Abstract
GABA, muscimol, and baclofen were microinjected into the rostral (rNTS) and caudal solitary tract nucleus (cNTS) in 24 anesthetized cats. Electromyograms (EMGs) of diaphragm (DIA) and abdominal muscles (ABD), blood pressure and esophageal pressure (EP) were recorded and analysed. Bilateral microinjections of 1 mM GABA (total 66 ± 4 nl), 1 mM baclofen (64 ± 4 nl) and unilateral microinjections of 0.5 mM muscimol (33 ± 1 nl) in the rNTS significantly reduced cough number (CN), amplitudes of ABD EMGs, expiratory EP, and prolonged the duration of the cough inspiratory phase. GABA microinjections decreased the amplitudes of cough-related DIA EMGs and inspiratory EP; muscimol microinjections decreased the cough DIA EMG on the contralateral side. Only microinjections of GABA into the cNTS suppressed CN. In some cases, microinjections prolonged the inspiratory phase, lowered respiratory rate, changed the depth of breathing, and increased blood pressure and heart rate. Our results confirm that GABA-ergic inhibitory mechanisms in the rNTS can regulate coughing in the anesthetized cat.
Keywords: Muscimol, Baclofen, Microinjection, Airway defense, Cough control
1. Introduction
The nucleus of the solitary tract (NTS) in the dorsomedial medulla is the entry point of afferent signals from various visceral mechanosensors, chemosensors, C-fibers, including cough afferents. The NTS comprises second-order neurons receiving and transmitting afferent information for the integration of visceral reflexes via reciprocal connections with higher centres in the central nervous system (Babic et al., 2015).
The synaptic profile of NTS neurons receiving afferent excitatory drive is highly complex. It involves distinct neurotransmitters and neuromodulators, including glutamate, adenosine triphosphate and acetylcholine (Zoccal et al., 2014). Various complex respiratory/cardiovascular functions are mediated/modulated by gamma-aminobutyric acid (GABA) receptors in the NTS (Wang et al., 2010). In general, GABA release and activation of postsynaptic GABAA receptors play a crucial role in controlling neuronal excitability in adult mammals (Watanabe et al., 2002). Fast GABA-mediated synaptic inhibitory neurotransmission requires that GABAA receptors are expressed and assembled at appropriate postsynaptic sites near GABA-releasing nerve terminals (MacDonald and Olsen, 1994; Kittler et al., 2002).
Cough-related activity has been found in neurons located in the NTS (Haji et al., 2012). The multiplicity of neuronal phenotypes and responses to perturbation of NTS neurons (Lipski et al., 1991; Paton, 1998; Paton et al., 1999) reveals a complex role for the NTS in regulation of cough excitability, its sequencing, motor drive and the spatiotemporal control of cough motor pattern (Poliacek et al., 2017a,b). Mutolo et al. (2007, 2009), Mutolo (2017), Cinelli et al. (2016) have repeatedly shown in the rabbit that pharmacological perturbation of the NTS can alter the duration of the inspiratory and expiratory phases of coughing, which is strong evidence for a role of this medullary area in cough rhythmogenesis.
However, several studies support significant species differences in the anatomical arrangement of NTS elements that modulate coughing. Poliacek et al. (2017a) showed that disruption of excitatory amino acid transmission with microinjection of a broad-spectrum antagonist, kynurenic acid, in the feline rNTS, but not the cNTS, had inhibitory effects on coughing. Further, in these experiments kynurenic acid profoundly affected temporal control of this behaviour such that activation of airway mechanoreceptors that normally produce repetitive coughing induced prolonged apneusis instead.
Conversely, work in the rabbit supports an important role of the cNTS but not the rNTS in regulation of cough. Mutolo et al. (2007, 2009, 2017) and Cinelli et al. (2016) in rabbit studies demonstrate effects of neuroactive and cough modulating drugs on neurons in the cNTS for modulation of cough expression and execution.
More recently, Cinelli et al. (2016) investigated the role of GABA receptors in the cNTS of the rabbit in the production of coughing. GABA receptors, especially the GABAA subtype, are important in the production of rhythmic respiratory behaviors (Bongianni et al., 2010; Anderson et al., 2016; Marchenko et al., 2016); but may not be essential for breathing to occur (Janczewski et al., 2013). Microinjection of the GABAA receptor antagonist, bicuculline, into the cNTS in the rabbit enhanced coughing and shortened cough inspiratory but not expiratory phase duration (Cinelli et al., 2016). Based on these previous data and the fact that systemically administered GABA-ergic agents suppress cough (Bolser et al., 1994; Nosalova, 1998; Mutolo et al., 2008) we attempted to modulate cough responses by local delivery of GABA, the GABAA receptor agonist muscimol and the GABAB receptor agonist baclofen in the NTS. It was hypothesized that GABAA as well as GABAB agonists in the rNTS would suppress coughing induced by mechanical stimulation of the tracheobronchial airways in cats. Further, we speculated that these GABA receptor agonists would perturb cough phase timing, consistent with a role of synaptic inhibition in the NTS in the regulation of cough rhythmogenesis. We also expected a limited effect of GABA-ergic agents on cough when administered in cNTS in the cat model.
2. Material and methods
2.1. Ethical approval
Animal care as well as all procedures were performed in accordance with the Animal Welfare Guidelines of the Comenius University and the legislation for animal use and welfare of Slovak Republic and European Union (Directive 2010/63/UE).
2.2. Animal preparation and recording procedures
The experiments were carried out on 24 male cats (4.06 ± 0.16 kg). The animals were anesthetized with sodium pentobarbital (Pfannenschmidt GmbH; initial dose 38 mg/kg, i.p., 1–3 mg/kg i.v. supplementary as needed). At the beginning of the experiment atropine (Biotika; 0.15 mg/kg, i.v.) was administered to reduce the mucosal secretion in the airways and hydrocortisone (VUAB Pharma a.s.; 2 mg/kg, i.v.) to reduce brain swelling. The level of anaesthesia was assessed regularly by the absence of reflex withdrawal of the hind limb in response to noxious pinching of the paw. The other criteria were the presence of palpebral reflex and jaw tone. The cats spontaneously breathed oxygen-enriched air (30–40%). The trachea, femoral vein and artery were cannulated. During the experiments respiratory rate (RR), end-tidal CO2 concentration (ETCO2), arterial blood pressure, and rectal temperature were continuously monitored. The animal’s temperature was maintained within the range 37.5–38.5 °C, using a heating pad and a lamp. Arterial blood samples were removed periodically to perform gas and pH analysis. For the measurement of intrathoracic pressure (esophageal pressure, EP) a soft balloon was inserted into the esophagus. The electromyograms (EMG) were recorded by bipolar insulated wire hook electrodes bilaterally from the diaphragm (DIA) (percutaneously) and expiratory transversus abdominis and/or external oblique abdominal (ABD) muscles. We performed the occipital craniotomy and a partial cerebellectomy while the animals were placed prone in a stereotaxic frame.
2.3. Stimulation procedures
The cough reflex was elicited mechanically by a soft polyethylene fiber via the tracheal cannula (tracheobronchial cough). The stimulation lasted 10 s; the fiber was moved periodically 4–5 times back and forth. The mechanical stimulation was always performed by the same person, with the same stimulation pattern during the trials.
2.4. Microinjection procedures
The drugs were dissolved in artificial cerebrospinal fluid (aCSF; ALZET Osmotic Pumps, California, USA; Poliacek et al., 2017a,b). Microinjections were performed in the cNTS targeting the commissural subnucleus and the NTS rostral to the obex (rNTS) targeting the ventrolateral subnucleus. The following neuroactive drugs were used: GABA (1 mM, Sigma Aldrich, Co.), a selective GABAA receptor agonist muscimol (0.5 mM, Sigma Aldrich, Co.) and a selective GABAB receptor agonist baclofen (1 mM, BIOTREND AG Zurich).
The position of the micropipette was set under stereotaxic control in the required region of NTS: 1.3–1.1 mm caudal to the obex, 1.1–1.2 mm lateral to the midline and 1.3–1.8 mm below the dorsal medullary surface for the cNTS; coordinates (rostral/lateral/depth) for the rNTS were 1.2/2.3–2.5/1.6–1.8 mm, respectively (Fig. 1; see also Poliacek et al., 2017b). The pH of the solutions was adjusted to 7.4. A single glass micropipette (tip diameter 20–60 μm) was used for pressure-injection of the solutions. The volume of injectate was measured using a microscope scale by monitoring the fluid meniscus in the pipette barrel. In order to identify the microinjection sites all solutions contained fluorescent latex beads (Life Technologies Eugene, Oregon, USA) which were examined histologically.
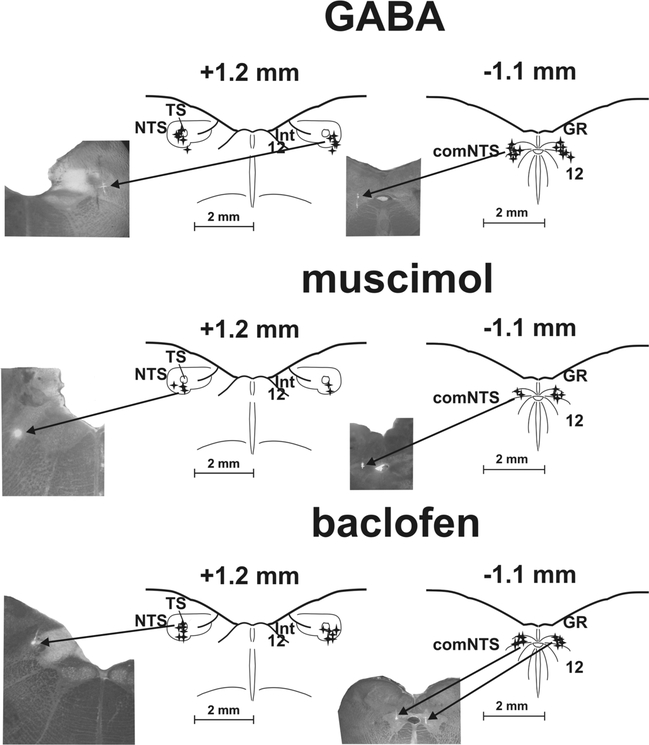
Fig. 1.
Reconstruction of microinjection sites.
Stars represent highlighted positions of the micropipette tip during microinjections of GABA, muscimol and baclofen in the rostral (rNTS; +1.2 mm to the obex) and caudal nucleus of the solitary tract (cNTS; −1.1 mm to the obex) as determined by fluorescent marker. Reference points: comNTS, 12, central canal, medullary surface for the cNTS; TS, NTS, 12, Int, the bottom of the 4th ventricle, medullary surface for the rNTS.
Microinjections of GABA. All 14 microinjection locations were found within or near the commissural subnucleus of the NTS in the cNTS. All 12 microinjections were positioned in or near ventrolateral subnucleus of the NTS in the rNTS.Microinjections of muscimol. All 6 locations where muscimol was delivered in or near comNTS were identified. In the rNTS 5 out of 6 microinjection locations were positively identified in the ventrolateral region of the NTS.
Microinjections of baclofen. All 10 Baclofen microinjections were positioned in or near comNTS in the cNTS. All 12 rNTS microinjection were identified in the area of TS and ventrolateral region of the NTS. 12: hypoglossal ncl., comNTS: commissural subnucleus of the NTS, GR: gracile ncl., Int: ncl. intercalatus, NTS: ncl. tractus solitarius, TS: tractus solitaries
Inset photographs demonstrate the process. The arrows ‘injection’ point out light spots of a spread of fluorescent marker.
2.5. Histology
For histological processing, the brainstem was surgically removed after the experiment and fixed in 4% paraformaldehyde, followed by a 30% sucrose solution. The sections, transverse slices 100 μm thick (cut using freezing microtome), were examined by light and fluorescent microscopy to localize the sites of injection. The position of micropipette tip during microinjection was considered to correspond to the location with the highest intensity of staining. An additional criterion for determination of micropipette tip position was the track caused by the micropipette insertion. If the microinjection spot was more than 0.2 mm from the target area then that animal was excluded from analysis. These structures and coordinates are consistent with published data (Berman, 1968; Dietrich et al., 1982; Claps and Torrealba, 1988; Maley, 1996) and our own adjustments (Poliacek et al., 2017a,b). Control aCSF microinjections in rNTS (and related analysis of data) were performed as described in our previous experiments (Poliacek et al., 2017a,b). A summary of histological analysis is presented in Fig. 1.
2.6. Data processing and analysis
All the EMGs were amplified, filtered (300–3000 Hz; GRASS Instruments, USA), digitized (12-bit multi-function plug-in ISA card, sampling frequency of 20 kHz), and recorded along with blood pressure and EP waveforms (Windaq, Dataq Instruments, Ohio, USA). EMGs were subsequently rectified and integrated (moving average) with a time constant of 200 ms (Spike software, CED, Cambridge, England).
The number of cough efforts (CN) induced during the mechanical stimulation of trachea (the average CN per 10 s duration stimulation trial), the DIA and ABD EMGs’ amplitudes (moving averages), and the amplitudes of EP during appropriate phases were analysed.
In the temporal analysis (Poliacek et al., 2016, 2017a), the duration of cough-related DIA (TDIA) and ABD (TABD) activations, augmenting part of DIA (CTI =inspiratory cough phase), the time from the maximum of DIA activity to the end of cough-related ABD activity (CTE1 = active expiratory cough phase), the time from the maximum of DIA activity to the end of the cough cycle (CTE =cough expiratory phase) were measured. Further, the time between maxima of DIA and ABD activity (peaks), the quiescent period of the cough cycle (cough TE2 phase =CTE2), the duration of all cough-related EMG activity (CTactive), and the whole cough cycle duration were analysed in each stimulation period (CTtot). In the analysis of cardiorespiratory data, RR (expressed in breaths/min), respiratory phase durations – inspiratory, post-inspiratory, quiescent expiratory (TI, TE1, TE2), respiratory-related amplitudes of DIA and EP, heart rate, mean blood pressure were measured. These parameters were taken during 3 standard consecutive breathing cycles just before the 1st microinjection and then 1 min after the last microinjection in the protocol. If necessary, they were also measured at about 20 min post-microinjections and in the recovery period. If more pronounced cardiorespiratory effects of microinjections occurred later than within 1 min post-injection (a few cases), we also analysed an additional time point (2–10 min post-injection). Blood gases and blood pH analysis were performed before and after microinjections and in the recovery period.
The results are expressed as means ± SE (standard error). A repeated measures ANOVA; ordinary ANOVA or Kruskal-Wallis test and/ or paired t-test or Wilcoxon matched-pairs test were applied as appropriate. The differences of variables were considered significant at p < 0.05.
2.7. Experimental protocol
Approximately 20 cough stimulation trials separated by 1 min, were executed to establish a stable cough baseline. Then 3–5 control preinjection trials were made. Another sequence of trials was performed at most 7 min after the microinjection (starting approximately 1 min after the last microinjection) followed by additional trials in the later postinjection intervals, usually at 20–40 min and more than 1–2 h postmicroinjections. If CN and/or cough intensity did not return to control values (i.e. “not-recovered) the data from that animal was not included in the final data analysis.
Magnitudes of the moving averages during coughing were normalized relative to the mean intensities of control pre-injection coughs (the average magnitudes of all control coughs for each particular EMG). All parameters were averaged over each group (3–5) of related trials. After long intervals in the protocol, in which mechanical stimuli were not applied to the trachea we often observed a reduced cough response during the first trial after this time period. We eliminated this cough trial from the analysis due to unstable and reduced coughing. This transient reduction in cough excitability after a delay or gap between tracheobronchial stimulation trials occurred regularly even in vehicle treated animals (Poliacek et al., 2010).
3. Results
Thirty-six sequences of NTS microinjections under 6 protocols were included into the analysis: A) 7 GABA, 6 muscimol and 5 baclofen microinjections in the cNTS; and B) 6 GABA, 6 muscimol and 6 baclofen microinjections in the rNTS (Fig. 1). Single protocols were conducted in 12 cats, and in 12 animals multiple protocols were run with at least 30 to 70 min between (baclofen or muscimol). Protocols were only included if clear recovery occurred.
3.1. GABA microinjections
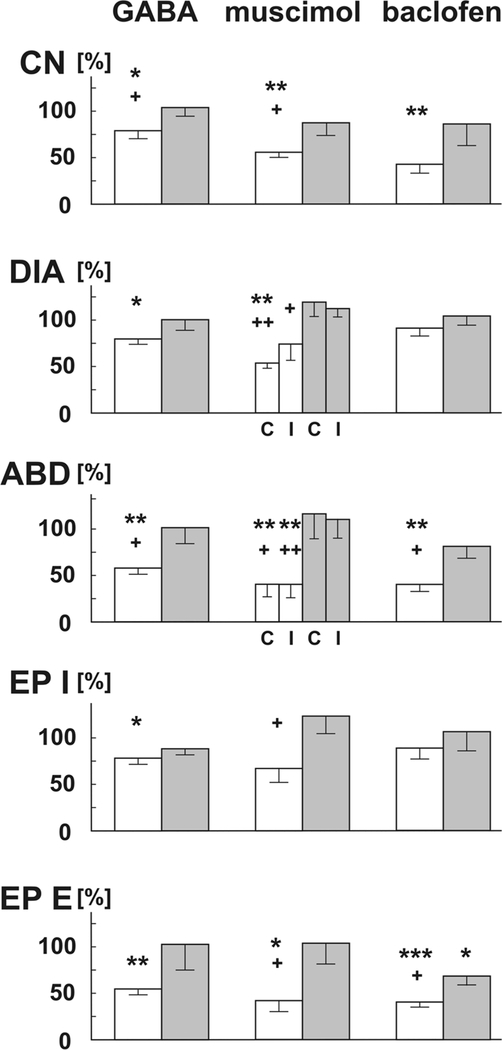
Bilateral rNTS microinjections of 1mM GABA (66 ± 4 nl total volume, 6 cats) significantly reduced CN (ANOVA p=0.03) from 6.0 ± 1.0 to 4.4 ± 0.4 (p < 0.05), which returned to 5.9 ± 0.8 (p < 0.05) during recovery. There was also a significant reduction in DIA and ABD EMG amplitudes, which resulted in a significant decrease in inspiratory and expiratory EP amplitudes during the cough (Fig. 2). There were also significant increases in CTI, TDIA, and CTtot (Table 1). No other temporal cough characteristics changed significantly. On average recovery of coughing occurred at 20–30 min (Fig. 2, Table 1).
Fig. 2.
Changes in tracheobronchial cough induced by microinjections of GABA, muscimol and baclofen into the rostral NTS
Open bars represent percentages of control (100%) within 7 min post-microinjections, gray bars recovery data (20–200 min following microinjections depending on the time when the cough recovery occurred). GABA and baclofen microinjections were bilateral, muscimol was delivered unilaterally. CN: number of coughs; DIA: amplitudes of the diaphragm EMG; C: contralateral to the microinjection; I: ipsilateral to the microinjection; ABD: amplitudes of the abdominal EMG; EP I: inspiratory amplitudes of esophageal pressure; EP E: expiratory amplitudes of esophageal pressure during cough. *, **, ***: p < 0.05, 0.01, 0.001 compared to the control data; +, ++: p < 0.05, 0.01 compared to the recovery data, respectively.
Table 1:
Metrics of temporal control of cough after microinjections of GABA, muscimol and baclofen into the rostral NTS.
| Control | Injection 7 min | Recovery | p value | |
|---|---|---|---|---|
| GABA, n=6 | ||||
| CTI [s] | 1.18 ± 0.26 | 1.41 ± 0.32 *+ | 1.22 ± 0.25 | 0.026 |
| CTE [s] | 1.53 ± 0.30 | 1.83 ± 0.21 | 1.50 ± 0.23 | 0.08 |
| CTE1 [s] | 0.90 ± 0.24 | 0.97 ± 0.19 | 0.99 ± 0.18 | 0.47 |
| CTE2 [s] | 0.63 ± 0.11 | 0.87 ±0.12 | 0.51 ± 0.10 | 0.06 |
| TDIA [s] | 1.40 ± 0.31 | 1.63 ± 0.36 *+ | 1.41 ± 0.28 | 0.022 |
| TABD [s] | 0.85 ± 0.21 | 0.93 ± 0.18 | 1.01 ± 0.16 | 0.36 |
| CTtot [s] | 2.72 ± 0.56 | 3.24 ± 0.51 *++ | 2.72 ± 0.48 | 0.009 |
| CTactive [s] | 2.08 ± 0.49 | 2.37 ± 0.51 | 2.21 ± 0.43 | 0.10 |
| peaks [s] | 0.29 ± 0.05 | 0.32 ± 0.05 | 0.28 ± 0.04 | 0.051 |
| Muscimol, n=6 | ||||
| CTI [s] | 0.90 ± 0.08 | 1.19 ± 0.16 * | 1.13 ± 0.17 * | 0.019 |
| CTE [s] | 2.44 ± 0.86 | 2.48 ± 0.45 | 2.13 ± 0.53 | 0.73 |
| CTE1 [s] | 0.60 ± 0.02 | 0.70 ± 0.06 | 0.71 ± 0.12 | 0.28 K-W |
| CTE2 [s] | 1.84 ± 0.85 | 1.78 ± 0.41 | 1.42 ± 0.49 | 0.63 |
| TDIA [s] | 1.02 ± 0.08 | 1.39 ± 0.19 * | 1.27 ± 0.19 * | 0.02 |
| TABD [s] | 0.77 ± 0.10 | 0.77 ± 0.10 | 1.02 ± 0.13 | 0.24 |
| CTtot [s] | 3.34 ± 0.86 | 3.66 ± 0.44 | 3.26 ± 0.55 | 0.68 |
| CTactive [s] | 1.49 ± 0.08 | 1.88 ± 0.17 | 1.84 ± 0.23 | 0.06 |
| peaks [s] | 0.24 ± 0.01 | 0.31 ± 0.02 **++ | 0.23 ± 0.02 | 0.006 OA |
| Baclofen, n = 6 | ||||
| CTI [s] | 0.89 ± 0.11 | 1.52 ± 0.24 *+ | 0.98 ± 0.09 | 0.027 |
| CTE [s] | 1.64 ± 0.23 | 1.67 ± 0.23 + | 1.12 ± 0.10 * | 0.02 |
| CTE1 [s] | 0.68 ± 0.11 | 0.74 ± 0.08 | 0.66 ± 0.07 | 0.65 |
| CTE2 [s] | 0.96 ± 0.18 | 0.93 ± 0.22 ++ | 0.46 ± 0.08 ** | 0.006 |
| TDIA [s] | 1.03 ± 0.12 | 1.79 ± 0.25 *+ | 1.15 ± 0.10 | 0.013 |
| TABD [s] | 0.93 ± 0.18 | 0.95 ± 0.25 | 0.92 ± 0.19 | 0.98 |
| CTtot [s] | 2.53 ± 0.30 | 3.20 ± 0.42 + | 2.10 ± 0.17 | 0.025 |
| CTactive [s] | 1.57 ± 0.20 | 2.27 ± 0.29 | 1.64 ± 0.11 | 0.11 |
| peaks [s] | 0.22 ± 0.01 | 0.37 ± 0.03 ***++ | 0.27 ± 0.02 * | 0.0003 |
Recovery data are 20–200 min post-microinjections depending on the time when the cough recovery occurred. p value is for repeated measures ANOVA analysis, OA is for ordinary ANOVA, K-W for Kruskal-Wallis test. GABA and baclofen microinjections were bilateral, muscimol was delivered unilaterally.
CTI: cough inspiratory phase duration; CTE: cough expiratory phase duration; CTE1: cough active expiratory phase duration; CTE2: cough quiescent portion of expiratory phase duration; TDIA: the duration of cough-related inspiratory diaphragm EMG activity; TABD: the duration of cough-related abdominal muscle EMG activity; CTtot: total cough cycle duration; CTactive: active cough phase duration = CTI + CTE1; peaks: the duration between maxima of DIA and ABD cough-related EMG activity.
: p < 0.05
p < 0.01
0.001 compared to the control data
: p < 0.05
: p < 0.01
0.01 compared to the recovery data, respectively.
Additionally, there was a significant increase in EP (from 0.22 ± 0.09 to 0.30 ± 0.05 kPa; p < 0.05) during breathing and a reduction in mean arterial blood pressure (from 19.9 ± 1.1 to 19.0 ± 1.1 kPa; p < 0.01). No significant changes in other cardiorespiratory parameters were observed.
When GABA was microinjected in the cNTS (66 ± 3 nl total volume, 7 cats), we saw a significant reduction in CN (Fig.) from 5.8 ± 0.9 to 4.7 ± 0.9 (ANOVA p=0.05) which recovered to 6.0 ± 0.7 (relative changes, Kruskal-Wallis test p=.01: 79 ± 6%, p < 0.05, recovery 101 ± 8%, p < 0.05). There was additionally a significant prolongation of eupneic TI (from 1.51 ± 0.32 to 1.73 ± 0.38; p < 0.05) and a reduction in RR (from 19.1 ± 3.5 to 16.9 ± 2.8; breaths/min; p < 0.05). No significant changes in other cough and cardiorespiratory characteristics were observed (data not shown).
3.2. Muscimol microinjections
Unilateral rNTS microinjections of 0.5mM muscimol (33 ± 1 nl, 6 cats) significantly affected CN (ANOVA p=0.002) as shown by a reduction from 6.8 ± 0.6 to 3.7 ± 0.3 (p < 0.01) and recovery back to 5.6 ± 0.5 (p < 0.05). There was also a significant reduction in contralateral DIA and bilateral ABD EMG amplitudes, and a reduction in peak expiratory EP during cough (Fig. 2). Additionally, CTI, TDIA and peaks were prolonged (Table 1). Reduced CN (4.8 ± 0.4; p < 0.05), amplitudes of ABD EMG (ipsilateral 34 ± 15%, p < 0.01; contralateral 46 ± 20%, p < 0.05) and expiratory EP (47 ± 18%, p < 0.05) persisted for ∼20–40 min post-microinjection. Recovery was observed after 1 h post microinjections (Fig. 2).
No significant changes in cardiorespiratory characteristics were observed (although a statistically non-significant 20% reduction in RR and 25% increase in DIA and EP amplitude during eupnea occurred).
Following unilateral microinjections of muscimol in the cNTS (38 ± 6 nl, 6 cats) no significant changes were seen across cough or cardiorespiratory parameters.
3.3. Baclofen microinjections
Bilateral rNTS microinjections of 1mM baclofen (64 ± 4 nl, 6 cats) reduced CN (ANOVA p=0.014) from 8.5 ± 1.8 to 3.6 ± 0.9 (p < 0.05), which recovered to 5.8 ± 0.8. There were also significant reductions in the amplitudes of ABD EMG and expiratory EP during cough (Fig. 2). Among metrics of cough temporal characteristics, CTI, peaks, and TDIA were significantly prolonged. Recovery time varied across animals, the shortest being 30 min (1 animal), continuing to 1 h in 3 animals, and up to 3 h in 2 animals. Interestingly, recovery CTE and CTE2 values were shortened when compared to control and early postmicroinjection data (Table 1).
There was a decrease in RR (from 20.3 ± 1.5 to 16.7 ± 1.5 breaths/min; p < 0.05) and an increase in eupneic TI following bilateral microinjections of baclofen into the rNTS (from 0.86 ± 0.11 to 1.49 ± 0.18 s; p < 0.05).
No significant cough changes were observed when baclofen was microinjected in the cNTS (62 ± 1 nl, 5 cats). However, we saw a 13% reduction in eupneic DIA activity (p < 0.05); and an increase in mean blood pressure (from 18.6 ± 1.4 to 20.0 ± 1.4 kPa; p < 0.05) and heart rate (from 194 ± 8 to 206 ± 11 beats per min; p < 0.05).
4. Discussion
This is the first study to investigate the role of NTS active GABAergic inhibition on tracheal cough in the cat. Consistent with our previous reports (Poliacek et al., 2017a,b) there were pronounced changes in the cough response when GABA-ergic agents were microinjected in the rNTS, while limited cough changes were induced by the same microinjections in the cNTS.
The differences in effects induced by microinjections of muscimol and baclofen (results, Fig. 2) are consistent with the selectivity of these drugs acting on GABAA vs. GABAB receptors, respectively. We did not conduct dose-responses in this study. Therefore, it is inappropriate to compare relative efficacies of these drugs on the various metrics of coughing. However, the value of our study lies in the fact that these metrics were perturbed by several different GABA receptor subtype agonists and the relative participation of the rostral and caudal NTS in these effects. We cannot rule out the participation of cNTS GABA-ergic mechanisms in the regulation of coughing in the cat but our data are consistent with a lower sensitivity of neurons in this area to exogenously applied GABA receptor agonists.
Muscimol is a partial agonist at the GABAA-ρ (GABAC) receptor (Woodward et al., 1993). The role of GABAC receptors in the regulation of the excitability NTS neurons that participate in the control of respiratory motor drive is poorly understood. The effects of muscimol that we observed cannot be attributed solely to GABAA receptors at this point.
The effects induced by our rNTS microinjections on the cough and cardiorespiratory changes after the delivery of GABA-ergic agents correspond well with the results obtained after the microinjections of muscimol and bicuculline in the cNTS of rabbits (Cinelli et al., 2016). This fact supports our previous suggestion (Poliacek et al., 2017a,b) that differences in the anatomical organization of cough related neuronal structures in the NTS of cat and rabbit. We believe that similar outcomes on the cat (present study) and rabbit (Cinelli et al., 2016) are due to the action on analogous functional elements located differently in these species.
Microinjections of GABA, muscimol and baclofen reduced CN as well as expiratory cough efforts (Figs. 2 and 3) and these results are consistent with previous findings (Poliacek et al., 2017a,b; Mutolo, 2017; Mutolo et al., 2008; Cinelli et al., 2016). Present findings support the concept that neuronal structures of the rNTS are involved in the control of the cough reflex. However, other neuronal components regulating cough have been found and/or proposed in the NTS including functionally-identified elements contributing to a cough gating mechanism (Haji et al., 2013; Poliacek et al., 2017b) and a cough rhythmogenic circuit (Poliacek et al., 2017a). Further, it also has been proposed that this area harbors interneurons contributing to feed forward inhibition shaping motor drive during repetitive coughs (Pitts et al., 2016). A cough gating mechanism in the NTS (Haji et al., 2012; Poliacek et al., 2017b) could contribute to reduced coughing if the structure is under GABA-ergic control. Both GABAA and GABAB receptors contributed to these changes (Fig. 2). Active GABA-ergic inhibition is involved in gating of neuronal activity in the NTS (e.g. Wang et al., 1982) including control of afferent drives onto 2nd order interneurons in this region of the medulla (Ezure et al., 1999; Miyazaki et al., 1999; Fernandes et al., 2011) and possibly by a direct action at the 2nd order cough neurons (Cinelli et al., 2016). The reduction of cough afferent drive due to unilateral cooling of the vagus nerve resulted in a qualitatively similar suppression of coughing (Simera et al., 2016).
The magnitude of cough DIA EMG was only reduced after microinjections of GABA and muscimol. This observation is consistent with a significant contribution of GABAA receptors to the control of the inspiratory burst. Axonal projections from inspiratory premotoneurons in the area of ventrolateral NTS primarily terminate in the phrenic motor nucleus on the contralateral side (Bianchi and Gestreau, 2009). Note that the magnitude of reduction of ABD motor drive during cough was similar on both sides in spite of unilateral application of muscimol (Fig. 2). Baclofen had a very limited effect on the magnitude of cough DIA motor drive. Indeed, microinjections of baclofen in the rNTS induced only changes in cough phase timing unlike GABA where inspiratory efforts during breathing were reduced (see results).
We saw a prolongation of cough related inspiratory DIA bursts and CTI with all the rNTS microinjections (Table 1). The effects are likely due to a reduced excitability circuits terminating the cough inspiratory phase. Significant prolongation of respiratory TI occurred only with baclofen suggesting that the GABAB receptor mechanism is of a higher importance for the control of TI, while during coughing all GABA-ergic inhibition shapes CTI. The action of codeine in this area resulted in reduced cough DIA amplitudes, but with no changes in the duration of inspiratory timing (Poliacek et al., 2017b), although codeine mediated cough inhibition might be non-specific in cat. Nonspecific blockage of neurotransmission with kynurenic acid, however, induced extreme increases in TDIA and CTI along with TI − apneustic breathing (Poliacek et al., 2017a). Prolongations of TDIA and CTI resulted from reduced cough afferent drive by unilateral vagal cooling (Simera et al., 2016) that would be consistent with a possible inhibition of 2nd order cough neurons induced by the microinjections in the present study. Another potential mechanism of CTI prolongation represents the activity of P cells which are 2nd order neurons from slowly-adapting pulmonary stretch receptors. We have shown that this afferent pathway may modulate cough, in particular reduced afferent drive from pulmonary stretch receptors prolonged CTI (Poliacek et al., 2016). It is likely that the rNTS microinjections inhibited the activity of P cells.
There were no direct alterations in expiratory phase durations and TABD due to the microinjections of GABA-ergic agents in our study (Table 1). A reduction in vagal afferent input (unilateral vagal cooling) resulted in a marked prolongation of CTE and CTE1 (Simera et al., 2016). The determining component of CTtot is CTE2 (Wang et al., 2009; Pitts et al., 2016). Our microinjections altered CTE2 very little (Table 1). However, CTtot significantly increased due to microinjections of GABA (Table 1), mainly due to changes in CTI.
There were significant changes in the interval between maxima of DIA and ABD activation (peaks) induced by microinjections of muscimol and baclofen (Table 1). This finding is consistent with the proposed role of rNTS structures in cough rhythmogenesis as well as the development of the cough inspiratory phase and the transition from inspiration to expiration (Poliacek et al., 2017a), Indeed, a reduced cough afferent drive by unilateral vagal cooling (Simera et al., 2016) as well as blockage of glutamate neurotransmission in the region (Poliacek et al., 2017a) resulted in marked prolongation of the time interval between inspiratory and expiratory peaks.
The differential effects of our microinjections on cough relative to the breathing motor pattern suggest that there is distinct control of these two behaviors in the rNTS; presumably cough specific neurons contribute to these effects (Haji et al., 2012). GABA microinjections in the rNTS resulted in increased respiratory amplitudes of EP while inspiratory cough efforts were reduced (Fig. 2). Conversely, microinjections of baclofen induced slower RR and prolonged TI and these observations are consistent with changes in cough (Table 1). The bilateral microinjections of GABA and bicuculline (a GABAA-receptor antagonist) induced no respiratory changes in rats (Chung et al., 2006), while bilateral microinjections of muscimol or baclofen into the ventrolateral portion of the NTS in rats resulted in the prolongation of respiratory TI (Wasserman et al., 2002). Further, TE1 +TE2 shortened after GABA microinjections in anesthetized, vagotomised rats (Wasserman et al., 2000). Muscimol was unilaterally microinjected in our study. Preliminary experiments with bilateral microinjections and/or higher muscimol concentrations resulted in excessive, frequently irreversible cardiorespiratory changes (and a loss of cough). Muscimol delivered unilaterally induced limited changes in cough excitability and presumably inhibited a population of neurons that was insufficient in number to reach threshold for an effect (Catelli et al., 1987). The reduction of RR after muscimol application into the rNTS (see also Cinelli et al., 2016) is consistent with the effects of baclofen and with a respiratory accelerating (and inspiration terminating) function of stretch receptors. The terminals of these receptors are located in the area of our rNTS microinjections and is near the location of P cells (Berger and Dick, 1987). Inhibition of P cells can also explain increased EP amplitudes during breathing observed following GABA microinjections.
Consistent with our previous results (Poliacek et al., 2017a,b), microinjections of GABA-ergic agents in the cNTS induced no other cough changes besides the reduction in CN after GABA. Presently, the only known modulation of cough in anesthetized cats during which CN was specifically reduced is the occurrence of aspiration reflexes immediately before coughing (Poliacek et al., 2009).
Histological analysis showed that our microinjections were successful in targeting the rNTS and cNTS (Fig. 1). In the rNTS, areas that were affected by our microinjections were largely the ventrolateral and in some cases partially also dorsal, interstitial, intermediate, ventral and medial subnuclei, while in the cNTS mainly the commissural subnucleus of the NTS but sometimes partially also hypoglossal nucleus (Fig. 1) (see Lipski et al., 1988; Poliacek et al., 2017a,b).
Our results are consistent with: 1) the existence of complex neuronal structures in the rNTS employed in the control of coughing, which likely involve primary cough afferent, secondary modulatory afferent, central integrative as well as inspiratory motor circuits, 2) an important role of GABA-ergic inhibition mediated by both A and B type of receptors in the rNTS of the cat on the reduction of coughing and the control of cough inspiratory timing, 3) a limited role of GABAB receptors in the rNTS in the control of inspiratory cough efforts, 4) a limited effect of GABA-ergic neurotransmission in the cNTS on cough in cat. Further, our findings support significant species differences in the cough circuitry between cats and rabbits.
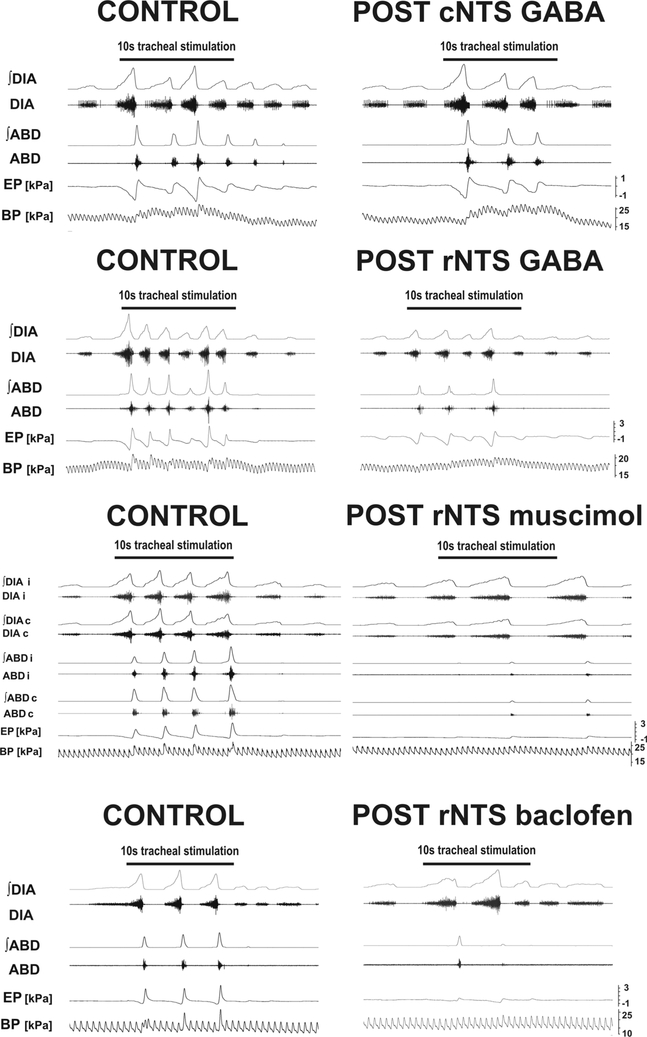
Fig. 3.
Representative examples of coughing changes induced by microinjections of GABA-ergic agents into the caudal (cNTS) and rostral NTS (rNTS).
GABA in cNTS only reduced cough number. In the rNTS cough number, expiratory cough efforts and for GABA and muscimol also inspiratory cough efforts were decreased. Muscimol microinjections were unilateral and the effects on ABD were equal, while suppression of DIA was more pronounced at the contralateral side. Post-microinjection cough trials were executed within 2–5 min for GABA and 2–7min post-injection time window for muscimol and baclofen. ∫ : moving average; DIA: the diaphragm EMG; ABD: the abdominal muscles EMG; EP: esophageal pressure; BP: arterial blood pressure; i: ipsilateral; c: contralateral.
Acknowledgements
This work was supported by VEGA 1/0253/15, VEGA 1/0072/16, and VEGA 1/0166/17, NIH OT2OD023854, R01HL131716, and HL111215. This work was supported by the Slovak Research and Development Agency under the contract N0 APVV 0189–11.
References
- Anderson TM, Garcia AJ 3rd, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM, 2016. A novel excitatory network for the control of breathing. Nature 536 (7614), 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic T, Ambler J, Browning KN, Travagli RA, 2015. Characterization of synapses in the rat subnucleus centralis of the nucleus tractus solitarius. J. Neurophysiol 113 (2), 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Dick TE, 1987. Connectivity of slowly adapting pulmonary stretch receptors with dorsal medullary respiratory neuropns. J. Neurophysiol 58 (6), 1259–1274. [DOI] [PubMed] [Google Scholar]
- Berman AL, 1968. The Brain Stem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates, 1 ed. University of Wisconsin Press, Madison, Wisconsin. [Google Scholar]
- Bianchi AL, Gestreau C, 2009. The brainstem respiratory network: an overview of a half century of research. Respir. Physiol. Neurobiol 168, 4–12. [DOI] [PubMed] [Google Scholar]
- Bolser DC, DeGennaro FC, O’Reilly S, Chapman RW, Kreutner W, Egan RW, Hey JA, 1994. Peripheral and central sites of action of GABA-B agonists to inhibit the cough reflex in the cat and guinea pig. Br. J. Pharmacol 113 (4), 1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Cinelli E, Pantaleo T, 2010. Respiratory responses induced by blockades of GABA and glycine receptors within the Bötzinger complex and the preBötzinger complex of the rabbit. Brain Res. 1344, 134–147. [DOI] [PubMed] [Google Scholar]
- Catelli JM, Giakas WJ, Sved AF, 1987. GABAergic mechanisms in nucleus tractus solitarius alter blood pressure and vasopressin release. Brain Res. 403 (2), 279–289. [DOI] [PubMed] [Google Scholar]
- Chung S, Ivy GO, Reid SG, 2006. GABA-mediated neurotransmission in the nucleus of the solitary tract alters resting ventilation following exposure to chronic hypoxia in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 291 (5), R1449–R1456. [DOI] [PubMed] [Google Scholar]
- Cinelli E, Iovino L, Bongianni F, Pantaleo T, Mutolo D, 2016. GABAA- and glycinemediated inhibitory modulation of the cough reflex in the caudal nucleus tractus solitarii of the rabbit. Am. J. Physiol. Lung Cell. Mol. Physiol 311 (3), L570–L580. [DOI] [PubMed] [Google Scholar]
- Claps A, Torrealba F, 1988. The carotid body connections: a WGA-HRP study in the cat. Brain Res. 455, 123–133. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Lowry OH, Loewy AD, 1982. The distribution of glutamate, GABA and aspartate in the nucleus tractus solitarius of the cat. Brain Res. 237, 254–260. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Miyazaki M, 1999. Electrophysiological and pharmacological analysis of synaptic inputs to pulmonary rapidly adapting receptor relay neurons in the rat. Exp. Brain Res 128 (4), 471–480. [DOI] [PubMed] [Google Scholar]
- Fernandes LG, Jin YH, Andresen MC, 2011. Heterosynaptic crosstalk: GABA-glutamate metabotropic receptors interactively control glutamate release in solitary tract nucleus. Neuroscience 174, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Ohi Y, Kimura S, 2012. Cough-related neurons in the nucleus tractus solitarius of decerebrate cats. Neuroscience 218, 100–109. [DOI] [PubMed] [Google Scholar]
- Haji A, Kimura S, Ohi Y, 2013. A model of the central regulatory system for cough reflex. Biol. Pharm. Bull 36 (4), 501–508. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL, 2013. Role of inhibition in respiratory pattern generation. J. Neurosci 33 (13), 5454–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, McAinsh K, Moss SJ, 2002. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol. Neurobiol 26, 251–268. [DOI] [PubMed] [Google Scholar]
- Lipski J, Bellingham MC, West MJ, Pilowski P, 1988. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J. Neurosci. Methods 26 (2), 169–179. [DOI] [PubMed] [Google Scholar]
- Lipski J, Ezure K, Wong She RB, 1991. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J. Physiol 443, 55–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald RL, Olsen RW, 1994. GABAA receptor channels. Annu. Rev. Neurosci 17, 569–602. [DOI] [PubMed] [Google Scholar]
- Maley BE, 1996. Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chem. Senses 21 (3), 367–376. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Koizumi H, Mosher B, Koshiya N, Tariq MF, Bezdudnaya TG, Zhang R, Molkov YI, Rybak IA, Smith JC, 2016. Perturbations of respiratory rhythm and pattern by disrupting synaptic inhibition within pre-Bötzinger and Bötzinger complexes. eNeuro 3 (2) pii: ENEURO. 0011–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Tanaka I, Ezure K, 1999. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Exp. Brain Res 129 (2), 191–200. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Fontana GA, Pantaleo T, 2007. The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res. Bull 74, 284–293. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Cinelli E, Pantaleo T, 2008. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am. J. Physiol. Regul. Integr. Comp. Physiol 295, 243–251. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Cinelli E, Pantaleo T, 2009. Role of excitatory amino acids in the mediation of tracheobronchial cough induced by citric acid inhalation in the rabbit. Brain Res. Bull 80, 22–29. [DOI] [PubMed] [Google Scholar]
- Mutolo D, 2017. Brainstem mechanisms underlying the cough reflex and its regulation. Respir. Physiol. Neurobiol 243, 60–76. [DOI] [PubMed] [Google Scholar]
- Nosalova G, 1998. Actions of drugs affecting the cough reflex. Bratisl. Lek. Listy 99, 531–535. [PubMed] [Google Scholar]
- Paton JF, Li YW, Kasparov S, 1999. Reflex response and convergence of pharyngoesophageal and peripheral chemoreceptors in the nucleus of the solitary tract. Neuroscience 93 (1), 143–154. [DOI] [PubMed] [Google Scholar]
- Paton JF, 1998. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J. Neurophysiol 79 (5), 2365–2373. [DOI] [PubMed] [Google Scholar]
- Pitts T, Morris KF, Segers LS, Poliacek I, Rose MJ, Lindsey BG, Davenport PW, Howland DR, Bolser DC, 2016. Feed-forward and reciprocal inhibition for gain and phase timing control in a computational model of repetitive cough. J. Appl. Physiol 121 (1), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliacek I, Jakus J, Simera M, Barani H, Visnovcova N, Halasova E, Tomori Z, 2009. Excitability and rhythmicity of tracheobronchial cough is altered by aspiration reflex in cats. J. Physiol. Pharmacol 60 (Suppl. 5), 105–110. [PubMed] [Google Scholar]
- Poliacek I, Wang C, Corrie LW, Rose MJ, Bolser DC, 2010. Microinjection of codeine into the region of the caudal ventral respiratory column suppresses cough in anesthetized cats. J. Appl. Physiol 108 (4), 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliacek I, Simera M, Veternik M, Kotmanova Z, Pitts T, Hanacek J, Plevkova J, Machac P, Visnovcova N, Misek J, Jakus J, 2016. The course of lung inflation alters the central pattern of tracheobronchial cough in cat-the evidence for volume feedback during cough. Respir. Physiol. Neurobiol 229, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliacek I, Pitts T, Rose MJ, Davenport PW, Simera M, Veternik M, Kotmanova Z, Bolser DC, 2017. a. Microinjection of kynurenic acid in the rostral nucleus of the tractus solitarius disrupts spatiotemporal aspects of mechanically induced tracheobronchial cough. J. Neurophysiol 117 (6), 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliacek I, Simera M, Veternik M, Kotmanova Z, Bolser DC, Machac P, Jakus J, 2017b. Role of the dorsomedial medulla in suppression of cough by codeine in cats. Respir. Physiol. Neurobiol 246, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simera M, Poliacek I, Veternik M, Babalova L, Kotmanova Z, Jakus J, 2016. Changes in vagal afferent drive alter tracheobronchial coughing in anesthetized cats. Respir. Physiol. Neurobiol 230, 36–43. [DOI] [PubMed] [Google Scholar]
- Wang L, Boyarsky LL, Frazier DT, 1982. The effect of transmitter antagonists on phasic respiratory neurons. J. Neurosci. Res 8 (4), 657–664. [DOI] [PubMed] [Google Scholar]
- Wang C, Saha S, Rose MJ, Davenport PW, Bolser DC, 2009. Spatiotemporal regulation of the cough motor pattern. Cough 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jordan D, Ramage AG, 2010. Both GABAA and GABAB receptors mediate vagal inhibition in nucleus tractus solitarii neurones in anaesthetized rats. Auton. Neurosci 152 (1–2), 75–83. [DOI] [PubMed] [Google Scholar]
- Wasserman AM, Sahibzada N, Hernandez YM, Gillis RA, 2000. Specific subnuclei of the nucleus tractus solitarius play a role in determining the duration of inspiration in the rat. Brain Res. 880 (1–2), 118–130. [DOI] [PubMed] [Google Scholar]
- Wasserman AM, Ferreira M Jr., Sahibzada N, Hernandez YM, Gillis RA, 2002. GABAmediated neurotransmission in the ventrolateral NTS plays a role in respiratory regulation in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol 283 (6), R1423–R1441. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H, 2002. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol 213, 1–47. [DOI] [PubMed] [Google Scholar]
- Woodward RM, Polenzani L, Miledi R, 1993. Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acid A and gamma-aminobutyric acid B receptor agonists and antagonists. Mol. Pharmacol 43 (4), 609–625. [PubMed] [Google Scholar]
- Zoccal DB, Furuya WI, Bassi M, Colombari DSA, Colombari E, 2014. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front. Physiol 5, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]