Abstract
OBJECTIVE
The aim of this study was to describe the clinical presentation, imaging appearance, and differential outcomes based on tumor location in 7 patients with desmoplastic infantile astrocytoma and desmoplastic infantile gangliogliomas (DIA/DIG).
METHODS
Data of 7 patients with histopathology-proven DIA/DIGs and preoperative imaging were retrospectively reviewed, and age, sex, clinical presentation, imaging characteristics, tumor location, surgical procedure, postoperative morbidity, and overall mortality were recorded.
RESULTS
Two subgroups of patients with DIA/DIGs were found to exist based on whether their tumor was located in the cerebral hemispheres or suprasellar region. Nearly all patients presented with rapidly enlarging head circumference regardless of tumor location. However, ocular abnormalities, including nystagmus and preference for downward gaze, were specific for patients with suprasellar disease. These patients experienced significant postoperative complications and had poor long-term outcomes. In contrast, patients with hemispheric tumors underwent more extensive resection than patients with suprasellar tumors, had uneventful postoperative courses, and had no documented long-term comorbidities.
CONCLUSIONS
Postoperative course and long-term outcome for patients with DIA/DIGs were correlated to the anatomical location and radiographic appearance of their tumor at presentation, despite having histologically and molecularly indistinguishable, WHO grade I tumors.
Keywords: pediatric neurosurgery, neuroradiology, desmoplastic infantile astrocytoma, desmoplastic infantile ganglioglioma, outcomes, oncology
DESMOPLASTIC infantile astrocytoma (DIA) and desmoplastic infantile ganglioglioma (DIG) are rare, benign CNS tumors that typically present within the first 2 years of life.1,29,33,34,39 While both tumors are characterized histologically by prominent desmoplastic stroma, DIA is characterized by a neoplastic population restricted to astrocytes, while DIG includes a population of neoplastic neuronal cells of variable maturity. In light of reportedly similar clinical, radiological, and pathological features, DIG and DIA are considered to be part of the same entity.14,39 Classically, patients with DIA/DIG present with rapidly enlarging head circumference.17,32,34,36 On MRI, DIA/DIGs are typically described as large supratentorial cysts with a cortical-based enhancing tumor nodule with a dural attachment.1,5,34 Resection is curative, and chemotherapy and/or radiation therapy is unnecessary in the majority of cases.1,3,26,34,35 Indeed, DIA/DIGs are now categorized together in the 2016 WHO classification, are designated grade I, and generally have a good prognosis with long disease-free survival.1,5,15,26,32,34,35,39 How- ever, there have been a few case reports of DIA/DIGs with atypical presentations, including primary vermian localization,1 primary spinal localization,25 metastatic spread,32 multiple cerebral lesions,1,18,28,32 and even occurrence in patients older than 6 years.9,25,26 Based on these reports, patients with atypical presentations of DIA/DIGs may have markedly different clinical courses and long-term outcomes than patients with more classic presentations. Thus, additional information is needed to better understand the spectrum of clinical presentations, management strategies, postoperative course, and long-term outcomes of patients with DIA/DIGs, particularly those with atypical presentations.
Here, we present a single-institution retrospective study of 7 patients with histologically confirmed DIA/DIGs, wherein 2 clinically distinct groups were evident after the review. Our findings indicate that there may be differential outcomes of patients with DIA/DIGs based on the tumor’s location.
Methods
We performed a retrospective review of the electronic medical records for all patients with a histological diagnosis of DIA/DIG who underwent surgery at our institution. Age at diagnosis, sex, clinical presentation, MRI characteristics, tumor location, surgical procedure, postoperative morbidity, and overall mortality were recorded, and our results are summarized in Tables 1–3. Study approval was granted by the Mayo Clinic institutional review board. The study population was limited to children 2 years and younger. Exclusion criteria were patients without preoperative electronic imaging and patients older than 2 years. Cases were accrued from the Mayo Clinic, Rochester Campus, between 1997 and 2016. Preoperative MRI was performed in all cases with and without intravenous contrast. All images were reviewed by a board-certified neuroradiologist. Surgically excised specimens were fixed in formalin and embedded in paraffin. Histological examination included standard H & E and Masson’s trichrome stains. Immunohistochemical staining was performed for additional evaluation of the tumors, including IDH1-R132H (H09 clone, Dianova), BRAF-V600E (VE1 clone, Spring), H3K27-me3 (C36B11 clone, Cell Signaling), and INI-1 (clone 25, BD Transduction). All histopathological sections and stains were reviewed by a board-certified neuropathologist at presentation and at rereview as part of this study. IDH1-R132H, BRAF-V600E, trimethylated H3K27, and INI1 staining was done as part of this study. All other stains, including GFAP, synaptophysin, neurofilament protein, S100, Ki-67, and collagen IV were done at the time of initial surgery and pathologic evaluation.
Table 1.
DIA/DIG patient characteristics
| Case No. | Age (mos), Sex | Presentation | Hydrocephalus | Location | Resection |
|---|---|---|---|---|---|
| 1 | 3, F | REHC, nystagmus | Yes | Suprasellar | Partial |
| 2 | 2, M | REHC, downward gaze | Yes | Suprasellar | Partial |
| 3 | 4, M | REHC, nystagmus | Yes | Suprasellar | Partial |
| 4 | 5, M | REHC | Yes | Hemispheric | Total |
| 5 | 4, F | REHC | Yes | Hemispheric | Total |
| 6 | 4, F | REHC | Yes | Hemispheric | Total |
| 7 | 9, M | Seizures | No | Hemispheric | Total |
REHC = rapidly enlarging head circumference.
Table 3.
Postoperative course for DIG/DIA patients
| Case No. | Location | Event |
|---|---|---|
| 1 | Suprasellar | Multiple VP shunt surgeries |
| 2 | Suprasellar | Multiple VP shunt surgeries, Szs, DI |
| 3 | Suprasellar | VP shunt surgery, Szs, DI, stroke |
| 4 | Hemispheric | VP shunt surgery |
| 5 | Hemispheric | None |
| 6 | Hemispheric | Sz |
| 7 | Hemispheric | None |
DI = central diabetes insipidus; Sz = seizure; VP = ventriculoperitoneal.
Results
Clinical Presentation Differs Based on Tumor Location in Our Cohort
Between 1997 and 2016, 7 patients younger than 2 years underwent operative intervention for histopathology-proven DIA/DIGs at our institution. All clinical characteristics are detailed in Table 1. There were 4 male and 3 female patients. Age at presentation for these patients ranged from 2 to 9 months with a mean age of 4.4 months. Six patients presented with rapidly enlarging head circumference secondary to obstructive hydrocephalus, and 1 patient presented with new-onset seizure. On clinical examination, it was noted that 3 patients had visual disturbances/impairments; 2 had nystagmus, and 1 had a predilection for downward gaze. MRI was performed in all patients prior to surgery. Four patients were found to have tumors in the cerebral hemispheres, whereas 3 patients had lesions in the suprasellar region (Table 1, Fig. 1). Importantly, eye movement abnormalities on clinical examination correlated with tumor location, as observed on MRI, since all 3 patients with suprasellar disease were noted to have either nystagmus or downward gaze. Furthermore, it was observed that patients with suprasellar disease tended to present at a younger age than patients with hemispheric disease (3 months vs 5.5 months), although this relationship was not statistically significant (p = 0.15, 2-tailed Student t-test).
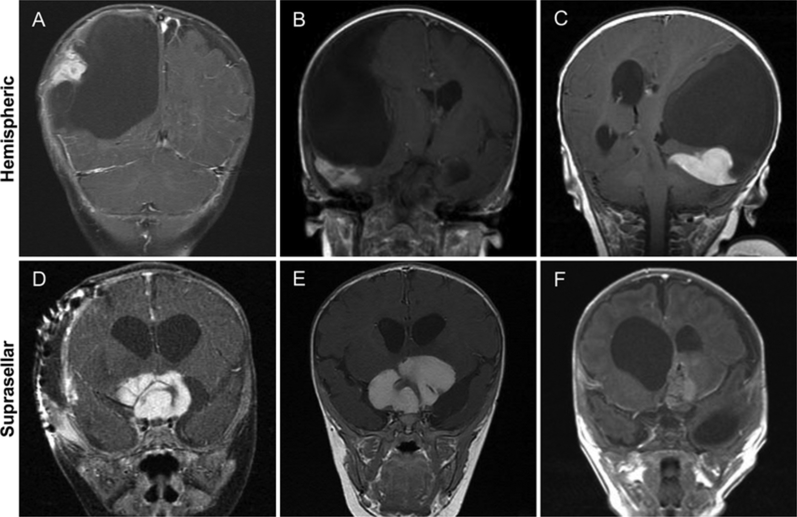
FIG. 1.
A–C: Coronal postcontrast T1-weighted MR images of classically described hemispheric DIGs showing massive cysts with cortically enhancing peripheral nodularity. D–F: Coronal postcontrast T1-weighted MR images of suprasellar DIGs showing large, homogeneously enhancing tumors with small associated cysts.
MRI Reveals Distinct Tumor Characteristics Based on Location
Preoperative MRI indicated that all 4 patients with hemispheric lesions had imaging characteristics consistent with what has been previously described in the literature for DIA/DIGs.2–4,8,13,23,36,39 Accordingly, these tumors were large, peripheral supratentorial tumors with a primary cystic component and a cortical-based enhancing tumor nodule (Fig. 1). Of the 4 tumors that were hemispheric in location, 2 were found to affect the temporal lobe while the remaining tumors were situated in the frontoparietal region. Conversely, suprasellar tumors had discretely different appearances on MRI. Suprasellar tumors were large, yet not as massive as hemispheric tumors, and appeared as homogeneously enhancing masses with no cystic component, and all occurred with hydrocephalus (Fig. 1). Based on preoperative imaging, the differential diagnosis for these lesions included primary glial neoplasm, namely optic pathway glioma and pilocytic astrocytoma, teratoma, atypical rhabdoid teratoid tumor, primitive neuroectodermal tumor, neuroblastoma, and central neurocytoma. Notably, DIA/DIG was not in the original differential diagnosis, as there were no distinctive imaging characteristics identified that readily distinguished suprasellar DIA/DIG.
Hemispheric and Suprasellar DIA/DIGs Are Histologically Indistinguishable
DIA/DIGs located in the hemispheric and suprasellar compartments showed identical histological features, without any identifiable differences in architecture, cytology, or proliferative activity. All tumors were negative for IDH1-R132H and had retained INI1 expression, while BRAF V600E was either negative or showed only very focal expression. Interestingly, while there appeared to be loss of trimethylated-H3K27 expression in 4 tumors, this was observed in 1 suprasellar and 3 hemispheric tumors, and did not show definite association with tumor location or patient outcomes (Table 2).
Table 2.
Immunocytological characterization of suprasellar and hemispheric DIAs/DIGs
| Case No. | Location | Diagnosis | IDH1-R132H | BRAF-V600E | Trimethylated H3K27 | INI-1 |
|---|---|---|---|---|---|---|
| 1 | Suprasellar | DIA | Negative | Negative | Retained | Retained |
| 2 | Suprasellar | DIG | Negative | Negative | Lost | Retained |
| 3 | Suprasellar | DIG | Negative | Negative | Retained | Retained |
| 4 | Hemispheric | DIG | Negative | Focal, weak | Lost | Retained |
| 5 | Hemispheric | DIG | Negative | Negative | Retained | Retained |
| 6 | Hemispheric | DIG | Negative | Focal, weak | Lost | Retained |
| 7 | Hemispheric | DIG | Negative | Negative | Lost | Retained |
Tumor Location Predicts Operative and Postoperative Course in Our Cohort
The extent of resection differed between patients with hemispheric and suprasellar disease. All 4 patients with hemispheric tumors underwent gross-total resection, whereas the 3 patients with suprasellar disease underwent subtotal resection limited by local invasion and/or circumferential adherence to surrounding neurovascular structures (Table 1, Figs. 2 and 3).
FIG. 2.
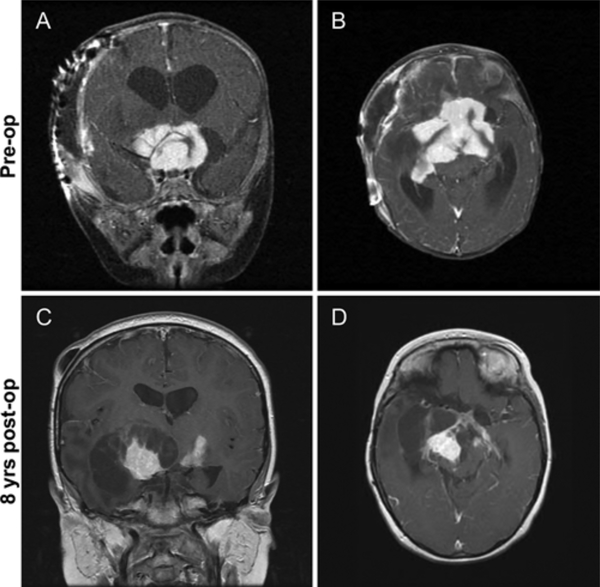
Case 1. A and B: Preoperative coronal (A) and axial (B) postcontrast T1-weighted images obtained in a 3-month-old girl with a large, homogeneously enhancing suprasellar tumor, which presented with rapidly enlarging head circumference, nystagmus, and bilateral optic nerve atrophy. The patient underwent subtotal tumor resection and subsequently received chemotherapy and radiation therapy. C and D: Coronal (C) and axial (D) postcontrast T1-weighted images obtained in the same patient 8 years after the first resection, with recurrent tumor growth and cystic transformation. A second partial resection was subsequently performed.
FIG. 3.
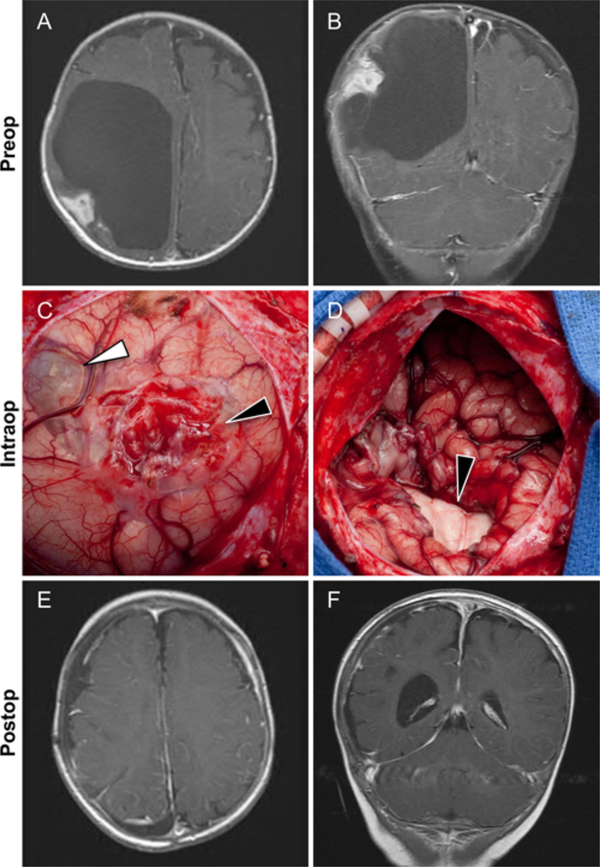
Case 6. A and B: This 4-month-old girl presented with a rapidly enlarging head circumference. Axial (A) and coronal (B) postcontrast T1-weighted images showing a large cystic tumor with an enhancing peripheral cortical-based nodule in the right parietal lobe. C: Intraoperative image obtained in the same patient after opening the dura and wide exposure of right parietal lobe. The solid part of the tumor appears as an extra-axial superficial cortical component (black arrowhead). The cystic portion is also visible (white arrowhead). D: Intraoperative image obtained in the same patient after removal of the tumor. There is significant collapse of the right brain hemisphere. Also seen is the medial wall of the bed of the tumor (black arrowhead). E and F: Axial (E) and coronal (F) postcontrast T1-weighted images obtained in the same patient 1 year after surgery. Neither the cystic or peripheral nodular components are present. Figure is available in color online only.
Postoperatively, 4 patients required at least 1 additional operation to divert ventricular and/or subdural fluid, including 1 with hemispheric and all 3 patients with suprasellar disease. Three patients developed postoperative seizures, 2 of whom were in the suprasellar group. Notably, one of the patients with a suprasellar DIG who experienced postoperative seizures progressed to develop a chronic, medically refractory seizure disorder and subsequently underwent a partial temporal lobectomy, while the hemispheric DIA/DIG patients subsequently were able to discontinue antiepileptic medications. Two patients with suprasellar tumors developed central diabetes insipidus in the postoperative period, and 1 patient with suprasellar disease experienced an ischemic stroke of the left internal carotid artery. The postoperative course of these patients is described in Table 3.
Tumor Location Correlates With Long-Term Outcomes in Our Cohort
To date, the patients with hemispheric DIA/DIG have been observed for a minimum of 2 years and a maximum of 18 years, and none has developed long-term sequelae or significant comorbidities from their disease or from surgery (Table 4). In contrast, all 3 patients with suprasellar tumors developed moderate to severe visual impairment. Additionally, 2 patients have significant developmental delay, and 2 patients developed pan-hypopituitarism and hemiparesis. Two patients with suprasellar tumors were noted to have significant radiographic evidence of tumor progression (Fig. 3). Of these, 1 patient received chemotherapy for approximately 6 years with vincristine, cisplatin, etoposide, cyclophosphamide, valproic acid, and carboplatin and 4500 cGy whole-brain radiation therapy with a 540-cGy boost. This patient is currently living 9 years after the initial diagnosis. A second patient with suprasellar disease is currently alive 5 years after the diagnosis, while the third patient died 4 months after diagnosis.
Table 4.
Long-term outcomes for DIG/DIA patients
| Case No. | Location | FU | Comorbidities | Status |
|---|---|---|---|---|
| 1 | Suprasellar | 9 yrs | Visual impairment, hemiparesis, panhypopituitarism, developmental delay, Sz disorder, tumor progression, chemo & radiation | Alive |
| 2 | Suprasellar | 5 yrs | Visual impairment, developmental delay | Alive |
| 3 | Suprasellar | 4 mos | Visual impairment, panhypopituitarism, tumor progression | Deceased |
| 4 | Hemispheric | 2 yrs | None | Alive |
| 5 | Hemispheric | 18 yrs | None | Alive |
| 6 | Hemispheric | 2 yrs | None | Alive |
| 7 | Hemispheric | 15 yrs | None | Alive |
FU = follow-up.
Discussion
DIA and DIG are rare, with a reported incidence of 0.1%–1.25% of all pediatric CNS tumors. These tumors usually present within the first 2 years of life, most often by 4–6 months of age.17,33,39 Males are more commonly affected by DIA/DIG than females (1.5–1.7:1), and patients almost always present with rapidly enlarging head circumference and, sometimes, vomiting and seizures.3,17,29,34 Classically, DIA/DIGs have been described as massive cystic lesions that emanate from firm nodules that are invariably supratentorial and often attached to the overlying dura.2–4,8,13,23,36,39 The tumor nodules tend to be homogeneously enhancing, hypodense on T2-weighted imaging, and are not associated with significant vasogenic edema.3 Although there are several studies demonstrating multifocal disease, progression, metastasis, and/or malignant transformation to glioblastoma,7,8,16,21,24,32,37 these more aggressive forms of DIA/DIGs tend to be exceptions rather than the rule.17 Generally, DIA/DIGs are considered benign and are often cured by resection alone.22,30 There have been reports of DIA/DIGs spontaneously regressing after subtotal resection without further surgical intervention or subsequent cytotoxic therapy.31
Histologically, these tumors are characterized by neoplastic neuroepithelial cells within a prominent, dense connective tissue network.6,17,26,27,38,39 While DIG and DIA are considered to be the same entity clinically,14,39 they differ histologically in that the neoplastic cells in DIAs appear restricted to astrocytes, while DIGs include a neuronal component or variable maturity DIAs.6,38,39 Distinguishing DIG and DIA requires demonstration of a neoplastic neuronal component.6,17,22,38,39 Significant progress has been made in understanding the molecular and genetic pathways underlying DIA/DIGs, yet the genetic and molecular pathways and the cellular origin of these tumors is not yet established.26,29 A few studies have investigated this issue by interrogating known tumor suppressor genes and oncogenes using immunohistochemistry, chromogenic in situ hybridization, or genome-wide DNA copy number analysis with multiplex ligation-dependent probe amplification. However, because of the rarity of DIA/DIG, these studies lack sufficient sample size to be extrapolated to entire patient populations. Nonetheless, several known oncogenes have been weakly and inconsistently implicated in the pathogenesis of these tumors, including HGFR/MET,14 EGFR,20 and MYC amplification,20 as well as BRAF V600E mutation.10,19 Notably, none of the tumors in our series showed widespread immunoreactivity for BRAF V600E on immunohistochemical analysis. In fact, we were unable to identify any discrete genetic alterations between the hemispheric and suprasellar DIA/DIGs using an immunohistochemical screening approach. Future studies that assess the DIA/DIG genome in an unbiased fashion from a larger sample will be helpful in determining the genetic underpinnings of this disease.
The findings herein pertaining to suprasellar DIA/DIG are analogous to optochiasmatic/suprasellar pilocytic astrocytoma. That is, optochiasmatic/suprasellar disease is a poor prognostic factor in patients with pilocytic astrocytoma.12 The mainstay of treatment for most hemispheric DIA/DIGs and pilocytic astrocytomas of the cortex and cerebellum is gross-total resection.11,30 The initial approach for pilocytic astrocytomas of the optochiasmatic/suprasellar/hypothalamic region is frequent imaging and chemotherapy for progressive lesions, rather than surgery, in order to prevent potentially serious surgical complications, including hypothalamic dysfunction, blindness, and injury to vascular structures. Surgery is typically reserved for patients with tumors that have extended into the third ventricle, causing obstructive hydrocephalus or lesions that are not responsive to therapy. In these patients, opening CSF pathways and surgical debulking is appropriate. In our cohort, all 3 patients with suprasellar DIA/DIGs presented with obstructive hydrocephalus and, therefore, debulking was performed to obtain tissue for diagnosis and to open CSF pathways. Based on our limited experience, we believe that the goal of surgery in these patients should be safe maximal tumor resection.
It is worth noting that the decision to operate on the patients with suprasellar DIA/DIGs was based mostly on their effects related to obstructive hydrocephalus and not necessarily the presumed histological diagnosis. Indeed, as discussed above, DIA/DIG was not initially in the differential diagnosis, as there were no distinctive imaging characteristics identified that readily distinguished DIA/DIG from other pediatric tumors of the suprasellar region. Rather, the diagnosis of DIA/DIG was not made until after tissue was obtained. Thus, it is very possible that some suprasellar DIA/DIGs without obstruction are being “missed” and conservatively managed because they are felt to be optic pathway gliomas, suprasellar pilocytic astrocytomas, or something else.
To date, only one other patient with suprasellar DIA/DIG without multifocal CNS lesions has been reported on.18 In our series of 7 patients with histologically proven DIA/DIGs, 4 patients presented with rapidly enlarging head circumference and were found to have massive, hemispheric cystic lesions with a peripherally located, homogeneously enhancing solid component. These patients experienced full recovery and disease cure following gross-total tumor resection, having a presentation and clinical course identical to what is most often reported in the literature. However, we observed a more unusual presentation in the remaining 3 patients, who presented with ocular manifestations (e.g., nystagmus and downward gaze) in addition to rapidly enlarging head circumference. All 3 were found to have large, homogeneously enhancing suprasellar tumors without cystic changes. These patients experienced significant perioperative complications and long-term comorbidities following subtotal tumor resection. Perioperative complications for these patients with suprasellar tumors included seizures (2 of 3) and central diabetes insipidus (2 of 3). Long-term comorbidities for these patients included moderate to severe visual impairment (3 of 3), panhypopituitarism (2 of 3), hemiparesis (2 of 3), disease progression (2 of 3), developmental delay (2 of 3), seizure disorder (1 of 3), tumor progression (2 of 3), and death (1 of 3).
Conclusions
Here, we describe in detail our experience with a newly recognized clinical variant of DIA/DIG located in the suprasellar region. We are the first to directly compare the clinical course of patients with suprasellar tumors and classically described hemispheric disease. Our study reveals that tumor location predicts perioperative course and long-term outcomes in patients with DIA/DIG, as patients with hemispheric disease have excellent outcomes whereas patients with suprasellar disease have considerably worse outcomes. Our study also highlights that DIA/DIG should be part of the radiographic differential diagnosis for children presenting in the first 2 years of life with a large, homogeneously enhancing, noncystic, suprasellar tumor.
Acknowledgments
R.M.N. is supported by a training grant from the NIH (F30 CA189339). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- DIA
desmoplastic infantile astrocytoma
- DIG
desmoplastic infantile ganglioglioma.
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Supplemental Information
Previous Presentations
Portions of this work were presented at the 15th International Symposium on Pediatric Neuro-Oncology, Toronto, ON, Canada, June 24–27, 2012.
References
- 1.Abuharbid G, Esmaeilzadeh M, Hartmann C, Hermann EJ, Krauss JK: Desmoplastic infantile astrocytoma with multiple intracranial and intraspinal localizations at presentation. Childs Nerv Syst 31:959–964, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Alexiou GA, Stefanaki K, Sfakianos G, Prodromou N: Desmoplastic infantile ganglioglioma: a report of 2 cases and a review of the literature. Pediatr Neurosurg 44:422–425, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bader A, Heran M, Dunham C, Steinbok P: Radiological features of infantile glioblastoma and desmoplastic infantile tumors: British Columbia’s Children’s Hospital experience. J Neurosurg Pediatr 16:119–125, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Basaran R, Cakir FB, Isik N, Sav A, Elmaci I: Desmoplastic infantile ganglioglioma: report of an unusual case with a cranial defect. J Pediatr Neurosci 9:48–51, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beppu T, Sato Y, Uesugi N, Kuzu Y, Ogasawara K, Ogawa A: Desmoplastic infantile astrocytoma and characteristics of the accompanying cyst. Case report. J Neurosurg Pediatr 1:148–151, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cerdá-Nicolás M, Lopez-Gines C, Gil-Benso R, Donat J, Fernandez-Delgado R, Pellin A, et al. : Desmoplastic infantile ganglioglioma. Morphological, immunohistochemical and genetic features. Histopathology 48:617–621, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Darwish B, Arbuckle S, Kellie S, Besser M, Chaseling R: Desmoplastic infantile ganglioglioma/astrocytoma with cerebrospinal metastasis. J Clin Neurosci 14:498–501, 2007 [DOI] [PubMed] [Google Scholar]
- 8.De Munnynck K, Van Gool S, Van Calenbergh F, Demaerel P, Uyttebroeck A, Buyse G, et al. : Desmoplastic infantile ganglioglioma: a potentially malignant tumor? Am J Surg Pathol 26:1515–1522, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Derinkuyu BE, Ucar M, Borcek AO, Damar C, Oztunali C, Gul Alimli A, et al. : Non-infantile variant of desmoplastic ganglioglioma: conventional and advanced MR imaging characteristics. Neuroradiol J 28:259–263, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dougherty MJ, Santi M, Brose MS, Ma C, Resnick AC, Sievert AJ, et al. : Activating mutations in BRAF characterize a spectrum of pediatric low-grade gliomas. Neuro Oncol 12:621–630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Due-Tønnessen BJ, Lundar T, Egge A, Scheie D: Neurosurgical treatment of low-grade cerebellar astrocytoma in children and adolescents: a single consecutive institutional series of 100 patients. J Neurosurg Pediatr 11:245–249, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz Paredes A, et al. : Pilocytic astrocytomas in children: prognostic factors—a retrospective study of 80 cases. Neurosurgery 53:544–553, discussion 554–555, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Geramizadeh B, Kamgarpour A, Moradi A: Desmoplastic infantile ganglioglioma: report of a case and review of the literature. J Pediatr Neurosci 5:42–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gessi M, Zur Mühlen A, Hammes J, Waha A, Denkhaus D, Pietsch T: Genome-wide DNA copy number analysis of desmoplastic infantile astrocytomas and desmoplastic infantile gangliogliomas. J Neuropathol Exp Neurol 72:807–815, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Gupta K, Singla N: Desmoplastic infantile ganglioglioma with focal cortical dysplasia: a rare double pathology in an infant with history of seizures. Neuropathology 36:475–479, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Hoving EW, Kros JM, Groninger E, den Dunnen WF: Desmoplastic infantile ganglioglioma with a malignant course. J Neurosurg Pediatr 1:95–98, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hummel TR, Miles L, Mangano FT, Jones BV, Geller JI: Clinical heterogeneity of desmoplastic infantile ganglioglioma: a case series and literature review. J Pediatr Hematol Oncol 34:e232–e236, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Jurkiewicz E, Grajkowska W, Nowak K, Kowalczyk P, Walecka A, Dembowska-Bagińska B: MR imaging, apparent diffusion coefficient and histopathological features of desmoplastic infantile tumors-own experience and review of the literature. Childs Nerv Syst 31:251–259, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Koelsche C, Sahm F, Paulus W, Mittelbronn M, Giangaspero F, Antonelli M, et al. : BRAF V600E expression and distribution in desmoplastic infantile astrocytoma/ganglioglioma. Neuropathol Appl Neurobiol 40:337–344, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Lönnrot K, Terho M, Kähärä V, Haapasalo H, Helén P: Desmoplastic infantile ganglioglioma: novel aspects in clinical presentation and genetics. Surg Neurol 68:304–308, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Milanaccio C, Nozza P, Ravegnani M, Rossi A, Raso A, Gambini C, et al. : Cervico-medullary desmoplastic infantile ganglioglioma: an unusual case with diffuse leptomeningeal dissemination at diagnosis. Pediatr Blood Cancer 45:986–990, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Ng TH, Fung CF, Ma LT: The pathological spectrum of desmoplastic infantile gangliogliomas. Histopathology 16:235–241, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Nikas I, Anagnostara A, Theophanopoulou M, Stefanaki K, Michail A, Hadjigeorgi C: Desmoplastic infantile ganglioglioma: MRI and histological findings case report. Neuroradiology 46:1039–1043, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Prakash V, Batanian JR, Guzman MA, Duncavage EJ, Geller TJ: Malignant transformation of a desmoplastic infantile ganglioglioma in an infant carrier of a nonsynonymous TP53 mutation. Pediatr Neurol 51:138–143, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Rasalkar DD, Paunipagar BK, Ng A: Primary spinal cord desmoplastic astrocytoma in an adolescent: a rare tumour at rare site and rare age. Hong Kong Med J 18:253–255, 2012 [PubMed] [Google Scholar]
- 26.Romero-Rojas AE, Diaz-Perez JA, Lozano-Castillo A: Desmoplastic infantile ganglioglioma with late presentation. A clinical, radiological and histopathological analysis. Neuroradiol J 26:649–654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rout P, Santosh V, Mahadevan A, Kolluri VR, Yasha TC, Shankar SK: Desmoplastic infantile ganglioglioma—clinicopathological and immunohistochemical study of four cases. Childs Nerv Syst 18:463–467, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Setty SN, Miller DC, Camras L, Charbel F, Schmidt ML: Desmoplastic infantile astrocytoma with metastases at presentation. Mod Pathol 10:945–951, 1997 [PubMed] [Google Scholar]
- 29.Soffietti R, Rudà R, Reardon D: Rare glial tumors. Handb Clin Neurol 134:399–415, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama K, Arita K, Shima T, Nakaoka M, Matsuoka T, Taniguchi E, et al. : Good clinical course in infants with desmoplastic cerebral neuroepithelial tumor treated by surgery alone. J Neurooncol 59:63–69, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Takeshima H, Kawahara Y, Hirano H, Obara S, Niiro M, Kuratsu J: Postoperative regression of desmoplastic infantile gangliogliomas: report of two cases. Neurosurgery 53:979–984, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Taranath A, Lam A, Wong CK: Desmoplastic infantile ganglioglioma: a questionably benign tumour. Australas Radiol 49:433–437, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Taratuto AL, Monges J, Lylyk P, Leiguarda R: Superficial cerebral astrocytoma attached to dura. Report of six cases in infants. Cancer 54:2505–2512, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Tiwari B, Sinha VD, Singhvi S, Bhandari A: Infantile desmoplastic astrocytoma: magnetic resonance imaging suggestive of pathology. J Neurosci Rural Pract 7:458–459, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.To CY, Rajah G, Klein E, McNaughton M, Ham S, Haridas A, et al. : Desmoplastic infantile ganglioglioma with associated giant aneurysm—case report. Childs Nerv Syst 31:1413–1418, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Trehan G, Bruge H, Vinchon M, Khalil C, Ruchoux MM, Dhellemmes P, et al. : MR imaging in the diagnosis of desmoplastic infantile tumor: retrospective study of six cases. AJNR Am J Neuroradiol 25:1028–1033, 2004 [PMC free article] [PubMed] [Google Scholar]
- 37.Uro-Coste E, Ssi-Yan-Kai G, Guilbeau-Frugier C, Boetto S, Bertozzi AI, Sevely A, et al. : Desmoplastic infantile astrocytoma with benign histological phenotype and multiple intracranial localizations at presentation. J Neurooncol 98:143–149, 2010 [DOI] [PubMed] [Google Scholar]
- 38.VandenBerg SR: Desmoplastic infantile ganglioglioma and desmoplastic cerebral astrocytoma of infancy. Brain Pathol 3:275–281, 1993 [DOI] [PubMed] [Google Scholar]
- 39.VandenBerg SR, May EE, Rubinstein LJ, Herman MM, Perentes E, Vinores SA, et al. : Desmoplastic supratentorial neuroepithelial tumors of infancy with divergent differentiation potential (“desmoplastic infantile gangliogliomas”). Report on 11 cases of a distinctive embryonal tumor with favorable prognosis. J Neurosurg 66:58–71, 1987 [DOI] [PubMed] [Google Scholar]