Abstract
Background:
Cardiovascular bypass grafting is an essential treatment for complex cases of atherosclerotic disease. Because the availability of autologous arterial and venous conduits is patient-limited, self-assembled cell-only grafts have been developed to serve as functional conduits with off-the-shelf availability. The unacceptably long production time required to generate these conduits, however, currently limits their clinical utility. Here, we introduce a novel technique to significantly accelerate the production process of self-assembled engineered vascular conduits.
Methods:
Human aortic smooth muscle cells and skin fibroblasts were used to construct bi-level cell sheets. Cell sheets were wrapped around a 22.5 Gauge angiocath needle to form tubular vessel constructs. A thin, flexible membrane of clinically-approved biodegradable tissue glue (Dermabond Advanced™) served as a temporary, external scaffold, allowing for immediate perfusion and endothelialization of the vessel construct in a bioreactor. Subsequently, the matured vascular conduits were used as femoral artery interposition grafts in rats (n=20). Burst pressure, vasoreactivity, flow dynamics, perfusion, graft patency, and histological structure were assessed.
Results:
Compared to engineered vascular conduits formed without external stabilization, glue membrane-stabilized conduits reached maturity in the bioreactor in one-fifth the time. After only 2 weeks of perfusion, the matured conduits exhibited flow dynamics similar to that of control arteries, as well as physiologic responses to vasoconstricting and vasodilating drugs. The matured conduits had burst pressures exceeding 500 mmHg and had sufficient mechanical stability for surgical anastomoses. The patency rate of implanted conduits at 8 weeks was 100%, with flow rate and hindlimb perfusion similar to that of sham controls. Grafts explanted after 8 weeks showed a histological structure resembling that of typical arteries, including intima, media, adventitia, and internal and external elastic membrane layers.
Conclusions:
Our technique reduces the production time of self-assembled, cell sheet-derived engineered vascular conduits to 2 weeks, thereby permitting their use as bypass grafts within the clinical time window for elective cardiovascular surgery. Furthermore, our method utilizes only clinically-approved materials and can be adapted to various cell sources, altogether simplifying the path toward future clinical translation.
Keywords: coronary artery bypass grafting, bioengineered vascular graft, cell sheet, tissue glue, bioreactor
Introduction
Cardiovascular disease remains the most common cause of death worldwide, and the development of long-term revascularization therapies continues to be highly relevant.1 For cases of complex atherosclerotic disease, bypass grafting has proven to be the gold standard in both the cardiac and peripheral vascular arenas.2,3 In coronary artery bypass grafting (CABG), for example, autologous arterial or venous grafts have relatively high 10-year patency rates of 85% and 60%, respectively.4,5 However, their availability is patient-limited, and the progressive nature of cardiovascular disease often leads to a need for repeat revascularization procedures when autologous graft options are few.5 In recent decades, conduits made of synthetic materials have been developed, although studies have shown unacceptable long-term patency rates of only 30% after 2 years.6 Therefore, a new generation of bypass conduits that combines the patency and function of autologous grafts with the “off-the-shelf” availability of synthetic grafts could dramatically improve the long-term survival and clinical outcomes of patients with cardiovascular disease.
Recently, several methods of engineering biological vessel grafts have been developed.6,7 The application of smooth muscle cells and fibroblasts as cellular components of the graft wall results in sufficient durability for surgical anastomosis and permits an in vivo perfusion pressure similar to that of native arteries.8–10 Implanted conduits are partially re-populated with host cells when used as interposition grafts in animal models.11 The patency of engineered vascular grafts may also be increased by in vitro perfusion with human umbilical vein endothelial cells (HUVECs), which assemble into a continuous endothelial monolayer within the graft.12,13 Most of these approaches employ permanent or biodegradable scaffolds to stabilize the vascular construct and accelerate the production process.7,14,15 However, artificial scaffolds dramatically reduce vasoreactivity and decrease long-term patency rates.6 Moreover, most scaffolds are composed of materials that are not approved by the United States Food and Drug Administration (FDA), constituting a major obstacle for clinical translation.
To avoid these complications, cell-only approaches to engineering vessels have been tested using spheroids or cell sheets without artificial stabilization.16 For example, cell sheet-derived vessels have already been employed clinically as hemodialysis shunts.17
Engineering protocols for manufacturing cell-only vascular conduits comprise two maturation phases: first the cell sheets are cultured around a mandrel to allow for stabilization of the vascular constructs in a tubular geometry.16 Subsequently, the tubular constructs are perfused, which facilitates maturation of the wall structure and allows for endothelialization.16 This process requires an excessively long production time of several months, hindering the clinical application of cell sheet-derived conduits for bypass grafting in both the urgent and elective settings.
In this study, we hypothesize that a novel technique of stabilizing cell sheet-derived vessel grafts results in functional engineered vascular conduits (EVCs) with outstanding patency rates and significantly reduced production time. A flexible membrane derived from clinically-approved tissue glue is used as an outer, biodegradable scaffold to stabilize the EVC and allow for immediate perfusion with a peristalsis pump. In this bioreactor system, constant flow through the EVC lumen facilitates the maturation of the vessel wall such that the EVC is ready for in vivo use as a bypass graft within 14 days.
Methods
The data and analytical methods will be made available to other researchers by the corresponding author upon reasonable request.
Cell Sheet Production
Patient-derived human aortic smooth muscle cells (passage 4–8, Thermo Fisher Scientific) were plated at 1.5×105 cells/cm2 in a 35-mm Upcell dish (Thermo Scientific Nunc), which is coated with temperature-responsive polymers. The cells were cultured in smooth muscle growth media (SMGM, Life Technologies) at 37°C and 5% CO2 for 24 hours before human skin fibroblasts (passage 4–8, Thermo Fisher Scientific) were added as a second cell layer directly atop the confluent layer of smooth muscle cells.18 After 24 hours of co-culturing, the dish was transferred into a 20°C incubator, at which point the confluent bi-level cell sheets (diameter approximately 1 cm) detached spontaneously from the temperature-responsive culture plate.18
EVC Construction from Cell Sheets and Tissue Glue Membranes
After cell sheet lifting, 8–10 cell sheets were transferred into a 10 cm petri dish filled with 50 mL of pre-warmed (37°C) SMGM (Fig. 1A). Next, a 22.5 Gauge (G) angiocath needle was submerged into the solution and the cell sheets were placed above the needle and aligned longitudinally (Fig. 1B). The needle was then lifted out of the media and rotated such that the cell sheets formed a tube-like construct wrapped around the needle (Fig. 1C).
Figure 1.
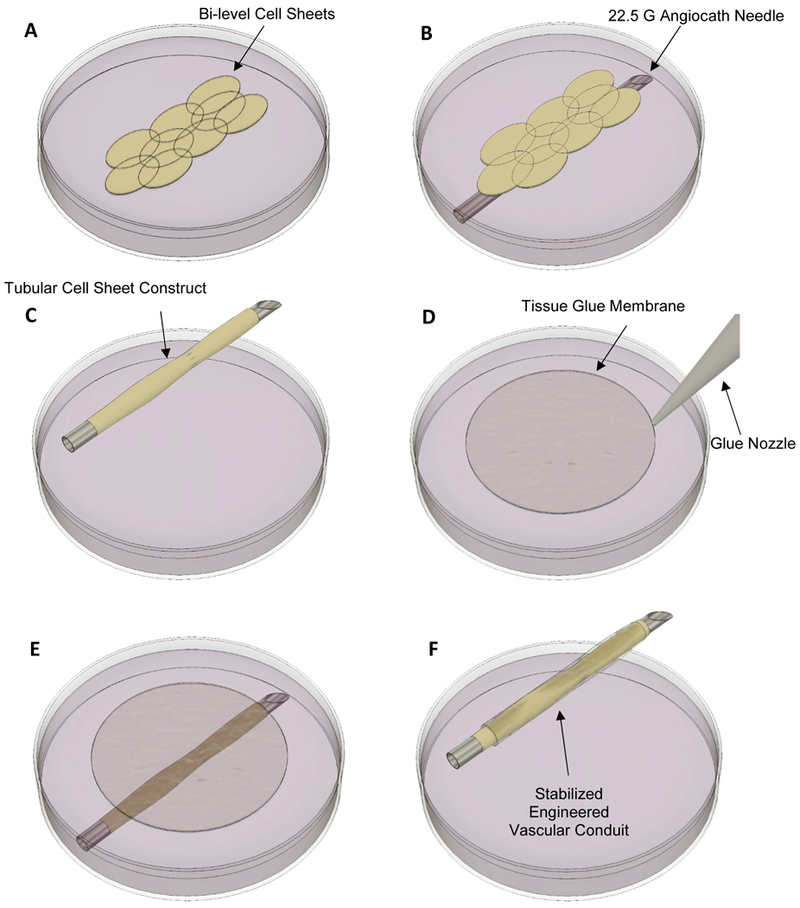
Engineering process of the vascular conduit. (A) Bi-level cell sheets are transferred into a 10 cm petri dish filled with smooth muscle growth media. (B) Cell Sheets are placed on top of a 22.5 G angiocath needle. (C) The cell sheets are wrapped around the needle to form a tube-like construct. (D) Tissue glue is added to a separate 10 cm petri dish and forms a flexible glue membrane on the surface of the media. (E) The glue membranes are wrapped around the cell sheet construct. (F) The final construct consists of an inner layer of cell sheets stabilized by a flexible tissue glue membrane.
Flexible glue membranes were used to stabilize the cell sheet construct. In order to develop these flexible glue membranes, a 10 cm petri dish was filled with 50 mL of pre-warmed SMGM. Subsequently, 30 μL of 2-octyl cyanoacrylate (Dermabond Advanced™, Ethicon), an FDA-approved tissue sealant, was added to the dish of growth media, resulting in rapid polymerization of the glue on the media surface (Fig. 1D). Finally, the flexible glue membranes were immediately wrapped around the tubular cell sheet construct in 3 continuous layers (Fig. 1E), producing the immature EVC (Fig. 1F).
Maturation of EVCs using a peristalsis pump bioreactor system
The EVC was connected via two 22.5 G needles to a bioreactor system (Fig. 2A), which consisted of a peristalsis pump (Econo Gradient Pump, BioRad), a growth media reservoir (50 mL), and 4 French connection tubing placed in an incubator (37°C, 5% CO2). Dermabond was used to secure the EVCs around the needle and prevent sliding of the EVC edges. The flow rate was set at 1 mL/min for the entire 14-day period of in vitro perfusion. The media outflow from the EVC was directed into the media reservoir without any additional resistance (zero-pressure outflow system). On day 1, the EVC was perfused with HUVECs at a concentration of 106 cells/mL in endothelial cell growth media for 24 hours. For this purpose, the media reservoir was bypassed with 4 French tubing to reduce media volume and the quantity of HUVECs needed. Following endothelialization, the EVC was perfused with SMGM for another 13 days. After 14 total days of in vitro perfusion, a 1.8 cm segment of the vessel construct was cut free. 1.5 cm of this segment was used for burst pressure testing and assessment of flow dynamics, while the remaining 0.3 cm was used for vasoreactivity testing. The pre-defined criteria for EVC maturity consisted of burst pressure exceeding 500 mmHg, which ensured sufficient durability for subsequent surgical procedures. A summary of the experimental timeline is illustrated in Figure 2B.
Figure 2.
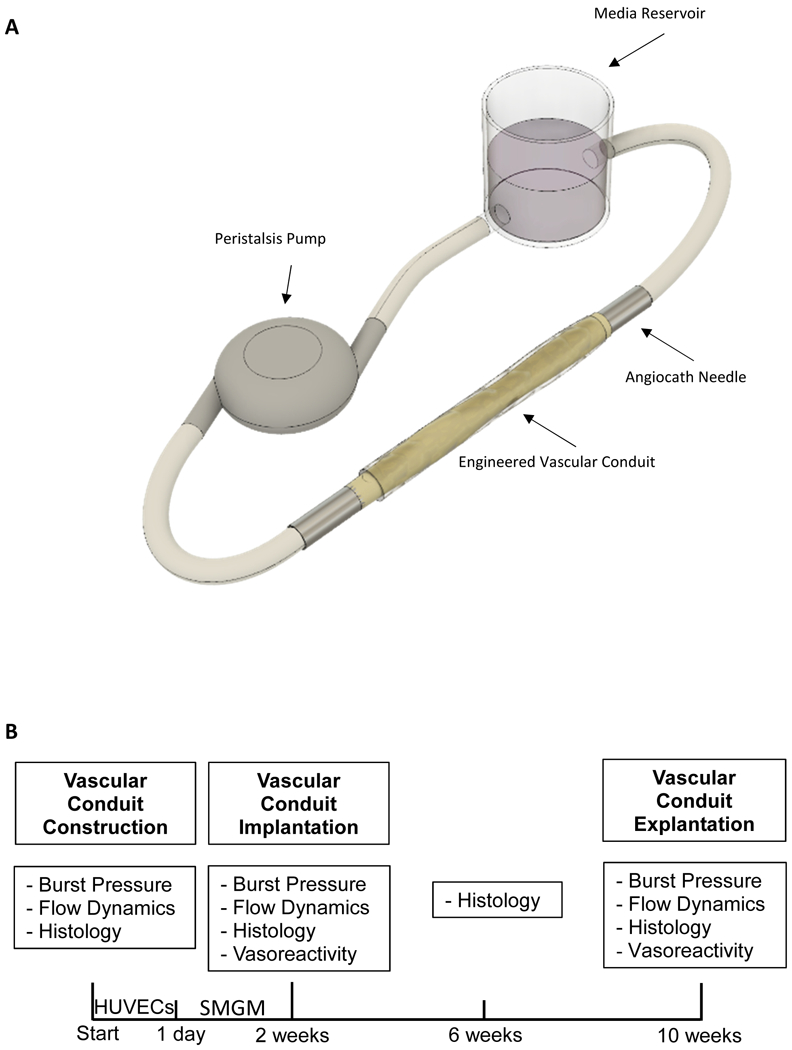
(A) The engineered vascular conduit (EVC) is connected to a perfusion system consisting of a peristalsis pump, a media reservoir, two 22.5 G needles, and connection tubing. (B) The experimental timeline is illustrated. Immediately after construction of the EVC, burst pressure, flow dynamics, and histology were assessed. The EVC was perfused with human umbilical vein endothelial cells (HUVECs) for 1 day and subsequently with smooth muscle growth media (SMGM) for 13 days. Upon completion of the in vitro perfusion/maturation period, burst pressure, flow dynamics, histology, and vasoreactivity were assessed. The mature EVC was implanted as a femoral artery interposition graft in a rat model. Four weeks later, EVC histology was again analyzed. Finally, 8 weeks after implantation, the EVC was explanted and burst pressure, flow dynamics, histology, and vasoreactivity were analyzed.
EVC Vasoreactivity, Burst Pressure, and Flow Dynamics
In order to assess vasoreactivity, 0.3 cm EVC segments were first washed in phosphate buffered saline (PBS) for 5 minutes. Subsequently, the EVCs were exposed to 1 μM epinephrine (vasoconstrictor, Sigma-Aldrich) or 1 μM S-Nitroso-N-acetyl-DL-penicillamine (vasodilator, Sigma-Aldrich) for 3 minutes.19 The EVCs were then rewashed in PBS and exposed to the other agent for 3 minutes. The maximum outer diameter of the EVCs was determined after each PBS wash and after each exposure to epinephrine or penicillamine.
In order to assess burst pressure, 1.5 cm EVC segments were connected to a 22.5 G cannula on one side and to a closed-end pressure sensor on the other. Both vessel ends were secured with 6–0 nylon suture to avoid failure of the connecting site. The EVC was then filled with pre-warmed PBS (37°C) at a flow rate of 0.1 mL/min until bursting, defined as leakage of PBS through the vessel wall due to mechanical failure. Following EVC-femoral artery anastomosis, anastomotic strength was measured in a similar fashion with failure defined as leakage of PBS through the anastomotic suture line.20
For assessment of flow dynamics, grafts were connected to a perfusion pump, and pressure sensors were applied to both ends of the EVC to measure the pressure drop over the 1.5 cm distance.
Animal Care and Biosafety
Homozygous nude rats were obtained from Charles River (male, 6–8 weeks old, 250–300 g). Food and water were provided ad libitum. All animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85–23, Revised 1996). The experimental protocol was approved by the Institutional Animal Care and Use Committee at Stanford University (Protocol 28921).
Surgical Application of EVCs as Femoral Artery Interposition Grafts
The EVCs were applied as femoral artery interposition grafts.21,22 Nude rats were anesthetized with 2% isoflurane (Fluriso™, VetOne) in 70% N2 and 30% O2 at a flow rate of 2 L/min. The left and right femoral arteries were exposed through bilateral 2 cm skin incisions made immediately below the femoral ligament. The femoral artery was separated from the femoral nerve and vein, and any branches were ligated. On one side (alternating between left and right), the artery was clipped twice and explanted over a distance of 2.5 cm beginning 0.5 cm distal to the femoral ligament. An EVC was then implanted as an interposition graft. The EVC-femoral artery anastomoses were performed using 5 interrupted 10–0 nylon sutures. In a subgroup of animals, the EVC-femoral artery anastomosis was performed with 8 interrupted stitches to test anastomotic strength at 1 and 3 days after implantation, compared to a femoral artery-femoral artery anastomosis performed using the contralateral femoral artery as an interposition graft and sewn using the same technique. After implantation of the EVC, the animals were allocated into 2 groups by simple randomization: in one group a segment of the contralateral femoral artery was excised without interposition graft replacement. In these animals, the proximal and distal ends of the femoral artery were ligated permanently with 6–0 polypropylene suture, resulting in hindlimb ischemia. In the other group, the contralateral artery was left untouched as a sham control. Eight weeks after the procedure, the animals were euthanized, and EVCs and native femoral arteries were explanted and either cryopreserved for histological analysis or harvested for functional and mechanical testing.
Invasive Flow Probe Measurements and Laser Doppler Flowmetry
Immediately after EVC implantation and immediately before EVC explantation, absolute blood flow was measured invasively with a flow probe. The flow probe was placed around the interposition graft and the contralateral femoral artery.
Perfusion of the hindlimb was further assessed by laser doppler imaging (PeriScan PIM 3, Perimed) at 1 day in vivo and weekly thereafter until 8 weeks in vivo. Animals were anesthetized with 2% isoflurane in 70% N2 and 30% O2. For each animal, three repeated images were taken to minimize the effect of variable anesthesia depth on peripheral perfusion. Flow data is expressed as unitless doppler signal intensity.
Scaffold Degradation, Formaldehyde Release, and Tissue Reaction
EVCs and native nude rat femoral arteries were dried for 1 day and subsequently stained with Basic Yellow 40 (BVDA America Inc., New Bedford, MA, USA), a fluorescent dye for cyanoacrylate. The surface area of the vessels covered with cyanoacrylate was visualized under ultraviolet light.
Formaldehyde release from scaffolds composed of 2-octyl cyanoacrylate (Dermabond Advanced) was compared to that of N-butyl cyanoacrylate (Vetbond Tissue Adhesive 1469, 3M, MN, USA), a tissue adhesive with a shorter alkyl chain leading to higher levels of formaldehyde release. Formaldehyde levels were measured using a fluorometric detection kit (Abcam, USA). 30 μL of tissue adhesive was submerged in 6 mL PBS and incubated at 37°C for 4 weeks. PBS was exchanged after 14 days, mimicking the transition from in vitro perfusion to in vivo implantation. 300 μL aliquots of PBS were removed from the samples and analyzed according to the manual of the detection kit.
The direct toxicity of Dermabond in vivo was assessed with a TUNEL assay (Sigma, USA) to detect cell death in situ and visualized with confocal microscopy (Leica). Cryosections of tissue blocks containing the EVC and surrounding tissue were analyzed for DNA fragmentation. TUNEL-positive cells were quantified in 10 microscopic fields (40x magnification) and compared to similar tissue sections from rat femoral arteries after sham surgery.
Cryosections of EVCs with surrounding tissue and similar sections from rat femoral arteries after sham surgery were stained with an antibody against Signal Regulatory Protein (SIRP, Abcam), a transmembrane glycoprotein expressed by granulocytes and macrophages. After confocal microscopy, granulocytes and macrophages were quantified as described above to assess the degree of inflammation around the scaffold.
Tensile Testing of EVCs
Explanted EVCs and native nude rat femoral arteries were cut to a length of 2.5 cm. The samples were placed in PBS at room temperature and transferred to a uniaxial tensile testing system within 1 hour after explantation (5944 materials testing system, 100 N load cell, Instron Corp., Norwood, MA). The specimen ends were held between pieces of 100 grit sandpaper and secured in serrated tensile grips. Following the application of a 0.02 N preload, samples were preconditioned at a rate of 1% strain/sec from 0–2% strain for 10 cycles. Specimens were then stretched to failure at 1% strain/sec. The resulting stress-strain curves were analyzed for tensile elastic modulus (Young’s modulus).
Histology and Immunohistochemistry
Immediately after EVC construction, before EVC implantation, and at 1 day, 4 weeks, and 8 weeks after EVC implantation, EVCs were embedded in optimum cutting temperature compound and 10 μm cryosections were prepared. The cryosections were stained with hematoxylin-eosin (HE) stain to assess the vessel wall structure, and with Van Gieson stain to detect elastic fibers and assess the formation of an inner and outer elastic membrane in the EVCs. The cryosections were also stained with an antibody to von Willebrand factor (vWF, 1:500 dilution, Abcam) to assess the endothelial layer, with an antibody against smooth muscle actin (SMA, 1:200, Abcam) to assess the formation of the tunica media, and with an antibody against fibroblast surface protein (FSP, Abcam, 1:200) to assess separation of smooth muscle cells and fibroblasts between the time of cell sheet wrapping and final EVC maturation. Furthermore, the population of human cells and host nude rat cells present in the EVC after implantation was revealed with antibodies against human leukocyte antigen-A (HLA-A) and rat-specific monomorphic determinant of major histocompatibility complex I (MHC I). Cell nuclei were counterstained with 6-diamidino-2-phenylindole (DAPI, Invitrogen). Intima-media thickness was measured from the lumen wall to the media-adventitia interface. The images were examined by light microscopy (HE, Van Gieson, 20x) and confocal microscopy (all immunostains, 20x, 40x).
Statistical Analysis
Data were presented as mean ± standard deviation and analyzed using one-way ANOVA followed by Tukey’s multiple comparisons test, or two-way ANOVA for repeated measures followed by Sidak’s multiple comparisons test, or unpaired t-test, or Fisher’s exact test. The statistical method used to analyze each data set is indicated in the respective figure legends. All statistical calculations were performed using SPSS software (version 11.0, SPSS Inc, Chicago, IL). P<0.05 was considered statistically significant.
Results
Rapid EVC production
Cell sheet-derived conduits constructed without additional stabilization could not be perfused due to poor wall integrity and persistent leakage. Indeed, these unstabilized cell sheets formed a tube-like construct in just 20% of attempts, and those that formed into tubular constructs required 32±9 days wrapped around the angiocath needle to become ready for perfusion and maturation in the bioreactor (Fig. 3A). In contrast, the addition of glue membranes (3 layers, diameter 0.3 mm each) around the wrapped cell sheet construct allowed for consistent tubular vessel-like formation, as well as immediate connection of this immature EVC to the bioreactor system. The ideal thickness of the glue layer was extensively investigated. A thickness less than 0.5 mm did not provide sufficient stability, resulting in leakage. Thicker layers of tissue glue, however, prevented maturation of a compact smooth muscle layer (tunica media), thus prolonging the production process and diminishing vasoreactivity. The addition of 3 glue membrane layers, each 0.3 mm thick, showed the best results with regards to stability and vessel maturation.
Figure 3.
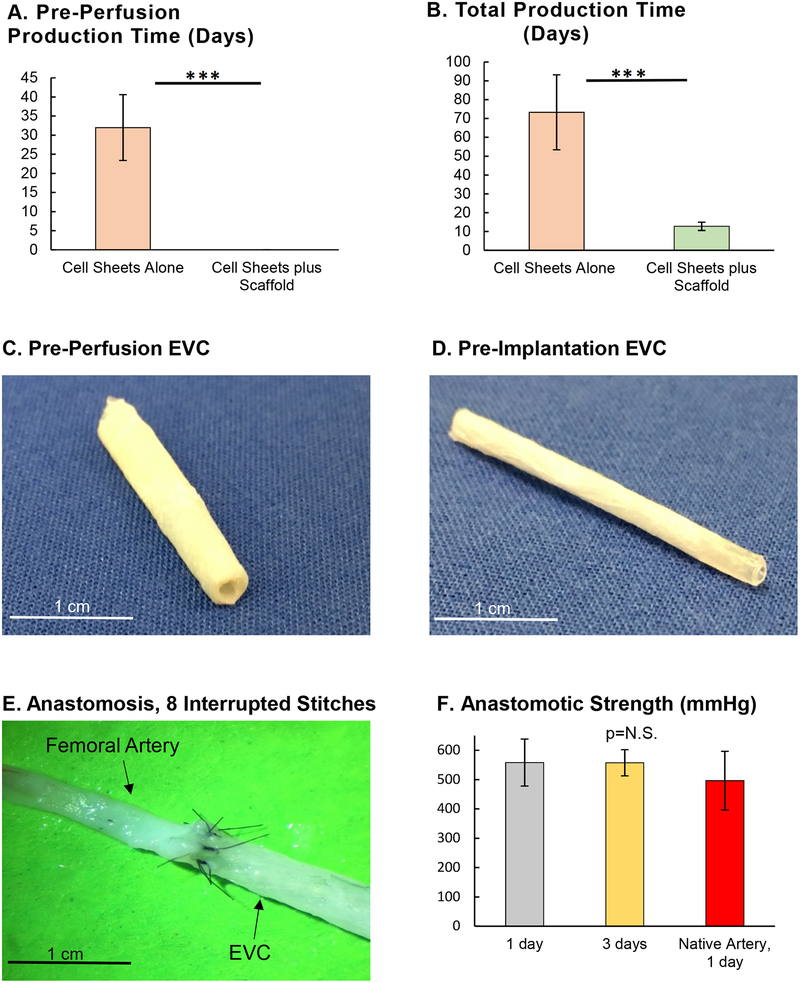
(A) Time elapsed from construction of the engineered vascular conduit (EVC) until ready for in vitro perfusion, n=4 for both groups (Student’s t-test). (B) Time elapsed from construction of the EVC until ready for in vivo surgical implantation, n=4 for both groups (Student’s t-test). (C) Gross anatomy of the EVC immediately after construction and before in vitro perfusion. (D) Gross anatomy of the EVC after 14 days of in vitro perfusion and before in vivo implantation. (E) Proximal anastomosis between the EVC and the femoral artery of a nude rat, using 8 interrupted 10–0 nylon stitches. (F) Anastomotic strength of the EVC-femoral artery anastomosis at 1 and 3 days after in vivo implantation, compared to a femoral artery-femoral artery anastomosis using the contralateral femoral artery of the nude rat as an interposition graft, n=5 for all groups (one-way ANOVA with Tukey’s Test). *** indicates p<0.001.
The EVCs fulfilled the pre-defined criteria for conduit maturation (i.e. burst pressure >500 mmHg) after 12.8±2.2 days of in vitro perfusion via the bioreactor system, compared to 73.3±19.9 days for conduits made of cell sheets without additional stabilization (Fig. 3B). As a result, the in vitro perfusion time for the EVC was set at 14 days for all subsequent experiments. Gross anatomy of the EVC pre- and post-perfusion are shown in Figures 3C and 3D. After in vitro perfusion, the EVC possessed sufficient wall integrity to hold 10–0 nylon suture without leakage or tearing (Fig. 3E). The strength of the EVC-femoral artery anastomosis at 1 and 3 days after implantation was similar to that for a femoral artery-femoral artery anastomosis (EVC-femoral artery at 1 day: 558±80 mmHg vs. EVC-femoral artery at 3 days: 557±45 mmHg vs. femoral artery-femoral artery: 496.4±100 mmHg, Fig. 3F).
EVC Flow Dynamics, Vasoreactivity, Burst Pressure, and Tensile Elasticity
During the in vitro perfusion/maturation period, EVC flow dynamics improved significantly to a level similar to that of native femoral arteries (Fig. 4A). Prior to perfusion, the pressure drop over the 1.5 cm EVC segment was 5.9±0.8 mmHg, compared to 3.0±0.6 mmHg after 14 days of perfusion (p<0.01). The pressure drop over a 1.5 cm segment of native femoral artery was 2.3±0.7 mmHg. Importantly, the pre-perfusion measurement was conducted before the formation of a continuous endothelial layer. Over the in vivo testing period, EVC flow dynamics did not change significantly (pressure drop after 8 weeks in vivo: 2.3±0.5 mmHg).
Figure 4.

(A) Pressure drop over a 1.5 cm engineered vascular conduit (EVC) segment at various time points after construction, n=5 for all samples. (B) Measurements of outer vessel diameter (n=5 for all samples) after 3 minutes wash in phosphate buffered salines (PBS), epinephrine (vasoconstrictor, 1 03BCM) or penicillamine (vasodilator, 1 μM) for EVCs pre-implantation, after 8 weeks in vivo, and for a native femoral artery from a nude rat. (C) Burst pressures for EVCs at various time points after construction, compared to a construct composed of glue membranes only, as well as a rat femoral artery. EVCs with burst pressures below 500 mmHg after 14 days of in vitro perfusion were excluded (i.e. not included in the pre-implantation sample), n=5 for all samples, p<0.01 for Pre-Implantation vs. all other samples, and p<0.01 for Scaffold only or Pre-Perfusion vs. 8 weeks in vivo or native femoral artery. N.S. = not statistically significant. (D) Percentage of EVCs that met the pre-defined criteria for implantation (burst pressure of 500 mmHg) after 14 days of in vitro perfusion vs. 14 days without perfusion, n=15 for both groups. (E) Young’s modulus from uniaxial tensile testing for EVCs immediately prior to implantation and after 8 weeks in vivo, compared to a native femoral artery. p<0.05 for Pre-Perfusion vs. both other groups, n=4 for all samples. One-way ANOVA followed by Tukey’s Test (A, B, C, E), Fisher’s exact test [95% confidence interval] (D). * indicates p<0.05. ** indicates p<0.01.
The structural maturation of the EVC was associated with an increased sensitivity to vasoconstrictors and vasodilators such as epinephrine and penicillamine, respectively. Immediately prior to implantation, the outer diameter of the EVC was reduced by 21% when directly exposed to 1 μM epinephrine solution and increased by 20% when directly exposed to 1 μM penicillamine solution (PBS: 1.12±0.15 mm vs. epinephrine: 0.88±0.12 mm vs. penicillamine: 1.35±0.12 mm, Fig. 4B). After 8 weeks in vivo, the sensitivity of EVCs to epinephrine and penicillamine was more pronounced and was similar to that of native femoral arteries (epinephrine: EVC 0.66±0.09 mm vs. femoral artery 0.70±0.07 mm; penicillamine: EVC 1.60±0.10 mm vs. femoral artery 1.56±0.09 mm), with both EVCs and native femoral arteries sharing a baseline outer diameter of 1.1 mm.
The mechanical stability of the EVCs increased rapidly throughout the construction process and throughout in vivo testing. The burst pressure increased significantly over the 14-day period of in vitro perfusion/maturation for EVCs that fulfilled the pre-defined burst pressure of 500 mmHg (Pre-Perfusion: 330±38 mmHg vs. Pre-Implantation: 632±115 mmHg, p<0.01, Fig. 4C). After 8 weeks in vivo, the burst pressure increased further to a level similar to that of a nude rat femoral artery (946±76 vs. 1004±81 mmHg, respectively, p=N.S.). The dramatic effect of immediate perfusion was substantiated by the maturation rate of 73% for perfused EVCs compared to 7% for unperfused conduits constructed the same way (Fig. 4D).
The tensile modulus of the EVCs increased significantly between the time of construction and final explantation after 8 weeks in vivo, at which time the mature EVCs showed similar elasticity to native femoral arteries from nude rats (Young’s modulus pre-perfusion: 0.31±0.21 MPa vs. 8 weeks in vivo: 0.88±0.27 MPa vs. native femoral artery: 0.99±0.32 MPa, Fig. 4E).
Surgical Application of EVCs as a Femoral Artery Interposition Graft
Mature EVCs could be surgically applied as an interposition graft after a segment of femoral artery was excised (Fig. 5A). Notably, the scaffold membranes alone without cellular components could not tolerate suture passes without being damaged and therefore could not be sewn to the femoral artery without leakage. This further substantiates the critical role of cellular organization and wall formation through in vitro perfusion. The time required to complete a femoral artery bypass grafting procedure utilizing the EVC as an interposition graft did not differ from that utilizing the contralateral femoral artery as an interposition graft (EVC: 49±9 min vs. native femoral artery graft: 52±13 min, Fig. 5B).
Figure 5.
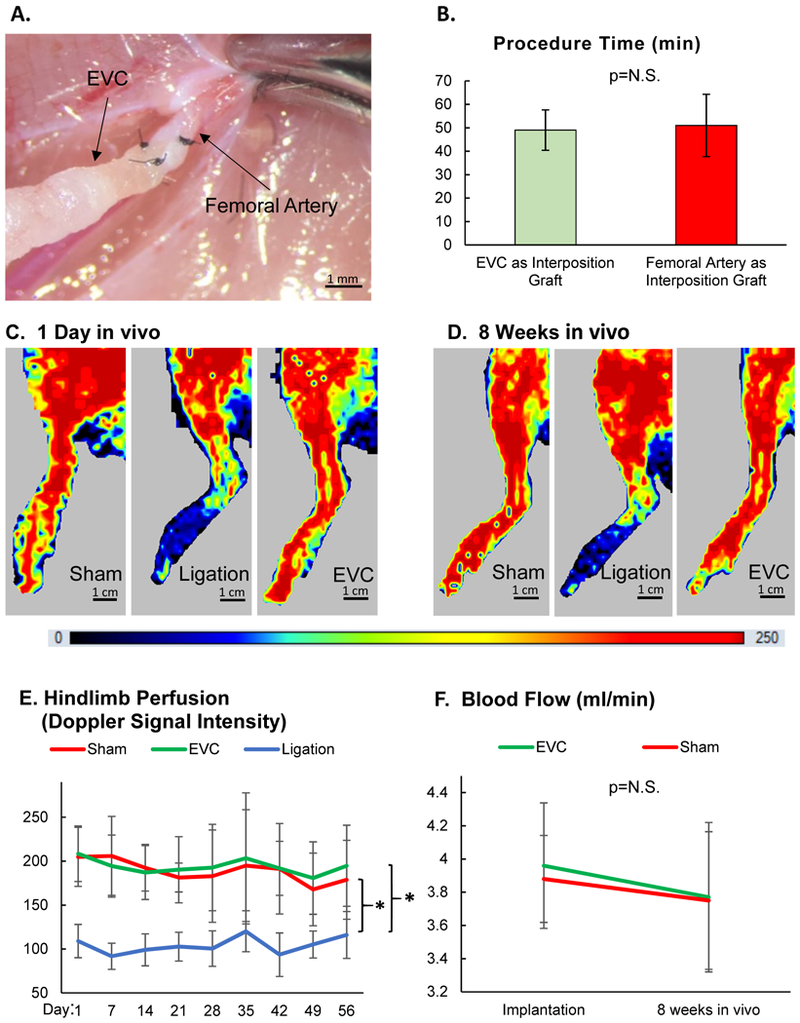
Engineered vascular conduits (EVCs) were tested as interposition grafts after femoral artery excision in rats. On the opposite side, animals were assigned to 2 groups: excision of the femoral artery followed by ligation of the proximal and distal ends without graft replacement, or sham surgery. (A) EVCs were anastomosed to the femoral artery as an interposition graft in end-to-end fashion (10–0 nylon suture, 5 stitches). (B) Time required to complete a femoral artery bypass grafting procedure utilizing the EVC or contralateral femoral artery as an interposition graft, n=5 for both groups (Student’s t-test). N.S. = not statistically significant. (C) Representative laser doppler images of hindlimbs at 1 day after sham surgery, ligation of the femoral artery, or EVC implantation. (D) Representative laser doppler images of hindlimbs at 8 weeks after sham surgery, ligation of the femoral artery, or EVC implantation. (E) Level of hindlimb perfusion following sham surgery, ligation of the femoral artery, or EVC implantation according to laser doppler imaging, n=20 animals (unitless doppler signal intensity, two-way ANOVA for repeated measures). (F) Blood flow in the EVC and native contralateral femoral artery was measured invasively using a flow probe immediately following graft implantation and after 8 weeks in vivo, n=5 for both groups (two-way ANOVA for repeated measures). * indicates p<0.05. N.S. = not statistically significant.
Hindlimb perfusion following femoral artery excision with or without EVC graft replacement was monitored using laser doppler flowmetry over 8 weeks in vivo (Figs. 5C–D). Excision of the femoral artery without graft replacement led to laser doppler signal reduction by 50% (Fig. 5E). In contrast, the implantation of an EVC as a femoral artery interposition graft restored hindlimb perfusion to the level of sham controls. These ratios did not significantly change over the course of the 8-week in vivo study. Invasive flow probe measurements performed during the EVC implantation and explantation procedures confirmed similar levels of blood flow between EVCs and contralateral femoral artery grafts at both timepoints (implantation: 3.96±0.38 vs. 3.88±0.26 mL/min, respectively; explantation: 3.77±0.45 vs. 3.75±0.41 mL/min, respectively, Fig. 5F).
Histological Maturation of the EVC
During in vitro perfusion with HUVECs, the immature EVCs developed a continuous endothelial lining, as evident by positive vWF staining (Figs. 6A–B). Following implantation in vivo, the EVCs became increasingly organized in intimal and medial wall structure (Figs. 6C–E). Importantly, the endothelial monolayer remained intact over the entire in vivo period. By 8 weeks in vivo, the anatomy of the EVC closely approximated that of a native nude rat femoral artery (Figs. 6E–F). The histological maturation of the EVC was also assessed using HE staining, which illustrated the progressive evolution of a compact, well-organized, and arterialized wall structure by 8 weeks in vivo (Figs. 6G–L).
Figure 6.
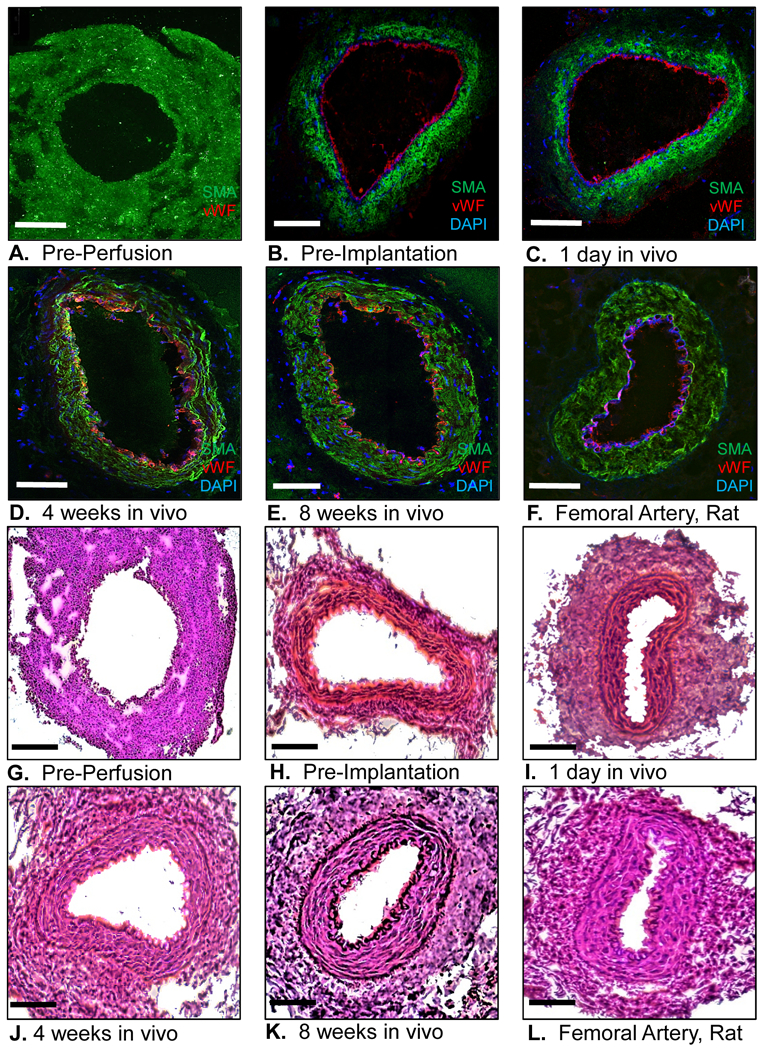
(A-F) Engineered vascular conduit (EVC) immediately before in vitro perfusion, immediately before implantation in vivo, and after 1 day, 4 weeks, and 8 weeks after implantation in vivo, compared to a native femoral artery of a nude rat. Human smooth muscle actin (SMA) antibody: green fluorescent protein; von Willebrand factor (vWF) antibody: Texas red; 4′,6-diamidino-2-phenylindole (DAPI): blue; confocal microscopy 20x. (G-L) Hematoxylin-Eosin staining of the EVC immediately before in vitro perfusion, immediately before implantation in vivo, and after 1 day, 4 weeks, and 8 weeks after implantation in vivo, compared to a native femoral artery of a nude rat. Light microscopy, 20x. Scale bar represents 500 μm.
While immature EVCs were anatomically composed of loosely-stacked bi-level cell sheets with alternating smooth muscle cell layers and fibroblast layers (Fig. 7A), the EVC wall at 8 weeks in vivo was structured concentrically with smooth muscle cells (SMA positive) and fibroblasts (FSP positive) aligned in organized layers (Fig. 7B). Van Gieson staining further revealed the existence of an internal and external elastic lamina at the interfaces of the tunica intima and media, and the tunica media and adventitia, respectively (Fig. 7C). Notably, at 8 weeks in vivo, the tunica media and adventitia were observed to be composed of a combination of human (HLA-A positive) and host rat-derived cells (rat-specific MHC I positive, Fig. 7D).
Figure 7.
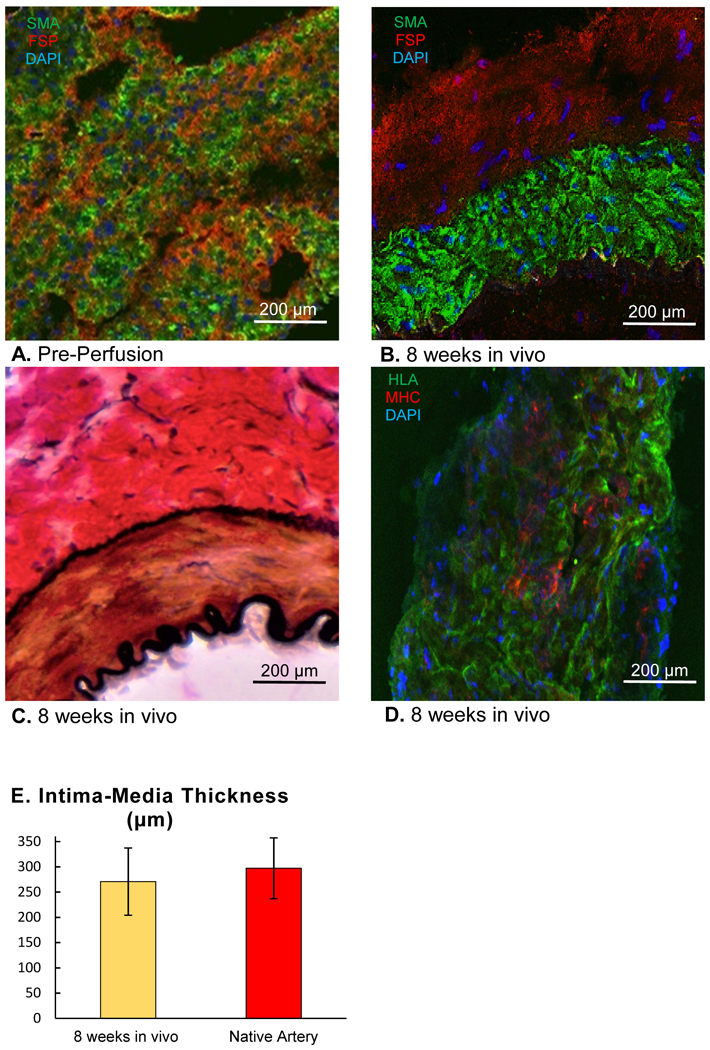
(A) Immunohistochemical staining of a wall segment of the engineered vascular conduit (EVC) immediately after construction (pre-perfusion) with antibodies against smooth muscle actin (SMA, green fluorescent protein) and fibroblast surface protein (FSP, Texas Red), 4′,6-diamidino-2-phenylindole (DAPI): blue. (B) Immunohistochemical staining of a wall segment of the EVC after 8 weeks in vivo with antibodies against smooth muscle actin (SMA, green fluorescent protein) and fibroblast surface protein (FSP, Texas Red), DAPI: blue. (C) Van Gieson staining of a wall segment of the EVC after 8 weeks in vivo. Light Microscopy, 20x. (D) Immunohistochemical staining of a wall segment of the EVC after 8 weeks in vivo with antibodies against human leukocyte antigen A (HLA, green fluorescent protein) and rat-specific major histocompatibility complex I (MHC I, Texas Red). Confocal Microscopy, 20x (B, C, D). (E) Intima-media thickness of EVCs 8 weeks after implantation in vivo compared to that of native femoral arteries, n=5 for both groups. Student’s t-test.
The intima-media thickness, representing a quantitative measure of vascular remodeling, was similar between EVCs at 8 weeks after implantation and native femoral arteries (EVC: 271±67 μm vs. native femoral artery: 297±60 μm, Fig. 7E). Furthermore, no signs of thrombosis or endothelial damage were observed in any of the cross-sectional slices, consistent with the 100% patency rate of the EVCs as femoral artery interposition grafts.
Dermabond Degradation, Formaldehyde Release, and Tissue Reaction
The EVCs were completely covered with cyanoacrylate over the 14-day period of in vitro perfusion and on day 1 after implantation (Fig. 8A). During the first 4 weeks of in vivo maturation, the EVC surface area covered with scaffold decreased to 42.2±7.8% (Fig. 8B). By 8 weeks after implantation, cyanoacrylate coverage of all EVCs was similar to that of native nude rat femoral arteries, indicating complete degradation of scaffold material from the EVC surface (Fig. 8A–B).
Figure 8.
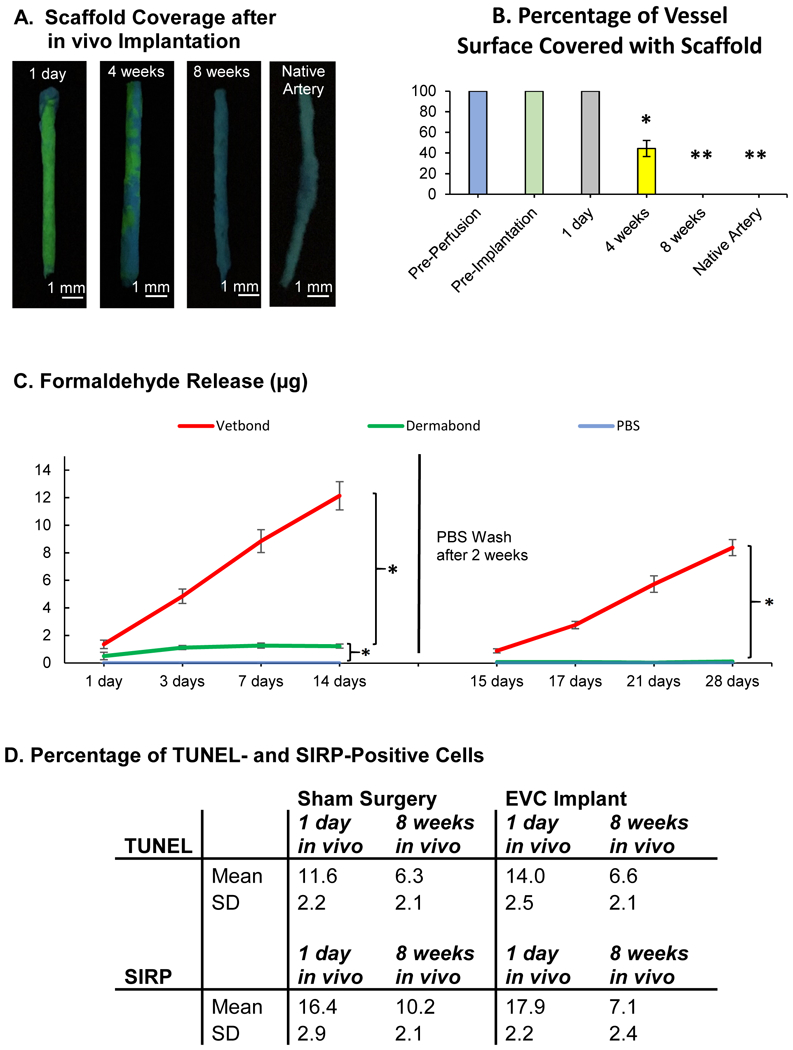
(A) Engineered vascular conduits (EVCs) and native arteries were stained with a fluorescent dye (Basic Yellow 40) to label the cyanoacrylate scaffold at 1 day, 4 weeks, and 8 weeks after implantation in vivo. UV light, Basic Yellow 40: yellow-green fluorescent. (B) Percentage of the EVC surface covered with scaffold immediately before in vitro perfusion, immediately before implantation, and at 1 day, 4 weeks, and 8 weeks after implantation in vivo. p<0.05 for 4 weeks in vivo vs. all other groups, and p<0.01 for Pre-Perfusion, Pre-Implantation, and 1 day in vivo vs. 8 weeks in vivo and Native Artery, n=5 for all samples (one-way ANOVA followed by Tukey’s Test). (C) Fluorometric measurements of cumulative formaldehyde release after polymerization of N-butyl cyanoacrylate (Vetbond) and N-octyl cyanoacrylate (Dermabond, EVC scaffold), compared to phosphate buffered saline (PBS), n=5 for all samples (two-way ANOVA for repeated measures). (D) Percentage of cells staining positive for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL, cell death) and signal regulatory protein α (SIRP, granulocytes, macrophages) in standardized tissue sections (1 cm2 × 10 μm) at 1 day and 8 weeks after EVC implantation in vivo or after sham surgery, n=4 for all samples p= not statistically significant (Student’s t-test). * indicates p<0.05. ** indicates p<0.01. SD = standard deviation.
During the initial 2 weeks of in vitro incubation of cyanoacrylate in PBS, the cumulative formaldehyde release was 1.23±0.15 μg for Dermabond compared to 12.14±1.02 μg for Vetbond (Fig. 8C). Formaldehyde release from Dermabond was highest within the first 2 days after polymerization and decreased gradually thereafter. Upon changing PBS on day 14, however, subsequent formaldehyde release from Dermabond was consistently below 1 μg and not significantly higher than that from PBS alone. This finding is consistent with prior studies showing minimal formaldehyde release from 2-octyl cyanoacrylate after 2 weeks in PBS.23 In contrast, Vetbond released considerable amounts of formaldehyde throughout the entire time course of the experiment.
There was no significant difference in the quantity of TUNEL-positive cells or granulocytes and macrophages between EVC implantation and sham surgery after 1 day or 8 weeks in vivo (Fig. 8D). These findings indicate minimal direct cytotoxicity and an insignificant localized inflammatory reaction to the scaffold.
Discussion
Over the last decade, two fundamentally different approaches have defined the field of bioengineered vascular grafts: one approach is based on scaffold-mediated graft stabilization, while the other is focused on self-assembly of grafts from cell sheets or spheroids.6 In this study, we show that the self-assembly process of cell sheets can be significantly accelerated through the use of temporary, external glue membranes. While glue membranes provide immediate stability to otherwise fragile cell sheet-derived tubular constructs, they do not interfere with the histological maturation of the vessel wall and hence are not part of the self-assembly process itself. In fact, we observed significant vascular maturation of our EVCs including formation of a compact media with SMA-positive cells, a process which was previously described for cell sheet-derived vascular grafts.16
This approach of external stabilization has direct consequences for the structure and physiology of the EVC. While mechanical stability can be easily achieved by using scaffolds inside the vessel wall, these pre-structured approaches usually diminish the flexibility of the smooth muscle layer and thereby reduce or abolish vascular sensitivity to hormones and other signaling molecules. In contrast, our data shows that cell sheet-derived EVCs develop a physiologic response to vasoconstrictors and vasodilators in coordination with increasing mechanical stability–a significant sign of biological self-assembly of the vessel wall. Furthermore, after 8 weeks in vivo, the EVCs demonstrated vasoreactivity, burst pressure, and tensile elasticity at the level of native nude rat femoral arteries.
Cyanoacrylate was selected as temporary, external scaffold to facilitate expeditious clinical translation of the vessel engineering technique. 2-octyl cyanoacrylate (Dermabond™) is already FDA-approved for skin sealing, and various formulations of cyanoacrylate are currently being used internally to control gastric variceal bleeding.24,25 Dermabond™ has also been successfully tested for anastomosing small vessels without sutures.26 However, one concern regarding the use of cyanoacrylate for vessel engineering is the stiffness of the final polymer construct.23 To this end, we ensured that the glue was not polymerized on the surface of the cell sheets but only on the surface of SMGM. This technique resulted in thin (0.3mm) and flexible glue membranes. These membranes could be easily wrapped around the cell sheet tube in a layer of defined thickness and provided external stability for immediate perfusion of the engineered vessel.
The in vivo application as femoral artery interposition grafts provided surgical and functional insights into potential clinical applications of the EVCs. During the implantation procedure, EVCs were handled in the same way as native femoral artery grafts and showed similar anastomotic strength at 1 and 3 days after implantation. In fact, the number of stitches needed to anastomose the EVCs was reduced presumably due to cyanoacrylate bridging the edges of the vessel walls. The hindlimb model allowed for a comparison of perfusion and function with no difference found between implanting an EVC or the native contralateral femoral artery as an interposition graft. Of note, it is important to consider that the inner diameter of our EVC is about 1 mm – smaller than most biological vessel grafts tested.6
Cell sheet-engineered vessels have already been tested as arterio-venous shunts in dialysis patients.17,27,28 This is one of the rare situations where the excessively long production time of cell sheet-derived vessel grafts (around 3–4 months) does not prohibit application in the clinical setting. For cardiovascular bypass grafting surgery, however, delaying an operation by several months in order to allow a conduit to mature is often unacceptable. The technique of external stabilization as described in this study reduces the production time by 70–80%, and thereby permits vascular conduit engineering within the clinical time window needed for cardiovascular bypass surgery.
One future step towards clinical translation of the EVC is the application of patient-specific cell sources to circumvent immunologic rejection. There are already established protocols to isolate and culture fibroblasts and endothelial cells from the patient’s skin and small veins for cell sheet production.16 Additionally, our laboratory described a protocol to trans-differentiate mesenchymal stem cells, which can be acquired from peripheral blood, into smooth muscle cells.29 Another step towards clinical application will be the upscaling of the EVC to the size of a human cardiovascular bypass graft. This process will involve the use of an angiocath needle with an outer diameter of at least 3 mm and construction with more cell layers.
A potential limitation of our current study is the relatively short application period of 8 weeks. During this experiment, however, the patency rate of the tested EVCs was 100% without any anticoagulation treatment. Furthermore, the EVCs matured significantly and resembled the histological structure of native nude rat femoral arteries after 8 weeks in vivo, indicating their potential for long-term revascularization. Another limitation of this study is that our in vitro model of formaldehyde release may not perfectly reflect the systemic release of formaldehyde during scaffold degradation in vivo. Nevertheless, we did not observe any significant adverse effects localized around the EVC which further supports the biocompatibility of the scaffold.
In conclusion, our technique reduces the production time of self-assembled cell sheet-derived vessel grafts to 2 weeks, thereby permitting the use of these bioengineered conduits within the clinical time window for elective cardiovascular bypass surgery. Furthermore, our method utilizes only clinically-approved materials and can be adapted to various cell sources, altogether simplifying the path toward future clinical translation.
Clinical Perspective
What is new?
We demonstrate a technique to manufacture fully-functioning engineered vascular conduits (EVCs) derived from smooth muscle cell and fibroblast bi-level cell sheets and stabilized by an FDA-approved tissue sealant (Dermabond Advanced™) within 2 weeks.
We show that EVCs can be handled in the same way as native arteries for the purpose of vascular bypass grafting.
We confirm that graft patency, vasoreactivity, tensile elasticity, burst pressure, and histological structure of EVCs are all similar to that of native femoral arteries after 8 weeks in vivo as a femoral artery interposition graft.
What Are the Clinical Implications?
The demonstrated technique has high translational potential, using a scaffold made from FDA-approved material that is degraded within 8 weeks in vivo without evidence of cytotoxic or pro-inflammatory effects.
The EVC construction time of only 2 weeks fits the necessary time window for application in elective cardiovascular bypass surgery.
The excellent surgical durability and rapid production potential of the EVC overcomes significant challenges associated with current scaffold-based and cell-only techniques, including poor long-term patency rates and substantially longer engineering time, respectively.
Acknowledgements:
None.
Funding Sources: This work was funded by the NIH (R01HL089315–01 to YJW), Neurowind e.V. (Fellowship to DvB) and the American Heart Association (14POST20380744 to ABG, 17POST33410497 to MJP, and 18POST33990223 to HW).
Footnotes
Disclosures: None.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, Hart JC, Herrmann HC, Hillis LD, Hutter AM, Lytle BW, Marlow RA, Nugent WC, Orszulak TA, American College of Cardiology, American Heart Association. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:e340–437. [PubMed] [Google Scholar]
- 3.Alexander JH, Smith PK. Coronary-Artery Bypass Grafting. N Engl J Med. 2016;374:1954–1964. [DOI] [PubMed] [Google Scholar]
- 4.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W, VA Cooperative Study Group #207/297/364. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. [DOI] [PubMed] [Google Scholar]
- 6.Pashneh-Tala S, MacNeil S, Claeyssens F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng Part B Rev. 2016;22:68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108:9214–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Hu J, Jiao J, Liu Z, Zhou Z, Zhao C, Chang L-J, Chen YE, Ma PX, Yang B. Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials. 2014;35:8960–8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A, Fiorillo A, Avila H, Wystrychowski W, Zagalski K, Maruszewski M, Jones AL, Cierpka L, de la Fuente LM, L’Heureux N. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30:1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quint C, Arief M, Muto A, Dardik A, Niklason LE. Allogeneic human tissue-engineered blood vessel. J Vasc Surg. 2012;55:790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gui L, Dash BC, Luo J, Qin L, Zhao L, Yamamoto K, Hashimoto T, Wu H, Dardik A, Tellides G, Niklason LE, Qyang Y. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials. 2016;102:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torikai K, Ichikawa H, Hirakawa K, Matsumiya G, Kuratani T, Iwai S, Saito A, Kawaguchi N, Matsuura N, Sawa Y. A self-renewing, tissue-engineered vascular graft for arterial reconstruction. J Thorac Cardiovasc Surg. 2008;136:37–45. [DOI] [PubMed] [Google Scholar]
- 13.Rémy-Zolghadri M, Laganière J, Oligny J-F, Germain L, Auger FA. Endothelium properties of a tissue-engineered blood vessel for small-diameter vascular reconstruction. J Vasc Surg. 2004;39:613–620. [DOI] [PubMed] [Google Scholar]
- 14.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–436. [DOI] [PubMed] [Google Scholar]
- 15.Syedain ZH, Meier LA, Bjork JW, Lee A, Tranquillo RT. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 2011;32:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NAF, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, L’heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. The Lancet. 2009;373:1440–1446. [DOI] [PubMed] [Google Scholar]
- 18.Shudo Y, Cohen JE, Macarthur JW, Atluri P, Hsiao PF, Yang EC, Fairman AS, Trubelja A, Patel J, Miyagawa S, Sawa Y, Woo YJ. Spatially oriented, temporally sequential smooth muscle cell-endothelial progenitor cell bi-level cell sheet neovascularizes ischemic myocardium. Circulation. 2013;128:S59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H-F, Liu JY, Andreadis ST, Swartz DD. Hair follicle-derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng Part A. 2011;17:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colen LB, Gonzales FP, Buncke HJ. The relationship between the number of sutures and the strength of microvascular anastomoses. Plast Reconstr Surg. 1979;64:325–329. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Tediashvili G, Pecha S, Reichenspurner H, Deuse T, Schrepfer S. Vein interposition model: A suitable model to study bypass graft patency. J Vis Exp. 2017;119: 54839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borschel GH, Huang Y-C, Calve S, Arruda EM, Lynch JB, Dow DE, Kuzon WM, Dennis RG, Brown DL. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005;11:778–786. [DOI] [PubMed] [Google Scholar]
- 23.Pascual G, Sotomayor S, Rodríguez M, Pérez-Köhler B, Kühnhardt A, Fernández-Gutiérrez M, San Román J, Bellón JM. Cytotoxicity of Cyanoacrylate-Based Tissue Adhesives and Short-Term Preclinical In Vivo Biocompatibility in Abdominal Hernia Repair. PLoS ONE. 2016;11:e0157920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattamal GJ. US FDA perspective on the regulations of medical-grade polymers: cyanoacrylate polymer medical device tissue adhesives. Expert Rev Med Devices. 2008;5:41–49. [DOI] [PubMed] [Google Scholar]
- 25.Ríos Castellanos E, Seron P, Gisbert JP, Bonfill Cosp X. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database Syst Rev. 2015;5:CD010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang EI, Galvez MG, Glotzbach JP, Hamou CD, El-ftesi S, Rappleye CT, Sommer K-M, Rajadas J, Abilez OJ, Fuller GG, Longaker MT, Gurtner GC. Vascular anastomosis using controlled phase transitions in poloxamer gels. Nat Med. 2011;17:1147–1152. [DOI] [PubMed] [Google Scholar]
- 27.Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L, L’Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg. 2014;60:1353–1357. [DOI] [PubMed] [Google Scholar]
- 28.Peck MK, Dusserre N, Zagalski K, Garrido SA, Wystrychowski W, Glickman MH, Chronos NAF, Cierpka L, L’Heureux N, McAllister TN. New biological solutions for hemodialysis access. J Vasc Access. 2011;12:185–192. [DOI] [PubMed] [Google Scholar]
- 29.Shudo Y, Cohen JE, Goldstone AB, MacArthur JW, Patel J, Edwards BB, Hopkins MS, Steele AN, Joubert L-M, Miyagawa S, Sawa Y, Woo YJ. Isolation and trans-differentiation of mesenchymal stromal cells into smooth muscle cells: Utility and applicability for cell-sheet engineering. Cytotherapy. 2016;18:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]