Abstract
The potent cytotoxicity and unique mode of action make the enediyne antitumor antibiotic C-1027 an exquisite drug candidate for anticancer chemotherapy. However, clinical development of C-1027 has been hampered by its low titer from the original producer Streptomyces globisporus C-1027. Here we report three new C-1027 alternative producers, Streptomyces sp. CB00657, CB02329, and CB03608, from The Scripps Research Institute actino-mycetes strain collection. Together with the previously disclosed Streptomyces sp. CB02366 strain, four C-1027 alternative producers with C-1027 titers of up to 11-fold higher than the original producer have been discovered. The five C-1027 producers, isolated from distant geographic locations, are distinct Streptomyces strains based on morphology and taxonomy. Pulsed-field gel electrophoresis and Southern analysis of the five C-1027 producers reveal that their C-1027 biosynthetic gene clusters (BGCs) are all located on giant plasmids of varying sizes. The high nucleotide sequence similarity among the five C-1027 BGCs implies that they most likely have evolved from a common ancestor.
Graphical Abstract
The enediynes are some of the most fascinating natural products, not only for their unprecedented molecular architectures but also for their extraordinary biological activities.1,2 Since the establishment of the enediyne chromophore structure of neocarzinostatin (NCS) in 1985, 13 enediyne natural products have been isolated and many more potential enediyne producers have been discovered by genome mining.3,4 Among the 13 known enediyne natural products (Figure S1), two, i.e., NCS in the poly(styrene-co-maleic acid)-conjugated form as in SMANCS and calicheamicin as the payload in the two recently approved antibody−drug conjugates (ADCs) Besponsa and Mylotarg, have been used as anticancer drugs, and another two, i.e., uncialamycin and C-1027, are currently in various stages of preclinical studies.3,4
C-1027, also known as lidamycin, was first isolated in 1989 from Streptomyces globisporus C-1027 as a chromoprotein complex, consisting of the CagA apoprotein and the C-1027 enediyne chromophore, with extremely high cytotoxicity.5–7 Encoded by the cagA gene within the C-1027 biosynthetic gene cluster, the CagA apoprotein binds with the C-1027 enediyne chromophore to form a protein−small-molecule complex that(i) stabilizes the highly labile C-1027 chromophore and (ii) transports the bound enediyne chromophore out of the cell by an efflux pump.8,9 As the prototype for nine-membered enediynes, the C-1027 biosynthetic pathway has been extensively studied, leading to the discovery of many unprecedented chemistries and new modes of action for the enediyne natural products.1,10 Thus, in addition to causing oxygen-dependent DNA double-strand breaks, a mechanism common to all enediynes, C-1027 and its engineered analogues can also induce oxygen-independent DNA interstrand cross-links.11–13 This unprecedented mode of action makes C-1027 and its analogues excellent drug candidates to target solid tumors and other cancer cells in a hypoxic environment.13,14 Given the high structural complexity and inherent instability of the enediyne chromophore, total synthesis of C-1027 is challenging, and microbial fermentation remains the practical way to supply this compound to support both mechanistic and clinical studies. However, the S. globisporus wild-type strain produces C-1027 in a low titer.15,16 Therefore, finding alternative producers of C-1027 with significantly higher C-1027 titers would greatly facilitate both fundamental studies and clinical advancement, as well as eventual commercialization, of this promising anticancer drug candidate.
Alternative producers of microbial natural products provide new opportunities to circumvent the recalcitrant issues often associated with the original wild-type producers, such as low production titers, unfavorable growth characteristics for fermentation optimization, and poor genetic amenability for recombinant DNA technologies.17 During an effort to survey potential enediyne producers from the actinomycetes strain collection at The Scripps Research Institute (TSRI), we discovered 81 potential enediyne producers from 3400 strains.3 Phylogenetic analysis of the translated sequences of a 1 kb internal fragment of pksE from the 81 new enediyne polyketide synthase (PKS) cassettes led to 28 distinct clades, upon applying a 90% amino acid identity cutoff (Figure S2). Four potential enediyne producers, Streptomyces sp. CB00657, CB02329, CB02366, and CB03608, were found to locate in the same clade as the original C-1027 producer S. globisporus. Genome sequencing of CB02366 showed that its enediyne biosynthetic gene cluster (BGC) has an identical genetic organization to the C-1027 BGC from S. globisporus, with amino acid sequence identities ranging from 83% to 99% (Figure S3). Preliminary fermentation confirmed CB02366 as a C-1027 alternative producer with a C-1027 titer significantly higher than that from the original producer S. globisporus.3
Here we systematically investigated the four S. sp. CB00657, CB02329, CB02366, and CB03608 strains, in comparison with S. globisporus, and confirmed them all as C-1027 producers. Morphological and taxonomic analysis of the five C-1027 producers showed that they are distinct Streptomyces strains collected from distant geographic locations. C-1027 titers in the alternative producers are significantly higher (up to 11-fold) than that from the original producer S. globisporus. Pulsed-field gel electrophoresis (PFGE) and Southern analysis of the five C-1027 producers revealed that their C-1027 BGCs all resided on giant plasmids of varying sizes.
■RESULTS AND DISCUSSION
Fermentation optimization of S. sp. CB00657, CB02329, and CB03608 from the same clade of S. globisporus and S. sp. CB02366 confirmed them all as C-1027 producers. As S. sp. CB02366 had been confirmed previously as a C-1027 alternative producer,3 we first investigated whether the other three strains, i.e., S. sp. CB00657, CB02329, and CB03608, from the same clade can also produce C-1027 (Figures S2, S4). The three strains were cultivated in the C-1027 production medium,15,16 with S. globisporus and S. sp. CB02366 as controls, and C-1027 biosynthetic potential was determined by measuring the production of heptaene, a major metabolite produced by the enediyne PKS, and the C-1027 chromophore as described previously.15,16 Production of heptaene and the C-1027 chromophore was observed from all five strains (Figure 1A–C), confirming the discovery of four C-1027 alternative producers from 3400 strains of the TSRI actinomycetes strain collection (Figures S2, S4). The identity of the produced C-1027 chromophore by the four C-1027 alternative producers was further confirmed by HR-ESIMS analysis, in comparison with the C-1027 chromophore produced by S. globisporus (Figure S4).
Figure 1.
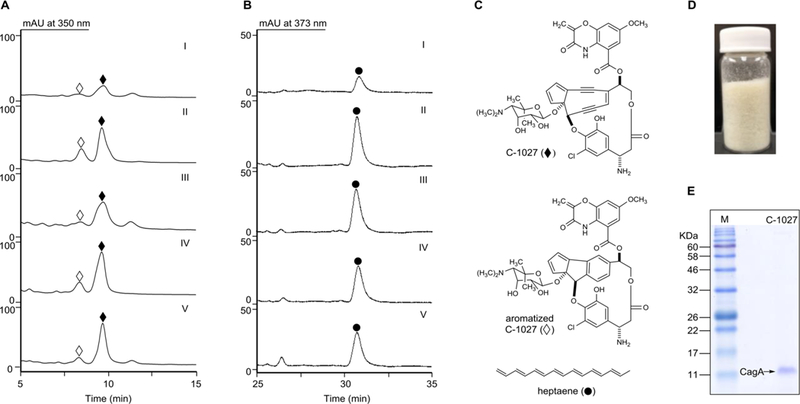
C-1027 and heptaene production by the five C-1027 producers. (A) HPLC analysis of the C-1027 chromophore (⧫) and aromatized C-1027 chromophore (◊) in (I) S. globisporus, (II) S. sp. CB00657, (III) CB02329, (IV) CB02366, and (V) CB03608. (B) HPLC analysis of heptaene (●) production in (I) S. globisporus, (II) S. sp. CB00657, (III) CB02329, (IV) CB02366, and (V) CB03608. (C) Structures of C-1027 chromophore (⧫), aromatized C-1027 chromophore (◊), and heptaene (●). (D) C-1027 chromoprotein (~720 mg) purified from 2 L fermentation of S. sp. CB02366. (E) SDS-PAGE analysis of the purified C-1027 chromoprotein from S. sp. CB02366. M, molecular weight standards; C-1027, the purified C-1027 chromoprotein complex with the predicted molecular weight of 10 501.5 Da for CagA.
Scale-up fermentation of S. sp. CB02366 afforded pure C-1027 chromoprotein, from which the C-1027 chromophore standard was prepared to determine C-1027 titers in the five producers. A 2 L fermentation of S. sp. CB02366 was carried out under the same conditions as described above.15,16 After fermentation, the C-1027 chromoprotein was sequentially purified by ammonium sulfate precipitation, ion-exchange chromatography using DEAE-cellulose, a second ammonium sulfate precipitation, size-exclusion chromatography (Sephadex G-200), and lyophilization to afford 720 mg of pure C-1027 chromoprotein (Figure 1D). The purified C-1027 chromoprotein migrated as a single band with an apparent molecular weight (MW) of ~11 000 (the predicted MW of CagA is 10 501) upon SDS-PAGE, indicating its homogeneity (Figure 1E). The pure C-1027 chromoprotein, as a 1:1 noncovalent complex of CagA and the C-1027 chromophore, was weighed and extracted with MeOH, and the resultant C-1027 chromophore was used as standard to determine C-1027 titers in the five producers by HPLC.
C-1027 production in the four alternative producers was significantly higher than the original producer S. globisporus. Among the four alternative producers, C-1027 titer in S. sp. CB03608 was the highest (67 ± 9 mg/L of the C-1027 chromophore on the basis of HPLC analysis), followed by CB02366 (64 ± 13 mg/L), CB00657 (52 ± 16 mg/L), and CB02329 (41 ± 10 mg/L). Comparing to the S. globisporus wild-type strain (6.2 ± 1.1 mg/L, which agreed well with the titer of 5.6 ± 1.3 mg/L reported previously15,16), the C-1027 alternative producers produced up to 11-fold more C-1027 (Figure 1A). According to the 1:1 ratio of the CagA apoprotein (MW of 10 501) to the C-1027 chromophore (MW of 844.2) in the C-1027 chromoprotein complex, the estimated titers of the C-1027 chromoprotein in the alternative producers range from approximately 550 to 900 mg/L. Based on the purified amount (720 mg from 2 L) and the calculated production (1730 mg in 2 L) of the C-1027 chromoprotein from S. sp. CB02366, the overall recovery yield for the purification procedure was approximately 42%.
The highest heptaene production was detected in S. sp. CB00657 (29 ± 4 mg/L), followed by CB02329 (24 ± 5 mg/L), CB02366 (17 ± 4 mg/L), and CB03608 (15 ± 3 mg/L) (Figure 1B). Heptaene production in the alternative producers was 2.3- to 4.4-fold higher than the S. globisporus wild-type strain (6.6 ± 1.7 mg/L).15,16 Interestingly, S. sp. CB02366 and CB03608 produced more C-1027 than S. sp. CB00657 and CB02329, while the latter two strains produced more heptaene than the former two. Of the five C-1027 producers, S. sp. CB03608 showed the highest ratio of C-1027 chromophore to heptaene (4.5:1), followed by CB02366 (3.7:1), CB00657(1.8:1), and CB02329 (1.7:1), with S. globisporus having the lowest ratio (0.9:1). As heptaene is a shunt product of enediyne biosynthesis,15,16 higher C-1027 to heptaene ratios would suggest that the C-1027 biosynthetic machineries in the alternative producers are more efficient in channeling the nascent enediyne biosynthetic intermediates into maturation of the C-1027 enediyne chromophore, a property that now could be considered in engineering C-1027 overproducers.
Morphological and taxonomic study of the five C-1027 producers showed that they are distinct Streptomyces strains. Close morphological examination revealed that the four C-1027 alternative producers were distinct from the original producer S. globisporus strain, in particular for their spore pigmentation and colony margin on the ISP4 agar plate (Figure 2A). S. sp. CB00657 produced white spores resembling the original C-1027 producer, while the spores were light gray for S. sp. CB02329 and CB03608 and greenish for S. sp. CB02366. Taxonomic analysis based on concatenated sequences of the selected housekeeping genes (16S rRNA gene, rpoB, and trpB) allowed us to classify these strains as Streptomyces species. Among the five producers, S. sp. CB00657 and CB03608 were the closest relatives and were closer to S. globisporus than S. sp. CB02366 and CB02329. S. sp. CB02366 was close to Streptomyces californicus, while S. sp. CB02329 resided by itself on a clade distant from the other four C-1027 producers (Figure 2B).
Figure 2.
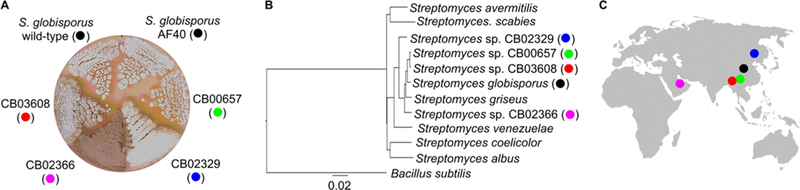
Morphology, taxonomy, and geographic distribution of the five C-1027 producers (color-coded). (A) Morphology of the four C-1027 alternative producers S. sp. CB00657, CB02329, CB02366, and CB03608 in comparison with the S. globisporus wild-type and AF40 mutant strains.(B) Taxonomic analysis of the four C-1027 alternative producers supporting their classification as Streptomyces species. The phylogenetic tree was constructed using the concatenated sequences of 16S rRNA, rpoB, and trpB of each strain, with representative Streptomyces species as controls and Micromonospora rifamycinica as an outgroup. (C) Geographic locations where the five C-1027 producers were isolated.
Geographically, the five C-1027 producers were isolated from soil samples collected at distant locations. S. globisporus was isolated in the 1980s from a soil sample collected in central China.5 The four C-1027 alternative producers were isolated in recent years, in an effort to build The Scripps Research Institute actinomycetes strain collection.3,18 S. sp. CB00657 and CB03608 were isolated from a soil sample collected in the urban area of Kunming City, 1900 m above sea level, and a soil sample collected in an unexplored primary evergreen broadleaf forest, 1400−2200 m above sea level, in Yunnan Province, respectively. S. sp. CB02329 was isolated from the fertile black soil of the Northeast Plain, 400 m above sea level, in the northeast of China, while S. sp. CB02366 was isolated from a sample collected on the beach, 10−50 m above sea level, in Dubai, the United Arab Emirates (Figure 2C). The discovery of multiple C-1027 producers from geographically distant locations and different soil types is interesting and may provide insight into the ecological functions of C-1027 in these producers.
The C-1027 biosynthetic gene clusters in the C-1027 producers are located on giant plasmids of varying sizes. Since the C-1027 BGC in S. globisporus resides on a linear plasmid of ~167 kb, SGLP1,19,20 and plasmids can facilitate the dissemination of BGC among streptomycetes, we sought to examine if the C-1027 BGCs in the alternative producers are also plasmid-born and, if so, whether these plasmids are evolutionarily related. PFGE analysis of the genomic DNAs from the five C-1027 producers, along with S. globisporus AF40,9 was undertaken to check the genetic location of the C-1027 BGCs. The S. globisporus AF40 mutant was obtained previously by treating the S. globisporus wild-type strain with acriflavine and was proposed to lose its ability to produce C-1027 by curing the SGLP1 plasmid; the latter proposal, however, has not been confirmed experimentally.9 We therefore decided to include the AF40 mutant in the PFGE analysis to confirm its genotype, thereby serving as a negative control. Mycelia of the five producers, together with the AF40 mutant, were embedded in agarose, treated with lysozyme and proteinase K, and analyzed by PFGE. No plasmid was observed in S. globisporus AF40, confirming the loss of the SGLP1 plasmid in this strain (Figure 3A). The five C-1027 producers contained plasmids with varying sizes. For S. globisporus, the size of SGLP1 was approximately 140 kb on the gel, slightly smaller than the predicted size of 167 kb,19 which could be attributed to the inconsistent migration of large DNA fragments with high G+C content in PFGE analysis.21 Among the four alternative producers, S. sp. CB02366 contained two plasmids with sizes of ~90 and ~250 kb, respectively, while S. sp. CB00657, CB02329, and CB03608 each contained a giant plasmid, with sizes ranging from ~95 to ~220 kb (Figure 3A). Since the SGLP1 plasmid in S. globisporus was found to be a linear plasmid,19,20 the four alternative producers were investigated under the standard conditions for the presence of linear plasmids.22 However, under PFGE analysis without the treatment of proteinase K, the genomic DNA degraded rapidly, thereby forfeiting our attempt to determine if the giant plasmids in the alternative producers are circular or linear.
Figure 3.
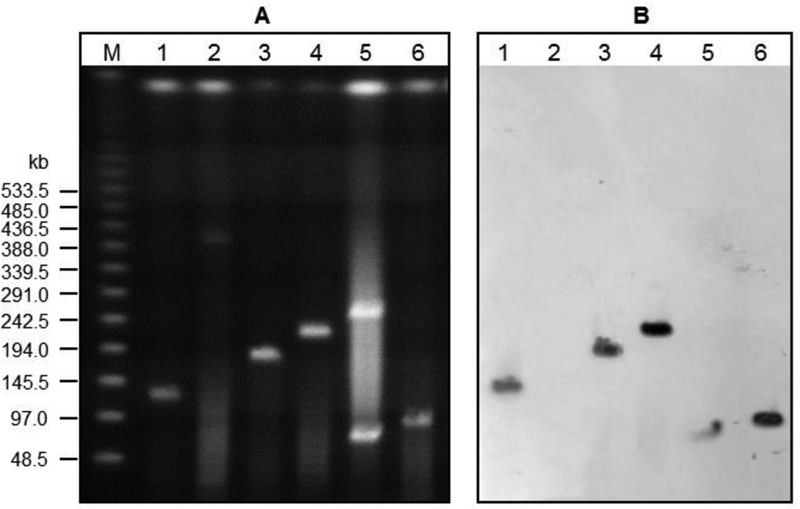
C-1027 BGCs residing on giant plasmids of varying size in the five C-1027 producers. (A) PFGE analysis of the five C-1027 producers showing the existence of giant plasmids of varying sizes. (B) Southern analysis revealing that the C-1027 BGCs all reside on giant plasmids in the five producers. M, Lambda PFG ladder (NEB); lane 1,S. globisporus wild-type; lane 2, S. globisporus AF40; lane 3, CB00657; lane 4, CB02329; lane 5, CB02366; and lane 6, CB03608.
Southern analysis showed that the C-1027 BGCs in all five producers are located on giant plasmids. Alignment of the 1-kb E5/pksE region and the cagA genes in the C-1027 BGCs revealed >92% nucleotide sequence identities (Figure S5). The high sequence similarities among the C-1027 producers allowed the design of probes to localize the C-1027 BGCs by Southern analysis. Thus, a 600 bp probe within the E5/pksE region was chosen and amplified by polymerase chain reaction (PCR) with genomic DNA of S. globisporus as a template.23 Upon Southern analysis, specific hybridizations to giant plasmids were observed, revealing that the C-1027 BGCs are located on giant plasmids; no hybridization was detected to the chromosomes (Figure 3B). For S. sp. CB02366, hybridization was only seen with the smaller plasmid, and therefore the C-1027 BGC must have resided on the 90 kb plasmid.
Pathway-specific regulatory genes within the C-1027 BGCs present opportunities to further improve C-1027 titers in the alternative producers. It has been shown that C-1027 production in the original producer S. globisporus can be improved by manipulating the pathway-specific regulators sgcR and sgcR1.15,16 In the absence of complete sequence for the C-1027 BGCs in S. sp. CB00657, CB02329, and CB03608, we amplified the sgcR and sgcR1 homologues in these strains by PCR using primers based on consensus sequences in the C-1027 BGCs from S. globisporus and S. sp. CB02366 and sequenced the resultant products. The SgcR and SgcR1 regulators showed 94% to 99% and 93% to 99% amino acid identity, respectively, among the five C-1027 producers (Figure S6), suggesting that they most likely have the same functions in the alternative producers as those in S. globisporus. As inactivation of the sgcR repressor gene in S. globisporus wild-type resulted in about a 3-fold increase in C-1027 production in the ΔsgcR mutant strain S. globisporus SB1022, and over-expression of the sgcR1 activator gene in the SB1022 mutant yielded the recombinant strain SB1023, which further improved the C-1027 production by 7-fold in comparison with the wild-type,15,16 it is very tempting to speculate that C-1027 production in the alternative producers could be further improved by applying similar strategies.
The C-1027 BGCs from the five producers most likely evolved from a common ancestor. Genome sequencing showed that the C-1027 BGCs from S. globisporus and S. sp. CB02366 are highly homologous. A pairwise alignment of the entire C-1027 BGCs from S. globisporus and S. sp. CB02366 revealed 93% identity between these two loci. Alignment of the cagA genes and the partial internal sequences of pksEs showed high identities (above 92%) among the five C-1027 producers (Figure S5). As the enediyne BGCs from strains located on the same clade are highly similar in both the nucleotide sequences and the genetic organization, as exemplified by the C-1027 BGCs from S. globisporus and S. sp. CB02366,3 we speculate that the C-1027 BGCs in all five alternative producers have similar genetic organization. The latter would suggest that the C-1027 BGCs from the five producers most likely evolved from a common ancestor. As the five producers are taxonomically distinct, the C-1027 BGCs could be acquired via horizontal transfer of the giant plasmids among different Streptomyces hosts, which have been observed in many secondary metabolite BGCs.20 After acquiring the C-1027 BGC, the plasmid could further evolve by insertion or deletion of small fragments between the biosynthetic genes, mutation of nucleotides in the open reading frames, as exemplified by Figure S7, or interaction with the linear chromosomes of their hosts,24,25 leading to varying sizes of the plasmids observed in five producers in this study.
Natural products have been an invaluable source of many clinically useful therapeutic agents. In the past few decades, manipulation of natural product biosynthetic machinery has offered great opportunities to activate cryptic pathways, improve the natural product titers, and generate novel natural product analogues.26 As many microbial strains are not amenable to standard genetic approaches, the discovery of alternative producers with better genetic amenability will provide a solution to circumvent the challenges in the original producer. Our recently developed strain prioritization method allows us to quickly survey producers of targeted metabolites from large strain collection.3,27,28 In the current study, genome mining of the TSRI actinomycetes strain collection led to the discovery of four C-1027 alternative producers, with C-1027 titers 11-fold higher (up to ~900 mg/L for the chromoprotein complex and ~67 mg/L for the C-1027 enediyne chromophore) than the original producer. Since further improvement of C-1027 titer by manipulation of a pathway-specific regulator has been demonstrated in S. globisporus,15,16 C-1027 production in the alternative producers may be further increased using the same strategy. Engineered strains with high C-1027 titers will not only facilitate the supply of C-1027 for current preclinical studies, future clinical trials, and eventual commercialization,8,14 but also advance the biosynthetic studies of enediyne natural products, as well as engineered production of designer C-1027 analogues with improved pharmacokinetic properties or new modes of action.11–13
The finding that the C-1027 BGCs all reside on giant plasmids of varying size in the five producers also provides a rare opportunity to dissect the intricate cross-talks between BGCs and their microbial hosts. The five C-1027 producers were isolated from geographically distant regions and are taxonomically distinct, but possess high sequence similarities in their C-1027 BGCs. Regulation of C-1027 biosynthesis in S. globisporus has been studied previously;15,16 however, interaction of the pathway-specific regulators with the regulators from the Streptomyces hosts remains elusive. As the giant plasmids harboring the C-1027 BGCs can be cured by chemical mutagenesis9 or be mobilized between different hosts,20,24,25 introduction of the same C-1027 BGC-containing giant plasmid into various hosts, upon curing the endogenous C-1027 BGC-containing plasmids, or introduction of various C-1027 BGC-containing giant plasmids into the same host will generate a combinatorial library of recombinant strains. Comparative studies of C-1027 production in these recombinant strains promise to reveal new insight into how microbial hosts influence the production of BGC-encoded natural products. These studies could further inspire the development of new strategies to improve the production of clinically important natural products or to activate cryptic BGCs for the discovery of new natural products.
■EXPERIMENTAL SECTION
General Experimental Procedures.
HRESIMS data were obtained on an Agilent 6230 TOF LC/MS equipped with a Poroshell 120 EC-C18 column (Agilent, 50 mm × 4.6 mm, 2.7 μm). HPLC was performed on a Varian semipreparative HPLC system (Woburn, MA, USA). All fermentations were carried out in Innova 44 incubator shakers (New Brunswick Scientific). Pulsed-field gel electrophoresis was conducted on the CHEF-DR III pulsed field electrophoresis systems (BioRad). Protein purification was performed using anÄKTA FPLC system (GE Healthcare Biosciences). DEAE-Cellulose resin was purchased from Sigma-Aldrich. PCR primers were synthesized by Sigma-Aldrich. Q5 high-fidelity DNA polymerase from NEB was used for PCR reaction. The DNA gel extraction kit was purchased from Omega Bio-Tek. DNA sequencing was carried out by Eton Bioscience.
Bacterial Strains.
The Streptomyces strains (Table S1) were grown at 28 °C on ISP4 agar medium to obtain spores and to compare their morphology.22 For the genomic DNA isolation and seed culture preparation, the Streptomyces strains were cultured in 250 mL baffled flasks containing 50 mL of tryptic soy broth (TSB) liquid medium and cultivated at 28 °C and 250 rpm for 36 to 48 h. Genomic DNA was isolated using standard protocols.22 Kocuria rhizophila ATCC 934129 (Table S1) was cultured in Luria−Bertani (LB) broth and used for the antimicrobial assay and bioactivity-guided purification of C-1027.8,9,15,16
DNA Sequencing and Phylogenetic Analysis.
Partial fragments of pksE, 16S rRNA, rpoB, and trpB of the four C-1027 alternative producers (S. sp. CB00657, CB02329, CB02366, and CB03608) were amplified by PCR with primers summarized in Table S2 and sequenced. All DNA sequences were deposited in the NCBI database for strains S. sp. CB00657, CB03608, CB02329, and CB02366, respectively, with the following accession codes: pksE, KT736389, KT736385, KT736327, KT736331; 16S rRNA, KT722843− KT722846; rpoB, KT736406−KT736409; trpB, KT793915, KT793901, KT793863, KT793837. For other genes in S. sp. CB00657, CB03608, and CB02329: cagA: MG544843, MG544842, MG544844; sgcR: MG544836, MG544838, MG544837; sgcR1: MG544839, MG544841, MG544840. Phylogenetic analysis of the concatenated gene sequences was conducted with MEGA 6.0 using the neighbor-joining method in the Jukes−Cantor model (based on 1000 bootstrap replications),30 with concatenated sequences of 16S rRNA, rpoB, and trpB from representative Streptomyces strains as controls and Micromonospora rifamycinica as an outgroup.31
Production and Analysis of C-1027 and Heptaene.
The C-1027-producing strains were cultured in A9 medium using a two-step fermentation procedure as previously described.8,15,16 After fermentation, the fermentation broth was adjusted to pH 4.0 using 0.1 M HCl and centrifuged to remove precipitates. The supernatant was saturated to 85% with (NH4)2SO4 and incubated at 4 °C for 4 h. The precipitated proteins were then collected by centrifugation, dissolved in 0.1 M potassium phosphate, pH 8.0, and extracted with ethyl acetate to isolate the C-1027 chromophore. The extract was concentrated under vacuum and redissolved in methanol for HPLC analysis, which was carried out on a Varian HPLC system equipped with a Prostar 330 PDA detector. Analysis of C-1027 production, using a Beckman ODS column (5 μm, 150 × 4.6 mm, Ultrasphere),3 and of heptaene production, using a C18 column (5 μm, 250 mm × 4.6 mm, Alltech),15,16 followed previously described HPLC programs. The identity of the C-1027 chromophore was confirmed by HPLC analysis in comparison with an authentic standard and by HRESIMS analysis. For purification of the C-1027 chromoprotein, as a standard for titer determination, a previously described protocol was followed.8,15,16
PFGE Analysis of the Giant Plasmids.
PFGE analysis of the giant plasmids was performed according to standard procedures with minor modifications.22 Spores of the Streptomyces strains were collected from ISP4 agar plates and inoculated into 250 mL baffled flasks containing 50 mL of yeast extract−malt extract (YEME) liquid medium (S. sp. CB02366 was cultured in TSB liquid medium due to the poor growth in the YEME liquid medium). The mycelia were harvested and suspended in 25 mM Tris-HCl buffer, pH 8.0, containing 25 mM ethylenediaminetetraacetic acid (EDTA) and 0.3 M sucrose, to OD600 = 3.0. After embedding the mycelia in low melting point agarose, the blocks were incubated at 37 °C for 90 min in lysozyme solution (40 min for S. sp. CB02366). After treatment with proteinase K solution and the wash steps, the blocks were loaded into the 1% agarose gel containing 100 μM thiourea. PFGE was performed on a CHEF-DR III chamber (BioRad) in 0.5× Tris/borate/EDTA buffer containing 100 μM thiourea. Lambda PFG Ladder (New England Biolabs) was used as the size standard. Electrophoresis was run at 4.5 V/cm using a ramped switch time from 5 to 120 s for 48 h at 15 °C. After electrophoresis, the gel was stained in 400 mL of deionized water containing 20 μL of GelRed nucleic acid gel stain for 2−16 h (the staining step was omitted for Southern analysis). Southern analysis for the C-1027 BGCs was performed with the DIG Easy hybridization kit from Roche according to the manufacturer’s instructions. A 600 bp E5-pksE fragment, amplified by PCR using the primers C1027southernprobeF and C1027southernprobeR (Table S2), was used as a probe.
Pairwise Alignment of the C-1027 Biosynthetic Gene Clusters in S. globisporus and S. sp. CB02366.
Pairwise alignment of the C-1027 biosynthetic gene clusters was performed using BioEdit7.2.5 and displayed in the graphic view.32 Deletion or insertion of nucleotides in the DNA sequences was manually annotated in Microsoft Word.
Supplementary Material
■ACKNOWLEDGMENTS
We thank Dr. Y. Li, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences, Beijing, China, for the S. globisporus wild-type and AF40 mutant strains. I.C. and S.F. were supported in part by a German Research Foundation postdoctoral fellowship and a postdoctoral fellowship from Tianjin University of Traditional Chinese Medicine. This work was supported in part by NIH grants GM115575 and CA204484. This is manuscript #29624 from The Scripps Research Institute.
Footnotes
■ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jnat-prod.7b01013.
Additional tables and figures (PDF)
The authors declare no competing financial interest.
■REFERENCES
- (1).Van Lanen SG; Shen B Curr. Top. Med. Chem 2008, 8, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Smith AL; Nicolaou KC J. Med. Chem 1996, 39, 2103–2117. [DOI] [PubMed] [Google Scholar]
- (3).Yan X; Ge H; Huang T; Hindra; Yang D; Teng Q; Crnovcic I; Li X; Rudolf JD; Lohman JR; Gansemans Y; Zhu X; Huang Y; Zhao L-X; Jiang Y; Van Nieuwerburgh F; Rader C; Duan Y; Shen B mBio 2016, 7, e02104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yan X; Chen JJ; Adhikari A; Yang D; Crnovcic I; Wang N; Chang CY; Rader C; Shen B Org. Lett 2017, 19, 6192–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hu JL; Xue YC; Xie MY; Zhang R; Otani T; Minami Y; Yamada Y; Marunaka TJ Antibiot 1988, 41, 1575–1579. [DOI] [PubMed] [Google Scholar]
- (6).Zhen YS; Ming XY; Yu B; Otani T; Saito H; Yamada YJ Antibiot 1989, 42, 1294–1298. [DOI] [PubMed] [Google Scholar]
- (7).Yoshida K; Minami Y; Azuma R; Saeki M; Otani T Tetrahedron Lett 1993, 34, 2637–2640. [Google Scholar]
- (8).Li W; Li X; Huang T; Teng Q; Crnovcic I; Rader C; Shen B Bioorg. Med. Chem 2016, 24, 3887–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Liu W; Shen B Antimicrob. Agents Chemother 2000, 44, 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Liu W; Christenson SD; Standage S; Shen B Science 2002, 297, 1170–1173. [DOI] [PubMed] [Google Scholar]
- (11).Kennedy DR; Gawron LS; Ju J; Liu W; Shen B; Beerman TA Cancer Res 2007, 67, 773–781. [DOI] [PubMed] [Google Scholar]
- (12).Kennedy DR; Ju J; Shen B; Beerman TA Proc. Natl. Acad. Sci. U. S. A 2007, 104, 17632–17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Beerman TA; Gawron LS; Shin S; Shen B; McHugh MM Cancer Res 2009, 69, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Chen Y; Yu D; Zhang C; Shang B; He H; Chen J; Zhang H; Zhao W; Wang Z; Xu X; Zhen Y; Shao RG Mol. Carcinog 2015, 54, 123–131. [DOI] [PubMed] [Google Scholar]
- (15).Chen Y; Yin M; Horsman GP; Huang S; Shen BJ Antibiot 2010, 63, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chen Y; Yin M; Horsman GP; Shen BJ Nat. Prod 2011, 74, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Krug D; Müller R Nat. Prod. Rep 2014, 31, 768–783. [DOI] [PubMed] [Google Scholar]
- (18).Xie P; Ma M; Rateb ME; Shaaban KA; Yu Z; Huang SX; Zhao LX; Zhu X; Yan Y; Peterson RM; Lohman JR; Yang D; Yin M; Rudolf JD; Jiang Y; Duan Y; Shen BJ Nat. Prod 2014, 77, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Li X; Lei X; Zhang C; Jiang Z; Shi Y; Wang S; Wang L; Hong BJ Biotechnol 2016, 222, 9–10. [DOI] [PubMed] [Google Scholar]
- (20).Kinashi HJ Antibiot 2011, 64, 19–25. [DOI] [PubMed] [Google Scholar]
- (21).Gravius B; Cullum J; Hranueli D Biotechniques 1994, 16, 52. [PubMed] [Google Scholar]
- (22).Kieser T; Bibb MJ; Buttner MJ; Chater KF; Hopwood DA Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- (23).Liu W; Ahlert J; Gao Q; Wendt-Pienkowski E; Shen B; Thorson JS Proc. Natl. Acad. Sci. U. S. A 2003, 100, 11959–11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Yamasaki M; Kinashi HJ Bacteriol 2004, 186, 6553–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Chater KF; Kinashi H Streptomyces Linear Plasmids: Their Discovery, Functions, Interactions with Other Replicons, and Evolutionary Significance In Microbial Linear Plasmids; Meinhardt F; Klassen R, Eds.; Springer: Berlin, Heidelberg, 2007; pp 1–31. [Google Scholar]
- (26).Katz L; Baltz RH J. Ind. Microbiol. Biotechnol 2016, 43, 155–176. [DOI] [PubMed] [Google Scholar]
- (27).Hindra; Huang T; Yang D; Rudolf JD; Xie P; Xie G; Teng Q; Lohman JR; Zhu X; Huang Y; Zhao LX; Jiang Y; Duan Y; Shen B J. Nat. Prod 2014, 77, 2296–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Pan G; Xu Z; Guo Z; Hindra; Ma M; Yang D; Zhou H; Gansemans Y; Zhu X; Huang Y; Zhao L-X; Jiang Y; Cheng J; Van Nieuwerburgh F; Suh J-W; Duan Y; Shen B Proc. Natl. Acad. Sci. U. S. A 2017, 114, E1113110.1073/pnas.1716245115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Tang JS; Gillevet PM Int. J. Syst. Evol. Microbiol 2003, 53, 995–997. [DOI] [PubMed] [Google Scholar]
- (30).Tamura K; Stecher G; Peterson D; Filipski A; Kumar S Mol. Biol. Evol 2013, 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Huang H; Lv J; Hu Y; Fang Z; Zhang K; Bao S Int. J. Syst. Evol. Microbiol 2008, 58, 17–20. [DOI] [PubMed] [Google Scholar]
- (32).Hall TA Nucl. Acids. Symp. Ser 1999, 41, 95–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


