Abstract
The alcohol preference model is one of the most widely used animal models relevant to alcoholism. Stressors increase alcohol consumption. Here we present a protocol for a rapid and useful tool to test alcohol preference and stress-induced alcohol consumption in mice. In this model, animals are given two bottles, one with a diluted solution of ethanol in water, and the other with tap water. Consumption from each bottle is monitored over a 24-h period over several days to assess the animal’s relative preference for the ethanol solution over water. In the second phase, animals are stressed by restraining them for an hour daily and their subsequent preference of tap water or the ethanol solution is evaluated. Preference is measured by the volume and/or weight or liquid consumed daily, which is then converted to a preference ratio. The alcohol preference model was combined with the conditioned place preference paradigm to determine alcohol conditioning and preference following the deletion of CB2 cannabinoid receptors in dopaminergic neurons in the DAT-Cnr2 Cre-recombinant conditional knockout (cKO) mice in comparison with the wild-type control mice.
Keywords: Alcohol, Stress, Mouse model, Behavior, Cannabinoid, Conditioned place preference
Background
Many aspects of alcoholism and alcohol consumption can be studied through animal models. Alcohol induces positive reinforcement, and animals can seek alcohol and even work for it. However, alcohol can also be a negative reinforcement, since it is capable of reducing anxiety. No animal model is able to duplicate the complex features of alcoholism. Oral ethanol self-administration is widely used for examining specific aspects of behavior and physiology relevant for understanding alcoholism (Mardones and Segovia-Riquelme, 1983; Cunningham et al., 2000 ). Mice can be genetically manipulated at cell type specific levels and therefore are valuable for research into the cell type specific genetic determinants of alcoholism.
The alcohol preference model is one of the most widely used animal models relevant to alcoholism. This model meets important criterion, which is that the ethanol should be self-administered orally (Cicero, 1980; Crabbe et al., 2010 ). An animal’s genotype exerts a strong influence on self-administration in this model. Some mouse strains, like the inbred strain of mouse C57BL/6J ( Rhodes et al., 2005 ), present a genetically influenced high preference for ethanol and they voluntarily consume it orally ( Yoneyama et al., 2008 ; Barkley-Levenson and Crabbe, 2012). The conditioned place paradigm (CPP) is widely used to explore the effects of addictive substances including alcohol, taking advantage of learned associations. Therefore, alcohol CPP measures the association of alcohol with a particular environment to determine whether mice can acquire alcohol CPP.
Stress can interact with ongoing ethanol consumption to trigger increased intake (e.g., self-medicating behavior), thereby increasing initial susceptibility to alcohol use disorders. Among the stressors, a restraint model of acute and chronic stress can increase ethanol consumption ( Yang et al., 2008 ).
New advances and accumulating evidence support a role for the endocannabinoid system in the effects of alcohol. The endocannabinoid system consists of two cannabinoid receptors, CB1Rs and CB2Rs, with endocannabinoids and the enzymes for the biosynthesis and inactivation of the endocannabinoids. Our goal here was to summarize the protocol used to measure alcohol preference in combination with stress-induced alcohol consumption. We also provide evidence that the endocannabinoid system plays a role in alcohol preference following dopaminergic neuron specific deletion of CB2Rs in the mouse model ( Liu et al., 2017 ).
Materials and Reagents
50 ml Polypropylene Centrifuge Tubes with Attached Caps (Boekel Scientific, catalog number: 120021)
Bottles (see Figures 1B and 1C) with Sipper Caps (Chewy, catalog number: 101445)
-
Mice: Adult (7 weeks or older) mice (C57BL/6J) (THE JACKSON LABORATORY, catalog number: 000664)
Note: Alternate strains and ages of mice may also be used. Mice are housed alone, each in their respective cages, in an environment with controlled temperature (around 23 °C) and humidity under a 12-12 h light-dark cycle with free access to food. See Animal considerations in Notes for more details.
100% Alcohol and dilutions: 8%, 16% and 32% (Sigma-Aldrich, catalog number: 1012768)
Figure 1. Photos showing experimental set-up for the evaluation of alcohol preference.
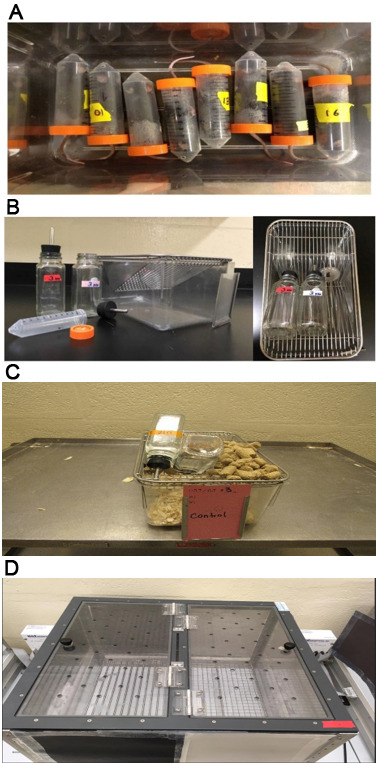
A. Tubes in which naïve or conditional knockout mice and wild-type controls are subjected to acute stress. B. Tubes, water bottles, and a clean empty mouse cage. C. Image of the control cage. D. Image of the apparatus for conditioned place preference.
Equipment
-
Mouse polycarbonate home cages (7.5 in W x 11 in L x 5 in H) with standard woodchip mouse bedding (Fisher Scientific, catalog number: 01-286-13A)
Manufacturer: Tecniplast, catalog number: 1290D00SU.
Note: Standard cage changes are allowed during the habituation period (see below). However, it is recommended to avoid cage changes during the period of data collection to prevent leakage. The cage can be cleaned between phases. One cage for each subject mouse.
-
Stainless steel wire cage lid modified to allow space for two bottles and the food (Fisher Scientific, catalog number: 01-286-13A)
Manufacturer: Tecniplast, catalog number: 1290D00SU.
Drill with small (1 mm) drill bit (for making three small holes in top of 50 ml Polypropylene Centrifuge Tubes, to allow the mouse to breathe, and a single larger hole in the cap to insert the tail)
Thermo Scientific weighing scale (Thermo Fisher Scientific, catalog number: 20031)
Software
Activity Monitor software (Med Associates, St. Albans, VT) (for automated data collection)
GraphPad PRISM 6.0 software (GraphPad Software. Inc., San Diego, CA, USA) (for data analysis)
Procedure
All experiments were conducted during dark phase, under dim light illumination.
-
Experimental setup
Animals: Naïve C57Bl/6Js were used, and as wild-type controls for the DAT-Cnr2 conditional knockout (cKO) mice. The generation and breeding of the cKO mice was previously reported ( Liu et al., 2017 ).
Experimental space should be quiet and dimly lit, under controlled humidity and temperature.
All the cages should be placed on a rack designated for this experiment. The rack should be stable to avoid movement and to prevent leakage. The experimenter should be careful when removing the bottles to minimize the amount of leakage. Leakage should be avoided to reduce undesired variability.
The experimental area should be clean, and all the measurements should be conducted at the same time of the day.
One of the cages will be the “control cage”. This cage will have everything described above except a mouse.
All cages should be labeled with the information for the specific experiment, such as the mouse’s strain, age, and gender. Food should always be kept on the right side for consistency.
-
Setting up 2-bottle choice test
-
Fill all the bottles with 150 ml of water and put on the sipper tap.
Note: Make sure there are no water leaks by inverting the bottles.
Label each bottle with the number of the subject mouse (1, 2, 3…) in permanent marker. Label the cages with the same number.
-
Place the 2 bottles onto the wire lid (Figure 2), with minimal shaking to avoid dripping. Both bottles should be placed on one side of the divided wire rack, leaving the other side for food. Put food pellets on the other side of the wire lid.
Note: Make sure that the food is always on the right side, to be consistent. Mice were adapted to the testing room, with the same conditions as in the colony room (22 ± 2 °C; humidity: 55 ± 5%; 12 h dim/artificial light/12 h dark cycle: light off at 7 PM).
-
-
Habituation to the 2-bottle
Place a single mouse in the proper mouse caging (see Equipment) under controlled conditions (see Materials and Reagents).
Isolate the subjects into individual cages and give them two bottles filled with 150 ml water. Allow mice to acclimate for at least 4 days under these conditions.
-
Setting up 2 bottle choice for alcohol preference
Remove the bottles and clean them.
Mix water with 100% alcohol to 16%.
-
For each mouse, fill one bottle with 150 ml of water and another bottle with alcohol solution. Put the sipper cap on the bottle.
Note: Make sure there are no water leaks by inverting the bottles.
Label each bottle with the assigned number of the mouse, and water or alcohol in permanent marker (e.g., 1 H2O/1 EtOH).
Record weight with cap on the bottle and write down the value on a record sheet.
-
Evaluation of alcohol preference
-
Place the 2 bottles onto the wire lid, with minimal shaking to avoid dripping. Both bottles should be placed on one side of the divided wire rack, leaving the other side for food. Put food pellets on the other side of the wire lid.
Note: Make sure that the food is always on the right side, to be consistent.
Assess water and alcohol solution consumption daily for 5 days. Record weight. Monitor for any problems with liquid flow or spillage, e.g., wet bedding below the sipper tube. Switch positions (left vs. right) of bottles daily.
The “control cage” has just two bottles of water without a mouse. These bottles are weighed to determine the amount of spillage when bottles are inverted, and to later correct the data of the other groups.
-
-
Acute stress-induced alcohol preference (5 days)
Set up 2 bottle choices for alcohol preference (described above).
After removing the bottles for daily weighing on the first day, mice are randomly assigned to be in the experimental or control group. Label the cages with “control” or “experimental” (above where the mouse’s number is) and place them by group on the shelf. The experimental group of mice are restrained in a 50 ml conical tube for an hour. After that, they are released and the bottles for both groups (control and experimental) and placed in the cages again.
Repeat these steps for 5 consecutive days. The bottles are weighed each day, and the mice are weighed before and after each phase.
The general timeline of the alcohol preference is depicted from Procedures D-F over the 5-day period.
-
Chronic mild stress (CMS) induced alcohol consumption (from 4-7 weeks) (Table 1)
Set up separate groups of the wild-type and selected cKO mice (n = 6 per group) for experimental and control groups in separate rooms. All animals are housed individually, except for the non-stressed control groups.
For the CMS regime, mice are subjected to various stressors according to a semi-random schedule Table 1 for 4-7 weeks as planned.
The stress regime for each week consisted of food deprivation for 12 h, water deprivation for 12 h, damp bedding for 12 h, overnight stroboscopic illumination, tail suspension for 10 min, tube restrain for 30 min, as described above, loud music overnight, wire mesh to replace bedding 12 h, 30 min introduction of an intruder mouse to the cage and lights off or on. These stressors were paired morning or overnight.
All non-CMS groups in the separate room were given food and water at all times.
-
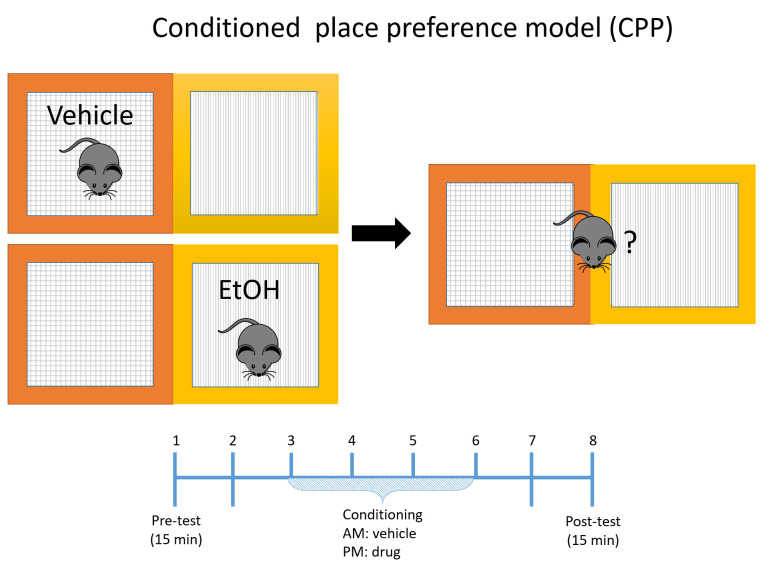
Conditioned place preference (CPP)
Alcohol CPP training and testing are conducted using an infra-red photobeam detector open field apparatus (ENV-510) from Med Associates (St. Albans, VT, USA).
Alcohol CPP training and testing are conducted using an infra-red photobeam detector open field apparatus (ENV-510) from Med Associates (St. Albans, VT, USA) equipped with the two-compartment place preference inserts (ENV-512), as shown in Figure 1D. The floor of compartment-1 has parallel rods (3-mm radius, 8 mm center to center spacing) with black cardboard paper covering the outside. Compartment-2 has a stainless steel wire mesh (6 x 6) floor with white cardboard paper covering the outside of the walls. Each compartment is 13 cm (width) x 15 cm (depth).
Perform all conditioning sessions and preference tests in each group in three phases (Figure 3) (pre-conditioning, conditioning and post-conditioning phases).
During the post-conditioning phase on the test day, mice are allowed to explore both compartments for 15 min.
The CPP score is defined as the time spent in the alcohol paired compartment minus the time spent in the saline-paired compartment during the CPP test.
Figure 2. Setup of 2 bottle choice for alcohol preference and water is available.
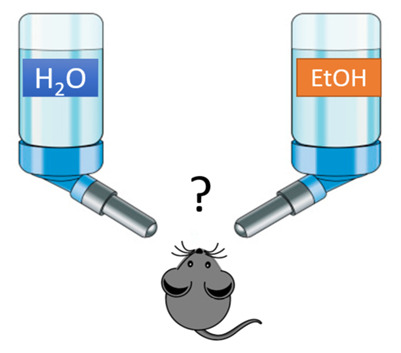
Table 1. Chronic Mild Stressors (from 4-7 weeks).
| Week 1 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
|---|---|---|---|---|---|---|---|
| 8:30 am | Water deprivation | Damp Bedding |
Tube Restraint |
Water Deprivation | Damp Bedding |
Tube Restraint |
Water Deprivation |
| 5:30 pm |
Tail Suspension |
Food Deprivation | Strobe Light |
Tail Suspension |
Food Deprivation | Strobe Light | Anhedonia Test |
| WEEK 2 | DAY 8 | DAY 9 | DAY 10 | DAY 11 | DAY 12 | DAY 13 | DAY 14 |
| 8:30 am |
Tail Suspension |
Tube Restraint |
Water Deprivation | Damp Bedding |
Tube Restraint |
Water Deprivation | Damp Bedding |
| 5:30 pm | Food Deprivation | Strobe Light |
Tail Suspension |
Food Deprivation | Strobe Light |
Tail Suspension |
Anhedonia Test |
| WEEK 3 | DAY 15 | DAY 16 | DAY 17 | DAY 18 | DAY 19 | DAY 20 | DAY 21 |
| 8:30 am | Food Deprivation | Water Deprivation | Damp Bedding | Damp Bedding | Empty Bottle | Water Deprivation | Damp Bedding |
| 5:30 pm | Strobe Light |
Tail Suspension |
Tube Restraint |
Food Deprivation | Strobe Light |
Tail Suspension |
Anhedonia Test |
| WEEK 4 | DAY 22 | DAY 23 | DAY 24 | DAY 25 | DAY 26 | DAY 27 | DAY 28 |
| 8:30 am |
Tail Suspension |
Tube Restraint |
Water Deprivation | Damp Bedding |
Tube Restraint |
Water Deprivation | Anhedonia Test |
| 5:30 pm | Food Deprivation | Strobe Light |
Tail Suspension |
Food Deprivation | Strobe Light |
Tail Suspension |
Sacrifice |
Figure 3. Graphical presentation of the CPP paradigm used.
All conditioning sessions and preference tests were performed at the same time daily. Animals were allowed to habituate in the CPP apparatus for 45 min before the conditioning phase.
Data analysis
All data are transcribed in a spreadsheet. Set up a column for the raw weight data and next to it a column for the corrected weight data. The corrected weight data is the amount of spillage (of water and alcohol) each day from the control cage. This number will be subtracted from the raw weight data. Each day, the spillage amount must be subtracted from the raw water and alcohol weight data to determine the corrected weight.
To calculate the amount of alcohol or water consumed per day, the bottle weights were averaged for each day. These values were then subtracted from the averages of the bottle weights for each group on that day. Subtracting the new bottle weight from that of the previous day gave the amount of alcohol or water drank by the mice overnight.
Day 0 is when the bottles were initially filled with 150 ml of water/alcohol and therefore the consumption for that day was 0.
The alcohol preference ratio was determined by dividing the amount of alcohol consumed by total liquid (alcohol + water) consumed.
The activity monitor recorded time spent in each of the compartments, using the photo electric beam break counts. The time that each mouse spent in the compartment was recorded and the CPP score was defined as the time spent in the alcohol paired compartment minus the time spent in the saline-paired compartment during the CPP test. The activity monitor software (Med Associates, St. Albans, VT) was used for automated data collection.
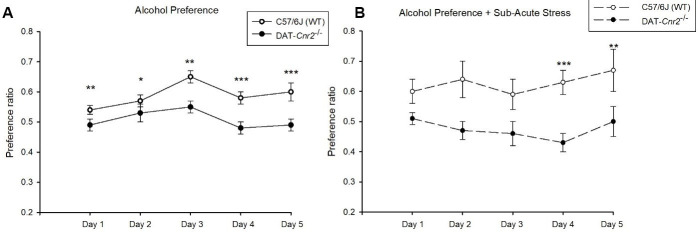
Representative data: Figure 4 is representative data for Alcohol Preference Test. In this test, the mice were individually housed and habituated with two fluid bottles available for three days. We used two groups: 12 male C57/6J mice as wild-type and 12 male DAT-Cnr2 conditional knockout (cKO) mice that do not express the CB2R in midbrain dopamine neurons. On the day of the experiment, all the animals were weighed. One bottle was filled with 150 ml of tap water and the other was filled with 150 ml of 16% alcohol solution. They were weighed with the tops on and placed on top of the cages (labeled correctly). For the alcohol preference test, the bottles were weighed for each animal for five consecutive days at 10 AM. The positions of the bottles in the different cages were randomized with respect to which side of the cages they were placed. In the second part of the experiment, each group was then split into two groups. One group (n = 6) was stressed by putting them in a 50 ml tube for one hour each day for 5 consecutive days. The rest of the animals remained in their cages. All the bottles were weighed for each animal for five consecutive days at the same time, and all the bottles were placed on top of the cages at 11 AM. In all the experiments, the ratio of alcohol to water consumed and the total fluid consumption were calculated to obtain a preference ratio. The alcohol preference ratio was determined by dividing the amount of alcohol consumed by total fluid (alcohol + water) consumed, with and without the sub-acute stress. The wild-type mice preferred alcohol over water, as evident by the significant increase (as determined by Repeated Measurement ANOVA). The DAT-Cnr2 cKO mice (mice did not show an alcohol-CPP induction, Figure 5), and do not show a significant increase of the preference for alcohol (Figure 4). Wild-type mice preferred more alcohol than the DAT-Cnr2 mice during the 5-day test with acute stress (as determined by Student’s t-test comparison between genotypes). These results suggest that the DAT-Cnr2 KO mice are resistant to alcohol consumption even in acute stress conditions. This supports the previous report that CB2 receptor agonists is involved in the development and enhancement of alcohol preference in stressed but not in non-stressed control mice ( Ishiguro et al., 2007 ; Onaivi et al., 2008 ).
Figure 4. Preference ratio for alcohol over water in normal conditions (A) and in response to an acute stress protocol (B) exhibited by wild-type mice (white circles) and DAT-Cnr2 cKO mice (black circles) during 5 consecutive days.
Data presented as the Mean ± standard error of the mean (SEM) daily consumption ratio over 5 days. Significant difference between genotypes paired t-test (*P < 0.05, **P < 0.01, ***P < 0.001).
Figure 5. Deletion of CB2Rs in dopamine neurons (DAT-Cnr2 cKO) mice and alcohol preference.
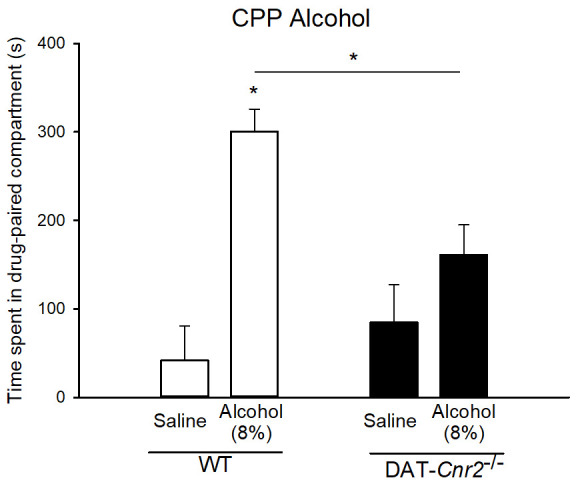
8% alcohol-induced CPP (P < 0.05) in wild-type mice but the DAT-Cnr2 cKO mice did not show significant difference in post-conditioning phase between alcohol and saline.
Notes
Animal considerations:
It is important to conduct preliminary studies to characterize the behavioral phenotype with particular equipment, environment, and animals. We measured baseline behaviors of wild-type, heterozygous, and homozygous of the DAT-Cre and DAT-Cnr2 mouse littermates to avoid genetic confounds. This is especially true in the case of varying mouse strains, either inbred or outbred, or if mice have been surgically or behaviorally manipulated prior to alcohol preference test.
If required, the baseline of consumption can be established by weighing both bottles filled with 150 ml of water and weighed with the tops on and placed on top of the cages for three days.
Acknowledgments
This protocol is in memory of our co-author, Norman Schanz, whose sudden and tragic loss will be difficult to replace in the animal laboratory. This research was supported by William Paterson University. ACA was supported by the Mexican National Council of Science and technology (CONACYT # CVU332502/232728). HI was supported by Public Interest Trust Research Aid Fund for Stress-Related Diseases (with Commemoration of Imai Kimi), KAKENHI (18023009, 20023006, and 20390098). ESO was supported by NIH grant DA032890. QRL is supported by IRP/NIA/NIH. We thank the Dean, Dr. Venkat Sharma, of the College of Science and Health at William Paterson University for student worker support in maintaining our mice in the animal laboratory. We are also thankful for the technical assistant of Sneha Tammareddy, Eugene Dennis, Branden Sanabria, Paola Velandia, Steve Gross, and Monika Chung while working in the laboratory of ESO. This protocol and figures are adapted from our previous work ( Liu et al., 2017 ).
Competing interests
The authors have no conflicts of interest to declare.
Ethics
The experimental procedures followed the Guide for the Care and Use of Laboratory Animals and were approved by William Paterson University Animal Care and Use Committee (IACUC).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Barkley-Levenson A. M. and Crabbe J. C.(2012). Bridging animal and human models: Translating from(and to) animal genetics. Alcohol Res 34(3): 325-335. [PMC free article] [PubMed] [Google Scholar]
- 2. Cicero T.(1980). Alcohol self-administration, tolerance and withdrawal in humans and animals: theoretical and methodological issues. In: Rigter, H. and Crabbe J. C.(Eds.) Alcohol tolerance and dependence. Amsterdam 7 Elsevier; /North Holland Biomedical Press, pp 1-51. [Google Scholar]
- 3. Crabbe J. C., Phillips T. J. and Belknap J. K.(2010). The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet 40(6): 737-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cunningham C. L., Fidler T. L. and Hill K. G.(2000). Animal models of alcohol's motivational effects. Alcohol Res Health 24(2): 85-92. [PMC free article] [PubMed] [Google Scholar]
- 5. Ishiguro H., Iwasaki S., Teasenfitz L., Higuchi S., Horiuchi Y., Saito T., Arinami T. and Onaivi E. S.(2007). Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J 7(6): 380-385. [DOI] [PubMed] [Google Scholar]
- 6. Liu Q. R., Canseco-Alba A., Zhang H. Y., Tagliaferro P., Chung M., Dennis E., Sanabria B., Schanz N., Escosteguy-Neto J. C., Ishiguro H., Lin Z., Sgro S., Leonard C. M., Santos-Junior J. G., Gardner E. L., Egan J. M., Lee J. W., Xi Z. X. and Onaivi E. S.(2017). Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci Rep 7(1): 17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mardones J. and Segovia-Riquelme N.(1983). Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol Teratol 5(2): 171-178. [PubMed] [Google Scholar]
- 8. Onaivi E. S., Carpio O., Ishiguro H., Schanz N., Uhl G. R. and Benno R.(2008). Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann N Y Acad Sci 1139: 426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhodes J. S., Best K., Belknap J. K., Finn D. A. and Crabbe J. C.(2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84(1): 53-63. [DOI] [PubMed] [Google Scholar]
- 10. Yang X., Wang S., Rice K. C., Munro C. A. and Wand G. S.(2008). Restraint stress and ethanol consumption in two mouse strains. Alcohol Clin Exp Res 32(5): 840-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoneyama N., Crabbe J. C., Ford M. M., Murillo A. and Finn D. A.(2008). Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42(3): 149-160. [DOI] [PMC free article] [PubMed] [Google Scholar]