Abstract
Lactic acid bacteria have wide applications in food processing. Lactic acid bacteria produced exopolysaccharides (EPS) which could be used as possible replacer for commercial stabilizer and thickeners produced by nonfood grade bacteria. Seventy‐two samples of Oil and Raphia palm sap were collected in eighteen locations across South‐Western Nigeria and screened for exopolysaccharide production in 6% sucrose agar using streaked plate method. Four hundred EPS‐producing bacteria (EPB) isolated were clustered based on morphological characteristics into two broad groups and preliminary screened for EPS‐producing capacity. Twenty representative of EPB were selected from the broad groups for tentative identification by API 50CHL and 10 high yielding EPB were selected for large‐scale EPS production. Each strain was inoculated into 6% sucrose broth with 3% (v/v) preculture grown overnight in a 1.5 ml flask and incubated at 37°C for 72 hr. The EPSs were purified and freeze‐dried prior to quantification of yields. EPS‐producing bacteria were identified as Leuconostoc lactis, Lactobacillus fermentum, Lactobacillus delbrueckii ssp. lactis, L. delbrueckii ssp. delbrueckii, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus crispatus, and Leuconostoc mesenteroides ssp. mesenteroides/dextranicum. EPS yield ranged from 132–810.75 mg/L and EPS‐producing potential of lactic acid bacteria (LAB) strains ranged; 36% (132–245 mg/L), 36% (250–460 mg/L), and 28% (461–820 mg/L). L. plantarum had the highest EPS yield of 810.75 mg/L whereas L. crispatus had the least yield 242.5 mg/L. These results suggest that majority of LAB in palm wine saps are gum‐producing bacteria. Leuconostoc and Lactobacillus were the most abundant LAB found in this study while L. plantarum could have applications as potential starter cultures for the production of exopolysaccharides (EPS) at industrial level.
Keywords: gum, Lactic acid bacteria, Oil palm sap, raphia palm sap
1. INTRODUCTION
Lactic acid bacteria (LAB) are gram‐positive and heterogeneous group of bacteria that produce lactic acid as their main fermentation product and are generally recognized as safe (GRAS) (Konings, Kok, Kuipers, & Poolman, 2000; Muñoz, Moreno‐Arribas, & De las Rivas, 2011). LAB are food grade organisms and are important to food and dairy industries, because the lactic acid and other organic acids produced by these bacteria act as natural preservatives and flavor enhancers. LAB are recognized as probiotics which are able to stimulate immune responses and prevent infections against enteropathogenic bacteria (Reid, 1999).
Microbial gums are biodegradable polymers with high molecular weight which are biosynthesized by a wide range of bacteria, yeasts, and fungi (Welman, Maddox & Archer, 2003; Vijayabaskar, Babinastarlin, Shankar, Sivakumar, & Anandapandian, 2011; Welman & Maddox, 2003). Exopolysaccharides are produce by some certain selected microorganisms in the course of fermentation and the resulting exopolysaccharides are usually isolated from the fermentation broth by appropriate procedure (Sutherland, 2001). LAB are well‐known for their ability to produce exopolysaccharide (EPS) (Badel, Bernardi, & Michaud, 2011; Chawla, Bajaj, Shrikant, & Singhal, 2009; De Vuyst & Degeest, 1999; Sutherland, 2001). Lactic acid bacteria produce several types of polysaccharides like many other bacteria that could be classified according to their location relative to the cell. The exopolysaccharide produces by lactic acid bacteria are of two groups; homopolysaccharides and heteropolysaccharides classified based on the composition of their monosaccharides and their biosynthetic pathway (Jolly, Vincent, Duboc, & Neeser, 2002). The microbial ecology of palm wine reveal the presence of bacteria species of the genus Micrococcus, Leuconostoc, Lactobacillus, and Acetobacter; while the yeasts usually implicated are Saccharomyces and Candida spp. (Faparusi & Bassir, 1971; Okafor, 1987; Uzochukwu, Ngoddy, & Balough, 1994). Several LAB have been reported to be responsible for the consistency and soluble white coloration of palm wine through their production of gums, largely dextrans and levans, in the fermentation process of the beverage (Uzochukwu, Ngoddy, & Balough, 1991; Uzochukwu, Ngoddy, Balough, Tucknott, & Lewis, 1994).
According to Hussein, Ibrahim, Asker, & Mahmoud, 2010; EPSs produced by LAB could be used as thickeners, stabilizers, emulsifiers, bodying agents, gelling agents, or fat replacers in several food products and as better alternatives to EPSs produced by Xanthomonas compestris, a pathogen of plants and nonfood grade bacterial. The quantities of EPSs produced by different species and strains of LAB varies between 50 and 350 mg/L depending on growth culture conditions (Cerning, 1995; Ruas‐Madiedo, Hugenholtz, & Zoon, 2002). The low yield of EPS produced by LAB has been attributed to the media composition, conditions of growth, and the method of isolation of EPS. These factors play a key role in media sugar composition and polymer yield (Grobben et al., 1998; Looijesteijn, van Casteren, Tuinier, Doeswijk‐Voragen, & Hugenholtz, 2000; Torino, Sesma, & de Valdez, 2000). The low yields EPSs production by majority of LAB species has reduced their commercial viability and limited industrial applications. Studies have showed that some LAB species have potentials to produce high yields EPSs roughly 40 g/L (Kaditzky & Vogel, 2008; Korakli, Pavlovic, Ganzle, & Vogel, 2003; Minervini et al., 2010). Industrial applications of natural polymers for various uses have increased their demand and this has led to an increased attention toward exopolysaccharides and this has re‐enforced the search for LAB strains with potentials for production of high yields EPS. Exopolysaccharides (EPS) produced by LAB might serve as an excellent source of food grade polysaccharides and as natural alternatives to commercial ones of plant or animal origin.
This study was carried out to identify gum‐producing LAB species and strains responsible for white coloration in palm wine. Isolated LAB will be characterized at phenotypic levels and the determination of strain to help in the selection of autochthonous strains which could be potentially used as starter cultures for the production EPS at the industrial level.
2. MATERIALS AND METHODS
2.1. Collection of palm sap
Freshly tapped Raphia and Oil palm‐wine were randomly collected in sterile plastic containers from three locations and four palm wine samples from different tappers per location in each of the six states of South‐western Nigeria; Ogun, Oyo, Lagos, Osun, Ondo, and Ekiti. The sterile plastic containers were immediately stored in an ice pack (4°C) and samples were analyzed within a day of collection.
2.2. Gum‐producing bacteria isolation
The method described by Uzochukwu et al. (1991) was used to isolate gum‐producing bacteria from palm wine. A loopful of palm wine sample were streaked on 6% sucrose agar and incubated for 24 hr at 35°C. After incubation for 24 hr at 35°C, mucoid colonies were identified as gum‐producing bacteria. Distinct colonies were subcultured severally and pure cultures were stored in 6% sucrose agar slants and stored at 4°C.
2.3. Tentative identification of gum‐producing LAB
Pure cultures of gum‐producing bacteria were streaked each on De Man, Rogosa and Sharpe (MRS) agar (Heywood, Lancashire, United kingdom) supplemented with 0.005% cycloheximide (Sigma‐Aldrich) and were incubated at 37°C for 48 hr. Successive subculturing on the same media was done to obtain pure cultures. Pure cultures were stored in sucrose broth containing 15% glycerol (v/v) at −80°C as stock culture. Presumptive LAB were phenotypically characterized by Gram‐staining, cell morphology, and catalase activity by following standard procedures. Only Gram‐positive, catalase negative rod and cocci isolated were selected. Carbohydrate fermentation pattern of lactic acid bacteria were determined in duplicate using API 50CH®system (API system, BioMerieux, Mercy ĺ Etoile, France) following the manufacturer's instructions. The results were recorded after 24 and 48 hr of incubation at 37°C and the result interpretation for identification was determined by API Labplus software provided by Bio‐Merieux in Central laboratory, LUTH (Lagos University teaching Hospital, Nigeria).
2.4. Microbial gum production
Each of gum‐producing LAB were grown in 50 ml sucrose broth to screen the species/strains of LAB for EPS quantification. Ten representative LAB species/strains with high amount of EPS productivity were selected and used for the production of Exopolysaccharides. 'Cultivation were performed in basal medium (v\v, 6% sucrose, 0.5% peptone, 0.05% K2HPO4, 0.025% MgSO4) in 1.5 L flasks with 1 L working volume. The medium was inoculated with 3% (v/v) overnight culture of isolated bacteria and incubated at 32°C for 72 hr and under noncontrol pH 5.6. Growth was monitored by absorbance measurement at 650 nm using a spectrophotometer (Spectrum lab S23A; Globe Medical, England).
2.5. Isolation and quantification of EPS
The modified methods described by Ricciardi et al. (2002) and Minervini et al. (2010) were used to isolate and quantify EPS. Modifications included dialysis of EPS with water and isopropanol (Figure 1). Cells were harvested from the fermented culture broth by centrifugation at 11,000×g for 30 min in a preweighed tube. Prior to centrifugation, the suspension was stirred with glass rod and heated at 80°C (Heating block) to extract EPS associated to bacteria cells. EPS were precipitated with three volumes of chilled ethanol (95%, v/v) and kept overnight at 4°C for complete precipitation. The precipitated crude was collected by centrifugation at 10,000×g, 4°C (J2‐HS; Beckman, USA) for 30 min, and EPS pellets were dried in an oven at 105°C to a constant weight. EPS pellets were dissolved in 50 ml distilled water, precipitated twice with isopropanol and then freeze‐dried (Telster, Cryodos‐8mode, Spain), and quantified by weight. The quantification of EPS was done using dry weight method.
Figure 1.
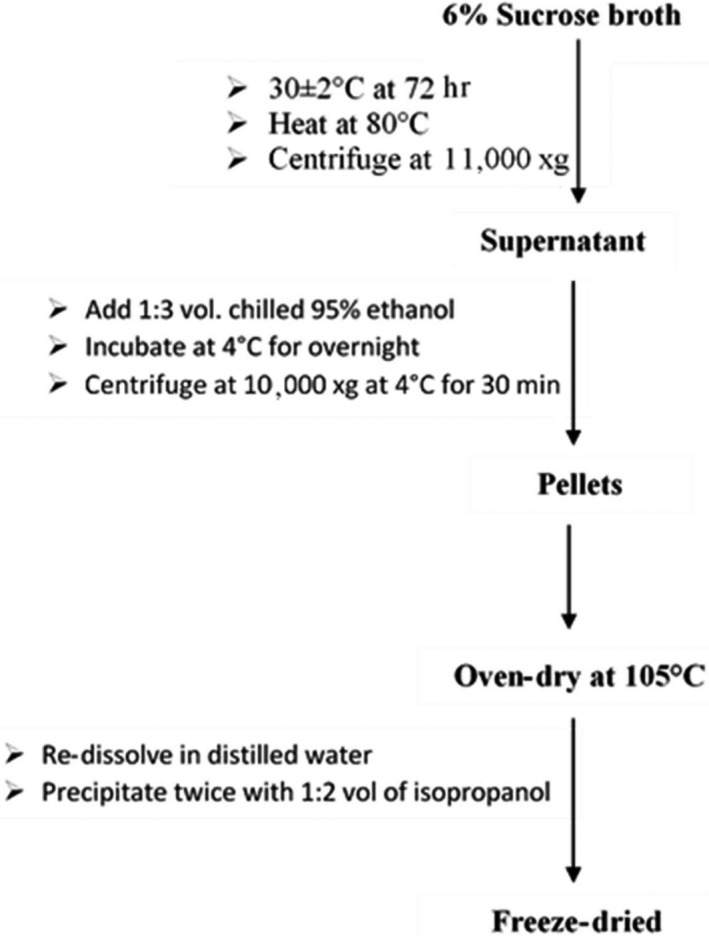
Flow chart of 6% (w/v) sucrose broth for isolation and purification of EPS produced. Source: Ricciardi et al. (2002) and Minervini et al. (2010)
3. RESULTS AND DISCUSSIONS
Four hundred (400) gum‐producing bacteria (GPB) were isolated from palm wine samples based on their ability to form mucoid colonies with a glistening and slimy appearance on 6% sucrose agar plates. The formation of mucoid or watery colonies on selected agar media plates and macroscopic detection as the basis of identification of GPB has been reported by several authors (Anbuselvi, Kumar, & Padmaja, 2012; Morin, 1998; Uzochukwu et al., 1991). Four hundred GPB isolates identified from the different palm wine samples were Gram positive, catalase and oxidase negative reactions, thus, considered as presumptive LAB. All GPB isolated from palm wine samples fit the classification of lactic acid bacteria as Gram positive, catalase negative and oxidase negative (Manel, Sana, Nedia, Moktar, & Ali, 2011; Salminen & Von Wright, 1993). The bacteria isolates were cluster into two major broad groups on the basis of their macro‐morphology and micro‐morphology. Ten representative GPB isolates were randomly chosen per group for tentative identification with API 50CHL. Several studies have reported that LAB isolated from palm wine and other fermented food had been initially differentiated on the basis of their cultural and cellular morphology prior to been subjected to various physiological and biochemical tests (Adebayo‐Tayo & Abiodun, 2008; Savadogo et al., 2004).
The identity of twenty gum‐producing bacteria using API 50CHL biochemical profiles is shown in Tables 1 and 2. The result shows that twenty representative GPB isolates were identified as twelve bacteria species belonging to Lactobacillus and Leuconostoc species. Consequently, 75% belongs to Lactobacillus species and 25% leuconostoc species. L. mesenteroides ssp. Mesenteroides/dextranicum (15%) and L. plantarum (15%) were the dominant species. The percentage of identification ranged from 76.3% to 99.9%. Lactobacillus acidophilus had the lowest percentage identity (76.3%) and Lactobacillus brevis had the highest percentage identity (99.9%). The API 50CHL system, which utilizes the characteristics of bacterial sugar fermentation and enzymatic activities, has been commonly used for the identification of lactic acid bacteria (Dickson, Riggio, & Macpherson, 2005; Le Jeune & Lonvaud‐Funel, 1994; Manel et al., 2011). Studies have also reported the dominance of Lactobacillus and Leuconostoc species in palm wine (Amoa‐Awua, Sampson, & Tano‐Debrah, 2007; Uzochukwu et al., 1991). Earlier studies had reported the presence of Lactobacillus and Leuconostoc genera in raphia palm wine, coconut palm sap, date palm wine (Atputharajah, Widanapathirana, & Samarajeewa, 1986; Okafor, 1987; Shamala & Streekantiah, 1988). Similarly, Manel et al. (2011) reported L. mesenteroides and L. delbrueckii as the dominated lactic acid bacteria in Tunisian date palm sap. The capacity of most Lactobacillus and Leuconostoc strains to produce and secrete large quantities of extracellular enzyme, invertase would enable the utilization and conversion of sucrose into glucose and fructose, and finally organic acids and alcohol (Naknean, Meenune, & Roudaut, 2010) thus enhancing their growth and proliferation over other strains. Uzochukwu et al. (1994), Uzochukwu, Ngoddy, and Balough (1994), Uzochukwu et al. (1999, 2002) and Lasekan, Buettner, and Christlbauer (2007) reported that Lactobacillus and Leuconostoc strains are responsible for the consistency and soluble white coloration of palm wine through their production of gums, largely dextrans and levans, in the fermentation of the beverage. Lactobacillus and Leuconostoc species uses sucrose as a sole source of carbon under aerobic and/or anaerobic environment (Valepyn, Berezina, & Paquot, 2012). This could also be an advantage to its proliferation in the sucrose‐rich palm wine sap.
Table 1.
Identification of Gum‐producing bacteria isolates using API 50CHL
| Isolate code | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IM 06 | IBAii 03 | IFEi 05 | MOD01 | FAT 10 | IJE 05 | BAD 02 | AIY 13 | OMO13 | MOW 13 | |
| LAR | − | − | − | − | − | − | + | + | − | + |
| RIB | − | + | − | − | − | − | + | − | − | − |
| DXY | − | + | − | − | − | − | − | − | − | − |
| GAL | − | + | − | + | − | − | + | − | − | − |
| MNE | − | + | − | − | − | − | + | − | − | + |
| RHA | − | − | − | − | − | − | + | − | − | − |
| MAN | − | − | + | − | − | + | + | − | − | − |
| SOR | − | − | − | − | − | − | + | − | − | − |
| MDG | + | − | − | − | − | − | + | − | − | − |
| NAG | + | + | + | + | + | + | + | + | + | + |
| AMY | − | − | − | − | − | − | + | − | − | − |
| ARB | − | − | − | − | − | − | + | − | − | − |
| ESC | − | − | − | − | + | − | + | − | − | + |
| SAL | − | − | − | − | + | − | + | − | + | − |
| CEL | − | − | − | − | − | − | + | − | − | − |
| MAL | + | + | − | + | + | − | + | + | + | − |
| LAC | − | − | − | − | − | − | − | + | − | − |
| MEL | − | + | − | − | − | − | − | + | − | − |
| TRE | − | + | + | + | + | + | + | − | + | + |
| MLZ | − | − | − | − | − | − | + | − | − | − |
| RAF | − | + | − | − | + | − | − | + | + | − |
| TUR | + | − | − | − | + | − | + | − | + | + |
| GNT | − | + | − | − | − | − | − | − | − | + |
| Organism | Leuconostoc lactis | Lactobacillus fermentum | Lactobacillus delbrueckii ssp. lactis | Lactobacillus delbrueckii ssp. delbrueckii | Lactobacillus acidophilus | Lactobacillus delbrueckii ssp. lactis | Lactobacillus plantarum | Leuconostoc lactis | L. acidiophilus | Leuconstoc mesenter. ssp. mesent./dextrac. |
+: positive; w: weakly positive; −: negative after 48 hr of incubation at+: positive; w: weakly positive; −: negative after 48 hr of incubation at 37°C. All strains fermented: Glucose, Fructose, N‐Acetylglucosamin and D‐Saccharose, LARA‐L: arabinose; GAL: D galactose; MNE: D mannose; RHA: L rhamnose; MAN: D mannitol; LAC: D lactose; SOR: D sorbitol; MDG: methy‐αD‐glucopyranoside; NAG: N‐Acetylglucosami; TRE: D trehalose; INU: inuline; MLZ: D melezitose; RAF: D raffinose; TUR: D turanose; GNT: potassium gluconate.
Table 2.
Identification of Gum‐producing bacteria isolates using API 50CHL
| Isolate code | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IMO 02 | ILU 03 | IKIii 04 | IFE 01 | FAT 01 | MOD 03 | IBA 06 | MOW 01 | MOW 08 | KEL 07 | |
| LAR | + | + | + | − | − | + | + | + | − | + |
| RIB | + | − | + | − | − | − | + | − | + | + |
| DXY | + | + | + | − | − | − | − | − | − | − |
| GAL | + | + | + | + | + | − | + | − | + | + |
| GLU | + | + | + | − | + | + | + | + | + | + |
| FRU | + | + | + | − | + | + | + | + | + | + |
| MNE | + | + | + | − | + | + | + | + | + | + |
| RHA | − | − | − | − | − | − | + | − | + | + |
| MAN | + | − | + | + | − | − | + | − | + | + |
| SOR | + | − | − | − | − | − | + | − | + | + |
| MDG | − | − | − | − | − | − | − | − | + | + |
| AMY | + | + | + | + | − | − | + | − | + | + |
| ARB | + | + | + | + | + | − | + | − | + | + |
| ESC | + | + | + | − | − | + | + | + | + | + |
| SAL | + | + | + | − | + | − | + | − | + | + |
| CEL | + | + | + | − | − | − | + | − | + | + |
| MAL | + | + | + | − | + | − | + | − | + | + |
| LAC | + | − | + | + | − | − | + | − | − | − |
| SAC | + | + | + | − | + | + | + | + | + | + |
| TRE | + | − | + | − | − | + | + | + | + | + |
| AMD | − | − | − | + | + | − | − | − | − | − |
| GEN | + | + | + | − | + | − | + | − | − | − |
| TUR | + | − | + | + | − | + | + | + | + | + |
| GNT | + | − | + | − | − | + | + | + | − | − |
| Organism | Lactobacillus pentosus | Lactobacillus coprophilus | Lactobacillus brevis | Lactobacillus cripatus | Lactobacillus delbrueckii ssp. delbrueckii | Leuconstoc mesenteroides ssp. mesent./dextracum. | Lactobacillus plantarum | Leuconstoc. Mesenteroides ssp mesent./ dextracum | Lactobacillus rhamnosus | L. plantarum |
+: positive; w: weakly positive; −: negative after 48 hr of incubation at+: positive; w: weakly positive; −: negative after 48 hr of incubation at 37°C. All strains fermented: Glucose, Fructose, N‐Acetylglucosamin and D‐Saccharose. LARA‐L, arabinose; GAL: D galactose; GLU: D glucose; FRU: D fructose; MNE: D mannose; RHA: L rhamnose; MAN: D mannitol; LAC: D lactose; SOR: D sorbitol; MDG: methy‐αD‐glucopyranoside; NAG: N‐Acetylglucosamin; AMY: amygdaline; ARB: arbutine; ESC: esculine citrate; SAL: salicine.
The synthesis of exopolysaccharide by gum producing lactic acid bacteria isolated from palm wine is presented in Table 3. The EPS yield production ranged from 242.5–810.75 mg/L. Cultures shown to be the same isolated species did not show similar exopolysaccharide production. The L. plantarum strains yield ranged from 677.5 to 810.75 mg/L; L. delbrueckii ssp. lactis 346.21 to 458.125 mg/L. The highest exopolysaccharide producing isolate strain was L. plantarum (810.75 mg/L) whereas, the least exopolysaccharide producing isolate was L. crispatus (242.5 mg/L). The values obtained for EPS production and cell dry weight were comparable with data obtained in previous experiments with lactic acid bacteria species/strains (Cerning, 1990; Cerning, Bouillanne, Landon, & Desmazeaud, 1992; Korakli et al., 2003; Minervini et al., 2010; Mozzi, Savoy de Giori, Oliver, & Font de Valdez, 1996; Vogel, 2008). Other studies have also reported that some microorganisms are capable of producing and excreting over 40 g/L of EPSs under conditions of stress (Lin & Cheng‐Chien, 2007; Papinutti, 2010; Ravella et al., 2010). The comparison of EPS yields to cell dry weight results showed that EPS production was growth associated. The growth associated biosynthesis of exopolysaccharides from the selected lactic acid bacteria species/strains was supported by sufficient amount of carbon/nitrogen ratio, and a direct relationship between growth condition (temperature and pH) and exopolysaccharides yields. Sucrose was first converted to glucose and glucose was completely converted into lactic acid to yield the necessary energy, enough nitrogen is necessary for the biosynthesis of essential cell components, while the cells actively produce exopolysaccharides in the presence of an appropriate carbon source (Cerning et al., 1994). Growth associated exopolymer production has been observed for several lactic acid bacteria strains (De Vuyst, Vanderveken, Van de Ven, & Degeest, 1998; Ricciardi et al., 2002; Van Den Berg et al., 1995) and other bacteria (Sunil, Amarsinh, Trishala, & Tejswini, 2013; Valepyn et al., 2012). The increase in yields of exopolysaccharides biosynthesis obtained in this study could be attributed to the fermentation temperature and pH. The growth temperature of 32°C and a pH 5.6 was used for the biosynthesis of exopolysaccharides by the selected lactic acid bacteria species/strains. The results obtained in this study are in agreement with previous studies that reported increased exopolysaccharide production at 32 or 37°C compared to a higher growth temperature of 42°C was observed for some Streptococcus thermophiles and L. delbrueckii subspecies bulgaricus strains (Gancel & Novel, 1994; Mozzi, Oliver, Savoy de Giori, & Font de Valdez, 1995). Increased in EPS yield at low temperatures has also been observed in many LAB (De Vuyst & Degeest, 1999; Minervini et al., 2010; Ricciardi & Clementi, 2000). The optimal pH for EPS production has been found to vary in different strains of LAB (De Vuyst & Degeest, 1999; Ricciardi & Clementi, 2000). Previous studies reported that some S. thermophiles, L. delbrueckii ssp. bulgaricus, and L. lactis strains, exopolysaccharide biosynthesis improved considerably when the pH was kept constant at about 6.0 (Cerning, 1995; Mozzi et al., 1996). Other studies have also reported that the optimal pH for EPS production is often close to 6.0 (De Vuyst et al., 1998; Gassem, Schmidt, & Frank, 1997). Similarly, Minervini et al. (2010) reported noncontrolled pH of 5.6 as the optimal pH for the biosynthesis of exopolysaccharides by Lactobacillus curvatus DPPMA10. On the contrary, Kumar, Mody, and Jha (2007) reported that there is no single set of culture conditions that guarantees high EPS yields since microorganisms differs in their temperature and pH optima, which are among the critical factors for maximum EPS production.
Table 3.
Production of EPS in 6% sucrose broth from bacteria isolated from palm wine
| S/n | Representative isolate | Microorganism identity | 6% Sucrose broth (mg/L) | Cell dry weight (mg/L) |
|---|---|---|---|---|
| 1 | IMa 06 | Leuconostoc lactis | 670.61 | 700.23 |
| 2 | IBAb 03 | Lactobacillus fermentum | 566.875 | 540.71 |
| 3 | IFEa 05 | Lactobacillus delbr. ssp. lactis | 458.125 | 470.43 |
| 4 | MODa 01 | Lactobacillus delbrueckii ssp. lactis | 346.21 | 330.95 |
| 5 | FATd 01 | Lactobacillus delbr. ssp. delbrueckii | 258.125 | 220.64 |
| 6 | FATa 10 | Lactobacillus acidophilus | 351.25 | 390.98 |
| 7 | BADa 02 | Lactobacillus plantarum | 677.5 | 560.86 |
| 8 | IFEc 10 | Lactobacillus crispatus | 242.5 | 240.15 |
| 9 | MOWa 05 | Leuc mesenteroides ssp. mesenteroides | 683.75 | 610.45 |
| 10 | BADa 02 | L. plantarum | 810.75 | 740.13 |
IMa 06: IMOTA; IBAb 03: IBAFO; IFEa 05: IFE; MODa 01: MODAKIKI; FATa 10: FATUNLA; IJEa 05: IJESA ISU‐EKITI; BADa 03: BADAGARY; AYIa 13: AIYEPE; OMOa 02: OMOTOSHO; MOWa 05: MOWE; ILUa 03: ILUOMOBA; IKIb 04: IKIRE; KELb 07: KELEB. Subscript a: palm wine tapper one; b: palm wine tapper two; c: palm wine tapper three; d: palm wine tapper four.
Heating treatment of the samples as the first step in the polysaccharide isolation procedure is crucial for completing recovery of the EPS. This could also be the reason for high yield of exopolysaccharides obtained in this study. Studies have shown that heating treatment of the samples as the first step in the polysaccharide isolation procedures resulted in recovery of the exopolysaccharide (Ricciardi et al., 2002; Wang et al., 2010). However, heating treatment should be used only where the exopolysaccharide is thermally stable (Kumar et al., 2007; Rimada & Abraham, 2003). Exopolysaccharides yield generated in this study has shown that the presence of sucrose as a sole carbon source may have had strongly influenced in the total amount of polysaccharide produced. This is in agreement with Stredansky, Conti, Navarini, and Bertocchi (1999) hypothesis that one important factor influencing the yield of synthesized EPS could be the similarity of the substrate to the natural habitat of the microorganisms. Studies have also shown that growth conditions and media composition can affect the quality and quantity of polymer (Mozz et al., 1999; Looijestein et al., 2000; Torino et al., 2000; Smith and de Valdez, 2000, Bramhachari et al., 2007). Studies have also shown that growth conditions and media composition can affect the quality and quantity of polymer (Looijestein et al., 2000; Torino et al., 2000; Smith and de Valdez, 2000, Bramhachari et al., 2007). Other authors have reported that most LAB strains preferred sucrose as their carbon source and in most of these studies an increased microbial growth with corresponding increase in EPS had also been observed (Minervini et al., 2010; Ruas‐Madiedo & de los Reyes‐Gavilan, 2005; Smitinont et al., 1999). However, studies also showed the results are sugar dependence because in each case the most suitable carbohydrate is largely dependent on the strain tested.
4. CONCLUSIONS
Lactobacillus and Leuconostoc species are the dominant gum producing bacteria species in palm wine sap. All 10 strains of LAB selected in this study showed higher yield of EPS that could serve as alternative organisms to produce commercial quantity and L. plantarum could be of importance at the industrial level as starter cultures for the production EPS.
CONFLICTS OF INTEREST
None declared.
ACKNOWLEDGMENT
I acknowledge that there is no conflict of interest statement concerning the journal guideline. Standard methods were used in conducting this study. No human subject was used. Standard and already established methods were used for this study. No human subject was used for this study hence consent was not obtained. As stated above, No human or animal subject was employed in this study.
Adamu‐Governor OL, Shittu TA, Afolabi OR, Uzochukwu SVA. Screening for gum‐producing Lactic acid bacteria in Oil palm (Elaies guineensis) and raphia palm (Raphia regalis) sap from South‐West Nigeria. Food Sci Nutr. 2018;6:2047–2055. 10.1002/fsn3.750
REFERENCES
- Adebayo‐Tayo, B. C. , & Abiodun, A. O. (2008). Screening of lactic acid bacteria strains isolated from some nigerian fermented foods for EPS production. World Applied Sciences Journal, 4(5), 741–747. [Google Scholar]
- Amoa‐Awua, W. K. , Sampson, E. , & Tano‐Debrah, K. (2007). Growth of yeasts, lactic and acetic acid bacteria in palm wine during tapping and fermentation from felled oil palm (Elaeis guineensis) in Ghana. Journal of Applied Microbiology, 102, 599–606. [DOI] [PubMed] [Google Scholar]
- Anbuselvi, S. , Kumar, M. S. , & Padmaja, M. V. (2012). A comparative study on biosynthesis of xanthan gum using three different Xanthomonas strains isolated from diseases plants. International Journal of Pharmarceutical and Biological Science, 3(3), B1–B6. [Google Scholar]
- Atputharajah, J. D. , Widanapathirana, S. , & Samarajeewa, U. (1986). Microbiology and biochemistry of natural fermentation of coconut palm sap. Food Microbiology, 3, 273–280. 10.1016/0740-0020(86)90009-2 [DOI] [Google Scholar]
- Badel, S. , Bernardi, T. , & Michaud, P. (2011). New perspective for Lactobacilli exopolysaccharides. Biotechnology Advances, 29, 54–66. 10.1016/j.biotechadv.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Bramhachari, P. V. , Kishor, P. B. , Ramadevi, R. , Kumar, R. , Rao, B. R. , & Dubey, S. K. (2007). Isolation and characterization of mucous exopolysaccharide (EPS) produced by Vibrio furnissi strain VBOS3. Journal of Microbiology and Biotechnology, 17(1), 44–51. [PubMed] [Google Scholar]
- Cerning, J. (1990). Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiology Reviews, 87, 113–130. 10.1111/j.1574-6968.1990.tb04883.x [DOI] [PubMed] [Google Scholar]
- Cerning, J. (1995). Production of exopolysaccharides by lactic acid bacteria and dairy propionibacteria. Lait, 75, 463–472. 10.1051/lait:19954-536 [DOI] [Google Scholar]
- Cerning, J. , Bouillanne, C. , Landon, M. , & Desmazeaud, M. (1992). Isolation and characterization of exopolysaccharides from slime‐forming mesophilic lactic bacteria. Journal of Dairy Science, 75, 692–699. 10.3168/jds.S0022-0302(92)77805-9 [DOI] [Google Scholar]
- Cerning, J. , Renard, C. M. G. C. , Thaibault, J. F. , Bouillanne, C. , Landon, M. , Desmazeaud, M. , & Topisirovic, L. (1994). Carbon source requirements for exopolysaccharide production by Lactobaccillus casei CG11 and partial structure analysis of the polymer. Applied and Environmental Microbiology, 60, 3914–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla, P. R. , Bajaj, I. B. , Shrikant, A. S. , & Singhal, R. S. (2009). Microbial cellulose: Fermentative production and applications. Food Technology and Biotechnology, 47, 107–124. [Google Scholar]
- Chervaux, C. , Ehrlich, S. D. , & Maguin, E. (2000). Physiological study of Lactobacillus delbrueckii subspecies bulgaricus strains in a novel chemically defined medium. Applied and Environmental Microbiology, 66, 5306–5311. 10.1128/AEM.66.12.5306-5311.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst, L. , & Degeest, B. (1999). Heteropolysaccharides from lactic acid bacteria. FEMS Microbiology Reviews, 23, 153–177. 10.1111/j.1574-6976.1999.tb00395.x [DOI] [PubMed] [Google Scholar]
- De Vuyst, L. , Vanderveken, F. , Van de Ven, S. , & Degeest, B. (1998). Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in a milk medium and evidence for their growth‐associated biosynthesis. Journal of Applied Microbiology, 84, 1059–1068. 10.1046/j.1365-2672.1998.00445.x [DOI] [PubMed] [Google Scholar]
- Dickson, E. M. , Riggio, M. P. , & Macpherson, L. (2005). A novel species‐specific PCR assay for identifying Lactobacillus fermentum . Journal of Medical Microbiology, 54, 299–303. 10.1099/jmm.0.45770-0 [DOI] [PubMed] [Google Scholar]
- Faparusi, S. I. , & Bassir, O. (1971). Microflora of fermenting palm‐wine. Journal of Food Science and Technology, 8, 206–212. [Google Scholar]
- Gancel, F. , & Novel, G. (1994). Exopolysaccharide production by Streptococcus salivartus ssp thermophiles cultures. I. condition of production. Journal of Dairy Science, 77, 685–688. 10.3168/jds.S0022-0302(94)77000-4 [DOI] [Google Scholar]
- Gassem, M. A. , Schmidt, K. A. , & Frank, J. F. (1997). Exopolysaccharide production from whey lactose by fermentation with Lactobacillus delbrueckii ssp. bulgaricus . Journal of Food Science, 62, 171–173. 10.1111/j.1365-2621.1997.tb04393.x [DOI] [Google Scholar]
- Grobben, G. J. , Chin‐Joe, I. , Kitzen, V. A. , Boels, I. C. , Boer, F. , Sikkerna, J. , … De Bont, J. A. M. (1998). Enhancement of exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 with a simplified defined medium. Applied and Environmental Microbiology, 64, 1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein, A. S. , Ibrahim, G. S. , Asker, M. M. S. , & Mahmoud, M. G. (2010). Exopolysaccharide from Lactobacillus helveticus: Identification of chemical structure and effect on biscuit quality. Czech Journal of Food Science, 28(3), 225–235. 10.17221/CJFS [DOI] [Google Scholar]
- Jolly, L. , Vincent, S. J. F. , Duboc, P. , & Neeser, J. R. (2002). Exploiting exopolysaccharides from lactic acid bacteria. Antonie Leeuwenhoek, 82, 367–374. 10.1023/A:1020668523541 [DOI] [PubMed] [Google Scholar]
- Kaditzky, S. , & Vogel, R. F. (2008). Optimization of exopolysaccharide yields in sourdoughs fermented by lactobacilli. European Food Research and Technology, 228, 291–299. [Google Scholar]
- Kaditzky, S. , & Vogel, R. F. (2008). Optimization of exopolysaccharide yields in sourdough fermented by Lactobacilli. European Food Research and Technology, 227, 433–442. 10.1007/s00217-007-0738-1 [DOI] [Google Scholar]
- Konings, W. N. , Kok, J. , Kuipers, O. P. , & Poolman, B. (2000). Lactic acid bacteria: The bugs of the new millennium. Current Opinion in Microbiology, 3(3), 276–282. 10.1016/S1369-5274(00)00089-8 [DOI] [PubMed] [Google Scholar]
- Korakli, M. , Pavlovic, M. , Ganzle, M. G. , & Vogel, R. F. (2003). Exopolysaccharide and ketose production by Lactobacillus sanfranciscensis LTH 2590. Applied and Environmental Microbiology, 69, 2073–2079. 10.1128/AEM.69.4.2073-2079.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. S. , Mody, K. , & Jha, B. (2007). Bacterial polysaccharides – A perception. Journal of Basic Microbiology, 47(2), 103–117. 10.1002/(ISSN)1521-4028 [DOI] [PubMed] [Google Scholar]
- Lasekan, O. , Buettner, A. , & Christlbauer, M. (2007). Investigation of important odorants of palm wine. Food Chemistry, 105, 15–23. 10.1016/j.foodchem.2006.12.052 [DOI] [Google Scholar]
- Le Jeune, C. , & Lonvaud‐Funel, A. (1994). Lactobacillus hilgardii and Lactobacillus brevis DNA analysis by restriction fragment length polymorphism (RFLP). Food Microbiology, 11, 195–202. 10.1006/fmic.1994.1023 [DOI] [Google Scholar]
- Lin, T. Y. , & Cheng‐Chien, M. F. (2007). Exopolysaccharide production as affected by lactic acid bacteria and fermentation time. Food Chemistry, 100, 6768–6775. [Google Scholar]
- Looijesteijn, P. J. , van Casteren, W. H. M. , Tuinier, R. , Doeswijk‐Voragen, C. H. L. , & Hugenholtz, J. (2000). Influence of different substrate limitations on the yield, composition and molecular mass of exopolysaccharides produced by Lactococcus lactis subsp cremoris in continuous cultures. Journal of Applied Microbiology, 89, 116–122. 10.1046/j.1365-2672.2000.01082.x [DOI] [PubMed] [Google Scholar]
- Manel, Z. , Sana, M. , Nedia, K. , Moktar, H. , & Ali, F. (2011). Microbiological analysis and screening of lactic acid bacteria from Tunisian date palm sap. Africa Journal of Microbiology Research, 5(19), 2929–2935. [Google Scholar]
- Minervini, F. , De Angelis, M. , Surico, R. F. , Di Cagno, R. , Ganzle, M. , & Gobbetti, M. (2010). High efficient synthesis of exopolysaccharides by Lactobacillus curvatus DPPMA 10 during growth in hydrolyzed wheat flour agar. International Journal of Food Microbiology, 141, 130–135. 10.1016/j.ijfoodmicro.2010.03.014 [DOI] [PubMed] [Google Scholar]
- Morin, A. (1998). Screening of exopolysaccharides‐producing microorganisms, factors influencing the production and recovery of microbial polysaccharides In Dumitriu S. (Ed.), Polysaccharides‐ structural diversity and functional versatility (pp. 275–296). New York, NY: Marcel Dekker Inc. Publication. [Google Scholar]
- Mozz, F. , Rollan, G. , de Giori, G. S. , & de Valdez, G. F. (1999). Effect of galactose and glucose on the exopolysaccharide production and activities of biosynthetic enzymes in Lactobacillus casei CRL87. Journal of Applied Microbiology, 91, 160–167. [DOI] [PubMed] [Google Scholar]
- Mozzi, F. , Oliver, G. , Savoy de Giori, G. S. , & Font de Valdez, G. F. (1995). Influence of temperature on the production of exopolysaccharides by thermophilic lactic acid bacteria. Milchwissenschaft, 50, 80–82. [Google Scholar]
- Mozzi, F. , Savoy de Giori, G. S. , Oliver, G. , & Font de Valdez, G. F. (1996). Exopolysaccharide production by Lactobacillus casei under controlled pH. Biotechnology Letter, 18, 435–439. 10.1007/BF00143466 [DOI] [Google Scholar]
- Muñoz, R. , Moreno‐Arribas, M. V. , & De las Rivas, B. (2011). Chapter 8: Lactic acid bacteria In Carrascosa A. V., Muñoz R., & González R. (Eds.), Molecular wine microbiology (p. 226). London, UK: Elsevier. [Google Scholar]
- Naknean, P. , Meenune, M. , & Roudaut, G. (2010). Characterization of palm sap harvested in Songkhla province, Southern Thailand. International Food Research Journal, 17, 977–986. [Google Scholar]
- Okafor, N. (1987). Palmwine Yeast from parts of Nigerian. Journal of Science, Food and Agriculture, 23, 1399–1407. [Google Scholar]
- Papinutti, L. (2010). Effects of nutrients, pH and water potential on exopolysaccharides production by a fungal strain belonging to Ganoderma lucidum complex. Bioresource Technology, 101(6), 1941–1946. [DOI] [PubMed] [Google Scholar]
- Ravella, S. R. , Quinones, T. S. R. , Retter, A. , Heiermann, M. , Amon, T. , & Hobbs, P. J. (2010). Extracellular polysaccharide (EPS) production by a novel strain of yeast‐like fungus Aureobasidium pullulans . Carbohydrate Polymers, 82(3), 728–732. 10.1016/j.carbpol.2010.05.039 [DOI] [Google Scholar]
- Reid, G. (1999). The Scientific basis for probiotic strains of Lactobacillus. Applied and Environmental Microbiology, 65, 3763–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi, A. , & Clementi, F. (2000). Exopolysaccharides from lactic acid bacteria: Structure, production and technological applications. Italian Journal of Food Science, 12, 23–45. [Google Scholar]
- Ricciardi, A. , Parente, E. , Crudele, M. A. , Zanetti, F. , Scolari, G. , & Mannazzu, I. (2002). Exopolysaccharide production by Streptococcus thermophiles SY: Production and preliminary characterization of the polymer. Journal of Applied Microbiology, 92, 297–306. 10.1046/j.1365-2672.2002.01487.x [DOI] [PubMed] [Google Scholar]
- Rimada, P. S. , & Abraham, A. G. (2003). Comparative study of different methodologies to determine the exopolysaccharide produced by kefir grains in milk and whey. Lait, 83, 79–87. 10.1051/lait:2002051 [DOI] [Google Scholar]
- Ruas‐Madiedo, P. , & de los Reyes‐Gavilan, C. G. (2005). Methods for the screening, isolation and characterization of exopolysaccharides produced by lactic acid bacteria. Journal of Dairy Science, 88, 843–856. 10.3168/jds.s0022-0302(05)72750-8 [DOI] [PubMed] [Google Scholar]
- Ruas‐Madiedo, P. , Hugenholtz, J. , & Zoon, P. (2002). An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. International Dairy Journal, 12, 161–171. [Google Scholar]
- Salminen, S. , & Von Wright, A. (1993). Lactic acid bacteria. New York, NY: Marcel Dekker Inc.. [Google Scholar]
- Savadogo, A. , Cheik, A. T. O. , Paul, W. S. , Nicolas, B. , Aboubacar, S. O. , & Alfred, S. T. (2004). Identification of exopolysaccharides‐producing lactic acid bacteria from Burkina Faso fermented milk samples. Africa Journal of Biotechnology, 3(3), 189–194. [Google Scholar]
- Shamala, T. S. , & Streekantiah, K. R. (1988). Microbiological and biochemical studies on tradition palm wine fermentation. Food Microbiology, 5, 157–162. 10.1016/0740-0020(88)90014-7 [DOI] [Google Scholar]
- Smitinont, T. , Tansakul, C. , Tanasupawat, S. , Keeratipibul, S. , Navarini, L. , Bosco, M. , & Cescutti, P. (1999). Exopolysaccharide‐producing lactic acid bacteria strains from traditional Thai fermented foods: Isolation, identification and exopolysaccharide characterization. International Journal of Food Microbioloy, 51, 105–111. 10.1016/S0168-1605(99)00094-X [DOI] [PubMed] [Google Scholar]
- Stredansky, M. , Conti, E. , Navarini, L. , & Bertocchi, C. (1999). Production of bacterial exopolysaccharides by solid substrate fermentation. Process Biochemistry, 34, 11–16. 10.1016/S0032-9592(98)00056-9 [DOI] [Google Scholar]
- Sunil, T. P. , Amarsinh, A. B. , Trishala, B. G. , & Tejswini, R. N. (2013). Isolation, screening and optimization of exopolysaccharide producing bacterium from saline soil. Journal of Microbiology, Biotechnology and Research, 3(3), 24–31. [Google Scholar]
- Sutherland, I. W. (2001). Microbial polysaccharides from Gram ‐negative bacteria. International Dairy Journal, 11, 663–674. [Google Scholar]
- Torino, M. I. , Sesma, F. , & de Valdez, G. F. (2000). Semi‐defined media for the exopolysaccharide (EPS) production by Lactobacillus helveticus ATCC 15807 and evaluation of the components interfering with the EPS quantification. Milchwissenschaft, 55, 314–316. [Google Scholar]
- Uzochukwu, S. V. A. , Ngoddy, P. O. , & Balough, E. (1991). Standard and pure culture inoculum for natural and formulated palm sap Fermentation. Nigerian Food Journal, 9, 67–77. [Google Scholar]
- Uzochukwu, S. V. A. , Ngoddy, P. O. , & Balough, E. (1994). The role of microbial gums in the colour and consistency of palm wine. Journal of Food Quality, 17, 393–407. 10.1111/j.1745-4557.1994.tb00160.x [DOI] [Google Scholar]
- Uzochukwu, S. V. A. , Ngoddy, P. O. , Balough, E. , Tucknott, O. G. , & Lewis, M. J. (1994). Volatile components of oil palm wine and sap. Journal of Science, Food and Agriculture, 64, 405–411. 10.1002/(ISSN)1097-0010 [DOI] [Google Scholar]
- Uzochukwu, S. V. A. , Ngoddy, P. O. , Balough, E. , Tucknot, O. G. , & Lewis, M. J. (1999). Role of palm wine Yeast and Bacteria in Palm Wine Roma. Journal of Food Science and Technology, 36(4), 301–304. [Google Scholar]
- Uzochukwu, S. V. A. , Esther, Balogh. , Loefler, R. T. , & Ngoddy, P. O. (2002). Structural analysis by 13C‐ nuclear magnetic resonance spectroscopy of glucan extracted from natural palm wine. Food Chemistry, 76, 287–291. [Google Scholar]
- Valepyn, E. , Berezina, N. , & Paquot, M. (2012). Optimization of production and preliminary characterization of new exopolysaccharides from Gluconacetobacter hansenii LMG1524. Advanced Microbiology, 2, 488–496. 10.4236/aim.2012.24062 [DOI] [Google Scholar]
- Van Den Berg, D. J. C. , Robijn, G. W. , Janssen, A. C. , Giuseppin, M. L. F. , Vreeker, R. , Kamerling, J. P. , … Verrips, C. T. (1995). Production of a novel extracellular polysaccharide by lactobacillus sake 0‐1 and characterization of the polysaccharide. Applied and Environmental Microbiology, 61(8), 2840–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayabaskar, P. , Babinastarlin, S. , Shankar, T. , Sivakumar, T. , & Anandapandian, K. T. K. (2011). Quantification and characterization of exopolysaccharides from Bacillus subtilis (MTCC 121). Advanced Biology Research, 5(2), 71–76. [Google Scholar]
- Wang, Y. , Li, C. , Liu, P. , Ahmed, Z. , Xiao, P. , & Bai, X. (2010). Physical characterization of exopolysaccharide produced by Lactobacillus plantarum KF5 isolated from Tibet Kefir. Carbohydrate Polymer, 82, 895–903. 10.1016/j.carbpol.2010.06.013 [DOI] [Google Scholar]
- Welman, A. D. , Maddox, I. S. , & Archer, R. H. (2003). Screening and selection of exopolysaccharide‐producing strains of Lactobacillus delbrueckii subsp. bulgaricus. Journal of Applied Microbiology, 95, 1200–1206. [DOI] [PubMed] [Google Scholar]


