Abstract
The central nervous system is generated from progenitor cells that are recognized as neural stem cells (NSCs). NSCs are defined as undifferentiated neural cells that are characterized by the capacity for self-renewal and multipotency. Throughout neural development, NSCs undergo proliferation, migration, and cellular differentiation, and dynamic changes are observed in the composition of carbohydrate-rich molecules, including gangliosides. Gangliosides are sialic acid-containing glycosphingolipids with essential and multifaceted functions in brain development and NSC maintenance, which reflects the complexity of brain development. Our group has pioneered research on the importance of gangliosides for growth factor receptor signaling and epigenetic regulation of ganglioside biosynthesis in NSCs. We found that GD3 is the predominant ganglioside species in NSCs (>80%) and modulates NSC proliferation by interacting with epidermal growth factor receptor signaling. In postnatal brain, GD3 is required for long-term maintenance of NSCs. Deficiency in GD3 leads to developmental and behavioral deficits, such as depression. The synthesis of GD3 is switched to the synthesis of complex, brain-type gangliosides, namely, GM1, GD1a, GD1b, and GT1b, resulting in terminal differentiation and loss of “stemness” of NSCs. In this process, GM1 is augmented by a novel GM1-modulated epigenetic gene regulation mechanism of glycosyltransferases at a later differentiation stage. Consequently, our research suggests that stage-specific gangliosides play specific roles in maintaining NSC activities and in cell fate determination.
1. INTRODUCTION
Neural stem cells (NSCs) are fundamental cells that are capable of differentiating into various cell types in the nervous system. Gangliosides undergo dramatic qualitative and quantitative developmental changes that are correlated with cellular proliferation, differentiation, and biological functions of nerve cells. In this article, we introduce the expression pattern and potential functions of gangliosides in NSCs and differentiating neuronal cells. We also discovered a novel epigenetic mechanism that regulates ganglioside expression and may play a role in cell fate determination.
In early neural development, neuroepithelial cells (NECs) proliferate by repeating symmetric cell divisions at the wall of the neural tube; as NECs accumulate, the wall gradually becomes thicker. Proliferated NECs that are transformed into bipolar-shaped cells are known as radial glial cells (RGCs). The NEC elongates its fibers and becomes an RGC whose cell body lines the ventricular zone (VZ), and the apical surface meets the ventricles with the radial fibers reaching the pial surface. RGCs lose some of their epithelial properties in favor of certain glial characteristics, but RGCs retain some of the original properties of NECs, such as apicobasal polarity, contacting with the ventricular surface. Previously, RGCs were thought of as specialized glial cells whose major function was to guide migration of newborn neurons.1–7 Recently, RGCs have been recognized as the precursors of neurons and glia. An RGC generates another RGC and an intermediate progenitor cell (IPC) or immature neuron by asymmetric cell division.8–10 IPCs remain in the subventricular zone (SVZ) to proliferate and give rise to more neurons. Immature neurons migrate along with radial fibers into the cortical plate, and then become mature neurons. At first, RGCs produce inner-layer neurons that shift and become outer-layer neurons at later stages. RGCs also give rise to the major types of macroglia in the central nervous system (CNS), including oligodendrocytes and ependymal cells, and RGCs can eventually differentiate into astrocytes. Both NECs and RGCs are considered as NSCs.7,11 A specialized microenvironment, known as the NSC niche, maintains stem cells in a multipotent and undifferentiated state. The NSC niche contains a variety of stem/progenitor cells, such as NECs, RGCs, and IPCs. Altogether, these versatile progenitors cooperate for neurogenesis and gliogenesis in the developing CNS.
During development, neurons and glia are generated in the CNS in a limited time-dependent manner. At early developmental stages, a preplate consisting of the earliest-born neurons and possibly other cell types is formed between the VZ and meninges at the brain surface. The VZ is a highly packed cell layer formed by morphologically homogeneous RGCs, and the SVZ is a second proliferative layer. Newly generated neurons migrate radially outward of the VZ/SVZ and form a new laminar structure. This preplate is subsequently split into the marginal zone and subplate by waves of migrating neurons. During this development, the VZ becomes smaller, and after neurogenesis is completed, the VZ is replaced by an ependymal cell layer. Postnatally, most of the SVZ disappears except along the lateral wall of the lateral ventricles, where it is considered a site for the NSC niche in the adult stage.11–13 Astrocytes fulfill a range of essential functions, including regulation of neurogenesis, synaptogenesis, and development of the neural circuit.14–17 Toward the end of cortical development, most RGCs transform into astrocytes. Astrocytic-destined IPCs are reported to divide locally before terminal differentiation into astrocytes in the embryonic and postnatal stages.18,19 In rodents, on the day of birth [postnatal day (P) 0], most astrocyte precursors are found in the inner half of the cortical width. On P4, the majority of the astrocyte precursors are distributed in the outer half of the cortical width. The pattern of gliogenesis in the early postnatal rat thus shows an inside-out tendency, in analogy to neurogenesis.20,21 Whether astrocyte IPCs are involved in this astrocytic differentiation in vivo is not well known. Oligodendrocytes, the chief myelin-forming cells in the CNS, are derived from RGCs. The myelin structure provides efficient axon insulation and facilitates saltatory conduction of nerve impulses. Most of the oligodendrocytes present in the adult cortex of mammals are generated after birth.20,21
For most mammals, neurogenesis commences during early embryonic stages and is almost complete shortly after birth; it continues to occur at a much slower pace and in a limited manner throughout the entire adult life. In the adult brain of mammals, neurogenesis persists primarily in two germinal zones, the SVZ of the lateral ventricles22,23 and the subgranular zone in the dentate gyrus of the hippocampus.24,25 In the adult SVZ, four distinct cell types are present. Type B cells are RGC-like cells and are considered as NSCs. Type B NSCs are slow dividing (duration of cell cycle >15 days) and express glial fibrillary acidic protein (GFAP). Type C cells are transient amplifying cells that are rapidly proliferating (duration of cell cycle about 13 h) and express the transcription factor Mash1. Type A cells are neuronal precursors that have already committed to differentiate into neurons, and these cells express polysialic acid-neural adhesion molecules on the cell surface (duration of cell cycle about 13 h).26 Ependymal cells are lined on the wall of the ventricle and have multimotile cilia, which are important for controlling the flow of cerebrospinal fluid. Multipotency of the ependymal cells has been reported,27 although this is still not settled.22,28,29 Recently, ependymal cells have been shown to be the most quiescent type of NSCs whose cell cycle is strictly regulated and reinitiated under specific circumstances. In certain restricted situations, a subpopulation of ependymal cells may develop into neurons, and these cells are considered as NSCs.30,31 In the subgranular zone, five types of cells have been described.32 Type 1 cells are considered quiescent neural progenitors that are RGC-like cells and largely equivalent to Type B NSCs in the SVZ. Type 2 cells express nestin, and this cell type has been classified into two cell populations: Type 2a cells are amplifying neural progenitors that are similar to Type C transient amplifying cells in the SVZ; Type 2b and 3 cells are neuroblasts that express polysialic acid-neural adhesion molecules.33,34 The other type of cells is mature granule neurons. Recently, it has been reported that Mash1+ cells do not amplify and are therefore not Type 2a amplifying neural progenitors that can directly differentiate into early neuroblasts without mitosis.35
With regard to gangliosides, we have shown that GD3 is the predominant ganglioside in NSCs, and it can serve as a convenient cell surface marker of these cells.36 The interaction of GD3 with epidermal growth factor receptor (EGFR) plays a crucial role in maintaining the self-renewal capacity of NSCs by directing the EGFR through the recycling pathway rather than through the degradative pathway after endocytosis.37 We have reported that efficient histone acetylation of glycosyltransferase (GT) genes contributes to the developmental alteration of ganglioside expression in mouse brain.38 Furthermore, we have demonstrated that acetylation of histones H3 and H4 on the GM2/GD2S gene promoter leads to recruitment of trans-activation factors Sp1 and AP-2 during neuronal differentiation.39 More recently, we found that nuclear GM1 binds with acetylated histones on the promoters of the GM2/GD2S gene as well as on the neurogenic transcription factor, NeuroD1 gene, in differentiated neurons.40 Here, we will further discuss the importance of ganglioside regulation in neural development and neuronal differentiation of NSCs.
2. GLYCOSPHINGOLIPID EXPRESSION IN NEURAL DEVELOPMENT
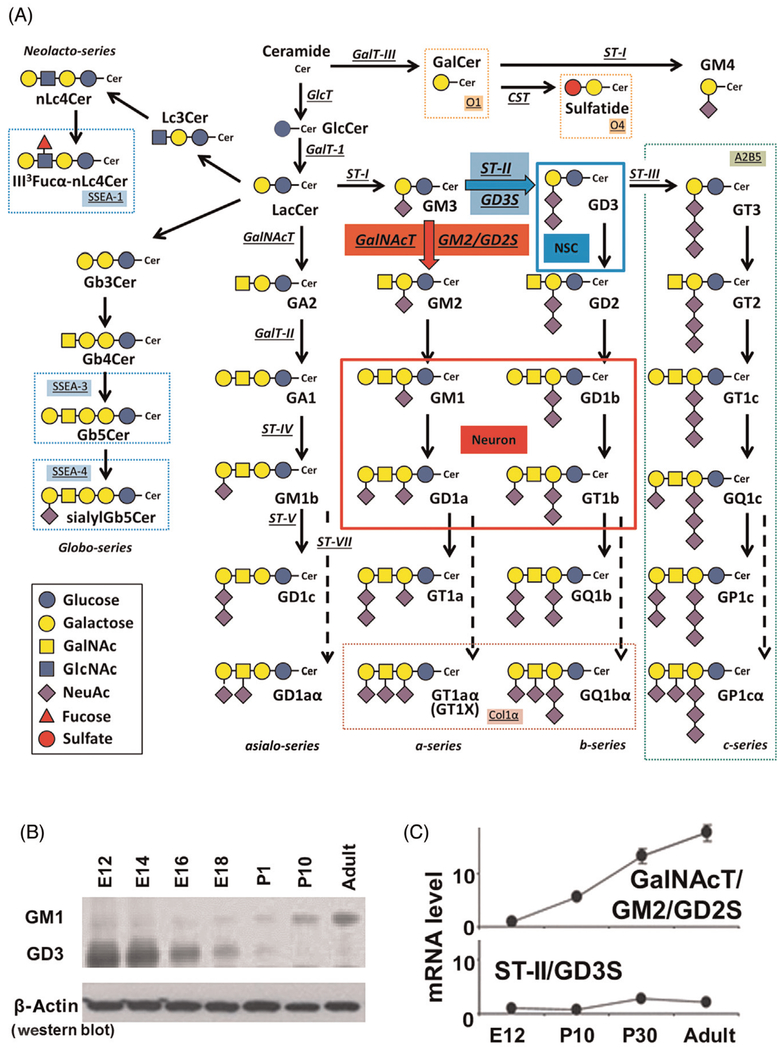
During neural development, dramatic and consistent changes in the composition of glycoconjugates, including glycolipids, glycoproteins, and proteoglycans, occur.41–43 Glycosphingolipids (GSLs) contain one or more monosaccharide residues bound by a glycosidic linkage to the hydrophobic moiety, ceramide. It is known that changes in the expression of glycolipids, including gangliosides that contain one or more sialic acid residues, in the nervous system correlate with neurodevelopmental events.44–47 For example, in fertilized eggs, the globo-series of glycolipids (e.g., stage-specific embryonic antigen, SSEA-3/SSEA-4; Fig. 1) are robustly expressed. As cell division proceeds, the lacto-series GSLs (e.g., lactoneotetraosylceramide, nLc4Cer) are expressed at embryonic day (E) 1.5, followed by the ganglio-series GSLs in the developing brain (from E7). The lipid portion of GSLs, including gangliosides, is ceramide, which is synthesized primarily in the endoplasmic reticulum (ER) from a sphingosine base and a fatty acid residue. Ceramide is transferred to the Golgi apparatus and glucosylated to glucosylceramide (GlcCer) and catalyzed by glucosyltransferase (GlcTor GlcCersynthase) on the cytosolic leaflet of the cis-Golgi. The expression of GlcCer is abundant in early embryonic brain and decreases from E16.41 The synthesized GlcCer is transported to the trans-Golgi by the phosphoinositol 4-phosphate adaptor protein 2 (FAPP2) and trans-located to the luminal side of the Golgi vesicle.48,49 On the other direction, FAPP2 also mediates GlcCer transportation from the cis-Golgi back to the ER.50 Then, GlcCer is flipflopped into the lumen of the ER, and GlcCer ultimately reaches the lumen of the Golgi by vesicular transport.51 At the luminal side of the Golgi, GlcCer is converted to lactosylceramide (LacCer). The sequential action catalyzed by sialyltransferases and galactosyltransferase can elongate the carbohydrate moieties to gangliosides. Another pathway from ceramide is catalyzed by galactosyltransferase III (GalT-III or GalCer-synthase) in the ER to form galactosylceramide (GalCer), sulfatide, and GM4.52 Each step is catalyzed by a unique, specifically controlled GT (Fig. 1A).
Fig. 1.
Glycosphingolipids and glycosyltransferases in the nervous system. (A) Structures and biosynthetic pathways of glycosphingolipids (GSLs). Cer, Ceramide; CST, cerebroside sulfotransferase (Gal3st1, sulfatide synthase); GalNAc-T, N-acetylgalactosaminyltransferase I (B4galnt1, GA2/GM2/GD2/GT2-synthase); GalT-I, galactosyltransferase I (B4galt6, lactosylceramide synthase); GalT-II, galactosyltransferase II (B3galt4, GA1/GM1/GD1b/GT1c-synthase); GalT-III, galactosyltransferase III (Ugt8a, galactosylceramide synthase); GlcT, glucosyltransferase (Ugcg, glucosylceramide synthase); ST-I, sialyltransferase I (St3gal5, GM3-synthase); ST-II, sialyltransferase II (St8Sia1, GD3-synthase); ST-III, sialyltransferase III (St8Sia3, GT3-synthase); ST-IV, sialyltransferase IV (St3gal2, GM1b/GD1a/GT1b/GQ1c-synthase); ST-V, sialyltransferase V (St8sia5, GD1c/GT1a/GQ1b/GP1c-synthase); ST-VII, sialyltransferase VII (St6galnac6, GD1aα/GT1aα/GQ1bα/GP1cα-synthase). Official symbols of genes are represented in italics in this figure legend. GD3 is the most abundant ganglioside in rodent NSCs. c-Series gangliosides are A2B5 antigens. GM1, GD1a, GD1b, and GT1b are the most abundant ganglioside species in adult mammalian brain and neurons. Oligodendrocyte markers O1 and O4 are GalCer and sulfatide, respectively. GT1aα and GQ1bα are cholinergic-specific antigens (Chol-1α). (B) Thin-layer chromatography (TLC) and (C) quantitative PCR studies demonstrate the structural complexity of gangliosides that develops with age. Note the dynamic changes from E12 up through the adult for expression of gangliosides (GM1 and GD3) and glycosyltransferases (GalNAcT; GD2/GM2S and ST-II; GD3S), indicative of developmental regulation.
Gangliosides are sialic acid-containing GSLs expressed primarily, but not exclusively, on the outer leaflet of the plasma membrane of cells in all vertebrates. In early embryonic rodent brains, the ganglioside pattern is characterized by the expression of a large quantity of simple gangliosides, such as GM3 and GD3. In later developmental stages, more complex gang-liosides prevail, particularly GM1, GD1a, GD1b, and GT1b, which account for more than 80% of the total gangliosides.41,43 Thus, the expression of neural gangliosides changes dramatically during cellular differentiation and brain development (Fig. 1B and C). This unique expression pattern of specific gangliosides can be used for specific cell lineage markers and may reflect the functional roles they play at specific developmental stages. Abundant evidence supports the notion that GSLs, including gangliosides, serve regulatory roles in cellular events, including proliferation and neural differentiation, as exemplified by neuritogenesis, axonogenesis, and synaptogenesis.41,45,53–57 The functional importance of gangliosides has been evaluated using specific enzyme gene knockout (KO) mice.
3. DEFICIENCY OF GLYCOSPHINGOLIPIDS
In recent years, with the advent of contemporary molecular genetics and biology, several mutant lines of genetically modified mice have been established in which the expression of gangliosides and other GSLs has been altered or depleted; this has greatly facilitated elucidating the biological functions of GSLs. For example, GM2/GD2-synthase (GM2/GD2S; N-acetylgalactosaminyl transferase, GalNAcT) is one of the keyenzymes needed for the synthesis of the major brain-type gangliosides, including GM1, GD1a, GD1b, and GT1b. Mice lacking this enzyme do not express GalNAc-containing gangliosides. Phenotypically, they are developmentally abnormal and exhibit neurological problems, such as axonal degeneration, sensory, motor and behavioral deficits, and other neurological dysfunctions.58–63 During brain development, gangliosides are assumed to modulate ceramide-induced apoptosis and to maintain cellular survival and differentiation.53 GM3-synthase (GM3S; sialyltransferase I, ST-I) is a critical enzyme for the synthesis of all complex gangliosides. Mutation of GM3-synthase is associated with human autosomal recessive infantile-onset symptomatic epilepsy syndrome,64 and an alteration of the GM2/GD2S gene has been reported in patients of hereditary spastic paraplegias.65 These studies clearly demonstrate that deletion of complex gangliosides can be associated with human diseases. A lack of b- and c-series gangliosides results in clear and subtle developmental and behavioral deficits with mice lacking these gang-liosides, which exhibited sudden death from audiogenic seizures.66 Both GM2/GD2S- and GM3S-deficient mice, which lack all gangliosides, die soon after weaning at 3 weeks of age.67 Taken together, these observations clearly indicate that GSLs, including gangliosides, have important biological functions in the developing nervous system.
4. GD3 ON NSC SELF-RENEWAL
b-Series ganglioside GD3 (CD60a) is expressed in the neural tube during early development and can be detected using a GD3-specific monoclonal antibody (mAb R24).68 Upon closer examination, GD3 is found to be robustly expressed on NECs in the neural tube and in RGCs of the VZ in embryos, and the SVZ of postnatal and adult rodents.36,69–72 Fig. 2A shows that GD3 is colocalized with nestin at the SVZ. GD3+ cells also express SSEA-1 in the SVZ of mouse brain.36 GD3 is the predominant ganglioside species in cultured mouse neurospheres, accounting for more than 80% of the total GSLs. In addition to GD3, another b-series ganglioside, GD2, has also been shown to be highly expressed in human NSCs.73 NSCs are costained with GD3 and SSEA-1, and GD3+ cells also express other NSC markers, such as Sox2, nestin, and Musashi-1. After cell differentiation, GD3 expression is decreased in cultured NSCs. For this reason, GD3 has been proposed to be a useful biomarker for mouse NSCs. Most living cells (80%) were positive for GD3 in E14 striata and in P2 and P10 SVZs. On the contrary, fewer cells (30%) are positive for GD3 in P30 and adult SVZs. Similar strong expression of GD3 is observed in NSCs isolated from embryonic, postnatal, and adult brains. Most GD3+ cells isolated by FACS were also positive for nestin and SSEA-1, but negative for differentiated cell markers. GD3+ cells can generate neurospheres more efficiently than GD3− cells. The GD3+ cells are capable of differentiating into neuronal and glial cells. Those results clearly suggest that GD3 is a useful cell surface marker for isolating living NSCs, especially from adult brain tissues in which NSCs are less abundant.36
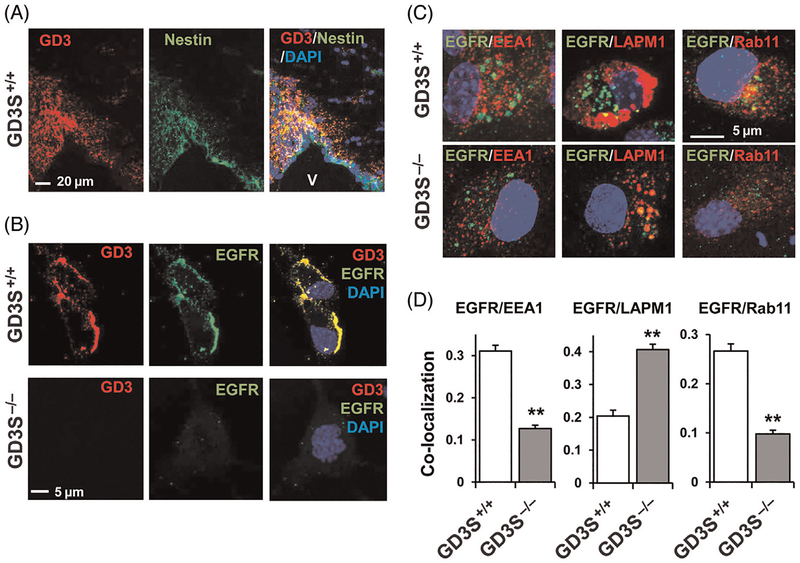
Fig. 2.
Interaction of GD3 with EGFR modulates self-renewal of murine NSCs. (A) GD3 (red) is colocalized with nestin (green) in SVZ (3-month-old wild-type mouse). (B) Cell surface EGFR expression (green) on GD3+/+ or GD3−/− NSCs. NSCs were cultured with 20 ng/mL EGF and 20 ng/mL FGF2. A decrease of EGFR expression is detected in GD3SKO NSCs. The decrease in EGFR expression is accompanied by an accelerated loss of the self-renewal capability in the GD3S-KO NSCs (data not shown). (C) GD3 regulates the endocytosis of EGFR. Colocalization of EGFR (green) with EEA1 (red), LAMP1 (red), and Rab11 (red) in monolayer NSCs under normal culture conditions (20 ng/mL EGF and 20 ng/mL FGF2). The colocalization coefficiency was measured by Zeiss LSM-enhanced colocalization software. Values are expressed as mean ± SEM; n = 7. **P < 0.01. EGFR showed significantly increased colocalization with the lysosomal marker LAMP1 in GD3S-KO NSCs. On the contrary, colocalization rates with a marker of recycling endosomes, Rab11 and the early endosomal marker, EEA1, showed decreases in GD3S-KO NSCs. V, Ventricle.
To clarify whether GD3 modulates the self-renewal capacity or cell fate determination of NSCs, NSCs from GD3-synthase (GD3S)-KO mice were analyzed. Surprisingly, no significant difference in the proliferation rate and expression of lineage-associated markers was found between GD3S+/+ and GD3S-KO NSCs that were cultured in the presence of FGF-2 but in the absence of EGF.74 GD3S-KO NSCs that were cultured with EGF, however, showed dramatically suppressed proliferation.37 The expression of nestin and EGFR was also strongly downregulated and the MAPK/ERK pathway signaling was impaired in GD3S-KO NSCs. In addition, EGFR degradation and the reduction of p-EGFR and p-ERK1/2 were correlated in the GD3SKO NSCs after EGF stimulation. Subsequently, a decrease of the MAPK/ERK proliferation pathway was found in GD3S-KO NSCs. Furthermore, more membrane EGFRs (Fig. 2B) were expressed on the cell surface of GD3S+/+ NSCs (53%) than in GD3S-KO NSCs (22%). Likewise, EGFR and GD3 were found to be colocalized in NSCs, and they interacted in the lipid raft region of the cell surface as well as in intracellular vesicles. Interestingly, EGFR was found to exist on nonlipid raft fractions in the GD3S-KO NSCs. Those findings provide convincing evidence that EGFR and GD3 are colocalized in lipid raft microdomains and support the notion that GD3 is essential for the maintenance of the self-renewal capability in NSCs by recruiting EGFR to the microdomains to sustain the EGF-induced downstream signaling.37
Endocytosis is a basic cellular process that is used by cells to internalize a variety of molecules. Cells are estimated to internalize, via endocytosis, about half their plasma membrane per hour.75 GSLs are recognized to undergo endocytosis. Once internalized, GSLs can be (1) recycled to the plasma membrane; (2) sorted to the Golgi complex, in which they can be reglycosylated; or (3) degraded in the lysosome. As EGFR expression was reduced in GD3S-KO NSCs, internalization of its ligand EGF was investigated. It was found that the number of NSCs with internalized biotinylated-fluorescent-EGF was significantly reduced in the absence of GD3. As it is known that EGFR can be recruited to the endosomes for recycling or sorted to lysosomes for degradation, it was found that in the GD3S-KO NSCs, EGFR exhibited increased colocalization with the LAMP 1 (lysosome) as well as diminished colocalization with a marker of recycling endosomes, Rab11 (Fig. 2C and D). The colocalization rate with EEA1 (an early endo-some marker) also showed a decrease in the GD3S-KO NSCs. Pursuing the endocytosed EGFR indicated that a large amount of EGFR in GD3S-KO NSCs underwent the endosomal–lysosomal degradative pathway, whereas a greater portion of EGFR was subject to the recycling pathway in the GD3S+/+ NSCs than in GD3S−/− NSCs. Hence, the interaction of GD3 and EGFR in NSCs is responsible for sustaining EGFR surface expression and downstream signaling to maintain the self-renewal of the cells.37
5. GD3 MAINTAINS POSTNATAL NSC POOLS
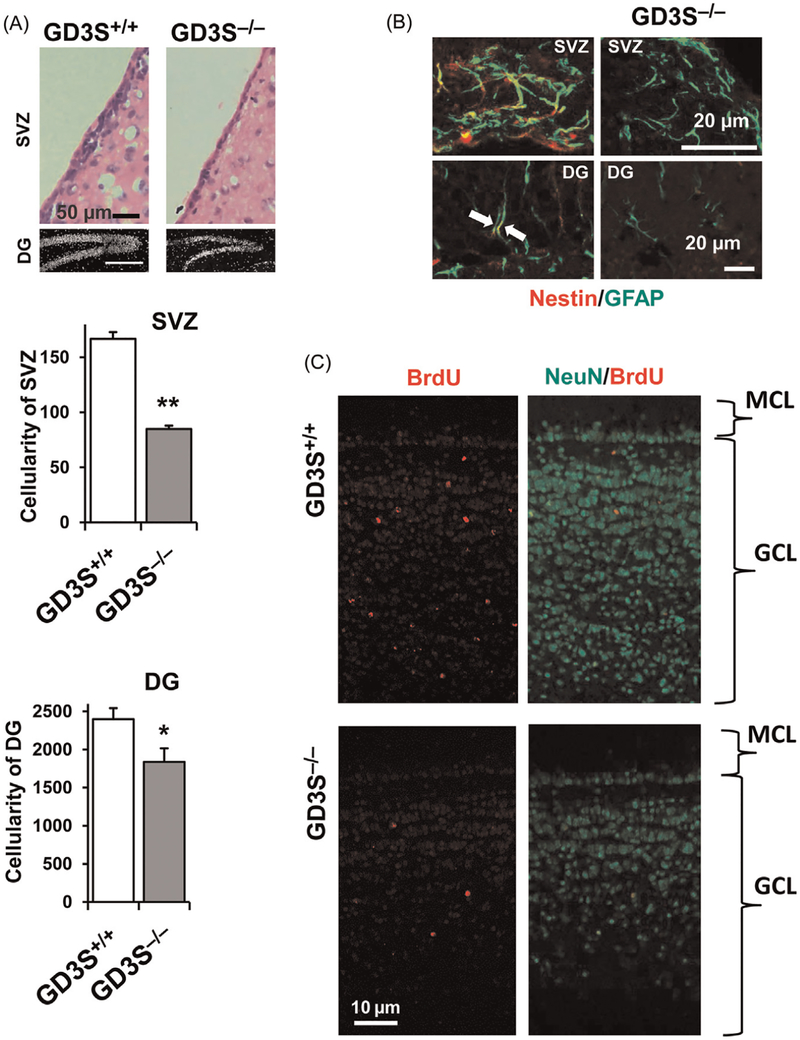
In postnatal brain, GM1, GD1a, GD1b, and GT1b are strongly expressed. On the contrary, the expression of GD3 is decreased and restricted. As shown in Fig. 2A, the expression of GD3 and nestin is found in identical cells at the SVZ of postnatal mice. In these nestin+ cells, GD3 is the predominant GSL species (>80%). We compared the histology and cellularity of SVZ and DG in 6-month-old wild-type (WT) and GD3SKO mice.76 The cellularity at the SVZ and DG of GD3S-KO mice was significantly reduced compared with that of their WT littermates (Fig. 3A). These findings indicated that there are progressive reductions of the cellular pool in the SVZ and DG in adult GD3S-KO mice. To investigate the population of adult NSCs, we counted the nestin and GFAP double-positive cells in the adult brain (Fig. 3B). We found that nestin+/GFAP+ radial glia-like NSCs were reduced in the SVZ of 1-month-old GD3S-KO mice(57.7 ± 16.2% of WT mice). In the 6-month-old SVZ, nestin+/GFAP+ cells were nearly absent from the GD3S-KO brains. Consistent with this observation, the number of nestin+/GFAP+ radial glia-like cells at the DG of GD3S-KO mice was also significantly reduced (45.7 ± 13.6% at 1-month-old mice; 83.2 ± 15.2% reduced at 6-month-old mice) compared with their WT littermates. To evaluate neurogenesis, bromo-deoxy-uridine (BrdU) was administered to 4-month-old mice (i.p.) and imunohistochemical analyses were performed at 4 weeks after BrdU injection (Fig. 3C). Newly generated neurons (NeuN+/BrdU+) in the olfactory bulb were significantly decreased in GD3S-KO mice. The density of NeuN in the granular cell layers was much lower in the GD3S-KO mice than in their WT littermates, which indicated a long-term insufficiency of the supply of new neurons to this region. At the SVZ and DG, the numbers of BrdU-incorporated cells were significantly reduced in the GD3S-KO mice compared with WT mice. Our study clearly demonstrates that GD3 plays a crucial role in the long-term maintenance of NSC populations in postnatal mouse brain. Moreover, impaired neurogenesis in the adult GD3S-KO mice can lead to depression-like behaviors.76 Thus, our results provide strong evidence linking ganglioside deficiency and neurogenesis defects to behavioral deficits.
Fig. 3.
NSC pools are reduced in GD3S-KO mouse brain in vivo. (A) Reduced cellularity in the SVZ and DG of adult GD3S-KO mouse brain (6-month-old mice). Hematoxylin and eosin staining was used to quantify the SVZ cellularity, and DAPI staining was used to trace the DG cellularity. Values are expressed as mean ± SEM; n = 4. *P < 0.05; **P < 0.01. (B) Nestin+/GFAP+ radial glia-like NSCs were reduced in the SVZ and DG of GD3S-KO mouse. In the 6-month-old SVZ, nestin+/GFAP+ cells were nearly absent from the GD3S-KO brains (B, right upper panel). Consistent with this observation, the number of nestin+/GFAP+ radial glia-like cells at DG of GD3S-KO mice was also significantly reduced (45.7 ± 13.6% at 1-month-old mice; 83.2 ± 15.2% reduced at 6-month-old mice) compared with their WT littermates. (C) Bromo-deoxy-uridine (BrdU) was administered to 4-month-old mice (i.p.) and imunohistochemical analyses were performed at 4 weeks after BrdU injection. Newly generated neurons (NeuN+/BrdU+) in the olfactory bulb (OB) were significantly decreased in GD3S-KO mice. The density of NeuN in the granular cell layers (GCL) was much lower in the GD3S-KO mice than in their WT littermates, which indicated a long-term insufficiency of the supply of new neurons to this region. MCL, Molecular cell layer.
6. A2B5 IN PROGENITOR CELLS
A2B5 antigens, including the c-series gangliosides, are well-known markers for immature progenitor cells in the nervous system and are the first GSL antigens expressed in cells of the glial lineage.77–80 A2B5 is a mouse mAb originally developed using chicken embryonic retina cells as the immunogen.77 The antigens recognized by mAb A2B5 have been established as the c-series gangliosides, including GQ1c, GT1c, and GT3,78,80 and the anti-body recognizes the Neu5Acα2–8-Neu5Acα2–8Neu5Acα-or α2,8-trisialosyl (triSia) structure.81 These c-series gangliosides are phylogenetically conserved and developmentally regulated, and are most abundant in the brain of lower vertebrates, such as fish, and the brain of mammalian embryos, but not adult brains.82–86 In adult zebrafish brain, c-series gangliosides comprise up to 70% of the total gangliosides.87 Accordingly, damaged zebrafish brains have high regeneration capacity, and the brain is rich in A2B5+ cells.88 In proliferating NSCs of mammals, GT3-synthase (GT3S or ST-III), an enzyme for the synthesis for c-series gangliosides (A2B5 antigens), is highly expressed.89,90 Some GFAP-expressing cells are considered as NSCs as well as astrocytes, and A2B5+/GFAP+ cells have a lipid composition distinct from mature astrocytes, but they are more similar to stem/progenitor cells.91 In maturing mammalian brain, the concentration of c-series gangliosides decreases drastically,46,82–84 and this decrease in the synthesis of c-series gangliosides is compensated for by a pathway shift in favor of the accretion of a- and b-series gangliosides.41,45,46 Glial-restricted precursors (GRPs) have been recognized by the expression of the A2B5 epitope.92 It is uncertain, however, whether GRPs exist in vivo. A2B5 antigens in these progenitors have been identified as GT3 and O-acetyl GT3.93 In the developing mouse brain, it has been reported that mAb A2B5 also reacts with four glycoproteins in addition to c-series gangliosides.81
7. CHOLINERGIC-SPECIFIC ANTIGEN (CHOL-1α)
The presence of neuronal gangliosides may also be functionally specific. For example, Chol-1α gangliosides (GT1aα and GQ1bα) are normally minor species in the brain and serve as unique markers of cholinergic neurons.94,95 They have been shown immunocytochemically in human CNS and may find applications in human neuropathology. The expression of Chol-1α gangliosides in rat brain regions, such as the hippocampus, is developmentally regulated, and their concentrations increase with aging.96 Ando et al.97 have further shown that treatment of anti-Chol-1α mAb inhibits the release of acetylcholine from synaptosomes. Interestingly, the memory and learning abilities of rats given anti-Chol-1α mAb are remarkably suppressed. On the contrary, addition of Chol-1α gangliosides of the synaptosomal preparation induces high affinity choline uptake into synaptosomes and enhances synthesis of acetylcholine. Thus, Chol-1α gangliosides may participate in maintaining cognitive functions, such as memory and learning. In addition, Chol-1α gangliosides have been shown to alleviate the decreased synaptic functions of aged brains.98,99 NSCs have been found to express both gangliosides, GT1aα and GQ1bα, in vitro.41 These findings suggest that Chol-1α antigens may play an important role in cholinergic synaptic transmission and participate in cognitive function.
8. EPIGENETIC REGULATION OF NEURONAL DIFFERENTIATION BY GM1
During neuronal differentiation, the concentration of GD3, which is the predominant ganglioside in NSCs, is rapidly decreased. Concomitantly, the levels of GM1, GD1a, GD1b, and GT1b continuously increase in young animals, reaching a plateau during adulthood.38,41,100 This pattern change follows closely with the upregulation of GM2/GD2S expression.41 The dramatic changes in the expression profile of gangliosides clearly reflect the biological needs of GalNAc-containing ganglio-series gangliosides at particular stages during brain development. During neuronal development, GM1-expressing cells are considered as neuronal progenitor cells and neurons.101–103
Recently, our laboratory has demonstrated for the first time that histone acetylation in chromatin of the 5′-region of the mouse GM2/GD2S gene can regulate mRNA expression, and this epigenetic modification is highly correlative to the stage-specific alterations of GM2/GD2S mRNA levels in mouse brain during development.38,39 In an initial study, we investigated the epigenetic regulation of two key GTs, GM2/GD2S and GD3S, in embryonic, postnatal, and adult mouse brains. Interestingly, the temporal expression patterns of GM2/GD2S and GD3S mRNAs are correlated with histone H3 and H4 acetylation (AcH3/AcH4) of the gene’s 5′-flanking region in chromatin.38 These observations suggest that the 5′-region of the GM2/GD2S and GD3S genes could be targets for epigenetic regulation by histone acetylation. Furthermore, we have demonstrated that acetylation of histones H3 and H4 on the GM2/GD2S gene promoter leads to recruitment of the trans-activation factors Sp1 and AP-2.
When the cellular histone deacetylase (HDAC) activity is globally inhibited by short-chain fatty acids, such as valproic acid and butyric acid, neuronal differentiation of NSCs is promoted with extensive neurite outgrowth.38,39 More GM2/GD2S or GD3S mRNA can be detected by the application of valproic acid in the NSC culture, which is triggered due to a loading boost of the transcription factors Ap-2 and Sp1 on the promoter region.39 Individually knocking down HDAC1 and HDAC2 gene expression increases the levels of AcH3 and AcH4 on the GM2/GD2S gene. However, the GM2/GD2S gene does not show any corresponding change in the promoter-binding abilities for the transcription factors or mRNA expression level by individually knocking down. Intriguingly, when both HDAC1 and HDAC2 are knocked down, the expression of GM2/GD2S mRNA is upregulated and Ap-2- or Sp1-loading is significantly increased, reflecting the elevated level of histone acetylation on the GM2/GD2S gene. This is not the case for the GD3S gene, however, which remains unchanged with respect to the levels of mRNA, the binding of transcription factors, or the acetylated histone status. Our results clearly indicate that transcription of GM2/GD2S and GD3S can be regulated by different HDAC isoforms because double-knockdown by si-HDAC1 and si-HDAC2 leads to GM2/GD2S gene trans-activation, but not GD3S.39
It is known that GM1 enhances neurite outgrowth in primary neuronal cultures. It has been shown that GM1 is also present in the nuclear membranes and upregulation of GM1 in the nuclear membrane accompanies the process of neurite outgrowth. This observation prompted Ledeen and coworkers to propose that the elevated GM1 has a modulatory effect on Ca2+ homeostasis in the nucleus, which is mediated by a tight association of GM1 with Na+/Ca2+exchanger.104,105 In earlier studies, it has been reported that GM1 induces tubulin mRNA accumulation during GM1-stimulated neurite outgrowth.106 Interestingly, NSCs cultured with a supplement of GM1 for 7 days also exhibit a significantly enhanced neurogenic effect.39 Additional GM1, but not GD3, enhanced GM2/GD2S expression of mRNA, whereas the mRNA level of GD3S remained unchanged. The presence of ectopic GM1 renders an enrichment of the acetylated histones on the gene loci of GM2/GD2S, but not GD3S, which is accompanied by the recruitment of transcription factors AP-2 and Sp1 within the gene promoter region. It is possible that in this enhanced neurogenic process, exogenous GM1 induces NSCs to transcribe more GM2/GD2S mRNA with a higher level of AcH4 on the GM2/GD2S’s promoter region where more transcription factors are recruited. On the contrary, the GD3S gene does not show significant changes in mRNA expression and AcH4 binding by addition of GM1. This result may represent a potential mechanism accounting for the correlations between the ganglioside pattern shift and epigenetic modifications of ganglioside synthase expression over neuronal differentiation and neural development. In this scenario, GM1 generates a positive feedback loop for NSCs to promote neuronal differentiation and to produce more GM1 and brain-type gangliosides, such as GD1a, GD1b, and GT1b. In this regard, GM1 is considered to play a crucial role in modulating the pathway switch in ganglioside expression in the developing brain.
The nuclear envelope, including the nuclear lamina and nuclear pore complexes, is an important structure to maintain chromatin architecture and cell-specific gene expression.107 Our confocal microscopy study reveals that nuclear GM1 is colocalized with lamin B1 (a protein of the nuclear lamina) or nucleoporin (a protein of the nuclear pore complex) on the nuclear periphery of induced neurons from NSCs.40 Coimmunoprecipitation experiments show that nuclear GM1 interacts with AcH3 and AcH4, both active epigenetic marks, but not with H3K27me3, an inactive epigenetic mark. Chromatin immunoprecipitation assays reveal that the promoter regions of the GM2/GD2S and NeuroD1 genes are associated with GM1. In situ hybridization assays have revealed that GM1 and the promoter region of the GM2/GD2S gene are in close proximity in the nucleus of a neuron. Despite the low expression of nuclear GM1 in NSCs, GM1 and the GM2/GD2S promoter do not colocalize therein. This result suggests that the interaction of GM1 and the GM2/GD2S gene promoter occurs in a differentiation stage-specific manner.40
9. CONCLUSION AND FUTURE STUDIES
Gangliosides are recognized as significant brain lipids that play important functions in cell–cell recognition, adhesion, and signal transduction. Brain gangliosides undergo dramatic qualitative and quantitative developmental changes that are correlated with cellular proliferation, differentiation, and play critical biological functions in the nervous system. Future studies should focus on gaining a better understanding of the molecular basis of the dramatic changes in ganglioside expression and their associated functional roles in neural cell fate determination and the regulatory mechanisms of GTs that account for the GSL pathway switch from simple to complex ganglio-sides during neural differentiation. Changes in GT expression in NSCs rationalize the dramatic changes of ganglioside expression during differentiation. The synthesis of GD3 is switched to the synthesis of complex gang-liosides (GM1, GD1a, GD1b, and GT1b), resulting in terminal differentiation and loss of the “stemness” of NSCs. In the adult, NSCs are enriched in the SVZ of the lateral ventricles and the subgranular layer of the DG in the hippocampus. There is a growing awareness that incorporation of NSCs is associated with not only improved learning and memory, but also in functional recovery from brain injuries. This latter observation also indicates that events impacting adult NSC homeostasis may significantly contribute to the pathogenic mechanisms associated with neurodegenerative diseases. Finally, our preliminary experiments showing that intraventricular infusion of ganglioside GD3 augments NSC pools in the adult mouse brain (unpublished data) suggest a mechanism for regulating adult neurogenesis. Future studies in this regard will contribute greatly to regenerative and reparative biology.
Although recent evidence of GSLs including gangliosides has shed light on their roles in modulating signaling pathways during cellular differentiation and reprograming, mice deficient in some of these molecules show only subtle phenotypic abnormalities compared with the WT animals in early development. Clearly, the biological function of one glycoconjugate can be substituted by another, albeit with less efficiency. However, the aberrant ganglioside expression becomes progressively more serious in the adult stage and pathogenic conditions. The “biological redundancy” can be considered for more important functions of these molecules. Future studies will clarify the cell- and stage-specific function and importance of gangliosides in maintaining NSC activities and in neural differentiation.
Cellular signaling events that mediate lipid metabolism are among the most crucial processes controlling maintenance of brain function at the structural level. Progressive imbalance of cell membrane lipid composition is a physicochemical property altered with normal aging, and further disruptions to these processes are observed in neurodegenerative diseases. Ganglioside expression profiles are associated not only with cell differentiation of stem cells, but also with pathogenic mechanisms of neurodegenerative diseases in CNS, such as Alzheimer’s disease, Parkinson disease, Huntington’s disease, amyotrophic lateral sclerosis, and multiple sclerosis.108–111 In addition, the aberrant and elevated expression of gang-liosides has also been observed in many types of cancer cells, thereby promoting tumor survival.112,113 Novel epigenetic regulatory mechanisms for GSLs will contribute to a better understanding of the pathway switch observed during neuronal differentiation of NSCs. In addition, epigenetic regulation of GSLs will provide clues underlying the pathogenic mechanisms, which are useful in developing novel strategies for disease treatment and tissue damage repair. Futures studies will prove extremely fruitful in this regard.
ACKNOWLEDGMENTS
This work was supported by a VA Merit Review Award (1 I01BX001388 to RKY), NIH grants (RO1 NS100839, NS26994, and NS11853 to RKY), and Mizutani Foundation for Glycoscience (150026 to YI). The authors declare no conflicts of interest.
REFERENCES
- 1.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. NatRevNeurosci. 2001;2(4):287–293. [DOI] [PubMed] [Google Scholar]
- 2.Fishell G, Kriegstein A. Cortical development: new concepts. Neuron. 2005;46(3): 361–362. [DOI] [PubMed] [Google Scholar]
- 3.Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. CurrOpinNeurobiol. 2003;13(1):34–41. [DOI] [PubMed] [Google Scholar]
- 4.Fujita S The discovery of the matrix cell, the identification of the multipotent neural stem cell and the development of the central nervous system. Cell Struct Funct. 2003;28(4):205–228. [DOI] [PubMed] [Google Scholar]
- 5.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6(10):777–788. [DOI] [PubMed] [Google Scholar]
- 6.Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron. 2007;54(3):357–369. [DOI] [PubMed] [Google Scholar]
- 7.Shimojo H, Ohtsuka T, Kageyama R. Dynamic expression of notch signaling genes in neural stem/progenitor cells. FrontNeurosci. 2011;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127(24):5253–5263. [DOI] [PubMed] [Google Scholar]
- 9.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31(5):727–741. [DOI] [PubMed] [Google Scholar]
- 10.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409 (6821):714–720. [DOI] [PubMed] [Google Scholar]
- 11.Franco SJ, Muller U. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron. 2013;77(1):19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinto L, Gotz M. Radial glial cell heterogeneity—the source of diverse progeny in the CNS. ProgNeurobiol. 2007;83(1):2–23. [DOI] [PubMed] [Google Scholar]
- 13.Qian X, Shen Q, Goderie SK, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28(1):69–80. [DOI] [PubMed] [Google Scholar]
- 14.Jayakumar AR, Tong XY, Curtis KM, et al. Decreased astrocytic thrombospondin-1 secretion after chronic ammonia treatment reduces the level of synaptic proteins: in vitro and in vivo studies. JNeurochem. 2014;131(3):333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodnight RB, Gottfried C. Morphological plasticity of rodent astroglia. J Neurochem. 2013;124(3):263–275. [DOI] [PubMed] [Google Scholar]
- 16.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai HH, Li H, Fuentealba LC, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337(6092):358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajos F, Woodhams PL, Basco E, Csillag A, Balazs R. Proliferation of astroglia in the embryonic mouse forebrain as revealed by simultaneous immunocytochemistry and autoradiography. Acta Morphol Acad Sci Hung. 1981;29(4):361–364. [PubMed] [Google Scholar]
- 19.Ichikawa M, Shiga T, Hirata Y. Spatial and temporal pattern of postnatal proliferation of glial cells in the parietal cortex of the rat. Brain Res. 1983;285(2):181–187. [DOI] [PubMed] [Google Scholar]
- 20.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468(7321):214–222. [DOI] [PubMed] [Google Scholar]
- 22.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6): 703–716. [DOI] [PubMed] [Google Scholar]
- 23.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. JNeurosci. 1997;17(13):5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. JNeurosci. 2001;21(18):7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383(6601):624–627. [DOI] [PubMed] [Google Scholar]
- 26.Morshead CM. Adult neural stem cells: attempting to solve the identity crisis. Dev Neurosci. 2004;26(2–4):93–100. [DOI] [PubMed] [Google Scholar]
- 27.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1): 25–34. [DOI] [PubMed] [Google Scholar]
- 28.Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19(11): 4462–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. ProcNatlAcad SciUSA. 2000;97(25):13883–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlen M, Meletis K, Goritz C, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. NatNeurosci. 2009;12(3):259–267. [DOI] [PubMed] [Google Scholar]
- 31.Coskun V, Wu H, Blanchi B, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105(3):1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filippov V, Kronenberg G, Pivneva T, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23(3):373–382. [DOI] [PubMed] [Google Scholar]
- 33.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci USA. 2006;103(21):8233–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54(8):805–814. [DOI] [PubMed] [Google Scholar]
- 35.Lugert S, Vogt M, Tchorz JS, Muller M, Giachino C, Taylor V. Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nat Commun. 2012;3:670. [DOI] [PubMed] [Google Scholar]
- 36.Nakatani Y, Yanagisawa M, Suzuki Y, Yu RK. Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology. 2010;20(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Yu RK. Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. ProcNatlAcadSciUSA. 2013;110(47): 19137–19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki Y, Yanagisawa M, Ariga T, Yu RK. Histone acetylation-mediated glycosyltransferase gene regulation in mouse brain during development. J Neurochem. 2011;116(5): 874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai YT, Yu RK. Epigenetic activation of mouse ganglioside synthase genes: implications for neurogenesis. JNeurochem. 2014;128(1):101–110. [DOI] [PubMed] [Google Scholar]
- 40.Tsai YT, Itokazu Y, Yu RK. GM1 ganglioside is involved in epigenetic activation loci of neuronal cells. NeurochemRes. 2016;41(1–2):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. JNeurochem. 2007;103(6):2327–2341. [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17(7):57R–74R. [DOI] [PubMed] [Google Scholar]
- 43.Yu RK, Macala LJ, Taki T, Weinfield HM, Yu FS. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988;50(6): 1825–1829. [DOI] [PubMed] [Google Scholar]
- 44.Xu YH, Barnes S, Sun Y, Grabowski GA. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J LipidRes. 2010;51(7):1643–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu RK, Nakatani Y, Yanagisawa M. The role of glycosphingolipid metabolism in the developing brain. J LipidRes. 2009;50(suppl):S440–S445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu RK, Itokazu Y. Glycolipid and glycoprotein expression during neural development. Adv Neurobiol. 2014;9:185–222. [DOI] [PubMed] [Google Scholar]
- 47.Itokazu Y, Tsai YT, Yu RK. Epigenetic regulation of ganglioside expression in neural stem cells and neuronal cells. GlycoconjJ. 2017;34(6):749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Angelo G, Polishchuk E, Di Tullio G, et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449(7158):62–67. [DOI] [PubMed] [Google Scholar]
- 49.D’Angelo G, Uemura T, Chuang CC, et al. Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature. 2013;501(7465):116–120. [DOI] [PubMed] [Google Scholar]
- 50.Halter D, Neumann S, van Dijk SM, et al. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J CellBiol. 2007;179(1):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chalat M, Menon I, Turan Z, Menon AK. Reconstitution of glucosylceramide flip-flop across endoplasmic reticulum: implications for mechanism of glycosphingolipid bio-synthesis. JBiolChem. 2012;287(19):15523–15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu RK, Tsai YT, Ariga T. Functional roles of gangliosides in neurodevelopment: an overview of recent advances. Neurochem Res. 2012;37(6):1230–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bieberich E, MacKinnon S, Silva J, Yu RK. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J Biol Chem. 2001;276(48):44396–44404. [DOI] [PubMed] [Google Scholar]
- 54.Fang Y, Wu G, Xie X, Lu ZH, Ledeen RW. Endogenous GM1 ganglioside of the plasma membrane promotes neuritogenesis by two mechanisms. Neurochem Res. 2000;25(7): 931–940. [DOI] [PubMed] [Google Scholar]
- 55.Wu G, Fang Y, Lu ZH, Ledeen RW. Induction of axon-like and dendrite-like processes in neuroblastoma cells. J Neurocytol. 1998;27(1):1–14. [DOI] [PubMed] [Google Scholar]
- 56.Wu G, Lu ZH, Xie X, Li L, Ledeen RW. Mutant NG108–15 cells (NG-CR72) deficient in GM1 synthase respond aberrantly to axonogenic stimuli and are vulnerable to calcium-induced apoptosis: they are rescued with LIGA-20. J Neurochem. 2001;76(3):690–702. [DOI] [PubMed] [Google Scholar]
- 57.Yu RK, Bieberich E, Xia T, Zeng G. Regulation of ganglioside biosynthesis in the nervous system. JLipidRes. 2004;45(5):783–793. [DOI] [PubMed] [Google Scholar]
- 58.Furukawa K, Aixinjueluo W, Kasama T, et al. Disruption of GM2/GD2 synthase gene resulted in overt expression of 9-O-acetyl GD3 irrespective of Tis21. J Neurochem. 2008;105(3):1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheikh KA, Sun J, Liu Y, et al. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci USA. 1999;96(13):7532–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugiura Y, Furukawa K, Tajima O, Mii S, Honda T. Sensory nerve-dominant nerve degeneration and remodeling in the mutant mice lacking complex gangliosides. Neuroscience. 2005;135(4):1167–1178. [DOI] [PubMed] [Google Scholar]
- 61.Susuki K, Baba H, Tohyama K, et al. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia. 2007;55(7):746–757. [DOI] [PubMed] [Google Scholar]
- 62.Takamiya K, Yamamoto A, Furukawa K, et al. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci USA. 1996;93(20):10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu G, Lu ZH, Kulkarni N, Amin R, Ledeen RW. Mice lacking major brain ganglio-sides develop parkinsonism. Neurochem Res. 2011;36(9):1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simpson MA, Cross H, Proukakis C, et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet. 2004;36(11):1225–1229. [DOI] [PubMed] [Google Scholar]
- 65.Boukhris A, Schule R, Loureiro JL, et al. Alteration of ganglioside biosynthesis responsible for complex hereditary spastic paraplegia. Am J Hum Genet. 2013;93(1):118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawai H, Allende ML, Wada R, et al. Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J Biol Chem. 2001;276(10):6885–6888. [DOI] [PubMed] [Google Scholar]
- 67.Yamashita T, Wada R, Sasaki T, et al. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci USA. 1999;96(16):9142–9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosner H, al-Aqtum M, Rahmann H. Gangliosides and neuronal differentiation. Neurochem Int. 1992;20(3):339–351. [DOI] [PubMed] [Google Scholar]
- 69.Bannerman PG, Oliver TM, Xu Z, Shieh A, Pleasure DE. Effects of FGF-1 and FGF-2 on GD3 immunoreactive spinal neuroepithelial cells. J Neurosci Res. 1996;45(5):549–557. [DOI] [PubMed] [Google Scholar]
- 70.Cammer W, Zhang H. Ganglioside GD3 in radial glia and astrocytes in situ in brains of young and adult mice. J Neurosci Res. 1996;46(1):18–23. [DOI] [PubMed] [Google Scholar]
- 71.Cammer W, Zhang H. Carbonic anhydrase II in microglia in forebrains of neonatal rats. J Neuroimmunol. 1996;67(2):131–136. [DOI] [PubMed] [Google Scholar]
- 72.Goldman JE, Hirano M, Yu RK, Seyfried TN. GD3 ganglioside is a glycolipid characteristic of immature neuroectodermal cells. J Neuroimmunol. 1984;7(2–3):179–192. [DOI] [PubMed] [Google Scholar]
- 73.Yanagisawa M, Yoshimura S, Yu RK. Expression of GD2 and GD3 gangliosides in human embryonic neural stem cells. ASN Neuro. 2011;3(2). pii: e00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu RK, Yanagisawa M. Glycosignaling in neural stem cells: involvement of glycoconjugates in signal transduction modulating the neural stem cell fate. J Neurochem. 2007;103(suppl 1):39–46. [DOI] [PubMed] [Google Scholar]
- 75.Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96(1):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J, Cheng A, Wakade C, Yu RK. Ganglioside GD3 is required for neurogenesis and long-term maintenance of neural stem cells in the postnatal mouse brain. J Neurosci. 2014;34(41):13790–13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eisenbarth GS, Walsh FS, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci USA. 1979;76(10):4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasai N, Yu RK. The monoclonal antibody A2B5 is specific to ganglioside GQ1c. Brain Res. 1983;277(1):155–158. [DOI] [PubMed] [Google Scholar]
- 79.Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983;3(6):1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saito M, Kitamura H, Sugiyama K. The specificity of monoclonal antibody A2B5 to c-series gangliosides. J Neurochem. 2001;78(1):64–74. [DOI] [PubMed] [Google Scholar]
- 81.Inoko E, Nishiura Y, Tanaka H, et al. Developmental stage-dependent expression of an alpha2,8-trisialic acid unit on glycoproteins in mouse brain. Glycobiology. 2010;20(7): 916–928. [DOI] [PubMed] [Google Scholar]
- 82.Ando S, Yu RK. Isolation and characterization of two isomers of brain tetrasialoganglio-sides. J Biol Chem. 1979;254(23):12224–12229. [PubMed] [Google Scholar]
- 83.Freischutz B, Saito M, Rahmann H, Yu RK. Activities of five different sialyltransferases in fish and rat brains. J Neurochem. 1994;62(5):1965–1973. [DOI] [PubMed] [Google Scholar]
- 84.Freischutz B, Saito M, Rahmann H, Yu RK. Characterization of sialyltransferase-IV activity and its involvement in the c-pathway of brain ganglioside metabolism. J Neurochem. 1995;64(1):385–393. [DOI] [PubMed] [Google Scholar]
- 85.Rosner H, Greis C, Henke-Fahle S. Developmental expression in embryonic rat and chicken brain of a polysialoganglioside-antigen reacting with the monoclonal antibody Q 211. Brain Res. 1988;470(2):161–171. [DOI] [PubMed] [Google Scholar]
- 86.Yu RK, Ando S. Structures of some new complex gangliosides of fish brain. Adv Exp Med Biol. 1980;125:33–45. [DOI] [PubMed] [Google Scholar]
- 87.Viljetic B, Labak I, Majic S, Stambuk A, Heffer M. Distribution of mono-, di- and trisialo gangliosides in the brain of Actinopterygian fishes. Biochim Biophys Acta. 2012;1820(9):1437–1443. [DOI] [PubMed] [Google Scholar]
- 88.Kishimoto N, Shimizu K, Sawamoto K. Neuronal regeneration in a zebrafish model of adult brain injury. Dis Model Mech. 2012;5(2):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Itokazu Y, Yu RK. Amyloid beta-peptide 1–42 modulates the proliferation of mouse neural stem cells: upregulation of fucosyltransferase IX and notch signaling. Mol Neurobiol. 2014;50:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koon NA, Itokazu Y, Yu RK. Ganglioside-dependent neural stem cell proliferation in Alzheimer’s disease model mice. ASN Neuro. 2015;7(6). pii: 1759091415618916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Itokazu Y, Tajima N, Kerosuo L, et al. A2B5+/GFAP+ cells of rat spinal cord share a similar lipid profile with progenitor cells: a comparative lipidomic study. Neurochem Res. 2016;41(7):1527–1544. [DOI] [PubMed] [Google Scholar]
- 92.Rao MS, Subbarao V. Effect of dexamethasone on ciprofibrate-induced cell proliferation and peroxisome proliferation. Fundam ApplToxicol. 1997;35(1):78–83. [DOI] [PubMed] [Google Scholar]
- 93.Farrer RG, Quarles RH. GT3 and its O-acetylated derivative are the principal A2B5-reactive gangliosides in cultured O2A lineage cells and are down-regulated along with O-acetyl GD3 during differentiation to oligodendrocytes. J Neurosci Res. 1999;57(3):371–380. [PubMed] [Google Scholar]
- 94.Ando S, Hirabayashi Y, Kon K, Inagaki F, Tate S, Whittaker VP. A trisialoganglioside containing a sialyl alpha 2–6 N-acetylgalactosamine residue is a cholinergic-specific antigen, Chol-1 alpha. J Biochem. 1992;111(3):287–290. [DOI] [PubMed] [Google Scholar]
- 95.Hirabayashi Y, Nakao T, Irie F, Whittaker VP, Kon K, Ando S. Structural characterization of a novel cholinergic neuron-specific ganglioside in bovine brain. J Biol Chem. 1992;267(18):12973–12978. [PubMed] [Google Scholar]
- 96.Derrington EA, Borroni E. The developmental expression of the cholinergic-specific antigen Chol-1 in the central and peripheral nervous system of the rat. Brain Res Dev Brain Res. 1990;52(1–2):131–140. [DOI] [PubMed] [Google Scholar]
- 97.Ando S, Tanaka Y, Kobayashi S, et al. Synaptic function of cholinergic-specific Chol-1alpha ganglioside. Neurochem Res. 2004;29(4):857–867. [DOI] [PubMed] [Google Scholar]
- 98.Ando S, Tanaka Y, Waki H, Kon K, Iwamoto M, Fukui F. Gangliosides and sialylcholesterol as modulators of synaptic functions. Ann NY Acad Sci. 1998;845:232–239. [DOI] [PubMed] [Google Scholar]
- 99.Ando S Glycoconjugate changes in aging and age-related diseases. Adv Neurobiol. 2014;9:415–447. [DOI] [PubMed] [Google Scholar]
- 100.Hirschberg K, Zisling R, van Echten-Deckert G, Futerman AH. Ganglioside synthesis during the development of neuronal polarity. Major changes occur during axonogenesis and axon elongation, but not during dendrite growth or synaptogenesis. J Biol Chem. 1996;271(25):14876–14882. [DOI] [PubMed] [Google Scholar]
- 101.Androutsellis-Theotokis A, Walbridge S, Park DM, Lonser RR, McKay RD. Cholera toxin regulates a signaling pathway critical for the expansion of neural stem cell cultures from the fetal and adult rodent brains. PLoS One. 2010;5(5):e10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liour SS, Dinkins MB, Su CY, Yu RK. Spatiotemporal expression of GM1 in murine medial pallial neural progenitor cells. J Comp Neurol. 2005;491(4):330–338. [DOI] [PubMed] [Google Scholar]
- 103.Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci. 2003;23(1):240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ledeen RW, Wu G, Lu ZH, Kozireski-Chuback D, Fang Y. The role of GM1 and other gangliosides in neuronal differentiation. Overview and new finding. Ann NY Acad Sci. 1998;845:161–175. [DOI] [PubMed] [Google Scholar]
- 105.Ledeen RW, Wu G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem Sci. 2015;40(7):407–418. [DOI] [PubMed] [Google Scholar]
- 106.Rybak S, Ginzburg I, Yavin E. Gangliosides stimulate neurite outgrowth and induce tubulin mRNA accumulation in neural cells. Biochem Biophys Res Commun. 1983;116(3):974–980. [DOI] [PubMed] [Google Scholar]
- 107.Talamas JA, Capelson M. Nuclear envelope and genome interactions in cell fate. Fron Genet. 2015;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu RK, Ueno K, Glaser GH, Tourtellotte WW. Lipid and protein alterations of spinal cord and cord myelin of multiple sclerosis. J Neurochem. 1982;39(2):464–477. [DOI] [PubMed] [Google Scholar]
- 109.Yu RK, Ledeen RW, Eng LF. Ganglioside abnormalities in multiple sclerosis. J Neurochem. 1974;23(1):169–174. [DOI] [PubMed] [Google Scholar]
- 110.Ariga T The pathogenic role of ganglioside metabolism in Alzheimer’s disease-cholinergic neuron-specific gangliosides and neurogenesis. Mol Neurobiol. 2017;54(1): 623–638. [DOI] [PubMed] [Google Scholar]
- 111.Ariga T Pathogenic role of ganglioside metabolism in neurodegenerative diseases. J NeurosciRes. 2014;92(10):1227–1242. [DOI] [PubMed] [Google Scholar]
- 112.Ladisch S, Liu Y. Dynamic aspects of neural tumor gangliosides. Adv Neurobiol. 2014;9:501–515. [DOI] [PubMed] [Google Scholar]
- 113.Groux-Degroote S, Guerardel Y, Delannoy P. Gangliosides: structures, biosynthesis, analysis, and roles in cancer. Chembiochem. 2017;18(13):1146–1154. [DOI] [PubMed] [Google Scholar]