Abstract
Toxoplasmosis is an infectious zoonotic disease caused by protozoan Toxoplasma gondii. Detection of T. gondii infection with touchy and particular strategies is a key advance to control and prevent toxoplasmosis. Genotyping can explain the virulence, epidemiology and setting up new methodologies for diagnosis and control in human and animals. The point of this study was to assess the seroprevalence of T. gondii in sheep and goat in Egypt and to comprehend the genetic variety of T. gondii isolates circling in Egypt. Blood samples were gathered from 113 ewes and 95 she-goats from three Egyptian governorates (Cairo, Giza and Al-Sharkia). Also blood and tissue samples were gathered from 193 sheep and 51 goats from Cairo and Giza abattoirs. All samples were assayed serologically utilizing ELISA and OnSite Toxo IgG/IgM Rapid test cassettes (OTRT) tests and the tissue samples of the seropositive animals were digested and microscopically examined then bio-assayed in mice as viability test. All the T. gondii isolates undergo molecular identification using PCR and genotyped utilizing nPCR/RFLP analysis of SAG2 gene. The total seropositivity of live sheep and goat was 47.15 and 39.2% utilizing ELISA and OTRT respectively. Concerning abattoirs, seropositivity, positive microscopic examination, mice viability from sheep samples were 47.1%, 37.3% and 44.1% respectively while that of goats were 45.5%, 33.3% and 48.6% respectively. Eighteen T. gondii isolates were affirmed utilizing PCR. Genotyping confirmed 10 isolates (55.5%) as type II, 6 (33.3%) as type III and 2 (11.1%) as atypical genotypes. Type II and III are the genotypes mostly circling among small ruminants in Egypt and this is most significance for the public health in Egypt.
Keywords: Toxoplasma gondii, SAG2 gene, Genotyping, Sheep, Goat, Egypt
Introduction
Toxoplasmosis is a zoonotic infectious disease caused by an obligate intracellular parasite Toxoplasma gondii which has the ability to infect all warm blooded vertebrates. Late epidemio- logical investigations uncovered that about half of the total world’s population is infected with T. gondii (Dubey et al. 2008). Intrauterine infections targeting the placenta and fetus happen in humans and small ruminants causing placentitis, fetal decease and resorption, abortion, premature birth, and stillbirth with the high feasibility of cysts in the meat of affected animals (Innes et al. 2009).
In Egypt, numerous investigations exhibited late abortion due to T. gondii in small ruminant with sero-prevalence of 47.8% and 51.4% in sheep and 35.1% and 39.4% in goat utilizing Latex Agglutination Test (LAT) and ELISA individually (Fereig et al. 2016), Moreover, Hasanain et al. (2013) demonstrated that 61.4%, 80.4%, 34.4% and 19.3% of sheep, swine, buffaloes and cows in Egypt were positive for toxoplasmosis. The high level of toxoplasmosis in the Egyptian farm animals discloses that up to 57.1% of ready to eat Egyptian meat meals were positive to toxoplasmosis using PCR as endorsed by Abd El-Razik et al. (2014). Regarding human, Hasanain et al. (2013) confirmed that 60.7% of aborted women and 37.8% of asymptomatic occupational personnel in Egypt as positive for Toxo IgG.
Location of T. gondii infection with delicate and particular methods is a key advance to control and avert toxoplasmosis in human and animals. Disease can be analyzed by the immediate discovery of the parasite in blood, body liquids, or in tissues by intensification of particular nucleic acid sequences (PCR), histologic showing of the parasite or its antigens, or by segregation of the organism (Remington et al. 2001).
Serological identification of toxoplasmosis affection should be possible utilizing an assortment of methods, of those the latex agglutination (LAT) & ELISAs are broadly utilized as reference tests for the seroprevalence of toxoplasmosis in various animal species (Matsuo et al. 2014).
Genotyping can clear up the fundamental pathogenic components of an organism such as the virulence (Armand et al. 2017). Previously, T. gondii strains have been characterized into three hereditary Types by their virulence impact on mice. Type I strains are poor forming either meat tissue cyst or environmental oocysts and highly fatal to mice with great zoonotic importance; Type II strains are very cystogenic (cyst-forming) and all around shed oocysts. In this way, they basically spread through meat cysts or sporulated oocysts and Type III strains are animal strains, very rarely seen in human toxoplasmosis and contain elements of both Type I and II (Elfadaly et al. 2017a, b; Sroka et al. 2017).
The fundamental point of this investigation was to assess the seroprevalence of T. gondii-particular antibodies in sheep and goat in Egypt and to characterize T. gondii from ovine and caprine tissue samples by a highly delicate multilocus nPCR/RFLP investigation relying upon gnwtic loci (5′ SAG2 and 3′ SAG2) so as to comprehend genetic diversity of strains coursing in Egypt.
Materials and methods
Samples
Blood samples were gathered from 113 ewes and 95 she-goats from little holders at Cairo, Giza and Al-Sharkia governorates of Egypt for sera separation and serological testing. We obtained the consent of the owners for sample collection and publication of this survey, prior to the study.
Both blood and the corresponding tissue samples were gathered from 193 sheep and 51 goats from Al-Basateen and Al-Moneib abattoir at Cairo and Giza governorates of Egypt. The animals were kept under complete hygienic measures complying with the Egypt legislation for the protection of animals.
Serological assay
Sera were separated, marked and kept at − 20 °C until examined serologically against toxoplasmosis. The samples were screened serologically utilizing The OnSite Toxo IgG/IgM Rapid test cassettes (OTRT) (a lateral flow chromatographic immunoassay using T. gondii recombinant antigen), CTK Biotech Co., USA, Cat. No. Ro2330-C) to recognize the presence of the anti-Toxoplasma antibodies in the serum samples, following manufacturer’s guidelines.
ID Screen® Toxoplasmosis Indirect Multi-species ELISA for the discovery of anti-Toxoplasma gondii antibodies in serum samples (France, Cat. No. TOXOS-MS-2P). This plate is coated with Toxoplasma gondii P30 antigen. Animals that demonstrated positive for both serological tests, their meat samples were additionally tested (digestion of meat samples).
Digestion of meat samples
Tissue samples were set up as pronounced by Shaapan and Elfadally (2015), 20 g of the equal mutton tissue samples from diaphragm, heart and thigh muscles gathered from abattoirs in Egypt. The collective tissue samples were divided into two groups, the 1st was frozen at − 80 °C for additional DNA extraction while the 2nd group was presented to pepsin digestion. The microscopically examination for the presumed samples containing bradyzoites were intra-peritoneal inoculated in 2 mice for each sample for viability test.
Viability test
The test was performed by Elfadaly et al. (2015), The suspended residue of each processed tissue samples from diaphragm and thigh muscles was inoculated separately into 2–3 seronegative Swiss Webster Albino mice about 1 month-old weighing approximately 25–30 gm were used in this study from Laboratory Animal House, (intra-peritoneally or subcutaneously, 1 ml /mouse) obtained from Laboratory Animal House, National Research Centre, Egypt. The inoculated mice were followed up every day perception of clinical signs or dead mice previously 48 h of inoculation. Relying on the virulence of the isolate, the method will be proceeded. If ascites happened, peritoneal exudates were gathered within 72–84 h DPI and microscopic examination for tachyzoites was finished. If mice didn’t demonstrate ascites, they were sacrificed within 15th day post-inoculation, samples were gathered from heart, lung, liver, spleen and kidney for histo-pathological examination as indicated by Ajzenberg et al. (2002). LD50 and LD100 were noted for each species isolates.
Virulent RH Toxoplasma gondii strain
T. gondii RH virulent strain was kept up in Zoonotic Diseases Department, National Research Center, Egypt by nonstop intraperitoneal passages into Swiss strain albino mice every 3 days (McLeod et al. 1988).
Polymerase chain reaction (PCR)
The genomic DNA from the isolated T. gondii strains were extracted using GF-1 Tissue DNA extraction kit (Cat.No.GF-TD-050, Vivantis Co., Malaysia) with elution of DNA in 50 μL of elution buffer and PCR Amplification of B1 Gene (Burg et al. 1989)with the Expected PCR product of 193 bp.
Genotype analysis
Equal volumes (2 μL) of the eighteen DNA Samples were analyzed at the SAG2 locus by a nested PCR approach that independently intensified the 5′ and 3′ ends of the locus (Prince et al. 1990). Primers separately amplify the 5′ and 3′ ends of the T. gondii SAG2 locus as 242-bp and 222-bp products, respectively. The amplified fragments were purified with Gene JET Gel Extraction Kit (K0691, Thermo Fisher Co., USA) then digestion of the 5′ amplification products with Sau3AI (RV1192, Vivantis Co., Malaysia) recognized type III strains from type I and II strains, and digestion of the 3′ amplification products with HhaI (RE1224, Vivantis Co., Malaysia) differentiated type II strains from type I and III strains.
Statistical analysis
Data are presented as % of total sum, percent of total N, measures of association and variance were performed using SPSS® software. Paired sample t test and paired sample correlations were also performed. P value P < 0.05 is considered significant.
Results
Clinical signs in mice
The infected animals 10 mice turned out to sick with varied between raised and rough hair coat, pendulous abdomen, severe ascites, dullness, tachypnea by resting with fore legs on walls of the cages and evidence of early emaciation and dehydration diminished activity and weight reduction showed up as early as 2 weeks after inoculation. Moreover, neurological signs compatible with toxoplasmic encephalitis (TE) through tottering gait and paralysis were seen in infected mice. None of these neurological signs developed in the negative control groups.
Morphological studies of the isolated T. gondii developmental stage
Tachyzoites was acquired from the peritoneal exudates of formerly inoculated mice 2–3 days earlier during maintenance of the T. gondii strain or acquired from mice inoculated with infected processed animal ‘ tissues after 6–8 days from inoculation.
Serological examination
Blood samples were gathered from 113 ewes and 95 she-goats from small holders at Cairo, Giza and Al-Sharkia governorates of Egypt for sera separation and serological screening. With respect to serological examination utilizing the OnSite Toxo IgG/IgM Rapid test cassettes (OTRT), the overall seroprevalence of the infection was of IgG type, and there was no seropositive IgM.
Regarding the seroprevalence of T. gondii in 113 sheep and 95 goats from different governorates of Egypt (Cairo, Giza and Al-Sharkia) using OTRT and ELISA tests, the highest incidence of the disease was in Al-Sharkia (58.3%), trailed by Giza (43.8%) and then Cairo (28.1%) as appeared in Table 1.
Table 1.
Seroprevalence of T. gondii in local ewes and she-goats in different governorates of Egypt
| Governorate | Sheep positive (OTRT) | Goat positive (OTRT) | Total (%) | Sheep positive (ELISA) | Goat positive (ELISA) | Total (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | No | % | |||
| Cairo | 10/25 | 40 | 10/32 | 31.2 | 35.1 | 7/25 | 28 | 9/32 | 28.1 | 28.1 |
| Giza | 20/33 | 60.6 | 9/22 | 40.1 | 52.7 | 17/33 | 51.5 | 8/22 | 36.3 | 43.8 |
| Al-Sharkia | 36/55 | 65.4 | 24/41 | 56.1 | 62.5 | 34/55 | 61.8 | 22/41 | 53.6 | 58.3 |
| Total | 66/113 | 58.4 | 43/95 | 45.2 | 52.4 | 58/113 | 51.3 | 39/95 | 41 | 46.6 |
Concerning the seroprevalence of T. gondii in sheep and goat, the incidence in sheep (51.3%, 58.4%) was higher than that of goat (41%, 45.2%) utilizing ELISA and OTRT respectively (Table 1). The prevalence using OTRT in sheep and goat (52.4%) was higher than that of ELISA (46.6%) as appeared in Table 2.
Table 2.
Prevalence of T. gondii infection in ewes and she-goat using different serological methods
| TEST | Sheep positive reactors | Goat positive reactors | Total | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| OTRT | 66/113 | 58.4 | 43/95 | 45.2 | 109/208 (52.4%) |
| ELISA | 58/113 | 51.3 | 39/95 | 41 | 97/208 (46.6%) |
From Tables 1, 2, 3, When Analysis of variance was performed depending on the results of the three governorates, there is a non-significant difference between breeds and between seropositive and negative with either ELISA or OTRT. Moreover the two tests (ATRT and ELISA) were nearly similar where no significant difference was observed when paired t test was performed and any one of them is enough depending on the high correlation of the paired t test (r = 0.956; P < 0.0001).
Table 3.
Prevalence of T. gondii infection in Sheep andgoat at slaughter house
| TEST | Sheep positive reactors | Goat positive reactors | Total | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| OTRT | 105/193 | 54.4 | 28/51 | 53 | 133/244 (54.5%) |
| ELISA | 94/193 | 48.7 | 22/51 | 43.1 | 116/244 (47.5%) |
| Positive by both | 91/193 | 47.1 | 20/51 | 39.2 | 111/244(45.5%) |
In the current work, blood and the coordinating tissue samples were gathered from 193 sheep and 51 goats from Cairo and Giza abattoirs (El-Moneib, El-Warak and El-Basatin abattoirs). The sero-prevalence of infection in sheep (48.7%, 54.4%) was higher than that of goat (43.1%, 53%) using ELISA and OTRT individually (Table 3). The seropositivity of OTRT in sheep and goat (54.5%) was higher than that of ELISA (47.5%) as appeared in Table 3.
Seropositive, microscopic examination, mice viability, LD100 and LD50 of small ruminants at slaughter house were displayed in Table 4
Table 4.
Seropositive, microscopic examination, mice viability, LD100 and LD50 and genotyping of small ruminants at slaughter house N.B. the seropositive samples hereby are those samples positive in both serological tests (OTRT and ELISA)
| Species | No. of samples | Seropositive/total (%) | Microscopic/total (%) | Mice viability/microscopic (%) | LD50 (%) | LD100 (%) | Genotyping | |||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | Atypical | |||||||
| Sheep | 193 | 91/193 (47.1%) | 34/91 (37.3%) |
15/34 (44.1%) |
15/34 (44.1%) |
0 (0%) |
0 | 7 | 6 | 2 |
| Goat | 51 | 20/51 (39.2%) |
3/20 (15%) |
3/3 (100%) |
3/3 (100%) |
0 (0%) |
0 | 3 | 0 | 0 |
| Total | 244 | 111/244 (45.5%) | 37/111 (33.3%) |
18/37 (48.6%) |
18/37 (48.6%) |
0 (0%) |
18 | |||
Microscopic examination
The microscopic examination was just performed on the digested tissue samples of the positive sera. The percentage values were 37.3% in sheep and 15% in goats (Table 4).
Viability test with LD50 and LD100 in mice
As indicated by mice viability, goats were recorded the most species harboring T. gondii tissue cysts (100%), followed by sheep (44.1%). The outcomes in the present investigation signified a total 18 of small ruminants isolates (15 sheep and 3 goat) were effectively passed into mice with comparative morbidity and mortality ratios (Table 4). The mice viability test of T. gondii was just identified to the microscopic positive tissue samples which contain bradyzoites like protozoa, the recorded values and rates were 15/34 (44.1%) in sheep and 3/3 (100%) in goat(Table 4). Additionally, The T gondii LD50 and LD100 were recorded fluctuated percentage values 44.1% and 0% and 100%, 0% with the relating to sheep, goats respectively with total percentages inside small ruminants 72%, 0% respectively.
The measures of association of seropositive samples of two breeds was significantly (P = 0.023) different than seronegative samples. Also vialbility test of seropositive samples was significantly high for goats (P = 0.001). The seropositive samples was significantly (P = 0.059) higher than the microscopic with high correlation (r = 0.94; P = 0.051). The seropositive was more significant (P = 0.051) than the viability and both correlated (r = 0.96; P = 0.032). n contrast the microscopic was not significantly different than the viability test (> 0.05) and their correlation was not significant (r = 0.89).
Polymerase chain reaction (PCR)
Utilizing PCR as a confirmatory test, all the 18 (7.38%) T. gondii isolates were affirmed as 15 (6.15%) from sheep and 3 (1.23%) from goat with PCR product (193 bp) as shown with few samples in Fig. 1.
Fig. 1.
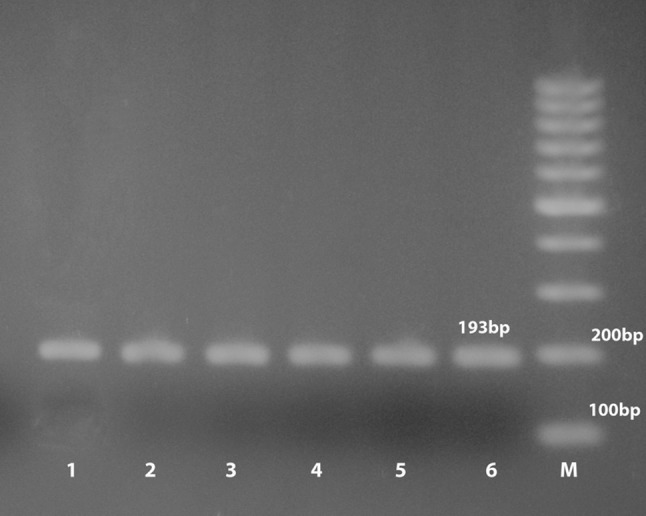
Detection of T. gondii isolates DNA by PCR. M, 100 bp ladder; Lane 1, Positive control; Lanes 2–6, selected positive local T. gondii DNA (193 bp)
Genotype analysis
Primers were chosen to independently amplify the 5′ and 3′ ends of the T. gondii SAG2 locus as 242 and 222 bp products, respectively as shown in Figs. 2 and 3. All the eighteen DNA samples were amplified at both sites with the exception of two samples which demonstrated negative amplifications at the 5′ end of SAG2 gene (atypical genotypes).
Fig. 2.
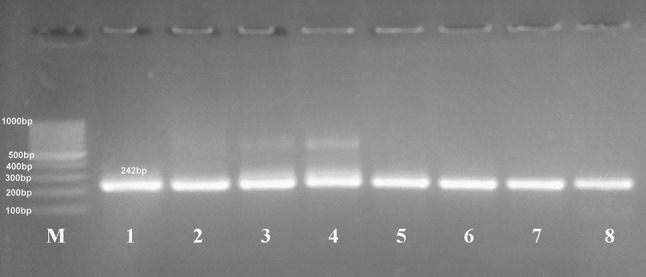
Nested PCR amplification of 5′ end of SAG2 gene resolved in 2% agarose gel electrophoresis shows amplification products of samples (lanes 2–8) at 242 bp, lane 1 = the positive control (RH strain), (M) marker = 100 bp DNA ladder
Fig. 3.
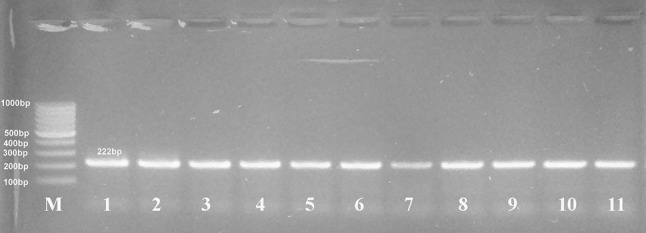
Nested PCR amplification of 3′ end of SAG2 gene resolved in 2% agarose gel electrophoresis shows amplification products of samples (lanes 2–11) at 222 bp, lane 1 = the positive control (RH strain), (M) marker = 100 bp DNA ladder
Digestion of the 5′ amplification products of reference isolates with Sau3AI recognized allele 3 (type III strains) from alleles 1 and 2 (type I and II strains) (Fig. 4a) and digestion of the 3′ amplification products of reference isolates with Hha distinguished allele 2 (type II strains) from alleles 1 and 3 (type I and III strains) (Fig. 4b). In this investigation, 10 samples (55.5%) were typed as type II and 6 (33.3%) as type III and 2 (11.1%) as atypical genotypes from samples using PCR-RFLP analysis as shown in Table 4, Figs. 5 and 6.
Fig. 4.
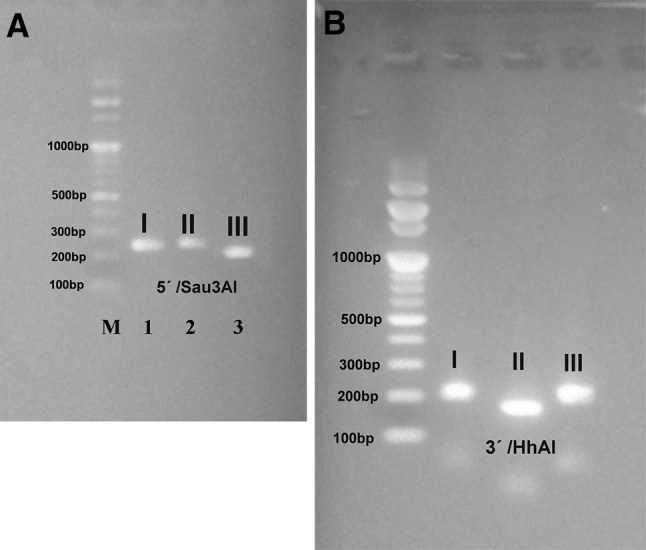
SAG2 nested PCR analysis. (A) Sau3AI restriction analysis of the 5′ amplification products from type I (RH), II (Me 49), and III (VEG) strains. (B) HhaI restriction analysis of the 3′ amplification products from type I, II, and III strains
Fig. 5.
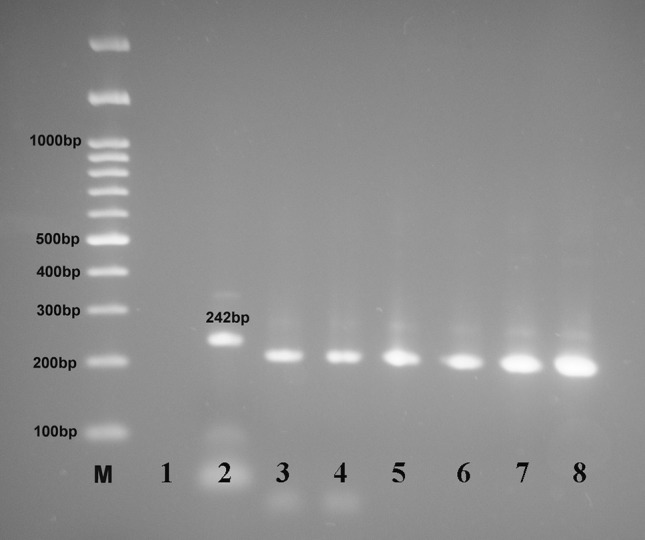
The restriction pattern of 5′ SAG2 amplified products by Sau3AI enzyme showing digested PCR products for samples in lanes 3–8, lane 1 = negative control, lane 2 = RH positive control, 242 bp (undigested), (M) marker = 100 bp DNA ladder
Fig. 6.
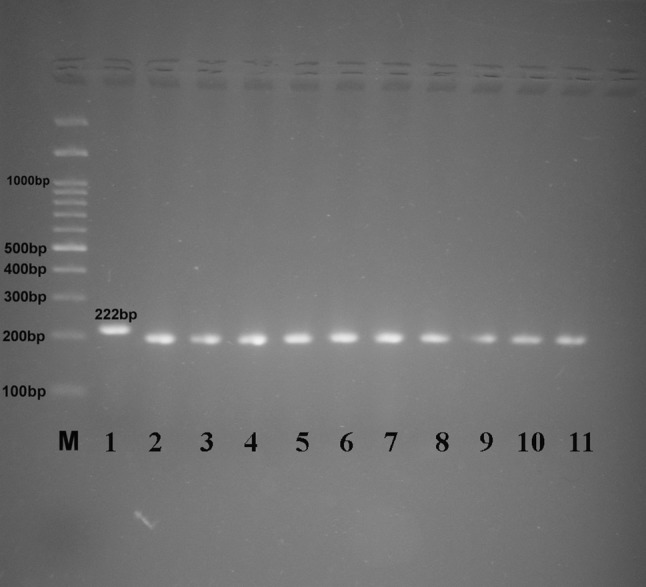
The restriction pattern of 3′ SAG2 amplified products by HhAI enzyme showing digested PCR products for samples in lanes 2–11, lane 1 = RH positive control, 222 bp (undigested), lane 12 = negative control, (M) marker = 100 bp DNA ladder
Discussion
Sheep and goat are most genuinely influenced by Toxoplasma gondii among farm animals and show high seroprevalences in numerous regions of the world up to 92 and 75%, respectively (Tavassoli et al. 2013). In Egypt, sheep and goats are viewed as the most highly liable hosts of toxoplasmosis with high rate of abortion, premature birth with resorption or mummification, stillbirth and neonatal passing and the great viability of cysts in the meat of infected animals (Fereig et al. 2016).
In Egypt, the seroprevalence of toxoplasmosis in sheep and goat was explored by numerous authors concerning different governorates such as Sadek et al. (2015) in Assiut (39%), Fereig et al. (2016) in Kafr El Sheikh and Minoufiya (38.7% and 28.7%), Ghoneim et al. (2010) in Al-fayium (98.4% and 41.7%), Younis et al. (2015) in Dakahlia (52% and 50%), Shaapan et al. (2008) and Hasanain et al. (2013) in Egypt (42.8 and 61.4% of sheep respectively).
In the present investigation, the seroprevalence of toxoplasmosis in live female sheep and goat (Table 1) was the most astounding in Sharkia (58.3%), trailed by Giza (43.8%) and Cairo (28.1%) utilizing ELISA and OTRT. These variable seroprevalence may rely upon the encouraging environmental surroundings for the development of oocysts, size of animal populations, which are reared in little or extensive gatherings (Fereig et al. 2016).
At present, a seroprevalence of 52.4% was accounted for ewes at various farms of Egypt (Table 2). It was parallel to different countries for example in Sudan (57.5%) Brazil (53.3%) as revealed by Khalil and Elrayah (2011) and Cosendey-KezenLeite et al. (2014), however higher than that of Saudia Arabia (23.4%) (Sanad and Al-Ghabban 2007), Kuwait (17.8%) (Alazemi 2014), Tunisia (1.8%) (Gharbi et al. 2013), Ghana (33.2%) (Van der Puije et al. 2000), South Africa (8%) (Hammond-Aryee et al. 2015), Argentina (17.3%) (Cosendey-KezenLeite et al. 2014), Iran (35%) (Tavakoli Kareshk et al. 2017). However, our outcomes were lower than that of Mor and Arslan (2007) in Turkey (95.7%), Bangladesh (69.9%) (Rahman et al. 2014), Greece (61.3%) (Anastasia et al. 2013) and Italy (60.6%) (Mancianti et al. 2013).
Regarding female goats in Egypt, a seropositivity of 46.6% was recorded (Table 2) which was higher than that of Fortes et al. (2017) in Brazil (33.3%), Tavakoli Kareshk et al. (2017) in Iran (13–30%), Zou et al. (2015) in China (17.6%), Issa (2017) in Iraq (13.04%) and Hossain et al. (2018) in Bangladesh (6.66%).
Similar outcomes were acquired for serum samples gathered from sheep (47.1%) and goat (39.2%) at slaughter house (Table 3). The prevalence of T. gondii infection was inferior in goats than sheep as concurred by Sharif et al. (2017). This might be credited to the dissimilarities in susceptibility to T. gondii and the feeding behaviors of these species (Bahrieni et al. 2008).
There are various serological techniques are accessible for the recognition of IgG and IgM antibodies; these are Sabin–Feldman Dye Test, Complement Fixation Test, Indirect Fluorescent Antibody Assay, Indirect Hemagglutination Assay, Modified Agglutination Test, Latex Agglutination Test (LAT) and Enzyme-Linked Immunosorbent Assay (ELISA) (Pal 2007).
Therefore, OTRT (Tables 1, 2, 3) in spite of the fact that this kit was created for human yet it was likewise utilized for animal as announced by Hossain et al. (2018). The test is a lateral flow chromatographic immunoassay for the simultaneous discovery and separation of IgG and IgM anti-T. gondii in serum . The general seroprevalence of the infection utilizing OTRT was just of IgG type, and there was no seropositive IgM (Tables 1-3). This can be clarified that IgG emerge within 1–2 weeks after infection and hold on through life, while IgM antibodies rise quickly inside the first week of infection, thus accordingly later on diminish and vanish at variable rates. A negative IgM test result basically shows old disease (Abu-Madi et al. 2008). Tests for the avidity of IgG antibodies have turned out consistent to decide the time of infection (Hamed et al. 2017).
These varieties in seropositivity might be identified with the approach utilized, geographical location,, climate situations, management practices, hygiene, the existence of felids, environmental pollution at each farm (Andrade et al. 2013), size of the farm and the age of the animals at the stage of sampling (Hamilton et al. 2015).
In the present study, positivity for Toxoplasma IgG antibodies is viewed as high hazard for public and animals’ health together, implies raising sheep on oocysts dirty unsanitary conditions and mirror the need of confine control measures against stray felines in the area of sheep.
The microscopic examination here was just performed on the digested tissue samples of the sheep and goats positive sera from abattoirs. The rate was 37.3% in sheep and 15% in goats. (Table 4). This was higher than that of Sadek et al. (2015) in Egypt who detailed a frequency of 8.62% and 12.77% in raw sheep and goat milk utilizing microscopical examination.
The mice viability test of T. gondii was just identified with the microscopic positive tissue samples which contain bradyzoites, goats were recorded the noteworthy species harboring T gondii tissue cysts (100%), trailed by sheep (44.1%). These outcomes concurs with that of Elfadaly et al. (2017a), b who detailed a level of 44% and 37.8% in goats and sheep respectively. The high rate of isolation of viable tissue cysts here shows an incredible general public health implication as it is an impression of the high level of pollution of the environment by oocysts of felines and the open air management system of animals as demonstrated by Gebremedhin et al. (2014).
Also, The T gondii LD50 and LD100 demonstrated changed rates (44.1%, 0% and 100%, 0%) to sheep and goats respectively with total percentages of 72% and 0% in sheep and goat. This was totally not quite the same as Elfadaly et al. (2017a), b comes about that demonstrated an aggregate rates of LD50 of 30.3%, and LD100 of 9.1% in small ruminants.
The infected mice turned out to be sick with ruffled fur, diminished actions and weight reduction showed up as early as 2 weeks after inoculation with neurological signs perfect with toxoplasmic encephalitis (TE). This was as opposed to that of Gebremedhin et al. (2014) that detailed that most isolates caused sub-clinical disease in mice with 2 sheep and 1 goat isolates were mouse-virulent and furthermore from Brazil where, 9 of the 16 T. gondii isolates from sheep (Ragozo et al. 2008) and 10 of the 12 T. gondii isolates from goats (Ragozo et al. 2009) were mice-virulent.
PCR assay is a fundamental route for the determination of Toxoplasma gondii infection and have higher precision, sensitivity and specificity than conventional diagnostic techniques (Kompalic-Cristo et al. 2007). Several PCR measures have been produced for the recognition of Toxoplasma gondii DNA by amplification of target such as B1 gene, PCR enhancing this gene target has demonstrated great specificity for Toxoplasma gondii DNA identification (Reischl et al. 2003).
In the present study, PCR relying upon B1 gene amplification affirmed all the 18 positive isolates (7.38%)in the mice viability test as 15 sheep (6.15%) and 3 (1.23%) goats. Despite the fact that PCR was not connected on serologically negative animals but rather this rate (7.38%) still was higher than 2% announced by Ahmed et al. (2014) in sheep milk samples in the rural areas at Sharkia, Egypt, and furthermore it was higher than that detailed by Khayeche et al. (2014) in sheep (5.7%) in Tunisia and that of Halova et al. (2013) in sheep (3.6%) in Ireland. The variety in the outcomes of PCR may be ascribed to the distinction in the territory, management, hereditary substance of the host and the pathogen (Tenter et al. 2000). The outcomes of PCR (7.38%) was lower than that of ELISA (45.5%). This demonstrates the existence of Toxoplasma-specific antibodies alone is not adequate for recognizing Toxoplasma infection and ELISA joined with the PCR is fundamental for precise determination of Toxoplasmosis (Hasanain et al. 2013).
The identification of the genotype of T. gondii is vital for better comprehension of its epidemiological perspectives such as sources of infection, methods of transmission to people, its clinical signs (Sibley et al. 2009), building up new approaches for diagnosis, as well as prevention and control of this parasite in human and animals (Nassef et al. 2015).
Diverse techniques for Toxoplasma genotyping have been created in the previous years (Liu et al. 2009). Multilocus PCR-RFLP was the strategy for decision to recognize the genotypes of T. gondii isolates, for the most part for its effortlessness, great sensitivity and applicability (Armand et al. 2017). It distinguishes between the three clonal genotypes of T. gondii adding to some atypical genotypes (Tavakoli Kareshk et al. 2017).
In this investigation, 10 samples (55.5%) were keyed as type II and 6 (33.3%) as type III and 2 (11.1%) as atypical genotypes utilizing PCR-RFLP examination (Table 4 and Fig. 5and 6). The Nested PCR amplification of the SAG2 locus applied here (5′SAG2 and 3′SAG2 locus), followed by RFLP analysis, allowed simple to perform, touchy, quick definition of T. gondii to a particular genotype as concurred by Su et al. (2010).
The negative amplifications in two samples could be because of presence of mutations or may be due to polymorphisms in the primer binding locations of these isolates, raising the likelihood of recombinant or mixed genotypes as affirmed by Eldeek et al. (2017).
These results was parallel to that of Elfadaly et al. (2017a), b that uncovered T. gondii genotype II (59%), III (31.8%) and I (4.5%) from sheep in Egypt. Higher opportunity of zoonotic spread was normal as genotype-II which is the principle women isolates has been recognized in meat of Egyptian producing animals. This was confirmed in Egypt by Abdel-Hameed and Hassanein (2008) where T. gondii type II was found in 87% of the considered human isolates with incidence of abortion and intrauterine fetal demise. Moreover, El Bolaky et al. (2015) announced the predominance of type II from pregnant women with obstetric complexities.
Genotype II is the most well-known in Europe and North America while Type III is discovered once in a while worldwide and infrequently (Sroka et al. 2017). In Africa, type II was related with abortion in small ruminants especially in Tunisia and Ethiopia (Alghamdi et al. 2015; Gebremedhin et al. 2014). The same in Europe for example, France (Halos et al. 2010), Switzerland (Berger-Schoch et al. 2011), Italy (Chessa et al. 2014), UK (Aspinall et al. 2002) and Denmark (Jungersen et al. 2002). Besides, in Asia, such as China (Zhou et al. 2009).
All in all, Type II and III are the transcendent genotypes mainly circulating among small ruminants in Egypt. This might be because of its capability to outcompete other genotypes as well as its capacity to make high quantities of cysts (Robert-Gangneux and Dardé 2012). This demonstrate constant introduction of sheep and goats to infection because of substantial ecological pollution with oocysts shed form the huge number of infected stray cats in the farms with poor managing situations (Abdel-Rahman et al. 2012). The high variety of current T. gondii genotypes of greatest significance for the public health as small ruminants, which is being broadly consumed by individuals, could be the fundamental wellspring of T. gondii for people.
Along these lines, the control and prevention of toxoplasmosis in Egypt can be refined by controlling the cats’ population, in this way staying away from the spread of oocysts in the environment (Hove et al. 2005).
Further studies ought to be directed in different parts of the country utilizing more gene markers to give a more extensive view through the animal sources of T. gondii for human as there is an opportunity that more virulent parasite strains may flow in animal reservoirs and consequently transmit to humans.
Acknowledgements
The authors acknowledge financial support from the Science and Technology Development Fund (STDF), Egyptian Ministry of Higher Education and Scientific Research (Project No. 24196).
Authors’ contribution
Dr. HAH was responsible for collection of serum samples and serological examination of sera. Dr. HAE and AMAB were responsible for isolation and routine identification of T. gondii and the mice viability testing, Dr. YAS and Dr. HAE carried out the routine PCR analysis while Dr. KAAE and Dr. AMY were responsible for the genotyping of the T. gondii isolates and participated in drafting the manuscript. All authors read and approved the final manuscript.
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication. The authors also declare that the trials conducted in this work fulfill with the existing country laws.
Ethical statement
The study was approved Ethically by the Medical Research Ethical Committee, National Research Centre, Egypt under registration number 1-2 /0- 2 -1-0.2012.
References
- Abd El-Razik KA, El Fadally HA, Barakat AMA, Abu Elnaga ASM. Zoonotic hazards T. gondii viable cysts in ready to eat Egyptian meat-meals. World J Med Sci. 2014;11:510–517. [Google Scholar]
- Abdel-Hameed DM, Hassanein OM. Genotyping of Toxoplasma gondii strains from female patients with toxoplasmosis. J Egypt Soc Parasitol. 2008;38:511–520. [PubMed] [Google Scholar]
- Abdel-Rahman MAM, EL-Manyawe SM, Khateib AM, Saba S. Occurrence of Toxoplasma antibodies in caprine milk and serum in Egypt. Assiut Vet Med J. 2012;58:145–152. [Google Scholar]
- Abu-Madi MA, Al-Molawi N, Behnke JM. Seroprevalence and epidemiological correlates of Toxoplasma gondii infections among patients referred for hospital-based serological testing in Doha, Qatar. Parasite Vector. 2008;1:39–43. doi: 10.1186/1756-3305-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed HA, Shafik SM, Ali MEM, Elghamry ST, Ahmed AA. Molecular detection of Toxoplasma gondii DNA in milk and risk factors analysis of seroprevalence in pregnant women at Sharkia. Egypt Vet World. 2014;7:594–600. doi: 10.14202/vetworld.2014.594-600. [DOI] [Google Scholar]
- Ajzenberg D, Banuls AA, Tibayrenc M, Darde ML. Microsatellite analysis of T. gondii shows considerable polymorphism structured into main clonal groups. Int J Parasitol. 2002;32:27–38. doi: 10.1016/S0020-7519(01)00301-0. [DOI] [PubMed] [Google Scholar]
- Alazemi MS. Prevalence of anti-Toxoplasma gondii antibodies in aborted ewes in Kuwait. J Egypt Soc Parasitol. 2014;44:393–396. doi: 10.12816/0006478. [DOI] [PubMed] [Google Scholar]
- Alghamdi J, Elamin MH, Alhabib S. Prevalence and genotyping of Toxoplasma gondii among Saudi pregnant women in Saudi Arabia. Saudi Pharm J. 2015;82:1–7. doi: 10.1016/j.jsps.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasia D, Elias P, Nikolaos P, Charilaos K, Nektarios G. T. gondii and N. caninum seroprevalence in dairy sheep and goats mixed stock farming. Vet Parasitol. 2013;198:387–390. doi: 10.1016/j.vetpar.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Andrade MMC, Carneiro M, Medeiros AD, Neto VA, Vitor RWA. Seroprevalence and risk factors associated with ovine toxoplasmosis in northeast Brazil. Parasite. 2013;20:1–6. doi: 10.1051/parasite/2013019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand B, Solhjoo K, Kordshooli MS, Davami MH, Pourahmad M, Orfaee V. T. gondii type I, predominant genotype isolated from sheep in South of Iran. Vet World. 2017;10:386–392. doi: 10.14202/vetworld.2017.386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall TV, Marlee D, Hyde JE, Sims PF. Prevalence of Toxoplasma gondii in commercial meat products as monitored by polymerase chain reaction—food for thought? Int J Parasitol. 2002;32:1193–1199. doi: 10.1016/S0020-7519(02)00070-X. [DOI] [PubMed] [Google Scholar]
- Bahrieni M, Fasihi Harandi M, Beigzadeh M, Kamyabi H, Zia-Ali N. Risk factors analysis associated with seropositivity to Toxoplasma gondii in sheep and goats in southeastern Iran using modified agglutination test (MAT) Iran J Parasitol. 2008;3:38–43. [Google Scholar]
- Berger-Schoch A, Herrmann D, Schares G, Müller N, Bernet D, Gottstein B, Frey C. Prevalence and genotypes of Toxoplasma gondii in feline faeces (Oocysts) and meat from sheep, cattle and pigs in Switzerland. Vet Parasitol. 2011;177:290–297. doi: 10.1016/j.vetpar.2010.11.046. [DOI] [PubMed] [Google Scholar]
- Burg JL, Grover CM, Pouletty P, Boothroyd JC. Direct and sensitive detection of a pathogenic protozoan, T. gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessa G, Chisu V, Porcu R, Masala G. Molecular characterization of Toxoplasma gondii type II in sheep abortion in Sardinia, Italy. Parasite. 2014;21:1–3. doi: 10.1051/parasite/2014007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosendey-KezenLeite RI, De Oliveira FC, Fraz ~ ao-Teixeira E, Dubey JP, De Souza GN, Ferreira AM, Lilenbaum W. Occurrence and risk factors associated to T. gondii infection in sheep from Rio de Janeiro, Brazil. Trop Anim Health Prod. 2014;46:1463–1466. doi: 10.1007/s11250-014-0667-5. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sundar N, Hill D, Velmurugan GV, Bandini LA. High prevalence and abundant atypical genotypes of Toxoplasma gondii isolated from lambs destined for human consumption in the USA. Int J Parasitol. 2008;38:999–1006. doi: 10.1016/j.ijpara.2007.11.012. [DOI] [PubMed] [Google Scholar]
- El Bolaky HAE, Hassanain MEA, Younis AIH, Abu Sarea EY, El Fadaly HA, Abd El Wahab WM. Bio-typing of Toxoplasma gondii isolates from complicated pregnant Egyptian women. MRJMMS. 2015;3:453–458. [Google Scholar]
- Eldeek HEM, Ahmad AA, El-Mokhtar MA, Abdel Kader AMM, Mandour AM, Mounib MEM. Toxoplasma genotyping in congenital toxoplasmosis in Upper Egypt: evidence of type I strain. J Parasitol Res. 2017;116:2393–2406. doi: 10.1007/s00436-017-5541-8. [DOI] [PubMed] [Google Scholar]
- Elfadaly HA, Hasanain MA, Shapan RM, Hasanain NA, Barakat AM. Corticosteroids opportunist higher T. gondii brain cysts in latent infected mice. Int J Zool Res. 2015;11:169–176. doi: 10.3923/ijzr.2015.169.176. [DOI] [Google Scholar]
- Elfadaly HA, Hassanan NA, Shaapan RM, Hassanain MA, Barakat AM, Abdelrahman KA. Molecular detection and genotyping of Toxoplasma gondii from Egyptian isolates. Asian J Epidemiol. 2017;10:7–44. [Google Scholar]
- Elfadaly HA, Hassanain MA, Shaapan RM, Hassanain NA, Barakat AM. Detection of Toxoplasma gondii from wastage nourished small ruminant and poultry: zoonotic significance. Int J Zool Res. 2017;13:6–11. [Google Scholar]
- Fereig RM, Mahmoud HYAH, Mohamed SGA, AbouLaila MR, Abdel-Wahab A, Ahmed Osman S, Zidan SA, El-Khodary SA, Mohamed AA, Nishikawa Y. Seroprevalence and epidemiology of Toxoplasma gondii in farm animals in different regions of Egypt. Vet Parasitol Reg Stud Rep. 2016 doi: 10.1016/j.vprsr.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Fortes MS, Lopes-Mori FMR, Caldart ET, Constantino C, Evers F, Pagliari S, de Almeida JC, Barros LD, Freire RL, Garcia JL, Headley SA, Navarro IT. Caprine toxoplasmosis in Southern Brazil: a comparative seroepidemiological study between the indirect immunofluorescence assay, the enzyme-linked immunosorbent assay, and the modified agglutination test. Trop Anim Health Prod. 2017 doi: 10.1007/s11250-017-1450-1. [DOI] [PubMed] [Google Scholar]
- Gebremedhin EZ, Abdurahaman M, Tessema TS, Tilahun G, Cox E, Goddeeris B, Dorny P, De Craeye S, Dardé M, Ajzenberg D. Isolation and genotyping of viable T. gondii from sheep and goats in Ethiopia destined for human consumption. Parasite Vector. 2014;7:1–8. doi: 10.1186/1756-3305-7-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbi M, Zribi L, Jedidi M, Chakkhari H, Hamdi S, Rhayem S. Prevalence of T. gondii infection in Tunisian sheep. Bull Soc Pathol Exot. 2013;106:184–187. doi: 10.1007/s13149-013-0290-4. [DOI] [PubMed] [Google Scholar]
- Ghoneim NH, Shalaby SI, Hassanain NA, Zeedan GS, Soliman YA, Abdalhamed AM. Comparative study between serological and molecular methods for diagnosis of toxoplasmosis in women and small ruminants in Egypt. Foodborne Pathog Dis. 2010;7:17–22. doi: 10.1089/fpd.2008.0223. [DOI] [PubMed] [Google Scholar]
- Halos L, Thébault A, Aubert D, Thomas M, Perret C, Geers R, Alliot A, Escotte-Binet S, Ajzenberg D, Dardé ML, Durand B. An innovative survey underlining the significant level of contamination by T. gondii of ovine meat consumed in France. Int J Parasitol Parasites. 2010;40:193–200. doi: 10.1016/j.ijpara.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Halova D, Mulcahy G, Rafter P, Turcekova L, Grant T, De Waal T. Toxoplasma gondii in Ireland: seroprevalence and novel molecular detection method in sheep, pigs, deer and chickens. Zoonoses Public Health. 2013;60:168–173. doi: 10.1111/j.1863-2378.2012.01514.x. [DOI] [PubMed] [Google Scholar]
- Hamed AMR, Omar SH, Basyoni MMA, El Antably AS, El Khateeb EA, El Kateb Y. Comparative and analytical study on active toxoplasmosis to assess the IgG avidity in correlation to serological profile in a cohort of Egyptian patients. Comp Clin Path. 2017;26:1157–1163. doi: 10.1007/s00580-017-2501-8. [DOI] [Google Scholar]
- Hamilton CM, Kelly PJ, Bartley PM, Burrells A, Porco A, Metzler D, Crouch K, Ketzis JK, Innes EA, Katzer F. Toxoplasma gondii in livestock in St. Kitts and Nevis, West Indies. Parasite Vector. 2015;8:166. doi: 10.1186/s13071-015-0776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Aryee K, van Helden LS, van Helden PD. The prevalence of antibodies to Toxoplasma gondii in sheep in the Western Cape, South Africa. Res Commun. 2015 doi: 10.4102/ojvr.v82i1.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanain MA, El-Fadally HA, Hassanain NA, Shapan RM, Barakat AM, Abd El-Razik KA. Serological and molecular diagnosis of toxoplasmosis in human and animals. WJMS. 2013;9:243–247. [Google Scholar]
- Hossain B, Ali Y, Rahman H, Munsi N, Talukder H. Sero-diagnosis of toxoplasmosis by using lateral flow chromatographic assay. Int J Nat Soc Sci. 2018;5:25–29. [Google Scholar]
- Hove T, Lind P, Mukaratirwa S. Seroprevalence of T. gondii infection in goats and sheep in Zimbabwe. Onderstepoort J Vet Res. 2005;72:267–272. doi: 10.4102/ojvr.v72i4.175. [DOI] [PubMed] [Google Scholar]
- Innes EA, Bartley PM, Buxton D, Katzer F. Ovine toxoplasmosis. Parasitology. 2009;136:1887–1894. doi: 10.1017/S0031182009991636. [DOI] [PubMed] [Google Scholar]
- Issa NA. Infection rate of toxoplasmosis in angora goats of duhok province-Iraq. BASJVET. 2017;16:144–158. [Google Scholar]
- Jungersen G, Jensen L, Rask M, Lind P. Non-lethal infection parameters in mice separate sheep type II Toxoplasma gondii isolates by virulence. Comp Immunol Microbiol Infect Dis. 2002;25:187–195. doi: 10.1016/S0147-9571(01)00039-X. [DOI] [PubMed] [Google Scholar]
- Khalil MM, Elrayah IE. Seroprevalence of Toxoplasma gondii antibodies in farm animals (camels, cattle, and sheep) in Sudan. Vet Med Anim Health. 2011;3:36–39. [Google Scholar]
- Khayeche M, Mhadhbi M, Gharbi M, Nasfi I, Darghouth MA. Detection of Toxoplasma gondii infection of sheep slaughtered in the governorate of Sousse on the occasion of the Muslim sacrifice feast (Eid Al-Adha) and analysis of risk factors. Bull Soc Pathol Exot. 2014;107:60–63. doi: 10.1007/s13149-014-0325-6. [DOI] [PubMed] [Google Scholar]
- Kompalic-Cristo A, Frotta C, Suárez-Mutis M, Fernandes O, Britto C. Evaluation of a real-time PCR assay based on the repetitive B1 gene for the detection of Toxoplasma gondii in human peripheral blood. J Parasitol Res. 2007;101:619–625. doi: 10.1007/s00436-007-0524-9. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang Z, Huang S, Zhu X. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasite Vector. 2009;20158:292. doi: 10.1186/s13071-015-0902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancianti F, Nardoni S, D’Ascenzi C, Pedonese F, Mugnaini L, Franco F, Papini R (2013) Seroprevalence, detection of DNA in blood and milk, and genotyping of Toxoplasma gondii in a goat Sadek population in Italy. Biomed Res Int 1–20 [DOI] [PMC free article] [PubMed]
- Matsuo K, Kamai R, Uetsu H, Goto H, Takashima Y, Nagamune K. Seroprevalence of Toxoplasma gondii infection in cattle, horses, pigs and chickens in Japan. Parasitol Int. 2014;63:638–639. doi: 10.1016/j.parint.2014.04.003. [DOI] [PubMed] [Google Scholar]
- McLeod R, Frenkel JK, Estes RG, Mack DG, Eisenhauer PB, Gibori G. Subcutaneous and intestinal vaccination with tachyzoites of Toxoplasma gondii and acquisition of immunity to peroral and congenital toxoplasma challenge. J Immunol. 1988;140:1632–1637. [PubMed] [Google Scholar]
- Mor N, Arslan MO. Kars yoresindeki koyunlarda Toxoplasma gondii in seroprevalansi’. Kafkas Univ Vet Fak Derg. 2007;13:165–170. [Google Scholar]
- Nassef NE, Abd El-Ghaffara MM, El-Nahasa NS, Hassanainb MA, Shams El-Dina SA, Ammar AIM. Seroprevalence and genotyping of Toxoplasma gondii in Menoufia governorate. Menoufia Med J. 2015;28:617–626. [Google Scholar]
- Pal M. Zoonoses. 2. Jaipur: Satyam Publishers; 2007. [Google Scholar]
- Prince JB, Auer KL, Huskinson J, Parmley SF, Araujo FG, Remington JS. Cloning, expression, and cDNA sequence of surface antigen p22 from Toxoplasma gondii. Mol Biochem Parasitol. 1990;43:97–106. doi: 10.1016/0166-6851(90)90134-8. [DOI] [PubMed] [Google Scholar]
- Ragozo AMA, Yai LEO, Oliveira LN, Dias RA, Dubey JP, Gennari SM. Seroprevalence and isolation of Toxoplasma gondii from sheep from Sao Paulo State, Brazil. J Parasitol. 2008;94:1259–1263. doi: 10.1645/GE-1641.1. [DOI] [PubMed] [Google Scholar]
- Ragozo AM, Yai LE, Oliveira LN, Dias RA, Goncalves HC, Azevedo SS, Dubey JP, Gennari SM. Isolation of Toxoplasma gondii from goats from Sao Paulo State, Brazil. J Parasitol. 2009;95:323–326. doi: 10.1645/GE-1854.1. [DOI] [PubMed] [Google Scholar]
- Rahman M, Azad MT, Nahar L, Rouf SM, Ohya K, Chiou SP. Age-specificity of Toxoplasma gondii seroprevalence in sheep, goats and cattle on subsistence farms in Bangladesh. J Vet Med Sci. 2014;76:1257–1259. doi: 10.1292/jvms.14-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischl U, Bretagne S, Kruger D, Ernault P, Costa JM. Comparison of two DNA targets for the diagnosis of toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect Dis. 2003;3:7. doi: 10.1186/1471-2334-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington JS, Klein J, editors. Infectious diseases of the fetus and newborn infant. 5. Philadelphia: W.B. Saunders; 2001. pp. 205–346. [Google Scholar]
- Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek OA, AbdeL-Hameed ZM, Kuraa HM. Molecular detection of Toxoplasma gondii DNA in raw goat and sheep milk with discussion of its public health importance in Assiut governorate. Assiut Vet Med J. 2015;61:166–177. [Google Scholar]
- Sanad MM, Al-Ghabban AJ. Serological survey on toxoplasmosis among slaughtered sheep and goats in Tabouk, Saudi Arabia. J Egypt Soc Parasitol. 2007;37:329–340. [PubMed] [Google Scholar]
- Shaapan RM, El-Nawawi FA, Tawfik MA. Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Vet Parasitol. 2008;153:359–362. doi: 10.1016/j.vetpar.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Shaapan RM, Elfadally HA. Latency in toxoplasmosis. Saarbrücken: LAP Lambert Academic Publishing; 2015. p. 72. [Google Scholar]
- Sharif M, Gholami S, Ziaei H, Daryani A, Laktarashi B. Seroprevalence of Toxoplasma gondii in cattle, sheep and goats slaughtered for food in Mazandaran province, Iran, during 2005. Vet J. 2017;174:422–424. doi: 10.1016/j.tvjl.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Khan A, Ajioka JW, Rosenthal BM. Genetic diversity of Toxoplasma gondii in animals and humans. Philos Trans R Soc Lond B Biol Sci. 2009;364(1530):2749–2761. doi: 10.1098/rstb.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS: Statistical package for social science, PC software, version16 for Windows Inc, Chicago, IL, USA Copyright© SPSS 2007
- Sroka J, Kusyk P, Bilska-Zając E, Karamon J, Dutkiewicz J, Wójcik-Fatla A, Zając V, Stojecki K, Różycki M, Cencek T. Seroprevalence of Toxoplasma gondii infection in goats from the south-west region of Poland and the detection of T. gondii DNA in goat milk. Folia Parasitol. 2017;64:023. doi: 10.14411/fp.2017.023. [DOI] [PubMed] [Google Scholar]
- Su C, Shwab EK, Zhou P, Zhu XQ, Dubey JP. Moving towards an integrated approach to molecular detection and identification of T. gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- Tavakoli Kareshk A, Mahmoudvand H, Keyhani A, Tavakoli Oliaee R, Mohammadi MA, Babaei Z, Hajhosseini MA, Zia-Ali N. Molecular detection and genetic diversity of T. gondii in different tissues of sheep and goat in Eastern Iran. Trop Biomed. 2017;34:681–690. [PubMed] [Google Scholar]
- Tavassoli M, Esmaeilnejad B, Malekifard F, Soleimanzadeh A, Dilmaghani M. Detection of Toxoplasma gondii DNA in sheep and goat milk in northwest of Iran by PCR-RFLP. Jundishapur J Microbiol. 2013;6:e8201. doi: 10.5812/jjm.8201. [DOI] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Puije WN, Bosompem KM, Canacoo EA, Wastling JM, Akanmori BD. The prevalence of anti-T. gondii antibodies in Ghanaian sheep and goats. Acta Trop. 2000;76:21–26. doi: 10.1016/S0001-706X(00)00084-X. [DOI] [PubMed] [Google Scholar]
- Younis EE, Abou-Zeid NZ, Zakaria M, Mahmoud MR. Epidemiological studies ontoxoplasmosis in small ruminants and equines in Dakahlia governorate, Egypt. Assiut Vet Med J. 2015;61:22–31. [Google Scholar]
- Zhou P, Zhang H, Lin RQ, Zhang DL, Song HQ, Su C, Zhu XQ. Genetic characterization of Toxoplasma gondii isolates from China. Parasitol Int. 2009;58:193–195. doi: 10.1016/j.parint.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Zou F, Yu X, Yang Y, Hu S, Chang H, Yang J, Duan G. Seroprevalence and risk factors of Toxoplasma gondii infection in buffaloes, sheep and goats in Yunnan Province, south western China. Iran J Parasitol. 2015;10:648–651. [PMC free article] [PubMed] [Google Scholar]


