Abstract
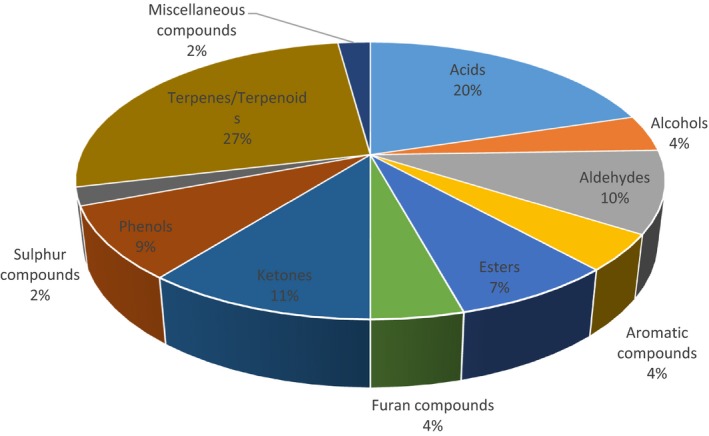
The objective of this study was to investigate the volatile flavor compounds of nkui, a Cameroonian food, using solid phase microextraction (SPME) and a two‐dimensional gas chromatography time of flight mass spectrometry GC×GC‐TOF‐MS system. Using SPME, volatile compounds were extracted from nkui and analyzed by GC×GC‐TOF‐MS. The data retrieved revealed the presence of flavor volatiles including acids (20%), alcohols (4%), aldehydes (10%), aromatic compounds (4%), esters (7%), furans (4%), ketones (11%), terpenes and terpernoids (27%). Although the terpene compounds were the most predominant, an ester (linalyl acetate) had the highest percentage of 19%, conferring a sweet, green and citrus flavor. Results obtained from this study suggest that the characteristic flavor of nkui was due to the combination of different volatile flavor compounds, which contributed to its aroma. Considering the medicinal importance of these compounds, their presence positions nkui as a vital food source with health benefits and medicinal properties.
Keywords: flavor, GC×GC‐TOF‐MS, nkui, SPME
1. INTRODUCTION
Plant parts are frequently used as spices, food additives, and meals. Nkui is a traditional heavily spiced Cameroonian soup, consumed as food and utilized in traditional medicine for nursing mothers. It is made from the combination of different stems and spices including Afrostyrax lepidophyllus Mildbr., Capsicum frutescens Linn., Fagara leprieurii Guill. et Perr., Fagara tessmannii Engl., Mondia whitei Hook. F. Skell., Pentadiplandra brazzeana Baill., Solanum gilo Raddi., Tetrapleura tetraptera Thaub., and Xylopia parviflora A. Rich. Benthane (Tchoupang et al., 2016). Nkui has a rich, distinct, and pleasant flavor, perceived during consumption and attributed to its abundant volatile compounds.
Flavor is a major factor that determines consumer selection, perception, acceptance of a particular food product and thus plays a significant role in the food. The flavor of any food is thus largely dependent on the number, quantity, and characteristics of the different volatile compounds it contains (Jelen, Majcher, & Dziadas, 2012). Such is extended to food substances and subsequent preparations from them. Despite the rich flavor and other volatile components present in nkui, previous studies that investigated nkui for these compounds have not been presented in the literature.
A comprehensive understanding and knowledge of volatile compounds in foods is quite complex and requires an efficient extraction and subsequent analytical technique. While several extraction techniques including solid phase extraction (SPE), stir bar sorptive extraction (SBSE), pressured hot water (PHW), and liquid–liquid extraction (LLE) are known (Gbashi, Adebo, Piater, Madala, & Njobeh, 2017; Goncalves et al., 2016), challenges around cost, environmental friendliness, ease of extraction, and cross‐contamination are their major challenges. The emergence of solid phase microextraction (SPME) has provided an efficient, robust, selective, cost effective, and environmentally friendly extraction technique and their combination with an effective detection technique offer enormous potentials (George et al., 2018; Kusano, Kobayashi, Iizuka, Fukushima, & Saito, 2016). The effectiveness of SPME is also demonstrated in its ability to integrate extraction, concentration, and analyte injection into a single process, ensuring sample throughput (Goncalves et al., 2016).
GC×GC‐TOF‐MS is sensitive chromatographic technique for both separation and detection of compounds. Its capabilities with includes increased mass spectral identification and deconvolution algorithms, identification abilities, enabled detection of thousands of peaks and the provision of additional data makes it a suitable analytical platform. Its compatibility with SPME for profiling of volatile flavor compounds has been reported (Ding, Wu, Huang, & Zhou, 2016; Goncalves et al., 2016). The aim of this study was thus to investigate the flavor components in nkui using SPME and subsequent analysis on GC×GC‐TOF‐MS.
2. MATERIALS AND METHODS
2.1. Nkui sampling and composition
Different plant materials that make up nkui were collected, identified by the National Herbarium in Cameroon, and deposited as specimens. The specimens were issued voucher numbers and the different plant parts and quantities used to make up the nkui are presented in Table 1.
Table 1.
Composition and proportion of materials making up nkui
| No. | Scientific name | Local name | Specimen no. | Part used | Quantity (g) |
|---|---|---|---|---|---|
| 1 | Afrostyrax lepidophyllus Mildbr. (Huaceae) | Mbadum | 31115 HNC | Fruit | 5 (5.55)a |
| 2 | Capsicum frutescens Linn. (Solanaceae) | Soga | 34726 HNC | Fruit | 1 (1.11) |
| 3 | Fagara leprieurii Guill. et Perr. (Rutaceae) syn. Zanthoxylum leprieurii Guill. et Perr. | Manyanje | 42992 HNC | Fruit | 3 (3.33) |
| 4 | Fagara tessmannii Engl. (Rutaceae) | Nga'ncu | 38960 HNC | Fruit | 7 (7.78) |
| 5 | Mondia whitei Hook. F. Skell. (Pleriplocaceae) | Dmte | 34180 HNC | Root | 35 (38.89) |
| 6 | Pentadiplandra brazzeana Baill. (Pentadiplandraceae) | Ndu'fe | 41536 HNC | Root | 11 (12.22) |
| 7 | Solanum gilo Raddi. (Solanaceae) | Seban | 14602 | Fruit | 6 (6.66) |
| 8 | Tetrapleura tetraptera Thaub. (Leguminosae) | Dumnkag | 31310 HNC | Fruit | 2 (2.22) |
| 9 | T. tetraptera Thaub. (Leguminosae) | Kubdum | 31310 HNC | Stem bark | 19 (21.11) |
| 10 | Xylopia parviflora A. Rich. Benthane (Annonaceae) | Mbatu'u | 42349 HNC | Fruit | 1 (1.11) |
Values in parentheses represent percentages.
2.2. SPME sampling
The extraction of nkui flavor components was done using a 50/30 μm SPME fiber coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) (Supelco, Inc., Bellefonte, PA). Briefly, 20 g of the sample was placed in a head‐space vial heated at 40°C for 20 min. Sampling was then done by exposing the fiber to the headspace of the sample for 20 min, after which the SPME device was transferred to the GC×GC‐TOF‐MS equipment for analysis. The process was repeated four times, though before each analysis the fibers were thermally cleaned and conditioned by heating them at 270°C in a stream of helium. A SPME fiber (Supleco, South Africa) was used to sample the volatile compound and immediately injected into a GC‐MS system for analysis.
2.3. GC×GC‐TOF‐MS analysis
Solid phase microextraction analyte samples were analyzed using a LECO Pegasus 4D Time of Flight mass spectrometer (LECO Corporation, St Joseph, MI, USA) equipped with a modified Agilent 7890A Gas Chromatograph (Agilent Technologies, Inc., Wilmington, DE, USA), a LECO GC×GC modulator and secondary oven (LECO Corporation, St Joseph, MI, USA) and a split/splitless inlet. The columns set used were: Rxi‐5 SilMS (29.5 m × 0.25 mm × 0.25 μm) as a primary column and Rxi 17 Sil MS (0.95 m × 0.25 mm × 0.25 μm) as the secondary column (Restek, Bellefonte, PA, USA). Helium was used as a carrier gas at a constant flow rate of 1 ml/min and an inlet temperature of 250°C. An initial oven temperature of 40°C was set and held for 0.5 min and then slowly ramped at 10°C/min to 250°C and then held for 0.5 min at 250°C. The modulator and secondary oven were run at an offset temperature of 5°C above the primary oven. The mass spectrometer was set up under the following conditions: no solvent delay because it was a SPME analysis; transfer line temperature at 250°C; Electron ionization at −70 eV; source temperature at 250°C; stored mass range: 45–600 μ; acquisition rate: 10 spectra/s for GC×GC‐TOF‐MS; detector offset voltage was set at 300 V.
Retention time alignment, matched filtration, peak detection, and peak matching were done on ChromaTOF software (LECO, USA). Subsequent identification was done by comparison with mass spectral databases (NIST, Adams, and EO libraries). A semi quantification of each compound was calculated on the basis of peak areas and relative concentration presented in %.
2.4. Statistical analysis
Average of the replicate data obtained from the GC×GC‐TOF‐MS analysis was computed and the result presented as mean ± standard deviation.
3. RESULTS AND DISCUSSION
Although a large number of volatile compounds are in foods, only a fraction of them eventually enhance flavor and aroma, as the perception of these sensory qualities is mainly driven by a combination of active volatile compounds sensed in the retronasal and/or orthonasal cavity (Bertuzzi, McSweeney, Rea, & Kilcawley, 2018). As these compounds have different characteristics, their concentrations can also affect the aroma of the finished food product. To identify the significant compounds that contribute to the flavor of nkui, this study investigated its flavor profile using SPME‐GC×GC‐TOF‐MS. Although a total of 250 compounds were obtained from the GC profiles, only 92 flavor related compounds were identified and subsequently divided into groups. This consisted of acids (8), alcohols (14), aldehydes (5), aromatic compounds (9), esters (9), furans (4), ketones (12), sulfur compounds (2), terpenes/terpenoids (22), and miscellaneous compounds (2) (Table 2). The structures of the major flavor compounds identified in the nkui are provided in Figure 1.
Table 2.
Volatile flavor compounds identified in nkui by SPME‐GC×GC‐TOF‐MS, their group classification and flavor descriptors
| Name | RT (s) | Quantity (%) | Flavor descriptorsa |
|---|---|---|---|
| Acids | |||
| Butanoic acid | 692.6 | 0.002 ± 0.00 | Butter, acidic, fruity, rose |
| Acetic acid | 716.6 | 1.712 ± 0.79 | Vinegar, sour, pungent |
| Propanoic acid | 806.7 | 0.002 ± 0.00 | Acidic, diary, fruity |
| 3,3‐dimethylacrylic acid | 857.5 | 0.002 ± 0.02 | Green, phenolic, diary |
| Butanoic acid, 3‐methyl‐ | 929.9 | 0.002 ± 0.00 | Cheese, dairy, creamy |
| Hexanoic acid | 1,077.3 | 0.009 ± 0.00 | Fatty, cheesy, fruity |
| Hexanoic acid, 2‐ethyl‐ | 1,160.8 | 0.003 ± 0.00 | Oily rancid, sweat‐like |
| Nonanoic acid | 1,317.1 | 0.004 ± 0.00 | Waxy, fatty cheesy |
| Alcohols | |||
| 1‐octen‐3‐ol | 728.8 | 0.035 ± 0.01 | Sweet, Mushroom, earthy, fungal |
| 4‐thujanol, stereoisomer | 739.5 | 0.224 ± 0.31 | Cooly, minty |
| Bicyclo[3.1.1]hept‐3‐en‐2‐ol, 4,6,6‐trimethyl‐, [1s‐(1à,2á,5à)]‐ | 792.7 | 0.069 ± 0.05 | Pine, ozone |
| Linalool | 827.9 | 17.849 ± 7.18 | Citrus, orange, floral |
| 1,2‐propanediol | 856.4 | 0.028 ± 0.02 | Odorless/fatty aroma |
| 1,5,7‐octatrien‐3‐ol, 3,7‐dimethyl‐ | 882.1 | 0.021 ± 0.02 | Moldy |
| Terpinen‐4‐ol | 896.0 | 0.159 ± 0.09 | Woody, earthy, musty |
| Pinocarveol | 918.9 | 0.012 ± 0.01 | Camphoreous, pine, woody |
| Trans‐piperitol | 942.2 | 0.067 ± 0.05 | Herbal |
| Trans‐carveol | 948.2 | 0.028 ± 0.03 | Caraway, solvent, spearmint |
| Farnesol | 972.9 | 0.013 ± 0.04 | Floral, juicy, green |
| 2,6‐octadien‐1‐ol, 3,7‐dimethyl‐, (E)‐ | 1,084.0 | 4.438 ± 5.98 | Floral |
| Nerolidol | 1,233.3 | 0.022 ± 0.01 | Green, floral, fruity |
| Thymol | 1,341.4 | 0.002 ± 0.00 | Medicinal spicy |
| Aldehydes | |||
| Nonanal | 664.6 | 0.053 ± 0.03 | Green, fat, citrus |
| Bicyclo[3.1.1]hept‐2‐ene‐2‐carboxaldehyde, 6,6‐dimethyl‐ | 891.1 | 0.031 ± 0.01 | Spicy, herbaceous |
| 2,6‐octadienal, 3,7‐dimethyl‐ | 941.1 | 0.139 ± 0.01 | Lemon |
| Cinnamaldehyde | 1,064.0 | 0.008 ± 0.00 | Cinnamon, spicy |
| Lilac aldehyde D | 1,151.9 | 0.069 ± 0.03 | Floral, lilac |
| Aromatic compounds | |||
| Benzene, 1‐methyl‐4‐(1‐methylethyl)‐ | 516.6 | 8.212 ± 9.10 | Spicy, balsamic, musty |
| Benzene, 1‐methyl‐2‐(1‐methylethyl)‐ | 519.2 | 1.764 ± 0.08 | Green, rubber |
| Benzene, 1‐methyl‐4‐(1‐methylethenyl)‐ | 708.7 | 0.077 ± 0.02 | Spicy, balsamic, musty |
| Benzaldehyde | 790.3 | 0.031 ± 0.01 | Nutty, bitter, woody |
| Linalyl anthranilate | 839.9 | 13.372 ± 0.11 | Fresh, linalool, orange, blossom |
| Benzaldehyde, 4‐(1‐methylethyl)‐ | 1,023.4 | 0.025 ± 0.00 | Nutty, bitter |
| Benzenemethanol, à,à,4‐trimethyl‐ | 1,081.3 | 0.046 ± 0.04 | Sweet, fruity, cherry |
| Quinoxaline, 5‐methyl‐ | 1,169.0 | 0.014 ± 0.01 | Nutty, peanut, roasted |
| Benzenemethanol, 4‐(1‐methylethyl)‐ | 1,270.1 | 0.007 ± 0.01 | Cumin, spicy, floral |
| Esters | |||
| Ethyl acetate | 129.3 | 0.783 ± 0.03 | Fruity, brandy‐like |
| Isononyl acetate | 674.8 | 0.004 ± 0.00 | Herbal, woody |
| (Z)‐3‐hexenyl acetate | 701.7 | 0.015 ± 0.08 | Green, fruity, apple pear |
| 1,6‐octadien‐3‐ol, 3,7‐dimethyl‐, acetate | 837.4 | 10.345 ± 8.59 | Bergamot, lavender |
| Linalyl acetate | 840.3 | 19.088 ± 0.12 | Sweet, green, citrus |
| Terpinyl propionate | 887.1 | 1.501 ± 1.12 | Floral, lavender |
| Geranyl formate | 968.3 | 0.079 ± 0.00 | Green floral |
| Neryl acetate | 987.1 | 0.849 ± 0.04 | Green, citrus like |
| 2,6‐octadien‐1‐ol, 3,7‐dimethyl‐, acetate | 1,013.1 | 2.344 ± 1.54 | Floral, rosy, sweet |
| Furan compounds | |||
| Furanoid | 715.5 | 0.320 ± 0.02 | Earthy, floral, sweet, woody |
| 2‐furanmethanol, 5‐ethenyltetrahydro‐à,à,5‐trimethyl‐, cis‐ | 744.6 | 0.725 ± 1.33 | Earthy, floral, sweet |
| Rosefuran epoxide | 875.7 | 0.010 ± 0.01 | Green, earthy, citrus |
| Furan, 2‐ethyl‐5‐methyl‐/2‐ethyl‐5‐methylfuran | 1,260.1 | 0.006 ± 0.01 | Gassy, burnt |
| Ketones | |||
| 6‐methyl‐5‐hepten‐2‐one | 597.9 | 0.632 ± 0.37 | Green, vegetable, musty, mushroom |
| 2‐nonanone | 659.1 | 0.464 ± 0.04 | Fruity, herbaceous |
| Bicyclo[2.2.1]heptan‐2‐one, 1,7,7‐trimethyl‐, (1s)‐ | 781.4 | 0.032 ± 0.04 | Camphoreous |
| 4‐isopropylcyclohex‐2‐en‐1‐one | 800.2 | 0.017 ± 0.01 | Spicy, cummy, caraway |
| Cyclohexanone, 5‐methyl‐2‐(1‐methylethylidene)‐, (r)‐ | 886.2 | 0.029 ± 0.03 | Peppermint, camphor |
| Umbellulone | 902.9 | 0.001 ± 0.00 | Minty, pungent |
| Cryptone | 925.4 | 0.013 ± 0.01 | |
| 2‐acetyl‐3,5‐dimethylpyrazine | 933.2 | 0.080 ± 0.06 | Nutty, roasted, hazelnut |
| Piperitone | 976.8 | 0.075 ± 0.05 | Herbal, minty |
| Ethyl maltol | 1,201.5 | 0.003 ± 0.00 | Sweet |
| 5‐methyl‐3,5‐octadien‐2‐one | 1,227.5 | 0.021 ± 0.02 | Buttery, woody |
| 7‐Oxabicyclo[4.1.0]heptan‐2‐one, 6‐methyl‐3‐(1‐methylethylidene)‐ | 1,426.3 | 0.126 ± 0.18 | Herbal, minty |
| Phenols | |||
| Phenol | 1,198.1 | 0.026 ± 0.01 | Sweet, medicinal |
| Phenol, 5‐methyl‐2‐(1‐methylethyl)‐ | 1,319.1 | 0.025 ± 0.07 | Aromatic, Sweet, medicinal |
| Phenol, 2‐methyl‐5‐(1‐methylethyl)‐ | 1,349.2 | 0.078 ± 0.08 | Pungent |
| Phenol, 3‐(1‐methylethyl)‐ | 1,352.3 | 0.029 ± 0.00 | Sweet, medicinal |
| Phenol, 5‐methyl‐2‐(1‐methylethyl)‐ | 1,319.1 | 0.025 ± 0.07 | Aromatic, Sweet, medicinal |
| Sulphur compounds | |||
| Trisulfide, dimethyl | 639.9 | 0.351 ± 0.16 | Sulphurous, alliaceous, eggy |
| Disulfide, methyl (methylthio)methyl | 1,085.7 | 3.578 ± 6.59 | Sulphurous, onion |
| Terpenes/Terpenoids | |||
| Eucalyptol (1,8‐cineole) | 423.3 | 0.895 ± 0.05 | Minty, eucalyptoid |
| Cyclohexene, 1‐methyl‐4‐(1‐methylethylidene)‐ | 530.4 | 0.261 ± 0.12 | Fresh, sweet, woody, citrus, pine |
| 3,7‐dimethyl‐1,3,6‐octatriene | 739.8 | 0.453 ± 0.08 | Woody, tropical, floral |
| δ‐Elemene | 749.3 | 0.013 ± 0.04 | Sweet herbal, woody |
| Tricyclo[4.4.0.0(2,7)]dec‐3‐ene, 1,3‐dimethyl‐8‐(1‐methylethyl)‐ | 768.8 | 1.013 ± 0.94 | Woody, spicy, honey |
| Methacrolein | 782.4 | 0.010 ± 0.01 | Floral |
| Naphthalene, 1,2,3,4,4a,5,6,8a‐octahydro‐7‐methyl‐4‐methylene‐1‐(1‐methylethyl)‐, (1à,4aà,8aà)‐ | 815.9 | 0.047 ± 0.02 | Fresh, woody |
| α‐zingiberene | 817.6 | 0.914 ± 0.01 | Spice, fresh |
| trans‐à‐Bergamotene | 850.1 | 0.224 ± 0.09 | Woody, warm, tea |
| α‐Santalene | 852.8 | 1.144 ± 0.24 | Woody |
| β‐Caryophyllene | 866.2 | 1.075 ± 1.04 | Spicy, peppery, woody |
| α‐Cubebene | 901.7 | 0.010 ± 0.00 | Herbal, earthy |
| Epi‐β‐santalene | 906.3 | 0.008 ± 0.00 | Woody |
| Terpinyl acetate<delta‐> | 918.4 | 0.014 ± 0.01 | Sweet, herbaceous |
| α‐Caryophyllene | 933.7 | 0.389 ± 0.07 | Musty, green |
| Farnesene<(E)‐β‐ | 950.1 | 0.002 ± 0.01 | Fruity, woody, citrus, sweet |
| Bisabolene<(Z)‐α‐ | 1,028.3 | 0.089 ± 0.10 | Sweet, spicy, balsamic |
| cis‐Carveol | 1,095.4 | 0.070 ± 0.07 | Spicy, caraway |
| α‐Phellandrene | 1,099.0 | 1.993 ± 2.96 | Peppery |
| 2,3‐dioxabicyclo[2.2.2]oct‐5‐ene, 1‐methyl‐4‐(1‐methylethyl)‐ | 1,146.5 | 0.264 ± 0.18 | Fatty, herbaceous |
| Limonene dioxide | 1,152.2 | 0.065 ± 0.02 | Citrus‐like, green |
| Carvacrol | 1,341.5 | 0.004 ± 0.02 | Pungent |
| Miscellaneous compounds | |||
| Butylhydroxytoluen (BHT) | 1,136.8 | 0.016 ± 0.02 | Mild, phenolic, camphor |
| Tris(methyl thio) methane | 1,194.3 | 0.623 ± 0.87 | Earthy, mushroom, musty |
Notes. RT: retention time (in s).
Flavor compounds were identified using the following references (Adams, R. P. 1995. Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Carol Stream, IL; Weyerstahl, P., Marschall, H., Thefeld, K., & Subba, G. C. 1998. Constituents of the essential oil from the rhizomes of Hedychium gardnerianum Roscoe. Flavour and Fragrance Journal, 13, 377–388; Jirovetz, L., Buchbauer, G., Stoyanova, A. S., Georgiev, E. V., & Damianova, S. T. 2003. Composition, quality control, and antimicrobial activity of the essential oil of long‐time stored dill (Anethum graveolens L.) seeds from Bulgaria. Journal of Agricultural and Food Chemistry, 51, 3854–3857; Behera, S., Nagarajan, S., & Rao, L. J. M. 2004. Microwave heating and conventional roasting of cumin seeds (Cuminum cyminum L.) and effect on chemical composition of volatiles. Food Chemistry, 87, 25–29; Burdock, G. A. (2010). Fenaroli's handbook of flavor ingredients 6th ed. CRC Press, NW, Boca Raton. http://www.google.com/patents/US4301184; http://www.thegoodscentscompany.com; http://www.perflavory.com).
Figure 1.
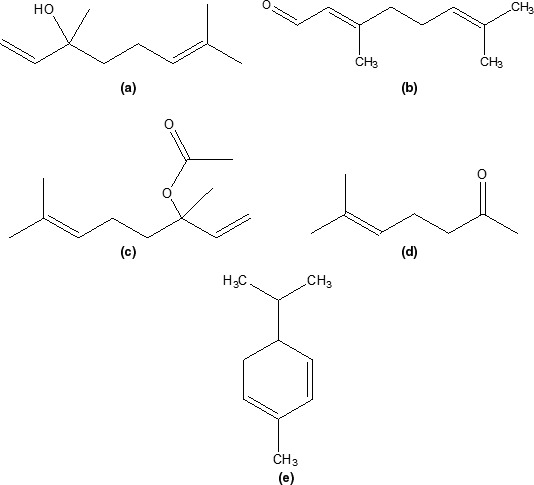
Major compounds in nkui: (a) Linalool, (b) 2,6‐octadienal,3,7‐dimethyl, (c) Linalyl acetate, (d) 6‐methyl‐5‐hepten‐2‐one, (e) à‐phellandrene
Alcohols are present in plants likely formed through physical damage, during storage, and processing (Eriksson, 1967; Goncalves et al., 2016). The alcohols identified in nkui constituted a total of 4% (Figure 2). Of the 14 alcohols recovered, the most abundant one was linalool exhibiting a floral, citrus like aroma. Other significant alcohol compounds found were 1,6‐octadien‐3‐ol, 3,7‐dimethyl‐, acetate, 2,6‐octadien‐1‐ol, 3,7‐dimethyl‐(E)‐ and its ester 2,6‐octadien‐1‐ol, 3,7‐dimethyl‐, acetate contributing a bergamot, floral, and sweet flavor to nkui (Table 2). Linalool (Figure 1) is a terpene alcohol which occurs in plants, spices, tree barks and possess anti‐inflammatory and chemoprotective activity (Peana et al., 2002; Ravizza, Gariboldi, Molteni, & Monti, 2008). Its presence in nkui could possibly be linked to the therapeutic and estrogenic use of the soup for lactating mothers in Cameroon, as demonstrated in an in vivo study by Tchoupang et al. (2016).
Figure 2.
Percentage representation of the flavor compounds in nkui
The largest group of compounds found in the nkui were terpenes and terpenoids (Figure 2). Terpenes constitute the largest class of natural products and are major sources of natural flavor additives in foods, fragrances, cosmetics, and in alternative medicines (Singh & Sharma, 2015). They are usually endogenous in plants as secondary metabolites and could also be formed through biosynthesis by certain microorganisms. The major compounds in this category were à‐phellandrene (2%) (Figure 1), α‐santalene (1.14%), and β‐caryophyllene (1.07%) (Table 2), all causing a peppery, woody, and spicy flavor to nkui. Other terpenes found in nkui were α‐zingiberene, trans‐à‐bergamotene, α‐caryophyllene, and eucalyptol (Table 2).
According to Takahashi, Sumitani, Inada, and Mori (2002), aldehydes could be formed through the degradation of polyunsaturated fatty acids (PUFA), either by enzymatic action or autoxidation and lipid peroxidation. Few aldehyde compounds were observed in this study (Table 2), with the most significant in terms of % occurrence being 3,7‐dimethyl‐2,6‐octadienal, with a lemon odor. The presence of 3,7‐dimethyl‐2,6‐octadienal has also been reported in several plants and fruits (Burdock, 2010). Similarly, ketone compounds with different chain lengths are abundant in nature and largely contribute to the flavors in plants and spices. Terpenes and their epoxides could possibly react to form ketones and alcohols (Preedy, 2009). Ketones generally occurred in low amounts with relative quantities of 0.63% and 0.46% for 6‐methyl‐5‐hepten‐2‐one and 2‐nonanone, respectively (Table 2). 6‐methyl‐5‐hepten‐2‐one (Figure 1) is an important flavor compound previously reportedly found in plants (Duke, 2000). Other ketone compounds contribution to the overall flavor profile of nkui are piperitone, 5‐methyl‐3,5‐octadien‐2‐one and cryptone (Table 2).
Seven esters were identified in this study with linalyl acetate and terpinyl propionate being the predominant ones, with 19% and 1.5%, respectively, found (Table 2). Esters are characterized by pleasant fruit odors that contribute to the aromatic, sweet, and honey notes in foods (Burdock, 2010). Linalyl acetate (Figure 1) is a naturally occurring phytochemical commonly found in spices and plants. It is an ester of linalool having a sweet, green, and citrus smell (Table 2). Its production from linalool has been hypothesized as being catalyzed by an alcohol acetyl transferase and biosynthesis via the nonmevalonate (1‐deoxyxylulose phosphate) terpene pathway (Harada, Ueda, & Iwata, 1985; Zaks, Davidovich‐Rakanati, Bar, Inbar, & Lewinsohn, 2008). Similar to linalool, linalyl acetate have also reported to have anti‐inflammatory, anti‐hypertensive, and analgesic effects (Peana et al., 2002).
Qualitatively, the acids were the sixth most important group of compounds identified in the SPME‐GC×GC‐TOF‐MS analysis of nkui (Figure 2). In terms of percentage abundance, the major acid observed was acetic acid (1.7%) known to induce a vinegar, sour and pungent odor. Two sulfur compounds identified in this study were disulfide, methyl (methylthio)methyl (3.6%) and trisulfide, dimethyl (0.35%) conferring a sulphurous and alliaceous smell. Also referred to as 2,3,5‐trithiahexane (TTH), disulfide, methyl (methylthio)methyl has been reported as volatile components in Brassica species conferring a sulfury, allecoius odor (Spadone, Matthey‐Doret, & Blank, 2006). Other significant aromatic, bicyclic and furan compounds found were benzene, 1‐methyl‐4‐(1‐methylethyl)‐ (8.2%), 7‐oxabicyclo[4.1.0]heptan‐2‐one, 6‐methyl‐3‐(1‐methylethylidene)‐ (0.13%) and 2‐furanmethanol, 5‐ethenyltetrahydro‐à,à,5‐trimethyl‐, cis‐ (0.73%), respectively (Table 2). These compounds influenced the earthy, herbal, spicy, and minty flavor in nkui.
4. CONCLUSION
The present work represents a comprehensive study on the volatile flavor profile of nkui. Using SPME‐GC×GC‐TOF‐MS, this study was able to establish the volatile profile of nkui allowing complementary qualitative information. Findings from this study showed the presence of major flavor‐related volatile compounds all contributing to the flavor and aroma of nkui. These major compounds were linalyl acetate, linalool, linalyl anthranilate, and 1,6‐octadien‐3‐ol,3,7‐dimethyl, acetate. Such data demonstrate the diverse range of flavors present in nkui and could possibly be potential sources of natural flavors for their use in the food and fragrance industries. Nonetheless, it is recommended to quantitatively analyze these volatile compounds, especially the major ones using reference standards and should be explored in future studies.
ETHICAL REVIEW
This study does not involve any human or animal testing.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ACKNOWLEDGMENTS
The authors acknowledge Mr. Alexander Whaley of LECO Africa for kind assistance with the use of their equipment and application laboratory.
Adebo OA, Njobeh PB, Desobgo SCZ, Pieterse M, Kayitesi E, Ndinteh DT. Profiling of volatile flavor compounds in nkui (a Cameroonian food) by solid phase extraction and 2D gas chromatography time of flight mass spectrometry (SPME‐GC×GC‐TOF‐MS). Food Sci Nutr. 2018;6:2028–2035. 10.1002/fsn3.736
Contributor Information
Oluwafemi A. Adebo, Email: oluwafemiadebo@gmail.com.
Steve C. Z. Desobgo, Email: desobgo.zangue@gmail.com
REFERENCES
- Bertuzzi, A. S. , McSweeney, P. L. H. , Rea, M. C. , & Kilcawley, K. N. (2018). Detection of volatile compounds of cheese and their contribution to the flavor profile of surface‐ripened cheese. Comprehensive Reviews in Food Science and Food Safety, 2, 371–390. 10.1111/1541-4337.12332 [DOI] [PubMed] [Google Scholar]
- Burdock, G. A. (2010). Fenaroli's handbook of flavor ingredients (6th ed.). Boca Raton, FL: CRC Press. [Google Scholar]
- Ding, X. , Wu, C. , Huang, J. , & Zhou, R. (2016). Characterization of interphase volatile compounds in Chinese Luzhou flavor liquor fermentation cellar analyzed by head space‐solid phase micro extraction coupled with gas chromatography mass spectrometry (HS‐SPME/GC/MS). LWT‐Food Science and Technology, 66, 124–133. 10.1016/j.lwt.2015.10.024 [DOI] [Google Scholar]
- Duke, J. A. (2000). Handbook of phytochemical constituents of GRAS herbs and other economic plants: Herbal reference library. Boca Rota, FL: CRC Press. [Google Scholar]
- Eriksson, C. E. (1967). Pea lipoxidase, distribution of enzyme and substrate in green peas. Journal of Food Science, 32, 438–441. 10.1111/j.1365-2621.1967.tb09705.x [DOI] [Google Scholar]
- Gbashi, S. , Adebo, O. A. , Piater, L. , Madala, N. E. , & Njobeh, P. B. (2017). A review on subcritical water extraction in biological materials. Separation and Purification Reviews, 46, 21–34. 10.1080/15422119.2016.1170035 [DOI] [Google Scholar]
- George, M. J. , Njobeh, P. B. , Gbashi, S. , Adegoke, G. O. , Dubery, I. A. , & Madala, N. E. (2018). Rapid screening of volatile organic compounds from Aframomum danielli seeds using headspace solid phase microextraction coupled to gas chromatography mass spectrometry. International Journal of Analytical Chemistry, 2018, 1–7. 10.1155/2018/8976304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, J. L. , Figueira, J. A. , Rodrigues, F. P. , Ornelas, L. P. , Branco, R. N. , Silva, C. L. , & Camara, J. S. (2016). A powerful methodological approach combining headspace solid phase microextraction, mass spectrometry and multivariate analysis for profiling the volatile metabolomic pattern of beer starting raw materials. Food Chemistry, 160, 266–280. [DOI] [PubMed] [Google Scholar]
- Harada, M. , Ueda, Y. , & Iwata, T. (1985). Purification and some properties of alcohol acyltransferase from banana fruit. Plant Cell Physiology, 26, 1067–1074. 10.1093/oxfordjournals.pcp.a077002 [DOI] [Google Scholar]
- Jelen, H. I. , Majcher, M. , & Dziadas, M. (2012). Sample preparation for food flavor analysis (flavors/off‐flavors). Comprehensive Sampling and Sample Preparation, 4, 119–145. 10.1016/B978-0-12-381373-2.00130-7 [DOI] [Google Scholar]
- Kusano, M. , Kobayashi, M. , Iizuka, Y. , Fukushima, A. , & Saito, K. (2016). Unbiased profiling of volatile organic compounds in the headspace of Allium plants using an in‐tube extraction device. BMC Research Notes, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peana, A. T. , D'Aquilla, P. S. , Panin, F. , Serra, G. , Pippia, P. , & Moretti, M. D. L. (2002). Anti‐inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine, 9, 721–726. 10.1078/094471102321621322 [DOI] [PubMed] [Google Scholar]
- Preedy, V. (2009). Beer in health and disease prevention. London, UK: Academic Press. [Google Scholar]
- Ravizza, R. , Gariboldi, M. B. , Molteni, R. , & Monti, E. (2008). Linalool, a plant‐derived monoterpene alcohol, reverses doxorubicin resistance in human breast adenocarcinoma cells. Oncology Reports, 20, 625–630. [PubMed] [Google Scholar]
- Singh, B. , & Sharma, R. A. (2015). Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. Biotechnology, 5, 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadone, J. C. , Matthey‐Doret, W. , & Blank, I. (2006). Formation of methyl (methylthio)methyl disulfide in broccoli (Brassica oleracea (L.) var. italica). Developments in Food Science, 43, 309–314. 10.1016/S0167-4501(06)80074-9 [DOI] [Google Scholar]
- Takahashi, H. , Sumitani, H. , Inada, Y. , & Mori, D. (2002). Identification of volatile compounds of Kombu (Laminaria spp.) and their odor description. Nippon Kagaku Kaishi, 49, 228–237. 10.3136/nskkk.49.228 [DOI] [Google Scholar]
- Tchoupang, E. N. , Ateba, S. B. , Zingue, S. , Zehl, M. , Krenn, L. , & Njamen, D. (2016). Estrogenic properties of spices of the traditional Cameroonian dish “Nkui” in ovariectomized Wistar rats. Journal of Complementary and Integrative Medicine, 13, 151–162. [DOI] [PubMed] [Google Scholar]
- Zaks, A. , Davidovich‐Rakanati, R. , Bar, E. , Inbar, M. , & Lewinsohn, E. (2008). Biosynthesis of linalyl acetate and other terpenes in lemon mint (Mentha aquatica var. citrata, Lamiaceae) glandular trichomes. Israel Journal of Plant Sciences, 56, 23–244. [Google Scholar]


