Abstract
Tobacco use is the leading preventable cause of death worldwide and a major risk factor for cardiovascular disease (CVD). Both prevention of smoking initiation among youth and smoking cessation among established smokers are key for reducing smoking prevalence and the associated negative health consequences. Proven tobacco cessation treatment includes pharmacotherapy and behavioral support, which are most effective when provided together. First-line medications (varenicline, bupropion, and nicotine replacement) are effective and safe for patients with CVD. Clinicians who care for patients with CVD should give as high a priority to treating tobacco use as to managing other CVD risk factors. Broader tobacco control efforts to raise tobacco taxes, adopt smoke-free laws, conduct mass media campaigns, and restrict tobacco marketing enhance clinicians’ actions working with individual smokers.
Keywords: smoking, nicotine, tobacco use disorder, smoking prevention, smoking cessation
Condensed abstract:
Tobacco use is the leading preventable cause of death worldwide and a major risk factor for cardiovascular disease. Both prevention of smoking initiation among youth and smoking cessation among established smokers are key for reducing smoking prevalence and the associated negative health consequences. Clinicians who care for patients with CVD should give as high a priority to treating tobacco use as they do to managing other CVD risk factors. Broader tobacco control policy efforts to raise tobacco taxes, adopt smoke-free laws, conduct mass media campaigns, and restrict tobacco marketing serve to enhance clinicians’ actions working with individual smokers.
Introduction
Tobacco use causes over 6 million annual deaths globally and is the leading preventable cause of death worldwide (1). Over 480,000 individuals die from cigarette smoking or secondhand tobacco smoke exposure annually in the United States (2), and a smoker’s life expectancy is at least 10 years shorter than a nonsmoker’s (3). Each year, >150,000 U.S. adults age 35 and older die from smoking-related cardiovascular diseases (CVD) (4), making smoking responsible for about 20% of CVD deaths in this population. The magnitude of the risk attributable to tobacco use provides a compelling reason why clinicians who care for patients with CVD need to put as high a priority on addressing tobacco use as they do on managing other cardiovascular risk factors such as hypertension or hyperlipidemia.
Worldwide, >1 billion individuals use tobacco products, but the prevalence varies considerably by gender and geography.(1) The 2015 Global Burden of Disease Study, representing 195 countries and territories, estimated that 25% of men and 5.4% of women worldwide smoked daily.(1) Although tobacco use was previously more common in high-income countries, the burden has now shifted to low and middle-income countries, where an estimated 80% of today’s smokers live.(5) In the U.S., adult cigarette consumption has been declining since the 1960s when the health consequences of tobacco use first became widely known, launching a variety of tobacco control policies by federal, state, and local governments (Figure 1).(4) Combustible cigarettes are the most common tobacco product used by U.S. adults.(6) In 2016, 15.5% of adults reported currently smoking cigarettes; of these, 76% smoked daily and 24% smoked less often than daily.(7) Smoking prevalence is disproportionately higher among adults who have less education, lower incomes, and comorbid psychiatric and other substance use disorders.(7,8) Cigarette smoking rates among adolescents are currently at their lowest level in decades, with 8.0% of high school students and 2.2% of middle school students in 2016 reporting cigarette smoking in the past 30 days, the definition for current smoking among adolescents.(9)
Figure 1. Per capita cigarette consumption per year among U.S. adults from 1900 to 2012.
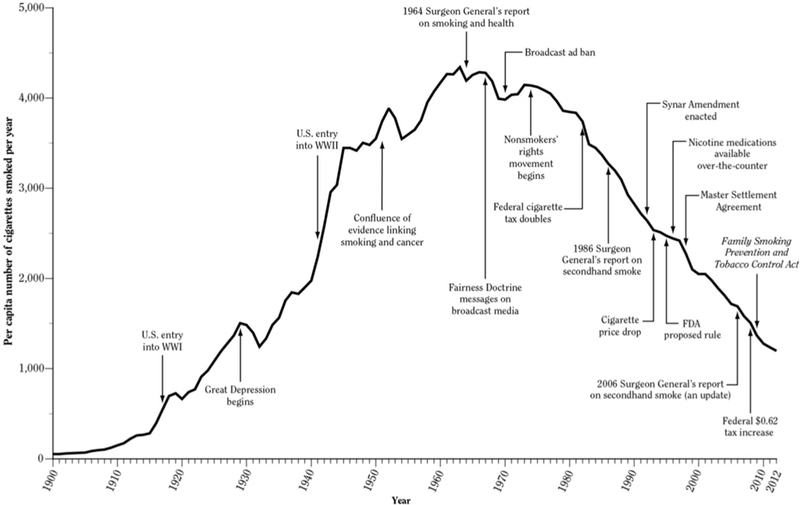
Cigarette consumption has been declining since the 1960s, during which time tobacco control policies have been adopted at local and national levels. From: US Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department ofHealth and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2014.
Increasingly, cigarette smokers are using more than one type of tobacco product. In the 2013–2014 Population Assessment of Tobacco and Health survey, 40% of U.S. adolescent and adult tobacco users reported using multiple products (10). These include cigars, cigarillos, pipes, waterpipes (also called hookah or shisha), and smokeless tobacco. However, the most common non-cigarette tobacco product used by both youths and adults in the U.S. is the electronic cigarette (e-cigarette) (10). This product is a battery-powered device that heats a liquid, usually containing nicotine, to create an aerosol that the user inhales. It is the first of a new category of alternative tobacco products that aim to reduce the health risks of cigarettes by not burning tobacco to produce smoke. In 2015, approximately 3.5% of U.S. adults reported past-30-day e-cigarette use (6), while 11.3% of high school students and 4.3% of middle school students reported using e-cigarettes in the past 30 days (9).
An even newer alternative tobacco product is the heat-not-burn device which creates an aerosol by heating tobacco without burning it (11). It differs from e-cigarettes in heating tobacco, while e-cigarettes heat a solution containing nicotine, a humectant like propylene glycol or vegetable glycerin, and flavoring. Heat-not-burn products are already marketed in 30 countries, but are not yet sold in the U.S.(11).
Pathophysiological Effects of Tobacco Smoking on the Cardiovascular System
Epidemiologic risks of tobacco use for cardiovascular disease
Cigarette smoking is a major risk factor for many cardiovascular diseases. Current smokers have significantly higher odds of myocardial infarction (MI) (OR 2.87, 95% CI 2.58–3.19),(12) and a two- to three-fold higher risk of death from ischemic heart disease than never smokers.(13) Smokers with known coronary artery disease are also at higher risk of sudden cardiac death compared to never smokers (hazard ratio 2.47, 95% CI 1.46–4.19).(14) Smoking approximately doubles the risk of death from stroke for current smokers compared to never smokers.(13)
The association between smoking and CVD is non-linear such that even a few cigarettes a day disproportionately increases cardiovascular risk.(15) Smoking just one cigarette daily is also associated with a higher stroke risk.(15) These data indicate that there is no safe level of cigarette consumption, and that the goal of treatment should be complete cigarette abstinence.
Smoking acutely raises blood pressure, but epidemiological studies have generally not shown an association between smoking and hypertension.(16,17) Smoking has been associated with an increased risk of complications from hypertension, such as increased risk of death from hypertensive heart disease(18) and decline in renal function.(19)
Current smokers have two- to three-fold higher odds of peripheral artery disease (PAD) compared with nonsmokers,(20) and greater progression of arterial stiffness over 5–6 years.(21) In one study, smokers were more likely than nonsmokers with PAD to be hospitalized over one year and were more likely to be hospitalized for acute MI and coronary heart disease (CHD).(22) Smokers also have an increased risk of abdominal aortic aneurysm (AAA).(23) More years of smoking and being a current versus former smoker have been associated with higher odds of AAA.(23) Current smoking was associated with a faster aneurysm growth rate compared to former or never smoking in a 2012 meta-analysis.(24)
The incidence of atrial fibrillation is approximately 1.5 and 2 times higher in former and current smokers, respectively, compared to never smokers.(25) Ventricular arrhythmia risk is also higher among smokers.(26) Smokers have higher risk of heart failure and poorer outcomes compared to never smokers. Among adults aged 70–79 participating in the Healthy Aging and Body Composition Study, the risk of heart failure was higher for current smokers compared to never smokers.(27) The Studies of Left Ventricular Dysfunction study, which included patients with a left ventricular ejection fraction below 35%, found a higher relative risk of all-cause mortality and congestive heart failure(CHF)-related mortality among current smokers than current non-smokers.(28) That study also found higher risk of hospitalization for CHF and higher risk of MI among current smokers compared to non-smokers.
Individuals who continue to smoke after a cardiovascular event or revascularization have poorer outcomes, highlighting the urgency of providing tobacco use treatment in these settings. After MI, continuing smokers have higher risk of recurrent coronary events than smokers who quit (RR 1.51, 95% CI 1.10–2.07).(29) Continued smoking after a stroke or transient ischemic attack, compared to quitting smoking, increases the 5-year risk of another stroke, MI, or death.(30) Continued smoking after an intervention for coronary artery disease also leads to negative outcomes. Among 985 coronary artery bypass graft surgery (CABG) patients followed for a median of 20 years, continuing smokers had a greater risk of all-cause mortality (RR 1.68, 95% CI 1.33–2.13) and cardiac death (1.75, 1.30–2.37) than smokers who quit.(31) Continuing smokers were also more likely than those who quit to require another procedure (e.g. CABG or angioplasty). Among 5450 smokers followed for up to 16 years after successful percutaneous revascularization, persistent smoking was associated with increased risk of all-cause mortality (RR 1.76, 95% CI 1.37–2.26) and Q-wave MI (2.08, 1.16–3.72) compared to never smoking (32).
Secondhand tobacco smoke exposure
Secondhand tobacco smoke exposure (TSE) by nonsmokers is associated with an increased risk of both CHD and stroke. In a meta-analysis, the relative risk for CHD among nonsmokers with TSE compared to nonsmokers without TSE was 1.31 (95% CI 1.21–1.41).(33) In another meta-analysis, TSE by nonsmokers was associated with increased risk of stroke (RR 1.25, 95% CI 1.12–1.38) (34).
Smokeless tobacco
While smokeless tobacco has the advantage of avoiding exposure to the products of combustion, it exposes users to varying amounts of nicotine and carcinogenic tobacco-specific nitrosamines and metals.(35) Most epidemiologic data on the CVD risk of smokeless tobacco comes from Sweden where a substantial proportion of men use snus, a smokeless tobacco product lower in nitrosamines and other contaminants than most other smokeless tobacco products. Most studies have not found an increased risk of non-fatal CVD among smokeless tobacco users.(36) However, a 2009 meta-analysis using data from Sweden and the U.S. found that smokeless tobacco use was associated with increased risk for both fatal MI (RR 1.13, 95% CI 1.06–1.21) and stroke (1.40, 1.28–1.54).(37) Following MI, continuing snus users have a substantially higher two-year mortality compared to those who quit.(38) In the 52-country INTERHEART case-control study, chewing tobacco use was associated with increased odds of acute MI compared to never tobacco use,(39) and individuals who used both cigarettes and chewing tobacco had higher odds of acute MI than users of either product alone.
Pathophysiologic mechanisms of increased risk
Cigarette smoke contains over 7,000 toxic chemicals, including several components implicated in CVD and 69 known carcinogens.(4) The constituents of tobacco smoke contribute to CVD via multiple mechanisms, including adverse effects on hemodynamics, endothelial dysfunction, thrombosis, inflammation, lipid abnormalities, and arrhythmogenesis (Figure 2, Table 1). While nicotine is the primary constituent of cigarette smoke that causes addiction and produces the hemodynamic effects associated with smoking, most of the excess cardiovascular risk of smoking is attributable to the effect of other cigarette smoke constituents. The free radicals and reactive oxygen species from cigarette smoke cause endothelial dysfunction, platelet activation, and promote atherosclerosis through oxidization of low density-lipoprotein (LDL).(40) Particulate matter also causes oxidative stress, endothelial dysfunction, and platelet activation, and has effects on the autonomic nervous system.(41) These effects can be observed even after exposure to secondhand smoke.(33,42) Other chemicals in cigarette smoke that may contribute to CVD include carbon monoxide, carbonyls (such as acrolein), polycyclic aromatic hydrocarbons, and metals (43).
Figure 2. Mechanisms by which smoking causes cardiovascular disease.
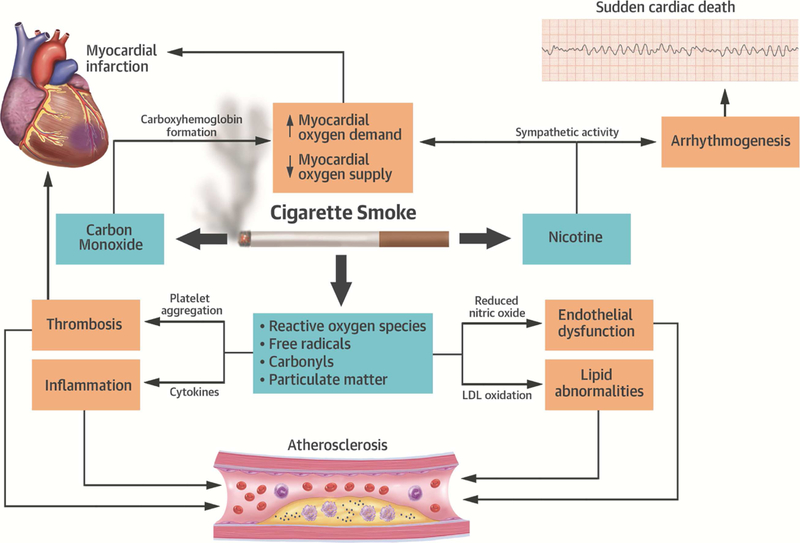
The major components of cigarette smoke that contribute to cardiovascular disease include nicotine, carbon monoxide, reactive oxygen species, free radicals, carbonyls (such as acrolein), and particulate matter.
Table 1.
Mechanisms of increased cardiovascular risk associated with cigarette smoking
| Cardiovascular effect | Pathophysiological mechanism |
|---|---|
|
Hemodynamic changes • ⬆ heart rate • ⬆ contractility • ⬆ blood pressure • ⬆ myocardial oxygen demand • ⬇ myocardial oxygen supply • Altered coronary blood flow |
• Nicotine activates the sympathetic nervous system(44,73) • In general, nicotine ⬆ coronary blood flow in response to increased myocardial work • Smoking leads to vasoconstriction in individuals with coronary artery disease(150) • Reduced oxygen delivery by red blood cells containing carboxyhemoglobin (from carbon monoxide in cigarette smoke)(44) |
| Endothelial dysfunction and damage | • Occurs as a consequence of oxidative injury and chronic inflammation • ⬇ availability of nitric oxide, which normally acts as an endothelial cell vasodilator, reduces leukocyte adhesion, and prevents platelet aggregation(151) |
| Hypercoagulability and thrombosis | • ⬆ platelet activation • ⬇ availability of nitric oxide • ⬆ blood viscosity due to polycythemia from chronic carbon monoxide exposure and relative hypoxia • ⬆ plasminogen activator inhibitor type 1(152) • ⬇ tissue plasminogen activator (tPA) release from the coronary vasculature(153) • ⬆ expression of tissue factor in atherosclerotic plaques(154) |
| Chronic inflammation | • Enhanced leukocyte adhesion to vascular endothelium(155) • ⬆ levels of inflammatory cytokines such as interleukin-6,(156) interleukin-1b, and tumor necrosis factor-alpha(157) • Smokers have higher levels of the inflammatory markers high-sensitivity C-reactive protein and fibrinogen(158) |
| Lipid abnormalities | • ⬇ high density lipoprotein • ⬆ low density lipoprotein and triglycerides(159) • ⬆ levels of oxidized low-density lipoprotein(160) |
| Arrhythmogenesis | • Nicotine may promote myocardial remodeling and fibrosis,(161) likely related to beta- adrenergic stimulation • Nicotine-medicated catecholamine release may contribute to ventricular arrhythmia and sudden cardiac death |
| Other | • ⬆risk of developing type 2 diabetes(162) likely due to greater insulin resistance among smokers than nonsmokers,(163) and believed to be mediated by the sympathomimetic effects of nicotine and nicotine-mediated activation of AMP-activated protein kinase(164) • ⬆ risk of coronary spasm(165) |
Taken together, the effects of cigarette smoke contribute to an environment that promotes plaque formation and thrombosis, produces an imbalance between myocardial blood supply and demand, and increases the likelihood of arrhythmogenesis, increasing the risk of cardiovascular events. Additionally, cigarette smoking appears to interact with genetic factors underlying CVD risk.(44) For example, the cardio-protective effects of a variation in a gene locus near the ADAMTS7 gene were diminished among those who smoked compared to never smokers.(45) Further studies on genetic and environmental factors may better characterize mechanisms of risk and identify individuals at higher risk for smoking-related CVD.
Factors Associated with Smoking Initiation and Cessation
Natural history of tobacco use
Tobacco use can be characterized as a chronic disease that begins in childhood because nearly all cigarette smokers report that they smoked their first cigarette during adolescence and then continue to use tobacco for decades.(46) Over 80% of U.S. adults who report ever trying a cigarette did so by age 18, and 98% did so by age 26.(47) Additionally, 96% of adults who ever smoked cigarettes daily report starting to smoke daily by age 26.(47) Initially, adolescents experiment with an occasional cigarette, and a subset of these individuals progress over varying periods of time to regular cigarette smoking. Once smoking becomes an established behavior, reducing the risk of tobacco-related disease requires smoking cessation. Promoting cessation is the primary task of clinicians who care for adults.
Quitting smoking is a dynamic process characterized by periods of regular smoking, reduced smoking, and no smoking.(48) Most smokers make numerous attempts to quit before they achieve success.(49) Among adult smokers responding to the 2015 National Health Interview Survey, 68% reported that they wanted to stop smoking and 55% had made a quit attempt in the past year, but only 7% had succeeded in quitting smoking.(50) However, most smokers continue to try to quit and eventually many of them succeed, because 59% of all living adults in the U.S. who have ever smoked have quit smoking as of 2016.(7) One reason for the low success rate is that despite the availability of effective evidence-based treatments for quitting smoking (51), most smokers (69%) do not use them in their attempts to quit (50).
Nicotine dependence
Tobacco use is tenacious because it is both a physical dependence on nicotine and a deeply-ingrained learned behavior. Nicotine binds to nicotinic acetylcholine receptors in the brain, leading to the release of neurotransmitters with rewarding and reinforcing effects.
Repeated use leads to the upregulation of nicotine receptors, tolerance, and physical dependence (4). The symptoms of nicotine dependence can develop rapidly, appearing after adolescents have only smoked a few cigarettes for a short period of time and well before adolescents have transitioned from initial experimentation to becoming regular daily cigarette smokers.(52) Nicotine dependence symptoms, in turn, are associated with future increases in cigarette consumption,(53) suggesting a bidirectional association between nicotine dependence and cigarette consumption. Stopping smoking is associated with nicotine withdrawal symptoms that include cravings, irritability, anxiety, restlessness, dysphoria, impaired concentration, and hunger.(54) Specific symptoms and the duration of withdrawal vary among smokers; while some smokers’ symptoms resolve within 2 weeks, others experience cravings even after 6 months of smoking abstinence (55).
The learned behavior of smoking derives from its association with specific triggers that prompt craving to smoke and contribute to repeated use. Triggers can include smoking in personal or social circumstances, drinking coffee or alcohol, seeing other smokers, or even seeing objects such as cigarette packs or advertisements. Many smokers report smoking in response to stress or other negative emotional states, and quitting smoking is particularly difficult for such smokers. This behavior is likely due both to the positive effects of nicotine and to the fact that smoking reverses the dysphoria and cognitive impairment induced by nicotine withdrawal in addicted smokers.
Smoking initiation
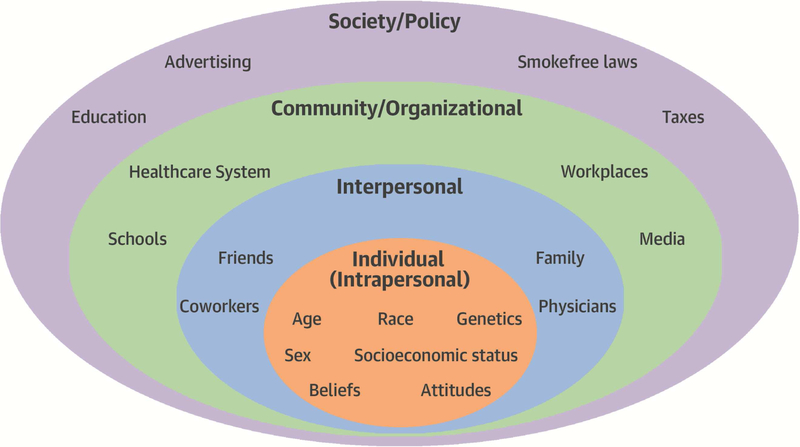
Multiple factors influence both the initiation and maintenance of tobacco use. The socioecological model provides a framework for categorizing these factors into levels of influence organized into an ascending order of intra-individual, interpersonal, community/organizational, and societal/policy levels (Central Illustration) (56).
Central Illustration. Socio-ecological model of factors associated with smoking initiation and continued smoking.
Various individual, interpersonal, community/organizations, and societal/policy factors play a role in why individuals start smoking and continue to smoke. Interventions targeting each of these factors can help to reduce smoking prevalence.
At the individual level, smoking among adolescents is more common in non-Hispanic whites than blacks or Hispanics and among adolescents with lower socioeconomic status as measured by their educational goals or parental educational attainment (47). Psychological factors associated with smoking initiation among adolescents and young adults include greater risk taking and impulsivity (46), stress (57), depressive symptoms (58), and anxiety (59). Genetic factors also influence both smoking initiation and development of nicotine dependence, and appear to play a larger role in the latter (60).
At the interpersonal level, the strongest effect on youth smoking is the influence of peers. Adolescents who smoke are often friends with other smokers, and those who do not smoke but have friends who smoke are more likely to start smoking.(61) Perceived prevalence of smoking among peers has also been associated with subsequent smoking among adolescents. A study of eighth graders found that nonsmokers who estimated that a higher percentage of eighth graders at their school were smokers were more likely to be smoking one year later.(62) Evidence for family influences on adolescent smoking is mixed: while some studies have found that parental smoking predicts smoking among adolescents, other studies have not (47).
Broader community and policy factors also influence smoking initiation. A robust body of evidence demonstrates that both the cost and marketing of tobacco products strongly influence smoking initiation. Higher prices have been clearly associated with reduced cigarette smoking prevalence.(63) This includes effects on both adult cigarette consumption and cigarette use by youth(64) and young adults.(65) Federal, state, or local governments can increase the price of cigarettes by raising tobacco excise tax rates. This is one of the strongest policy tools available to discourage smoking initiation.
Tobacco marketing, which includes both advertisements and promotional efforts such as sponsorship of events attractive to teens, is also strongly associated with both smoking initiation and continued smoking among adolescents.(47) Adolescents exposed to tobacco promotions and depictions of tobacco in media have more favorable tobacco-related attitudes and are more likely to start smoking.(66) In one study of adolescents who were not susceptible to smoking, those who had tobacco promotional items or who reported that they would use them were more likely to initiate cigarette smoking.(67)
Smoke-free laws and policies are also associated with a lower smoking prevalence by youth and young adults. Living in an area with 100% smoke-free laws in workplaces has been associated with lower odds of smoking initiation among adolescents and young adults (OR 0.66, 95% CI 0.44–0.99).(68) Adolescents and young adults who live in areas with 100% smoke-free bars are also less likely to be current smokers (OR 0.80, 95% CI 0.71–0.90).(68) Smoke-free laws have also been associated with the adoption of voluntary smoke-free policies in cars and homes,(69) which are associated with lower adolescent smoking prevalence.(70) Reducing the supply of cigarettes, such as by enforcing laws that prohibit tobacco sales to minors, can reduce illegal sales.(71) However, the effect of such interventions on policies on smoking behavior may be diminished by the fact that youth report getting cigarettes from a variety of sources and not only by purchasing tobacco products.(72) Recently, raising the legal age of tobacco purchase from 18 to 21 years has been advocated as a strategy to reduce adolescent tobacco use. A National Academy of Science Report concluded that “increasing the minimum age of legal access to tobacco products will likely prevent or delay initiation of tobacco use by adolescents and young adults” and supported current advocacy efforts to increase the legal age of smoking from 18 to 21 years (46).
Smoking cessation
Stopping smoking requires an individual to overcome nicotine dependence and also abandon a deeply-ingrained rewarding behavior. Barriers to cessation can be conceptualized using the socioecological model (Central Illustration) (56). At the intra-individual level, nicotine dependence is a major reason for difficulty quitting smoking (73). Dependence is stronger in certain smokers, such as smokers with low socioeconomic status, mental illness, and other substance use disorders (74). These individuals often have lower success in their attempts to quit, with or without treatment.
At the interpersonal level, the smoking status of the spouse and other household members is influential. Living with other smokers has been associated with reduced smoking cessation, while having a ban on smoking in the home has been associated with increased cessation.(75) In one study, a smoker’s chance of smoking was reduced by 67% if a spouse quit smoking and 36% if a friend quit smoking.(76) Another study found that smokers who reduced their number of smoking friends were more likely to quit smoking than smokers who had no change in smoking friends.(77) A smoker’s perception of strong social support for quitting from family and friends is also associated with greater success in quitting.(78).
Community and policy-level interventions can also promote smoking cessation. Smokers whose worksites have smoke-free policies are more likely to both reduce cigarette use and quit smoking compared to smokers without smoke-free worksites.(79) Cigarette prices are inversely associated with smoking behavior,(80) and increases in tobacco excise taxes that lead to higher prices for cigarettes can reduce smoking prevalence among adults.(81) Comprehensive tobacco control programs that include mass media campaigns to educate smokers about quitting resources can be effective in promoting cessation among adults.(82)
The Role of Preventive Action
Health benefits of smoking cessation
Quitting smoking reduces the risk of overall mortality among adult smokers, with health benefits observed even among smokers who quit after the age of 65 or after the development of a tobacco-related disease.(13,73,83) The greatest mortality benefit occurs among smokers who quit smoking by the age of 40, who reduce their risk of dying from smoking-related disease by 90%,(3) but mortality benefits also extend to smokers over the age of 70.(83) In addition to mortality benefits, smokers who quit reduce their risk of developing tobacco-related diseases, such as cardiac disease, pulmonary disease, and malignancy.(73) Thus, smoking cessation should be prioritized for smokers of all ages.
The benefits of smoking cessation begin within hours of quitting smoking with reductions in heart rate and blood carbon monoxide levels.(84) The effects of smoking on platelet activation decrease within days.(85) The risk of tobacco-related diseases declines at different rates. For CHD, the excess risk among former smokers declines rapidly, falling to half of the risk of continued smoking after 1 year of cessation and reaching the level of nonsmokers within 15 years.(84) The excess risk of stroke is almost completely eliminated by 5 to 15 years after cessation.(84) In contrast, the reduction in excess lung cancer risk occurs more gradually(86) and remains elevated even for long-time former smokers compared to never smokers.(84)
Smoking cessation reduces CVD risk rapidly even among smokers with pre-existing disease, making treating tobacco use a priority for secondary CVD prevention. Among smokers with CHD, quitting smoking was associated with a 36% reduction in cardiovascular mortality over two years compared to continued smoking in one systematic review.(87) Smokers who quit after MI, compared to those continuing to smoke, reduce subsequent mortality by 15% to 61%, have a better health-related quality of life and less angina.(88,89) Smoking cessation also has positive health effects for smokers with other tobacco-related diseases. For smokers with COPD, cessation slows the rate of decline in lung function,(90) reduces symptoms and the odds of exacerbation,(91,92) and lowers all-cause mortality.(93) For smokers with lung cancer, smoking cessation can reduce the risk of recurrence, development of a second primary cancer, and mortality (94).
Unlike the health benefits of smoking cessation, reducing cigarette consumption without eventually quitting cigarettes appears to produce a relatively small health benefit.(95) Smoking reduction has not been associated with decreased overall mortality or mortality from tobacco-related diseases (2,96). Smoking even one cigarette per day is associated with a higher risk of CHD and stroke (15). Therefore, smokers’ goal for improving health should be stopping all cigarette smoking.
Health benefits of reducing secondhand tobacco smoke exposure
To protect nonsmokers from the health risks of secondhand tobacco smoke exposure, governments and private-sector organizations have adopted a variety of laws and policies prohibiting smoking in public places, workplaces, restaurants, bars, and other locations. Comprehensive smoke-free policies have measurable clinical benefits. In a 2012 meta-analysis, comprehensive smoke-free laws were associated with reductions in hospitalizations or deaths from acute myocardial infarction (RR 0.85, 95% CI 0.82–0.88), other heart disease (0.61, 0.44–0.85), cerebrovascular accidents (0.84, 0.75–0.94), and respiratory disease (0.76, 0.68–0.85), and more comprehensive policies were associated with larger risk reduction.(97) A 2014 meta-analysis also found that smoke-free laws were associated with reductions in preterm birth and hospital visits for asthma, but no significant difference in low birthweight.(98)
Evidence-based Tobacco Control Policies
A substantial evidence base supports the effectiveness of public policies to reduce tobacco use. Most tobacco control policies act by reducing the demand for tobacco products. Such measures include increasing the price of cigarettes by raising tobacco excise taxes, adopting smoke-free policies for indoor areas, mandating health warning labels on tobacco packages, and supporting mass media campaigns to educate the public and promote cessation. Other measures aim to reduce the supply of tobacco to adolescents by raising the legal age of tobacco purchase and actively enforcing these laws.
The World Health Organization assembled evidence-based tobacco control measures into a formal policy document, the Framework Convention on Tobacco Control (FCTC), which is essentially a public health treaty. It took effect in 2005 and the 181 countries that have ratified the FCTC have agreed to adopt its package of tobacco control measures.(99,100) The MPOWER acronym was introduced to help countries implement these policies (Table 2).(101) The implementation of the FCTC has been associated with increased adoption of advertising bans(102) and smoke-free legislation.(103) Countries that implemented the measures to reduce demand at the highest level have observed reductions in smoking prevalence.(104) However, some countries have yet to implement any of these measures, and the U.S. has not ratified the FCTC. Increasing adoption and effective implementation of effective tobacco control policies is a key worldwide public health priority.(101) Given the importance of tobacco abstinence for cardiovascular health, cardiologists can act as tobacco control advocates to help reduce smoking initiation among youth and young adults, and promote smoking cessation among adults at-risk for or already affected by CVD.
Table 2.
WHO MPOWER Measures(101)
| Measure | Examples of tobacco control actions | |
|---|---|---|
| M | Monitor tobacco use and prevention policies |
Conduct surveys to monitor tobacco use by adults and adolescents |
| P | Protect people from tobacco smoke | Enact smoke-free laws and policies in public places, workplaces, restaurants, bars |
| O | Offer help to quit tobacco use | Insure that health care providers assess tobacco use, advise tobacco cessation, and offer behavioral and pharmacological assistance to quit. |
| W | Warn about the dangers of tobacco | Conduct mass media campaigns to educate children and adults about health risks and addictiveness of tobacco, promote use of cessation resources. Require warning labels on tobacco packages. |
| E | Enforce bans on tobacco advertising, promotion, and sponsorship |
Restrict tobacco industry sponsorship of events, distribution of free samples. |
| R | Raise taxes on tobacco | Increase tobacco excise tax rate to raise the price of tobacco products. |
| WHO, World Health Organization | ||
Harm reduction
Attaining complete abstinence, especially from combustible tobacco products, is the optimal way for a current smoker to reduce tobacco-related health risks, but not all tobacco users are willing or able to do so with current treatments. For these individuals, harm reduction is an alternate approach. This strategy recognizes that while nicotine is what maintains tobacco use, most of the health risks of tobacco use derive from other constituents of tobacco smoke. Thus, nicotine-containing products exist on a risk continuum from less harmful to more harmful (Figure 3) (105). Harm reduction could be achieved by moving the population from higher to lower risk nicotine products. The U.S. Food and Drug Administration (FDA) adopted this strategy in 2017 when it announced a plan to regulate nicotine and tobacco products with the goal of reducing the use of combustible cigarettes and their associated morbidity and mortality (105).
Figure 3. Risk continuum of nicotine-containing products.
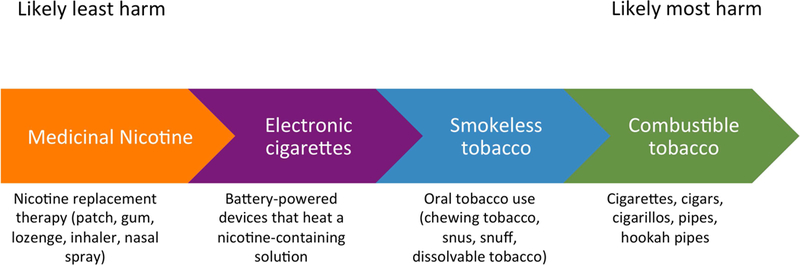
Nicotine comes in many forms with varying degrees of potential harm to users. Medicinal nicotine, such as that contained in nicotine replacement therapy, is least likely to cause harm, while combustible tobacco, such as cigarettes, is most likely to cause harm. It is not known where heat-not-burn tobacco (also known as heated tobacco) products fall on this spectrum given the limited evidence on their health effects.
One strategy that the FDA is exploring is mandating a reduction in cigarettes’ nicotine content to a minimally-addictive level, estimated to be <0.4 mg per gram of tobacco.(106) Smoking very low nicotine content (VLNC) cigarettes, compared to usual cigarettes, has been shown to reduce smokers’ nicotine exposure, dependence, and daily cigarette consumption.(107,108) A potential concern is that smokers will compensate for their reduced nicotine intake by smoking more cigarettes, thereby increasing their exposure to cigarette smoke’s many other toxicants. So far, studies have found little evidence of compensation, but smokers do seek out other sources of nicotine.(109) Having lower-risk nicotine-containing products, such as nicotine replacement therapy (NRT) or e-cigarettes, readily available to smokers of VLNC cigarettes will likely be essential for the strategy to succeed.(110) A simulation model found that reducing cigarettes’ nicotine content to minimally addictive levels would lead to a substantial reduction in tobacco-related mortality, despite uncertainty about the effects on smoking behaviors.(111)
A key component of the reduced nicotine strategy is to identify the appropriate regulatory approach to alternative sources of nicotine such as e-cigarettes.(112) Because they do not burn tobacco, e-cigarettes should pose less risk to users than cigarette smoke. A 2018 report from the U.S. National Academies of Sciences, Engineering, and Medicine concluded that e-cigarette aerosol exposes users to fewer toxicants than cigarette smoke and that e-cigarette use is likely to be less harmful than smoking cigarettes, although long-term health effects of e-cigarettes have yet to be determined.(113) The report found insufficient evidence at present of a risk from e-cigarettes on cardiovascular outcomes or measures of subclinical atherosclerosis.(113) Policies surrounding increased access to e-cigarettes for smokers trying to reduce their harm from cigarette smoking will need to be balanced against those limiting access to e-cigarettes by nonsmoking youth and young adults. Furthermore, these policies will need to continue to be reassessed as knowledge on the long-term health consequences of products such as e-cigarettes increases.
Guidance for Healthcare Providers
Screening and brief clinical interventions
According to national surveys, current smokers’ interest in quitting smoking is high but their use of evidence-based treatments and the success of individual quit attempts remains modest.(50) At outpatient visits, 63% of patients are screened for tobacco use and only 25% of identified smokers are provided cessation treatment in the form of medication or counseling.(114) Clinicians who care for patients with CVD can narrow this gap by routinely screening for tobacco use and not only providing advice to quit, but also helping current smokers use evidence-based tobacco cessation treatment. Even brief advice from a physician can increase the likelihood of successful smoking cessation.(115) A 2015 Cochrane meta-analysis found that psychosocial interventions for smokers with coronary heart disease increased smoking abstinence after 6–12 months (RR 1.22, 95% CI 1.13–1.32).(116) Additionally, clinicians can help nonsmokers avoid the health risks of secondhand tobacco smoke exposure on CVD by routine screening and advice to adopt smoke-free home and car policies.
A new diagnosis of CVD, acute MI, or acute coronary syndrome, or a cardiovascular procedure all serve as “teachable moments” that can motivate a smoker to attempt cessation. Among smokers hospitalized for cardiovascular disease, a Cochrane meta-analysis found that providing a smoking cessation intervention in the hospital and sustaining it post-discharge increased smoking cessation rates (RR 1.42, 95% CI 1.29–1.56).(117) However, only 7% of smokers with acute MI used smoking cessation medications in the early post-discharge period in a recent study (118).
Tobacco cessation treatment
Evidence-based smoking cessation treatments include medications and behavioral support. The combination of medication and counseling is the most effective approach because it allows management of both nicotine dependence and the conditioned behavior of smoking.(119) The U.S. Public Health Service’s 5As model provides a framework for brief office-based tobacco treatment. Its steps include includes asking about tobacco use, advising tobacco users to quit, assessing readiness to quit, assisting with quit attempts by providing medications or connecting individuals to counseling resources, and arranging follow-up to monitor success or roadblocks related to quitting.(120) An abridged 3-step model that recognizes that care is increasingly delivered by a health care team is helpful for busy clinicians; it includes asking about tobacco use, assisting those who use tobacco in trying to quit, and referring them to appropriate resources to help them accomplish their tobacco cessation goals (Figure 4) (121).
Figure 4. The Ask, Assist, Refer tobacco cessation intervention.

Providers can use this tool to screen for tobacco use among all patients, and refer them to appropriate resources to help with smoking cessation.
Tobacco cessation counseling can occur in-person or can be telephone-, mobile phone-, or web-based. In-person counseling can include individual(122) or group(123) settings. Telephone quit lines available in all U.S. states provide free services to smokers (1-800-QUIT-NOW). Proactive counseling in which counselors reach out to smokers over multiple sessions increases smoking cessation success.(124) Mobile phone-based interventions have also been effective in helping smokers quit.(125) In the U.S, the National Cancer Institute offers the SmokefreeTXT program (smokefree.gov/smokefreetxt). In contrast, acupuncture(126) and hypnotherapy(127) have not shown a consistent benefit for smoking cessation.
The medications approved by the U.S. FDA as smoking cessation aids are NRT (5 different products), varenicline, and bupropion (Table 3). Decisions on medications and combinations of medications should be customized based on patient preferences and side effect profiles. NRT helps to combat nicotine withdrawal symptoms by providing users with nicotine in either a long-acting (patch) or short-acting (gum, lozenge, inhaler, nasal spray) form. The nicotine patch provides nicotine transdermally throughout the day, while short-acting NRT acts more quickly but for a shorter duration. Use of NRT in any form increases smoking abstinence by 60% compared to placebo (RR 1.60, 95% CI 1.53–1.68).(128) To increase efficacy, the nicotine patch can be combined with short-acting NRT, which has been shown to be more effective than using one form of NRT (RR 1.34, 95% CI 1.18–1.51).(128)
Table 3.
Medications Approved by the U.S. Food and Drug Administration for Smoking Cessation
| Medication | Dosing | Common Side Effects | Available over the counter? |
|---|---|---|---|
| Nicotine patch | >10 cigarettes/day: 21mg to start, taper after 6 weeks ≤10 cigarettes/day: 14mg to start, taper after 6 weeks |
Redness/irritation at patch site, sleep disturbance |
Yes |
| Nicotine gum | Smokes first cigarette ≤30 minutes of waking: 4mg every 1-2 hours Smokes first cigarette >30 minutes of waking: 2mg every 1-2 hours |
Oral issues, nausea, heartburn, hiccups |
Yes |
| Nicotine lozenge | Smokes first cigarette ≤30 minutes of waking: 4mg every 1-2 hours Smokes first cigarette >30 minutes of waking: 2mg every 1-2 hours |
Oral issues, nausea, heartburn, hiccups |
Yes |
| Nicotine inhaler | 10mg cartridges 6-16 times per day |
Oropharyngeal irritation, cough | No |
| Nicotine nasal spray |
1-2 sprays per nostril per hour | Nasopharyngeal irritation, sneezing, coughing |
No |
| Varenicline | 0.5mg daily for 3 days, then 0.5mg twice daily for 4 days, then 1mg twice daily |
Nausea, abnormal dreams | No |
| Bupropion | 150mg daily for 3 days, then 150mg twice daily |
Insomnia, dry mouth | No |
Because of nicotine’s sympathomimetic properties, the safety of NRT use in patients with CVD has been studied. NRT was associated with an increase in cardiovascular events, but not major cardiovascular events, when compared to placebo in a network meta-analysis that included individuals both with and without heart disease.(129) A study that specifically focused on outpatient smokers with CVD found no significant increase in cardiovascular events (death, myocardial infarction, cardiac arrest, or hospital admission for worsening angina, arrhythmia, or heart failure) among smokers receiving nicotine patch versus placebo.(130)
Varenicline is a partial nicotinic receptor agonist that more than doubles smoking abstinence rates compared to placebo (RR 2.24, 95% CI 2.06–2.43).(131) The EAGLES trial, which randomized more than 8000 smokers in 16 countries to varenicline, bupropion, nicotine patch, or placebo in a 1:1:1:1 ratio, found a significantly higher 6-month abstinence rate for the varenicline group compared to all other groups.(132) In a randomized trial of smokers with stable CVD, treatment with varenicline increased the odds of continuous abstinence at 1 year over placebo (OR 3.14, 95% 1.94–5.11).(133) A randomized trial of smokers with acute coronary syndrome found a higher rate of continuous abstinence at 24 weeks and a higher point- prevalence abstinence rate at 52 weeks in those taking varenicline vs. placebo.(134,135)
Because varenicline has nicotine-like effects, concerns have been raised about a potential cardiovascular risk of the drug. The bulk of available evidence does not suggest an elevated CVD risk when varenicline is used in smokers with or without CVD. The CATS trial, which examined cardiovascular events in over 8000 smokers from the EAGLES trial, found no evidence of increased cardiovascular events compared to bupropion, nicotine patch, or placebo.(136) The trial was not limited to smokers with CVD, but participants were middle-aged smokers, many of whom had other CVD risk factors. Although one meta-analysis of earlier randomized trials had reported an increased risk of CVD events among smokers using varenicline,(137) subsequent larger meta-analyses(129,138–140) and several large retrospective cohort studies(141–143) found no evidence of a significantly increased CVD risk with varenicline use. Recently, an observational study using Canadian prescription and health outcome databases reported an increase in cardiovascular events among smokers taking varenicline compared to a self-controlled time interval,(144) but limitations of the study design have been noted (145).
Cytisine, a plant alkaloid, is a partial nicotinic receptor agonist, like varenicline. Cytisine has demonstrated efficacy for smoking cessation in multiple clinical trials,(146) and has been used for this purpose in Eastern Europe for several decades. Efforts to develop cytisine as a smoking cessation aid in the U.S. are underway.
Bupropion increases levels of norepinephrine and dopamine and is approved both as an antidepressant and for smoking cessation. Bupropion increases smoking cessation rates compared to placebo or control.(132,147) Two studies of smokers hospitalized with acute coronary events did not find increases in smoking cessation in smokers who received bupropion versus placebo.(148,149) Bupropion treatment does not appear to be associated with an increase in major adverse cardiovascular events.(129) Contraindications to use of bupropion include seizure disorder or having a high risk for seizure.
In 2009, concern about possible neuropsychiatric events with both varenicline and bupropion led the FDA to require both drugs’ packages to carry a boxed warning. Subsequently, the EAGLES trial, which included individuals with and without mild-moderate psychiatric illness, showed no increase in adverse neuropsychiatric events among smokers receiving varenicline or bupropion compared to those receiving NRT or placebo (132). In 2016, FDA removed the boxed warning requirement for varenicline and bupropion.
Acknowledgments
Funding: Dr. Kalkhoran’s work in preparation of this manuscript was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (K23HL136854). Dr. Rigotti’s work was also supported by NHLBI (R01 HL11821). The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Disclosures: Drs. Kalkhoran, Rigotti and Benowitz receive royalties from UpToDate for chapters on electronic cigarettes, smoking cessation, and the cardiovascular effects of nicotine. Dr. Rigotti has received a research grant from and been an unpaid consultant to Pfizer, and a paid consultant to Achieve Life Sciences. Dr. Benowitz has been a paid consultant to Pfizer, Inc. and Achieve Life Sciences, and has been an expert witness in litigation against tobacco companies.
Abbreviations
- CVD
cardiovascular disease
- e-cigarette
electronic cigarette
- MI
myocardial infarction
- CHD
coronary heart disease
- CHF
congestive heart failure
- AAA
abdominal aortic aneurysm
- CABG
coronary artery bypass grafting
- TSE
tobacco smoke exposure
- FDA
Food and Drug Administration
- NRT
nicotine replacement therapy
References
- 1.Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017;389:1885–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godtfredsen NS, Holst C, Prescott E, Vestbo J, Osler M. Smoking reduction, smoking cessation, and mortality: a 16-year follow-up of 19,732 men and women from The Copenhagen Centre for Prospective Population Studies. Am J Epidemiol 2002; 156:994–1001. [DOI] [PubMed] [Google Scholar]
- 3.Jha P, Ramasundarahettige C, Landsman V et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013;368:341–50. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. The health consequences of smoking— 50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014;17. [Google Scholar]
- 5.World Health Organization. Tobacco. Available at: http://www.who.int/mediacentre/factsheets/fs339/en/. Accessed March 6, 2018
- 6.Phillips E, Wang TW, Husten CG et al. Tobacco Product Use Among Adults - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamal A, Phillips E, Gentzke AS et al. Current Cigarette Smoking Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The NSDUH Report: Adults with Mental Illness or Substance Use Disorder Account for 40 Percent of All Cigarettes Smoked. Available at: https://www.samhsa.gov/data/sites/default/files/spot104-cigarettes-mental-illness-substance-use-disorder/spot104-cigarettes-mental-illness-substance-use-disorder.pdf. Accessed May 19, 2017
- 9.Jamal A, Gentzke A, Hu SS et al. Tobacco Use Among Middle and High School Students - United States, 2011–2016. MMWR Morb Mortal Wkly Rep 2017;66:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasza KA, Ambrose BK, Conway KP et al. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N Engl J Med 2017;376:342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabuchi T, Kiyohara K, Hoshino T, Bekki K, Inaba Y, Kunugita N. Awareness and use of electronic cigarettes and heat-not-burn tobacco products in Japan. Addiction 2016;111:706–13. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Hawken S, Ounpuu S et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case- control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- 13.Thun MJ, Carter BD, Feskanich D et al. 50-Year Trends in Smoking-Related Mortality in the United States. New England Journal of Medicine 2013;368:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldenberg I, Jonas M, Tenenbaum A et al. Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch Intern Med 2003;163:2301–5. [DOI] [PubMed] [Google Scholar]
- 15.Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. Bmj 2018;360:j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green MS, Jucha E, Luz Y. Blood pressure in smokers and nonsmokers: epidemiologic findings. Am Heart J 1986;111:932–40. [DOI] [PubMed] [Google Scholar]
- 17.Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension 2001;37:187–93. [DOI] [PubMed] [Google Scholar]
- 18.Carter BD, Abnet CC, Feskanich D et al. Smoking and mortality--beyond established causes. N Engl J Med 2015;372:631–40. [DOI] [PubMed] [Google Scholar]
- 19.Regalado M, Yang S, Wesson DE. Cigarette smoking is associated with augmented progression of renal insufficiency in severe essential hypertension. American Journal of Kidney Diseases 2000;35:687–694. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Mackay DF, Pell JP. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart 2014;100:414–23. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama H, Hashimoto H, Tanaka H et al. Continuous smoking and progression of arterial stiffening: a prospective study. J Am Coll Cardiol 2010;55:1979–87. [DOI] [PubMed] [Google Scholar]
- 22.Duval S, Long KH, Roy SS et al. The Contribution of Tobacco Use to High Health Care Utilization and Medical Costs in Peripheral Artery Disease: A State-Based Cohort Analysis. J Am Coll Cardiol 2015;66:1566–1574. [DOI] [PubMed] [Google Scholar]
- 23.Lederle FA, Johnson GR, Wilson SE, et al. The aneurysm detection and management study screening program: Validation cohort and final results. Archives of Internal Medicine 2000;160:1425–1430. [DOI] [PubMed] [Google Scholar]
- 24.Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 2012;99:655–65. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain AM, Agarwal SK, Folsom AR et al. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm 2011;8:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldenberg I, Moss AJ, McNitt S et al. Cigarette smoking and the risk of supraventricular and ventricular tachyarrhythmias in high-risk cardiac patients with implantable cardioverter defibrillators. J Cardiovasc Electrophysiol 2006;17:931–6. [DOI] [PubMed] [Google Scholar]
- 27.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV et al. Cigarette Smoking Exposure and Heart Failure Risk in Older Adults: The Health, Aging, and Body Composition Study. Am Heart J 2012;164:236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suskin N, Sheth T, Negassa A, Yusuf S. Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. Journal of the American College of Cardiology 2001;37:1677–1682. [DOI] [PubMed] [Google Scholar]
- 29.Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med 2002;137:494–500. [DOI] [PubMed] [Google Scholar]
- 30.Epstein KA, Viscoli CM, Spence JD et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology 2017;89:1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Domburg RT, Meeter K, van Berkel DF, Veldkamp RF, van Herwerden LA, Bogers AJ. Smoking cessation reduces mortality after coronary artery bypass surgery: a 20-year follow-up study. J Am Coll Cardiol 2000;36:878–83. [DOI] [PubMed] [Google Scholar]
- 32.Hasdai D, Garratt KN, Grill DE, Lerman A, Holmes DR, Jr. Effect of smoking status on the long-term outcome after successful percutaneous coronary revascularization. N Engl J Med 1997;336:755–61. [DOI] [PubMed] [Google Scholar]
- 33.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 2005;111:2684–98. [DOI] [PubMed] [Google Scholar]
- 34.Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health (Oxf) 2011;33:496–502. [DOI] [PubMed] [Google Scholar]
- 35.National Cancer Institute and Centers for Disease Control and Prevention. Smokeless Tobacco and Public Health: A Global Perspective: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Institutes of Health, National Cancer Institute. NIH Publication No. 14–7983, 2014. [Google Scholar]
- 36.Piano MR, Benowitz NL, Fitzgerald GA et al. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation 2010;122:1520–44. [DOI] [PubMed] [Google Scholar]
- 37.Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. Bmj 2009;339:b3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arefalk G, Hambraeus K, Lind L, Michaelsson K, Lindahl B, Sundstrom J. Discontinuation of smokeless tobacco and mortality risk after myocardial infarction. Circulation 2014;130:325–32. [DOI] [PubMed] [Google Scholar]
- 39.Teo KK, Ounpuu S, Hawken S et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet 2006;368:647–58. [DOI] [PubMed] [Google Scholar]
- 40.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509–15. [DOI] [PubMed] [Google Scholar]
- 41.Brook RD, Rajagopalan S, Pope CA, 3rd et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–78. [DOI] [PubMed] [Google Scholar]
- 42.US Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006;709. [Google Scholar]
- 43.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst 1999;91: 1194–210. [DOI] [PubMed] [Google Scholar]
- 44.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis 2003;46:91–111. [DOI] [PubMed] [Google Scholar]
- 45.Saleheen D, Zhao W, Young R et al. Loss of Cardioprotective Effects at the ADAMTS7 Locus as a Result of Gene-Smoking Interactions. Circulation 2017;135:2336–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Institute of Medicine Committee on the Public Health Implications of Raising the Minimum Age for Purchasing Tobacco Products, Bonnie RJ, Kwan LY, Stratton KR Public health implications of raising the minimum age of legal access to tobacco products: National Academies Press, 2015. [PubMed] [Google Scholar]
- 47.US Department of Health Human Services. Preventing tobacco use among youth and young adults: A report of the Surgeon General Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health 2012;3. [Google Scholar]
- 48.Hughes JR, Solomon LJ, Naud S, Fingar JR, Helzer JE, Callas PW. Natural history of attempts to stop smoking. Nicotine Tob Res 2014;16:1190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaiton M, Diemert L, Cohen JE et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting Smoking Among Adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2017;65:1457–1464. [DOI] [PubMed] [Google Scholar]
- 51.Fiore MC, Bailey WC, Cohen SJ et al. Treating tobacco use and dependence: clinical practice guideline Rockville, MD: US Department of Health and Human Services 2000:00–0032. [Google Scholar]
- 52.DiFranza J, Savageau J, Rigotti N et al. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control 2002;11:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doubeni CA, Reed G, Difranza JR. Early course of nicotine dependence in adolescent smokers. Pediatrics 2010;125:1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 1986;43:289–94. [DOI] [PubMed] [Google Scholar]
- 55.Herd N, Borland R. The natural history of quitting smoking: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction (Abingdon, England) 2009;104:2075–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q 1988;15:351–77. [DOI] [PubMed] [Google Scholar]
- 57.Tyas SL, Pederson LL. Psychosocial factors related to adolescent smoking: a critical review of the literature. Tobacco Control 1998;7:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaiton MO, Cohen JE, O’Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health 2009;9:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am J Public Health 1998;88:1518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet 2005;35:397–406. [DOI] [PubMed] [Google Scholar]
- 61.Peers Kobus K. and adolescent smoking. Addiction 2003;98 Suppl 1:37–55. [DOI] [PubMed] [Google Scholar]
- 62.Ellickson PL, Bird CE, Orlando M, Klein DJ, McCaffrey DF. Social context and adolescent health behavior: does school-level smoking prevalence affect students’ subsequent smoking behavior? J Health Soc Behav 2003;44:525–35. [PubMed] [Google Scholar]
- 63.Chaloupka FJ. Macro-social influences: the effects of prices and tobacco-control policies on the demand for tobacco products. Nicotine Tob Res 1999;1 Suppl 1:S105–9. [DOI] [PubMed] [Google Scholar]
- 64.Ross H, Chaloupka FJ. The effect of cigarette prices on youth smoking. Health Econ 2003;12:217–30. [DOI] [PubMed] [Google Scholar]
- 65.Chaloupka FJ, Wechsler H. Price, tobacco control policies and smoking among young adults. J Health Econ 1997;16:359–73. [DOI] [PubMed] [Google Scholar]
- 66.Wellman RJ, Sugarman DB, DiFranza JR, Winickoff JP. The extent to which tobacco marketing and tobacco use in films contribute to children’s use of tobacco: a meta-analysis. Arch Pediatr Adolesc Med 2006;160:1285–96. [DOI] [PubMed] [Google Scholar]
- 67.Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Berry CC. Tobacco industry promotion of cigarettes and adolescent smoking. Jama 1998;279:511–5. [DOI] [PubMed] [Google Scholar]
- 68.Song AV, Dutra LM, Neilands TB, Glantz SA. Association of Smoke-Free Laws With Lower Percentages of New and Current Smokers Among Adolescents and Young Adults: An 11-Year Longitudinal Study. JAMA Pediatr 2015;169:e152285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng KW, Okechukwu CA, McMillen R, Glantz SA. Association between clean indoor air laws and voluntary smokefree rules in homes and cars. Tob Control 2015;24:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farkas AJ, Gilpin EA, White MM, Pierce JP. Association between household and workplace smoking restrictions and adolescent smoking. Jama 2000;284:717–22. [DOI] [PubMed] [Google Scholar]
- 71.Stead LF, Lancaster T. Interventions for preventing tobacco sales to minors. Cochrane Database Syst Rev 2005:Cd001497. [DOI] [PubMed] [Google Scholar]
- 72.Dai H, Hao J. Temporal Trends of Sources of Cigarettes among U.S. High School Students: 2001–2015. Nicotine Tob Res 2018. [DOI] [PubMed] [Google Scholar]
- 73.US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010;2. [Google Scholar]
- 74.Goodwin RD, Pagura J, Spiwak R, Lemeshow A, Sareen J. Predictors of persistent nicotine dependence among adults in the United States. Drug Alcohol Depend 2011;118:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farkas AJ, Gilpin EA, Distefan JM, Pierce JP. The effects of household and workplace smoking restrictions on quitting behaviours. Tob Control 1999;8:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christakis NA, Fowler JH. Quitting in Droves: Collective Dynamics of Smoking Behavior in a Large Social Network. N Engl J Med 2008;358:2249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hitchman SC, Fong GT, Zanna MP, Thrasher JF, Laux FL. The Relation Between Number of Smoking Friends, and Quit Intentions, Attempts, and Success: Findings from the International Tobacco Control (ITC) Four Country Survey. Psychol Addict Behav 2014;28:1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mermelstein R, Cohen S, Lichtenstein E, Baer JS, Kamarck T. Social support and smoking cessation and maintenance. J Consult Clin Psychol 1986;54:447–53. [DOI] [PubMed] [Google Scholar]
- 79.Bauer JE, Hyland A, Li Q, Steger C, Cummings KM. A longitudinal assessment of the impact of smoke-free worksite policies on tobacco use. Am J Public Health 2005;95:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayne SL, Auchincloss AH, Stehr MF et al. Longitudinal Associations of Local Cigarette Prices and Smoking Bans with Smoking Behavior in the Multi-Ethnic Study of Atherosclerosis. Epidemiology 2017;28:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chaloupka FJ, Straif K, Leon ME. Effectiveness of tax and price policies in tobacco control. Tob Control 2011;20:235–8. [DOI] [PubMed] [Google Scholar]
- 82.Bala MM, Strzeszynski L, Topor-Madry R. Mass media interventions for smoking cessation in adults. Cochrane Database Syst Rev 2017;11 :Cd004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nash SH, Liao LM, Harris TB, Freedman ND. Cigarette Smoking and Mortality in Adults Aged 70 Years and Older: Results From the NIH-AARP Cohort. Am J Prev Med 2017;52:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. 2004. [Google Scholar]
- 85.Benowitz NL, Fitzgerald GA, Wilson M, Zhang Q. Nicotine effects on eicosanoid formation and hemostatic function: comparison of transdermal nicotine and cigarette smoking. J Am Coll Cardiol 1993;22:1159–67. [DOI] [PubMed] [Google Scholar]
- 86.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case- control studies. Bmj 2000;321:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: A systematic review. JAMA 2003;290:86–97. [DOI] [PubMed] [Google Scholar]
- 88.Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: Meta-analysis of cohort studies. Archives of Internal Medicine 2000;160:939–944. [DOI] [PubMed] [Google Scholar]
- 89.Buchanan DM, Arnold SV, Gosch KL et al. Association of Smoking Status With Angina and Health-Related Quality of Life After Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes 2015;8:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anthonisen NR, Connett JE, Kiley JP et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. Jama 1994;272:1497–505. [PubMed] [Google Scholar]
- 91.Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med 1999;106:410–6. [DOI] [PubMed] [Google Scholar]
- 92.Au DH, Bryson CL, Chien JW et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2009;24:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005;142:233–9. [DOI] [PubMed] [Google Scholar]
- 94.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. Bmj 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Begh R, Lindson-Hawley N, Aveyard P. Does reduced smoking if you can’t stop make any difference? BMC Med 2015;13:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tverdal A, Bjartveit K. Health consequences of reduced daily cigarette consumption. Tob Control 2006;15:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation 2012;126:2177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A. Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. Lancet 2014;383:1549–60. [DOI] [PubMed] [Google Scholar]
- 99.Organization. WH. WHO Framework Convention on Tobacco Control. Geneva: World Health Organization, 2003. [Google Scholar]
- 100.Drope J, Schluger N, Cahn Z et al. The Tobacco Atlas. Atlanta: American Cancer Society and Vital Strategies, 2018. [Google Scholar]
- 101.World Health Organization. WHO report on the global tobacco epidemic 2017: Monitoring tobacco use and prevention policies. 2017. [Google Scholar]
- 102.Hiilamo H, Glantz S. FCTC followed by accelerated implementation of tobacco advertising bans. Tob Control 2017;26:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uang R, Hiilamo H, Glantz SA. Accelerated Adoption of Smoke-Free Laws After Ratification of the World Health Organization Framework Convention on Tobacco Control. Am J Public Health 2016;106:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gravely S, Giovino GA, Craig L et al. Implementation of key demand-reduction measures of the WHO Framework Convention on Tobacco Control and change in smoking prevalence in 126 countries: an association study. The Lancet Public Health 2017;2:e166–e174. [DOI] [PubMed] [Google Scholar]
- 105.Gottlieb S, Zeller M. A Nicotine-Focused Framework for Public Health. New England Journal of Medicine 2017;377:1111–1114. [DOI] [PubMed] [Google Scholar]
- 106.Benowitz NL, Donny EC, Hatsukami DK. Reduced nicotine content cigarettes, e- cigarettes and the cigarette end game. Addiction 2017;112:6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Donny EC, Denlinger RL, Tidey JW et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med 2015;373:1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hatsukami DK, Kotlyar M, Hertsgaard LA et al. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction 2010;105:343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev 2015;24:472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hatsukami DK, Luo X, Dick L et al. Reduced nicotine content cigarettes and use of alternative nicotine products: exploratory trial. Addiction 2017;112:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Apelberg BJ, Feirman SP, Salazar E et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. N Engl J Med 2018;378:1725–1733. [DOI] [PubMed] [Google Scholar]
- 112.Fairchild AL, Lee JS, Bayer R, Curran J. E-Cigarettes and the Harm-Reduction Continuum. N Engl J Med 2018;378:216–219. [DOI] [PubMed] [Google Scholar]
- 113.National Academies of Sciences Engineering and Medicine Public health consequences of e-cigarettes. Washington, DC: The National Acadmies Press, 2018. [PubMed] [Google Scholar]
- 114.Jamal A, Dube SR, King BA. Tobacco Use Screening and Counseling During Hospital Outpatient Visits Among US Adults, 2005–2010. Prev Chronic Dis 2015;12:E132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev 2013:Cd000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barth J, Jacob T, Daha I, Critchley JA. Psychosocial interventions for smoking cessation in patients with coronary heart disease. Cochrane Database Syst Rev 2015:Cd006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rigotti NA, Clair C, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev 2012:Cd001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pagidipati NJ, Hellkamp A, Thomas L, Gulati M, Peterson ED, Wang TY. Use of Prescription Smoking Cessation Medications After Myocardial Infarction Among Older Patients in Community Practice. JAMA Cardiol 2017;2:1040–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2012;10:Cd008286. [DOI] [PubMed] [Google Scholar]
- 120.Fiore MC, Jaen CR, Baker T et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 121.Winickoff JP, Hippie B, Drehmer J et al. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) Intervention: A Decade of Lessons Learned. Journal of clinical outcomes management : JCOM 2012;19:414–419. [PMC free article] [PubMed] [Google Scholar]
- 122.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017;3:Cd001292. [DOI] [PubMed] [Google Scholar]
- 123.Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2017;3:Cd001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2013:Cd002850. [DOI] [PubMed] [Google Scholar]
- 125.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2016;4:Cd006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.White AR, Rampes H, Liu JP, Stead LF, Campbell J. Acupuncture and related interventions for smoking cessation. Cochrane Database Syst Rev 2014;1:Cd000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barnes J, Dong CY, McRobbie H, Walker N, Mehta M, Stead LF. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev 2010:Cd001008. [DOI] [PubMed] [Google Scholar]
- 128.Stead LF, Perera R, Bullen C et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2012;11:Cd000146. [DOI] [PubMed] [Google Scholar]
- 129.Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation 2014;129:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Joseph AM, Norman SM, Ferry LH et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med 1996;335:1792–8. [DOI] [PubMed] [Google Scholar]
- 131.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2012;4:Cd006103. [DOI] [PubMed] [Google Scholar]
- 132.Anthenelli RM, Benowitz NL, West R et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507–20. [DOI] [PubMed] [Google Scholar]
- 133.Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation 2010;121:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eisenberg MJ, Windle SB, Roy N et al. Varenicline for Smoking Cessation in Hospitalized Patients With Acute Coronary Syndrome. Circulation 2015. [DOI] [PubMed] [Google Scholar]
- 135.Windle SB, Dehghani P, Roy N et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. Cmaj 2018;190:E347–e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Benowitz NL, Pipe A, West R et al. Cardiovascular Safety of Varenicline, Bupropion, and Nicotine Patch in Smokers: A Randomized Clinical Trial. JAMA Intern Med 2018;178:622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. Cmaj 2011;183:1359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ware JH, Vetrovec GW, Miller AB et al. Cardiovascular safety of varenicline: patient- level meta-analysis of randomized, blinded, placebo-controlled trials. Am J Ther 2013;20:235–46. [DOI] [PubMed] [Google Scholar]
- 139.Sterling LH, Windle SB, Filion KB, Touma L, Eisenberg MJ. Varenicline and Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. Bmj 2012;344:e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kotz D, Viechtbauer W, Simpson C, van Schayck OC, West R, Sheikh A. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir Med 2015;3:761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Monarrez-Espino J, Galanti MR, Hansson J, Janszky I, Soderberg K, Moller J. Treatment with bupropion and varenicline for smoking cessation and the risk of acute cardiovascular events and injuries: a Swedish case-crossover study. Nicotine Tob Res 2017. [DOI] [PubMed] [Google Scholar]
- 143.Svanstrom H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. Bmj 2012;345:e7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gershon AS, Campitelli MA, Hawken S et al. Cardiovascular and Neuropsychiatric Events Following Varenicline Use for Smoking Cessation. Am J Respir Crit Care Med 2017. [DOI] [PubMed] [Google Scholar]
- 145.Kalhan R, Wilkins JT, Hitsman BL. Tobacco Smoking is a Medical Problem: We Ought to Treat It Like One. Am J Respir Crit Care Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2016:Cd006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2014;1:Cd000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eisenberg MJ, Grandi SM, Gervais A et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J Am Coll Cardiol 2013;61:524–32. [DOI] [PubMed] [Google Scholar]
- 149.Planer D, Lev I, Elitzur Y et al. Bupropion for smoking cessation in patients with acute coronary syndrome. Arch Intern Med 2011;171:1055–60. [DOI] [PubMed] [Google Scholar]
- 150.Czernin J, Waldherr C. Cigarette smoking and coronary blood flow. Prog Cardiovasc Dis 2003;45:395–404. [DOI] [PubMed] [Google Scholar]
- 151.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004;43:1731–7. [DOI] [PubMed] [Google Scholar]
- 152.Simpson AJ, Gray RS, Moore NR, Booth NA. The effects of chronic smoking on the fibrinolytic potential of plasma and platelets. Br J Haematol 1997;97:208–13. [DOI] [PubMed] [Google Scholar]
- 153.Newby DE, McLeod AL, Uren NG et al. Impaired coronary tissue plasminogen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation 2001;103:1936–41. [DOI] [PubMed] [Google Scholar]
- 154.Matetzky S, Tani S, Kangavari S et al. Smoking increases tissue factor expression in atherosclerotic plaques: implications for plaque thrombogenicity. Circulation 2000;102:602–4. [DOI] [PubMed] [Google Scholar]
- 155.Lehr HA, Frei B, Arfors KE. Vitamin C prevents cigarette smoke-induced leukocyte aggregation and adhesion to endothelium in vivo. Proceedings of the National Academy of Sciences 1994;91:7688–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Levitzky YS, Guo CY, Rong J et al. Relation of smoking status to a panel of inflammatory markers: the framingham offspring. Atherosclerosis 2008;201:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barbieri SS, Zacchi E, Amadio P et al. Cytokines present in smokers’ serum interact with smoke components to enhance endothelial dysfunction. Cardiovasc Res 2011;90:475–83. [DOI] [PubMed] [Google Scholar]
- 158.McEvoy JW, Nasir K, DeFilippis AP et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35:1002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. Bmj 1989;298:784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Grundy SM. Oxidized LDL and atherogenesis: relation to risk factors for coronary heart disease. Clin Cardiol 1993; 16:I3–5. [DOI] [PubMed] [Google Scholar]
- 161.Goette A, Lendeckel U, Kuchenbecker A et al. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart 2007;93:1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. Jama 2007;298:2654–64. [DOI] [PubMed] [Google Scholar]
- 163.Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet 1992;339:1128–30. [DOI] [PubMed] [Google Scholar]
- 164.Wu Y, Song P, Zhang W et al. Activation of AMPKalpha2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med 2015;21:373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sugiishi M, Takatsu F. Cigarette smoking is a major risk factor for coronary spasm. Circulation 1993;87:76–9. [DOI] [PubMed] [Google Scholar]