Abstract
Adult hippocampal neurogenesis has been implicated in cognitive processes, such as pattern separation, and in the behavioural effects of stress and antidepressants. Young adult-born neurons have been shown to inhibit the overall activity of the dentate gyrus by recruiting local interneurons, which may result in sparse contextual representations and improved pattern separation. We propose that neurogenesis-mediated inhibition also reduces memory interference and enables reversal learning both in neutral situations and in emotionally charged ones. Such improved cognitive flexibility may in turn help to decrease anxiety-like and depressive-like behaviour.
Mood and anxiety disorders are heterogeneous in terms of their aetiology and pathophysiology, which complicates the challenge of identifying specific neural and molecular correlates that may be targeted by future treatments. To gain an integrative understanding of the neurobiology that underlies psychiatric disorders, clinical neuroscience has begun to focus on understanding the complex neural circuitry that is involved in these conditions. Such an approach is a crucial step towards developing novel circuit-based therapeutic strategies.
The hippocampus has considerable importance for cognition and mood. One of the most fascinating features of this brain region is its unusual capacity for adult neurogenesis, a process by which new neurons are continuously generated in the dentate gyrus, where they develop into mature neurons and functionally integrate into the existing neural circuitry1. In humans, 700 new adult-born neurons are added to this circuitry each day in a middle-aged adult2. How such a relatively small number of young neurons functionally contributes to complex behaviours and psychiatric disorders remains unclear.
In this Review, we primarily discuss data from rodent studies that have investigated how adult-born neurons contribute to information encoding in the dentate gyrus both by integrating information as independent encoding units and by inhibiting the activity of mature granule cells. We discuss how this regulation of dentate gyrus function by adult neurogenesis affects the cognitive processes of reversal learning and cognitive flexibility, which may have important implications for both memory and mood. We conclude by proposing that targeting dentate gyrus activity and neurogenesis-dependent cognitive processes, such as pattern separation and cognitive flexibility, may be promising new leads in the treatment of patients with mood disorders.
The hippocampal dorsal–ventral axis
Animal and human studies have shown that the hippocampus is a heterogeneous structure with gradually segregated functional differences along its dorsoventral axis3–6. In rodents, lesion studies and optogenetics have been used to examine the differential functions of the dorsal and the ventral hippocampal poles. These studies have shown that lesions of the dorsal hippocampus primarily impair cognition and spatial learning and that optogenetic inhibition of dorsal dentate gyrus granule cells impairs contextual memory encoding7. In humans, the posterior hippocampus, which is analogous to the dorsal hippocampus in rodents, is larger in individuals who require a large capacity for processing spatial and contextual information, such as taxi drivers8. High activity in the posterior hippocampus has also been found in non-human primates after spatial learning9.
By contrast, lesions of the ventral hippocampus alter emotional behaviour, social interactions10 and stress resilience11–14, and optogenetic activation of ventral dentate gyrus granule cells decreases innate anxiety-like behaviour in rodents7. The human anterior hippocampus, which is analogous to the ventral hippocampus in rodents, is smaller in unmedicated patients with depression and larger in antidepressant-treated patients than in healthy individuals15. Non-human primates with increased anxiety-like behaviour and neuroendocrine activity also show an increase in metabolism in the anterior hippocampus16,17.
Dorsoventral differences are also observed for neurogenesis. Specifically, in rodents, the number of adult-born cells that express doublecortin, a marker of immature neurons, is higher in the dorsal than in the ventral dentate gyrus18–21. However, the number of mature adult-born neurons is higher in the ventral than in the dorsal dentate gyrus20,22, suggesting that, overall, more neurons may be added to the ventral dentate gyrus. In accordance with the functional segregation between the dorsal and the ventral hippocampus, dorsal neurogenesis is increased in rodents that undergo complex spatial and contextual stimulation in an enriched environment20,22,23. By contrast, chronic stress models, such as social defeat, social submissiveness and unpredictable chronic stress, are associated with a decrease in neurogenesis in the ventral dentate gyrus20,22,24,25. In addition, chronic treatment with antidepressants increases neurogenesis predominantly in the ventral dentate gyrus and counteracts the effects of stress in both rodents25 and humans26.
In the hippocampus, the basic cellular connectivity of the trisynaptic circuit is maintained throughout its dorsoventral extent4,27. However, the afferent and efferent connectivity of the hippocampus markedly differs between the dorsal and the ventral poles6. In line with its role in stress processing and mood, the ventral hippocampus is at the centre of a complex neural circuit that regulates anxiety and emotion (FIG. 1. Recent studies have shown that this neural circuit includes glutamatergic projections from the ventral hippocampus to several downstream processing structures that are involved in anxiety regulation, stress responses and reward seeking. For example, projections to the medial prefrontal cortex (mPFC) promote anxiety, context aversion and behavioural inhibition28,29, and bidirectional connections between the ventral hippocampus and the amygdala are implicated in fear processing and social interaction. These amygdala projections have also been proposed to consolidate memories with emotional content30. Moreover, projections from the ventral hippocampus to the nucleus accumbens regulate dopamine release from the ventral tegmental area and are implicated in pleasure and reward seeking31, as well as in regulating stress susceptibility and resilience32. The ventral hippocampus is also an important regulator of the neuroendocrine system, which inhibits hypothalamus–pituitary–adrenal (HPA) axis activity and glucocorticoid release. At the level of the neural circuitry, ventral hippocampal projections activate GABAergic interneurons in the bed nucleus of the stria terminalis, which in turn inhibit the production of corticotropinreleasing hormone from the paraventricular nucleus of the hypothalamus33–35. These ventral hippocampal projections may be important in the regulation of mood and anxiety (discussed in detail below).
Figure 1 |. The ventral hippocampus and the neural circuitry of mood and anxiety.
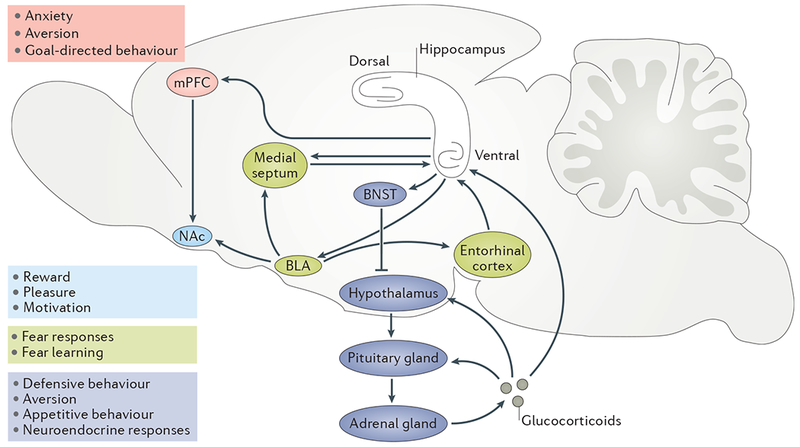
The figure shows a simplified representation of the ventral hippocampus and its place within the circuits that are implicated in mood and anxiety. The basolateral amygdala (BLA) provides cholinergic input to the medial septum and glutamatergic input to the entorhinal cortex186, which both send inputs to and can enhance ventral hippocampus activity to increase fear and anxiety-like responses187. Direct projections from CA1 in the ventral hippocampus provide feedforward inhibition by activating BLA interneurons188. The ventral hippocampus also sends glutamatergic project ions to GABAergic medium spiny neurons in the nucleus accumbens (NAc) to regulate NAc control over dopamine release from the ventral tegmental area (not shown). Ventral hippocampus-to-NAc projections promote reward-seeking behaviour in the absence of stress31 but induce anxiety-like and depressive-like behaviour during stress32. Direct projections from the ventral hippocampus to the medial prefrontal cortex (mPFC) promote anxiety and stress susceptibility29 and are also involved in antidepressant effects32. Glutamatergic projections from the ventral hippocampus activate GABAergic interneurons in the bed nucleus of the stria terminalis (BNST). These interneurons inhibit neurosecretory neurons in the paraventricular nucleus (PVN) of the hypothalamus. PVN neurons release corticotropin-releasing hormone, which stimulatesthe production of adreno-corticotropic hormone (ACTH) in the pituitary gland. ACTH stimulatesthe production and release of glucocorticoids from the adrenal cortex into the bloodstream. Glucocorticoids exert feedback inhibition on the hypothalamus–pituitary–adrenal axis by activating glucocorticoid and mineralocorticoid receptors in the pituitary gland, hypothalamus and hippocampus34. Consistently high levels of glucocorticoids during conditions of chronic stress can reduce hippocampal neurogenesis and cause neuronal atrophy127,189.
Dentate gyrus function
The ability of the dentate gyrus to generate new neurons has stimulated research on how these adult-born neurons influence hippocampal information processing. In a 12-week-old adult mouse, the number of 4–6-week-old adult-born neurons is approximately 10% of the total number of developmentaly born, mature granule cells36–38. These young adult-born neurons in their critical period of development have distinct electrophysiological properties, including high input resistance and a lack of GABAergic inhibition, which results in these cells having a greater propensity for hyperexcitability and exhibiting lower activation thresholds than mature granule cells. Young adult-born neurons also show enhanced plasticity and long-term potentiation, which are mediated by NR2B-containing NMDA receptors36,39,40. In addition, 4–6-week-old neurons make a unique contribution to hippocampus-dependent behaviours41. Below, we discuss how adult-born neurons may regulate complex aspects of information processing in the dentate gyrus microcircuit by encoding discrete spatial and temporal information as independent encoding units and by regulating the activity of mature granule cells.
Adult-born neurons are recruited by learning.
Rodent studies have suggested that hippocampus-dependent learning tasks that involve temporal-based associations or spatial contextual navigation promote and, indeed, require adult neurogenesis. By contrast, various studies have indicated that non-hippocampus-dependent learning does not require adult neurogenesis42–46 (but see also REFS 47–51). In line with a role for young adult-born neurons in novelty encoding and learning, navigating in novel and enriched environments also increases adult neurogenesis23,52,53. Studies using immediate early genes as markers of neuronal activity showed that 2–4-week-old adult-born cells are recruited during spatial learning, fear memory retrieval, or stressful and anxiogenic conditions54–57,58. In addition to contextual information encoding, computational models and electrophysiological studies suggest that young neurons may encode temporal information during their critical period59,60. As the population of young neurons matures, these cells leave their critical period, and events that are far apart in time would therefore be encoded by different, non-overlapping hyperexcitable cell populations. By contrast, closely related events would be encoded by a similar population of young neurons, which could be a potential mechanism for associating proximally dated memories59. In support of this idea, adult-born neurons that are activated by exposure to an enriched environment during the first 3 weeks of development are more likely to be reactivated by re-exposure to the same environment once they have reached maturity61. However, such a temporal coding model relies on the assumption that, owing to their hyperexcitability, young adult-born neurons are indeed preferentially activated by learning compared with mature granule cells58, a finding that is not yet well established62. Nevertheless, the addition of new neurons to the dentate gyrus may be an efficient method of encoding new information without disrupting previously stored memories63–65.
Adult-born neurons modulate dentate gyrus function.
In addition to their proposed role in encoding new information, adult-born neurons also modulate the activity of mature granule cells. For example, the ablation of neurogenesis by X-ray irradiation increases dentate gyrus gamma burst amplitude and increases the number of active mature granule cells during reversal learning tasks66,67. Conversely, increasing neurogenesis reduces dentate gyrus excitability68, and optogenetic stimulation of adult-born neurons reduces mature granule cell activity69.
In vivo, this inhibitory effect of young neurons is specific to anxiogenic conditions and neurogenesis-dependent tasks. For example, neurogenesis ablation only increases immediate early gene expression in the dentate gyrus during neurogenesis-dependent conflict situations67. Accordingly, optogenetic activation of adult-born neurons decreases dentate gyrus activity in conditions of novelty and anxiety, whereas the activation of a similar number of mature granule cells has no effect on the activity of the remaining mature cells69. However, in vitro slice electrophysiology has shown that, under baseline conditions, mature (8-week-old) adult-born neurons are stronger inhibitors of the dentate gyrus than young (4-week-old) neurons70. These discordant data emphasize the need to determine the exact age at which and the exact behavioural situations in which adult-born neurons exert unique inhibitory control over mature granule cells to modify the dentate gyrus network (BOX 1).
Box 1 |. The dentate gyrus inhibition hypothesis.
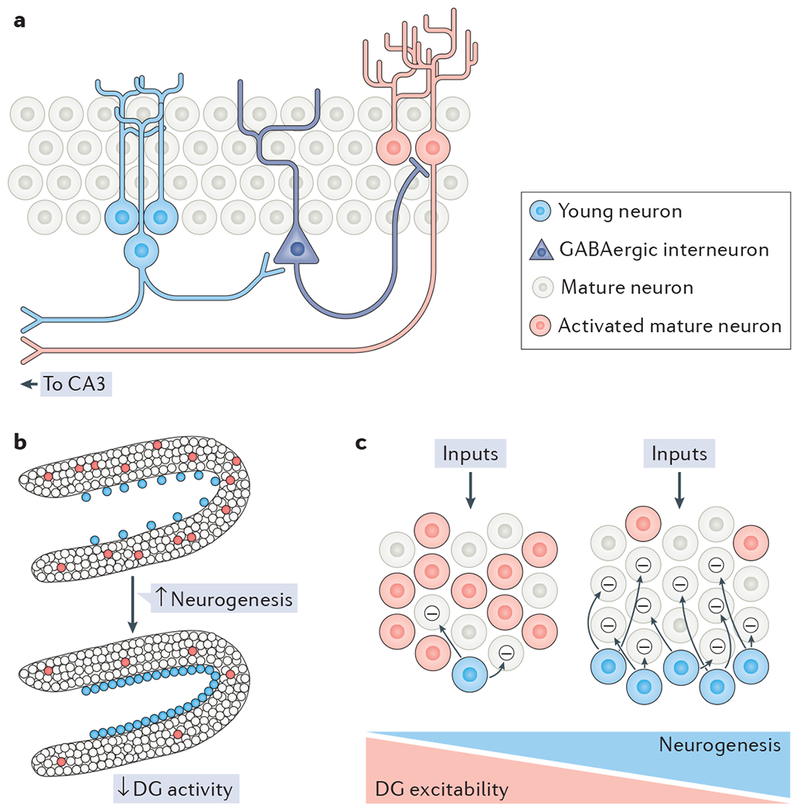
Adult-born neurons functionally integrate into the rodent dentate gyrus (DG) at 2–3 weeks of age but require more than 8 weeks to develop electrophysiological properties that are similar to those of developmentally born, mature granule cells. At the microcircuit level, presynaptic terminals onto hilar interneurons develop at ~4 weeks of age and recruit feedback inhibition onto mature granule neurons40,54 (see the figure, part a). Accordingly, increasing neurogenesis in mice through environmental enrichment or transgenic strategies inhibits mature granule cells and reduces the overall excitability of the DG68,69. In line with this requirement for neurogenesis in DG inhibition, optogenetic stimulation of young neurons activates GABAergic interneurons in the hilus, and these interneurons in turn inhibit the dense population of mature cells in vivo and in vitro69,70. Conversely, ablation of neurogenesis reduces inhibitory input to granule cells, increases DG activity, enhances input–output coupling and expands the pool of active cells during reversal learning tasks66,67 (see the figure, part b).
Pattern separation by the DG has been proposed to rely on sparse activation of the dense granule cell population. By inhibiting mature granule cells, adult-born neurons may decrease the activity of the DG during anxiogenic tasks and DG-dependent behaviours (see the figure, part c).
Considering that stress and glucocorticoids increase dentate gyrus synaptic currents in vitro71 and that stress increases dentate gyrus activity in vivo56, neurogenic inhibition of the dentate gyrus may be particularly relevant for stress-induced psychopathology, including anxiety and depression. Changing dentate gyrus activity may also ultimately determine the strength of hippocampal projections to downstream associated brain structures (FIG. 1). Therefore, neurogenesis may exert potent effects on behaviour by modulating the neuronal networks of mood and anxiety through activity changes in the hippocampus.
Neurogenesis, learning and memory
Much research has examined the effects of mood on memory encoding and retrieval72. However, less is known about how memory can influence mood and anxiety. We propose below that some of the effects of neurogenesis on mood and anxiety may not be independent of the effects of neurogenesis on cognition.
Neurogenesis and pattern separation.
A major task of a functional memory system is to encode highly similar pieces of information discretely and without interfering with previously established memories. The dentate gyrus has been proposed to accomplish this task by disambiguating similar contextual representations through the computational process of pattern separation73,74. In the dentate gyrus, neuronal inputs from the entorhinal cortex are first encoded by sparse populations of granule neurons that form distinct representations of mnemonic information with high similarity75–77. The addition of new neurons with enhanced plasticity to the dentate gyrus has indeed been proposed to be most efficient in encoding new information while avoiding the loss of memory retrieval properties64,65,78. This sparsely encoded information in the dentate gyrus is subsequently relayed to CA3 pyramidal neurons, which store these representations in auto-associative cellular networks that allow memory retrieval when partial cues of incomplete aspects of that memory are being detected (pattern completion)75,79,80.
Experimentally, ablating neurogenesis or the optogenetic silencing of adult-born neurons impairs the ability to discriminate highly similar contexts (high interference)7,51,77,81–83. Accordingly, increasing the number of adult-born neurons84–87 or their functional integration into the dentate gyrus microcircuit88 enhances the ability to discriminate highly similar contexts. By contrast, discriminating contexts that are very different (low interference) is not dependent on neurogenesis37,51,77,84,85,89.
According to computational models, sparse activity of the dentate gyrus is indeed necessary for pattern separation73,90–92. Young neurons themselves may thus be rather ineffective at separating similar events, as their hyperexcitability may make them broadly tuned to new, temporally related information37,59. By contrast, mature granule cells are proposed to be more efficient in the sparse encoding of contextual information owing to their higher excitation threshold37,63. It has thus been suggested that adult-born neurons may function as ‘pattern integrators’ that are able to associate similar events with each other, as opposed to carrying out pattern separation63. Such a pattern integration function, which may provide a means by which to encode temporal associations, may not conflict with a second role for young neurons in pattern separation. On the basis of the activation of local inhibitory circuits (BOX 1), highly excitable young cells may simultaneously inhibit the population of mature cells to achieve the sparse activation that is required for pattern separation. In this hypothetical dual model, young adult-born neurons would act as pattern integrators to encode temporal associations of information and as indirect ‘pattern separators’ that inhibit mature granule cells to facilitate sparse coding.
The dentate gyrus and emotional memory engratns.
To elucidate how fear and anxiety are encoded by the hippocampus, rodent studies have used contextual fear discrimination learning and indelible labelling of active cells using immediate early gene-dependent tagging strategies. When mice learn to associate a context with an aversive event, such as a footshock, that fear-memory is encoded by a cellular representation in the hippocampus. When mice are re-exposed to the same context in which they had previously received the footshock, a proportion of the cells that were active during encoding are also recruited during the retrieval of that memory. By contrast, exposure to a novel context, in which fear was never experienced, activates a different population of neurons in the dentate gyrus93–95.
These memory traces, or engrams, in the dentate gyrus are indeed necessary and sufficient to produce fear and anxiety-like behaviours. For example, optogenetic activation of cells that are labelled during the encoding of a fearful context can elicit a fear response in a neutral context in which fear was never experienced96. Conversely, the silencing of engrams of a fearful experience suppresses fear and anxiety-like behaviour during memory recall94. A fear memory can also be converted into a rewarding memory when the fear-encoding engram is activated during the experience of a reward. As a result, mice that associate the original fear engram with reward exhibit less freezing in the aversive context. Similarly, the memory of a reward can be replaced by an aversive memory when the reward engram is activated during exposure to a fearful context97. Activating these dentate memory engrams of positive experiences engages excitatory projections from the basolateral amygdala to the nucleus accumbens and ameliorates stress-induced anxiety-like and depressive-like symptoms98.
Neurogenesis and emotional memory engrams.
In agreement with the crucial role of neurogenesis in pattern separation, adult-born neurons are necessary for encoding information in non-overlapping memory engrams. Indeed, ablation of neurogenesis, either by hippocampal X-ray irradiation or by optogenetically silencing adult-born granule cells, impairs engram reactivation and weakens the memory of a fearful event37,41,94. However, ablation of neurogenesis impairs reactivation in CA3 but not in the dentate gyrus itself94, suggesting that neurogenesis regulates information processing across hippocampal subregions99.
In addition to pattern integration and pattern separation during memory encoding, neurogenesis has also been proposed to promote the clearance of previously established memories100,101. This post-encoding memory erasure may act concurrently with pattern separation to reduce proactive interference, a process by which previously learned information impedes the learning of new information that has a highly similar content. This memory erasure may depend on the ability to inhibit mature granule cells that store a previously encoded memory (FIG. 2) but may not necessarily compromise previously learned information that has already been transferred from the temporary memory storage of the hippocampus to the permanent cortical storage areas.
Figure 2 |. Neurogenesis facilitates cognitive flexibility by allowing the formation of new distinct memory traces.
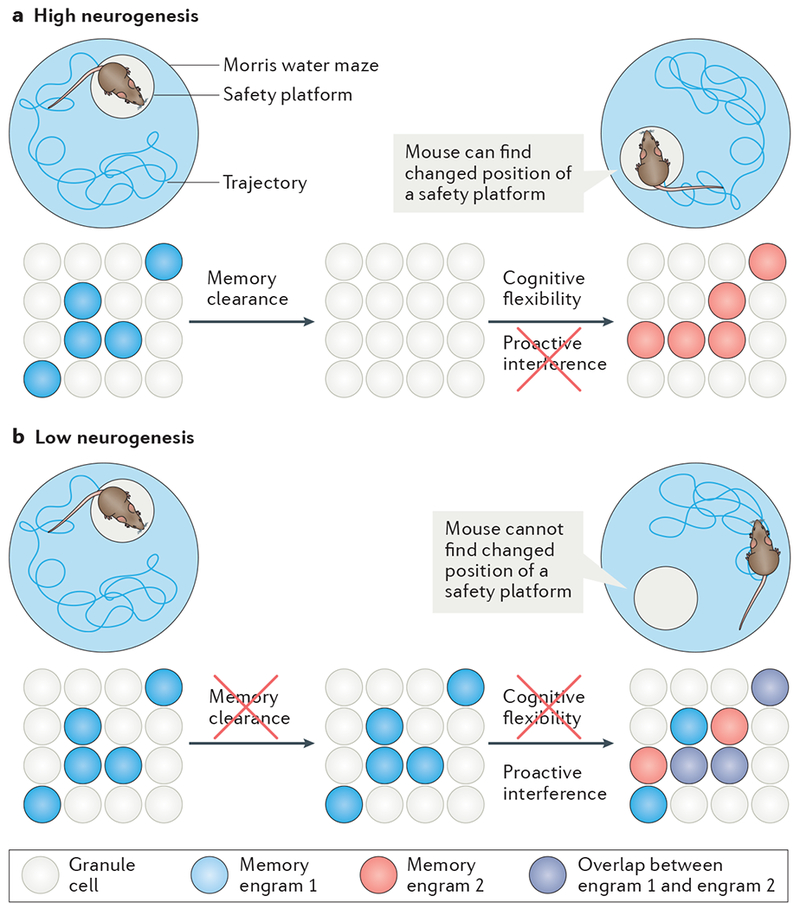
Contextual memories, such as where a safety platform is hidden in a Morris water maze, are encoded by neuronal ensembles in the dentate gyrus. These cellular representations of a memory are known as memory traces or engrams.
a | With high neurogenesis, engrams may be cleared faster and transmitted to downstream processing structures. Efficient memory clearance reduces proactive interference between the memory of the previous location and the ability to accurately encode a novel location. This may facilitate reversal learning and cognitive flexibility to promote the finding of a new safety platform once the location has been changed101,105,106.
b | With low neurogenesis, engrams of previous contexts may not be cleared sufficiently. As a result, novel contextual contingencies may not be encoded appropriately by distinct neuronal ensembles. This may increase proactive interference, reduce cognitive flexibility and impair the ability to find the new location of a hidden safety platform.
Neurogenesis and cognitive flexibility.
Although cognitive flexibility is mostly attributed to the PFC, human functional MRI (fMRI) and positron emission tomography studies102,103, as well as rodent reversal learning experiments67,104–107, have suggested that it may also require the hippocampus. Computational studies have also proposed that hippocampal neurogenesis may be necessary for cognitive flexibility, as it allows the avoidance of interference between novel and previously formed memories64,65,78,108–110. This was first experimentally demonstrated by studies showing that mice with reduced neurogenesis were impaired in finding a safety platform in the Morris water maze105,106. Accordingly, environmental enrichment, which increases neurogenesis23, also increased the ability of an animal to apply more efficient spatial search strategies in this task after a platform location was changed111. These findings were extended by studies using similar versions of the Morris water maze task112,113, touch-screen discrimination tasks107 or active place-avoidance tasks in which neurogenesis is required for learning that the position of a shock zone on a rotating platform has changed67. In all these experiments, neurogenesis was only required for learning the reversal of a rule and not for learning the initial rule (for example, neurogenesis was not required for initially finding the safety location). This suggests an emerging new function for neurogenesis in reversal learning and cognitive flexibility, in addition to its role in pattern separation during the encoding of new memories. In accordance with the memory clearance hypothesis78,100, neurogenesis may promote the erasure of previously learned associations; for example, where a safety platform is hidden in the Morris water maze task. ‘Forgetting’ the old association may consequently facilitate the learning of novel associations, such as the new location of the safety platform101 (FIG. 2). It is conceivable that such a process requires the inhibition of mature dentate gyrus granule cells to silence previously established engrams and to enable the formation of new memory traces and, ultimately, cognitive flexibility. We therefore propose a model by which neurogenesis-dependent inhibition of the dentate gyrus facilitates cognitive flexibility by allowing new, distinct memories to be formed faster (FIG. 2). Below, we discuss how this effect of neurogenesis on cognitive flexibility may contribute to mood and anxiety disorders.
Neurogenesis, mood and anxiety
The effects of stress on neurogenesis, depression and anxiety.
Adult neurogenesis has been extensively described as a key function of the hippocampus that is sensitive to the effects of stress33,35,114. Chronic stress procedures for 2–8 weeks, such as chronic social defeat24, unpredictable chronic mild stress20,115–117, chronic immobilization118, prenatal stress119,120 and early life stress121, reduce neurogenesis in rodents. Similar effects are also observed in non-human primates, in which social isolation or subordination reduces neurogenesis and causes anhedonia122–124 These stress effects on neurogenesis occur most prominently in the ventral portion of the hippocampus22,24 and seem to be relevant to the clinical situation, as patients with depression also exhibit decreased levels of neurogenesis125. Most rodent studies have demonstrated that reducing or enhancing neurogenesis modulates anxietylike and depressive-like behaviours only in response to stress and that neurogenesis does not modulate these behaviours if stress is absent115,117,126–128. However, some studies have suggested that even without prior stress, neurogenesis ablation increases innate anxiety-like and approach-avoidance behaviour126,129.
The crucial role of neurogenesis in learning and memory suggests that changes in neurogenesis, for example, as a result of chronic stress, may ultimately affect intra-hippocampal information processing and information relay to downstream connected brain structures, such as the amygdala and the hypothalamus, which are crucial for regulating anxiety and neuroendocrine function (FIG. 1). Indeed, changes in neurogenesis may affect basolateral amygdala activity after stress, although a causal relationship remains to be established118.
Neurogenesis and the neuroendocrine system.
Hyperactivity of the HPA axis and elevated glucocorticoid levels are potent mediators of stress effects on neurogenesis. For example, chronic stress does not impair neurogenesis in adrenalectomized rodents in which glucocorticoids are maintained at consistently low levels24. Conversely, chronic treatment with exogenous glucocorticoids precipitates anxiety-like and depressive-like behaviour and reduces neurogenesis in rodents through a glucocorticoid receptor-mediated effect127,128,130–132. Glucocorticoids have also been shown to reduce neurogenesis in in vitro human hippocampal stem cell culture models133,134. In addition to being particularly vulnerable to the effects of glucocorticoids, neurogenesis has been implicated in regulating the body’s response to, and recovery from, stress. Glutamatergic projections from the ventral hippocampus activate GABAergic inhibitory interneurons in the bed nucleus of the stria terminalis, which in turn inhibit hypothalamic paraventricular nucleus neurons and glucocorticoid release from the adrenal cortex33–35. Without neurogenesis, mice exhibit impaired HPA axis feedback inhibition and an increased glucocorticoid surge after acute stress compared with mice in which neurogenesis is intact135,136. These findings suggest that neurogenesis is required to maintain appropriate hippocampal inhibitory control over the HPA axis, and that impairments in neurogenesis (for example, after chronic stress) may exacerbate glucocorticoid abnormalities and reduce neurogenesis. The resulting persistent HPA axis hyperactivity may set in motion a vicious cycle, in which glucocorticoid abnormalities continue to reduce neurogenesis, ultimately leading to long-term disturbances in stress reactivity and sustained anxiety-like and depressive-like behaviour. This is supported by a rodent study showing that neurogenesis is required for spontaneous recovery from anhedonia, anxiety and cognitive flexibility impairments 4–6 weeks after exposure to chronic stress137.
Reconciling memory, cognitive flexibility and mood disorders.
The role of neurogenesis in pattern separation and cognitive flexibility may have important implications for the above-described development of mood and anxiety disorders. During the encoding of a stressful or fearful experience, a memory engram of the fear-associated context is formed in the dentate gyrus. Once the stressor is over, cognitive flexibility is required to learn that the same context has become safe and is now no longer associated with fear. This is often modelled by fear extinction paradigms in rodents, which have been suggested by some, but not all, studies to be dependent on neurogenesis138 (but see also REF 84). To enable fear extinction or cognitive flexibility at the cellular level, adult-born neurons may inhibit the mature granule cells that had previously encoded the fear association and that contain the memory engram of the fearful situation. Inhibition of these granule cells would allow the erasure of the engram encoding the fearful association and, at the same time, would enable sparse activity for pattern separation during the encoding of the new safe association. If neurogenesis is impaired, a previously fear-associated memory engram may not be sufficiently cleared from the dentate gyrus and the new, safe condition may not be discretely encoded. As a result, the perception of fear will persist owing to a lack of neurogenesis and impaired cognitive flexibility. A lack of cognitive flexibility may then contribute to persistent HPA axis abnormalities and the engagement of stress and anxiety circuits, potentially leading to chronic psychopathology (FIG. 3). This effect may be particularly relevant from a clinical perspective, as cognitive flexibility is impaired across many psychiatric disorders, including post-traumatic stress disorder139, obsessive–compulsive disorders140 and depression141. Increasing neurogenesis may thus be a potential therapeutic strategy to facilitate reversal learning and cognitive flexibility as a treatment for patients with depression and anxiety disorders.
Figure 3 |. Neurogenesis promotes efficient stress recovery.
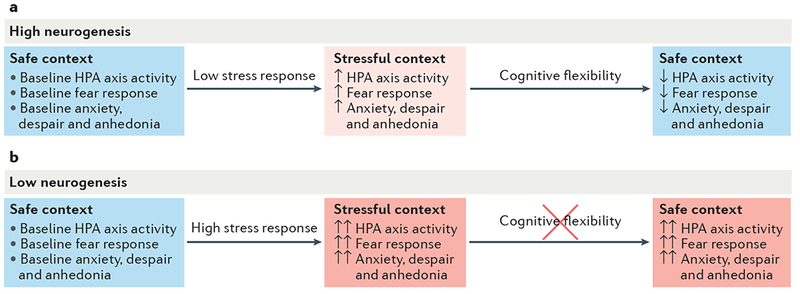
High or low levels of adult hippocampal neurogenesis do not influence baseline hypothalamus–pituitary–adrenal (HPA) axis activity, fear responses, innate anxiety, despair or anhedonia in rodents84,115,128. During stressful experiences, neuroendocrine responses and anxiety-like and depressive-like behaviour are less increased in mice with high levels of neurogenesis (part a) than in mice with low levels of neurogenesis135,136 (part b). Once the stressor has ended, neurogenesis facilitates reversal learning and cognitive flexibility137 to promote positive adaptation to novel, non-threatening environments. Therefore, increasing neurogenesis may facilitate fast recovery from HPA axis hyperactivity and anxiety-like and depressive-like behaviours and may prevent the development of chronic stress-induced psychopathology (part a).
Therapeutic potential of neurogenesis
Neurogenesis mediates the behavioural effects of antidepressants.
Major support for the therapeutic potential of adult-born neurons originates from evidence that pharmacologically diverse antidepressants increase neurogenesis in rodents117,142–144, non-human primates122, human post-mortem brain tissue26,145 and human hippocampal progenitor cells in vitro146. These neurogenic effects of antidepressants are most pronounced in the ventral dentate gyrus and are required for some of the behavioural effects of selective serotonin reuptake inhibitors (SSRIs) in rodents25,115,116,127. Non-pharmacological antidepressant strategies also potently increase neurogenesis and include interventions such as electroconvulsive shock therapy, environmental enrichment, physical exercise and calorific restriction23,53,123,142,147,148. Accordingly, neurogenesis is sufficient to confer antidepressant effects in chronically stressed mice128,149 and to facilitate spontaneous remission from depression-like symptoms137. Enhancing neurogenesis through pharmacological or non-pharmacological means may thus be a promising strategy to confer stress resilience or antidepressant effects in patients. Indeed, physical (and mental) exercise has been shown to be effective in the treatment of clinical depression150,151. However, a limitation of this approach is that not all antidepressant effects are dependent on neurogenesis117,127, and increasing neurogenesis is not always necessary to change behaviour117,126,152. Some pathological conditions, such as epilepsy, also increase neurogenesis, suggesting that an aberrant increase in neurogenesis may not always be beneficial153,154.
Glucocorticoid-signalling or neurotrophic-signalling mechanisms that regulate neurogenesis have been identified in adult neural stem cells and young neurons36,133,134,155–157. However, the clinical challenge remains to specifically target this defined population of adult-born cells pharmacologically. It is important to note in this context that the effects of SSRIs are, at least in part, mediated by serotonin 1A receptors (5HT1 ARs) that are located on mature granule cells but not on young adult-born neurons158. Whether these 5HTlAR-dependent effects of SSRIs are mediated by enhanced neurogenesis that results from the 5HT1AR-dependent release of neurotrophic factors from mature granule cells remains to be elucidated158. Of note, 5HT1ARs are Gi protein-coupled receptors that silence neuronal activity by opening G protein-coupled inward-rectifying potassium (GIRK) channels159. Therefore, it is possible that inhibiting dentate gyrus function may elicit antidepressant or anxiolytic effects, which is consistent with the proposed inhibitory role of young adult-born granule cells69 (BOX 1). This assertion is also supported by a study showing that blocking GABA type A receptors in the ventral, but not in the dorsal, dentate prevents the anxiolytic effects of exercise56. Directly targeting the activity of mature granule cells may expand our therapeutic opportunities. Genes that are expressed specifically in the dentate gyrus may be promising candidate targets for novel drugs to confer such inhibitory effects (for examples, see REF. 160).
Neurogenesis and dentate gyrus impairments as targetable endophenotypes.
The heterogeneity of depression is a major impediment to the correct diagnosis and treatment of patients161. This heterogeneity complicates the identification of novel genes and brain circuits because the complexity of depressive symptomatology probably includes several different endophenotypes with distinct neurobiological impairments. Depression might be more correctly classified using a ‘bottom-up approach’ in which patients are diagnosed and treated based on discrete impairments of known neurobiological origin162,163.
So far, no in vivo markers for neurogenesis in humans are available, although some promising tools are being developed. For example, magnetic resonance spectroscopy of the proton nuclear magnetic resonance peak at 1.28 ppm may prove useful for quantifying neural progenitor cells in the dentate gyrus164. Positron emission tomography markers, such as 3′-deoxy-3′-[18F]fluorothymidine, which detects changes in cell proliferation, have recently been used in rodents and could potentially be translated into clinical applications165,161. Cerebral blood volume has been proposed as an indirect measure of human neurogenesis, as it is sensitive to changes in angiogenesis that may correlate with neurogenesis in the dentate gryus167. In addition to these in vivo approaches, in vitro strategies could use patient-specific human induced pluripotent stem cells to reprogramme patient-specific skin or hair cells to neurons that have granule cell-like phenotypes168. These granule cells could then be probed in culture for their ability to proliferate and differentiate or potentially grown as 3D hippocampal organoids169, which may allow future drug screening for compounds with neurogenic potential on a patient-by-patient basis.
Identifying pattern separation and cognitive flexibility deficits in humans.
In addition to directly measuring levels of adult-born neurons, novel, neurogenesis-guided approaches could be aimed at identifying and treating dentate gyrus-dependent cognitive processes, such as pattern separation and cognitive flexibility, to determine the extent to which neurogenesis and dentate gyrus dysfunction contribute to disease manifestation.
In humans, neurocognitive testing, fMRI and in vivo hippocampal electrophysiological recordings have implicated the hippocampus in pattern separation. These studies have used hippocampal-dependent behavioural tasks that require discriminating small differences in highly similar object representations170,171, emotional discrimination tasks172, delayed match-to-sample tasks49,77,102,173 and virtual-reality memory tasks with a spatial navigation component174. Indeed, performance in these tasks is impaired in individuals with depression who, as described above, may exhibit reduced levels of hippocampal neurogenesis26,125,173,175–177. Behaviour-based tests for hippocampal function and pattern separation may be useful to help guide diagnosis and to identify specific patients who would benefit from therapeutic interventions aimed at enhancing neurogenesis. For example, set-shifting tasks, in which attention needs to be redirected from a reinforced stimulus to a new stimulus, could probe cognitive flexibility178,179. Although performance in set shifting has primarily been linked to the PFC, some studies suggest that perseverative errors may also partly depend on the hippocampus103,180–182. We propose that, to distinguish dentate gyrus-dependent and PFC-dependent cognitive flexibility in humans, tasks will need to be designed that require the use of contextual or temporal cues to engage the hippocampus.
Tests for hippocampal function could potentially also include virtual-reality tasks that are modelled on rodent behavioural tests of pattern separation or reversal learning. For example, virtual radial arm mazes have already been used in some clinical studies and could assess pattern separation or reversal learning deficits with a similar design to those already used in rodent studies77,183 (FIG. 4).
Figure 4 |. Potential methods of harnessing the function of adult-born neurons to treat dentate gyrus-dependent mood and anxiety disorders.
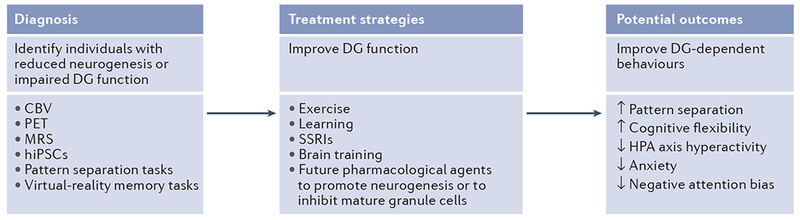
To harness adult hippocampal neurogenesis as a treatment for depression and anxiety, patients with reduced neurogenesis or patients with neurogenesis-dependent impairments in dentate gyrus (DG) function will first need to be identified (left panel). Possible diagnostic measures could include imaging technigues of cerebral blood volume (CBV) in the DG, which has been shown to correlate with levels of neurogenesis167; positron emission tomography (PET) using markers for neurogenesis that are currently being developed in mice165; and magnetic resonance spectroscopy (MRS) of the 1.28 ppm peak, which has been suggested to indicate levels of proliferating neural progenitor cells164. In vitro models, such as human induced pluripotent stem cells (hiPSCs), could be used to grow granule-like neurons from patient-specific skin or hair cells168. This may allow the testing of the effect of stress hormones or antidepressants on neurogenesis in culture146. Neurocognitive testing could also be used to identify patients with DG-dependent cognitive impairments. These tests could involve pattern separation tasks170–172 or virtual-reality memory tasks183 with a spatial navigation component, as patients with depression have an impaired performance in these tasks. Once patients with neurogenesis deficits or DG impairments have been identified, treatments could be used that have been shown to reliably increase neurogenesis in rodent models, such as exercise53, learning-based approaches23 and selective serotonin reuptake inhibitors (SSRIs)142. Cognitive strategies might include brain-training games, which have been shown to improve reversal learning and cognitive flexibility and to engage mood-relevant neural circuitry184. The development of future pharmacologicaltargets should be aimed at compoundsthat can increase neurogenesis orthat can inhibit mature granule cells and DG activity in patients. Enhancing neurogenesis and improving DG function in patients with confirmed impairments may increase cognitive flexibility and reduce hypothalamus–pituitary–adrenal (ElPA) axis hyperactivity, anxiety, negative attention bias and negative affect. Treating these impairments may facilitate efficient stress recovery and prevent or counteract the development of chronic psychopathology.
Treating pattern separation and cognitive flexibility deficits in humans.
Once patients have been classified on the basis of hippocampal pathology or hippocampus-dependent cognitive deficits, pharmacological interventions could include novel compounds, such as the anti-apoptotic and pro-neurogenic chemical P7C3, which has antidepressant effects in mice by increasing neurogenesis149. Similar compounds could also be developed to target dentate gyrus activity, as suggested above. Non-pharmacological strategies to increase neurogenesis should be informed by rodent studies, which have shown that neurogenesis can be enhanced by learning, aerobic exercise53,150, environmental enrichment23 and caloric restriction148. These strategies may indeed be useful therapeutic tools in humans to reduce anxiety disorders and depression. Novel therapeutic interventions could also target cognitive functions that are dependent on neurogenesis, such as reversal learning and cognitive flexibility, by using brain-training games that are designed to engage the hippocampus184,185 (FIG. 4)
Conclusions and perspectives
The hippocampus has repeatedly been implicated in learning and memory, as well as in the behavioural response to stress and in the pathophysiology of mood disorders. These cognitive and mood-related functions of the hippocampus are not independent processes. Adult neurogenesis in the dentate gyrus has been proposed to regulate information processing in the hippocampus, and young neurons may contribute to the circuitry both by integrating new information and by inhibiting the activity of the dense network of mature granule cells. This inhibitory effect of adult-born neurons may be important to erase previously established, fear-associated memories and to allow new, non-fear-associated memories to be formed instead (cognitive flexibility). At the same time, inhibition may facilitate sparse encoding of new information (pattern separation). Ultimately, memory clearance and sparse coding may reduce interference between the previous memory of a stressful or fearful context and the experience of a new safe context. Therefore, cognitive flexibility and pattern separation may be required to encode the novel context as safe and to facilitate recovery from anxiety and depressive symptoms by improving the ability to learn that the adverse context has changed.
In the clinic, identifying patients in whom dentate gyrus function and cognitive flexibility are impaired could be crucial to guide appropriate, individualized treatments. Indeed, increasing neurogenesis or enhancing cognitive flexibility may thus represent promising new treatment strategies for patients with compromised dentate gyrus function.
Acknowledgements
R.H. is supported by the Hope for Depression Research Foundation (HDRF, RGA-13-003), the US National Institutes of Health (R01 AG043688, R01 MH083862, R37 MH068542) and NYSTEM (C029157). C. A. is supported by a K99/R00 award from the US National Institutes of Health (K99MH108719).
Glossary
- Cognitive flexibility
A cognitive process of executive function by which previously learned behavioural strategies can be modified to adapt to changes in environmental contingencies. Enables adaptation to new situations by switching from previously held beliefs or thoughts to new response strategies
- Optogenetics
A research technique that allows the control of the activity of live neurons that have been genetically modified to express light-sensitive ion channels. Cell type-specific expression of photosensitive cation or anion channels can be used to acutely depolarize or hyperpolarize neurons with light in a spatially and temporally defined manner
- Trisynaptic circuit
The flow of incoming information within the hippocampus generally occurs via three synapses: from entorhinal cortex to dentate gyrus, from dentate gyrus to CA3, and from CA3 to CA1
- Critical period
The first 2–6 weeks in the development of adult-born neurons during which they display heightened excitability and plasticity
- Input resistance
In a neuron, the ratio of the input voltage to the input current, as determined by the number of open membrane ion channels. Young adult-born neurons display high input resistance due to a low density of membrane K+ channels during early development
- GABAergic inhibition
Inhibitory interneurons primarily release GABA, which activates ionotropic GABA type A receptors (GABAARs), or metabotropic GABABRs. GABAARs are Cl− channels that hyperpolarize mature neurons. In young adult-born neurons, GABAAR-mediated currents are depolarizing because of a reverse Cl− gradient.
- Immediate early genes
Genes the expression of which is rapidly and transiently increased following neuronal activation; for example, Fos, Arc and Zif268. Such genes are used as markers for neuronal activity or to indelibly label neurons that are active during a specific experience
- X-ray irradiation
Repeated exposure to 2.5–5 Gy of X-rays eliminates proliferating progenitor cells from the dentate gyrus and consequently ablates neurogenesis
- Entorhinal cortex
A medial temporal lobe area that is divided into lateral and medial entorhinal cortices and that provides the main excitatory input into the hippocampal dentate gyrus
- Hilar interneurons
Dentate gyrus interneurons are a diverse group of inhibitory neurons that are primarily located in the hilus and use GABA as their primary neurotransmitter.
- Engrams
Neuronal ensembles that are recruited during memory encoding to form a cellular representation of that memory (memory trace)
- Proactive interference
A neurobiological process by which previously learned information hinders the acquisition and distinct encoding of a new memory trace
- Endophenotypes
Specific aspects of complex diseases that have a measurable biological foundation. Can be used to stratify heterogeneous (psychiatric) illnesses.
- Negative affect
The experience of unpleasant emotions, poor self-confidence and lack of motivation
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Taupin P & Gage FH Adult neurogenesis and neural stem cells of the central nervous system in mammals. J. Neurosci. Res. 69, 745–749 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Spalding KL et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This important study provided evidence for continued neurogenesis in adulthood at rates that suggest that it may have an important role in human behaviour.
- 3.Kjelstrup KB et al. Finite scale of spatial representation in the hippocampus. Science 321, 140–143 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Thompson CL et al. Genomic anatomy of the hippocampus. Neuron 60, 1010–1021 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Fanselow MS & Dong HW Are the dorsal and ventral hippocampus functionally distinct structures? Neurones, 7–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strange BA, Witter MP, Lein ES & Moser EI Functional organization of the hippocampal longitudinal axis. Nat Rev. Neurosci. 15, 655–669 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Kheirbek MA et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that mature granule neurons in the dorsal dentate gyrus are important for learning, whereas granule neurons in the ventral dentate gyrus control anxiety.
- 8.Maguire EA et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl Acad. Sci. USA 97, 4398–4403 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo M, Fernandez I, Nakamura K & Gross CG Functional differentiation along the anterior–posterior axis of the hippocampus in monkeys. J. Neurophysiol. 80, 1002–1005 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Felix-Ortiz AC & Tye KM Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 34, 586–595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes KR Dorsal and ventral hippocampus lesions and maze learning: influence of preoperative environment. Can.J. Psychol. 19, 325–332 (1965). [DOI] [PubMed] [Google Scholar]
- 12.Stevens R & Cowey A Effects of dorsal and ventral hippocampal lesions on spontaneous alternation, learned alternation and probability learning in rats. Brain Res. 52, 203–224 (1973). [DOI] [PubMed] [Google Scholar]
- 13.Henke PG Hippocampal pathway to the amygdala and stress ulcer development. Brain Res. Bull. 25, 691–695 (1990). [DOI] [PubMed] [Google Scholar]
- 14.Moser E, Moser MB & Andersen P Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J. Neurosci. 13, 3916–3925 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boldrini M et al. Benzodiazepines and the potential trophic effect of antidepressants on dentate gyrus cells in mood disorders. Int. J. Neuropsychopharrnacol 17, 1923–1933(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shackman AJ et al. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc. Natl Acad. Sci. USA 110, 6145–6150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Leary OF & Cryan JF A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol. Sci. 35, 675–687 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Jinno S Topographic differences in adult neurogenesis in the mouse hippocampus: a stereology-based study using endogenous markers. Hippocampus 21,467–480 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Snyder JS, Radik R, Wojtowicz JM & Cameron HA Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus 19, 360–370 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanti A et al. Region-dependent and stage-specific effects of stress, environmental enrichment, and antidepressant treatment on hippocampal neurogenesis. Hippocampus 23, 797–811 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Piatti VC et al. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J. Neurosci. 31,7715–7728 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanti A, Rainer Q, Minier F, Surget A & Belzung C Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology.63, 374–384 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Kempermann G, Kuhn HG & Gage FH More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ & Herkenham M Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J. Neurosci. 33, 2961–2972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu MV & Hen R Functional dissociation of adult-born neurons along the dorsoventral axis of the dentate gyrus. Hippocampus 24, 751–761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boldrini M et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34, 2376–2389 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen L, Bisgaard CF, Nielsen ΗB & Wiborg O Transcriptome differentiation along the dorso–ventral axis in laser-captured microdissected rat hippocampal granular cell layer. Neuroscience 170, 731–741 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Adhikari A, Topiwala MA & Gordon JA Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65, 257–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padilla-Coreano N et al. Direct ventral hippocampal–prefrontal input is required for anxiety-related neural activity and behavior. Neuron 89, 857–866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson MP, Strange BA & Dolan RJ Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat. Neurosci. 7, 278–285 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Britt JP et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagot RC et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Corrirriun. 6, 7062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anacker C Adult hippocampal neurogenesis in depression: behavioral implications and regulation by the stress system. Curr. Top. Behav. Neurosci. 18, 25–43 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Anacker C, Zunszain PA, Carvalho LA & Pariante CM The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology 36, 415–425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anacker C et al. Neuroanatomic differences associated with stress susceptibility and resilience. Biol. Psychiatry 79, 840–849 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tannenholz L, Hen R & Kheirbek MA GluN2B-containing NMDA receptors on adult-born granule cells contribute to the antidepressant action of fluoxetine. Front. Neurosci. 10,242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danielson NB et al. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron 90, 101–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cameron HA & McKay RD Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435, 406–417 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Ge S, Yang CH, Hsu KS, Ming GL & Song H A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54, 559–566 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toni N & Schindei AF Maturation and functional integration of new granule cells into the adult hippocampus. Cold Spring Harb. Perspect. Biol. 8, aO 18903 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denny CA, Burghardt NS, Schachtei DM, Hen R & Drew M R. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus 22, 1188–1201 (2 012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gould E, Beylin A, Tanapat P, Reeves A & Shorn IJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265 (1999). [DOI] [PubMed] [Google Scholar]; This study showed that adult-born neurons are affected by associative memory formation.
- 43.Shors I J. et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Shors IJ, Townsend DA, Zhao M, Kozorovitskiy Y & Gould H Neurogenesis may relate to some but not all types of hippocampal-dependent learning .Hippocampus 12, 578–584 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leuner B et al. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 24, 7477–7481 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drapeau H et al. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl Acad. Sci. USA 100, 14385–14390 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ambrogini P et al. Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci. Lett. 359, 13–16(2004). [DOI] [PubMed] [Google Scholar]
- 48.Dobrossy MD et al. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry 8, 974–982 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS & Wang S Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16, 296–304 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Dupret D et al. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 5, e214 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kesner RP et al. The role of postnatal neurogenesis in supporting remote memory and spatial metric processing. Hippocampus 24, 1663–1671 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Kempermann G, Kuhn HG & Gage FH Experience-induced neurogenesis in the senescent dentate gyrus. J. Neurosci. 18, 3206–3212 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Praag H, Kempermann G & Gage FH Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Tashiro A, Sandler VM, Toni N, Zhao C & Gage FH NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442, 929–933 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Kirby ED et al. Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol. Psychiatry 17, 527–536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B & Gould E Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J. Neurosci. 33, 7770–7777 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stone SS et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J. Neurosci. 31, 13469–13484 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kee N, Teixeira CM, Wang AH & Frankland PW Preferential incorporation of adultgenerated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 10, 355–362 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Aimone JB, Wiles J & Gage FH Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 9, 723–727 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Rangel LM et al. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nat. Cornmun. 5, 3181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tashiro A, Makino H & Gage FH Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J. Neurosci. 27, 3252–3259 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stone SS et al. Functional convergence of developmentaly and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus 21,1348–1362 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Aimone JB, Wiles J & Gage FH Computational influence of adult neurogenesis on memory encoding. Neuron 61, 187–202 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Appleby PA & Wiskott L Additive neurogenesis as a strategy for avoiding interference in a sparsely-coding dentate gyrus. Network 20, 137–161 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Appleby PA, Kempermann G & Wiskott L The role of additive neurogenesis and synaptic plasticity in a hippocampal memory model with grid-cell like input. PLoS Cornput. Biol. 7, e 1001063 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lacefield CO, Itskov V, Reardon T, Hen R & Gordon JA Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus 22, 106–116 (2 012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burghardt NS, Park EH, Hen R & Fenton AA Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus 22, 1795–1808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ikrar T et al. Adult neurogenesis modifies excitability of the dentate gyrus. Front. Neural Circuits 7, 204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drew LJ et al. Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus 26, 763–778 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Temprana SG et al. Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells. Neuron 85, 116–130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karst H & Joëls M Effect of chronic stress on synaptic currents in rat hippocampal dentate gyrus neurons. J. Neurophysiol. 89, 625–633 (2003). [DOI] [PubMed] [Google Scholar]
- 72.McGaugh JL et al. Neuromodulatory systems and memory storage: role of the amygdala. Behav. Brain Res. 58, 81–90 (1993). [DOI] [PubMed] [Google Scholar]
- 73.Man D Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lorid. B 262, 23–81 (1971). [DOI] [PubMed] [Google Scholar]
- 74.Becker S A computational principle for hippocampal learning and neurogenesis. Hippocampus 15, 722–738 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Leutgeb JK, Leutgeb S, Moser MB & Moser EI Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007). [DOI] [PubMed] [Google Scholar]; This was the first in vivo electro physiology study to show that signals from the entorhinal cortex can be decorrelated both by the dentate gyrus and by the recruitment of nonoverlapping cell assemblies in CA3.
- 76.McHugh TJ et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Clelland CD et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first study to show that neurogenesis is necessary for behavioural pattern separation.
- 78.Wiskott L, Rasch MJ & Kempermann G A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus 16, 329–343 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Kheirbek MA, Klemenhagen KC, Sahay A & Hen R Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci. 15,1613–1620 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neunuebel JP, Yoganarasimha D, Rao G & Knierim JJ Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J. Neurosci. 33, 9246–9258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tronel S et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus 22, 292–298 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Creep DJ, Romberg C, Saksida LM, van Praag H & Bussey TJ Running enhances spatial pattern separation in mice. Proc. Natl Acad. Sci. USA 107, 2367–2372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Coba MP et al. TNiK is required for postsynaptic and nuclear signaling pathways and cognitive function. J. Neurosci. 32, 13987–13999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sahay A et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study developed the first mouse model to specifically increase neurogenesis and showed that mice with increased neurogenesis have an improved pattern separation ability.
- 85.Kent BA et al. The orexigenic hormone acyl-ghrelin increases adult hippocampal neurogenesis and enhances pattern separation. Psychoneuroendocrinology 51,431–439 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bekinschtein P et al. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus 24, 905–911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bekinschtein P et al. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 5, 759–768 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McAvoy KM et al. Modulating neuronal competition dynamics in the dentate gyrus to rejuvenate aging memory circuits. Neuron 91, 1356–1373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakashiba T et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Reilly RC & McClelland JL Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus 4, 661–682 (1994). [DOI] [PubMed] [Google Scholar]
- 91.Jung MW & McNaughton BL Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus 3, 165–182 (1993). [DOI] [PubMed] [Google Scholar]
- 92.McAvoy K, Besnard A & Sahay A Adult hippocampal neurogenesis and pattern separation in DG: a role for feedback inhibition in modulating sparseness to govern population-based coding. Front. Syst. Neurosci. 9, 120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng W, Aimone JB & Gage FH New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11,339–350 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denny CA et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tayler KK, Tanaka KZ, Reijmers LG & Wiltgen BJ Reactivation of neural ensembles during the retrieval of recent and remote memory. Curr. Biol. 23, 99–106 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Ramirez S et al. Creating a false memory in the hippocampus. Science 341,387–391 (2013). [DOI] [PubMed] [Google Scholar]; This study generated an artificial fear memory and was able to induce fearful behaviour by activating this memory.
- 97.Redondo RL et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramirez F, Moscarello JM, LeDoux JE & Sears RM Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci. 35, 3470–3477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kitamura T et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139, 814–827 (2009). [DOI] [PubMed] [Google Scholar]
- 100.Akers KG et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Epp JR, Silva Mera R, Köhler S, Josselyn SA & Frankland PW Neurogenesis-mediated forgetting minimizes proactive interference. Nat. Cornmun. 7, 10838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that neurogenesis minimizes proactive memory interference, which could be important for cognitive flexibility.
- 102.Monk CS et al. Human hippocampal activation in the delayed matching- and nonmatching-to-sample memory tasks: an event-related functional MRI approach. Behav. Neurosci. 116, 716–721 (2002). [DOI] [PubMed] [Google Scholar]
- 103.Takahashi H et al. Differential contributions of prefrontal and hippocampal dopamine D, and D2 receptors in human cognitive functions. J. Neurosci. 28, 12032–12038(2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park EH, Burghardt NS, Dvorak D, Hen R & Fenton AA Experience-dependent regulation of dentate gyrus excitability by adult-born granule cells. J. Neurosci. 35, 11656–11666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dupret D et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE’S, e 1959 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Garthe A, Behr J & Kempermann G Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE 4, e5464 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swan AA et al. Characterization of the role of adult neurogenesis in touch-screen discrimination learning. Hippocampus 24, 1581–1591 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rubin RD, Watson PD, Duff MC & Cohen NJ The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 8, 742 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McClelland JL, McNaughton BL, O’Reilly RC Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457 (1995). [DOI] [PubMed] [Google Scholar]
- 110.Hvoslef-Hide M & Oomen CA Adult neurogenesis and pattern separation in rodents: a critical evaluation of data, tasks and interpretation. Front. Biol. 11, 168–181 (2016). [Google Scholar]
- 111.Garthe A, Roeder I & Kempermann G Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus 26, 261–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kalm M, Karlsson N, Nilsson MK & Blomgren K Loss of hippocampal neurogenesis, increased novelty-induced activity, decreased home cage activity, and impaired reversal learning one year after irradiation of the young mouse brain. Exp. Neurol. 247, 402–409 (2013). [DOI] [PubMed] [Google Scholar]
- 113.Garthe A, Huang Z, Kaczmarek L, Filipkowski RK & Kempermann G Not all water mazes are created equal: cyclin D2 knockout mice with constitutively suppressed adult hippocampal neurogenesis do show specific spatial learning deficits. Genes Brain Behav. 13,357–364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lucassen PJ et al. Regulation of adult neurogenesis and plasticity by (early) stress, glucocorticoids, and inflammation. Cold Spring Harb. Perspect. Biol. 7, a021303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Santarelli L et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301,805–809 (2003). [DOI] [PubMed] [Google Scholar]; This was the first study to show that neurogenesis is necessary for some of the behavioural effects of antidepressants.
- 116.Surget A et al. Antidepressants recruit new neurons to improve stress response regulation. Mol. Psychiatry 16, 1177–1188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Surget A et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol. Psychiatry 64, 293–301 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Ramirez S et al. Activating positive memory engrams suppresses depression-like behaviour. Nature 522, 335–339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors of this study were able to induce anti-depressant-like behavioural effects by artificially activating memory engrams of positive events.
- 119.Coe CL et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol. Psychiatry 54, 1025–1034 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Lemaire V, Koehl M, Le Moal M & Abrous DN Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl Acad. Sci. USA 97, 11032–11037 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mirescu C, Peters JD & Gould H Early life experience alters response of adult neurogenesis to stress. Nat. Neurosci. 7, 841–846 (2004). [DOI] [PubMed] [Google Scholar]
- 122.Perera ID. et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS ONE 6, e 17600 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perera ID. et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 27, 4894–4901 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu MV et al. Impact of social status and antidepressant treatment on neurogenesis in the baboon hippocampus. Neuropsychopharmacology 39, 1861–1871 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lucassen PJ, Stumpel MW, Wang Q & Aronica E Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology 58, 940–949 (2010). [DOI] [PubMed] [Google Scholar]
- 126.Bessa JM et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol. Psychiatry 14, 764–773 (2009). [DOI] [PubMed] [Google Scholar]
- 127.David DJ .et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62, 479–493 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hill AS, Sahay A & Hen R Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology 40, 2368–2378 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Revest JM et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mot. Psychiatry 14,959–967 (2009). [DOI] [PubMed] [Google Scholar]
- 130.Murray E, Smith DW & Hutson PH Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur. J. Pharmacol. 583, 115–127 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Oomen CA, Mayer JL, de Kloet ER, Joels M & Lucassen PJ Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J. Neurosci. 26, 3395–3401 (2007). [DOI] [PubMed] [Google Scholar]
- 132.Mayer JL et al. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J. Neuroendocrinol. 18, 629–631 (2006). [DOI] [PubMed] [Google Scholar]
- 133.Anacker C et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc. Natl Acad. Sci. USA 110, 8708–8713 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anacker CA et al. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38, 872–883 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schloesser RJ, Manji ΗK & Martinowich K Suppression of adult neurogenesis leads to an increased hypothalamo–pituitary–adrenal axis response. Neuroreport 20, 553–557 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Snyder JS, Soumiei A, Brewer M, Pickel J & Cameron HA Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first study to demonstrate that mice with complete ablation of neurogenesis show elevated glucocorticoid responses and anxiety-like and depressive-like behaviour in response to acute moderate stress.
- 137.Mateus-Pinheiro A et al. Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Trans! Psychiatry 3, e210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Deng W, Saxe MD, Gallina IS & Gage FH Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–13542 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Keith J, Velezmoro R, O’Brien C Correlates of cognitive flexibility in veterans seeking treatment for posttraumatic stress disorder. J. Nerv. Ment. Dis. 203,287–293 (2015). [DOI] [PubMed] [Google Scholar]
- 140.Chamberlain SR et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am. J. Psychiatry 164, 335–338 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Deveney CM & Deldin PJ A preliminary investigation of cognitive flexibility for emotional information in major depressive disorder and non-psychiatric controls. Emotion 6, 429–437 (2006). [DOI] [PubMed] [Google Scholar]
- 142.Malberg JE, Eisch AJ, Nestlei EJ & Duman RS Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]; This was the first study to show that different antidepressant treatments increase adult hippocampal neurogenesis.
- 143.Banasi M, Soumiei A, Hery M, Mocaer E & Daszuta A Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol. Psychiatry 59, 1087–1096 (2006). [DOI] [PubMed] [Google Scholar]
- 144.Dagyte G et al. The novel antidepressant agomelatine normalizes hippocampal neuronal activity and promotes neurogenesis in chronically stressed rats. CNS Neurosci. Ther. 16, 195–207 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boldrini M et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol. Psychiatry 72, 562–571 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Anacker C et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mot. Psychiatry 16, 738–750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lee J, Duan W & Mattson MP Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 82, 1367–1375 (2002). [DOI] [PubMed] [Google Scholar]
- 148.Stangl D & Thuret S Impact of diet on adult hippocampal neurogenesis. Genes Nutt: 4, 271–282 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Walker AK et al. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mot. Psychiatry 20, 500–508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shors TJ, Olson RL, Bates ME, Selby EA & Alderman BL Mental and physical (MAP) training: a neurogenesis-inspired intervention that enhances health in humans. Neurobiol. Learn. Mem. 115, 3–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Craft LL & Perna FM The benefits of exercise for the clinically depressed. Prim. Care Companion J. Clin. Psychiatry 6, 104–111 (2 004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Meshi D et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat. Neurosci. 9, 729–731 (2006). [DOI] [PubMed] [Google Scholar]
- 153.Scharfman ΗE Functional implications of seizure-induced neurogenesis. Adv. Exp. Med. Biol. 548, 192–212 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scharfman ΗE & Hen R Neuroscience. Is more neurogenesis always better? Science 315, 336–338 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jin K et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl Acad. Sci. USA 99, 11946–11950 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Y et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Conboy L et al. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine-stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Mol. Psychiatry 1 6, 533–547 (2011). [DOI] [PubMed] [Google Scholar]
- 158.Samuels BA et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat. Neurosci. 18, 1606–1616 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study emphasized for the first time the role of mature granule neurons in the dentate gyrus as crucial mediators of the antidepressant response.
- 159.Madroñal N et al. Rapid erasure of hippocampal memory following inhibition of dentate gyrus granule cells. Nat. Cornmun. 7, 10923 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dalley RA, Ng LL & Guillozet-Bongaarts AL Dentate gyrus (DG). Allen Brain Atlas Community Site http://communitv.brain-map.org/download/attachments/798/DG.pdf?version=1 (2017). [Google Scholar]
- 161.Haslei G, Drevets WC, Manji ΗK & Charney DS Discovering endophenotypes for major depression. Neuropsychopharmacology 29, 1765–1781 (2004). [DOI] [PubMed] [Google Scholar]
- 162.Cuthbert BN & Insel TR Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Donaldson ZR & Hen R From psychiatric disorders to animal models: a bidirectional and dimensional approach. Biol. Psychiatry 77, 15–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Manganas LN et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science 318, 980–985 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rueger MA et al. Noninvasive imaging of endogenous neural stem cell mobilization in vivo using positron emission tomography. J. Neurosci. 30, 6454–6460 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tamura Y et al. Noninvasive evaluation of cellular proliferative activity in brain neurogenic regions in rats under depression and treatment by enhanced [l8F]FLT-PET imaging. J. Neurosci. 36, 8123–8131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pereira AC et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl Acad. Sci. USA 104, 5638–5643 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu DX et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep. 2, 295–310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fatehullah A, Tan SH & Barker N Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254 (2016). [DOI] [PubMed] [Google Scholar]
- 170.Lacy JW, Yassa MA, Stark SM, Muftuler LT & Stark CE Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem. 18, 15–18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bakker A, Kirwan CB, Miller M & Stark CE Pattern separation in the human hippocampal CA3 and dentate gyrus. Science 319,1640–1642 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; This fMRI study provided the first evidence for a role of the dentate gyrus-CA3 in pattern separation in humans.
- 172.Leal SL, Tighe SK„ Jones CK & Yassa MA Pattern separation of emotional information in hippocampal dentate and CA3. Hippocampus 24, 1146–1155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Becker S, Macqueen G & Wojtowicz JM Computational modeling and empirical studies of hippocampal neurogenesis-dependent memory: effects of interference, stress and depression. Brain Res. 1299, 45–54 (2009). [DOI] [PubMed] [Google Scholar]
- 174.Miller JF et al. Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science 342, 1111–1114 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Déry N, Goldstein A & Becker S A role for adult hippocampal neurogenesis at multiple time scales: a study of recent and remote memory in humans. Behav. Neuroses. 129, 435–449 (2015). [DOI] [PubMed] [Google Scholar]
- 176.Déry N et al. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front. Neuroses. 7, 66(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gould NF et al. Performance on a virtual reality spatial memory navigation task in depressed patients. Am. J. Psychiatry 1 64, 51 6–519 (2007). [DOI] [PubMed] [Google Scholar]
- 178.Channon S Executive dysfunction in depression: the Wisconsin Card Sorting Test. J. Affect. Disord. 39, 107–114 (1996). [DOI] [PubMed] [Google Scholar]
- 179.Degl’Innocenti A, Agren H & Bäckman L Executive deficits in major depression. Acta Psychiatr. Scand. 97, 182–188 (1998). [DOI] [PubMed] [Google Scholar]
- 180.Corcoran R & Upton D A role for the hippocampus in card sorting? Cortex 29, 293–304 (1993). [DOI] [PubMed] [Google Scholar]
- 181.McAlonan K & Brown VJ Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 146, 97–103 (2003). [DOI] [PubMed] [Google Scholar]
- 182.Brown VJ & Tait DS Attentional set-shifting across species. Curr. Top. Behav. Neurosci. 28, 363–395 (2016). [DOI] [PubMed] [Google Scholar]
- 183.Marsh R et al. Reward-based spatial learning in unmedicated adults with obsessive–compulsive disorder. Am. J. Psychiatry 172, 383–392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cole SW, Yoo DJ & Knutson B Interactivity and reward-related neural activation during a serious videogame. PLoS ONE 7, e33909 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mishra J, Anguera JA & Gazzaley A Video games for neuro-cognitive optimization. Neuron 90, 214–218 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Pikkarainen M, Rönkkö S, Savander V, Insausti R & Pitkänen A Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J. Comp. Neurol. 403, 229–260 (1999). [PubMed] [Google Scholar]
- 187.Felix-Ortiz AC et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bazelot M et al. Hippocampal theta input to the amygdala shapes feedforward inhibition to gate heterosynaptic plasticity. Neutvn 87, 1290–1303 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Watanabe Y, Gould E & McEwen BS Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 588, 341–345 (1992). [DOI] [PubMed] [Google Scholar]


