Sipos D, Forro B, Bartosova P, Holzer AS. Molecular characterization of Sphaerospora molnari (Myxozoa), the agent of gill sphaerosporosis in common carp (Cyprinus carpio carpio) Dis Aquat Organ. 2013;104:59–67. doi: 10.3354/dao02584. [DOI] [PubMed] [Google Scholar]
- Fomena A, Bouix G. Myxosporea (Protozoa: Myxozoa) of freshwater fishes in Africa: keys to genera and species. Syst Parasitol. 1997;37:161–178. doi: 10.1023/A:1005839220014. [DOI] [Google Scholar]
- Haaparanta A, Valtonen ET, Hoffmann RW. Pathogenecity and seasonal occurrence of Henneguya creplini (Protozoa, Myxosporea) on the gills of pesch Perca fluviatilis in Central Finland. Dis Aquat Organ. 1994;20:15–22. doi: 10.3354/dao020015. [DOI] [Google Scholar]
- Holzer AS, Sommerville C, Wooten R. Molecular Studies on the seasonal occurrence and development of five myxozoans in farmed Salmo trutta L. Parasitology. 2005;132:193–205. doi: 10.1017/S0031182005008917. [DOI] [PubMed] [Google Scholar]
- Hossain MD, Hossain MK, Rahaman MH, Akter A, Khanom DA. Prevalence of ectoparasites of carp fingerlings at Santaher, Bogra. Univ J Zool Rajshahi Univ. 2008;27:17–19. [Google Scholar]
- Kalavati C, Nandi NC. Handbook on myxosporidean parasites of Indian fishes. Zoological Survey of India: Kolkata; 2007. p. 293. [Google Scholar]
- Kalavati C, Narasimhamurti CC. Histopathological changes in the gills of Channa punctatus BL. infected with Henneguya waltairensis. Arch Protistenkd. 1985;129:199–202. doi: 10.1016/S0003-9365(85)80023-3. [DOI] [Google Scholar]
- Kaur H, Ahmad I. Morphological description of M. markiwi (Cnidaria: Myxosporea: Myxozoa) infecting gills of fingerlings of aquaculture ponds from Punjab, India. Species. 2016;17(56):141–149. [Google Scholar]
- Kaur H, Ahmad I. A report on two new myxozoan parasites infecting gills of fingerlings of Indian major carps cultured in nursery ponds in Punjab (India) J Parasit Dis. 2017;41(4):987–996. doi: 10.1007/s12639-017-0923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Attri R. Morphological and molecular characterization of Henneguya bicaudi n. sp. (Myxosporea: Myxobolidae) infecting gills of Cirrhinus mrigala (Ham.) in Harike Wetland, Punjab (India) Parasitol Res. 2015;114:4161–4167. doi: 10.1007/s00436-015-4647-0. [DOI] [PubMed] [Google Scholar]
- Kaur H, Katoch A. Gill disease caused by Thelohanellus bifurcata Basu and Haldar, 1999 a pathogenic myxozoan parasite in cultured Indian carp, Labeo rohita (Hamilton, 1822) in Punjab, India. J Anim Health Prod. 2014;2(2):19–24. doi: 10.14737/journal.jahp/2014/2.2.19.24. [DOI] [Google Scholar]
- Kaur H, Katoch A. Prevalence, site and tissue preference of myxozoan parasites infecting gills of cultured fish in Punjab (India) Dis Aquat Organ. 2016;118:129–137. doi: 10.3354/dao02959. [DOI] [PubMed] [Google Scholar]
- Kaur H, Singh R (2008) Observation on one new species of the genus Myxobolus (Myxozoa: Myxosporea: Bivalvulida) and redescription of Myxobolus magauddi (Bajpai, 1981) Landsberg and Lom, 1991 recorded from freshwater fishes of Kanjali Wetland of Punjab (India) Proc Natl Congr Parasitol, NEHU Shillong, pp 75–79
- Kaur H, Singh R. Two new species of Myxobolus (Myxosporea, Bivalvulida) from the Indian major carp Labeo rohita Hamilton, 1822. Protistology. 2010;6(4):264–270. [Google Scholar]
- Kaur H, Singh R. A synopsis of the species Myxobolus Bütschli, 1882 (Myxozoa: Bivalvulida) parasitizing Indian fishes and a revised key to myxosporean genera. Syst Parasitol. 2012;81:17–37. doi: 10.1007/s11230-011-9321-z. [DOI] [PubMed] [Google Scholar]
- Kaur H, Singh R. Biodiversity of myxozoan parasites infecting freshwater fishes of three main wetlands of Punjab, India. Protistology. 2012;7(2):79–89. [Google Scholar]
- Kaur H, Katoch A, Gupta M. Thelohanellus filli sp. n., a pathogenic myxosporean infecting gills of cultured carp, Labeo rohita (Hamilton 1822) in Punjab, India. Species. 2014;10:31–38. [Google Scholar]
- Kaur H, Dar SA, Singh R. One new and three already known myxosporean parasites of Indian major carps in Punjab (India) Species. 2015;4:17–24. [Google Scholar]
- Lom J, Dyková I. Practical key for determination of myxosporean genera. In: Svodova Z, Vykusova B, editors. Diagnostics, prevention and therapy of fish diseases and intoxications. Vodnany: Research Institute of Fish Culture and Hydrobiology; 1991. [Google Scholar]
- Lom J, Dyková I. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol. 2006;53:1–36. doi: 10.14411/fp.2006.001. [DOI] [PubMed] [Google Scholar]
- Lom J, Noble ER. Revised classification of the class Myxosporea Butschli, 1881. Folia Parasitol. 1984;31:193–205. [Google Scholar]
- Longshaw M, Frear PA, Feist SW. Descriptions, development and pathogenicity of myxozoan (Myxozoa: Myxosporea) parasites of juvenile cyprinids (Pisces: Cyprindiae) J Fish Dis. 2005;28:489–509. doi: 10.1111/j.1365-2761.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- Luna LG. Manual of histological staining method of the Armed Forces Institute of Pathology. New York: McGraw-Hill; 1968. p. 111. [Google Scholar]
- Madhavan R, Bandyopadhyay PK, Santosh B. Observations on two new species of Myxobolus Bütschli, 1882 from minor carps of Tripura, India. J Parasit Dis. 2013;37(1):56–61. doi: 10.1007/s12639-012-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins ML, Souza VN, Moraes FR, Moraes JRE, Costa AJ, Rocha UF. Pathology and behavioral effects associated with Henneguya sp. (Myxozoa: Myxobolidae) infections of captive pacu Piaractus mesopotamicus in Brazil. J World Aquac Soc. 1997;28:297–300. doi: 10.1111/j.1749-7345.1997.tb00646.x. [DOI] [Google Scholar]
- Molnár K. Site preference of fish myxosporeans in the gill. Dis Aquat Organ. 2002;48:197–207. doi: 10.3354/dao048197. [DOI] [PubMed] [Google Scholar]
- Molnár K. Redescription and histopathology of Myxobolus cyprinicola Reuss. 1906. An intestinal parasite of the common carp (Cyprinus carpio L.) Acta Protozool. 2002;41:279–283. [Google Scholar]
- Piper RG, Mc Elwain IB, Orme LE, Mc Caren JP, Fowler LG, Leonard JR. Fish hatchery management. Washington, DC: US Fish and Wildlife service; 1982. [Google Scholar]
- Saha H, Saha RK, Kamilya D, Kumar P. Low pH dissolved oxygen and high temperature induces Thelohanellus rohita (myxozoan) infestation in tropical fish, Labeo rohita (Hamilton) J Parasit Dis. 2012;37:264–270. doi: 10.1007/s12639-012-0177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaullah M, Ahmed ATA. Gill myxoboliasis of major carps in Bangladesh. J Fish Dis. 1980;3:349–354. doi: 10.1111/j.1365-2761.1980.tb00404.x. [DOI] [Google Scholar]
- Schmidt-Posthaus H, Steiner P, Muller B, Casanova-Nakayama A. Complex interaction between proliferative kidney disease, water temperature and concurrent nematode infection in brown trout. Dis Aquat Organ. 2013;104:23–34. doi: 10.3354/dao02580. [DOI] [PubMed] [Google Scholar]
- Seenappa D, Manohar L. Five new species of Myxobolus (Myxosporea: Protozoa), parasitic in Cirrhina mrigala (Hamilton) and Labeo rohita (Hamilton), with a note on a new host record for M. curmucae Seenappa and Manohar, 1980. J Protozool. 1981;28:358–360. doi: 10.1111/j.1550-7408.1981.tb02866.x. [DOI] [Google Scholar]
- Sitja-Bobadilla A, Alvarez-Pellitero P. Population dynamics of Sphaerospora dicentrarchi Sitja-Bobadilla et Alvarez-Pellitero, 1992 and S. testiculasis Sitja-Bobadilla et Alvarez-Pellitero, 1990 (Myxosporea: Bivalvulida) infections in wild and cultured sea bass (Dicentrarchus labrax L.) Parasitology. 1993;106:39–45. doi: 10.1017/S0031182000074795. [DOI] [PubMed] [Google Scholar]
- Svobodova Z, Lloyd R, Machova J, Vykusova B. Water quality and fish health. Eifac Tech Pap 54. Rome: FAO; 1993. p. 59. [Google Scholar]