Abstract
Objective
To assess associations between epigenetic maturity of extremely preterm babies (born at less than 28 weeks of gestation), neonatal interventions, and respiratory outcomes, including the administration of surfactant and postnatal corticosteroids, duration of assisted ventilation, and development of bronchopulmonary dysplasia (BPD).
Study design
DNA was extracted from neonatal blood spots collected after birth from 143 extremely preterm infants born 1991–1992 in Victoria, Australia and used to determined DNA methylation (DNAm). A DNAm based gestational age was determined using our previously published method. The residual of DNAm gestational age and clinically estimated gestational age (referred to as “gestational age acceleration”) was used as a measure to assess developmental maturity. Associations between gestational age acceleration and respiratory interventions and morbidities were determined.
Results
Infants with higher gestational age acceleration were less likely to receive surfactant (P =.009) or postnatal corticosteroids (P = .008), had fewer days of assisted ventilation (P = .01), and had less BPD (P = .02). Respiratory measures are known to correlate with gestational age; however, models comparing each with clinically estimated gestational age were improved by the addition of the gestational age acceleration measure in the model.
Conclusions
Gestational age acceleration correlates with respiratory interventions and outcomes of extremely preterm babies. Surfactant and postnatal corticosteroid use, assisted ventilation days, and BPD rates were all lower in babies who were epigenetically more mature than their obstetrically estimated gestational age. This suggests that gestational age acceleration is a clinically relevant metric of developmental maturity.
Preterm infants, especially those born very (<32 weeks of gestation) or extremely (<28 weeks of gestation) preterm are at an increased risk of developing acute and long-term health complications, including acute respiratory distress and failure.1–3 Most require respiratory interventions such as mechanical ventilation, continuous positive airway pressure, high flow oxygen, or treatment with surfactant or postnatal corticosteroids.3,4 Advances in these supportive therapies have improved neonatal survival, but many infants develop bronchopulmonary dysplasia (BPD) as a consequence of the developmental immaturity of their lungs and the respiratory interventions required.3,5, 6 BPD is a chronic lung disorder most often defined as a requirement for supplemental oxygen at 36 weeks of postmenstrual age.3,7,8 In addition, preterm infants may also face long-term neurodevelopmental and pulmonary morbidities.9,10
Gestational age is a major determinant of neonatal morbidity and mortality,11 including the risk of developing BPD. Gestational age is clinically determined either by the date of the last menstrual period or, more accurately, by early obstetrical ultrasound assessment.12,13 However, individual variations in outcome are observed for infants with the same gestational age, for reasons not clearly understood. Biological variability in development and maturity between babies of the same gestational age may be a contributing factor.
Epigenetic modifications such as variations in DNA methylation (DNAm) are important biological mechanisms regulating developmental processes.14 Methylation levels at cytosine-guanosine dinucleotides (CpG) vary at hundreds of thousands of locations genome wide and can be determined by array technologies.15 We have recently developed a method to predict gestational age at birth using DNAm at 148 CpG sites across the genome.16 DNAm gestational age is highly correlated with clinically estimated gestational age. The residual between DNAm age and clinical gestational age, known as gestational age acceleration, has previously been associated with birth weight, which may reflect developmental maturity.16 The aim of this study was to explore the relationships between gestational age acceleration and respiratory interventions and outcomes after birth in infants born extremely preterm. As higher gestational age acceleration may indicate increased developmental maturity, we hypothesized that it may be associated with less respiratory intervention in the neonatal intensive care unit and lower likelihood of developing BPD.
Methods
All surviving preterm babies born at <28 weeks of gestation by best obstetrical estimate in the state of Victoria, Australia, during 1991 and 1992 were enrolled in a longitudinal follow-up study. The study was approved by the Human Research Ethics Committees of the Royal Women’s Hospital, the Mercy Hospital for Women, Monash Medical Centre, and the Royal Children’s Hospital, Melbourne, Australia (HREC No.23034C). Details of this cohort have been previously described.17
The 143 subjects included in this analysis represent probands born in the state of Victoria at less than 28 weeks of gestational age in 1991–1992 and who survived to 18 years of age, and for whom consent was obtained for the current study. Initial enrollment into the above-mentioned study was performed with parental consent. The study probands had reached adulthood and gave informed consent for the analysis of their neonatal blood spots, which were collected at an average of 9.9 days after birth. Blood spots were stored at ambient temperatures after collection. Data relevant to this study were recorded during the perinatal period, including neonatal sex, surfactant administration, use of postnatal corticosteroids, duration of assisted ventilation (including intermittent positive pressure ventilation via an endotracheal tube or nasal continuous positive airway pressure), and incidence of BPD (defined as receiving oxygen at 36 weeks of postmenstrual age). Decisions to give surfactant (Exosurf was the only surfactant available) or postnatal corticosteroids (predominantly dexamethasone) were made by the treating clinicians.
DNA Methylation Analysis
DNA was extracted from dried blood spot cards using the ZR DNA Card Extraction Kit (Zymo Research; Irvine, California). Sample quality and DNA concentration were assessed through spectrophotometry (Nanodrop, Wilmington, Delaware). Samples with sufficient quality and concentration were processed on the Illumina Infinium HumanMethylation450 BeadChip (Illumina, San Diego, California) per manufacturer’s recommendation as in our previous work.18 Data were sub jected to standard quality control through the use of the R package CpGassoc.19 Data frames of the raw signal values were supplied as input to CpGassoc. Any data point with a detection P value above .001 was set to missing. CpG sites with >5% missing data and subsequently, samples with >5% missing data were excluded. After quality control, β values were determined by the ratio of methylation to total signal. Cellular heterogeneity (proportions of CD4, CD8, CD14, CD19, CD56, neutrophils, and eosinophils) was estimated as previously described.20
Determination of DNAm Gestational Age and Gestational Age Acceleration
DNAm gestational age was estimated using our previously published method,16 implemented in the publicly available software (https://github.com/akknight/PredictGestationalAge). Briefly, we estimate gestational age based on DNAm at 148 CpG sites across the genome. Prior to gestational age estimation, a data frame of all CpG sites passing quality control is supplied as input for the predictive algorithm, and undergoes a modified beta-mixture quantile normalization as described previously.16,21,22 DNAm gestational age was compared with clinically estimated gestational age. Gestational age acceleration was determined as the residual of a linear model regressing all DNAm gestational age values on clinically determined gestational age values, after adjusting for time to sample collection (9.9 ± SD of 3.9 days).16 Table I (available at www.jpeds.com) gives an overview of the definitions and descriptions of all measures used to describe gestational age and gestational age acceleration.
Table I.
Definitions and description of measures used to quantify gestational age and gestational age acceleration
| 1. Clinically estimated gestational age: Gestational age at birth as determined in routine clinical care through measurements on ultrasound before 20 wk, or menstrual history if no ultrasound dating was available |
| 2. DNAm gestational age: Gestational age at sample collection predicted from DNAm. This measure is highly correlated with gestational age, but differences between gestational age and DNAm gestational age are observed and may reveal an infant’s developmental maturity. |
| 3. Gestational age acceleration: Calculated as residual of an individual proband’s DNAm gestational age onto the regression curve of gestational age and DNAm gestational age for the complete cohort. This measure is used to assess an infant’s developmental maturity. A positive gestational age acceleration indicates a more mature infant than their gestational age suggests and a negative gestational age acceleration indicates a less mature infant than their gestational age suggests. It is important to note that gestational age acceleration is independent of gestational age. |
Statistical Analyses
To assess associations between gestational age acceleration and common neonatal complications, logistic regressions were performed to determine the associations between each binary outcome (surfactant administration, use of postnatal corticosteroids, and incidence of BPD) and gestational age acceleration, adjusting for cellular heterogeneity and neonatal sex. Linear regression was performed to evaluate the association between duration of assisted ventilation and gestational age acceleration, adjusting for cellular heterogeneity and neonatal sex. To assess the additional contribution of gestational age acceleration to models including gestational age for neonatal outcome predictions, linear regressions, adjusting for neonatal sex and cell type, with and without the inclusion of the gestational age acceleration term were compared. As male infants have been shown to fare worse, interaction terms were created for gestational age acceleration by multiplying gestational age acceleration and an indicator variable for sex. Models with significant interaction terms were further investigated in a subgroup analysis, where linear regressions were performed on male subjects and female subjects separately. CIs reflect the change in slope. To ensure individual CpG sites were not driving the associations between the outcomes and DNAm age, linear regressions were performed for each of the 148 CpG sites included in the predictor and each outcome with the false discovery rate controlled at 5%. All analyses were performed using R v 3.3.0 and the R package ppcor.23
Results
A total of 225 infants born at less than 28 weeks of gestational age in the state of Victoria during 1991 and 1992 survived to 18 years of age. Cohort demographics for the 143 extremely preterm survivors who participated in this study are presented in Table II. Almost one-half received exogenous surfactant, just over one-third were treated with postnatal corticosteroids, and 41% developed BPD.
Table II.
Cohort demographics (n = 143)
| General characteristics | |
|---|---|
| Male, n (%) | 66 (46.2) |
| Gestational age (wk), mean ± SD | 25.8 ± 1.1 |
| Male | 25.6 ± 1.1 |
| Female | 25.9 ± 1.1 |
| Postmenstrual age at sampling*, mean ± SD | 27.2 ± 1.2 |
| Male | 27.1 ±1.1 |
| Female | 27.3 ± 1.3 |
| DNAm age (wk), mean ± SD | 28.8 ± 2.1 |
| Male | 28.3±1.7 |
| Female | 29.2 ± 2.4 |
| Birth weight (g), mean ± SD | 883 ± 178 |
| Respiratory outcomes/interventions | |
| Surfactant administration, n (%) | 67 (46.8) |
| Males receiving surfactant, n (%) | 35 (52.2) |
| Corticosteroid administration, n (%) | 55 (38.4) |
| Males receiving corticosteroids, n (%) | 34(61.8) |
| Period of assisted ventilation (d), mean ± SD | 23.2 ± 17.3 |
| Male | 28.6 ± 18.6 |
| Female | 18.6 ± 14.8 |
| BPD, n (%) | 58 (40.6) |
| Males with BPD, n (%) | 30(51.7) |
Fractional week when blood sample collected plus gestational age.
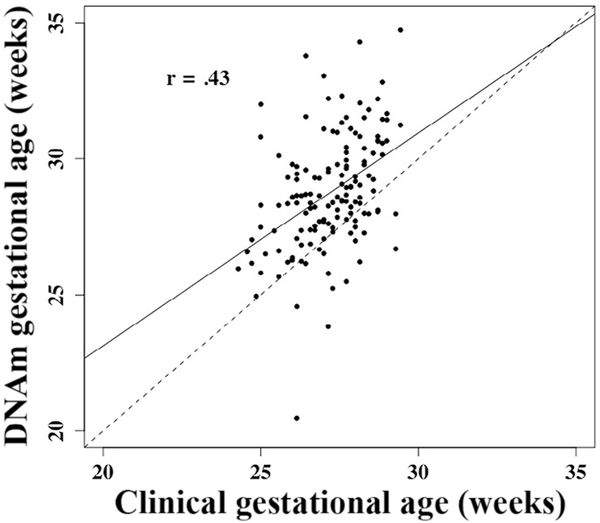
DNAm gestational age and subsequently gestational age acceleration were determined for each subject. Gestational age was correlated with DNAm gestational age (Figure 1 [available at www.jpeds.com]; r = 0.43, P = 8.7 × 10–8), but not gestational age acceleration (Figure 2 [available at www.jpeds.com]; r = 2.0 × 10–17, P = .99).
Figure 1.
Correlation between clinically estimated gestational age, adjusted for days to sample collection, and DNAm based gestational age (r = .43, P< .001). Both measured in weeks. Solid line represents the regression line; dotted line indicates equivalence.
Figure 2.
Gestational age acceleration is not associated with gestational age (r = 2 × 10–17, P > .99).
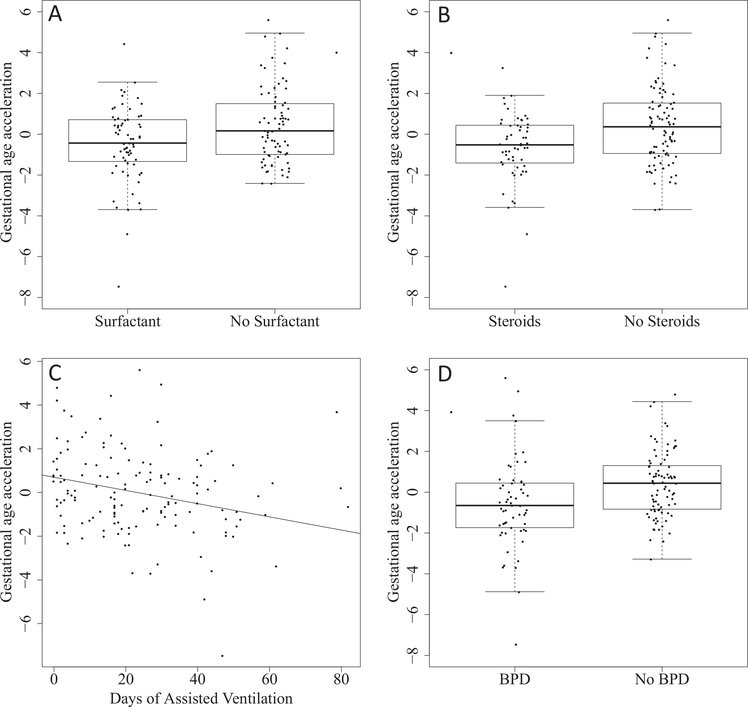
Extremely preterm infants who received surfactant had lower gestational age acceleration values compared with those who did not (mean difference –.057, 95% CI [–.099,–.015] weeks, P = .009; Figure 3, A); gestational age acceleration was lower in infants who were treated with postnatal corticosteroids compared with those who did not receive postnatal corticosteroids (mean difference –.056 weeks, 95% CI [–.098,–.015], P = .008; Figure 3, B), and was negatively associated with days of assisted ventilation (−1.79 days per week of gestational age acceleration, 95% CI [−3.28,−0.30], P = .02; Figure 3, C). In addition, infants who developed BPD had lower gestational age acceleration (mean difference –.055 weeks, 95% CI [–.098, –.012], P = .01; Figure 3, D).
Figure 3.
Respiratory interventions and outcomes of 143 preterm infants born at less than 28 weeks of gestation. A, Infants administered surfactant have a lower gestational age acceleration (P = .009); B, Infants administered postnatal corticosteroids have a lower gestational age acceleration (P = .008). C, Gestational age acceleration is negatively associated with days of associated ventilation (P = .02). D, Infants who develop BPD have a lower gestational age acceleration (P= .01).
For each respiratory intervention and outcome, the inclusion of the gestational age acceleration added significantly to the amount of variance explained by the model compared with gestational age alone (Table III).
Table III.
Coefficients of determination with and without inclusion of gestational age acceleration in the model
| Clinical intervention or outcome | Adjusted r* | Adjusted r, gestational age acceleration | P value for addition of gestational age acceleration |
|---|---|---|---|
| Surfactant administration | 0.35 | 0.40 | .004 |
| Corticosteroid administration | 0.36 | 0.42 | .002 |
| Period of assisted ventilation (d) | 0.61 | 0.64 | .009 |
| BPD | 0.34 | 0.40 | .004 |
P value for comparison of the models.
r is computed as the square root of R2 for linear regression models, and the square root of McFadden pseudo-R2 for logistic regression models.
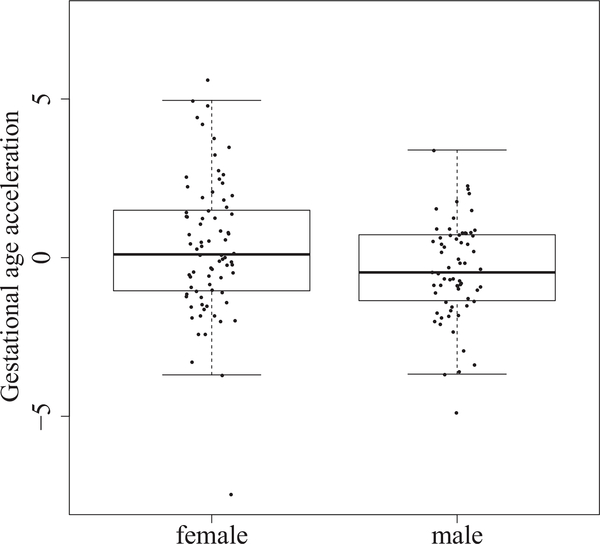
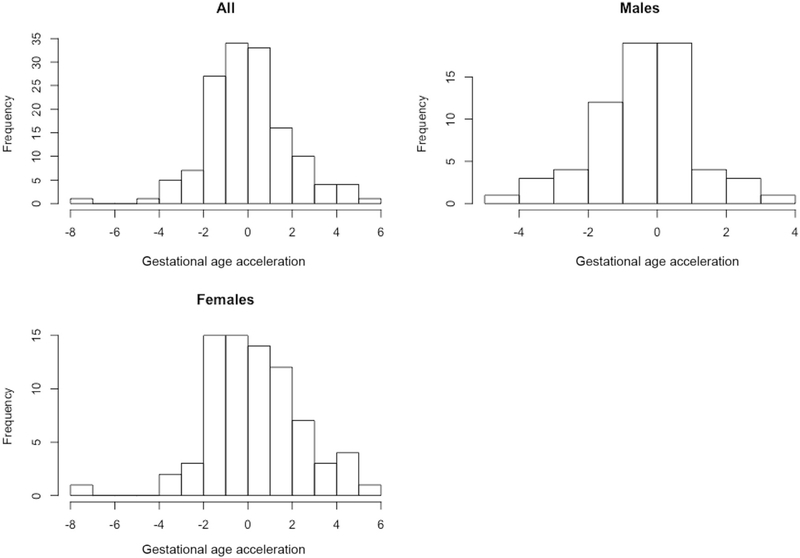
Previous studies have shown sex-specific differences in outcomes after preterm birth, prompting us to consider whether gestational age acceleration was associated with infant sex. Male subjects had a mean gestational age acceleration of −0.40 and female subjects had a mean gestational age acceleration of 0.35, indicating that female subjects were potentially developmentally more mature than male subjects (mean difference = 0.75 weeks, 95% CI [0.14, 1.36], P = .02; Figure 4); this is further illustrated in Figure 5 (available at www.jpeds.com) which shows the distribution of gestational age acceleration in the overall cohort. The interaction term for sex was significant only for BPD (P = .007). In subsequent subgroup analysis stratified by sex, development of BPD was significantly related to lower gestational age acceleration only in male subjects (mean difference −0.154 weeks, 95% CI [−0.230, –.079], P < .008) but not in female subjects (mean difference 0.27 weeks, 95% CI [–.05,.05], P= .9).
Figure 4.
Gestational age acceleration is associated with neonatal sex (P = .02), with male infants having lower gestational age acceleration.
Figure 5.
Histogram of the distribution of gestational age acceleration for the entire cohort and separately for male and female infants.
To ensure results were not driven by CpG sites comprising the predictor that were also independently associated with the outcome or intervention of interest, we used linear models to assess associations with each of the 148 CpG sites. Two CpG sites were experiment-wide significant. One site (cg15856055, ZNF511) was associated with BPD (P = .002), and 1 site was associated with days of assisted ventilation (P = .0001, cg27258399, HTRA4). No individual CpG site was significantly associated with surfactant or postnatal corticosteroid administration, supporting our hypothesis that the relationship between the outcome and gestational age acceleration was not due to associations with individual CpG sites.
Discussion
In our study, we used a metric based on DNAm (which we postulate represents developmental maturity in utero and up to 10 days after birth) to assess associations between the postulated measure of developmental maturity and respiratory outcomes that is independent of gestational age. We found that infants born at <28 weeks of gestational age, increased DNAm age was strongly related to reduced respiratory morbidity and fewer respiratory interventions after birth. Importantly, gestational age acceleration explained these outcomes over and above knowledge of gestational age at birth alone.
The clinical interventions selected for inclusion in this study mark several critical benchmarks in treating respiratory distress in infants. The demographic characteristics and frequency of respiratory interventions and BPD in our cohort are similar to other cohorts from this era.24,25 Infants admitted to the neonatal intensive care unit (NICU) often require respiratory support26 and the risk for BPD and long-term adverse effects on pulmonary function is generally higher the earlier the infant is born.11
The decision to treat an infant with surfactant was made by a neonatologist based on clinical criteria. Surfactant was administered in this cohort only as rescue treatment and infants had to be intubated, receiving intermittent positive airway pressure respiratory support, and require >50% oxygen. Our finding that infants who received surfactant had a lower gestational age acceleration than infants not administered surfactant, is consistent with developmental physiology, which implies that more immature preterm infants are expected to be more surfactant deficient.27
Infants experiencing more severe respiratory failure may require postnatal corticosteroids during their NICU course.28 Postnatal corticosteroids were widely used in the early 1990s after being shown to improve respiratory function and facilitate extubation of very immature preterm babies.28,29 This is consistent with our observation that infants who received postnatal corticosteroids had a lower gestational age acceleration than those who were never given corticosteroids. Practice changed in the late 1990s when it became evident that postnatal corticosteroid use carried significant long-term risks.28,30,31 They are now used with caution and are generally only administered to a select group of babies after prolonged periods of assisted ventilation28,30; the effect of this practice change is not captured in our cohort because of recruitment in the early 1990s.
Finally, this study examined 2 markers of long-term pulmonary outcomes associated with extremely preterm birth: duration of assisted ventilation and development of BPD. Extremely preterm infants require disproportionately more assisted ventilation with diminishing maturity. In 1 study, for each week of decrease in gestational age, survivors born <28 weeks of gestation required a mean of 13 days more assisted ventilation after birth.32 In the current study, we found that longer duration of assisted ventilation was associated with lower gestational age acceleration. Similarly, we also found that gestational age acceleration was lower in infants who developed BPD.
All 4 indicators of preterm birth-associated respiratory mortality were substantially correlated with gestational age acceleration. This supports our hypothesis that gestational age acceleration is a marker of developmental maturity, including that of the lung. Although there is some overlap between subjects with multiple interventions, these 4 indicators are not collinear. Figure 6 (available at www.jpeds.com), shows overlap between surfactant, corticosteroid use and BPD. This correlation of respiratory outcomes (−0.44 < r < 0.65) in this cohort is likely to reduce our power to detect statistically significant associations between gestational age acceleration and each outcome individually. However, even assuming a stringent Bonferroni correction for the number of outcomes tested (.05/4 = .0125), inclusion of gestational age acceleration in models predicting each of the 4 outcomes remains significant (Table III).
Figure 6.
Venn diagram demonstrating the overlap between surfactant, corticosteroid use and BDP.
Further research is needed to determine if these associations remain if samples are taken closer to birth, such as in cord blood, or even before birth, if in utero fetal blood sampling is performed. One recent study reported associations between gestational age acceleration estimated from cord blood methylation and several pregnancy-associated factors, including maternal preeclampsia and prenatal betamethasone treatment.33 Antenatal betamethasone was associated with increased gestational age acceleration, which is consistent with antenatal corticosteroids being used to accelerate maturity before birth.33
Male subjects had lower gestational age acceleration than female subjects. Male infants are more likely to experience complications because of respiratory disorders, have poorer psychomotor development when born preterm, and have higher mortality rates than female infants.34–38 Thus, this observation is consistent with our hypothesis that DNAm age is reflective of neonatal morbidity. Our previous study16 did not show a significant sex difference in accuracy based on the median error, but did not examine the association between sex and gestational age acceleration. However, a recent study also showed increased gestational age acceleration in female infants.33 Lack of associations with neonatal interventions in the analyses stratified by sex could be due to a reduction in power from decreased sample size in our study.
We assessed if any of the 148 CpG sites were associated with the outcome variables. Only 2 variables showed associations, one with BPD and one with days of assisted ventilation. No individual CpG site was significantly associated with surfactant or postnatal corticosteroid administration. We conclude that associations between CpG sites and variables examined did, thus, not adversely affect our study outcome.
Our study has several limitations. As this cohort was recruited between 1991 and 1992, clinical care given to these infants may vary from current practice. Surfactant therapy has “dramatically reduced mortality and morbidity”28; as surfactant was available as therapy to our 1991–1992 cohort, we believe that this major change in clinical practice was captured in our study. In our cohort, surfactant use was limited to infants requiring supplemental oxygen at over 50%; thus, surfactant was given to babies with more severe respiratory distress syndrome. Today, surfactant is given at a lower oxygen requirement; the potential effect of this change in practice could only be determined by repeating our study with a more contemporary cohort.
With a rate of 73% for our 1991–1992 cohort, the antenatal corticosteroid rate was similar to many current day cohorts.6 Since the 1990s, ventilation strategies have changed and new ventilator modes have been introduced, but we agree with Owen et al that “most interventions have had little effect on the risk of bronchopulmonary dysplasia.”28 We, therefore, believe that results from our 1991–1992 cohort remain relevant until superseded by more contemporary data.
A further limitation was that DNAm was measured from blood spots, which were collected at an average of 9.9 days after birth. Although we have adjusted for postnatal age in our analyses, methylation may have been affected by the days and care received between birth and sample collection and, thus, may not have been fully representative of developmental maturity at birth alone. Further information on how rapidly the epigenome changes after birth will be highly relevant. We have initiated a prospective study (The EpiPrem Study) that addresses longitudinal changes in genome wide DNAm and gestational age acceleration over several weeks of NICU care with multiple analyses between birth and term equivalence. We hope to identify unique epigenetic signatures that could assist in identifying patients who are at risk for specific acute and long-term morbidities associated with preterm birth.
We anticipate that assessment of gestational age acceleration will be informative in studies of long-term outcomes. As gestational age acceleration is independent of gestational age at birth, this metric provides another clinical and research tool to evaluate developmental maturity, regardless of the gestational age at which the infant was born.
Future studies should investigate the development and use of gestational age acceleration during pregnancy based on cells obtained from amniotic fluid or other fetal cells, which could assist to inform clinical decisions at the limits of viability.
If further studies determine that use of DNAm gestational age has clinical utility in predicting more neonatal morbidity, targeted assays to rapidly return results of the assessment of DNAm-based developmental maturity to clinicians would be needed. Technically, it should be possible to perform the DNAm analysis and provide results to clinicians within 48 hours. The potential clinical utility of such a DNAm based assessment of developmental maturity and its potential contribution to personalized neonatal care interventions and outcome predictions should be investigated further. ∎
Acknowledgments
The Victorian Infant Collaborative Study Group who designed the cohort study from which the participants were obtained comprised the following collaborators: Peter Anderson, PhD, Monash University, Melbourne, Australia; Catherine Callanan, RN, Premature Infant Follow-Up Program, the Royal Women’s Hospital, Parkville, Australia. Elizabeth Carse, FRACP, Monash Newborn, Monash Medical Centre, Melbourne, Australia; Noni Davis, FRACP, Premature Infant Follow-Up Program, the Royal Women’s Hospital, Parkville, Australia; Julianne Duff, FRACP, Premature Infant Follow-Up Program, the Royal Women’s Hospital, Parkville, Australia; Marie Hayes, RN, Monash Newborn, Monash Medical Centre, Melbourne, Australia. Marion McDonald, RN, Premature Infant Follow-Up Program, the Royal Women’s Hospital, Parkville, Australia; Gillian Opie, FRACP, Neonatal Services, Mercy Hospital for Women, Melbourne, Australia.
Supported by the National Health and Medical Research Council (NHMRC) of Australia (APP1083779 [to J.M.C., C.T., and J.L.C.] and 491246 [to L.D. and J.L.C.); the Centre of Research Excellence (APP1060733 [to L.D., P.D., and J.L.C]); and further funded by the Children’s Foundation (2014–134 [to J.M.C., L.D., and C.T.]). The Murdoch Childrens Research Institute is supported by the Victorian Government’s Operational Infrastructure Support Program. The authors declare no conflicts of interest.
Glossary
- BPD
Bronchopulmonary dysplasia
- CpG
Cytosine-guanosine dinucleotides
- DNAm
DNA methylation
- NICU
Neonatal intensive care unit
References
- 1.Natile M, Ventura ML, Colombo M, Bernasconi D, Locatelli A, Plevani C, et al. Short-term respiratory outcomes in late preterm infants. Ital J Pediatr 2014;40:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung JH, Kim SH, Kim YM, Kim JH, Kim MN, Lee HR, et al. Neonatal outcomes of twin pregnancies delivered at late-preterm versus term gestation based on chorionicity and indication for delivery. J Perinat Med 2016;44:903–11. [DOI] [PubMed] [Google Scholar]
- 3.Gleason CAJ, Juul SE. Avery’s diseases of the newborn. Philadelphia (PA): Saunders; 2012. [Google Scholar]
- 4.Roberts CT, Owen LS, Manley BJ, Froisland DH, Donath SM, Dalziel KM, et al. Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med 2016;375:1142–51. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Davis P, Dharmalingam A, Bowman E. Assisted ventilation and survival of extremely low birthweight infants. J Paediatr Child Health 1996;32:138–42. [DOI] [PubMed] [Google Scholar]
- 6.Doyle LW, Adams AM, Robertson C, Ranganathan S, Davis NM, Lee KJ, et al. Increasing airway obstruction from 8 to 18 years in extremely preterm/ low-birthweight survivors born in the surfactant era. Thorax 2017;72:712–9. [DOI] [PubMed] [Google Scholar]
- 7.Shahzad T, Radajewski S, Chao CM, Bellusci S, Ehrhardt H. Pathogenesis of bronchopulmonary dysplasia: when inflammation meets organ development. Mol Cell Pediatr 2016;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9. [DOI] [PubMed] [Google Scholar]
- 9.Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol 2006;30:227–32. [DOI] [PubMed] [Google Scholar]
- 10.Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol 2006;30:219–26. [DOI] [PubMed] [Google Scholar]
- 11.Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol 2016;215:e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch CD, Zhang J. The research implications of the selection of a gestational age estimation method. Paediatr Perinat Epidemiol 2007;21(Suppl 2):86–96. [DOI] [PubMed] [Google Scholar]
- 13.Mongelli M, Wilcox M, Gardosi J. Estimating the date of confinement: ultrasonographic biometry versus certain menstrual dates. Am J Obstet Gynecol 1996;174:278–81. [DOI] [PubMed] [Google Scholar]
- 14.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet 2013;14:204–20. [DOI] [PubMed] [Google Scholar]
- 15.Sandoval J, Heyn H, Moran S, Serra-Musach J, Pujana MA, Bibikova M, et al. Validation of a DNA methylation microarray for 450,000 CpG sites in the human genome. Epigenetics 2011;6:692–702. [DOI] [PubMed] [Google Scholar]
- 16.Knight AK, Craig JM, Theda C, Baekvad-Hansen M, Bybjerg-Grauholm J, Hansen CS, et al. An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biol 2016;17:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts G, Cheong J, Opie G, Carse E, Davis N, Duff J, et al. Growth of extremely preterm survivors from birth to 18 years of age compared with term controls. Pediatrics 2013;131:e439–45. [DOI] [PubMed] [Google Scholar]
- 18.Cruickshank MN, Oshlack A, Theda C, Davis PG, Martino D, Sheehan P, et al. Analysis of epigenetic changes in survivors of preterm birth reveals the effect of gestational age and evidence for a long term legacy. Genome Med 2013;5:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barfield RT, Kilaru V, Smith AK, Conneely KN. CpGassoc: an R function for analysis of DNA methylation microarray data. Bioinformatics 2012;28:1280–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 2012;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horvath S DNA methylation age of human tissues and cell types. Genome Biol 2013;14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2013;29:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S ppcor: an R package for a fast calculation to semi-partial correlation coefficients. Commun Stat Appl Methods 2015;22:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger TM, Fontana M, Stocker M. The journey towards lung protective respiratory support in preterm neonates. Neonatology 2013;104:265–74. [DOI] [PubMed] [Google Scholar]
- 25.Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia - trends over three decades. Paediatr Child Health 2013;18:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow S, Le Marseny R, Hossain S, Haslam R, Lui K. Report of the Australian and New Zealand Neonatal Network 2013. ANZNN. 2015.
- 27.Whitsett JA, Weaver TE. Alveolar development and disease. Am J Respir Cell Mol Biol 2015;53:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen LS, Manley BJ, Davis PG, Doyle LW. The evolution of modern respiratory care for preterm infants. Lancet 2017;389:1649–59. [DOI] [PubMed] [Google Scholar]
- 29.Mammel MC, Green TP, Johnson DE, Thompson TR. Controlled trial of dexamethasone therapy in infants with bronchopulmonary dysplasia. Lancet 1983;1:1356–8. [DOI] [PubMed] [Google Scholar]
- 30.Doyle LW, Ehrenkranz RA, Halliday HL. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev 2014;(1):CD001146. [DOI] [PubMed] [Google Scholar]
- 31.Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics 1998;101:E7. [DOI] [PubMed] [Google Scholar]
- 32.Doyle LW, Murton LJ, Kitchen WH. Escalating consumption of nursery resources by extremely immature infants. Aust N Z J Obstet Gynaecol 1987;27:201–4. [DOI] [PubMed] [Google Scholar]
- 33.Girchenko P, Lahti J, Czamara D, Knight AK, Jones MJ, Suarez A, et al. Associations between maternal risk factors of adverse pregnancy and birth outcomes and the offspring epigenetic clock of gestational age at birth. Clin Epigenetics 2017;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skiold B, Alexandrou G, Padilla N, Blennow M, Vollmer B, Aden U. Sex differences in outcome and associations with neonatal brain morphology in extremely preterm children. J Pediatr 2014;164:1012–8. [DOI] [PubMed] [Google Scholar]
- 35.Iturri P, Bairam A, Soliz J. Efficient breathing at neonatal ages: a sex and Epo-dependent issue. Respir Physiol Neurobiol 2016;245:89–97. [DOI] [PubMed] [Google Scholar]
- 36.Mage DT, Donner M. Female resistance to hypoxia: does it explain the sex difference in mortality rates? J Womens Health (Larchmt) 2006;15:786–94. [DOI] [PubMed] [Google Scholar]
- 37.Romeo DM, Brogna C, Sini F, Romeo MG, Cota F, Ricci D. Early psychomotor development of low-risk preterm infants: influence of gestational age and gender. Eur J Paediatr Neurol 2016;20:518–23. [DOI] [PubMed] [Google Scholar]
- 38.Neubauer V, Griesmaier E, Ralser E, Kiechl-Kohlendorfer U. The effect of sex on outcome of preterm infants - a population-based survey. Acta Paediatr 2012;101:906–11. [DOI] [PubMed] [Google Scholar]