Abstract
Wetland depressions without surface channel connections to aquatic systems are substantial sinks for nitrogen (N), phosphorus (P) and organic carbon (org. C). We assessed accretion, N, P and org.-C accumulation rates in 43 depressional wetlands across three ecoregions of the USA (Erie Drift Plain, EDP; Middle Atlantic Coastal Plain, MACP; Southern Coastal Plain, SCP) using caesium-137 (137Cs). The mean sediment accretion rate in minimally affected (reference) sites was 0.6 ± 0.4 mm year−1 and did not differ among ecoregions. Accumulation rates for N and org. C averaged 3.1 ± 3.1 g N m−2 year−1and 43.4 ± 39.0 g org. C m−2 year−1 respectively, and did not differ across minimally affected sites. Phosphorus accumulation rates were significantly greater in EDP (0.10 ± 0.10 g P m−2 year−1) than MACP (0.01 ± 0.01 g P m−2 year−1) or SCP (0.04 ± 0.04 g P m−2 year−1) sites. Land-use modality and wetland-type effects were analysed in SCP, with few differences being found. Depressional wetlands sequester substantive amounts of nutrients and C; their cumulative contributions may significantly affect landscape nutrient and C dynamics because of the abundance of wetland depressions on the landscape, warranting further investigation and potential watershed-scale conservation approaches.
Additional keywords: assimilation, caesium-137, ecosystem services, geographically isolated wetland, sequestration, upland embedded wetland
Introduction
Wetlands are among the first aquatic landscape elements to be exposed to exogenous materials such as nutrients (e.g. nitrogen (N), phosphorus (P)) or organic carbon (org. C) translocated or entrained by surface runoff and interstitial movement from the surrounding contributing areas. This influx of nutrients and org. C provides the necessary material for high rates of endogenous productivity. The ability of wetlands to process both exogenous and endogenous materials through physical or biogeochemical activities such as denitrification, organic-matter mineralisation and C or P sequestration is reflected by our attempts to replicate these ecosystem services through constructed wetlands, and has demonstrated the importance of maintaining and conserving existing wetlands. Conversely, the loss of wetlands from the landscape has been implicated in increased nutrient loading and altered hydrology of receiving waters (e.g. Hey 2002; Gleason et al. 2007; Evenson et al. 2015).
Freshwater wetlands, which comprise ~95% of the remaining 44.5 × 106 ha of wetlands in the conterminous United States (Dahl 2011), occur throughout the country, with high densities in certain ecoregions (Omernik 1987). A subset of freshwater wetlands, namely including those with no obvious surface-water connections to other waters, has been variously described as geographically isolated wetlands (GIWs), upland-embedded wetlands or wetland depressions (Tiner 2003a; Leibowitz 2015; Mushet et al. 2015), and these have been increasingly analysed by the scientific and regulatory community to better ascertain their functioning in the landscape and connectivity vis-à-vis other waters, in part because of recent USA Supreme Court decisions and clarifications to the USA Clean Water Act (see Alexander 2015; Marton et al. 2015; US EPA 2015; Rains et al. 2016). Recently, Lane and D’Amico (2016) conducted a geospatial analysis of wetland proximity to other waters at the scale of the conterminous United States and reported almost eight million potential closed basin depressional wetland systems (i.e. GIWs) covering almost 6.6 × 106 ha.
Wetland systems are also known for their biogeochemical processing capacity (Reddy and DeLaune 2008). However, owing in part to the breadth of wetland types described as depressional systems, the literature is sparsely populated with information on the ability of depressional wetlands to sequester nutrients and C, despite their relative frequency of occurrence (however, see Craft and Casey 2000; Dunne et al. 2007; Bhadha and Jawitz 2010; Badiou et al. 2011; Besasie and Buckley 2012; Bernal and Mitsch 2012; US EPA 2015). With the entrainment of pollutants and C in surface and near-surface waters, and subsequent movement to depressional wetlands, where these constituents are biogeochemically processed (see Marton et al. 2015), this relative dearth of information represents not only a gap in our knowledge but is also a critical piece in understanding the system-scale functioning of depressional wetland ecosystems.
Nutrient and C storage can be ascertained through standard assessments of wetland soil samples (e.g. total P concentration in mg P kg−1 of wetland soil), although this information does not provide nutrient accumulation or sediment accretion rates in such analyses, limiting assessments of the functioning of wetland systems. The use of thermonuclear bomb-derived radioisotopes for soil dating provides a method to estimate soil accretion and accumulation rates (Ritchie and McHenry 1990). The radioisotope caesium-137 (137Cs) is a product of the atmospheric nuclear weapon-testing era that is otherwise not found in nature. Atmospheric nuclear explosions began in 1945, with peak 137Cs in the atmosphere occurring ca 1964, a date typically used for 137Cs dating maxima. Caesium-137 has a half-life of 30.2 years, and the atmospheric 137Cs fall-out is strongly adsorbed to clay and organic matter particles. Assuming no bioturbation of the sediments, 137Cs can be used to quantify nutrient accumulation and sediment accretion rates. For instance, Craft and Casey (2000) assessed accretion rates of riverine and depressional wetlands in south-western Georgia by using 137Cs and lead-210 (210Pb) markers and quantified soil N, P and org. C. Other studies have used 137Cs and other bomb-based radioisotopes (e.g. 210Pb, in particular) for wetland soil sequestration-rate analyses (e.g. Reddy et al. 1993; Craft and Richardson 1998; Efremova et al. 2002; Bernal and Mitsch 2012; Craft 2012; Cabezas et al. 2014).
Wetlands known as geographically isolated or closed depressional systems occur throughout the United States in varying land uses (Tiner 2003b; Lane and D’Amico 2016), and, as such, are exposed to differing rates of atmospheric and terrestrial inputs of nutrients and C (and other entrained constituents). In the present study, we analysed and report nutrient (N, P) and organic-C accumulation rates and sediment accretion rates in depressional wetlands at the study sites spanning three ecoregions of the United States. Understanding the rates at which depressional wetland systems sequester or otherwise process nutrients and C can improve our understanding and assessment of the functions that these systems provide and help better manage watersheds for sustainable futures.
Materials and methods
Sites sampled
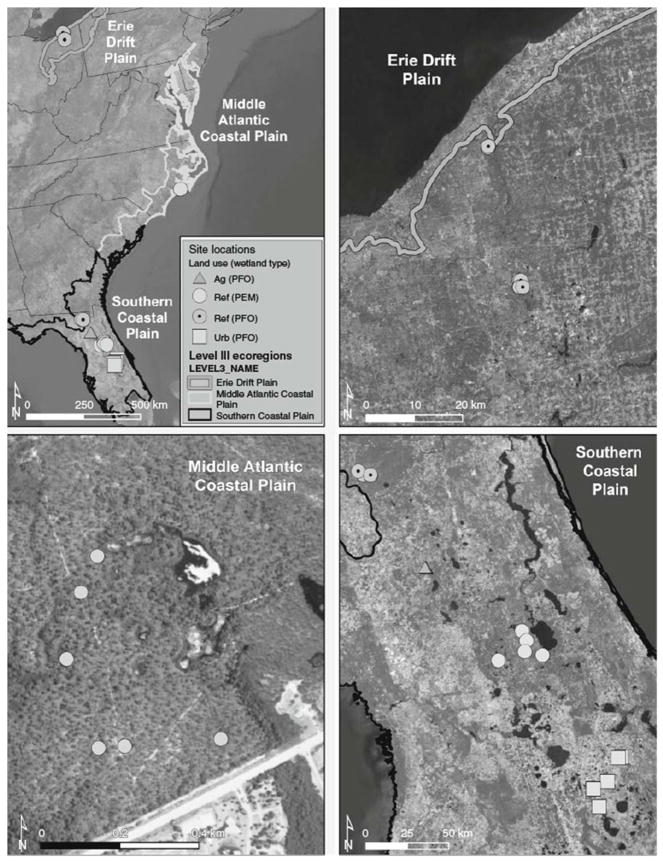
Our research addressed quantifying and contrasting differences in soil parameter and accretion rates both at sites minimally affected by human alterations and at those with a substantive human effect. For our analyses, we identified 30 minimally affected depressional wetland sites sampled once in 2010 across 3 different Omernik (1987) ecoregions, including 12 sites in the Erie Drift Plain (EDP) of Ohio, 6 sites in the Middle Atlantic Coastal Plain (MACP) and 12 sites in Florida’s Southern Coastal Plain (SCP, Fig. 1). These sites included both predominantly forested (i.e. palustrine, forested; PFO) and emergent marsh wetlands (i.e. palustrine, emergent marsh; PEM; sensu Cowardin et al. 1979), with 17 forested sites and 13 emergent marsh-wetland sites sampled; however, these were not distributed evenly among the studied ecoregions (i.e. no emergent marsh wetlands were sampled in the EDP, and no forested wetlands were sampled in the MACP).
Fig. 1.
Wetland depression sites sampled (n = 43) across multiple Omernik (1987) ecoregions include those in reference (ref), agricultural (ag) and urban (urb) land-use modalities, and both palustrine forested (PFO) and emergent marsh (PEM) wetlands.
To explore how differing land uses and site disturbance can affect accretion and accumulation rates, we added a small number of forested wetland sites sampled a single time in 2011 in urban settings (n = 6) and agricultural settings (n = 7) located in Florida within the SCP (see Fig. 1). The sites sampled (n = 43) ranged in size from 0.01 to 12.25 ha, with an average size of 1.02 ± 2.16. All were identified as depressional and non-floodplain wetlands, with no visible channels or regular surface-water connections to other waters, frequently described as ‘geographically isolated wetlands’ (Tiner 2003a; Leibowitz 2015; Mushet et al. 2015), although we acknowledge that these wetlands are unlikely to be completely functionally isolated (Cohen et al. 2016).
Sites denoted as minimally affected or ‘reference’ (n = 30) were located in local and state parks and national forests. These sites were typically hundreds of metres from visible alterations such as roads and had no known active management (e.g. burning, mowing, other manipulations). Urban wetlands were located within the Greater Orlando, Florida, area and appeared to be natural wetlands encroached on by development. That is, they were not planted nor built, but rather were built around over time. Urban sites were surrounded by medium- and high-density residential and industrial (i.e. warehouse) land uses (e.g. Lane et al. 2012; McCauley et al. 2013), with abundant roads or ditches creating systems disconnected from typical overland excess-capacity flows. The wetlands sampled in agricultural settings were located in a cattle-rearing educational research ranch where ranching operations allowed cattle unfettered access to the wetlands.
Soil sampling methodology
A single core sample was taken in an area representative of the approximate wetland centre at each location, by using a slide hammer and a 75 × 200-mm stainless-steel coring device with a butyrate liner. The core sampler was hammered into the wetland soil and the extracted core was frozen at −20°C. Because of resource limitations, only the first 100 mm of the frozen core was sectioned into 20-mm slices for analyses, each having a volume of ~88.3 cm3. This resulted in five 20-mm slices per site (i.e. Slices A–E; Zhang et al. 2016). Each slice was air-dried, homogenised, and then subsampled for radioisotope, nutrient and carbon analyses.
Carbon and nutrient analyses
Air-dried soil slices were weighed for bulk-density measures. Slices were analysed for percentage organic matter (%OM) by using the loss-on-ignition method wherein an air-dried subsample was heated at 105°C for 2 h to correct for soil moisture, cooled in a desiccator and weighed, and then heated to 350°C in a muffle furnace, and similarly cooled and weighed again. The %OM was calculated as the difference between the initial soil moisture-corrected sample and the final sample, divided by the initial soil moisture-corrected weight and multiplied by 100. Following Pribyl (2010), the abundance of org. C in each sample was assumed to be 50% the OM content. Percentage N was analysed with an elemental analyser, isotope ratio mass-spectrometer (Elemental Analyzer NC2500, CE Elantech, Lakewood, NJ, USA). Total P was colourimetrically analysed on a flow-injection analyser (Lachat Instruments, Loveland, CO, USA) using H2SO4 and persulfate digestion following Liess and Hillebrand (2005).
Caesium analyses and accretion-rate calculations
Caesium-137 concentration was determined by gamma spectroscopy analysis of the 661.7-keV photopeak for 137Cs (Canberra Genie 2000 Alpha Spectrometer HPGe Gamma detector, Meriden, CT, USA). Samples were prepared for gamma spectroscopy by air-drying and passing through a 250-μm standard sieve. Between 30 and 200 g of each sample was placed in the γ-detector and disintegrations per minute (pCi g−1 min−1) were counted over a period from 200 to 500 min. Peak 137Cs activity was ascribed to the slice with the greatest disintegrations per minute, attributed to deposition that occurred in 1964. As each slice was ~20 mm in depth, the midpoint was used for accretion analyses (e.g. 10 mm for Slice A, with a depth of 0–20 mm; 30 mm for Slice B, with a depth from 20 to 40 mm, and so on). Accretion rates were calculated by dividing the midpoint depth where the 137Cs peak occurred by the years since peak atmospheric deposition, attributed to 1964. For instance, if peak 137Cs occurred in Slice B, the nominal midpoint depth of 30 mm was given and divided by 46 years, resulting in an accretion rate of 0.65 mm year−1. Note that this example is for samples collected in 2010 and that, for samples collected in 2011, the denominator was 47 years. Phosphorus accumulation rates (AR) were calculated as follows, according to Craft and Casey (2000):
| (1) |
where P ARsite is the phosphorus accumulation rate (g P m−2 year−1) for a given site; ARsite is the accretion rate (mm year−1) for a site, based on 137Cs peak location within Slices A–E and years since global peak 137Cs deposition; BDsite is the average bulk density (g cm−3) for a site, including slice where peak 137Cs was found and those above; NCsite is the average nutrient concentration (mg P g−1) for a site, including slice where peak 137Cs was found and those above.
Nitrogen and organic-C accumulation rates were calculated similarly, as follows:
| (2) |
where N, org. C, ARsite is the nitrogen or org. C accumulation rate (g N (or org. C) m−2 year−1) for a given site; ARsite is the accretion rate (mm year−1) for a site, based on 137Cs peak location within Slices A–E and years since global peak 137Cs deposition; BDsite is the average bulk density (g cm−3) for a site including slice where peak 137Cs was found and those above; NCsiteN, org. C is the average fraction N or org. C at and above slice where peak 137Cs was found, multiplied by the average mass (g) of the same slices.
Statistical analysis
We tested for ecoregional effects (EDP v. MACP v. SCP reference sites, n = 30), within-region wetland type (PFO v. PEM of reference sites in the SCP, n = 12), and within-region land-use effects (urban v. agricultural v. reference in the SCP, n = 25) on soil parameters and accretion rates, using the non-parametric Kruskal–Wallis and Mann–Whitney U-tests when comparing between three or two groups respectively in SAS (SAS version 9.4, Cary, North Carolina, USA). Because of the small sample sizes and voids, we were not able to test for interactions (e.g. how wetland type and land use interact to affect accretion rates). When significant (P < 0.05) differences were found with the Kruskal–Wallis test, we subsequently determined differences among groups using Tukey’s honest significant difference test (Tukey’s HSD) using SAS.
Results
We collected and analysed 215 slices from the 43 sites located throughout the EDP, MACP and SCP ecoregions. Sites were analysed for soil parameters on a per-slice basis, and both accretion rates and nutrient accumulation rates were calculated using peak 137Cs. Five sites were not used in the accumulation or accretion analyses, because they either had no peak 137Cs identified within the top 100 mm (i.e. OH-08, a reference site; and FL2-01, FL2-02, FL2-12) or had two 137Cs peaks (i.e. FL2-09), suggesting bioturbation.
Reference sites in the EDP, MACP and ACP
Soil core characteristics for P, N and C across ecoregions
Reference sites (n = 30) were sampled in 2010 in the EDP, MACP and SCP. Mean core bulk density differed by ecoregion (Kruskal–Wallis χ2 = 10.4387, P = 0.0054), with the MACP having significantly higher bulk density than either the EDP or SCP, which did not differ from each other (Table 1). The average core soil P concentration (mg P g−1 soil; i.e. the average across the five 20-mm slices that comprise the 100-mm core) was significantly different among the ecoregions (Kruskal–Wallis χ2 = 16.6129, P = 0.002), being highest in the EDP (0.631 ± 0.484 mg P g−1), which was significantly greater than that in the SCP and almost 14 times higher than P concentrations in the MACP. Percentage N (Kruskal–Wallis χ2 = 11.5129, P = 0.0032) and org. C (Kruskal–Wallis χ2 = 11.1097, P = 0.0039) also differed among ecoregions; the MACP had the lowest average core %N (0.4 ± 0.2%), being significantly less than that in both the EDP and SCP, which did not differ from each other. Cores sampled from EDP and SCP had similarly high %org. C (14.5 ± 7.8% and 18.7 ± 9.3% respectively) relative to MACP (4.3 ± 2.8%), which was also significantly lower than that of the SCP and EDP.
Table 1. Reference-site soil characteristics (mean ± s.d.) by Omernik (1987) ecoregion.
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Variable | Erie Drift Plain (n = 12) | Middle Atlantic Coastal Plain (n = 6) | Southern Coastal Plain (n = 12) |
|---|---|---|---|
| Bulk density (g cm−3) | 0.41 ± 0.25a | 0.89 ± 0.20b | 0.37 ± 0.25a |
| Total phosphorus (mg Pg−1 soil) | 0.631 ± 0.484a | 0.046 ± 0.024b | 0.272 ± 0.208b |
| Percentage nitrogen | 1.2 ± 0.5b | 0.4 ± 0.2a | 1.4 ± 0.8b |
| Percentage organic carbon | 14.5 ± 7.8b | 4.3 ± 2.8a | 18.7 ± 9.3b |
Soil core characteristics for P, N and org. C and wetland typology in the SCP
Differences by wetland type in reference settings were tested in the SCP only (Table 2), and we found no difference in P concentration (mg P g−1) between forested and emergent marsh wetlands (Wilcoxon–Mann–Whitney Z = −1.2010, P = 0.2298). However, emergent marsh depressions had significantly greater %N (Wilcoxon–Mann–Whitney Z = −2.3219, P = 0.0202) and %org. C (Wilcoxon–Mann–Whitney Z = −2.3219, P = 0.0202) than did forested wetlands. Bulk density was significantly greater in the forested wetlands than in emergent marsh wetlands in the SCP (Wilcoxon–Mann–Whitney Z = 2.5621, P = 0.0104).
Table 2. Reference-site soil characteristics (mean ± s.d.) in the Southern Coastal Plain ecoregion.
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Variable (±s.d.) | Palustrine forested wetlands (n = 6) | Palustrine emergent marsh wetlands (n = 6) |
|---|---|---|
| Bulk density (g cm−3) | 0.55 ± 0.23a | 0.20 ± 0.08b |
| Total phosphorus (mg Pg−1 soil) | 0.253 ± 0.274a | 0.290 ± 0.139a |
| Percentage nitrogen | 0.8 ± 0.6a | 2.0 ± 0.5b |
| Percentage organic carbon | 13.0 ± 7.0a | 24.5 ± 7.8b |
Peak 137Cs and accretion rates
There were no significant differences in peak 137Cs or accretion rates among the ecoregions (Kruskal–Wallis χ2 = 4.4822, P = 0.1063, Table 3). Peak 137Cs was found for 11 of 12 EDP wetlands; the one outlier (OH-12) had an increasing 137Cs concentration at 100 mm from the surface, which was the maximum depth in our analyses. Four EDP wetlands had peaks in the first slice (0–20-mm depth) and four had peaks in the third (from 40 to 60 mm), whereas three wetlands had peaks in the fifth slice (from 80 to 100 mm) below the surface. Using the middle value of the slice depth (e.g. 10 mm for the first slice, 30 mm for the second, and so on), the average peak 137Cs depth in the EDP was 28.2 ± 16.6 mm and the accretion rate for EDP wetlands was calculated to be 0.6 ± 0.4 mm year−1. All but one of the six MACP wetlands had peak 137Cs in the first (from 0 to 10 mm) slice. The one outlier had a 137Cs peak in the slice that was ~20–40 mm below the surface (nominal peak of 30 mm). Across the six MACP samples, the average 137Cs peak depth was 13.3 ± 8.2 mm and the calculated accretion rate was 0.3 ± 0.2 mm year−1. Reference wetlands in SCP returned peak 137Cs values of 30.0 ± 19.1 mm. Most (5 of 11) sampled SCP wetlands had a 137Cs peak in the second slice (20–40 mm from the surface), although four SCP wetlands had the peak in the first slice. One sample had the peak in the fourth slice, 60–80 mm from the surface. The average accretion rate for SCP wetlands was determined to be 0.7 ± 0.4 mm year−1.
Table 3. Peak caesium-137 (137Cs) depths (mean ± s.d.) and calculated accretion rates across the ecoregions.
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Variable mean (±s.d.) | Erie Drift Plain (n = 11) | Middle Atlantic Coastal Plain (n = 6) | Southern Coastal Plain (n = 12) |
|---|---|---|---|
| 137Cs peak depth (mm) | 28.2 ± 16.6a | 13.3 ± 8.2a | 30.0 ± 19.1a |
| Accretion rate (mm year−1) | 0.6 ± 0.4a | 0.3 ± 0.2a | 0.7 ± 0.4a |
Contrasting peak 137Cs depths in SCP forested and emergent marsh wetlands found greater 137Cs peak depths in emergent marsh (33.3 ± 23.4 mm) than forested wetlands (26.7 ± 15.1 mm; Table 4), although these data were not significantly different. This led to slightly greater, although not significantly, accretion rates in emergent marshes (0.7 mm year−1) than in forested systems (0.6 mm year−1; Wilcoxon–Mann–Whitney Z = −0.3392, P = 0.7345).
Table 4. Peak caesium-137 (137Cs) and calculated accretion rates (mean ± s.d.) for palustrine forested and emergent marsh reference wetlands within the Southern Coastal Plain (SCP).
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Variable mean (±s.d.) | SCP palustrine forested wetlands (n = 6) | SCP palustrine emergent marsh wetlands (n = 6) |
|---|---|---|
| 137Cs peak depth (mm) | 26.7 ± 15.1a | 33.3 ± 23.4a |
| Accretion rate (mm year−1) | 0.6 ± 0.3a | 0.7 ± 0.5a |
Non-reference sites in the SCP
Soil-core characteristics for P, N and org. C in the SCP
When analysing non-reference sites sampled from the SCP in 2011 (Table 5), we found that agricultural sites had higher P concentrations than did urban sites, with the values in agricultural sites almost four times that of urban sites (0.446 ± 0.188 mg P g−1 and 0.123 ± 0.030 mg P g−1 respectively) and twice those of reference sites (0.272 ± 0.208 mg P g−1). These P concentrations were significantly different (Kruskal–Wallis χ2 = 10.2921, P = 0.0058); urban-site P concentration in soil differed from that in agricultural sites (Tukey’s HSD, P < 0.05), but there were no differences between urban sites and reference sites or reference and agricultural sites for P. No differences were found for %N (Kruskal–Wallis χ2 = 2.4629, P = 0.2919), %org. C (Kruskal–Wallis χ2 = 3.6514, P = 0.1611) or bulk density (Kruskal–Wallis χ2 = 5.0714, P = 0.0792) among sites sampled in various land-use modalities in the SCP.
Table 5. Soil characteristics (mean ± s.d.) of reference and non-reference sites in the Southern Coastal Plain ecoregion.
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Variable | Reference sites (n = 12) | Agricultural sites (n = 7) | Urban sites (n = 6) |
|---|---|---|---|
| Total phosphorus (mg Pg−1 soil) | 0.272 ± 0.208a,b | 0.446 ± 0.188a | 0.123 ± 0.030b |
| Percentage nitrogen | 1.4 ± 0.8a | 1.1± 0.7a | 0.8 ± 0.3a |
| Percentage organic carbon | 18.7 ± 9.3a | 19.4 ± 15.3a | 9.6 ± 3.3a |
| Bulk density (g cm−3) | 0.37±0.25a | 0.55 ± 0.33a | 0.68 ± 0.16a |
Peak 137Cs and accretion rates in the SCP
Three of 13 non-reference sites in the SCP did not have peaks within the first 100 mm from the surface, and one site had a peak in the first slice and a second peak in the fifth slice. Data from these four sites were not used in the accretion-rate analyses. Of the remaining non-reference sites (n = 9) contrasted with SCP reference sites (n = 12), we found that non-reference SCP sites were more prone to deeper 137Cs peak values, although there were no significant differences in peak 137Cs depth or accumulation rates among reference, agricultural or urban sites (Kruskal–Wallis χ2 = 0.2119, P = 0.8995; Table 6). Sites in agricultural settings had average 137Cs peak depths of 40.0 mm (± 25.8 mm), whereas those in urban settings had peak depths of 34.0 mm (± 16.7 mm); however, we used only four cores for agricultural accretion-rate calculations and five for urban sites. Calculated accretion rates were, thus, higher in agricultural settings (0.9 ± 0.5 mm year−1) than in urban or reference settings (both 0.7 ± 0.4 mm year−1), although this difference was not significant.
Table 6. Contrasting peak caesium-137 (137Cs, mean ± s.d.) and calculated accretion rates for Southern Coastal Plain reference and non-reference wetlands.
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Variable | Reference sites (n = 12) | Agricultural sites (n =4) | Urban sites (n = 5) |
|---|---|---|---|
| 137Cs peak depth (mm) | 30.0 ± 19.1a | 40.0 ± 25.8a | 34.0 ± 16.7a |
| Accretion rate (mm year−1) | 0.7 ± 0.4a | 0.9 ± 0.5a | 0.7 ± 0.4a |
Accumulation rates of N, P, org. C and sediment
Contrasting reference-site areal accumulation rates across ecoregions
We calculated areal nutrient (N, P) and org. C accumulation rates across the ecoregions sampled. Phosphorus accumulation rates differed among ecoregions (Kruskal–Wallis χ2 = 11.3706, P = 0.0034), driven by significantly greater accumulation rates for P in the EDP (Table 7). No differences in P accumulation rates were found between MACP or SCP wetlands, which averaged 0.03 ± 0.03 g P m−2 year−1 (n = 18). Accumulation rates for N and org. C were greater in both the MACP and SCP than in the EDP; however, these differences were not significant (%N Kruskal–Wallis statistic χ2 = 0.3580, P = 0.8361; %org. C Kruskal–Wallis χ2 = 0.9994, P = 0.6067). Across the reference sites sampled, accumulation rates for N and org. C averaged 3.1 ± 3.1 g N m−2 year−1 and 43.4 ± 39.0 g org. C m−2 year−1respectively.
Table 7. Nutrient (nitrogen, N, and phosphorus, P) and organic carbon accumulation rates (mean ± s.d.) calculated for the ecoregions analysed in the present study.
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Nutrient accumulation rate | Erie Drift Plain (n = 12) | Middle Atlantic Coastal Plain (n = 6) | Southern Coastal Plain (n = 12) |
|---|---|---|---|
| P (g m−2year−1) | 0.10 ± 0.10a | 0.01 ± 0.01b | 0.04 ± 0.04b |
| N (g m−2 year−1) | 2.7 ± 2.3a | 3.7 ± 3.5a | 3.2 ± 3.8a |
| Organic C (g m−2year−1) | 35.8 ± 24.9a | 44.4 ± 22.4a | 50.0 ± 54.5a |
Effect of wetland type on reference-site accumulation rates in the SCP
Accumulation rates between palustrine forested and palustrine emergent marsh reference wetlands in the SCP were contrasted. No significant differences were found for any of the comparisons (P: Wilcoxon–Mann–Whitney Z = 0.0801, P = 0.9362; N: Wilcoxon–Mann–Whitney Z = −0.4003, P = 0.6889; org. C: Wilcoxon–Mann–Whitney Z = 0.2402, P = 0.8102); however, we note that the org. C accumulation rate in forested wetlands was nearly twice that of emergent marshes (Table 8).
Table 8. Nutrient (phosphorus, P, and nitrogen, N) and organic carbon accumulation rates (mean ± s.d.) calculated within forested and emergent marsh wetlands sampled in the Southern Coastal Plain (SCP).
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Nutrient | Palustrine forested wetlands (n = 6) | SCP palustrine emergent marsh wetlands (n =6) |
|---|---|---|
| P (g m−2year−1) | 0.05 ± 0.05a | 0.04 ± 0.03a |
| N (g m−2year−1) | 3.9 ± 5.3a | 2.5 ± 1.5a |
| Organic C (g m−2year−1) | 66.1 ± 74.1a | 33.8 ± 20.2a |
Contrasting non-reference areal accumulation rates within the SCP
No significant differences were found for P accumulation rates between reference, agricultural, or urban sites sampled in the SDP (Table 9, Kruskal–Wallis χ2 = 5.3649, P = 0.0684). Results of the Kruskal–Wallis tests indicated significant differences between SCP sites for both N and organic C accumulation rates (Kruskal–Wallis χ2 = 8.5268, P = 0.0141, and Kruskal–Wallis χ2 = 7.1567, P = 0.0273 respectively). In both cases, reference sites had ~50% of the accumulation rates for N and org. C (see Table 9). However, analyses with Tukey’s HSD test did not successfully differentiate which of the three categories was significantly different.
Table 9. Nutrient (phosphorus, P, and nitrogen, N) and organic carbon accumulation rates (mean ± s.d.) calculated within forested and emergent marsh wetlands sampled in the Southern Coastal Plains ecoregion.
Values within a row followed by the same letter do not differ from one another at P = 0.05
| Nutrient | Reference sites (n = 12) | Agricultural sites (n = 4) | Urban sites (n = 5) |
|---|---|---|---|
| P (g m−2year−1) | 0.04 ± 0.04a | 0.26 ± 0.22a | 0.05 ± 0.02a |
| N (g m−2year−1) | 3.2 ± 3.8A | 7.2 ± 2.6A | 8.2 ± 5.1A |
| Organic C (g m−2year−1) | 50.0 ± 54.5A | 116.7 ± 43.9A | 109.5 ± 59.6A |
A significant group mean difference was found, but the source of the difference could not be discemed
Discussion
Analyses of the biogeochemical functions associated with depressional wetlands have identified substantial soil biogeochemical processing rates (see review by Marton et al. 2015), although few have used discrete bomb-based markers to quantify process rates in wetland depressions on a yearly basis (e.g. Freeland et al. 1999; Craft and Casey 2000; Bernal and Mitsch 2012). Understanding wetland nutrient and C-processing rates informs our understanding of the roles that wetlands play in both local- and landscape-level hydrological and biogeochemical dynamics. Armed with such knowledge, resource managers and the public can make well informed decisions on the impacts that wetland conservation or drainage can have on managed waterways. For instance, wetlands tend to have higher nutrient processing and assimilation rates than does the upland matrix they are embedded within (Cheesman et al. 2010, Marton et al. 2015). Altering wetland hydrology by drainage or filling in a wetland creates drier environments more akin to uplands, which can decrease the processing ability of wetlands. In addition, drainage activities in particular may bypass wetland processing and shunt nutrient-loaded waters to ditches and waterways (e.g. Arango and Tank 2008), with potential deleterious effects.
Outputs from research on a resource, such as depressional wetlands, with many different types found across the country (e.g. Tiner 2003a; Comer et al. 2005), can serve to improve models using such data at the landscape scale, while concurrently identifying data gaps. For instance, hydrologists have been exploring the hydrological implication of depressional wetland water storage (ostensibly from precipitation) on downstream systems, finding that the modelled effect of depressional wetlands is especially pronounced in certain times of the year (e.g. Golden et al. 2014; Fossey et al. 2015; Evenson et al. 2016). A logical next step in quantifying the effects of depressional wetlands on downstream systems is to assess the biogeochemical processing rates in wetland systems. Nutrients, as well as other solutes, are entrained in in-flows to wetlands. Quantifying the short-term processing rates (e.g. denitrification rates) as well as the longer-term accretion and accumulation rates begins to explain the watershed-scale, cumulative effects of wetlands. The US EPA (2015) found that it was likely that depressional wetlands that intersect flow paths from parts of the landscape prone to nutrient-laden runoff perform a significant service by removing N and P through biogenic and physical processes. The present study has supplemented the findings regarding longer-term depressional wetland processing and storage of N, P and org. C.
Contrasting findings by ecoregion
We analysed N, P and org. C concentrations and accumulation rates across three different ecoregions of the USA, using soil cores and 137Cs from nuclear-bomb fallout as a marker for wetland accretion and nutrient accumulation since 1964. Wetlands sampled in the MACP had approximately twice the bulk density of EDP or SCP sites, but were otherwise depauperate when contrasted with EDP and SCP sites for concentrations of N and org. C. MACP sites had less OM and were likely to have a greater sand concentration than did EDP or SCP sites (C. R. Lane and B. C. Autrey, pers. obs.), which was reflected by a decreased concentration in org. C and approximately one-third of the %N, as well as decreased P relative to that in the EDP and SCP (see Table 1). Higher sand content would also lead to lower soil moisture and, potentially, greater aerobic conditions, increasing C and P mineralisation rates. Values for peak 137Cs and accretion rates from the MACP were similarly lower than those from the EDP or SCP, being approximately half those of the other ecoregions; however, these differences were not significant (see Table 2). Our mean accretion rate across reference sites (0.6 ± 0.4 mm year−1) was approximately equal to that found in Georgia wetland depressions analysed by Craft and Casey (2000), but less than that reported for wetland depressions in Ohio (4.5 mm year−1; Bernal and Mitsch 2012). Bernal and Mitsch (2012) ascribed the high rates of accretion at their sites, which were also higher than those at riverine sites they analysed, to abundant deposition of recalcitrant tree litter and high rates of endogenous production.
Soil characteristics of the sites sampled may have also affected our accretion-rate results. For instance, we did not analyse soil pH in the present study; however, previous analyses have shown depressional wetland sites in SCP and MACP having soil pH values of <4.0 (Lane et al. 2015). Craft and Richardson (1998) reported that 137Cs dating techniques might not work effectively in acid organic soils because either H+ or aluminium species are preferentially absorbed over 137Cs. All but one of our MACP sites had the highest 137Cs levels in the first slice (i.e. also the region of the greatest OM, averaging 23.3 ± 15.1% OM in Slice A, and linearly decreasing by ~50% at each interval, to 1.3 ± 1.2% at Slice E, the deepest slice in the present analysis; Table 10), suggesting that 137Cs may have been translocated elsewhere in the soil profile, and was, perhaps, affecting the results from the MACP. However, wetland soils from depressional wetlands in the SCP, much like those from the MACP, were likely to have acidic pH values and organic soils, but also demonstrated clear 137Cs peaks (e.g. Fig. 2). The use of alternative radiometric dating methodologies, such as 210Pb, can help corroborate Cs-based analyses; however, this was not performed in the present study because of funding constraints.
Table 10. Soil characteristics for each soil slice by ecoregion (n = 30), with average values provided (s.d. in parentheses).
Note that only reference sites are reported for the Southern Coastal Plain
| Ecoregion and slice letter | Depth from surface (mm) | Bulk density (g cm−3) | Total phosphorus (mg g−1 soil) | Organic matter (%) | Nitrogen (%) |
|---|---|---|---|---|---|
| Erie Drift Plain | |||||
| A | 0–20 | 0.31 (0.24) | 0.576 (0.305) | 33.8 (13.8) | 1.4 (0.6) |
| B | >20–40 | 0.34 (0.21) | 0.596 (0.373) | 31.7 (14.6) | 1.4 (0.5) |
| C | >40–60 | 0.39 (0.23) | 0.579 (0.510) | 28.8 (15.2) | 1.2 (0.5) |
| D | >60–80 | 0.50 (0.33) | 0.723 (0.687) | 25.7 (16.2) | 1.1 (0.5) |
| E | >80–100 | 0.50 (0.35) | 0.712 (0.723) | 26.2 (18.8) | 1.0 (0.6) |
| Middle Atlantic Coastal Plain | |||||
| A | 0–20 | 0.56 (0.36) | 0.109 (0.063) | 23.3 (15.1) | 0.7 (0.3) |
| B | >20–40 | 0.70 (0.29) | 0.054 (0.031) | 10.3 (8.4) | 0.3 (0.2) |
| C | >40–60 | 0.90 (0.20) | 0.029 (0.013) | 5.0 (3.4) | 0.2 (0.2) |
| D | >60–80 | 1.08 (0.18) | 0.022 (0.012) | 2.4 (2.2) | 0.1 (<0.1) |
| E | >80–100 | 1.19 (0.10) | 0.016 (0.007) | 1.3 (1.2) | 0.6 (0.7) |
| Southern Coastal Plain | |||||
| A | 0–20 | 0.17 (0.06) | 0.429 (0.239) | 57.0 (15.5) | 1.8 (0.6) |
| B | >20–40 | 0.26 (0.20) | 0.325 (0.212) | 44.0 (21.6) | 1.5 (0.8) |
| C | >40–60 | 0.41 (0.32) | 0.225 (0.203) | 32.5 (25.7) | 1.3 (0.9) |
| D | >60–80 | 0.48 (0.33) | 0.204 (0.212) | 28.4 (22.6) | 1.2 (0.9) |
| E | >80–100 | 0.55 (0.40) | 0.176 (0.207) | 25.1 (21.5) | 1.2 (0.9) |
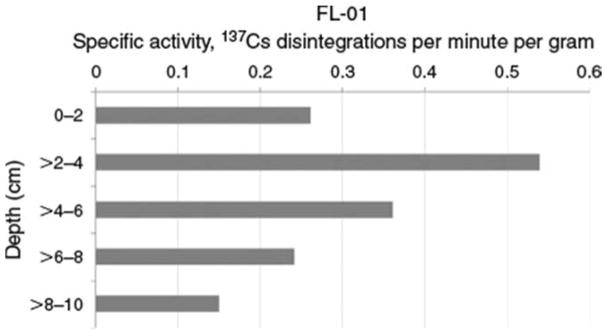
Fig. 2.
Example of disintegrations per minute typical of sites assessed in the present study; FL-01 is a palustrine forested wetland located in Osceola National Forest, Florida.
The mean nutrient and organic-C accumulation values differed across the ecoregions only sampled for P (see Table 7), with the EDP having mean P retention (0.10 g P per m−2 year−1) values 2.5–10 times greater than either the MACP or the SCP. The EDP has a shorter growing period and longer winters than do the MACP and SCP, with decreased microbial decomposition activity during the non-growing season, which may limit org. C and P mineralisation. Although clay content was not analysed, the soils in the EDP were also likely to have a substantial clay fraction (Fig. 3), which might have bonded more strongly to available P than did the wetland soils of the SCP and MACP, both of which often had sand interspersed within the sample (Kushlan 1990), with concomitantly fewer P sorption sites (Dunne et al. 2007; Cheesman et al.2010; Lane and Autrey 2016). In addition, soils with high clay fraction (e.g. the EDP, presumably) would create more tortuous pathways for near-surface interstitial water flow, thus concentrating P in the wetland depressions. This may explain the increase in P concentration with EDP wetland core depth (see Table 10), although increasing concentrations owing to soil compaction were likely to be a greater determinant. The EDP sites with high P concentration are unlike the wetlands sampled in the MACP and SCP, which are likely to be more intertwined with the local hydrology (sensu McLaughlin et al. 2014). For instance, Sun et al. (1995) reported that, depending on the season, forested depressions they hydrologically analysed in the SCP serve as recharge, flow-through or discharge wetlands, with substantive flow being generated across the periphery of the wetland. Thus, the repeated wetting and drying cycles de rigueur for depressional wetlands of the south-eastern USA, at least for portions of these ecosystems, would liberate absorbed P when anoxic conditions abound (e.g. during deluge conditions), and hydraulic heads could translocate P away from the wetland systems (Rains et al. 2016).
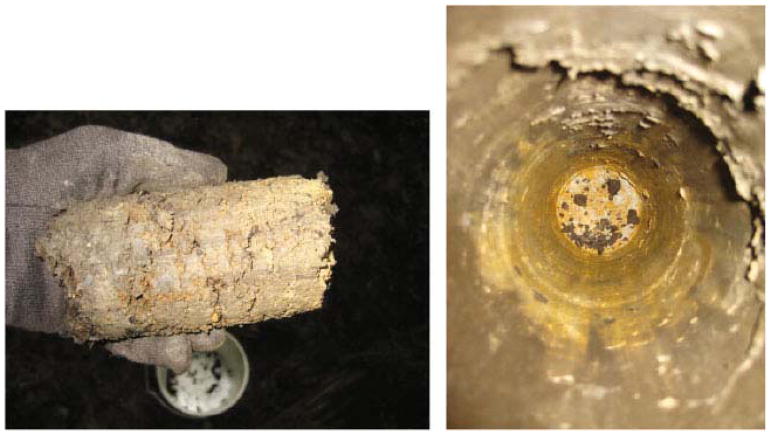
Fig. 3.
Cores sampled from sites in the Erie Drift Plain (EDP) appeared to have substantial clay fraction of the soil, which may provide sorption sites for available phosphorus, although this was not ascertained in the study. The left panel is an example of the cores extracted from EDP, whereas the right panel is a picture looking downward into the core hole.
Reference wetlands sampled in the present study (n = 30) had quantifiable P (0.06 ± 0.07 g P m−2 year−1), N (3.1 ± 3.1 g N m−2 year−1) and org. C (43.4 ± 39.0 g org. C m−2 year−1) accumulation rates. These values are approximate to other reported means for freshwater wetlands. For instance, Cabezas et al. (2014, p. 69) provided a lengthy table of org. C, N and P accumulation rates (g m−2 year−1) from the literature for fens, wetlands and other freshwater ecosystems, with the following conservative values (i.e. the median when a range is provided) of 0.9 g P m−2 year−1, 9.0 g N m−2 year−1 and 700.2 g C m−2 year−1. Deviation from these values would be expected, considering the range of hydrologic and soil (and sediment) characteristics in the review by Cabezas et al. (2014), including bogs, fens, floodplains, agricultural ponds, created wetlands and eutrophic lakes. A more approximate comparison for the present study might be that of Johnston (1991), who provided estimates of wetland accumulation rates for N (1.4–1.7 g N m−2 year−1) and P (0.30–0.57 g P m−2 year−1). Craft and Casey (2000), in their 137Cs analysis of the top 30 cm of soil in wetland depressions in south-western Georgia, USA, reported means (±s.e.) for P (0.08 ± 0.03 g P m−2 year−1), N (1.5 ± 0.6 g N m−2 year−1) and org. C (2 ± 8 g org. C m−2 year−1), demonstrating that there is substantial variation among wetland ecosystems. Craft (2012) reported 137Cs-derived P accretion rates of 0.29 ± 0.05 g P m−2 year−1 in tidal-freshwater forest wetlands, whereas Reddy et al. (1993) sampled nutrient-enriched and nutrient-limited areas of the Everglades, Florida, USA, and reported P accumulation rates ranging from 0.54 to 1.14 g P m−2 year−1 in enriched areas, and from 0.11 to 0.25 g P m−2 year−1 in nutrient-limited areas. The lower P-sequestration rates in the present study may reflect the small size, and the likely small catchment or contributing area, of the wetlands we sampled. Smaller contributing areas would result in less vegetative and organic soil material to be broken down and translocated (e.g. through precipitation, or aeolian events) from the terrestrial to the wetland ecosystem. However, there was no significant linear correlation between wetland size and P sequestration (Pearson r = −0.10, P = 0.6108), although we did not determine wetland catchments. Mean N accumulation rates in the present study (3.1 ± 3.1 g N m−2 year−1) were higher than those reported by Craft and Casey (2000) and Johnston (1991), but lower than the least nutrient-enriched areas of the Everglades sampled by Reddy et al. (1993; 5 g N m−2 year−1). Movement of N to wetlands occurs through atmospheric deposition and through breakdown of N-containing OM, among other processes (Reddy and DeLaune 2008). Sequestration of N occurs when OM is incorporated into the soil structure and not mineralised, or otherwise released and used by plants for growth, or importantly, by microbes as an electron acceptor. When wetland conditions are not conducive to biogeochemical transformation to N2, N accumulates in wetland systems and enters longer-term storage.
Our reference-site org.-C values (43.4 ± 39.0 g org. C m−2 year−1, n = 30) were ~21 times greater than those of Craft and Casey (2000), but much lower than average C sequestration in depressional forested (820 g C m−2 year−1) or emergent marshes (570 g C m−2 year−1) sampled to 80-cm depth by Marín-Muñiz et al. (2014) in coastal Mexico. The C values in the present study were also an order of magnitude lower than those reported by Bernal and Mitsch (2012) in wetland depressions in Ohio (317 ± 93 g C m−2 year−1; 98–99% of which the authors reported to be org. C). Bernal and Mitsch (2012, p. 1645) provided a literature-based assessment of average ‘isolated, depressional, or forested wetlands’ of 188 ± 33 g C m−2 year−1), with an increasing C accumulation being associated with longer hydroperiods and a greater organic-matter input (Eswaran et al. 1993). The smaller catchment areas and short hydroperiods reflective of the shallow wetland systems sampled in the present study may be reflected in the low C-sequestration values.
Wetland typology, land-use modality and soil characteristics
Wetland typology: forested and emergent marsh systems
Wetland vegetation can substantially affect, and be affected by, soil characteristics. Wetland structure responds to hydroperiod and concomitant factors such that the hydroperiod and hydropattern are frequently determinants of wetland typology (Sharitz and Gresham 1998; Miller and Zedler 2003; Foti et al. 2012). Differences in soil characteristics and biogeochemical processing rates have been reported between forested and emergent marsh wetlands (e.g. Ullah and Faulkner 2006; Dodla et al. 2008), including depressional wetland systems (e.g. Lane et al. 2015). We report greater SCP reference emergent marsh %N and %org. C, with values for forested wetlands of ~50% of those for emergent marsh systems (see Table 2). Although soil characteristics varied, the accretion rates between forested and emergent marsh wetlands did not differ, and, subsequently, the nutrient and organic-C accumulation rates, likewise, did not differ between wetland types, albeit these analyses were performed with a small sample size (n = 6 wetlands per wetland type). This may have resulted from the variability inherent within wetlands. Those classified in the field as ‘emergent marsh’ may, indeed, maintain a healthy abundance of forested or shrub scrub vegetation, for we classified sites on the basis of the prevalence of different wetland vegetation. However, vegetation litter breaks down at different rates depending on recalcitrant-C concentration, moisture levels and other factors (Battle and Golladay 2007). That no significant difference was found in accumulation or accretion rates among very different wetland systems composed of varying vegetation structure and type suggests that a worthy research activity would be the exploration of within-site variability in nutrient processing and accumulation rates (e.g. Cohen et al. 2008).
Wetland characteristics in differing land uses
We contrasted the concentration and accumulation rates for P, N and org. C across three land-use modalities in the SCP. Although we had low sample sizes for these analyses, we expected that nutrients would be higher in agricultural settings and lower in reference settings, with urban systems expressing no clear pattern because of the variability of the antecedent land-use before urbanisation (see McCauley et al. 2013). Only soil P differed by land use, with agricultural sites having the highest and urban sites having the lowest P concentrations, bracketing the reference-site values (see Table 5). Wetlands enriched with P through runoff from terrestrial systems may be subjected to repeated P mineralisation, vegetative and microbial uptake and incorporation into organic material, with very little P being likely to leave closed basin systems. Wetlands with elevated soil P may end up as sources for P in the future, should the wetland be connected to other systems through ditching or should inundation occur such that soil P is released into the water column. We hypothesise that the low P values for the urban sites might be related to urban hydrological factors, namely the propensity for urban sites to be frequently ‘hydrologically isolated’ from their variable source areas through proximity to impervious surfaces and frequent ditching (e.g. for mosquito control). This not only decreases the variable source area for these wetlands, thereby decreasing the possibility of P-laden soil entrainment with storm events, but also can alter the hydrology such that OM, a repository for P, is oxidised (Cheesman et al. 2010; Lane et al. 2015). Oxidised OM releases P, which can be either incorporated into vegetation structure or translocated from the system through overland or interstitial flow (see Sun et al. 1995).
No significant differences were observed for 137Cs peaks or accretion rates by land use, and although P concentrations were highest in agricultural systems, there was no difference in P accumulation rates, even though reference and urban sites accumulated ~20% the rate of agricultural sites. A significant difference was found for N and organic-C accumulation rates among the three land-use modalities, and even though our statistical tests failed to discern which modality differed (Table 9), it is clear that reference sites sequestered substantially less N and org. C, because both values are less than 50% the rate of urban and agricultural sites. This suggests that both elements could be limiting in the sampled reference wetland systems. As noted, N is frequently lost to denitrification. Decreased C at reference sites might reflect a high frequency of wetting and drying events or perhaps a higher likelihood of fires altering the C storage in reference depressional wetland systems. Natural areas in the south-east frequently maintain a high burn rate. For instance, Craft and Chiang (2002) reported a burn frequency in their study area of 1–3-years. Fires could be expected to enter the high-C pools of depressional wetland systems, at least occasionally, altering C storage. We did not investigate the historical record for sites with different land uses. Thus, we do not know when an urban area was built up, or whether particular agricultural lands were crop farmed or laid fallow for years. Similarly, our reference sites could have been in an actively logged area or otherwise affected by events occurring within approximately the past 50 years (i.e. since the 1964 atmospheric peak 137Cs). With a single core from our sites, and with small sampling sizes, stochastic events or simple chance could result in substantial differences by category (e.g. sampling a non-depositional area; sampling in a wetland that was historically burned).
Study limitations
Wetlands are inherently variable systems, and one core may not have fully captured the site variability. Hence, additional cores would certainly have been useful to bracket within-site spatial variability. In addition, we used the midpoint of the 20-mm slices where the peak 137Cs was found, for accretion and accumulation analyses. The potential temporal resolution of the 20-mm slice, a typical slice length in accumulation and accretion analyses using radioisotopes, did not allow us to discern whether the peak was at the top or bottom of the slice. We, therefore, used the midpoint (e.g. 10 mm, 30 mm, 50 mm, and so on) as the numerator for yearly accretion rates for Slices A, B and C,… (and so on). Although the statistical differences in accretion and accumulation rates among ecoregions, land use and wetland types described above would not change because of the non-parametric nature of the statistics employed, it is worth noting that there is a 35% decrease in reported accretion and, thus, accumulation rates when a more conservative accretion-rate location, namely, that of the top of the slice, is used. For instance, we calculated the overall accretion rate across all reference sites (n = 30) of 0.6 ± 0.4 mm year−1; this is revised downward to 0.4 ± 0.4 mm year−1 when, more conservatively, the top of the slice is used versus the midpoint.
In addition, Pribyl (2010) critically reviewed the limitations associated with assuming that 50% of the OM in soil samples is carbon, as we and others have assumed when these data are not available (e.g. Craft and Casey 2000). Although multiple factors affect the abundance of soil C in a sample, Pribyl (2010) noted that the range of org. C in OM may typically vary from ~35 to 63%. Therefore, although the statistics of our results for accumulation of org. C will not change because of their non-parametric nature, the accumulation of org. C in the wetlands may vary, ranging from 70% of the reported values to 126% of the reported values for org.-C accumulation rates, depending on the true value of the abundance of organic C in the sample. For instance, the reported accumulation rate of org. C in the EDP was 35.8 ± 24.9 g org. C m−2 year−1, assuming 50% org. C in the samples. This ranges from 25.0 to 45.1 g org. C m−2 year−1, depending on whether the value of 35 or 63% for the abundance of OM that is C is more appropriate for the particular soil-sample analysed. Nevertheless, the use of Pribyl’s (2010) standard 50% value for org. C in OM provides useful information to inform the management of depressional wetland resources.
Conclusions
Accumulation of nutrients and org. C occurs in wetland depressions, as detected through sediment accretion-rate analyses using 137Cs, a thermonuclear bomb-derived radioisotope. No differences in accretion rates were found across three ecoregions, or between wetland structural types or among land-use settings in a subset of the site samples. The lack of differentiation in accretion rates across ecoregions, which drives the nutrient and org.-C accumulation results, might indicate emerging geometric or variable source-area characteristics of wetland depressions at large spatial scales (e.g. Van Meter and Basu 2015; Cohen et al. 2016; Serran and Creed 2016). Contrasting the areal accumulation rates from reference settings across a limited number of samples in three ecoregions determined that only P rates varied significantly (i.e. in the EDP ecoregion), perhaps driven by an abundance of available sorption sites in the clayey wetland soils of the EDP. We did not find differences in accumulation rates between the typological wetland classes (e.g. forested or emergent marshes) from a subsample of sites in a given ecoregion either. The present study was conducted on a limited number of sites, but across a wide swath of the USA. The data are suggestive, should these analyses be typical of depressional wetlands within these ecoregions. For instance, estimates of the extent of these depressional wetland systems range from ~15 to 20% of the freshwater wetlands of the conterminous USA (Likens et al. 2000; Lane and D’Amico 2016). If one begins to assess both the nutrient accumulation rates of wetland depressions (e.g. as in the present paper and others referenced herein) as well as assimilation rates (e.g. through denitrification (e.g. Lane et al. 2015) or methane analyses (e.g. Badiou et al. 2011)), an emerging and substantive aggregate contribution of these small depressions is suggested (sensu Marton et al.2015; Cohen et al. 2016). Last, the findings suggest that additional analyses across greater spatial extents within the ecoregions would be useful to corroborate the results (e.g. greater sampling locations throughout the breadth of land-use and wetland typologies within a given ecoregion, as well as analyses within a given site).
Acknowledgments
We appreciate insightful comments from Jim Harvey and anonymous journal reviewers. This paper has been reviewed in accordance with the US Environmental Protection Agency’s peer and administrative review policies and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Statements in this publication reflect the authors’ professional views and opinions and should not be construed to represent any determination or policy of the US Environmental Protection Agency.
References
- Alexander LC. Science at the boundaries: scientific support for the Clean Water Rule. Freshwater Science. 2015;34:1588–1594. [Google Scholar]
- Arango CP, Tank JL. Land use influences the spatiotemporal controls on nitrification and denitrification in headwater streams. Journal of the North American Benthological Society. 2008;27:90–107. [Google Scholar]
- Badiou P, McDougal R, Pennock D, Clark B. Greenhouse gas emissions and carbon sequestration potential in restored wetlands of the Canadian prairie pothole region. Wetlands Ecology and Management. 2011;19:237–256. [Google Scholar]
- Battle J, Golladay S. How hydrology, habitat type, and litter quality affect leaf breakdown in wetlands on the Gulf Coastal Plain of Georgia. Wetlands. 2007;27:251–260. [Google Scholar]
- Bernal B, Mitsch WJ. Comparing carbon sequestration in temperate freshwater wetland communities. Global Change Biology. 2012;18:1636–1647. [Google Scholar]
- Besasie NJ, Buckley ME. Carbon sequestration potential at central Wisconsin Wetland Reserve Program sites. Soil Science Society of America Journal. 2012;76:1904–1910. [Google Scholar]
- Bhadha JH, Jawitz JW. Characterizing deep soils from an impacted subtropical isolated wetland: implications for phosphorus storage. Journal of Soils and Sediments. 2010;10:514–525. [Google Scholar]
- Cabezas A, Matthias P, Schönfelder I, Gelbrecht J, Zak D. Carbon, nitrogen, and phosphorus accumulation in novel ecosystems: shallow lakes in degraded fen areas. Ecological Engineering. 2014;66:63–71. [Google Scholar]
- Cheesman AW, Dunne EJ, Turner BL, Ramesh RK. Soil phosphorus forms in hydrologically isolated wetlands and surrounding pasture uplands. Journal of Environmental Quality. 2010;39:1517–1525. doi: 10.2134/jeq2009.0398. [DOI] [PubMed] [Google Scholar]
- Cohen MJ, Dunne EJ, Bruland GL. Spatial variability of soil properties in cypress domes surrounded by different land uses. Wetlands. 2008;28:411–422. [Google Scholar]
- Cohen MJ, Creed IF, Alexander L, Basu N, Calhoun A, Craft C, D’Amico E, DeKeyser E, Fowler L, Golden HE, Jawitz JW, Kalla P, Kirkman LK, Lane CR, Lang MW, Leibowitz SG, Lewis DB, Marton J, McLaughlin DL, Mushet D, Raanan-Kipperwas H, Rains MC, Smith L, Walls S. Do geographically isolated wetlands influence landscape function? Proceedings of the National Academy of Sciences of the United States of America. 2016;113:1978–1986. doi: 10.1073/pnas.1512650113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer P, Goodwin K, Tomaino A, Hammerson G, Kittel G, Menard S, Nordman C, Pyne M, Reid M, Sneddon L, Snow K. Biodiversity Values of Geographically Isolated Wetlands in the United States. Natureserve; Arlington, VA, USA: 2005. [Google Scholar]
- Cowardin LM, Carter V, Goulet FC, LaRoe ET. Classification of Wetlands and Deepwater Habitats of the United States. Fish and Wildlife Service; Washington DC., USA: 1979. [Google Scholar]
- Craft CB. Tidal freshwater forest accretion does not keep pace with sea level rise. Global Change Biology. 2012;18:3615–3623. [Google Scholar]
- Craft CB, Casey WP. Sediment and nutrient accumulation in floodplain and depressional freshwater wetlands of Georgia, USA. Wetlands. 2000;20:323–332. [Google Scholar]
- Craft CB, Chiang C. Forms and amounts of soil nitrogen and phosphorus across a longleaf pine-depressional wetland landscape. Soil Science Society of America Journal. 2002;66:1713–1721. [Google Scholar]
- Craft CB, Richardson CJ. Recent and long-term organic soil accretion and nutrient accumulation in the Everglades. Soil Science Society of America Journal. 1998;62:834–843. [Google Scholar]
- Dahl TE. Status and Trends of Wetlands in the Conterminous United States 2004 to 2009. Department of the Interior, Fish and Wildlife Service; Washington, DC, USA: 2011. [Google Scholar]
- Dodla SK, Wang JJ, DeLaune RD, Cook RL. Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. The Science of the Total Environment. 2008;407:471–480. doi: 10.1016/j.scitotenv.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Dunne EJ, Smith J, Perkins DB, Clark MW, Jawitz JW, Reddy KR. Phosphorus storages in historically isolated wetland ecosystems and surrounding pasture uplands. Ecological Engineering. 2007;31:16–28. [Google Scholar]
- Efremova TT, Sukhorukov FV, Efremov SP, Budashkina VV. Accumulation of Cs-137 in peatbogs on the Ob and Tom’ river interfluve. Eurasian Soil Science. 2002;35:91–98. [Google Scholar]
- Eswaran H, Van Den Berg E, Reich P. Organic carbon in soils of the World. Soil Science Society of America Journal. 1993;57:192–194. [Google Scholar]
- Evenson GR, Golden HE, Lane CR, D’Amico E. Geographically isolated wetlands and watershed hydrology: a modified model analysis. Journal of Hydrology. 2015;529:240–256. [Google Scholar]
- Evenson GR, Golden HE, Lane CR, D’Amico E. An improved representation of geographically isolated wetlands in a watershed-scale hydrologic model. Hydrological Processes. 2016;30:4168–4184. [Google Scholar]
- Fossey MR, Alain N, Bensalma F, Savary S, Royer A. Integrating isolated and riparian wetland modules in the PHYSITEL/HYDROTEL modelling platform: model performance and diagnosis. Hydrological Processes. 2015;29:4683–4702. [Google Scholar]
- Foti R, del Jesus M, Rinaldo A, Rodriguez-Iturbe I. Hydroperiod regime controls the organization of plant species in wetlands. Proceedings of the National Academy of Sciences, USA. 2012;109:19596–19600. doi: 10.1073/pnas.1218056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeland JA, Richardson JL, Foss LA. Soil indicators of agricultural impacts on northern Prairie wetlands: cottonwood Lake research area, North Dakota, USA. Wetlands. 1999;19:56–64. [Google Scholar]
- Gleason RA, Tangen BA, Laubhan MK, Kermes KE, Euliss NH. Open File Report 2007–1159. US Geological Survey; Reston, VA, USA: 2007. Estimating water storage capacity of existing and potentially restorable wetland depressions in a subbasin of the Red River of the North. [Google Scholar]
- Golden HE, Lane CR, Amatya DM, Bandilla KW, Raanan-Kiperwas H, Knightes CD, Ssegane H. Hydrologic connectivity between geographically isolated wetlands and surface water systems: a review of select modeling methods. Environmental Modelling & Software. 2014;53:190–206. [Google Scholar]
- Hey DL. Nitrogen farming: harvesting a different crop. Restoration Ecology. 2002;10:1–10. [Google Scholar]
- Johnston CA. Sediment and nutrient retention by freshwater wetlands: effects on surface water quality. Critical Reviews in Environmental Control. 1991;21:491–565. [Google Scholar]
- Kushlan JA. Freshwater marshes. In: Myers RL, Ewel JJ, editors. ‘Ecosystems of Florida’. University of Central Florida Press; Orlando, FL, USA: 1990. pp. 323–363. [Google Scholar]
- Lane CR, Autrey BC. Phosphorus retention of forested and emergent marsh depressional wetlands in differing land uses in Florida, USA. Wetlands Ecology and Management. 2016;24:45–60. [Google Scholar]
- Lane CR, D’Amico E. Potential geographically isolated wetlands of the Conterminous United States. Journal of the American Water Resources Association. 2016;52:705–722. [Google Scholar]
- Lane CR, D’Amico E, Autrey B. Isolated wetlands of the southeastern United States: abundance and expected condition. Wetlands. 2012;32:753–767. [Google Scholar]
- Lane CR, Autrey BC, Jicha T, Lehto L, Elonen C, Seifert-Monson L. Denitrification potential in geographically isolated wetlands of North Carolina and Florida, USA. Wetlands. 2015;35:459–471. [Google Scholar]
- Leibowitz S. Geographically isolated wetlands: why we should keep the term. Wetlands. 2015;35:997–1003. [Google Scholar]
- Liess A, Hillebrand H. Stoichiometric variation in C : N, C : P, and N : P ratios of littoral benthic invertebrates. Journal of the North American Benthological Society. 2005;24:256–269. [Google Scholar]
- Likens GE, Zedler J, Mitsch B, Sharitz R, Larson J, Fredrickson L, Pimm S, Semlitsch R, Bohlen C, Woltemade C, Hirschfeld M, Callaway J, Huffman T, Bancroft T, Richter K, Teal J and the Association of State Wetland Managers. Brief for Dr Gene Likens et al. as Amici Curiae of Writ of Certiorari to the United States Court of Appeals for the Seventh Circuit. Solid Waste Agency of Northern Cook County v. US Army Corps of Engineers, number 99-1178. 2000 Submitted by T. D. Searchinger and M. J. Bean, attorneys for Amici Curiae. Available at http://supreme.findlaw.com/supreme_court/briefs/99-1178/99-1178fo21/text.html [Verified 27 June 2017]
- Marín-Muñiz JL, Hernandez ME, Moreno-Casasola P. Comparing soil carbon sequestration in coastal freshwater wetlands with various geomorphic features and plant communities in Veracruz, Mexico. Plant and Soil. 2014;378:189–203. [Google Scholar]
- Marton JM, Creed I, Lewis D, Lane CR, Basu N, Cohen MJ, Craft C. Geographically isolated wetlands are important biogeochemical reactors on the landscape. Bioscience. 2015;65:408–418. [Google Scholar]
- McCauley LA, Jenkins DG, Quintana-Ascencio PF. Isolated wetland loss and degradation over two decades in an increasingly urbanized landscape. Wetlands. 2013;33:117–127. [Google Scholar]
- McLaughlin DL, Kaplan DA, Cohen MJ. A significant nexus: geographically isolated wetlands influence landscape hydrology. Water Resources Research. 2014;50:7153–7166. [Google Scholar]
- Miller RC, Zedler JB. Responses of native and invasive wetland plants to hydroperiod and water depth. Plant Ecology. 2003;167:57–69. [Google Scholar]
- Mushet D, Calhoun AJK, Alexander LC, Cohen MJ, DeKeyser ES, Fowler L, Lane CR, Lang MW, Rains MC, Walls SC. Geographically isolated wetlands: rethinking a misnomer. Wetlands. 2015;35:423–431. [Google Scholar]
- Omernik JM. Ecoregions of the Conterminous United States. Annals of the Association of American Geographers. 1987;77:118–125. [Google Scholar]
- Pribyl D. A critical review of the conventional SOC to SOM conversion factor. Geoderma. 2010;156:75–83. [Google Scholar]
- Rains MC, Leibowitz SG, Cohen MJ, Creed IF, Golden HE, Jawitz JW, Kalla P, Lane CR, Lang MW, McLaughlin DL. Geographically isolated wetlands are part of the hydrological landscape. Hydrological Processes. 2016;30:153–160. [Google Scholar]
- Reddy KR, DeLaune RD. Biogeochemistry of Wetlands: Science and Applications. CRC Press; Boca Raton, FL, USA: 2008. [Google Scholar]
- Reddy KR, Delaune RD, Debusk WF, Koch MS. Long-term nutrient accumulation rates in the Everglades. Soil Science Society of America Journal. 1993;57:1147–1155. [Google Scholar]
- Ritchie JC, McHenry JR. Application of radioactive fallout cesium-137 for measuring soil erosion and sediment accumulation rates and patterns: a review. Journal of Environmental Quality. 1990;19:215–233. [Google Scholar]
- Serran J, Creed IF. New mapping techniques to estimate the preferential loss of small wetlands on prairie landscapes. Hydrological Processes. 2016;30:396–409. [Google Scholar]
- Sharitz RR, Gresham CA. Pocosins and Carolina Bays. In: Messina MG, Conner WH, editors. Southern Forested Wetlands. Lewis Publishers; Boca Raton, FL, USA: 1998. pp. 343–377. [Google Scholar]
- Sun G, Riekerk H, Korhnak LV. Shallow groundwater table dynamics of cypress wetland pine upland systems in Florida flatwoods. Proceedings - Soil and Crop Science Society of Florida. 1995;54:66–71. [Google Scholar]
- Tiner RW. Geographically isolated wetlands of the United States. Wetlands. 2003a;23:494–516. [Google Scholar]
- Tiner RW. Estimated extent of geographically isolated wetlands in selected areas of the United States. Wetlands. 2003b;23:636–652. [Google Scholar]
- Ullah S, Faulkner SP. Denitrification potential of different land-use types in an agricultural watershed, lower Mississippi valley. Ecological Engineering. 2006;28:131–140. [Google Scholar]
- US EPA. Connectivity of Streams and Wetlands to Downstream Waters: a Review and Synthesis of the Scientific Evidence. US EPA Office of Research and Development; Washington, DC, USA: 2015. EPA/600/R-14/475F. [Google Scholar]
- Van Meter KJ, Basu NB. Signatures of human impact: size distributions and spatial organization of wetlands in the Prairie Pothole landscape. Ecological Applications. 2015;25:451–465. doi: 10.1890/14-0662.1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu X, Shao X, Chen C, Li X, Zhao F, Li G, Matsumoto E. Temporal variation of sedimentation rates and potential factors influencing those rates over the last 100 years in Bohai Bay, China. The Science of the Total Environment. 2016;572:68–76. doi: 10.1016/j.scitotenv.2016.07.172. [DOI] [PubMed] [Google Scholar]