Abstract
Purpose
Nonsurgical therapies, including biotherapy, chemotherapy, and liver-directed therapy, provided a limit survival benefit for PNET patients with hepatic metastases. With the development of liver resection technique, there was a controversy on whether to perform a liver resection for these patients.
Methods
A computerized search was made of the Medline/PubMed, EMbase, Cochrane Library, and SinoMed (CBM) before March 2018. A meta-analysis was performed to investigate the differences in the efficacy of liver resection and nonliver resection treatments based on the evaluation of morbidity, 30-day mortality, symptom relief rate, and 1-, 3-, and 5-year survival. Two investigators reviewed all included articles and extracted the data of them. The meta-analysis was performed via Review Manager 5.3 software.
Results
A total of 13 cohort studies with 1524 patients were included in this meta-analysis. Compared with the nonliver resection group, liver resection group had a longer 1-, 3-, and 5-year survival time and a higher symptom relief with an acceptable mortality and morbidity.
Conclusions
Liver resection is a safe treatment and could significantly prolong the long-term prognosis for highly selected patients with resectable liver metastases from PNET. Further randomized, controlled trials are needed.
1. Introduction
PNET (pancreatic neuroendocrine tumor), commonly known as islet cell tumors, is a rare malignant neoplasm comprising of <2% pancreatic tumors and its incidence is <1 per 10,000 person per year [1–3]. However, the incidence is increasing recently due to the advancements of imaging and endoscopic technique [4]. In contrast to pancreatic adenocarcinoma, PNET is a kind of relatively indolent tumor [5]. PNET is highly heterogeneous and could be separated with many different subtypes according to secreted hormones [6]. Owing to 50%-80% of PNETs are malignant (except for insulinomas), metastases always turn out during the progression of PNETs and liver is a frequent disseminate site [5, 6] Treatments of hepatic metastases include surgery (hepatic resection), intervention (embolization [HAE] and transcatheter arterial chemoembolization [TACE]), biotherapy (octreotide/interferon and peptide receptor radionuclide therapy [PRRT]), systemic chemotherapy (streptozotocin, 5-fluorouracil, and everolimus), and ablation. Chemoembolization means HAE combined with chemotherapeutic agents [5, 7, 8]. Among these approaches, liver surgery for metastatic disease has provided a potentially curative choice for patients with colorectal cancer [9]. With the safety enhancement of liver surgical techniques, hepatic resection is becoming an optimal option for PNET patients with liver metastases [10]. This meta-analysis was mainly to evaluate overall survival outcomes and postoperative symptom relief from PNET patients with liver metastases between liver resection and nonliver resection groups.
2. Materials and Methods
2.1. Search Strategy
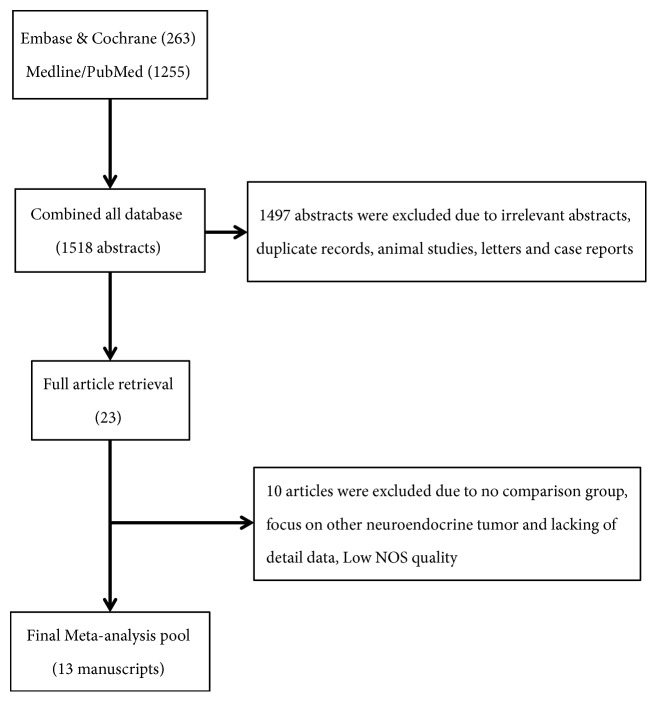
A computerized search was made of the Medline/PubMed, EMbase, Cochrane Library, and SinoMed (CBM) before March 2018. No language was limited. We used the following keywords: “pancreatic neuroendocrine tumors”, “hepatic metastases”, “liver metastases”, “hepatic metastases resection”, “liver metastases resection”, “hepatectomy”, “hepatic resection”, and “liver resection”, and we combined these keywords with “AND” “OR”. We also searched related references in the retrieved studies and reviewed articles from the database. For details, please see the flowchart of search history in Figure 1.
Figure 1.
PRISMA Flowchart describing literature search history.
2.2. Inclusion and Exclusion Criteria
The original studies included in the meta-analysis need to meet the following criteria: (1) cohort or comparative studies of patients with liver metastatic PNET undergoing hepatectomy; (2) at least 10 patients that should be reported; (3) at least 1-year overall survival (OS) data that should be available after hepatic resection; (4) NOS score≥6. Abstracts, letters, animal experiments, reviews without original data, case reports, and studies lacking control groups were excluded.
2.3. Data Extraction
Abstracts of all articles were identified by two reviewers (Xinzhe Yu & Jichun Gu) independently. If there were any discrepancies which could not be solved with discussion between the two authors, a third independent author (Chen Jin) would determine the eligibility and data of the study. We extracted such data from all articles as follows: first author, year of publication, study population characteristics, study design, inclusion and exclusion criteria, resection margin, procedure-related morbidity and mortality, OS, and median follow-up. All of the texts, tables, and figures were reviewed for data extraction. All patients in liver resection group were treated with the resection of primary tumors and liver metastases. Patients in nonliver resection group underwent nonliver resection with or without primary tumors resection.
2.4. Quality Assessment
A quality assessment of retrieved studies in this meta-analysis was carried out in the form of Newcastle-Ottawa Quality Assessment Scale (NOS System) for cohort studies [11]. The aspects of selection, comparability, and follow-up were assessed with every inclusive study. Any study that could obtain a score ≥ 7 may be recognized as high-quality study for inclusion.
2.5. Statistical Analysis
We conducted the meta-analysis following the MOOSE guidelines [12] with the Review Manager 5.3 software. The outcomes of liver resection group versus nonliver resection group were pooled with a random-effect or fixed-effect meta-analysis. In addition, heterogeneity among studies was evaluated by I2 and p value, with significance being set at p< 0.05 and I2 > 50%. A high value for I2 indicates heterogeneity. If there was substantial heterogeneity, random effects models would be used in analysis. Publication bias was evaluated with a funnel plot.
3. Results
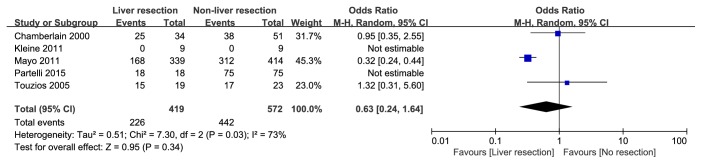
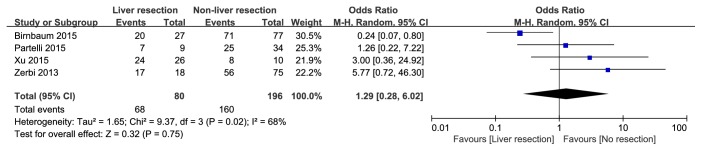
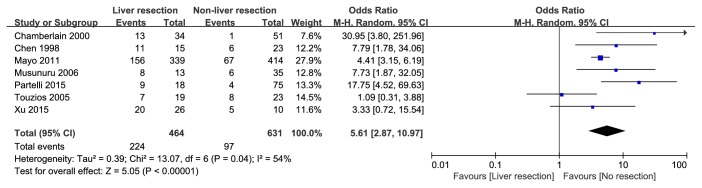
A total of 23 articles were selected for full-text. Among them, 9 studies were recognized as high quality studies because of NOS score ≥7 and 4 studies lacking some of the key data got 6 points, while the rest, 10 studies, were excluded due to NOS scores< 6. In summary, 13 cohort [13–25] studies were included and the details of the included and excluded [26–35] studies were shown in Table 1 and Supporting Table 1. There were 1524 patients in 13 cohort studies included in this meta-analysis, and 616 patients undergoing hepatic resection were divided into liver resection group while other 908 patients were with nonliver resection therapies as the control group. The details of baseline data from these studies were shown in Table 1. Furthermore, a meta-analysis of the site of hepatic metastases (unilobar/bilobar), the grading system (G1&G2/G3), and the occurrence time of hepatic metastases (synchronous/metachronous) was performed, and there is no significant difference on the occurrence time of hepatic metastases and the grading system between two groups (Figure 2: OR=0.63; 95% CI, 0.24, 1.64; p=0.34. Figure 3: OR=1.29; 95% CI, 0.28, 6.02; p=0.75) while the liver resection group have more unilobar hepatic metastases (Figure 4: OR=5.61; 95% CI, 2.87, 10.97; p<0.001).
Table 1.
Basic study characteristics of included trials.
| Author | Year | Country | Time Period | Patient Number | Age | Male/Female ratio | Grade | Hepatic resection (Y/N) | Hepatic Metastases | NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver resection | Non-liver resection | G1/G2/G3 | Unilobar/ | Synchronous/ | ||||||||
| Bilobar | Metachronous | |||||||||||
| Birnbaum et al | 2015 | France | 1995-2012 | 118 | 60 (31-82) | 57 (20-83) | 62/56 | 48/43/13 | 27/91 | 10/17 | 27/0 | 7 |
| Xu et al | 2015 | China | 2008-2013 | 36 | 49.9 ± 11.0 | 56.5 ± 12.5 | 14/22 | 6/26/4 | 26/10 | 25/11 | NR | 6 |
| Partelli et al | 2015 | Italy | 2000-2011 | 93 | 50 (45-59) | 51.5 (43-63) | 53/40 | 21/51/20 | 18/75 | 13/80 | 93/0 | 7 |
| Zerbi et al | 2013 | Italy | 2004-2007 | 45 | 56.9 | 60.6 | 26/19 | 13/19/11 | 9/36 | NR | NR | 6 |
| Kleine et al | 2011 | German | 1990-2009 | 15 | 55 (20-77) | NR | NR | 9/6 | NR | 15/0 | 7 | |
| Mayo et al | 2011 | America | 1985-2010 | 753 | 56 ± 12.6 | 57 ± 12.8 | 391/362 | NR | 339/414 | 223/530 | 273/480 | 8 |
| House et al | 2006 | America | 1988-2003 | 31 | 52 (31-71) | 41 (31-52) | 15/16 | NR | 26/5 | NR | NR | 9 |
| Osborne et al | 2006 | America | 2000-2004 | 120 | 56 ± 11.6 | 58 ± 11.1 | 64/56 | NR | 61/59 | NR | NR | 6 |
| Musunuru et al | 2006 | America | 1996-2004 | 48 | 56 (27-85) | NR | NR | 13/35 | 14/34 | 28/20 | 7 | |
| Touzios et al | 2005 | America | 1990-2004 | 42 | 58 ± 3 | 59 ± 3 | 17/25 | NR | 19/23 | 15/27 | 32/10 | 6 |
| Solorzano et al | 2001 | America | 1988-1999 | 100 | NR | NR | 56/44 | NR | 20/80 | NR | NR | 7 |
| Chamberlain et al | 2000 | America | 1992-1998 | 85 | 50 (20-79) | 54 (23-79) | 37/48 | NR | 34/51 | 14/71 | 63/22 | 7 |
| Chen et al | 1998 | America | 1984-1995 | 38 | 54 ± 4 | 59 ± 3 | 24/14 | NR | 15/23 | 17/21 | NR | 7 |
NR indicates no report.
Figure 2.
Forest plot for the occurrence time of hepatic metastases (synchronous/metachronous) of liver resection group and nonliver resection group in patients with liver metastases from pancreatic neuroendocrine tumor. There is no significant difference between two groups.
Figure 3.
Forest plot for the Grade classification (G1&G2/G3) of liver resection group and nonliver resection group in patients with liver metastases from pancreatic neuroendocrine tumor. There is no significant difference between two groups.
Figure 4.
Forest plot for the site of hepatic metastases (unilobar/bilobar) of liver resection group and nonliver resection group in patients with liver metastases from pancreatic neuroendocrine tumor. Liver resection group have more unilobar hepatic metastases.
Then a further literature review of 13 included studies was performed. (Table 2) In general, the median postoperative adjuvant therapy rate is of 42.00% (0%-68.00%). For postoperative outcomes, the morbidity was 33.5% (3.28%-44.44%) and the 30-day mortality was 3.32% (0%-5.30%). For long-term outcomes, compared with nonliver resection group whose median OS was 17 months (17-54.8), liver resection group could be prolonged to 84 months (36-123). Overall, almost all studies have an agreement on the idea that hepatic resection could provide a prognosis benefit for PNET patients with liver metastases and according to the data from these studies, there was no concern about the safety of liver resection.
Table 2.
All included literatures review.
| Study ID | Morbidity | 30-day mortality | Postoperative adjuvant therapy Rate | Median OS (m) | Median follow-up (m) | Non-liver resection treatments | Conclusion | |
|---|---|---|---|---|---|---|---|---|
| Hepatic resection | Non-liver resection | |||||||
| Birnbaum 2015 | 44.00% | 5.00% | NR | 90 | NR | NR | Resection of primary tumors | Resection of liver metastases improve survival |
| Xu 2015 | NR | NR | NR | 57.2 | 54.8 | 32 | Somatostatin analogues and chemotherapy | Resection of liver metastases could not prolong OS but could improve PFS |
| Partelli 2015 | 44.44% | NR | 68.00% | 97 | 36 | 41 | Somatostatin analogues, PRRT, chemotherapy | Resection of liver metastases improve survival |
| Zerbi 2013 | NR | NR | 48.68% | NR | 20.5 | 21 | Somatostatin analogues, PRRT, ablation, chemotherapy | Resection of liver metastases could be the first-choice treatment for malignent PNET |
| Kleine 2011 | 22.22% | NR | NR | NR | 37.8 | 40 | Resection of primary tumors | Resection of liver metastases may prolong OS |
| Mayo 2011 | NR | NR | NR | 123 | 33 | 26 | Intra-arterial therapy and resection of primary tumors | Hepatectomy most benefited those patients with low-volume ( <25%) liver metastasis or those with symptomatic high-volume liver metastasis |
| House 2006 | 25.00% | 0.00% | 11.54% | 78 | 17 | NR | Somatostatin analogues, chemotherapy, chemoembolization and resection of primary tumors | There is a survival benefit from complete surgical resection of metastatic islet cell tumors originating from the pancreas |
| Osborne 2006 | 3.28% | 1.64% | 65.57% | NR | NR | NR | Somatostatin analogues and chemotherapy, PRRT, embolization | Patients who undergo surgical cytoreduction of symptomatic neuroendocrine hepatic metastases enjoy prolonged survival when compared with their medically treated counterparts |
| Musunuru 2006 | NR | NR | 8.00% | NR | NR | 20 | Systemic hormonal and chemotherapy, ablation, hepatic artery embolization | In patients with liver-only neuroendocrine metastases, surgical therapy is associated with improved survival |
| Touzios 2005 | 42.00% | 5.30% | 36.00% | >96 | NR | NR | Somatostatin analogues, chemotherapy, PRRT, ablation and resection of primary tumors | Resection has been shown to be an excellent treatment and accumulating data document improved survival with resection of these tumors |
| Solorzano2001 | NR | NR | 60.00% | 36 | 21.6 | 32 | Chemotherapy, hepatic artery embolization | Aggressive management should probably be restricted to younger patients with limited extrahepatic disease |
| Chamberlain 2000 | NR | NR | NR | NR | NR | 27 | Hepatic artery embolization, Somatostatin analogues and chemotherapy and resection of primary tumors | Hepatic resection has a role in the man-agement of patients with NET metastases and may prolong survival |
| Chen 1998 | NR | NR | 0.00% | NR | 27 | 27 | Chemoembolization, chemotherapy, PRRT and resection of primary tumors | Hepatic resection for metastatic neuroen-docrine tumors may prolong survival |
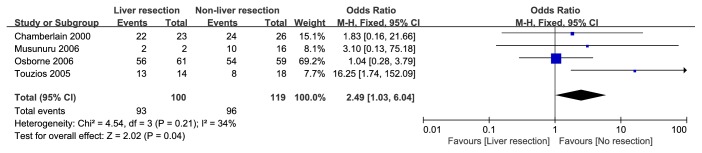
The median 1-year, 3-year, and 5-year OS rates of all patients in the liver resection group were 92.69%, 76.93%, and 67.54%, respectively, whereas the data of nonliver resection group was 77.31%, 40.94%, and 26.6%. In the retrieved studies, liver resection was not only related to a significant higher 1-year OS rate (OR=3.31; 95% CI, 2.34, 4.67; p<0.001), 3-year OS rate (OR=4.29; 95% CI, 2.71, 6.80; p<0.001), and 5-year OS rate (OR=5.30; 95% CI, 3.24, 8.67; p<0.001) (Table 3) but also created a chance for patients, no matter with functional or nonfunctional PNET, to have a higher symptom relief rate including hormonal symptoms, mechanical symptoms (Figure 5: OR=2.49; 95% CI, 1.03, 6.04; p=0.04). A funnel plot of these 13 studies was used to examine publication bias in the meta-analysis (Figure 6). As shown in Figure 6, this plot showed that there was no significant publication bias in this meta-analysis and unpublished data were not evaluated.
Table 3.
Survival outcomes of liver resection group versus nonliver resection group.
| Survival outcomes | No. Of studies | No. Of event for liver resection | No. Of event for non-liver resection | OR | 95% CI | P Value |
Heterogeneity P, I2 |
Meta-analysis model |
|---|---|---|---|---|---|---|---|---|
| 1-year overall survival | 13 | 571/616 | 702/908 | 3.31 | 2.34, 4.67 | <0.001 | 0.55, 0% | Fixed |
| 3-year overall survival | 12 | 467/607 | 357/872 | 4.29 | 2.71, 6.80 | <0.001 | 0.02, 52% | Random |
| 5-year overall survival | 12 | 410/607 | 232/872 | 5.30 | 3.24, 8.67 | <0.001 | 0.02, 53% | Random |
Figure 5.
Forest plot for the symptom relief (hormonal symptoms and mechanical symptoms) of liver resection group and nonliver resection group in patients with liver metastases from PNET (functioning or nonfunctioning). Liver resection group have a higher symptom relief rate.
Figure 6.
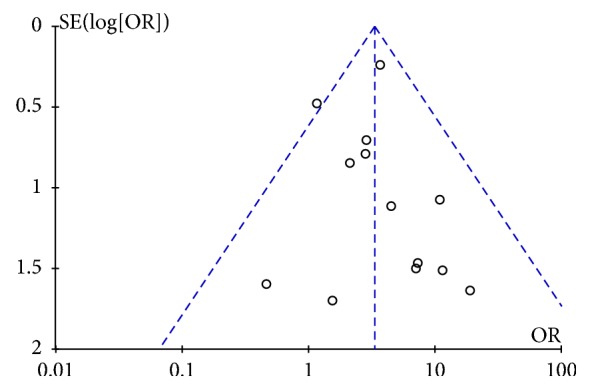
Funnel plot for evaluating publication bias-results from 13 studies.
4. Discussion
Nowadays, there are various treatments for PNET patients with liver metastases including biotherapy, chemotherapy, and intervention. However, no randomized, controlled studies demonstrated that any of them could prolong the OS [7, 36, 37]. Hepatic resection may be the only treatment, even a curative treatment, to prolong the OS. In this meta-analysis, we are mainly intended to compare the prognosis of hepatic resection group with nonliver resection group in PNET patients with liver metastases. According to the result, liver resection is a safe treatment with a low mortality rate (3.32%) and an acceptable morbidity rate (33.5%). The grading system is related to the proliferation capacity of the tumor measured by Ki-67 staining of the PNET specimens. According to previous studies, patients with G1 and G2 PNET have a better survival compared with G3 [38, 39]. In this meta-analysis, there was no difference between two groups about patients with G1, G2, or G3 (p=0.75). However, we could not further perform a subgroup analysis about the OS of patients with G1, G2, and G3 because of the lack of data. From 13 included cohort studies, liver resection provided a longer median survival, a higher 1-, 3-, and 5-year survival rate and postoperative symptom relief rate. Notably, 13 studies in our pool showed PNET patients with liver metastases undergoing hepatic resection had a median of 67.54% for 5-year survival rate, which is higher than 60% from previous reports [40].
There was one citation retrieved which suggested that 1-, 3-, and 5-year survival rate in liver resection group were lower than nonliver resection group (95, 93, and 87% versus 87, 84, and 66%, p =0.006) because there were no hepatic metastases in nonliver resection group [23]. The rest 12 studies included had a consensus on a prolonged OS and higher 1-, 3-, and 5-year survival rate in liver resection group and, with extending of follow-up duration, the difference was more obvious between the two groups. Although a conclusion that liver resection could prolong the OS for PNET patients with liver metastases was easily acquired, we could not overlook the fact that liver resection group have more unilobar hepatic metastases which means there were more patients with resectable liver metastases. Touzios et al. even suggested that patients with more than 50% liver involvement may not benefit from a liver resection (5-year survival rate: 67% versus 8%, p<0.05) [16]. In another word, liver resection could prolong the OS just for highly selected PNET patients with resectable hepatic metastases.
Due to the decrease of tumor bulk, the symptom relief rate was higher in liver resection group of this meta (p=0.04) [14, 16, 17, 19]. Some authors proposed that liver resection should be attempted if at least 90% of visible tumors could be removed, which means cytoreduction, to relieve symptoms for patients with malignant PNET [7, 41, 42]. Three of included studies (Chamberlain et al., Osborne et al., and Partelli et al.) clarified that cytoreduction could even offer a survival benefit [14, 17, 24]. However, in recent years, some authors consider that 90% debulking threshold is completely made up and advocates that 70% debulking threshold might be a better cut-off value. Morgan et al. discovered that there was no difference in OS between patients who had 100%, >90% or > 70% cytoreduction for all 44 PNET patients with liver metastases (p=0.75) [43]. Although there were small bowel and PNET for all 108 patients, Maxwell et al. proposed that patients with greater than 70% debulking had significantly improved OS compared with patients with less than 70% (median OS: not reached vs 6.5 years, p=0.009) [35]. Some included researches carry out liver resection for PNET patients with extrahepatic metastases but did not conduct indepth research on it [14, 16, 21]. Morgan et al. also did the same, yet all deaths in the series were due to liver failure instead of extra-hepatic disease [43]. Thus, 70% debulking threshold might be able to replace 90% for PNET patients with liver metastases and extra-hepatic metastases should not be an obstacle for surgical therapy [44].
Further, some reviews even suggested that the resection of primary tumors should be attempted even if there were metastases because it might decrease the rate of development of liver metastases and extends survival by preventing the development of progressive disease [7, 45, 46]. In this pooled meta-analysis, 7 studies performed resection of primary tumors in nonliver resection group, but we could not perform a subgroup analysis to support the view because of the lack of data [13, 14, 16, 18, 20, 21, 23]. Although we could not draw a conclusion that palliative resection would provide a better prognosis according to these results, we still should attach importance to the role of palliative resection in advanced PNET patients and launch some prospective researches.
Due to the high recurrence rate even after liver resection [38], postoperative adjuvant therapies, including TACE/HAE, systemic chemotherapy, somatostatin analogues, and PRRT, were recommended for PNET patients with hepatic metastases [47]. In this study, the median postoperative adjuvant therapy rate was 42.0% in liver resection group. Although all postoperative adjuvant therapies could relieve the symptoms, their effects on overall survival have not been proved [7, 17, 48–52]. Recently, everolimus (mammalian target of rapamycin [mTOR] inhibitor) yielded a longer progression-free survival (PFS) and a survival benefit of 6.3 months compared with placebo which brought a hope for advanced PNET patients [37, 53].
Our study has some limitations. There were no randomized controlled trials (RCTs) in retrieved studies and the overall level of clinical evidence was relatively low. The heterogeneity of treatment ranging from the sole resection of the primary tumor to multimodal therapy concepts (somatostatin analogues, chemotherapy, TACE/HAE, and resection of the primary tumor) in nonliver resection, and the heterogeneity of patients' characteristics between two groups might be a source of bias.
5. Conclusions
If PNET patients with resectable liver metastases were highly selected, liver surgical resection was an effective and safe treatment to provide a better long-term prognosis including prolonged OS and higher symptom relief. Therefore, there is an urgent need for a further randomized, controlled trial to solve this clinical issue.
Acknowledgments
This work was funded by (1) the National Natural Science Foundation of China (81472221); (2) Key Project of National Health and Planning Commission of the PRC on General Surgery 2012-2014; (3) Key Project of National Health and Planning Commission of the PRC on Oncology 2013-2015; (4) the Research Fund for the Doctoral Program of Higher Education of China (20130071110052 and 20110071110065).
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
Xinzhe Yu and Jichun Gu are equal contributors to this manuscript. Xinzhe Yu and Jichun Gu collected and analyzed data and write the manuscript. Ji Li, Deliang Fu, and Haoxuan Wu provided critical revision. Chen Jin designed the study, determined the eligibility of data, and approved the final version of the manuscript.
Supplementary Materials
Supporting Table 1: Basic study characteristics of excluded cohort studies.
References
- 1.Öberg K., Eriksson B. Endocrine tumours of the pancreas. Best Practice & Research Clinical Gastroenterology. 2005;19(5):753–781. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Halfdanarson T. R., Rabe K. G., Rubin J., Petersen G. M. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Annals of Oncology. 2008;19(10):1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Öberg K. Pancreatic endocrine tumors. Seminars in Oncology. 2010;37(6):594–618. doi: 10.1053/j.seminoncol.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J., Enewold L., Stojadinovic A., et al. Incidence rates of exocrine and endocrine pancreatic cancers in the United States. Cancer Causes & Control. 2010;21(6):853–861. doi: 10.1007/s10552-010-9512-y. [DOI] [PubMed] [Google Scholar]
- 5.Panzuto F., Boninsegna L., Fazio N., et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. Journal of Clinical Oncology. 2011;29(17):2372–2377. doi: 10.1200/jco.2010.33.0688. [DOI] [PubMed] [Google Scholar]
- 6.Ito T., Igarashi H., Jensen R. T. Pancreatic neuroendocrine tumors: Clinical features, diagnosis and medical treatment: Advances. Best Practice & Research Clinical Gastroenterology. 2012;26(6):737–753. doi: 10.1016/j.bpg.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metz D. C., Jensen R. T. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135(5):1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen R. T., Cadiot G., Brandi M. L., et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: Functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95(2):98–119. doi: 10.1159/000335591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNally S., Parks R. Surgery for Colorectal Liver Metastases. Digestive Surgery. 2013;30(4-6):337–347. doi: 10.1159/000351442. [DOI] [PubMed] [Google Scholar]
- 10.Gurusamy K. S., Ramamoorthy R., Sharma D., Davidson B. R. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases. Cochrane Database of Systematic Reviews. 2009;(2) doi: 10.1002/14651858.CD007060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Stroup D. F., Berlin J. A., Morton S. C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Journal of the American Medical Association. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Chen H., Hardacre J. M., Uzar A., Cameron J. L., Choti M. A. Isolated liver metastases from neuroendocrine tumors: Does resection prolong survival? Journal of the American College of Surgeons. 1998;187(1):88–93. doi: 10.1016/S1072-7515(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain R. S., Canes D., Brown K. T., et al. Hepatic neuroendocrine metastases: Does intervention alter outcomes? Journal of the American College of Surgeons. 2000;190(4):432–445. doi: 10.1016/S1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 15.Solorzano C. C., Lee J. E., Pisters P. W. T., et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery. 2001;130(6):1078–1085. doi: 10.1067/msy.2001.118367. [DOI] [PubMed] [Google Scholar]
- 16.Touzios J. G., Kiely J. M., Pitt S. C., et al. Neuroendocrine hepatic metastases: Does aggressive management improve survival? Annals of Surgery. 2005;241(5):776–785. doi: 10.1097/01.sla.0000161981.58631.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne D. A., Zervos E. E., Strosberg J., et al. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Annals of Surgical Oncology. 2006;13(4):572–581. doi: 10.1245/ASO.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 18.House M. G., Cameron J. L., Lillemoe K. D., et al. Differences in survival for patients with resectable versus unresectable metastases from pancreatic islet cell cancer. Journal of Gastrointestinal Surgery. 2006;10(1):138–145. doi: 10.1016/j.gassur.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Musunuru S., Chen H., Rajpal S., et al. Metastatic neuroendocrine hepatic tumors: Resection improves survival. JAMA Surgery. 2006;141(10):1000–1004. doi: 10.1001/archsurg.141.10.1000. [DOI] [PubMed] [Google Scholar]
- 20.Kleine M., Schrem H., Vondran F. W. R., Krech T., Klempnauer J., Bektas H. Extended surgery for advanced pancreatic endocrine tumours. British Journal of Surgery. 2012;99(1):88–94. doi: 10.1002/bjs.7681. [DOI] [PubMed] [Google Scholar]
- 21.Mayo S. C., De Jong M. C., Bloomston M., et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: A multicenter international analysis. Annals of Surgical Oncology. 2011;18(13):3657–3665. doi: 10.1245/s10434-011-1832-y. [DOI] [PubMed] [Google Scholar]
- 22.Zerbi A., Capitanio V., Boninsegna L., et al. Treatment of malignant pancreatic neuroendocrine neoplasms: Middle-term (2-year) outcomes of a prospective observational multicentre study. HPB. 2013;15(12):935–943. doi: 10.1111/hpb.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birnbaum D. J., Turrini O., Vigano L., et al. Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Annals of Surgical Oncology. 2015;22(3):1000–1007. doi: 10.1245/s10434-014-4016-8. [DOI] [PubMed] [Google Scholar]
- 24.Partelli S., Inama M., Rinke A., et al. Long-term outcomes of surgical management of pancreatic neuroendocrine tumors with synchronous liver metastases. Neuroendocrinology. 2015;102:68–76. doi: 10.1159/000431379. [DOI] [PubMed] [Google Scholar]
- 25.Xu X., Han X., Liu L., Ji Y., Li J., Lou W. Clinical analysis and comprehensive treatment of nonfunctional pancreatic neuroendocrine tumor with liver metastases: A retrospective single-center study. Pancreas. 2015;44(6):995–996. doi: 10.1097/MPA.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 26.Yao K. A., Talamonti M. S., Nemcek A., et al. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery. 2001;130(4):677–685. doi: 10.1067/msy.2001.117377. [DOI] [PubMed] [Google Scholar]
- 27.Elias D., Lasser P., Ducreux M., et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: A 15-year single center prospective study. Surgery. 2003;133(4):375–382. doi: 10.1067/msy.2003.114. [DOI] [PubMed] [Google Scholar]
- 28.Elias D., Goéré D., Leroux G., et al. Combined liver surgery and RFA for patients with gastroenteropancreatic endocrine tumors presenting with more than 15 metastases to the liver. European Journal of Surgical Oncology. 2009;35(10):1092–1097. doi: 10.1016/j.ejso.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 29.De Jong M. C., Farnell M. B., Sclabas G., et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: A dual-center analysis. Annals of Surgery. 2010;252(1):142–148. doi: 10.1097/SLA.0b013e3181dbb7a7. [DOI] [PubMed] [Google Scholar]
- 30.Mayo S. C., De Jong M. C., Pulitano C., et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Annals of Surgical Oncology. 2010;17(12):3129–3136. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 31.Poultsides G. A., Huang L. C., Chen Y., et al. Pancreatic neuroendocrine tumors: Radiographic calcifications correlate with grade and metastasis. Annals of Surgical Oncology. 2012;19(7):2295–2303. doi: 10.1245/s10434-012-2305-7. [DOI] [PubMed] [Google Scholar]
- 32.Krausch M., Raffel A., Anlauf M., et al. "cherry picking", a multiple non-anatomic liver resection technique, as a promising option for diffuse liver metastases in patients with neuroendocrine tumours. World Journal of Surgery. 2014;38(2):392–401. doi: 10.1007/s00268-013-2267-3. [DOI] [PubMed] [Google Scholar]
- 33.Du S., Ni J., Weng L., et al. Aggressive Locoregional Treatment Improves the Outcome of Liver Metastases from Grade 3 Gastroenteropancreatic Neuroendocrine Tumors. Medicine (United States) 2015;94(34):p. e1429. doi: 10.1097/MD.0000000000001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertani E., Falconi M., Grana C., et al. Small intestinal neuroendocrine tumors with liver metastases and resection of the primary: Prognostic factors for decision making. International Journal of Surgery. 2015;20:58–64. doi: 10.1016/j.ijsu.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell J. E., Sherman S. K., O'Dorisio T. M., Bellizzi A. M., Howe J. R. Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery. 2016;159(1):320–333. doi: 10.1016/j.surg.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plockinger U., Wiedenmann B. Endocrine tumours of the gastrointestinal tract. Management of metastatic endocrine tumours. Best Practice & Research: Clinical Gastroenterology. 2005;19:553–576. doi: 10.1016/j.bpg.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Yao J. C., Shah M. H., Ito T. Everolimus for advanced pancreatic neuroendocrine tumors. The New England Journal of Medicine. 2011;364(6):514–523. doi: 10.1056/nejmoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scigliano S., Lebtahi R., Maire F. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocrine-Related Cancer. 2009;16:977–990. doi: 10.1677/ERC-08-0247. [DOI] [PubMed] [Google Scholar]
- 39.Strosberg J., Nasir A., Coppola D., Wick M., Kvols L. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Human Pathology. 2009;40(9):1262–1268. doi: 10.1016/j.humpath.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Schurr P. G., Strate T., Rese K., et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: An institutional experience. Annals of Surgery. 2007;245(2):273–281. doi: 10.1097/01.sla.0000232556.24258.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carty S. E., Jensen R. T., Norton J. A. Prospective study of aggressive resection of metastatic pancreatic endocrine tumors. Surgery. 1992;112(6):1024–1032. [PubMed] [Google Scholar]
- 42.Sarmiento J. M., Heywood G., Rubin J., Ilstrup D. M., Nagorney D. M., Que F. G. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. Journal of the American College of Surgeons. 2003;197(1):29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 43.Morgan R. E., Pommier S. J., Pommier R. F. Expanded criteria for debulking of liver metastasis also apply to pancreatic neuroendocrine tumors. Surgery. 2018;163(1):218–225. doi: 10.1016/j.surg.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 44.Howe J. R., Cardona K., Fraker D. L., et al. The surgical management of small bowel neuroendocrine tumors. Pancreas. 2017;46(6):715–731. doi: 10.1097/MPA.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fraker D. L., Norton J. A., Alexander H. R., Venzon D. J., Jensen R. T. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Annals of Surgery. 1994;220(3):320–330. doi: 10.1097/00000658-199409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norton J. A., Fraker D. L., Alexander H. R., et al. Surgery increases survival in patients with gastrinoma. Annals of Surgery. 2006;244(3):410–419. doi: 10.1097/01.sla.0000234802.44320.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahlman H., Wängberg B., Jansson S., et al. Interventional treatment of gastrointestinal neuroendocrine tumours. Digestion. 2000;62(1):59–68. doi: 10.1159/000051857. [DOI] [PubMed] [Google Scholar]
- 48.Moertel C. G., Lefkopoulo M., Lipsitz S., Hahn R. G., Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil, or chlorozotocin in the treatment of advanced islet-cell carcinoma. The New England Journal of Medicine. 1992;326(8):519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 49.Venook A. P. Embolization and chemoembolization therapy for neuroendocrine tumors. Current Opinion in Oncology. 1999;11(1):38–41. doi: 10.1097/00001622-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 50.O'Toole D., Ruszniewski P. Chemoembolization and other ablative therapies for liver metastases of gastrointestinal endocrine tumours. Best Practice & Research Clinical Gastroenterology. 2005;19(4):585–594. doi: 10.1016/j.bpg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Shah T., Caplin M. Biotherapy for metastatic endocrine tumours. Best Practice & Research Clinical Gastroenterology. 2005;19(4):617–636. doi: 10.1016/j.bpg.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 52.Brabander T., Teunissen J. J. M., Van Eijck C. H. J., et al. Peptide receptor radionuclide therapy of neuroendocrine tumours. Best Practice & Research Clinical Endocrinology & Metabolism. 2016;30(1):103–114. doi: 10.1016/j.beem.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Yao J. C., Pavel M., Lombard-Bohas C., et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, Phase III RADIANT-3 study. Journal of Clinical Oncology. 2016;34(32):3906–3913. doi: 10.1200/JCO.2016.68.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table 1: Basic study characteristics of excluded cohort studies.