Abstract
Cancer remains a major public health concern and a significant cause of death worldwide. Identification of bioactive molecules that have the potential to inhibit carcinogenesis continues to garner interest among the scientific community. In particular, flavonoids from dietary sources are the most sought after because of their safety, cost-effectiveness, and feasibility of oral administration. Emerging data have provided newer insights into understanding the molecular mechanisms that are essential to identify novel mechanism-based strategies for cancer prevention and treatment. Dietary flavonoid fisetin (3,3′,4′,7-tetrahydroxyflavone) found in many fruits and vegetables has been shown in preclinical studies to inhibit cancer growth through alteration of cell cycle, inducing apoptosis, angiogenesis, invasion, and metastasis without causing any toxicity to normal cells. Although data from in-vitro and in-vivo studies look convincing, well-designed clinical trials in humans are needed to conclusively determine the efficacy across various cancers. This review highlights the chemopreventive and therapeutic effects, molecular targets, and mechanisms that contribute to the observed anticancer activity of fisetin against various cancers.
Keywords: Cancer, Chemoprevention, Diet, Fisetin, Flavonoid
1. Introduction
Cancer remains as one of the leading causes of death worldwide [1]. It is a major public health concern with about 1 658370 new cases expected to be diagnosed in 2015 [2]. Oncogenesis is a multistep process in which a normal cell acquires alteration at the cellular, genetic, and epigenetic level to progressively transform into a cancer cell. These transformations progress to hyperproliferation, unlimited replication potential, evading apoptosis, sustained angiogenesis, invasive potential, and metastasis [3]. Despite advances in diagnostic and therapeutic approaches over the years, cancer continues to be a formidable challenge [1]. This is largely because of the growing urbanization as well as cancer-associated lifestyle choices including unhealthy dietary habits. Among all cancers, lung cancer remains as the leading cause of deaths among men and women followed by prostate cancer (PCa) in men and breast cancer in women [4]. Mono-targeted therapies are currently available but can induce toxicity and side effects due to a specific single target. Other limitations of targeted therapies include ineffective targeting, resistance, and ever increasing cost of treatment [5, 6]. As cancer is a multifactor disease, it may require prevention/treatment approaches with compounds that are able to target multiple biochemical and molecular pathways. This appears to be the most practical method to decrease the disease incidence and burden [5, 7]. Cancer chemoprevention, a rapidly evolving field of preventive oncology, focusses on the use of synthetic, pharmaceutical, and/or natural agents to entirely inhibit, retard, or reverse the process of carcinogenesis [8]. Few of the many advantages of using natural agents for chemoprevention comprise of safety, efficacy, ease of availability, affordability, potential to overcome resistance to other traditional therapies, and anticancer drugs [7]. Fisetin is one such agent with a potential to target multiple signaling pathways in cancer cells without presenting any toxicity to normal cells.
2. Fisetin and cancer
Chemoprevention is an emerging, appealing, and an innovative strategy for the management of cancer. While fruits and vegetables are an abundant source of many cofactors, vitamins, minerals, the phytochemicals constituents known as flavonoids have special ability to target several key cellular events involved in the development of cancer [9, 10]. Flavonoids are the most abundant polyphenols consumed in human diet and are classified into flavonols, flavones, isoflavones, anthocyanidins, theaflavins, and thearubigins. Several studies have demonstrated that flavonoids possess anti-oxidant, anti-inflammatory, and chemopreventive properties. The compound 3, 7, 3′, 4′-tetrahydroxyflavone is a natural flavone commonly referred to fisetin. The chemical structure and basic properties of fisetin are shown in Fig. 1. Major fisetin containing food sources include strawberries, apples, persimmons, grapes, onions, and cucumbers [11] as shown in Table 1. Fisetin affects multiple molecular and signaling pathways depending on the cancer type as shown in Fig. 2. Among all the multiple biological effects fisetin possesses, its anticancer potential has been recently explored making it a promising agent for cancer prevention and therapy. This review summarizes the cellular effects of fisetin in an attempt to elucidate its preventive and therapeutic potential against various cancers.
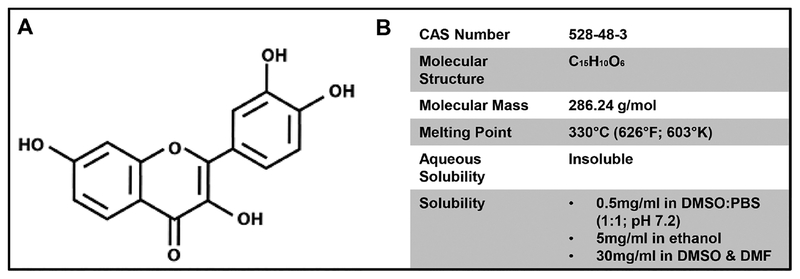
Figure 1.
Structure and basic properties of fisetin. (A) The chemical structure of fisetin (3, 7, 3′, 4′-tetrahydroxyflavone). (B) Major physical and chemical properties of fisetin.
Table 1.
Dietary sources of fisetin
| Dietary food sources | Fisetin concentration (μg/g) |
|---|---|
| Strawberry | 160 |
| Apple | 26.9 |
| Persimmon | 10.6 |
| Lotus root | 5.8 |
| Onion | 4.8 |
| Grape | 3.9 |
| Kiwi | 2.0 |
| Peach | 0.6 |
| Cucumber | 0.1 |
| Tomato | 0.1 |
The concentration of fisetin (dry weight basis) was measured in freeze-dried vegetables and fruits after acid hydrolysis of the parent glycosides. Adapted from Kimira et al. [53].
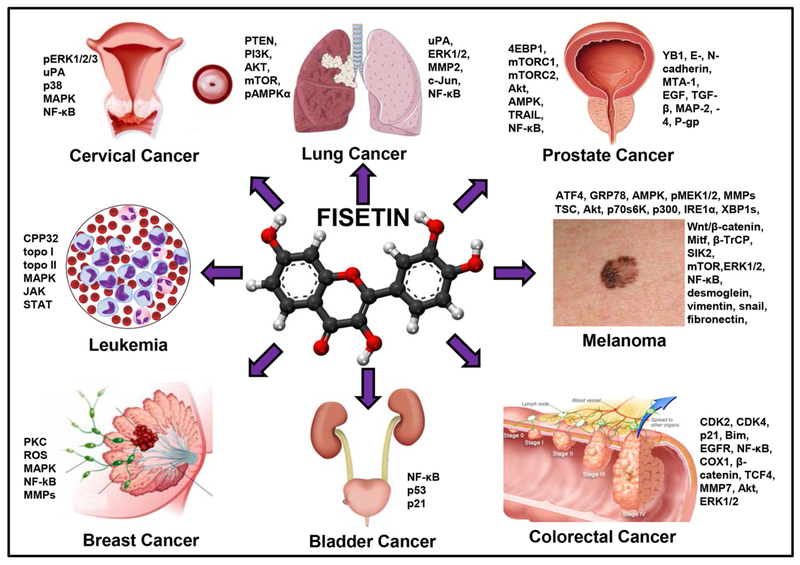
Figure 2.
Molecular targets of fisetin in various cancers. Fisetin interacts with multiple cellular targets by binding to and interacting with several molecular targets fisetin regulates a wide variety of cell functions. Fisetin disrupts Wnt signaling and results in cell cycle arrest. It inhibits the YB-1 binding protein to suppress epithelial to mesenchymal transition and thus prevents invasion and migration of cancer cells. By physically interacting with the mTOR molecule, fisetin inhibits signaling involved in cell survival thus explaining its inhibitory effect on cellular growth and proliferation. Fisetin binds to and disrupts microtubule dynamics and acts as a stabilizing agent with effects far superior to paclitaxel. However across all cancers, mTOR and NF-kB appear to be the most commonly affected pathways.
3. Fisetin and lung cancer
Lung cancer is the leading cause of cancer-related deaths in the United States. It accounts for 13% of all cancers diagnosed and 27% of all cancer-related deaths [1, 2]. An important obstacle to non-small cell (NSC) lung cancer chemotherapy is the development of resistance to a widely used chemotherapeutic drug cisplatin. Fisetin has shown antiproliferative, apoptotic, and antiangiogenic properties in lung cancer cells[12]. Fisetin was reported to reverse the acquired cisplatin-resistance in A549-CR lung cancer cells. Fisetin (40 μM) in combination with cisplatin (10 μM) showed intense suppression of cell viability and induction of apoptosis as compared to cells treated with fisetin and cisplatin alone, possibly via inactivating MAPK pathways as well as suppressing Survivin expression [13]. Fisetin was shown to induce apoptosis in NSC lung cancer via mitochondrial-mediated pathways. Fisetin induced DNA fragmentation, ROS generation, and apoptosis in NCI-H460 cells via a reduction in Bcl-2 and increase in Bax expression. Fisetin treatment increased cleavage of caspase-9 and caspase-3 thereby increasing caspase-3 activation [14]. Similarly, fisetin supplementation alleviated mitochondrial dysfunction and induced apoptosis by the upregulation of Bax/Bcl-2 ratio, thereby leading to cytochrome-c release and activation of caspase-9, caspase-3 leading to apoptotic cell death during benzo(a)pyrene (B(a)P)-induced lung cancer in vivo [15]. Fisetin (25 mg/kg body weight) decreased histological lesions and levels of lipid peroxidation and modulated the enzymatic and nonenzymatic anti-oxidants in B(a)P-treated Swiss Albino mice [16]. We observed that fisetin treatment (5–20 μM) inhibits cell growth and colony formation in A549 NSC lung cancer cells. Fisetin activated tumor suppressor PTEN and negatively regulated protein synthesis by phosphorylation of AMPKα. Fisetin also inhibited PI3K/Akt/mTOR signaling pathway in NSC lung cancer cells. Inhibitors of this pathway have entered preclinical and clinical trials and suggest fisetin as a promising candidate drug for therapeutic intervention in lung cancer [17]. Fisetin in combination with CPA was shown to inhibit angiogenesis using a Matrigel plug assay. Combination of fisetin (223 mg/kg) with CPA (30 mg/kg) produced a marked inhibition of tumor growth (92%) in LLC-bearing mice as compared to animals treated with fisetin or CPA alone. This study provided the first evidence that fisetin exhibits antiangiogenic and anticancer activities in mice bearing LLC [18]. Another study showed that fisetin inhibits adhesion, migration, and invasion in A549 lung cancer cells by downregulating uPA, ERK1/2, and MMP-2. Treatment with fisetin also decreased the nuclear levels of NF-kB, c-Fos, c-Jun, and AP-1 and inhibited NF-kB binding. Taken together, fisetin demonstrated promising results against lung cancer both in-vitro and in-vivo.
4. Fisetin and prostate cancer
Prostate cancer (PCa) is the most frequently diagnosed cancer in men with an estimated 220 800 new cases. It is the second-leading cause of cancer deaths in men with 27540 deaths in 2015 in the US alone [1, 2]. Treatment and prevention of PCa with fisetin is an active area of research. Cell culture studies show that fisetin exerts anti-proliferative effect on human PCa cells. Our laboratory has previously shown that treatment of LNCaP cells with fisetin caused inhibition of PCa by G1-phase cell cycle arrest, modulating CKI–cyclin–CDK network and induction of apoptosis [19]. Fisetin induced apoptosis and cell cycle arrest in LNCap cells, and inhibited androgen signaling and tumor growth in athymic nude mice implanted with androgen receptor-positive 22Rv1 cells [20]. Fisetin suppressed cell proliferation by hypophosphorylation of 4E-binding protein-1 and induced autophagic cell death in PCa cells through suppression of mTORC1 and mTORC2. Fisetin acts as a dual inhibitor of mTORC1/C2 and activated the mTOR repressor TSC2, commonly associated with inhibition of Akt and activation of AMPK [21]. TRAIL plays an important role in the defense against tumor cells. Fisetin sensitized TRAIL-resistant androgen-dependent LNCaP and the androgen-independent DU145 and PC3 PCa cells to TRAIL-induced death [22]. Additionally, in androgen-independent PCa cell lines the cytotoxic and apoptotic effects of TRAIL in combination with fisetin are lowest compared to androgen dependent LNCaP cells. Inhibition of NF-kB activation by fisetin in LNCaP cells augmented the apoptotic effect of TRAIL. These findings confirm that the downregulation of NF-kB sensitizes PCa cells to TRAIL in-vitro [22]. We recently reported that fisetin inhibits YB-1, an important transcription factor that promotes EMT in PCa. YB-1 is over-expressed in PCa whereas E-cadherin, a marker for EMT is downregulated. During PCa progression, forced YB1 expression induced a mesenchymal phenotype both in-vitro and in-vivo. Fisetin binds to the cold shock domain of YB-1 protein and was reported as an inhibitor of YB1 phosphorylation and MTA-1 expression. Fisetin also inhibits EGF and TGF-β induced YB-1 phosphorylation and EMT in PCa cells [23]. TMFol, a structural analogue of fisetin and quercetin exhibited chemopreventive efficacy superior to fisetin alone both in-vitro and in-vivo. TMFol inhibited cell growth in 22Rv1, TRAMP C2, PC-3, and LNCaP cells. It also slowed tumor development in nude mice bearing TRAMP C2 and 22Rv1 cells [24]. Recently, we showed that fisetin binds to β-tubulin and disrupts microtubule dynamics in PCa cells by enhancing tubulin polymerization. Fisetin was able to arrest cells in G2/M phase, inhibits cell proliferation, invasion, migration, cell viability, colony formation, and decrease the P-gp protein in multidrug resistant NCI/ADR-RES cells. These invitro results establish fisetin as a microtubule targeting agent and shows potential to be developed as an adjuvant with microtubule targeting based therapies [25]. Collectively, all these studies provide ample evidence that fisetin by targeting multiple pathways could be developed as an effective agent against PCa.
5. Fisetin and melanoma/skin cancer
Melanoma is the deadliest form of skin cancer due to its high likelihood of producing metastasis. With an estimated 73 870 new cases, melanoma will account for an estimated 13 340 skin cancer deaths in 2015 alone making it an important area for research [1, 2]. We reported for the first time that fisetin has the potential to inhibit human melanoma by disrupting the Wnt/β-catenin/Mitf signaling. In-vitro studies showed that the decrease in β-catenin levels, induction of β-TrCP, and a reduction of Mitf mRNA and protein levels are possible mechanisms for fisetin-mediated suppression of Wnt signaling in 451Lu human melanoma cells. Interestingly, the in-vitro effects were reflected in in-vivo studies which showed that fisetin significantly inhibited tumor growth in 451Lu melanoma xenografts that was associated with decreased Mitf levels [26]. Another study showed that a 4′MF, a mono-methyl analogue of fisetin is a potent inhibitor of SIK2 and strongly induced melanogenesis in B16F10 melanoma cells. By modulating SIK2 signaling with 4′MF, the authors reported CREB-mediated transcription via TORC1 activation in-vivo [27]. A nonmelanoma skin cancer study revealed that fisetin inhibited growth and induced apoptosis via increase in Bax, Bak, and Bad protein expressions in A431 cells. Fisetin treatment also resulted in G2/M arrest, modulation in Bcl-2 family proteins (Bcl-2, Bcl-xL, and Mcl-1), disruption of mitochondrial potential, and activation of caspases and cleavage of PARP [28]. Another group investigated the photochemopreventive effect of fisetin for the management of UVB-induced skin malignancies in SKH-1 mouse model of skin cancer. Their findings revealed that topical application of fisetin (250 and 500 nmol) to SKH-1 hairless mice skin after UVB exposure results in significant decrease in leukocyte infiltration, inflammatory markers (MPO, COX2, and PGE2), cytokines (TNF-α, IL-1β, and IL-6) and proliferation markers (PCNA and cyclin D1). Fisetin also inhibited the PI3K/AKT/NFkB signaling which is associated with UVB-induced inflammation, cell survival, and proliferation. Fisetin was found to cause no damage to the mouse skin and increased protein expression of p53 and p21 after UVB treatment [29]. The MAPK (BRAF-MEK-ERK) pathway is a key regulator of melanoma cell invasion and potential targets for melanoma treatment and prevention. Fisetin treatment (5–20 μM) resulted in inhibition of cell invasion in A375, SK-MEL-28, RPMI-7951, SKMEL-119, and Hs294T melanoma cells. BRAF mutated cells were found to be more sensitive due to a decrease in phosphorylation of MEK1/2 and ERK1/2. Fisetin inhibited the activation of IKK leading to a reduction in the activation of the NF-kB signaling pathway. Fisetin also stimulated melanoma cell phenotype transition from mesenchymal to epithelial as observed by decrease in mesenchymal markers (N-cadherin, vimentin, snail, fibronectin) and increase in epithelial markers (E-cadherin and desmoglein) [30]. We showed that fisetin inhibits A375 human melanoma cell growth in monolayer and 3D cultures. Fisetin inhibited melanoma progression in a 3D melanoma skin model with downregulation of mTOR, Akt, and upregulation of TSC. Fisetin inhibited A375 and 451Lu melanoma cell proliferation by binding to p70s6K with higher affinity than mTOR [30]. A combinational treatment study of melatonin and fisetin demonstrated enhanced antitumor activity of fisetin. The combination significantly led to inhibition of melanoma cell viability, migration, colony formation, and induced greater apoptosis when compared to fisetin alone. These effects were associated with activation of cytochrome-c/caspase-dependent apoptosis and inhibition of p300/NF-kB-mediated COX-2 and iNOS expression [31]. We showed involvement of ER stress and activation of extrinsic and intrinsic apoptotic pathways with fisetin in human melanoma. Fisetin inhibited ROS and augmented NO generation in A375 melanoma cells. Fisetin (20–80 μM) mediated apoptosis was accompanied with transient autophagy and induction of ER stress evidenced by increased IRE1α, XBP1s, ATF4, and GRP78 levels in A375 and 451Lu cells. Silencing of AMPK failed to prevent cell death indicating that fisetin-induced cytotoxicity is mediated through both AMPK-dependent and -independent mechanisms [32]. Recently, another combinatorial approach of using fisetin with sorafenib (RAF inhibitor) demonstrated inhibition of melanoma cell proliferation, induction of apoptosis and inhibition of tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. The combination treatment resulted in enhanced apoptosis, cleavage of caspase-3 and PARP, expression of Bax and Bak, inhibition of Bcl-2 and Mcl-1, and inhibition of expression of PI3K, phosphorylation of MEK1/2, ERK1/2, AKT, and mTOR. Fisetin treatment resulted in greater reduction of tumor growth and inhibition of the MAPK/PI3K pathways in A375 and SK-MEL-28 cell xenografts when compared to fisetin and sorafenib alone suggesting combination therapy to be more effective than monotherapy [33]. A follow-up study showed that combination of fisetin with sorafenib effectively inhibited EMT and augmented the anti-metastatic potential of sorafenib by reducing MMP-2 and MMP-9 proteins in melanoma cell xenografts [34]. Collectively these studies reflect the potential of fisetin alone as well as in combination with other established agents for management of melanoma.
6. Fisetin and colorectal cancer
Colorectal cancer is the third most common cancer in both men and women. With an estimated 93 090 cases of colon cancer and 39 610 cases of rectal cancer expected to be diagnosed, 49 700 deaths from colorectal cancer are expected to occur in 2015 [1, 2]. A comparative study of various flavonoids was performed showing their efficacy on proliferation, cytotoxicity, and apoptosis in Caco-2 and HT-29 human colon cancer cells [35]. Fisetin (0–60 μM) was shown to inhibit activity of CDKs dose-dependently leading to cell cycle arrest in HT-29 human colon cancer cells. Fisetin inhibited both cell growth and DNA synthesis at 72 h, cell cycle progression at 8 h, and G2/M phase arrest after 24 h. Fisetin treatment decreased activities of CDK2 and CDK4 via decreased levels of cyclin-E, cyclin-D1 and increase in p21 (CIP1/WAF1) levels. This study indicated that inhibition of cell cycle progression in HT-29 cells with fisetin treatment can be explained by modification of CDK activities [36]. Another follow-up study attempted to characterize the actual mechanism of fisetin mediated apoptosis in HCT-116 colon cancer cells. Fisetin (5–20 μM) induced DNA condensation, cleavage of PARP and activation of caspase-9, −7, and −3 in HCT-116 cells. Anti-apoptotic proteins Bcl-xL and Bcl-2 were downregulated whereas pro-apoptotic proteins Bak and Bim were upregulated that induced the mitochondrial translocation of Bax. Fisetin increased p53 protein levels, and the inhibition of p53 expression by small interference RNA resulted in a decrease in the fisetin-induced translocation of Bax to the mitochondria, release of monoand oligonucleosome in the cytoplasm, and PARP cleavage. This study provided a molecular basis for using fisetin as an apoptosis stimulatory agent via activation of caspases and induction of p53 resulting in translocation of Bax to mitochondria [37]. Overexpression of COX-2 and Wnt signaling has been known to play roles in colorectal cancer. We reported that fisetin (30–120 μM) induces apoptosis in colon cancer cells by inhibiting COX-2 and Wnt/EGFR/NF-kB -signaling pathways. Fisetin induced apoptosis and downregulation of COX-2 protein expression without affecting COX-1 and inhibited the secretion of prostaglandin E2 in HT-29 and HCT116 colon cancer cells. Fisetin treatment inhibited Wnt/EGFR/NF-kB signaling via downregulation of β-catenin, TCF-4, cyclin D1, and MMP-7 suggesting its promising role in colon cancer prevention [38]. In another study fisetin treatment was found to radiosensitize human colorectal cancer cells which are resistant to radiotherapy. Pre-treatment of p53-mutant HT-29 cells with fisetin enhanced the radiosensitivity by causing accumulation of cells in the radiosensitive G2/M phase, suppressing cellular DNA repair capacity, thereby increasing radiation-induced double-strand breaks. Fisetin pre-treatment augmented radiation-induced pro-apoptotic p38 MAPK activation and ultimate shut down of pro-survival signals [39]. A recent combinatorial study showed that 10–20 μM NAC enhances fisetin-mediated apoptosis in COLO25 colon cancer cells when compared to fisetin treatment alone. Combined treatment of fisetin with NAC increased cleaved caspase-3, PARP, reduced mitochondrial membrane potential with induction of caspase-9 in COLO25 cells. NAC sensitization to fisetin-induced apoptosis was also identified in various other cells like HCT-116, HT-29, and HCT-15 suggesting a novel strategy to treat colon cancer [40]. Fisetin is poorly soluble in water and it is difficult for intravenous administration. To address this issue, a novel approach was adapted by preparing nanoassemblies of polymeric micelles which can encapsulate fisetin leading to improved therapeutic effect in colon cancer. The polymeric micelle encapsulation demonstrated a sustained and prolonged in-vitro release, enhanced cytotoxicity, cellular uptake, and apoptosis. Fisetin micelles were also shown to exhibit increased tumor apoptosis, suppress proliferation, and anti-angiogenesis activities[41]. Taken together, fisetin demonstrated promising results against colon cancer.
7. Fisetin and bladder cancer
With an estimated 74 000 new cases and 16 000 deaths alone in 2015, bladder cancer remains as a concern. Bladder cancer incidence is about four times higher in men than in women and almost two times higher in white men than in black men [1, 2]. A recent study reported that fisetin induced apoptosis in human bladder cancer via upregulation of p53 and down-regulation of NF-kB activity, causing a change in the ratio of pro- and anti-apoptotic proteins. Results from this study revealed that fisetin inhibits the proliferation of T24 and EJ cells by inducing apoptosis and blocking cell cycle progression in the G0/G1 phase by significantly increasing p53, p21 proteins, and decreasing the protein levels of cyclinD1, cyclinA, CDK4, and CDK2. This in-vitro study showed that activation of p53 and inhibition of the NF-kB play important roles in the fisetin-induced apoptosis in bladder cancer cells [42]. A follow-up in-vivo study using a rat bladder cancer model induced by MNU suggested that p53 activation and NF-kB inhibition play important roles in the fisetin-induced apoptosis in bladder cancer. Furthermore, fisetin inhibited tumor growth and bladder carcinogenesis in MNU-initiated rats without any toxicity [43]. These findings identify the in-vivo chemo-preventive efficacy of fisetin and suggest that fisetin could be developed as an effective chemopreventive agent against bladder cancer.
8. Fisetin and breast cancer
With an estimated 231 840 new diagnoses and 40 730 deaths, breast cancer ranks as the second cause of cancer deaths in women after lung cancer [1, 2]. A study published in 2012 extensively investigated the cytotoxicity and apoptotic effects induced by fisetin in MCF-7 and MDA-MB-231 breast cancer cells. Fisetin was found to exhibit anticancer activity in caspase-3 deficient MCF-7 cells. Additionally, in MCF-7 cells, fisetin induced a novel form of atypical apoptosis and triggered plasma membrane rupture, mitochondrial depolarization, activation of caspase-7, −8, and −9, and PARP cleavage. These atypical features of apoptosis were due to caspase-3 deficiency in MCF-7 cells. Furthermore, fisetin inhibited autophagy which promoted cell death in MCF-7 cells [44]. These few preliminary studies suggest that bioactive actions of fisetin hold promise against breast cancer.
9. Fisetin and leukemia
A limited number of studies have examined the potential of fisetin against leukemia. Leukemia is a cancer of the bone marrow and blood and is classified into four main groups according to cell type and rate of growth: acute lymphocytic (ALL), chronic lymphocytic (CLL), acute myeloid (AML), and chronic myeloid (CML). With an estimated 54 270 new cases and 24 450 deaths, leukemia still remains a health concern [1, 2]. A study involving seven structurally related flavonoids was conducted to evaluate their biological activities on human leukemia cell line HL-60. Among all flavonoids, fisetin showed the most cytotoxic effects and a combination of wogonin and fisetin being the most-potent apoptosis inducers. The combination caused rapid and transient induction of caspase-3/CPP32, cleavage of PARP, and decrease in anti-apoptotic protein Mcl-1. However, the combination treatment did not have any effect on Bcl-2, Bcl-xL, and Bad. ROS production was decreased in apoptosis induced by the combination treatment along with enhanced Ca(2+)-dependent endonuclease activity. This study showed an interesting correlation between antioxidant activities of fisetin combined with wogonin towards flavonoids-induced apoptosis [45]. DNA topoisomerases (topo) are the target of several drugs commonly used in cancer chemotherapy. These drugs induce topo-DNA complexes with either topo I or topo II that eventually trigger cell death [46]. An increased risk for developing leukemia has been observed in patients treated with some topo II inhibitors. Effect of many flavonoids including fisetin was evaluated on topo I and topo II in K562 human leukemia cells at various concentrations and exposure times. Fisetin induced neither topo I- nor topo II-DNA complexes, but behaved as a catalytic inhibitor of both enzymes. These results suggest that fisetin acts as an inhibitor of DNA topoisomerases I and II in leukemia cells [46]. Recently, a combination effect of fisetin and hesperetin was reported elucidating their role and mechanism(s) of action in human HL-60 acute promyelocytic leukemia cells. Fisetin and hesperetin induced apoptosis along with inhibited cell proliferation, induced G2/M arrest, disrupted the mitochondrial potential, and increased caspase-3 activity. Microarray gene profiling of treated cells revealed some important biological pathways including MAPK, DNA binding signaling pathways and genes involved in cell proliferation, division, and apoptosis [47]. Another similar study demonstrated the effect of fisetin and hesperetin combination on human K562 CML cells. Combination treatment significantly modulated genes involved in cell proliferation, cell division, apoptosis, cell cycle, and various other cellular processes such as replication, transcription, and translation. Microarray gene profiling analysis revealed genes involved in JAK/STAT pathway, KIT receptor, and growth hormone receptor signaling to be potential candidates of fisetin-hesperetin combination for targeted CML therapy [47].
10. Fisetin and cervical cancer
With an estimated 12 900 new cases and 4100 deaths, cervical cancer is currently one of the leading causes of mortality in women [1, 2, 48]. A preliminary study reported that fisetin induced apoptosis in human cervical cancer HeLa cell via activation of caspase-3, −8, and cleavage of PARP. Additionally, fisetin treatment induced a sustained activation of pERK1/2. Inhibitor of ERK1/2 or transfection with mutant ERK1/2 expression vector reversed the fisetin induced apoptosis. This study showed that apoptosis induction by fisetin in HeLa cells is via ERK1/3-mediated activation of caspase-3/caspase-8 dependent pathway. In-vivo tumor xenograft experiments in mice revealed that fisetin significantly reduced tumor growth [49]. Another study reported that fisetin inhibits migration and invasion of SiHa and CaSki human cervical cancer cells. This study provided strong evidence for the molecular mechanism of fisetin in inhibiting aggressive phenotypes by downregulating uPA gene expression via interrupting the p38 MAPK-dependent NF-kB signaling pathway [50]. A novel combination approach of fisetin with sorafenib on human cervical cancer cell lines showed significant antitumor effect both in-vitro and in-vivo. The combination treatment induced apoptosis in HeLa and SiDR5-treated HeLa cells via caspase-3 and −8 activation and was accompanied by marked loss of mitochondrial potential. In addition, animal studies using a HeLa xenograft model demonstrated that combined fisetin and sorafenib treatment was clearly superior to sorafenib treatment alone. This combined fisetin and sorafenib treatment approach reveals a novel therapeutic strategy for future clinical development in advanced cervical cancer [51].
11. Future prospects and conclusion
Cancer is a heterogeneous disease that uses multiple signaling pathways to survive and continues to be one of the leading causes of death. Single-agent-targeted therapies have rarely cured patients with cancer. To effectively halt tumor development and progression, a drug that can target multiple deregulated proteins and pathways would be ideal. Dietary flavonoid fisetin has shown tremendous potential in preclinical settings to target multiple deregulated proteins, signaling pathways, and regulates wide variety of cell functions against various cancers. Fisetin disrupts Wnt signaling and results in cell cycle arrest. It inhibits the YB-1 binding protein to suppress epithelial to mesenchymal transition and thus prevents invasion and migration of cancer cells. By physically interacting with the mTOR molecule, fisetin inhibits signaling involved in cell survival thus explaining its inhibitory effect on cellular growth and proliferation. Fisetin binds to and disrupts micro-tubule dynamics and acts as a stabilizing agent with effects far superior to paclitaxel. To put the chemopreventive potential of fisetin to clinical use, well-designed clinical trial interventions at right doses (as shown in Table 2) in right population are required, which actually mirror the animal modeling data from which they were derived. Before actual clinical trials are conducted it is important that toxicological profile of fisetin is comprehensively investigated. Unfortunately, no toxicity data on fisetin currently exists to justify its potential use in humans. Also, the pharmacokinetic and bioavailability issues have to be studied in detail before phase I and II trials are initiated.
Table 2.
Fisetin dosage and efficacy in preclinical studies against various cancers
| Cancer | Animal models | Fisetin dosage | Outcome of the study | References |
|---|---|---|---|---|
| Lung cancer | B(a)P-induced | 25 mg/kg b.wt., orally | Inhibited tumor growth by modulating activities of mitochondrial enzymes and induced apoptosis | [15] |
| Lewis lung carcinoma cell xenograft | 223 mg/kg b.wt., i.p | Inhibited angiogenesis, tumor growth | [18] | |
| Prostate cancer | 22Rv1 cell xenograft | 40 mg/kg b.wt. (1 mg/animal), i.p. | Inhibited androgen signaling, tumor growth, and reduced serum PSA levels | [20] |
| NB26 cell xenograft | 40 mg/kg b.wt. (1 mg/animal), i.p | Inhibited YB1 phosphorylation and TGF-β induced EMT | [23] | |
| Melanoma | 451 Lu cell xenograft | 45 and 90 mg/kg b.wt. (1 mg and 2 mg/animal), i.p | Inhibited tumor growth, decreased Mitf levels | [26] |
| Skin cancer | SKH-1 hairless mice model | 250 and 500 nmol/animal; topical application | Reduced inflammation and proliferation with decreased leukocytic infiltration and cytokines | [29] |
| Bladder cancer | MNU-induced rat model | 200 mg/kg b.wt., i.p. | Inhibited carcinogenesis, induced apoptosis via p53 activation and NF-κB inhibition | [43] |
| Cervical cancer | HeLa cell xenograft | 4 mg/kg b.wt., orally | Reduced tumor growth, induced apoptosis via mitochondrial and DR5-dependent caspase signaling | [51] |
The debate on cancer chemoprevention attracts a lot of attention with dietary components showing promise in pre-clinical setting but lack of effect when used in clinical trials. Several critiques downgrade the importance phytochemicals based on their multiple biological effects, targets, and signaling pathways. However, we strongly feel that there is a lot of potential for cancer chemoprevention provided that if modeled in the right way, it can offer an effective alternate strategy for the management of cancer, especially for high-risk individuals [52]. Development of drug resistance is one of the most prominent limitations in the development of targeted therapies. A combination of targeted therapies with more traditional therapies could be a potential key to the problem of resistance. However, in overcoming drug resistance issues related to toxicity and high treatment cost remains an impediment in the development of FDA-approved anticancer drugs. There is enough strong preclinical evidence that fisetin shows tremendous promise as an anticancer agent and warrants clinical trials either to be administered alone or in combination with available anticancer drugs.
Acknowledgments
The original work from the author’s (H. Mukhtar) laboratory outlined in this review was supported by United States Public Health Service Grant RO1 CA 160867.
Abbreviations:
- AKT
protein kinase B
- AMPK
5′AMP-activated protein kinase
- AP
activator protein
- ATF
activating transcription factor
- Bax
Bcl-2-associated X Protein
- Bad
Bcl-2-associated death promoter
- Bcl
B-cell lymphoma
- β-TrCP
F-box/WD repeat-containing protein
- CDK
cyclin dependent kinase
- COX
cyclooxygenase
- CKI
CDK inhibitor
- CPA
cyclophosphamide
- CREB
cAMP response element-binding protein
- EGF
epidermal growth factor
- EMT
epithelial-to-mesenchymal transition
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- IKK
IkappaBalpha
- iNOS
inducible nitric oxide synthase
- IRE1
serine/threonine-protein kinase/endoribonuclease
- JAK
janus kinase
- Kit
tyrosine-protein kinase
- LLC
lewis lung carcinoma
- 4′MF
4′-O-methylfisetin
- MAPK
mitogen-activated protein kinases
- MCL-1
myeloid cell leukemia 1
- Mitf
microphthalmia-associated transcription factor
- MNU
N-Nitroso-N-methylurea
- MMP
matrix metalloproteinase
- MTA
metastasis-associated protein
- mTOR
mechanistic target of rapamycin
- NAC
N-acetyl-L-cysteine
- NSC
non-small cell
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PARP
poly ADP ribose polymerase
- PI3K
Phosphoinositide 3-kinase
- ROS
reactive oxygen species
- SIK
salt inducible kinase
- STAT
signal transducer and activator of transcription
- TGF
Transforming growth factor
- TOPO
DNA topoisomerase
- TRAIL
TNF-related apoptosis-inducing ligand
- TSC
tuberous sclerosis
- TMFol
3′,4′,5′-trimethoxyflavonol
- uPA
irinary plasminogen activator
- XBP-1
X-box binding protein 1
- YB1
Y box binding protein 1
Footnotes
The authors have declared no conflict of interest.
12 References
- [1].Torre LA, Bray F, Siegel RL, Ferlay J et al. , Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2015, 65, 87–108. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2015. CA: A Cancer Journal for Clinicians 2015, 65, 5–29. [DOI] [PubMed] [Google Scholar]
- [3].Singh SR, Cancer stem cells: recent developments and future prospects. Cancer Letters 2013, 338, 1–2. [DOI] [PubMed] [Google Scholar]
- [4].Kasala ER, Bodduluru LN, Madana RM, V AK et al. , Chemopreventive and therapeutic potential of chrysin in cancer: mechanistic perspectives. Toxicology Letters 2015, 233, 214–225. [DOI] [PubMed] [Google Scholar]
- [5].Teiten MH, Eifes S, Dicato M, Diederich M, Curcuminthe paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins 2010, 2, 128–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hasima N, Aggarwal BB, Cancer-linked targets modulated by curcumin. International Journal of Biochemistry and Molecular Biology 2012, 3, 328–351. [PMC free article] [PubMed] [Google Scholar]
- [7].Russo M, Spagnuolo C, Tedesco I, Russo GL, Phyto-chemicals in cancer prevention and therapy: truth or dare? Toxins 2010, 2, 517–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cragg GM, Pezzuto JM, Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Medical Principles and Practice : International Journal of the Kuwait University, Health Science Centre 2015, doi: 10.1159/000443404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beliveau R, Gingras D, Role of nutrition in preventing cancer. Canadian Family Physician Medecin De Famille Canadien 2007, 53, 1905–1911. [PMC free article] [PubMed] [Google Scholar]
- [10].Murthy NS, Mukherjee S, Ray G, Ray A, Dietary factors and cancer chemoprevention: an overview of obesity-related malignancies. Journal of Postgraduate Medicine 2009, 55, 45–54. [DOI] [PubMed] [Google Scholar]
- [11].Lall RK, Syed DN, Adhami VM, Khan MI et al. , Dietary polyphenols in prevention and treatment of prostate cancer. International Journal of Molecular Sciences 2015, 16, 3350–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arai Y, Watanabe S, Kimira M, Shimoi K et al. , Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. The Journal of Nutrition 2000, 130, 2243–2250. [DOI] [PubMed] [Google Scholar]
- [13].Zhuo W, Zhang L, Zhu Y, Zhu B et al. , Fisetin, a dietary bioflavonoid, reverses acquired Cisplatin-resistance of lung adenocarcinoma cells through MAPK/Survivin/Caspase pathway. American Journal of Translational Research 2015, 7, 2045–2052. [PMC free article] [PubMed] [Google Scholar]
- [14].Kang KA, Piao MJ, Hyun JW, Fisetin induces apoptosis in human nonsmall lung cancer cells via a mitochondria-mediated pathway. In Vitro Cellular & Developmental Biology. Animal 2015, 51, 300–309. [DOI] [PubMed] [Google Scholar]
- [15].Ravichandran N, Suresh G, Ramesh B, Manikandan R et al. , Fisetin modulates mitochondrial enzymes and apoptotic signals in benzo(a)pyrene-induced lung cancer. Molecular and Cellular Biochemistry 2014, 390, 225–234. [DOI] [PubMed] [Google Scholar]
- [16].Ravichandran N, Suresh G, Ramesh B, Siva GV, Fisetin, a novel flavonol attenuates benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association 2011, 49, 1141–1147. [DOI] [PubMed] [Google Scholar]
- [17].Khan N, Afaq F, Khusro FH, Mustafa Adhami V et al. , Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nons-mall cell lung cancer cells by a dietary flavonoid fisetin. International Journal of Cancer. Journal International Du Cancer 2012, 130, 1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Touil YS, Seguin J, Scherman D, Chabot GG, Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemotherapy and Pharmacology 2011, 68, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khan N, Afaq F, Syed DN, Mukhtar H, Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis 2008, 29, 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Khan N, Asim M, Afaq F, Abu Zaid M et al. , A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Research 2008, 68, 8555–8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Suh Y, Afaq F, Khan N, Johnson JJ et al. , Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis 2010, 31, 1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Szliszka E, Helewski KJ, Mizgala E, Krol W, The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. International Journal of Oncology 2011, 39, 771–779. [DOI] [PubMed] [Google Scholar]
- [23].Khan MI, Adhami VM, Lall RK, Sechi M et al. , YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget 2014, 5, 2462–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hill CU, Saad SE, Britton RG, Gescher AJ et al. , Inhibition of prostate cancer cell growth by 3′,4′,5′- trimethoxyflavonol (TMFol). Cancer Chemotherapy and Pharmacology 2015, 76, 179–185. [DOI] [PubMed] [Google Scholar]
- [25].Mukhtar E, Adhami VM, Sechi M, Mukhtar H, Dietary flavonoid fisetin binds to beta-tubulin and disrupts micro-tubule dynamics in prostate cancer cells. Cancer Letters 2015, 367, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Syed DN, Afaq F, Maddodi N, Johnson JJ et al. , Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/beta-catenin signaling and decreased Mitf levels. The Journal of Investigative Dermatology 2011, 131, 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumagai A, Horike N, Satoh Y, Uebi T et al. , A potent inhibitor of SIK2, 3, 3′, 7-trihydroxy-4′-methoxyflavon (4′-O-methylfisetin), promotes melanogenesis in B16F10 melanoma cells. PloS One 2011, 6, e26148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pal HC, Sharma S, Elmets CA, Athar M et al. , Fisetin inhibits growth, induces G(2)/M arrest and apoptosis of human epidermoid carcinoma A431 cells: role of mitochondrial membrane potential disruption and consequent caspases activation. Experimental Dermatology 2013, 22, 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pal HC, Athar M, Elmets CA, Afaq F, Fisetin inhibits UVB-induced cutaneous inflammation and activation of PI3K/AKT/NFkappaB signaling pathways in SKH-1 hairless mice. Photochemistry and Photobiology 2015, 91, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pal HC, Sharma S, Strickland LR, Katiyar SK et al. , Fisetin inhibits human melanoma cell invasion through promotion of mesenchymal to epithelial transition and by targeting MAPK and NFkappaB signaling pathways. Plos One 2014, 9, e86338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yi C, Zhang Y, Yu Z, Xiao Y et al. , Melatonin enhances the anti-tumor effect of fisetin by inhibiting COX-2/iNOS and NF-kappaB/p300 signaling pathways. PloS One. 2014, 9, e99943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Syed DN, Lall RK, Chamcheu JC, Haidar O et al. , Involvement of ER stress and activation of apoptotic pathways in fisetin induced cytotoxicity in human melanoma. Archives of Biochemistry and Biophysics 2014, 563, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pal HC, Baxter RD, Hunt KM, Agarwal J et al. , Fisetin, a phytochemical, potentiates sorafenib-induced apoptosis and abrogates tumor growth in athymic nude mice implanted with BRAF-mutated melanoma cells. Oncotarget 2015, 6, 28296–28311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pal HC, Diamond AC, Strickland LR, Kappes JC et al. , Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget 2015, 7, 1227–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kuntz S, Wenzel U, Daniel H, Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. European Journal of Nutrition 1999, 38, 133–142. [DOI] [PubMed] [Google Scholar]
- [36].Lu X, Jung J, Cho HJ, Lim DY et al. , Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. The Journal of Nutrition 2005, 135, 2884–2890. [DOI] [PubMed] [Google Scholar]
- [37].Lim do Y, Park JH, Induction of p53 contributes to apoptosis of HCT-116 human colon cancer cells induced by the dietary compound fisetin. American Journal of physiology. Gastrointestinal and Liver Physiology 2009, 296, G1060–G1068. [DOI] [PubMed] [Google Scholar]
- [38].Suh Y, Afaq F, Johnson JJ, Mukhtar H, A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis 2009, 30, 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen WS, Lee YJ, Yu YC, Hsaio CH et al. , Enhancement of p53-mutant human colorectal cancer cells radiosensitivity by flavonoid fisetin. International Journal of Radiation Oncology, Biology, Physics 2010, 77, 1527–1535. [DOI] [PubMed] [Google Scholar]
- [40].Wu MS, Lien GS, Shen SC, Yang LY et al. , N-acetyl-L-cysteine enhances fisetin-induced cytotoxicity via induction of ROS-independent apoptosis in human colonic cancer cells. Molecular Carcinogenesis 2014, 53(Suppl 1), E119–E129. [DOI] [PubMed] [Google Scholar]
- [41].Chen Y, Wu Q, Song L, He T et al. , Polymeric micelles encapsulating fisetin improve the therapeutic effect in colon cancer. ACS Applied Materials & Interfaces 2015, 7, 534–542. [DOI] [PubMed] [Google Scholar]
- [42].Li J, Cheng Y, Qu W, Sun Y et al. , Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic & Clinical Pharmacology & Toxicology 2011, 108, 84–93. [DOI] [PubMed] [Google Scholar]
- [43].Li J, Qu W, Cheng Y, Sun Y et al. , The inhibitory effect of intravesical fisetin against bladder cancer by induction of p53 and down-regulation of NF-kappa B pathways in a rat bladder carcinogenesis model. Basic & Clinical Pharmacology & Toxicology 2014, 115, 321–329. [DOI] [PubMed] [Google Scholar]
- [44].Yang PM, Tseng HH, Peng CW, Chen WS et al. , Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. International Journal of Oncology 2012, 40, 469–478. [DOI] [PubMed] [Google Scholar]
- [45].Lee WR, Shen SC, Lin HY, Hou WC et al. , Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochemical Pharmacology 2002, 63, 225–236. [DOI] [PubMed] [Google Scholar]
- [46].Lopez-Lazaro M, Willmore E, Austin CA, The dietary flavonoids myricetin and fisetin act as dual inhibitors of DNA topoisomerases I and II in cells. Mutation Research 2010, 696, 41–47. [DOI] [PubMed] [Google Scholar]
- [47].Adan A, Baran Y, The pleiotropic effects of fisetin and hesperetin on human acute promyelocytic leukemia cells are mediated through apoptosis, cell cycle arrest, and alterations in signaling networks. Tumour Biology : The Journal of the International Society for Oncodevelopmental Biology and Medicine 2015, 36, 8973–8984. [DOI] [PubMed] [Google Scholar]
- [48].Saslow D, Solomon D, Lawson HW, Killackey M et al. , American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA: A Cancer Journal for Clinicians 2012, 62, 147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chou RH, Hsieh SC, Yu YL, Huang MH et al. , Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. PloS One 2013, 8, e71983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ying TH, Yang SF, Tsai SJ, Hsieh SC et al. , Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Archives of Toxicology 2012, 86, 263–273. [DOI] [PubMed] [Google Scholar]
- [51].Lin MT, Lin CL, Lin TY, Cheng CW et al. , Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumour Biology : The Journal of the International Society for Oncodevelopmental Biology and Medicine 2015, doi: 10.1007/s13277-015-4526-4. [DOI] [PubMed] [Google Scholar]
- [52].Adhami VM, Bailey HH, Mukhtar H, Cancer chemoprevention is not a failure. Carcinogenesis 2014, 35, 2154–2155. [DOI] [PubMed] [Google Scholar]
- [53].Kimira M, Arai Y, Shimoi K, Watanabe S, Japanese in-take of flavonoids and isoflavonoids from foods. Journal of epidemiology / Japan Epidemiological Association 1998, 8, 168–175. [DOI] [PubMed] [Google Scholar]