Abstract
The gastrointestinal tract contains its own set of intrinsic neuroglial circuits—the enteric nervous system (ENS)—which detects and responds to diverse signals from the environment. Here, we address recent advances in the understanding of ENS development, including how neural-crest-derived progenitors migrate into and colonize the bowel, the formation of ganglionated plexuses and the molecular mechanisms of enteric neuronal and glial diversification. Modern lineage tracing and transcription-profiling technologies have produced observations that simultaneously challenge and affirm long-held beliefs about ENS development. We review many genetic and environmental factors that can alter ENS development and exert long-lasting effects on gastrointestinal function, and discuss how developmental defects in the ENS might account for some of the large burden of digestive disease.
The intrinsic innervation of the gastrointestinal (GI) tract, known as the enteric nervous system (ENS), consists of two major ganglionated plexuses: the myenteric plexus and the submucosal plexus (which itself is subdivided into two further plexuses in larger mammals)1–3. The ENS is the largest and most complex unit of the peripheral nervous system (PNS). Because most of its component neurons lack direct innervation from the CNS, the ENS is neither sympathetic nor parasympathetic but a third autonomic division that receives sympathetic and parasympathetic input4. The ENS is unique in containing intrinsic primary afferent neurons (IPANs) that allow it to regulate enteric behaviour without CNS input. Although the ENS can function independently, the ENS and CNS normally communicate bidirectionally. Intrinsic ENS pathways are largely responsible for the detailed patterns of GI motor and secretory activity, such as peristalsis and fluid or electrolyte secretion. The ENS also communicates with many other cell types, including intestinal epithelial, endocrine and immune cells, to influence various physiological responses at the level of the gut.
The ENS has been called, on presumed evolutionary grounds, ‘the first brain’, to imply that its basic pattern evolved with bilateria, before and independently of that of the brain itself5. This speculation draws attention to the reciprocal ENS-CNS connections and the neuronal projections from both the ENS and CNS that converge on visceral and prevertebral sympathetic ganglia. Rising interest in ENS development is reflected in excellent recent reviews6–17. Most of these reviews focus on one disorder, Hirschsprung disease (HSCR), which involves an absence of ganglia (aganglionosis), is wholly attributable to an ENS developmental abnormality and is usually fatal unless the aganglionic gut is removed18.
The absence of ganglia in HSCR and the potentially fatal bowel obstruction that results from it are, of course, noticeable. HSCR thus has motivated advances in knowledge and merits attention, although its incidence is only ~1 in 5,000 births. When ganglia are present, however, ENS defects are not easily diagnosed because the classification of the pathology of ENS disorders has only just begun19,20; moreover, the presence of ganglia does not necessarily mean that the ENS will function normally. Indeed, even after the aganglionic intestine has been removed from individuals with HSCR, the motility of the remaining ganglionated intestine may be abnormal21–23. Other life-threatening abnormalities of GI motility, such as chronic intestinal pseudo-obstruction, can also occur despite the presence of enteric ganglia24.
Because survival requires the ENS to be functional at birth to accommodate oral feeding25, ENS circuits necessary for motility must be assembled before birth. Before GI motility comes under the control of the ENS, only non-propulsive myogenic ‘ripples’ are present26–28. It is thus important to understand ENS development, not only for its intrinsic scientific value or to cope better with HSCR but also to gain insight into more common GI disorders, such as irritable bowel syndrome (IBS) and chronic constipation, that may arise from abnormalities of ENS development.
In this Review, we provide an overview of ENS development, including the colonization and migration of neural-crest-derived progenitors to the developing gut and their differentiation, organization and proliferation. We also describe recent efforts to determine the molecular mediators underlying these processes. The complexity of ENS development is paralleled by the adverse effects that occur when these developmental processes or molecular interactions are disrupted. Even minor perturbations in the activity of key molecules during ENS development can lead to long-lasting structural and functional consequences for the gut that could contribute to the pathogenesis of GI disorders.
Neural-crest colonization of the gut
Early work in chick embryos demonstrated that enteric ganglia failed to arise after the vagal neural crest (corresponding to somites 1–7) was ablated29, suggesting that the ENS is a neural-crest derivative and that vagal-crest-derived cells migrate to and colonize the bowel. Later experiments confirmed these suggestions by tracing migrating crest cells in chick embryos in which various regions of the crest were removed and replaced with quail crest30. Quail crest-derived cells from the vagal level colonized the entire bowel, and quail cells originating from the sacral crest (axial levels caudal to somite 28) contributed additional cells to the gut caudal to the umbilicus (known as the post-umbilical bowel)31 (Box 1; FIG. 1).
Box 1 |. The colonization of the gut by cells from the neural crest.
Recent chick–quail transplantation studies have revealed that there are regional differences within the vagal crest9. Crest-derived cells from somite levels 1–2 populate only the oesophagus; those from levels 3–5 colonize the bowel from the stomach to the hindgut; and migrating cells from levels 6–7 go only to the hindgut36. Conflicting results obtained by using a lipophilic dye as a tracer in fetal mice suggest that crest-derived cells from somite levels 6–7 colonize the oesophagus and not the hindgut, as in the avian studies173. It is not clear whether the conflict in these data is due to species or technical differences. Crest-derived cells from somite level 3 seem to be particularly invasive and can alone compensate for the ablation of the entire vagal crest67.
Recent genetic studies have suggested that much of what has traditionally been considered vagal crest has to be reconsidered56. The vagal crest has properties that are transitional between cranial and truncal37. Crest-derived cells next to somites 1–2 are thought to give rise to Schwann cells that migrate along descending vagus nerve fibres to the oesophagus and stomach and give rise to the enteric nervous system (ENS) in these regions56 (FIG. 1). These are truly vagal-crest-derived, because they migrate as components of the vagus nerves. By contrast, crest-derived cells from somite levels 3–7 are suggested to be like the more distal crest-derived cells of the trunk and actually to be contributors to sympathetic chains; moreover, their ventral migration seeds not only the sympathetic chain ganglia but also the ganglia of the entire GI tract. These data are interpreted to account for why abnormalities are seen in both enteric and sympathetic ganglia when the gene encoding the receptor tyrosine kinase ERBB3 is deleted56,174,175. The atrophy in half of the oesophageal ganglia when the nerve-associated form of the ERBB3 ligand, neuregulin 1 (considered to be essential for parasympathetic neuronal development), is deleted is considered to be evidence that the oesophageal neurons are parasympathetic. The ENS is thus postulated to have four origins (FIG. 1): crest-derived cells from axial levels 1–2, which migrate as Schwann cells to the oesophagus and stomach; a ‘sympatho-enteric’ component from axial levels 3–7, which migrates through the entire remainder of the bowel; the sacral crest (caudal to somite 28), which contributes to the post-umbilical gut; and Schwann cells that reach the bowel with extrinsic nerves65. Further studies, perhaps incorporating live imaging of genetically labelled cell populations, are needed to confirm or deny this interesting new hypothesis.
Fig. 1 |. Migration of neural-crest-derived progenitors to the primordial gut.
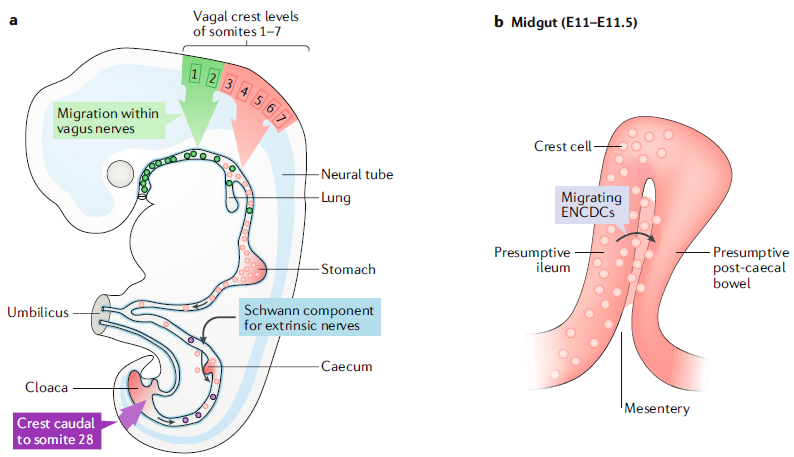
a | A diagram of a developing mouse fetus after the formation of the primordial foregut, midgut and hindgut. The vasculature defines regions of the fetal bowel: the coeliac trunk supplies the foregut, the superior mesenteric artery supplies the midgut and the inferior mesenteric artery, supplies the hindgut. The foregut includes the portion of the bowel from the pharynx to the entry of the pancreatic and bile ducts in the mid-duodenum; the midgut extends to the mid-transverse colon; and the hindgut extends to the ectoderm of the anal canal. The midgut grows rapidly and herniates transiently into the umbilical cord but then folds extensively and rotates upon returning to the abdomen. Neural-crest-derived cells were first shown to migrate to the bowel from the vagal level (corresponding to somites 1–7) and sacral axial level (dark red; caudal to somite 28). A third source of crest-derived precursors of neurons or glia (blue) enter the colon later in ontogeny, among the Schwann cell population found in innervating extrinsic nerve fibres. Most recently, molecular genetic studies have suggested that the vagal level of the crest is more complex than previously suspected. Crest-derived cells from axial levels 1–2 (green) migrate to the oesophagus within the descending fibres of the vagus nerves. Properties of the more caudal vagal crest, at levels 3–7 (red), are more like those of the truncal crest, which gives rise to sympathetic ganglia. This ‘sympatho-enteric’ crest colonizes the entire gut and is the major source of enteric neurons and glia. Sacral-crest-derived cells colonize only the post-umbilical gut. The proportion of enteric neurons derived from the sacral crest (purple) is relatively small and higher distally than proximally. In contrast to vagal-crest-derived cells, which migrate proximo-distally, sacral-crest-derived cells migrate in a distal-to-proximal direction. b | During the folding of the midgut, at embryonic day 11 (E11) to E11.5, the presumptive ileum is transiently located next to a loop of post-caecal bowel (presumptive ascending colon). The dorsal mesentery intervenes between them. A subset of cells within the descending vagal enteric neural-crest-derived cell (ENCDC) population (red) takes a shortcut through the mesentery (pink) to enter the still-to-be colonized gut distal to the caecum. These cells do not have to traverse the caecum. Part a is adapted with permission from REF9, Elsevier.
Further work involving transplantations at ectopic levels and various times suggested that crest cells do not migrate to the bowel because they are intrinsically programmed to get there. Rather, they migrate along defined pathways, and the microenvironments these cells encounter along these routes and in their final destinations influence how they differentiate32. These observations therefore suggested that cells in the premigratory crest are undetermined and multipotent.
Although crest cells from other axial levels implanted into the vagal region of an embryo can colonize the gut and give rise to enteric neurons and glia, vagal crest cells seem to be more effective colonizers9; for example, when vagal crest cells replace the sacral crest, the vagal-crest-derived cells are far more invasive than non-replaced sacral-crest cells and colonize a greater proportion of the gut33. One hypothesis to explain the difference between vagal-crest-derived cells and sacral-crest-derived cells that migrate to the bowel is that the crest-derived cells at somites 8 and below express roundabout (ROBO) receptors and thus avoid the proximal gut, where the repellent ligand for ROBO receptors, SLIT2, is expressed34,35 (FIG. 1a). SLIT2 does not similarly repel vagal-crest-derived cells, because they do not express ROBO and thus are able to enter the SLIT2-expressing bowel.
To reach the gut, vagal-crest-derived and sacral-crest-derived cells migrate ventrally away from the neuraxis. The earliest wave of vagal-crest-derived cells migrates to the pharyngeal arches and the cardiac outflow tract36,37. The later pathway taken by ENS precursors as well as additional cardiac-crest cells (at somites 1–3) leads preferentially through the mesenchyme of the anterior portions of each somitic sclerotome38,39. Unlike their ENS counterparts, cardiac-crest precursor cells express CXC-chemokine receptor 4 (CXCR4) and migrate towards the CXCR4 ligand, stromal cell-derived factor 1 (SDF1), in the developing heart40,41.
A repulsive ephrin receptor-ephrin ligand interaction helps to guide crest-derived cells leaving the neuraxis42,43; however, selective migration of crest-derived cells through somites persists in mice that lack ephrin signalling44,45. The preference of crest-derived cells for the anterior halves of somites probably depends more on another ligand-receptor interaction — namely, the repulsion of neuropilin-2-receptor-expressing cells by the semaphorin-3F-expressing mesenchyme of posterior half-somites46,47.
The migration through the vagal somitic mesenchyme seems to restrict the potential of crest-derived cells to ENS phenotypes and to eliminate lineages that are inappropriate in the gut, such as the melanocytic lineage48. Retinoic acid is produced in the somitic environment and acts on nuclear retinoic acid receptor-α (RARα) and RARγ within migrating crest-derived cells49. A retinoic-acid-driven upregulation of the expression of the receptor tyrosine kinase RET (discussed in detail below) drives the commitment of crest-derived cells to enteric lineages50. The ENS fails to arise in mice that are deficient in retinaldehyde dehydrogenase 2, one of the biosynthetic enzymes for retinoic acid51.
The migration of crest-derived cells ahead of descending vagus nerve fibres along the murine vagal pathway was first visualized in 1989 (REF52) and was later confirmed53. The nerve fibres move quickly along the shared pathway and overtake lagging vagal-crest-derived precursor cells by embryonic day 10 (E10). Precursors derived from the truncal crest (corresponding to somites 8–27) that are destined for dorsal root and sympathetic ganglia exhibit a similar pattern of migration, advancing along a pathway shared later by projections of spinal nerve fibres46,47. Interestingly, a subset of the migrating and proliferating vagal-crest-derived precursor population seems to be transiently catecholaminergic and gives rise to non-catecholaminergic neurons within the ENS52,54,55. A recently proposed alternative explanation for the presence of crest-derived cells among descending vagal fibres is that they are not uncommitted precursors but really Schwann cells that vagal axons guide to the oesophagus and stomach, where they transdifferentiate to give rise to neurons56.
Vagal enteric neural-crest-derived cells (ENCDCs) seem to migrate proximo-distally within the outer gut mesenchyme; however, a large subset takes a shortcut from the ileum through the dorsal mesentery (the mesenchymal attachment of the bowel to the body wall; FIG. 1) and thus avoids having to traverse the caecum57, which seems to slow the migration of traversing crest-derived cells58. ENCDCs migrate as chains and must remain in contact with one another for directional migration59.
Sacral-crest-derived cells, similar to their vagal counterparts, migrate to the bowel through the somitic mesenchyme; however, the sacral population waits alongside the hindgut from E11.5 before entering it with the invading extrinsic sacral innervation at approximately E14.5 (REFS53,60). In the gut, sacral-crest-derived cells migrate orally, countercurrent to the anally directed vagal population61,62. Sacral-crest-derived cells give rise to a minority of post-umbilical neurons (~20%), and their numbers are inversely proportional to the distance from the umbilicus60,63,64. The sacral innervation has recently become controversial in that a redefinition has been proposed (Box 2). Schwann cells, or truncal-crest-derived precursors that are currently indistinguishable from Schwann cells, enter the caudal midgut with extrinsic nerves and give rise to ~20% of the neurons of the colonic ENS65 (Box 1).
Box 2 |. What is the autonomic nervous system and how does the ENS relate to it?
Surprisingly, the nature of the motor component of the sacral innervation has recently been challenged. The physiologist John Newport Langley defined the autonomic nervous system as one that is entirely efferent and differs from the skeletal motor system by having at least one peripheral synapse to traverse before innervating its effectors4. In his original classification, Langley distinguished parasympathetic and sympathetic divisions on the basis of their outflows from the CNS. He considered the sacral autonomic outflow to be parasympathetic, which meant that it was similar anatomically and functionally to the cranial outflow. The cranial outflow to the gut is found in the vagus nerves.
The cranial and sacral parasympathetic neurons that actually innervate effectors are found in ganglia that are located within or very close to the innervated organs. By contrast, the thoracolumbar sympathetic outflow from the CNS is directed to prevertebral and paravertebral ganglia. Langley’s insights have not been disproved176; however, a recent report has now analysed cellular phenotypes to re-examine the autonomic classification177. The authors identified 15 phenotypic and developmental ‘features’ that distinguish the preganglionic and postganglionic neurons of cranial parasympathetic neurons from their thoracolumbar (sympathetic) analogues. For each of these features, the sacral and thoracolumbar outflows are ‘indistinguishable’. The conclusion of this molecular study, which is quite different from that of Langley4, is that the parasympathetic outflow may be routed exclusively through cranial nerves, whereas everything else — including sacral outflow — may be considered sympathetic and innervated by spinal nerves.
Langley, of course, classified the enteric nervous system (ENS) as a separate autonomic division, neither sympathetic not parasympathetic, because most enteric neurons receive no direct innervation from the CNS4; however, the ENS is now thought to receive an extrinsic innervation that includes sympathetic, parasympathetic and sensory components. The new definition of the autonomic nervous system outlined above177 does not include the ENS, but it would imply that the parasympathetic innervation of the bowel ends at the distal-most extent of the vagi, which is species-dependent but universally excludes the rectum178. The distal colon and rectum would therefore be considered to receive only a sympathetic innervation, although some of these newly defined sympathetic nerves (formerly considered sacral parasympathetics) are functionally antagonistic to the remainder. Furthermore, the new definition does not take into account the reciprocal connections of the sacral region to the spinal cord5 or the direct projections of the spinal cord to the bowel179, which, according to Langley’s old definition, are not at all sympathetic, because they do not synapse in prevertebral or paravertebral ganglia. In fact, given that molecular analyses have suggested that the ENS distal to the oesophagus is derived, in a major part, from sympatho-enteric progenitors and that the truly ‘vagal’ crest is limited to levels next to somites 1–2 (REF.56), the entire ENS may be considered to be a component of the sympathetic nervous system. This notion is confusing because the colorectum would be considered to receive functionally antagonistic sympathetic innervations177. The evident contradictions in this discussion can be rationalized, but particularly in view of the multiple physiological, neurochemical and anatomical differences between sympathetic and parasympathetic nervous systems176, there is good reason to retain the long-accepted physiological-anatomical definitions of the vagal crest29,180 and the autonomic nervous systems4 and to superimpose the new molecular information on that scaffold.
Progenitor differentiation
Regardless of origin, ENS progenitors that colonize the gut face a complex problem: they must proliferate to build and maintain a precursor pool, differentiate to form neurons and glia without depleting the progenitor pool and coordinate these processes with a lengthy migration along the entire extent of the developing gut. Progenitor proliferation is important to maintain an adequate mass of cells at the migratory wavefront and to sustain the distal-ward movement of precursor cells in the developing intestine66–68; therefore, differentiation must be carefully timed. Premature differentiation of progenitors into neurons or glia leads to inadequate colonization of the distal bowel69–72.
Various molecules influence progenitor differentiation, but progenitors express three central regulators that are essential for normal ENS development: the homeodomain transcription factor paired-like homeobox 2B (PHOX2B), transcription factor SOX1O and RET. In mice lacking PHOX2B, vagal-crest-derived progenitors enter the foregut at E10.5 but an ENS does not develop, presumably because PHOX2B is required for the normal expression of other important proteins, including RET and the transcription factors SOX10 and achaetescute homologue 1 (ASCL1; also known as ASH1)73,74. Heterozygosity for mutations in human PHOX2B (which are mostly loss-of-function mutations) causes congenital central hypoventilation syndrome (CCHS); 16% of individuals with CCHS have associated HSCR75,76. Thus, even PHOX2B haploinsufficiency is sufficient to exert major effects on ENS development and GI function.
All crest-derived progenitors express SOXIO as they delaminate from the neural tube and this initial phase of SOXIO expression is PHOX2B-independent. In the developing ENS, progenitors need this initial SOX10 expression to maintain potency and survival. A naturally occurring autosomal dominant mutation with incomplete penetrance in mice (Dom) was observed to cause deficiency of myenteric neurons, leading to megacolon77; Dom was later identified to be in the Sox10 locus78,79. Sox10 haploinsufficiency is sufficient to cause colonic hypoganglionosis and megacolon in mice80; moreover, human mutations causing SOX10 haploinsufficiency are associated with Waardenburg-Shah syndrome81.
Initially, neural-crest-derived progenitors need to express SOX10 in order to survive, maintain multipotency and turn on expression of PHOX2B and ASCL1 (REFS82,83). As progenitors differentiate, they must maintain SOX10 expression in order to generate glia80,82 and downregulate SOX10 levels to generate neurons83 (FIG. 2). This model of SOXIO function in neural-crest-derived progenitor differentiation is largely based on observations in the extra-enteric PNS. To determine whether SOX10 expression affects the differentiation of ENCDCs specifically, a recent study used genetic lineage tracing to examine enteric crest derivatives in Sox10Dom/+ mice84. Although the colons of Sox10Dom/+ mice exhibited varying lengths of distal aganglionosis, the ENS in the small intestines of these animals was grossly normal84. Close examination of the small intestinal ganglia in Sox10Dom/+ mice revealed normal numbers of neurons and glia but alterations in the types of neurons generated, leading to defects in GI motility84. These findings suggest that SOXIO plays a role in neuronal-subtype specification in the small intestine and that distinct levels of SOX10 activity might be required for neuronal specification and gliogenesis. Developmental defects in the cell-subtype composition of enteric ganglia resulting from reduced activity of SOXIO or other factors might represent an unrecognized aetiology of GI dysmotility.
Fig. 2 |. Molecular regulation of cell-type diversification in the enteric nervous system.
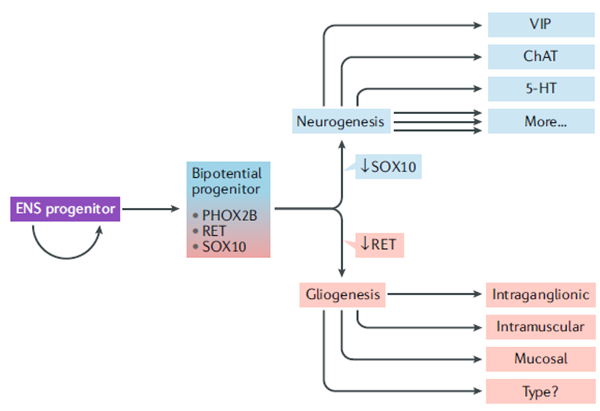
Enteric nervous system (ENS) progenitors must proliferate to maintain an adequate progenitor pool while a certain proportion become bipotential progenitors that generate cells that diverge along neurogenic or gliogenic trajectories. Neurogenic commitment requires downregulating transcription factor SOX10 and maintaining receptor tyrosine kinase RET expression, whereas gliogenesis requires maintaining SOX10 and downregulating RET. Paired-like homeobox 2B (PHOX2B) expression is maintained in all enteric neurons and some enteric glia. There are many neuronal subtypes in the ENS, including those marked by vasoactive intestinal peptide (VIP), choline acetyltransferase (ChAT), serotonin (5-HT) and many others. The full extent of enteric glial diversity remains unknown, but morphologically distinct glia are found in at least three distinct locations.
RET is a signalling co-receptor for the glial-derived neurotrophic factor (GDNF) family of ligands and is essential for ENS development. In mice lacking RET, the GI tract beyond the most proximal region of the stomach (near the oesophago-gastric junction) is almost entirely aganglionic85. In humans, heterozygous loss-of-function mutations in RET are the most common known cause of both familial and sporadic forms of HSCR86. One important role of GDNF signalling through RET and one of its co-receptors, GDNF family receptor-αl (GFRαl), is to guide enteric neural-crest-derived progenitors into and along the developing gut, at least as far as the caecum, where GDNF accumulates87. However, similar to SOXIO, RET plays multiple roles during ENS development, and some of these roles differ between the small and large intestines. When GFRα1 or RET is globally deleted in mice at E15.5 (after ENCDCs have completed their colonization of the gut), enteric neurons undergo apoptosis in colon but not in small intestine, causing colonic hypoganglionosis88,89. These data suggest that RET signalling is required selectively for the survival of newly born colonic neurons. RET expression is maintained in a subset of enteric neurons after fetal development in the small and large intestines90,91, but its functions in the postnatal ENS remain largely unknown. Another receptor tyrosine kinase, MET, is expressed in subsets of enteric neurons that are mutually exclusive of RET+ subsets90. Ablation of MET in the mouse PNS leads to defects in enteric neurite outgrowth and increased vulnerability to intestinal inflammation90. Similarly, mice lacking the RET co-receptor GFRα2, or its ligand, neurturin, exhibit reduced density of enteric nerve fibres and altered GI motility without aganglionosis92,93. Thus, receptor tyrosine kinases probably play many roles during and after ENS development that remain to be discovered.
Recent studies have shown that ENCDCs in the developing gut are not homogenous; cells close to the migrating wavefront co-express PHOX2B, SOXIO and RET, whereas cells trailing the wavefront are more heterogenous in their expression of these and other markers94,95. Single-cell RNA sequencing of Sox10-expressing cells in E12.5 mouse gut has revealed at least three transcriptional types of enteric neural-crest progenitors: one that co-expresses Phox2b, Sox10 and Ret, and two others that diverge along either gliogenic or neurogenic trajectories96. Consistent with this divergence, lineage tracing of the progeny of single Sox10+ progenitors in the small intestines of mice shows that some Sox10+ progenitors give rise only to neurons, some give rise only to glia and others give rise to both96. Deletion of Ret within individual Sox10-expressing progenitors leads to increased proliferation at the expense of neuronal differentiation in the clonal populations derived from these cells, suggesting that RET is required to activate the neurogenic programme96. Thus, differentiating ENCDCs seem to face a proverbial fork in the road: to generate neurons, they must downregulate SOXIO and maintain RET expression, and to generate glia, they must downregulate RET and maintain SOXIO expression (FIG. 2). How SOXIO and RET shift their roles from progenitor maintenance and guidance to neuronal or glial fate acquisition and subtype specification is still incompletely understood.
Although PHOX2B, RET and SOX1O are crucial, they are by no means the only molecules important for normal ENS development. A wide range of signalling pathways — including neuregulin 1 activation of receptor tyrosine kinase ERBB3, endothelin 3 activation of endothelin receptor type B, Notch proteins, Hedgehog, retinoids, bone morphogenetic proteins (BMPs) or the nuclear orphan receptor NR2F1 — all modulate enteric progenitor cell proliferation and differentiation to alter the balance of neurons and glia in the ENS. A comprehensive discussion of all of these pathways is beyond the scope of this Review, but they have been well described by others97–99. It is clear that ENCDC differentiation is finely tuned and can be altered by many genetic and environmental factors, with long-lasting structural and functional consequences.
Formation of ganglionated plexuses
Most of the ENS is organized into two interconnected plexuses of ganglia — the submucosal plexus in the submucosa, and the myenteric plexus between the circular and longitudinal muscle layers of the gut wall — each of which contain multiple subtypes of neurons involved in distinct circuits and functions. More than 30 years ago, myenteric neurons were observed to develop before submucosal neurons in mice; a similar pattern was later detected in human fetal tissues100 (FiG. 3a). These observations suggested that some neural progenitors migrate radially from the myenteric plexus towards the mucosa to give rise to the submucosal plexus. A birth-dating study with [3H]thymidine in mice confirmed that both peptidergic and non-peptidergic neurons in the myenteric plexus are born before their counterparts in the submucosal plexus101, supporting the ‘outside-in’ hypothesis of ENS development, which has become widely accepted in the field.
Fig. 3 |. The ‘outside-in’ development and columnar organization of the enteric plexuses.
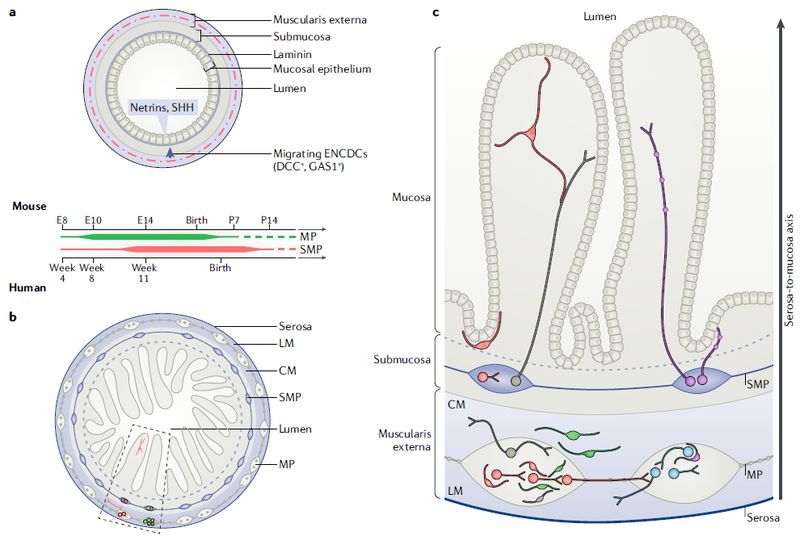
a | Schematic of the murine bowel during embryonic development illustrates the radial migration of enteric neural-crest-derived cells (ENCDCs) from the muscularis externa into the submucosa. The rudimentary mucosal epithelium expresses netrins, which serve as chemoattractants, and sonic hedgehog (SHH), which serves as a chemorepellent. The subset of ENCDCs that expresses the netrin receptor DCC migrates into the submucosa to populate the submucosal plexus (SMP). SHH signalling through the growth arrest-specific protein 1 (GAS1) receptor prevents them from migrating into the mucosa and extending premature projections to the epithelium. Laminin 111, which can convert netrin-DCC signalling from attractive to repulsive, is expressed in the basal lamina immediately underneath the epithelium and might serve as an additional cue that keeps ENCDCs from migrating beyond the submucosa into the epithelium. The timelines for myenteric plexus (MP) and SMP development in mice and humans are illustrated below from embryonic day 8 (E8) through postnatal day 14 (P14) and from week 4 of gestation, respectively. The timelines are based on birth-dating of enteric neuronal subtypes in mice; human data are much more limited and suggest that some ENCDCs have colonized the hindgut as early as week 8 of gestation and that SMP development lags behind MP development by ~3 weeks100. Timelines are illustrated as continuums without definitive end points because recent reports suggest that there is robust enteric neurogenesis in the mature murine gut131, and it remains unknown to what extent this occurs in humans. b | Schematic of the mature small intestine illustrating the laminar organization of the bowel and its integrated MP and SMP. The MP is located in between the circular (CM) and longitudinal (LM) layers of smooth muscle, and the SMP is located in the submucosa. Clonally related neurons and glia (shown in the same colours) are distributed in columns along the radial axis, as highlighted in the boxed area. c | The boxed area in part b is expanded to illustrate the observations made by lineage tracing of the progeny of individual SoxlO-expressing ENCDCs using the multicolour Confetti reporter and examining clonally related cells (which all express the same reporter colour) in mature bowel96. Three types of clones were observed: neuronal (N; depicted in blue), glial (G; green) and those that contained both neurons and glia (NG; red). The majority of clonally related cells were distributed in a columnar fashion, similar to the CNS. All three types of clones can be observed in the MP, but only NG and G contribute to the SMP (neurons and/or glia) and mucosa (glia). Sister cells are distributed in register along the radial (serosa-to-mucosa) axis of the bowel.
Lasrado and colleagues recently used new mouse genetic tools to formally test this hypothesis96. They mapped the fates of individual enteric neural-crest-derived progenitors in mice by expressing the Confetti transgene in Sox10-expressing cells at E12.5 and analysing reporter expression in adult small intestines96. Remarkably, most clonally related cells that expressed a single reporter colour were organized into columns along the radial axis, from serosa to mucosa (Fig. 3b,c). Individual Sox10+ precursors gave rise to clones containing neurons, glia or both (neuroglia (NG)). All three types of clones contributed to the myenteric plexus, but only the glia-containing and NG-containing clones contributed to the submucosal plexus and mucosa. These glial clones and NG clones, which spanned both plexuses, consisted of cells that were aligned along the radial axis of the gut (Fig. 3c). These findings suggest that submucosal neurons arise from bipotent precursors that originate within the myenteric plexus and that the topological organization of the ENS is columnar, similar to that of the cortex and the spinal cord. Moreover, clonally related neurons derived from E12.5 Sox10+ cells were more likely to show synchronous Ca2+ responses to single-pulse electrical stimulation of the plexus than were unrelated neurons, suggesting that sister cells are more likely to wire together96. Enteric neuronal subtypes are born during distinct developmental periods101, and cell types with similar birthdates might be more likely to wire together. Further studies will need to determine to what extent lineage relationships and developmental timing each contribute to enteric circuit formation.
The signals that guide differentiating progenitors from the myenteric plexus to the submucosal plexus and determine their connectivity have only begun to be identified. One important set of signals is the netrin family of secreted proteins, which are also involved in axon guidance in the CNS. The developing epithelium of the murine intestine expresses netrins, and a subset of ENCDCs expresses the netrin receptor DCC102. Mice lacking DCC do not have a submucosal plexus102, suggesting that DCC-mediated netrin signalling is required for enteric neural-crest-derived progenitors to migrate radially and populate the submucosal plexus (FIG. 3a). Interestingly, netrin-DCC signalling is also important in mice and chicks for driving the migration of ENCDCs from the primordial distal foregut into the pancreatic bud to give rise to pancreatic ganglia and, later (at approximately E15 in mice), in guiding vagal sensory axons to myenteric ganglia103. Laminin 111 is concentrated in the basal lamina of the intestinal epithelium104,105, promotes neuronal differentiation106 and converts the effect of netrin-DCC signalling from attraction into repulsion107; therefore, laminin 111 may be a signal that causes radially migrating ENCDCs to stop in the submucosa, short of the source of netrin in the epithelium108 (FIG. 3a). Recent transcriptional profiling of Sox10-expressing cells at two different time points in mouse ENS development revealed more than 150 signalling molecules and receptors expressed by differentiating enteric neural-crest-derived progenitors as well as the surrounding mesenchyme109. This rich resource is sure to contain many more candidate pathways that might influence the development of the submucosal plexus.
Once newly born neurons populate the two enteric plexuses, they must extend radial and longitudinal projections to connect these plexuses and form functional circuits that target the mucosa, the muscularis externa, interstitial cells of Cajal, extrinsic neurons, blood vessels, lacteals (lymphatic capillaries) and each other. The mechanisms that underlie enteric circuit formation and determine this connectivity remain poorly understood. The G protein-coupled receptor cadherin EGF LAG seven-pass G-type receptor (CELSR3), known for its role in planar cell polarity, is one of the few molecules shown to be important for establishing ENS connectivity. In mice lacking CELSR3 in the ENS, neuronal density and subtype specification are intact, but neuronal projections are disorganized, leading to abnormal GI motility and premature death110. The circuits of the ENS, which have yet to be meaningfully characterized, even in the adult bowel, represent one of the great challenges for future research. Because neurons of each type are intermixed in enteric ganglia, and functional segregation into spatially defined nuclei is lacking, these circuits are extremely difficult to map. It is likely that advances in functional imaging and synaptic tracing will accelerate this process.
Differentiating enteric neurons express many molecules known to be active in axon guidance, but most have not been functionally examined in the ENS109. One secreted signal that plays an important role in regulating mucosal projections is sonic hedgehog (SHH). During development, SHH is expressed in the intestinal epithelium, and ENCDCs in the myenteric plexus express one of its receptors, growth arrest-specific protein 1 (GAS1)111,112. In mice lacking SHH, the myenteric and submucosal plexuses are present by late fetal development, but the immunoreactivity of neural markers is also found ectopically in the mucosa throughout the small intestine111. Shh-null mice also have extensive non-neural defects in gut patterning and the epithelium, making it unclear whether this ENS defect is direct or indirect. Global or conditional deletion of Gas1 in the PNS of mice induces a similar ENS phenotype, with ectopic neurons and premature projections into the mucosa112,113. In combination with in vitro data showing that SHH repels enteric neurites in a GAS1-dependent manner113, these studies suggest that SHH secreted from the epithelium repels GASl-expressing enteric neural-crest-derived progenitors; thus, these progenitors are prevented from inappropriately colonizing the mucosa, and differentiating myenteric neurons are prevented from prematurely extending projections towards the epithelium (FIG. 3a). Additional studies will be needed to determine how the repulsive SHH cue is relieved to allow appropriate mucosal innervation by enteric neurons later in development and the identity of chemoattractive cues that further guide target selection. Overall, most of the signals responsible for enteric neuronal connectivity and synapse formation remain to be discovered. Observations from a new enteric neuron–glia co-culture model suggest that, as in the CNS, glial-derived factors, such as GDNF and purines, probably play an important role114.
Enteric neurons
Neuronal diversity in the ENS.
The ENS in mature mammals contains many different types of neurons3. These cells can be distinguished on the basis of their neurotransmitter content, electrophysiological characteristics, axonal projections and roles in enteric physiology115. Neurons in the myenteric plexus can broadly be categorized into at least three major types: IPANs; motor neurons that excite or inhibit smooth muscle; and interneurons that ascend, descend and/or project to the submucosal plexus3 (Fig. 4). At least 70% of all myenteric neurons are cholinergic, but this group is heterogeneous and can be subdivided on the basis of various co-transmitters. Most enteric neurons that inhibit smooth muscle contain nitric oxide synthase 1 (NOS1), but NOS1 is also found in some enteric interneurons. Calbindin marks IPANs specifically in the guinea pig ENS116 but is not such a clear IPAN marker in other species115; in mice, IPANs may also contain calretinin, which is also found in subsets of neurons that excite smooth muscle and in interneurons. One set of descending myenteric interneurons, which are relatively few in number but project very widely, expresses serotonin (5-HT)117,118. Many additional neuroactive molecules and neurotransmitters, such as GABA119 and dopamine120,121, characterize the ENS; however, their precise roles in ENS function and control of enteric physiology are not yet clear.
Fig. 4. Neuroglial diversification and cellular interactions in the enteric nervous system.
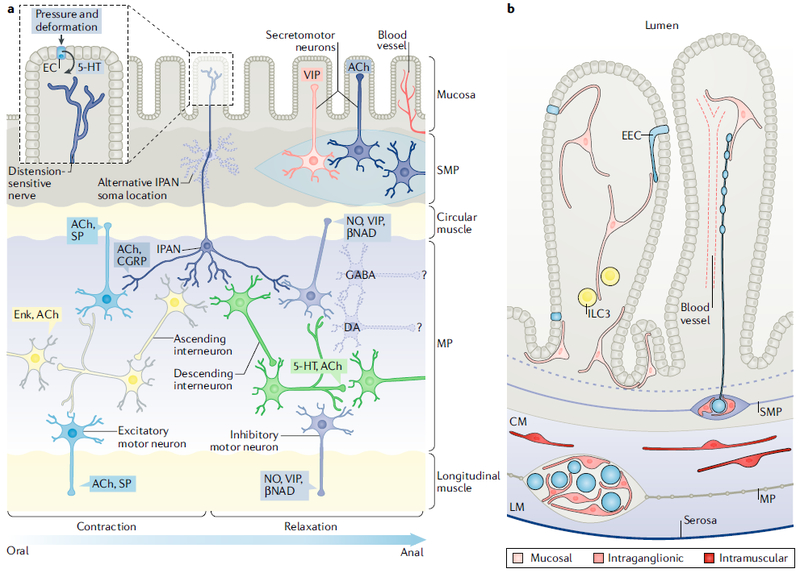
| a | Schematic illustrating the major neuronal subtypes found in the enteric plexuses and highlighting the subset that forms the peristaltic reflex microcircuit. Luminal distention or mucosal deformation triggers direct activation of mechanoreceptive endings of intrinsic primary afferent neurons (IPANs) as well as indirect activation of IPANs upon serotonin (5-HT) release by enterochromaffin cells (ECs) in the epithelium. IPANs release acetylcholine (ACh) to activate ascending and descending interneurons, which stimulate excitatory and inhibitory motor neurons, respectively. Motor neuron activity leads to oral contraction and anal relaxation of intestinal smooth muscle, which propels luminal contents in the proximal-distal direction. Ascending interneurons express enkephalin (Enk) and descending interneurons release ACh and 5-HT. Excitatory motor neurons secrete ACh and substance P (SP), whereas inhibitory motor neurons secrete nitric oxide (NO), vasoactive intestinal peptide (VIP) and the purine β-nicotinamide adenine dinucleotide (βNAD). Secretomotor and vasomotor neurons of the submucosal plexus (SMP) secrete ACh or VIP. GABAergic and dopaminergic (DA) neurons are also found in enteric plexuses, but their synaptic targets are unknown. b | Schematic of enteric glial subpopulations and their interactions with other cell types. Mucosal glia are found in the lamina propria immediately underneath the epithelium and have been reported to interact with enteroendocrine cells181 (EECs; specialized epithelial cells that release peptides and other signals in response to luminal stimuli, such as ingested nutrients or mechanical distention), a subset of immune cells known as group 3 innate lymphoid cells (ILC3s; important for antimicrobial defence and maintaining tolerance to commensal microbiota)182, nerve fibres and blood vessels. Intraganglionic glia are found within the myenteric plexus (MP) and SMP, closely apposing and partially ensheathing neurons (depicted as blue circles). A subset of intraganglionic glia is connected by gap junctions. Long, bipolar, intramuscular glia are found in the circular and longitudinal muscle layers (CM and LM, respectively), in close association with nerve fibres (not shown) that innervate the smooth muscle. CGRP, calcitonin gene-related peptide. Part a is adapted from REF156, Springer Nature Limited.
The submucosal plexus contains cholinergic and vasoactive intestinal peptide-expressing secretomo-tor neurons as well as IPANs122,123. The submucosal and myenteric plexuses reciprocally project to one another124, and myenteric serotonergic neurons stimulate the activity of submucosal cholinergic neurons that stimulate epithelial proliferation125,126. The high and not yet fully characterized phenotypic diversity of enteric neurons reflects the complexity of the many functions and behaviours that the ENS regulates or controls with or without input from the CNS5,127. Little is known at the transcriptional level about how enteric neuronal diversity is established. A recent advance is the discovery that SOX6 expression is required for the formation of murine gastric dopaminergic neurons109.
Cell-cycle regulation.
Various types of enteric neurons exit from the cell cycle at different times and in a reproducible sequence101. Early-born and late-born enteric neurons are intermixed in every ganglion of the myenteric plexus. Early-born enteric neurons include those that contain 5-HT and acetylcholine, whereas late-born neurons are peptidergic and/or express NOS1, calbindin or calretinin101,128,129. BMPs (BMP2 and BMP4) from the mesenchyme stimulate BMP receptors on enteric neurons to promote their differentiation and thus regulate the timing of withdrawal from the cell cycle129. Alterations in this timing and the number of amplifying cell divisions between commitment to the neuronal phenotype and the terminal mitosis may alter enteric neuronal specification. Consequently, the number of enteric neurons and the distribution of various enteric neuronal phenotypes are altered when BMPs (which promote withdrawal from the cell cycle) are deficient during the period of intense enteric neurogenesis (before postnatal day 21 (P21) in mice). Exposure of ENCDCs to a factor that promotes proliferation (such as GDNF, which, in excessive concentrations, can overly stimulate RET) exerts an opposite effect to that of BMPs and alters the normal pattern of enteric neuronal phenotypic expression130.
In mice, the late birth of neurons continues postnatally through about P21, after which the pace of neurogenesis abates101; however, recent work has suggested that enteric neurogenesis and neuronal turnover occur in adult mice at an astonishingly brisk rate: 88% of small intestinal myenteric neurons in adult mice may be less than 2 weeks old131. This robust, and previously unsuspected, turnover of enteric neurons requires a great deal of cell death. In the developing murine ENS, apoptosis occurs at a very low rate132, if at all133, and cell death is not a major phenomenon. This paucity of developmental apoptosis stands in contrast to other regions of the developing nervous system, where neurons are generated in excess and then cut back. Further studies will need to characterize these new observations of adult enteric neurogenesis and determine how the underlying molecular and cellular mechanisms compare to the programmes that lead to initial development and differentiation of enteric neurons and glia.
Neurotransmitter-mediated regulation of late neurogenesis.
Because enteric neurons are born in a reproducible, phenotype-related sequence101,128, terminally differentiated enteric neurons coexist with dividing precursors. The early-born serotonergic and cholinergic neurons are thus able to influence, through their activity, the precursors of late-born neurons before the latter commit to their terminally differentiated phenotypes121. Considerable evidence supports the idea that early-born enteric neurons, neurotransmitters and their transporters all modify ENS development. We concentrate on 5-HT as an example.
Serotonergic neurons synapse on dividing precursors in the developing ENS134, and the ENS is profoundly hypoplastic in mice that lack tryptophan hydroxylase 2, which is essential for 5-HT biosynthesis in neurons135. A hypoplastic ENS is also found in mice carrying a gain-of-function mutation (G56A) in Slc6a4, the gene encoding the serotonin transporter (SERT), which removes 5-HT from its receptors; the G56A-mutant SERT clears 5-HT before it can adequately activate its receptors126. By contrast, the ENS is hyperplastic in mice in which the effects of 5-HT are amplified, such as those that lack SERT or that have been exposed to a selective serotonin-reuptake inhibitor (SSRI) throughout gestation126. GI motility is abnormal when the ENS is either hyperplastic or hypoplastic.
5-HT and 5-HT4 receptor agonists stimulate enteric neurogenesis when they are applied to isolated ENCDCs in vitro, and this effect is blocked by 5-HT4 receptor antagonists135,136. 5-HT4 receptor agonists also stimulate enteric neurogenesis in the adult bowel in vivo, and 5-HT4 antagonists block this stimulation136–138. These data suggest that transmitter release from enteric serotonergic neurons is essential to support 5-HT4-receptor-mediated neurogenesis in the developing and mature ENS. Consistent with this suggestion, 5-HT4 receptor agonists rescue ENS development and GI motility in mice in which SERT is overactive126. The early-born enteric serotonergic and cholinergic neuronal phenotypes are of clinical interest, because antidepressants such as SSRIs, drugs of abuse (such as cocaine and opiates) and antispas-modics are commonly ingested during pregnancy, alter the function of these neurons and thus may exert unanticipated and long-lasting effects on the ENS of the offspring126,139.
In the brain, SERT is transiently expressed during development in glutamatergic neurons, which do not synthesize 5-HT; nevertheless, their uptake of 5-HT enables these ‘5-HT-absorbing’ neurons to utilize 5-HT to establish the innervation pattern of the sensory cortex140–143. SERT expression in the fetal ENS may also not be restricted to serotonergic neurons. Future studies will have to determine whether non-serotonergic 5-HT-absorbing cells, similar to those of the CNS, function in ENS development. Indeed, in the gut, SERT is expressed not only in neurons but also in fetal and adult enterocytes and is functional in the early fetal endoderm144. Mucosal SERT probably protects the developing ENS from the large amount of 5-HT that enterochromaffin cells constitutively release. Mucosal 5-HT would overwhelm the much smaller quantity of 5-HT secreted at enteric serotonergic synapses if it were able to reach the ENS118. 5-HT, therefore, is a multifunctional molecule, acting not only as a neurotransmitter, paracrine factor and hormone118 but also as a growth factor that regulates the genesis and diversity of enteric neurons.
Disrupted neurogenesis and disease.
Disruption of the ability of early-born enteric neurons to regulate neurogenesis during development is a novel but plausible mechanism that may contribute to disorders of the mature bowel. IBS, a functional condition of aberrant motility and visceral pain, often begins in childhood145,146. Children with IBS show evidence of serotonergic dysfunction (higher 5-HT content and decreased SERT in intestinal mucosa) in the bowel147, which could exert lasting effects on coincident neuronal development. In animal models, exposure to early-life adversity, such as maternal deprivation, induces long-term upregulation of enteric cholinergic neuronal activity; furthermore, in rats, chemical irritation of the developing colon148 or neonatal stress (caused by maternal separation)149,150 induces adult visceral hypersensitivity. IBS also frequently follows intestinal infection and persists long after infection-induced inflammation has cleared151, supporting the idea that experience-dependent alterations in the microenvironment of developing and even adult enteric neurons cause lasting changes in the ENS152.
Enteric neurons become active and the ENS becomes functional surprisingly early in murine prenatal development27,153. The effects of this early neuronal activity may not only prepare animals for oral nutrition but also contribute to sculpting ENS development. These effects need not all be prenatal, as ENS development persists after birth154; enteric neurons continue to be born in the postnatal and mature bowel65,136,137,155. Psychosocial trauma, stress, drugs (therapeutically administered or abused), infection and inflammation alter neuronal activity and SERT expression and thus also change the availability of 5-HT. Dysregulation of 5-HT concentrations may represent a common pathway for various stimuli — from stress to infection or inflammation — to influence the properties of the mature ENS. The effects of environment-induced alterations in 5-HT signalling on the ENS may also provide insight into the genesis of GI dysfunction that sometimes accompanies CNS disorders, such as autism spectrum disorder in individuals with SERT mutations126,156.
Enteric glia
Molecular and morphological identity of enteric glia.
Enteric glia were long considered to be analogous to Schwann cells in the extra-enteric PNS on the basis of their common origin in the neural crest. As ultrastructural analyses and immunohistochemical studies later suggested that enteric glia were more similar to astrocytes, the dogma shifted and enteric glia came to be considered the astrocytes of the gut. The astrocyte marker glial fibrillary acid protein (GFAP) and the calcium-binding protein S100β were commonly used to identify enteric glia. Transcriptional profiling of enteric glia has now challenged this dogma. Although there is no myelination in the ENS, almost all enteric glia express myelin proteolipid protein (PLP1) and, overall, express more genes in common with myelinating glia than with astrocytes157. Enteric glia exhibit a hybrid pattern of astrocyte and oligodendrocyte marker gene expression, suggesting that although they have features that are common to both of these other types of cells, they do not closely resemble either one157. These findings could mean either that enteric glia are a completely unique subtype of glia or that enteric glia are heterogeneous and subsets of enteric glia exhibit features of different populations of extra-enteric glia.
Glial heterogeneity in the ENS was first reported more than 20 years ago when investigators microinjected cells with membrane-impermeant dyes and described two morphological subtypes of glia in the enteric plexuses158. Outside the plexuses, at least two additional morphological types were identified: intramuscular glia, which are associated with nerve fibres coursing through the muscularis externa, and mucosal glia, in the lamina propria underneath the epithelium159 (FIG. 4). There are no known molecular markers that distinguish these morphological subtypes, but whereas most enteric glia express SOX10, PLP1 and S100β, only a subset expresses GFAP157,160. Ablation of Gfap-expressing enteric glia leads to fulminant intestinal inflammation — an observation initially interpreted to suggest that glia are essential for preventing inflammation and maintaining intestinal epithelial barrier integrity161,162; however, recent studies in which Plp1-expressing enteric glia were ablated revealed more extensive glial loss but neither inflammation nor epithelial barrier defects163. These different outcomes may be explained by GFAP expression in rare non-glial cells in the intestinal epithelium and/or by non-cell-autonomous toxicity of the methods used to ablate glia in the older studies163. Better definition of enteric glial subtypes and their functions remains an important task to be accomplished.
Functional subtypes.
In addition to molecular marker expression, some reported functional differences between enteric glia also provide clues to their underlying diversity. Even within a single myenteric ganglion, individual glia exhibit distinct Ca2+ responses160, and a subset of enteric glia within the myenteric plexus is dye-coupled to each other through gap junctions164, making these cells capable of transmitting calcium waves. Interfering with these Ca2+ dynamics alters GI motility and secretion, suggesting that Ca2+ signalling, at least in a subset of glia, is important for GI homeostasis165–167. Myenteric glia also respond uniquely to inflammatory insults, such as exposure to lipopolysaccharide (LPS). When rats were systemically challenged with LPS, GFAP was selectively upregulated in the myenteric plexus but not in the mucosa or submucosa; in vitro, glia isolated from the myenteric plexus that are exposed to LPS secrete the pro-inflammatory cytokine interferon-γ168. The functional importance of this glial interferon-γ response in vivo remains to be determined; nevertheless, these data suggest that enteric glial populations can respond differently to systemic stress.
Mucosal glia in the lamina propria are in a markedly different microenvironment from that of glia within the enteric plexuses and probably constitute a unique enteric glial subset. In support of this idea, the development of mucosal glia in mice was found to be uniquely sensitive to weaning; delayed weaning was associated with delayed glial colonization of the lamina propria169. To examine the dynamics of mucosal gliogenesis, the multicolour Confetti reporter was expressed within Sox10-expressing glia; this revealed that mucosal glia are born in the myenteric plexus and migrate into the mucosa169. Remarkably, this radial migration along the serosa-to-lumen axis is dependent on the microbiome. Germ-free mice and mice exposed to broad-spectrum antibiotics have far fewer mucosal glia in the small intestine than do control mice, and this deficit is restored by microbial exposure169. The numbers, nerve fibre density, subtype specification and excitability of enteric neurons are all altered in germ-free mice170–172, suggesting that microorganisms modulate ENS development in many ways. Whether glial migration into the mucosa reflects a direct or indirect interaction between glia and the microbiome is thus unclear. At a minimum, mucosal glia seem to be functionally distinct from other enteric glia; at a maximum, given the wide use of antibiotics in medicine, the findings above may have far-reaching implications if the mechanisms of enteric glial migration prove to be evolutionarily conserved in humans.
Conclusions
The complexity of the ENS, the multiplicity of its projections, its ability to function independently of CNS input and the essentiality of its functions are reflected in a very complicated pattern of development. Important advances have recently been made in understanding this pattern. The remarkable plasticity of crest-derived precursors enables internal and external environments to participate in sculpting the ENS. Internal cues are found along the migration pathways that crest-derived cells follow to and within the bowel as well as in enteric ganglia. As development proceeds, neurons arise, coexist with and regulate the development of remaining precursor cells. By modulating the activity of early-born neurons, the external environment, including the enteric microbiome, can regulate enteric neuronal and glial diversification and thus sculpt the ENS. The extreme complexity of ENS development provides a remarkable abundance of possibilities for abnormalities to arise.
HSCR is currently the only disorder widely known to result from abnormal ENS development. That situation is not likely to persist after modern tools of neuro-biological research, such as single-cell transcriptomics, light sheet imaging and connectomics, are brought to bear on the study of GI pathophysiology. Further studies of animal models of human disease, coupled with reverse genetic investigations in which disrupted human genes are expressed in mice, will rapidly help to reveal which disorders have their pathophysiological roots in ENS development.
Acknowledgements
M.R. receives research support from the US National Institutes of Health (NIH; DK098903), the Paul Marks Scholars Program, the American Gastroenterological Association–Takeda Pharmaceuticals International Research Scholar Award in Neurogastroenterology, and Ivan and Phyllis Seidenberg. M.D.G. is supported by grants NS 15547, NS099270 and DK093094 from the NIH and by the Einhorn Family Charitable Trust.
Glossary
- Ganglionated plexuses
Nerve networks interspersed with ganglia (aggregates of neuronal cell bodies) at the internodes of connecting nerve strands
- Bilateria
Animals with bilateral symmetry
- Hirschsprung disease
(HSCR). A congenital absence of ganglia from a segment of gut that may be short or long
- Chronic intestinal pseudo-obstruction
A gut motility disorder giving rise to a functional but not mechanical obstruction to the transit of intestinal contents
- Somites
Bilaterally paired blocks of paraxial mesoderm that form along the head-to-tail axis of the developing embryo in segmented animals
- Sacral crest
Neural crest at an axial level caudal to somite 28
- Proximal gut
The region of the bowel closest to the mouth, including the oesophagus, stomach, and duodenum rostral to the ampulla of Vater (corresponding to the fetal foregut)
- Neuraxis
The main axis of the CNS along the anterior-posterior dimension
- Pharyngeal arches
A series of outpouchings of mesoderm on both sides of the developing pharynx
- Mesenchyme
The embryonic connective tissue. It is of mesodermal origin in most of the body, but in the region of the head and neck it is derived from the neural crest
- Sclerotome
The portion of a somite that gives rise to bone or other skeletal tissue
- Melanocytic lineage
Neural-crest-derived precursors that commit to developing as melanin-producing cells
- Retinaldehyde dehydrogenase 2
Enzyme that catalyses the synthesis of retinoic acid from retinaldehyde
- Hindgut
Most caudal region of the fetal bowel; the region supplied by the inferior mesenteric artery
- Megacolon
Massively dilated colon, such as that which occurs proximal to the pseudo-obstruction caused by an aganglionic region of colon
- Waardenburg-Shah syndrome
Syndrome characterized by hearing loss and pigment abnormalities that can be associated with enteric aganglionosis
- Confetti transgene
A genetic multicolour-reporting system typically used in mice that enables expression of one of four different fluorescent proteins, the expression of which results from stochastic recombination in individual cells and is maintained in their progeny, allowing cell lineage to be traced
- Interstitial cells of Cajal
Connective tissue cells of the gut derived from a mesenchymal precursor common to intestinal smooth muscle. They are innervated and serve as pacemakers to intestinal smooth muscle
- Enterocytes
Simple columnar epithelial cells that constitute the majority of intestinal epithelial cells and have both absorptive and secretory functions
- Lamina propria
Loose areolar connective tissue of the intestinal mucosa. It lies under the mucosal epithelium and is demarcated from the submucosa by a thin layer of smooth muscle called the muscularis mucosa
- Dye-coupled
Relating to two cells joined by gap junctions that permit membrane-impermeant dye (and, often, electrical charge) to pass from one cell to another
- Lipopolysaccharide
(LPS). A component of the outer membrane of Gram-negative bacteria. Also known as endotoxin, it is a ligand for Toll-like receptor 4
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Neuroscience thanks R. O. Heuckeroth, V. Pachnis and the other anonymous reviewer(s) for their contribution to the peer review of this work.
References
- 1.Gershon MD Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci.33, 446–456 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Gershon MD & Tack J The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Furness JB The Enteric Nervous System. (Blackwell Publishing, Malden, MA, 2006). [Google Scholar]
- 4.Langley JN The Autonomic Nervous System, Part 1. (W. Heffer, Cambridge, 1921). [Google Scholar]
- 5.Furness JB & Stebbing MJ The first brain: species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol. Motil https://doi.org/10.1111/nmo.13234 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Ganz J Gut feelings: studying enteric nervous system development, function, and disease in the zebrafish model system. Dev. Dyn 247, 268–278 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Stamp LA Cell therapy for GI motility disorders: comparison of cell sources and proposed steps for treating Hirschsprung disease. Am. J. Physiol. Gastrointest. LiverPhysiol 312, G348–G354 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Zhao X & Pack M Modeling intestinal disorders using zebrafish. Methods Cell Biol. 138, 241–270 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Nagy N & Goldstein AM Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol 66, 94–106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sergi CM, Caluseriu O, McColl H & Eisenstat DD Hirschsprung’s disease: clinical dysmorphology, genes, micro-RNAs, and future perspectives. Pediatr. Res 81, 177–191 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Obata Y & Pachnis V The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 151, 836–844 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young HM, Stamp LA & McKeown SJ ENS development research since 1983: great strides but many remaining challenges. Adv. Exp. Med. Biol 891, 53–62 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Bondurand N & Southard-Smith EM Mouse models of Hirschsprung disease and other developmental disorders of the enteric nervous system: old and new players. Dev Biol. 417, 139–157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torroglosa A et al. Epigenetics in ENS development and Hirschsprung disease. Dev. Biol 417, 209–216 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Uesaka T, Young HM, Pachnis V & Enomoto H Development of the intrinsic and extrinsic innervation of the gut. Dev. Biol 417, 158–167 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Heuckeroth RO & Schafer KH Gene-environment interactions and the enteric nervous system: neural plasticity and Hirschsprung disease prevention. Dev. Biol 417, 188–197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avetisyan M, Schill EM & Heuckeroth RO Building a second brain in the bowel. J. Clin. Invest 125, 899–907 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam PK Hirschsprung’s disease: a bridge for science and surgery. J. Pediatr. Surg 51, 18–22 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Knowles CH et al. Quantitation of cellular components of the enteric nervous system in the normal human gastrointestinal tract — report on behalf of the Gastro 2009 International Working Group. Neurogastroenterol. Motil 23, 115–124 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Knowles CH et al. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut 59, 882–887 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Langer JC et al. Guidelines for the management of postoperative obstructive symptoms in children with Hirschsprung disease. Pediatr Surg. Int 33, 523–526 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Gosain A et al. Guidelines for the diagnosis and management of Hirschsprung-associated enterocolitis. Pediatr. Surg. Int 33, 517–521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng LS, Schwartz DM, Hotta R, Graham HK & Goldstein AM Bowel dysfunction following pullthrough surgery is associated with an overabundance of nitrergic neurons in Hirschsprung disease. J. Pediatr. Surg 51, 1834–1838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauro A, De Giorgio R & Pinna AD Advancement in the clinical management of intestinal pseudoobstruction. Expert Rev. Gastroenterol. Hepatol 9, 197–208 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Furness JB The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol. Motil 20 (Suppl. 1), 32–38 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Roberts RR et al. The first intestinal motility patterns in fetal mice are not mediated by neurons or interstitial cells of Cajal. J. Physiol 588, 1153–1169 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foong JP et al. Changes in nicotinic neurotransmission during enteric nervous system development. J. Neurosci 35, 7106–7115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foong JP, Nguyen TV, Furness JB, Bornstein JC & Young HM Myenteric neurons of the mouse small intestine undergo significant electrophysiological and morphological changes during postnatal development. J. Physiol 590, 2375–2390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yntema CL & Hammond WS The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J. Comp. Neurol 101, 515–542 (1954).This paper establishes definitively that the ENS is a neural-crest derivative; all subsequent work on the origin of the ENS is based on this observation.
- 30.Le Douarin NM A biological cell labeling technique and its use in experimental embryology. Dev. Biol 30, 217–222 (1973). [DOI] [PubMed] [Google Scholar]
- 31.Le Douarin NM & Teillet MA The migration of neural crest cells to the wall of the digestive tract in avian embryo. J. Embryol. Exp. Morphol 30, 31–48 (1973).This manuscript establishes lineage tracing in ENS experimental development and provides confirmation of the neural-crest origin of the ENS and that the ENS is derived from vagal and sacral axial levels.
- 32.Le Douarin NM Cell line segregation during peripheral nervous system ontogeny. Science 231, 1515–1522 (1986). [DOI] [PubMed] [Google Scholar]
- 33.Burns AJ, Delalande JM & Le Douarin NM In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development 129, 2785–2796 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Zuhdi N et al. Slit molecules prevent entrance of trunk neural crest cells in developing gut. Int. J. Dev. Neurosci 41, 8–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Bellard ME, Rao Y & Bronner-Fraser M Dual function of Slit2 in repulsion and enhanced migration of trunk, but not vagal, neural crest cells. J. Cell Biol 162, 269–279 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo BR & Erickson CA Regional differences in neural crest morphogenesis. Cell Adh. Migr 4, 567–585 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo BR & Erickson CA Vagal neural crest cell migratory behavior: a transition between the cranial and trunk crest. Dev. Dyn 240, 2084–2100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serbedzija GN, Fraser SE & Bronner-Fraser M Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development 108, 605–612 (1990). [DOI] [PubMed] [Google Scholar]
- 39.Rickmann M, Fawcett JW & Keynes RJ The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J. Embryol. Exp. Morphol 90, 437–455 (1985). [PubMed] [Google Scholar]
- 40.Escot S, Blavet C, Hartle S, Duband JL & Fournier-Thibault C Misregulation of SDF1-CXCR4 signaling impairs early cardiac neural crest cell migration leading to conotruncal defects. Circ. Res 113, 505–516 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Escot S et al. Disruption of CXCR4 signaling in pharyngeal neural crest cells causes DiGeorge syndrome-like malformations. Development 143, 582–588 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Wang HU & Anderson DJ Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron 18, 383–396 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Krull CE et al. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr. Biol 7, 571–580 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Adams RH et al. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell 104, 57–69 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Davy A, Aubin J & Soriano P Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 18, 572–583 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gammill LS, Gonzalez C, Gu C & Bronner-Fraser M Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development 133, 99–106 (2006).This study solves the mystery of why crest-derived cells migrate preferentially through the anterior halves of somites, even in animals lacking ephrin signalling.
- 47.Roffers-Agarwal J & Gammill LS Neuropilin receptors guide distinct phases of sensory and motor neuronal segmentation. Development 136, 1879–1888 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs-Cohen RJ, Wade PR & Gershon MD Suppression of the melanogenic potential of migrating neural crest-derived cells by the branchial arches. Anat. Rec 268, 16–26 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Cui J, Michaille JJ, Jiang W & Zile MH Retinoid receptors and vitamin A deficiency: differential patterns of transcription during early avian development and the rapid induction of RARs by retinoic acid. Dev. Biol 260, 496–511 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Simkin JE, Zhang D, Rollo BN & Newgreen DF Retinoic acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS ONE 8, e64077(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niederreither K et al. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 130, 2525–2534 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Baetge G & Gershon MD Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: relationship to the development of enteric neurons. Dev. Biol 132, 189–211 (1989). [DOI] [PubMed] [Google Scholar]
- 53.Anderson RB, Stewart AL & Young HM Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res. 323, 11–25 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Baetge G, Pintar JE & Gershon MD Transiently catecholaminergic (TC) cells in the bowel of fetal rats and mice: precursors of non-catecholaminergic enteric neurons. Dev. Biol 141, 353–380 (1990). [DOI] [PubMed] [Google Scholar]
- 55.Obermayr F, Stamp LA, Anderson CR & Young HM Genetic fate-mapping of tyrosine hydroxylase-expressing cells in the enteric nervous system. Neurogastroenterol. Motil 25, e283–291 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Espinosa-Medina I et al. Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proc. Natl Acad. Sci. USA 114, 11980–11985 (2017).This manuscript presents the revolutionary new concept that enteric neurons in the most proximal bowel are derived from Schwann cells guided to the gut in the descending vagus nerves and that the rest of the ENS is analogous, from a developmental point of view, to the sympathetic nervous system.
- 57.Nishiyama C et al. Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat. Neurosci 15, 1211–1218 (2012).This paper demonstrates, unexpectedly, that ENCDCs take a short cut, migrating through the mesentery to get from the small intestine to the colon, thereby avoiding the long and evidently slower trip through the caecum.
- 58.Coventry S, Yost C, Palmiter RD & Kapur RP Migration of ganglion cell precursors in the ileoceca of normal and lethal spotted embryos, a murine model for Hirschsprung disease. Lab. Invest 71, 82–93 (1994). [PubMed] [Google Scholar]
- 59.Young HM et al. Colonizing while migrating: how do individual enteric neural crest cells behave? BMC Biol. 12, 23(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Chan AK, Sham MH, Burns AJ & Chan WY Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology 141, 992–1002 e1–6 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Pomeranz HD & Gershon MD Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev. Biol 137, 378–394 (1990). [DOI] [PubMed] [Google Scholar]
- 62.Pomeranz HD, Rothman TP & Gershon MD Colonization of the post-umbilical bowel by cells derived from the sacral neural crest: direct tracing of cell migration using an intercalating probe and a replication-deficient retrovirus. Development 111, 647–655 (1991). [DOI] [PubMed] [Google Scholar]
- 63.Burns AJ & Le Douarin NM The sacral crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125, 4335–4347 (1998). [DOI] [PubMed] [Google Scholar]
- 64.Kapur RP Colonization of the murine hindgut by sacral crest-derived neural precursors: experimental support for an evolutionarily conserved model. Dev. Biol 227, 146–155 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Uesaka T, Nagashimada M & Enomoto H Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J. Neurosci 35, 9879–9888 (2015).This work shows that Schwann cell precursors on extrinsic nerves innervating the gut give rise to up to 20% of enteric neurons in the large intestine, revealing a new role for extrinsic innervation and another source of enteric neurons independent of enteric neural-crest-derived progenitors.
- 66.Simpson MJ, Zhang DC, Mariani M, Landman KA & Newgreen DF Cell proliferation drives neural crest cell invasion of the intestine. Dev. Biol 302, 553–568 (2007).This paper establishes the importance of cell proliferation as a driving force enabling crest-derived cells to colonize the gut.
- 67.Barlow AJ, Wallace AS, Thapar N & Burns AJ Critical numbers of neural crest cells are required in the pathways from the neural tube to the foregut to ensure complete enteric nervous system formation. Development 135, 1681–1691 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Young HM et al. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev. Biol 270, 455–473 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Wu JJ, Chen J-X, Rothman TP & Gershon MD Inhibition of in vitro enteric neuronal development by endothelin-3: mediation by endothelin B receptors. Development 126, 1161–1173 (1999). [DOI] [PubMed] [Google Scholar]
- 70.Hearn CJ, Murphy M & Newgreen D GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev. Biol 197, 93–105 (1998). [DOI] [PubMed] [Google Scholar]
- 71.Fujiwara N et al. Altered expression of laminin alpha1 in aganglionic colon of endothelin receptor-B null mouse model of Hirschsprung’s disease. Pediatr. Surg. Int 34, 137–141 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Bergeron KF et al. Upregulation of the Nr2fl-A830082KI2Rik gene pair in murine neural crest cells results in a complex phenotype reminiscent of Waardenburg syndrome type 4. Dis. Model. Mech 9, 1283–1293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pattyn A, Morin X, Cremer H, Goridis C & Brunet JF The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399, 366–370 (1999). [DOI] [PubMed] [Google Scholar]
- 74.Nagashimada M et al. Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression. J. Clin. Invest 122, 3145–3158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amiel J et al. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat. Genet 33, 459–461 (2003). [DOI] [PubMed] [Google Scholar]
- 76.Weese-Mayer DE et al. Idiopathic congenital central hypoventilation syndrome: analysis of genes pertinent to early autonomic nervous system embryologic development and identification of mutations in PHOX2b. Am. J. Med. Genet. A 123A, 267–278 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Lane PW & Liu HM Association of megacolon with a new dominant spotting gene (Dom) in the mouse. J. Hered 75, 435–439 (1984). [DOI] [PubMed] [Google Scholar]
- 78.Herbarth B et al. Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc. Natl Acad. Sci USA95, 5161–5165 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Southard-Smith EM, Kos L & Pavan WJ Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat. Genet 18, 60–64 (1998).This work identifies a loss-of-function mutation in the Sox10 gene as the causative mutation in Dom mice, which have colonic aganglionosis, establishing Sox10 as an important gene in ENS development.
- 80.Britsch S et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 15, 66–78 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pingault V et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet 18, 171–173 (1998). [DOI] [PubMed] [Google Scholar]
- 82.Paratore C, Goerich DE, Suter U, Wegner M & Sommer L Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development 128, 3949–3961 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Kim J, Lo L, Dormand E & Anderson DJ S0X10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38, 17–31 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Musser MA, Correa H & Southard-Smith EM Enteric neuron imbalance and proximal dysmotility in ganglionated intestine of the Soxl0Dom/+ Hirschsprung mouse model. Cell. Mol. Gastroenterol. Hepatol 1, 87–101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F & Pachnis V Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994).This classic paper establishes incontrovertibly that RET expression is essential for development of the ENS distal to the oesophagus and proximal stomach.
- 86.Heanue TA & Pachnis V Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat. Rev. Neurosci 8, 466–479 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Barlow A, de Graaff E & Pachnis V Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron 40, 905–916 (2003). [DOI] [PubMed] [Google Scholar]
- 88.Uesaka T et al. Conditional ablation of GFRα 1 in postmigratory enteric neurons triggers unconventional neuronal death in the colon and causes a Hirschsprung’s disease phenotype. Development 134, 2171–2181 (2007). [DOI] [PubMed] [Google Scholar]
- 89.Uesaka T, Nagashimada M, Yonemura S & Enomoto H Diminished Ret expression compromises neuronal survival in the colon and causes intestinal aganglionosis in mice. J. Clin. Invest 118, 1890–1898 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Avetisyan M et al. Hepatocyte growth factor and MET support mouse enteric nervous system development, the peristaltic response, and intestinal epithelial proliferation in response to injury. J. Neurosci 35, 11543–11558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barrenschee M et al. Site-specific gene expression and localization of growth factor ligand receptors RET, GFRα 1 and GFRα2 in human adult colon. Cell Tissue Res. 354, 371–380 (2013). [DOI] [PubMed] [Google Scholar]
- 92.Heuckeroth RO et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 22, 253–263 (1999). [DOI] [PubMed] [Google Scholar]
- 93.Rossi J et al. Alimentary tract innervation deficits and dysfunction in mice lacking GDNF family receptor α2. J. Clin. Invest 112, 707–716 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young HM, Ciampoli D, Hsuan J & Canty AJ Expression of Ret-, p75NTR-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev. Dyn 216, 137–152 (1999). [DOI] [PubMed] [Google Scholar]
- 95.Taylor CR, Montagne WA, Eisen JS & Ganz J Molecular fingerprinting delineates progenitor populations in the developing zebrafish enteric nervous system. Dev. Dyn 245, 1081–1096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lasrado R et al. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356, 722–726 (2017).This paper presents an elegant genetic lineage tracing study that shows that the ENS develops in an outside-in manner, with the two plexuses being populated by clonal populations of neurons and glia organized into a columnar topology along the serosa-to-lumen, or radial axis, of the gut.
- 97.Lake JI & Heuckeroth RO Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol 305, G1–G24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Charrier B & Pilon N Toward a better understanding of enteric gliogenesis. Neurogenesis 4, e1293958(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Obermayr F, Hotta R, Enomoto H & Young HM Development and developmental disorders of the enteric nervous system. Nat. Rev. Gastroenterol. Hepatol 10, 43–57 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Wallace AS & Burns AJ Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 319, 367–382 (2005). [DOI] [PubMed] [Google Scholar]
- 101.Pham TD, Gershon MD & Rothman TP Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J. Comp. Neurol 314, 789–798 (1991).This manuscript demonstrates for the first time that enteric neurons of different chemically defined subtypes are born in a reproducible sequence during ontogeny.
- 102.Jiang Y, Liu M & Gershon MD Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev. Biol 258, 364–384 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Ratcliffe EM et al. Enteric neurons synthesize netrins and are essential for the development of the vagal sensory innervation of the fetal gut. Dev. Neurobiol 71, 362–373 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simo P et al. Dual and asynchronous deposition of laminin chains at the epithelial-mesenchymal interface in the gut. Gastroenterology 102, 1835–1845 (1992). [DOI] [PubMed] [Google Scholar]
- 105.Simon-Assmann P et al. The laminins: role in intestinal morphogenesis and differentiation. Ann. NY Acad. Sci 859, 46–64 (1998). [DOI] [PubMed] [Google Scholar]
- 106.Chalazonitis A, Tennyson VM, Kibbey MC, Rothman TP & Gershon MD The α1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immunoselected from the fetal mouse gut. J. Neurobiol 33, 118–138 (1997). [DOI] [PubMed] [Google Scholar]
- 107.Höpker V, Shewan D, Tessier-Lavigne M, Poo M & Holt C Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 401, 69–73 (1999). [DOI] [PubMed] [Google Scholar]
- 108.Ratcliffe EM, D’Autreaux F & Gershon MD Laminin terminates the netrin/DCC mediated attraction of vagal sensory axons. Dev. Neurobiol 68, 960–971 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Memic F et al. Transcription and signaling regulators in developing neuronal subtypes of mouse and human enteric nervous system. Gastroenterology 154, 624–636 (2018).This gene expression study of ENS progenitors and surrounding mesenchyme isolated from fetal gut provides an excellent resource of transcription factors, signalling molecules and other molecules thought likely, on the basis of highly suggestive expression patterns, to play a role in ENS specification and circuit formation.
- 110.Sasselli V et al. Planar cell polarity genes control the connectivity of enteric neurons. J. Clin. Invest 123, 1763–1772 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramalho-Santos M, Melton DA & McMahon AP Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127, 2763–2772 (2000). [DOI] [PubMed] [Google Scholar]
- 112.Biau S, Jin S & Fan CM Gastrointestinal defects of the Gas1 mutant involve dysregulated Hedgehog and Ret signaling. Biol. Open 2, 144–155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jin S, Martinelli DC, Zheng X, Tessier-Lavigne M & Fan CM Gas1 is a receptor for sonic hedgehog to repel enteric axons. Proc. Natl Acad. Sci. USA 112, E73–E80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Le Berre-Scoul C et al. A novel enteric neuron-glia coculture system reveals the role of glia in neuronal development. J. Physiol 595, 583–598 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qu ZD et al. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 334, 147–161 (2008). [DOI] [PubMed] [Google Scholar]
- 116.Pompolo S & Furness JB Ultrastructure and synaptic relationships of calbindin-reactive, Dogiel type II neurons, in the myenteric ganglia of guinea-pig small intestine. J. Neurocytol 17, 771–782 (1988). [DOI] [PubMed] [Google Scholar]
- 117.Furness JB & Costa M Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea pig small intestine. Neuroscience 7, 341–350 (1982). [DOI] [PubMed] [Google Scholar]
- 118.Gershon MD 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes, Obes 20, 14–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krantis A GABA in the mammalian enteric nervous system. News Physiol. Sci 15, 284–290 (2000). [DOI] [PubMed] [Google Scholar]
- 120.Li ZS, Schmauss C, Cuenca A, Ratcliffe E & Gershon MD Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci 26, 2798–2807 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li ZS, Pham TD, Tamir H, Chen JJ & Gershon MD Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J. Neurosci 24, 1330–1339 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mongardi Fantaguzzi C, Thacker M, Chiocchetti R & Furness JB Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 336, 179–189 (2009). [DOI] [PubMed] [Google Scholar]
- 123.Kirchgessner AL, Tamir H & Gershon MD Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J. Neurosci 12, 235–249 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kirchgessner AL & Gershon MD Projections of submucosal neurons to the myenteric plexus of the guinea pig intestine: In vitro tracing of microcircuits by retrograde and anterograde transport. J. Comp. Neurol 277, 487–498 (1988). [DOI] [PubMed] [Google Scholar]
- 125.Gross ER, Gershon MD, Margolis KG, Gertsberg ZV & Cowles RA Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143, 408–417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Margolis KG et al. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J. Clin. Invest 126, 2221–2235 (2016).This manuscript, working through an analysis of the effects of gain-of-function and loss-of-function of SERT activity, demonstrates how early-born serotonergic neurons can sculpt the developing ENS by affecting the development of neurons born after them.
- 127.Wood JD Nonruminant Nutrition Symposium: neurogastroenterology and food allergies. J. Animal Sci 90, 1213–1223 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Bergner AJ et al. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J. Comp. Neurol 522, 514–527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chalazonitis A et al. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J. Comp. Neurol 509, 474–492 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang H et al. The timing and location of glial cell line-derived neurotrophic factor expression determine enteric nervous system structure and function. J. Neurosci 30, 1523–1538 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kulkarni S et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl Acad. Sci. USA 114, E3709–E3718 (2017).This exciting and controversial recent paper provides evidence that there is robust and continuous generation and replacement of enteric neurons in healthy adult mice that is fuelled by nestin-expressing progenitor cells.
- 132.Corpening JC, Cantrell VA, Deal KK & Southard-Smith EMA Histone2BCerulean BAC transgene identifies differential expression of Phox2b in migrating enteric neural crest derivatives and enteric glia. Dev. Dyn 237, 1119–1132 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gianino S, Grider JR, Cresswell J, Enomoto H & Heuckeroth RO GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 130, 2187–2198 (2003).This work investigates the role of programmed cell death in ENS development and finds no evidence of cell death in healthy mice or altered enteric neuronal number in mice lacking key molecules in the apoptotic cascade such as apoptosis regulator BAX and BH3-interacting domain death agonist (BID).
- 134.Gershon MD, Sherman D & Gintzler A An ultrastructural analysis of the developing enteric nervous system of the guinea pig small intestine. J. Neurocytol 10, 271–296 (1981). [DOI] [PubMed] [Google Scholar]
- 135.Li Z et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci 31, 8998–9009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu MT, Kuan YH, Wang J, Hen R & Gershon MD 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J. Neurosci 29, 9683–9699 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Belkind-Gerson J et al. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm. Bowel Dis 21, 870–878 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goto K et al. Activation of 5-HT4 receptors facilitates neurogenesis from transplanted neural stem cells in the anastomotic ileum. J. Physiol. Sci 66, 67–76 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z et al. Cholinergic neuronal regulation of enteric neurogenesis, GI motility, and mucosal homeostasis: insights from mice that under-or overexpress the presynaptic choline transporter. Gastroenterology 150, S343(2016). [Google Scholar]
- 140.Gaspar P, Cases O & Maroteaux L The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci 4, 1002–1012 (2003). [DOI] [PubMed] [Google Scholar]
- 141.D’Amato RJ, Largent BL, Snowman AM & Snyder SH Selective labeling of serotonin uptake sites in rat brain by [3H]citalopram contrasted to labeling of multiple sites by [3H]imipramine. J. Pharmacol. Exp. Ther 242, 364–371 (1987). [PubMed] [Google Scholar]
- 142.Lebrand C et al. Transient developmental expression of monoamine transporters in the rodent forebrain. J. Comp. Neurol 401, 506–524 (1998). [PubMed] [Google Scholar]
- 143.Chen X et al. Disruption of transient serotonin accumulation by non-serotonin-producing neurons impairs cortical map development. Cell Rep. 10, 346–358 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rothman TP, Ross LL & Gershon MD Separately developing axonal uptake of 5-hydroxytryptamine and norepinephrine in the ileum of the rabbit. Brain Res. 115, 437–456 (1976). [DOI] [PubMed] [Google Scholar]
- 145.Chitkara DK, van Tilburg MA, Blois-Martin N & Whitehead WE Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am. J. Gastroenterol 103, 765–774 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chitkara DK et al. Recollection of childhood abdominal pain in adults with functional gastrointestinal disorders. Scand. J. Gastroenterol 44, 301–307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Faure C, Patey N, Gauthier C, Brooks EM & Mawe GM Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 139, 249–258 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Al-Chaer ED, Kawasaki M & Pasricha PJ A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 119, 1276–1285 (2000). [DOI] [PubMed] [Google Scholar]
- 149.Gareau MG, Jury J, Yang PC, MacQueen G & Perdue MH Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr. Res 59, 83–88 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Gareau MG, Jury J & Perdue MH Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am. J. Physiol. Gastrointest. Liver Physiol 293, G198–203 (2007). [DOI] [PubMed] [Google Scholar]
- 151.Spiller R & Garsed K Postinfectious irritable bowel syndrome. Gastroenterology 136, 1979–1988 (2009). [DOI] [PubMed] [Google Scholar]
- 152.Dinan TG, Cryan J, Shanahan F, Keeling PW & Quigley EM IBS: an epigenetic perspective. Nat. Rev. Gastroenterol. Hepatol 7, 465–471 (2010). [DOI] [PubMed] [Google Scholar]
- 153.Hao MM et al. Enteric nervous system assembly: functional integration within the developing gut. Dev. Biol 417, 168–181 (2016). [DOI] [PubMed] [Google Scholar]
- 154.Foong JP Postnatal development of the mouse enteric nervous system. Adv. Exp. Med. Biol 891, 135–143 (2016). [DOI] [PubMed] [Google Scholar]
- 155.Laranjeira C et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J. Clin. Invest 121, 3412–3424 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rao M & Gershon MD The bowel and beyond: the enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol 13, 517–528 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rao M et al. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 63, 2040–2057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hanani M & Reichenbach A Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 278, 153–160 (1994). [DOI] [PubMed] [Google Scholar]
- 159.Gulbransen BD & Sharkey KA Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol 9, 625–632 (2012). [DOI] [PubMed] [Google Scholar]
- 160.Boesmans W, Lasrado R, Vanden Berghe P & Pachnis V Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63, 229–241 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Bush TG et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201 (1998). [DOI] [PubMed] [Google Scholar]
- 162.Aube AC et al. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 55, 630–637 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rao M et al. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153, 1068–1081 (2017).This work uses genetic ablation to eliminate the majority of enteric glia in young adult mice and shows that enteric glia are required for the regulation of GI motility in a sex-dependent manner but are dispensable for epithelial functions such as barrier formation and cell proliferation.
- 164.Maudlej N & Hanani M Modulation of dye coupling among glial cells in the myenteric and submucosal plexuses of the guinea pig. Brain Res. 578, 94–98 (1992). [DOI] [PubMed] [Google Scholar]
- 165.McClain JL et al. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146, 497–507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McClain JL, Fried DE & Gulbransen BD Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell. Mol. Gastroenterol. Hepatol 1, 631–645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grubisic V & Gulbransen BD Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J. Physiol 595, 3409–3424 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rosenbaum C et al. Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS ONE 11, e0151335(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kabouridis PS et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85, 289–295 (2015).Using genetic lineage tracing, the authors of this paper show that mucosal glia are continually renewed from cells migrating out of the enteric plexuses and that this migration is dependent upon the microbiota.
- 170.Collins J, Borojevic R, Verdu EF, Huizinga JD & Ratcliffe EM Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 26, 98–107 (2014).This paper is among the first studies to examine the consequences of an absent microbiota on ENS development; it shows that the early postnatal ENS in the small intestines of germ-free mice has altered neuron numbers and subtype composition, which are associated with defects in bowel motility.
- 171.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA & Kunze WA The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil. 25, 183–e88 (2013). [DOI] [PubMed] [Google Scholar]
- 172.McVey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J & Kunze WA The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil. 27, 627–636 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F & Pachnis V Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development 122, 349–358 (1996). [DOI] [PubMed] [Google Scholar]
- 174.Birchmeier C ErbB receptors and the development of the nervous system. Exp. Cell Res 315, 611–618 (2009). [DOI] [PubMed] [Google Scholar]
- 175.Chalazonitis A, DAutreaux F, Pham TD, Kessler JA & Gershon MD Bone morphogenetic proteins regulate enteric gliogenesis by modulating ErbB3 signaling. Dev. Biol 350, 64–79 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Horn JP The sacral autonomic outflow is parasympathetic: Langley got it right. Clin. Auton. Res 28, 181–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Espinosa-Medina I et al. The sacral autonomic outflow is sympathetic. Science 354, 893–897 (2016).This work analyses the sacral autonomic outflow and concludes on genetic grounds that the sacral outflow is actually sympathetic and not parasympathetic as previously thought on the basis of physiological evidence; the physiological and genetic evidence still have to be reconciled.
- 178.Altschuler SM, Escardo J, Lynn RB & Miselis RR The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104, 502–509 (1993). [DOI] [PubMed] [Google Scholar]
- 179.Payette RF et al. Origin and morphology of nerve fibers in the aganglionic colon of the lethal spotted (ls/ls) mutant mouse. J. Comp. Neurol 257, 237–252 (1987). [DOI] [PubMed] [Google Scholar]
- 180.Le Douarin NM & Kalcheim C The Neural Crest. (Cambridge Univ. Press, Cambridge, 1999). [Google Scholar]
- 181.Bohorquez DV et al. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS ONE 9, e89881(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ibiza S et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535, 440–443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


