Abstract
Vaccines are the most effective tool for preventing infectious diseases; however, subunit vaccines, considered the safest type, suffer from poor immunogenicity and require adjuvants to create a strong and sustained immune response. As adjuvants, pathogen-associated molecular patterns (PAMPs) offer potent immunostimulatory properties and defined mechanisms of action through their cognate pattern recognition receptors (PRRs). Their activity can be further enhanced through combining two or more PAMPs, particularly those that activate multiple immune signaling pathways. However, the cytosolic localization of many PRRs requires intracellular delivery of PAMPs for optimal biological activity, which is particularly true of the stimulator of interferon genes (STING) PRR. Using acetalated dextran (Ace-DEX) microparticles (MPs) encapsulating STING agonist 3′3′-cyclic GMP-AMP (cGAMP) combined with soluble PAMPS, we screened the effect of codelivery of adjuvants using primary mouse bone marrow derived dendritic cells (BMDCs). We identified that codelivery of cGAMP MPs and soluble Toll-like receptor 7/8 (TLR7/8) agonist resiquimod (R848) elicited the broadest cytokine response. cGAMP and R848 were then coencapsulated within Ace-DEX MPs via electrospray. Using the model antigen ovalbumin, we observed that Ace-DEX MPs coencapsulating cGAMP and R848 (cGAMP/R848 Ace-DEX MPs) induced antigen-specific cellular immunity, and a balanced Th1/Th2 humoral response that was greater than cGAMP Ace-DEX MPs alone and PAMPs delivered in separate MPs. These data indicate that polymeric Ace-DEX MPs loaded with STING and TLR7/8 agonists represent a potent cellular and humoral vaccine adjuvant.
Keywords: acetalated dextran, vaccine adjuvants, microparticles, STING, cGAMP adjuvant
Graphical Abstract
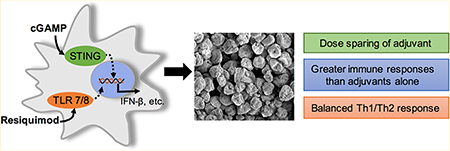
INTRODUCTION
Vaccination is widely regarded as the most efficient strategy for preventing morbidity and mortality associated with infectious disease. Historically, protection has been achieved using live attenuated vaccines (LAV) or inactivated pathogens, which can induce long-lasting and potent immunity. However, LAVs have safety concerns such as risk of reversion to a more virulent strain, which limits their ability to be broadly applied to populations such as the youth, elderly, and immunocompromised.1,2 Inactivated pathogen vaccines overcome some of the safety concerns associated with LAVs; however, they are less immunogenic, and like LAVs, they fail to provide protection against pathogens with high levels of antigenic variability. A safer alternative to LAVs or inactivated pathogen vaccines are subunit vaccines, which contain only part of the pathogen. Subunit antigens tend to be poorly immunogenic, requiring the administration of an adjuvant to generate protective immunity.3 While existing FDA-approved adjuvants such as aluminum salts (alum) or MF59 can generate humoral immunity and T helper cell type 2 (Th2)-skewed immune responses, they frequently fail to induce significant T helper cell type 1 (Th1)-biased immunity. A Th1 response is crucial for protective cellular immunity against intracellular pathogens, like viruses.4 Furthermore, the mechanisms of alum and MF59 are poorly understood.5 Therefore, vaccine adjuvants with known mechanisms that mount strong Th1 humoral and cellular responses are needed.
Pathogen-associated molecular patterns (PAMPs) have garnered a great deal of interest as vaccine adjuvants, because they have known mechanisms and can invoke a Th1 immune response. PAMPs consist of one or more structures that are sensed by pattern recognition receptors (PRRs) on host cells and can activate immune responses mediated through one or more signaling pathways. Three common classes of PRRs are Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and stimulator of interferon genes (STING). Some PRRs are found on the plasma membrane (e.g., TLR2, TLR4), where they are readily accessible to extracellularly delivered PAMPs, such as MPL, an FDA-approved agonist for TLR4. In contrast, others PRRs are located on phagasomal membranes or in the cytosol and require intracellular delivery of PAMPs such as R848, murabutide, and poly I:C, which activate TLR7/8, NOD2, and TLR3, respectively. These cytosolic PRRs predominantly coordinate Th1-biased humoral and cellular immunity.6 Of these cytosolic PRRs, STING activation can result in potent Th1-biased immune responses. One ligand that binds directly to and activates STING is 3′,3′-cyclic GMP-AMP (cGAMP). This variety of cGAMP, which is a cyclic dinucleotide (CDN) made by bacteria, has a higher STING binding affinity than other bacterial CDNs7 and has been shown to generate robust transcriptional activation of the type-I interferon (IFN) pathway.8 While cGAMP is known to be a potent STING agonist, the cellular membrane is a formidable obstacle to overcome in order for this hydrophilic charged small molecule to bind to its cytosolic PRR.
To improve delivery of cytosolic PAMPs such as GAMP and other STING agonist cyclic dinucleotides (CDNs), carriers such as liposomes9–12 and polymeric particles13,14 have been applied in preclinical studies. However, liposomes are difficult to scale for vaccine applications,15 and the polymers employed to date require polymer blends (e.g., polyethylenimine/hyaluronic acid)12 or are known to be cytotoxic (e.g., poly beta-amino esters).13 Polyesters (e.g., poly lactic-co-glycolic acid [PLGA]) are an another attractive polymer type, but disadvantages such as slow degradation rates within phagosomal compartments of antigen-presenting cells (APCs) and acidic hydrolytic byproducts limit their clinical efficacy.16,17
To overcome the shortcomings of these delivery vehicles, we have utilized the biopolymer acetalated dextran (Ace-DEX), which is derived from the FDA-approved water-soluble polysaccharide, dextran.18–20 Ace-DEX is unique due to its simple synthesis, in which acetal groups are formed along the glucose backbone of dextran, making the polymer acid-sensitive and organic-soluble. In aqueous environments, acetal groups are hydrolytically cleaved, generating pH-neutral and biocompatible degradation byproducts ethanol, acetone, and dextran. Ace-DEX has been formulated into microparticles (MPs), which offer micron particle size-dependent passive delivery to phagocytic APCs,20,21 resulting in minimal off-target delivery and efficient trafficking to lymph nodes.22,23 Ace-DEX MPs have demonstrated rapid degradation over a period of hours to days within low pH lysosomal conditions, with sustained degradation over a period of days to months at physiological pH.18,24 Ace-DEX MPs have illustrated dose sparing of several PAMPs24–28 as well as subunit vaccines against Bacillus anthracis, Burkholderia pseudomallei, and influenza.27,29,30
Previously, through electro-hydrodynamic spraying (electro-spray), we have generated cGAMP-loaded Ace-DEX MPs ranging in size from 1.5 to 3 μm.22 A comparative study evaluating cGAMP encapsulated in liposomes, PLGA MPs, and Ace-DEX MPs demonstrated a significantly higher expression of type-I IFN in response to Ace-DEX MPs than the other formulations. Additionally, cGAMP Ace-DEX MPs generated a robust cellular response and a balanced Th1/Th2 humoral immune response, which provided long-term (7 months) protection against a lethal influenza challenge. However, despite this success, we noted that the breadth of cytokines elicited by cGAMP Ace-DEX MPs was relatively narrow and did not include cytokines known to contribute to robust and long-lasting immune responses, most notably IL-1β and IL-12.31–34
One approach for eliciting broader and more protective cytokine responses is codelivery of PAMPS for concurrent activation of PRRs. During natural infection, pathogens often stimulate multiple PRRs,35 illustrating the importance of vaccines that can mimic this response to provide protection. An example of a multi-PRR stimulating vaccine is the yellow fever 17D (YF-17D) vaccine. The FDA-approved LAV YF-17D vaccine is one of the most effective vaccines known to date and activates multiple TLRs to generate a potent and balanced Th1/Th2 response.36 The broadly stimulating capacity of YF-17D vaccine yields a potent vaccine that only needs to be administered once for life-long protection. This is because cross-talk between multiple PRRs can be both quantitatively and qualitatively different than the contribution of each individual pathway.37
This current study first investigated the formulation of cGAMP MPs using multiple techniques and evaluated in vitro combinations of soluble PAMP adjuvants for synergistic potential. The optimal ratio of this multiadjuvant system was determined and then formulated into a single MP delivery vehicle containing two PAMPs. An in vitro comparison of a single adjuvant and combinational adjuvants using Ace-DEX and PLGA MPs was performed. The adjuvant systems also were compared in vivo to determine the degree of immune response enhancement.
MATERIALS AND METHODS
Animal Ethics Statement.
All studies were conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee at the University of North Carolina. All animals were maintained in pathogen-free facilities.
Chemicals.
All materials were purchased from Sigma-Aldrich (St. Louis, MO) and used as received, unless otherwise indicated. Vaccine grade ovalbumin (OVA), Alhydrogel 2%, cGAMP, and resiquimod (R848) were purchased from Invivogen (San Diego, CA).
Synthesis of Acetalated Dextran.
Ace-DEX was synthesized according to Kauffman et al. using dextran from Leuconostoc mesenteroides (molecular weight = 70 kDa).38 Briefly, lyophilized dextran and pyridinium p-toluenesulfonate (0.0617 mmol) were dissolved in anhydrous dimethyl sulfoxide (DMSO). Dissolved dextran was reacted with 2-ethoxypropene (Matrix, Columbia, SC) under nitrogen gas at room temperature for 30 min and quenched with an excess of triethylamine (TEA). The reaction volumes were then precipitated in basic water (0.04% TEA by volume in water) and centrifuged, and the resulting pellet was frozen and lyophilized overnight. The following day, the product was dissolved in ethanol and centrifuged to further purify the polymer. The supernatant was precipitated in basic water, centrifuged again, frozen, and lyophilized to yield 70 kDa Ace-DEX polymer. The polymer’s relative cyclic acetal coverage was determined to be 40%, as measured by 1H NMR spectroscopy (Inova 400 MHz spectrometer) following degradation of the polymer in 10% v/v deuterium chloride in deuterium oxide.
Formulation of Microparticles Using Ace-DEX or PLGA by Electrospray or Emulsion.
To prevent endotoxin contamination, all dishes and glassware were soaked in 1.0 M sodium hydroxide overnight, washed with isopropanol, and dried before use. Encapsulation of cGAMP or R848 within Ace-DEX microparticles (cGAMP Ace-DEX MPs or R848 Ace-DEX MPs, respectively) or cGAMP and R848 coencapsulated within the same MPs (cGAMP/R848 Ace-DEX MPs) were fabricated using electrospray following the setup of Junkins et al.22 The same methods were used to make poly(lactic-co-glycolic acid) ([PLGA], 85:15, 50–75 kDa, ester terminated) particles coencapsulating cGAMP and R848 (cGAMP/R848 PLGA MPs).22 Egg phosphatidylcholine (EggPC, Avanti Polar Lipids, Alabaster, AL) was added postfabrication to increase MP suspendability.
Alternatively, fabrication of MPs by solvent evaporation was performed using a modified version of the water-in-oil-in-water emulsion procedure previously reported.39
The endotoxin content of all MPs was measured prior to treatment according to Gallovic et al.39 and contained <0.25 EU/mg, within the recommended levels for preclinical subunit vaccine formulations.40
Imaging of Microparticles.
cGAMP, R848, and combination cGAMP/R848 MPs were imaged using a Hitachi S-4700 Cold Cathode Field Emission Scanning Electron Microscope (SEM). SEM images were used to confirm MP size and morphology.
Quantification of cGAMP and R848 Loading within Microparticles.
cGAMP loading was determined using high performance liquid chromatography (HPLC) according to Junkins et al.22 R848 loading was determined by its autofluorescence (ex/em: 260/360 nm) according to Duong et al.28 Blank MPs were used as a background correction.
cGAMP and R848 Release Profiles from Micro-particles.
cGAMP, R848, or cGAMP/R848 Ace-DEX MPs were suspended in phosphate-buffered saline (PBS; pH 7.4) at 37 °C on a shaker plate operating at 200 rpm. At predetermined time points, aliquots were removed and centrifuged (30 min at 21 000g, 4 °C). The pellets were analyzed similarly to the loading protocols outlined above for either cGAMP or R848 MPs, respectively. Blank MPs collected at each time point were subjected to the same process and used as background subtractions.
Flow Cytometry of Multiple Fluorophore Micro-particles.
Ace-DEX encapsulating fluorophores BODIPY 493/503, Texas Red, and BODIPY 630/650 (Thermo Fisher Scientific) were coencapsulated within the same MPs using the electrospray setup as described above. MPs were suspended in FACs buffer (PBS containing 4% fetal calf serum and 0.05% sodium azide), and flow cytometric analysis was performed using an LSR II flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Blank MPs were used to generate a gated population.
Bone Marrow Derived Dendritic Cell Culture.
Bone marrow derived dendritic cells (BMDCs) were cultured as described previously.41 Briefly, bone marrow obtained from 8-to 12-week-old C57BL/6 mice were washed and seeded in BMDC differentiation media containing mouse GM-CSF (Peprotech, Rocky Hill, NJ). After 7 days, nonadherent cells were seeded in GM-CSF-free media overnight prior to treatment.
Cytokine ELISAs.
Tumor necrosis factor (TNF), IL-6, IL-1β, IL-2, and IL-12p70 levels were measured by ELISAs purchased from BD Biosciences (San Jose, CA; Santa-Cruz, Dallas, TX), while interferon gamma (IFN-γ) was measured using ELISAs purchased from eBioscience (Thermo Fisher, Waltham, MA). ELISAs were performed according to the manufacturer’s protocol. IFN-β was measured via an ELISA described previously.22
In Vitro Assessment of Microparticle Activity.
For in vitro screening of PAMPs with cGAMP MPs, BMDCs were treated with soluble agonists: murabutide (10 μg/mL), monophosphoryl lipid A (MPL, 1 μg/mL), poly(dA:dT) (10 μg/mL), CpG (1 μg/mL), poly(I:C) (10 μg/mL), or R848 (0.01 μg/mL) alone or in combination with cGAMP MPs (1 μg/mL cGAMP) or an equivalent amount of blank MPs. Cell supernatants were collected 22 h later for cytokine analysis. Experiments with PLGA MPs and Ace-DEX MPs for comparison of coloaded and individually loaded particles were performed as with the adjuvant combinations. All experiments were performed in triplicate on four separate cultures of BMDCs.
In Vivo Immunization Studies.
Age (8–16 weeks) and sex matched (male and female) C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were immunized by intramuscular (i.m.) injections on days 0 and 21 with either PBS or 10 μg of soluble OVA (low endotoxin) mixed with an MP group (Supplemental Table S1) in a total volume of 50 μL (n = 4–5/group). Alum controls received soluble OVA plus Alhydrogel 2% mixed at a 1:1 ratio by volume, also given as a 50 μL i.m. injection. Serum was collected on day 28 for antibody titers. Mice received a second vaccine boost on day 35 and were then sacrificed on day 42.
Antigen-Specific Serum Endpoint-Binding Titers.
Antigen-specific serum antibody-binding titers (endpoint) were determined by a standard ELISA as previously described.42 Briefly, serial dilutions of test sera were performed in plates coated with OVA (Invivogen) at 2.5 μg/mL. Following incubation and washing, horseradish-peroxidaseconjugated antimouse Ig-specific antibodies (Southern Biotech, Birmingham, AL) were added to plates at a 1:4000 dilution. After incubation and washing, 3,3′,5,5′-tetramethylbenzidine substrate solution (KPL, Gaithersburg, MD) was added and stopped with 2 N H2SO4 solution. The plates were read at an optical density (OD) of 450 nm (SynergyH1 plate reader, BioTek, Winooski, VT). Endpoint titers are reported as the log of the reciprocal of the highest serum dilution, at which the OD value was equal to or greater than 3 times the average background OD of the plate.
Splenocyte Restimulation.
On day 42, splenocytes were isolated from mice that were immunized as described above per Junkins et al.22 IFN-γ and IL-2 ELISpots were performed according to manufacturer’s protocols (Thermo Fisher Scientific) using 2 × 105 splenocytes stimulated with 10 μg/mL SIINFEKL peptide (Anaspec, Fremont, CA) for 36 h. Plates were dried, and spots were quantified using an ELISpot Reader System (AID, Strassberg, Germany). Alternatively, 2 × 105 splenocytes were stimulated with 10 μg/mL whole OVA protein for 36 h. Supernatants were collected and analyzed for IL-2 and IFN-γ by ELISAs.
Statistical Analysis.
All data were analyzed using Graph-Pad Prism software. Group comparisons were analyzed using one-way analysis of variance (ANOVA), with differences between groups assessed using Tukey’s post hoc test. A p-value of less than 0.05 was considered statistically significant. Data are reported as indicated in figure captions.
RESULTS AND DISCUSSION
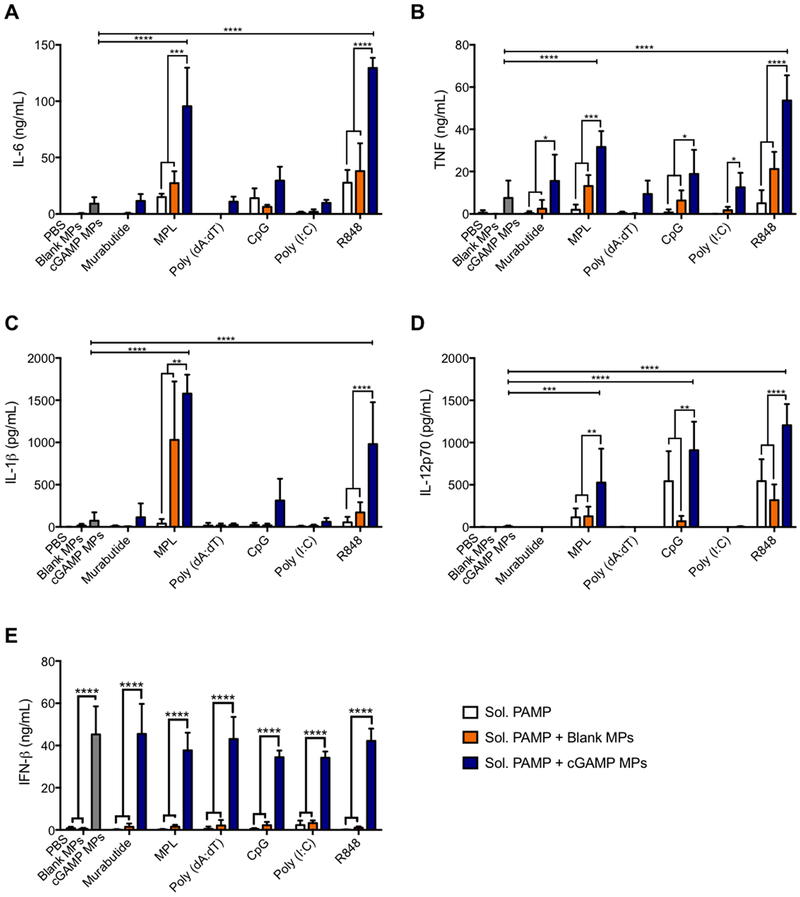
Previously, our laboratory has demonstrated that Ace-DEX MPs encapsulating cGAMP were safe and potent inducers of type-I interferon (IFN), and vaccination using a cGAMP MP adjuvant protected mice against a lethal influenza challenge with dose sparing over soluble cGAMP.22 In that work, cGAMP MPs alone generated high levels of IFN-β, IL-6, and TNF,22 which are all key contributors in generating a robust immune response; however, cGAMP MPs lacked the ability to produce IL-1β and IL-12p70, which are critical during viral infections and play pivotal roles in generating adaptive immune responses.43,44 Additionally, recombinant IL-1β31,45,46 and IL-1247,48 have been used successfully as vaccine adjuvants in preclinical model systems. Therefore, in our current study, we aimed to identify a combination of PAMP adjuvants that can elicit a broader cytokine profile, including IL-1β and IL-12p70. Murine BMDCs were treated with cGAMP MPs alone or in combination with a variety of soluble PAMPs. We tested several PAMPs that target TLRs either on the plasma membrane (FDA-approved TLR4 agonist MPL) or phagosomal membranes (TLR3 agonist poly I:C, TLR7/8 agonist R848, or TLR9 agonist CpG). Poly(dA:dT), which is detected by cytosolic DNA sensors, and NOD2 agonist murabutide were also evaluated in combination with cGAMP MPs. Among the group of PAMPs examined, MPL and R848 showed the greatest impact on cytokine responses (Figure 1). In combination with cGAMP MPs, both PAMPs induced significant levels of IL-1β and IL-12p70, which were not achieved with either cGAMP MPs or soluble PAMPs alone. Furthermore, the combinations significantly enhanced production of IL-6 and TNF compared to each stimulus on their own.
Figure 1.
Combined treatment of cGAMP microparticles (MPs) with various soluble pathogen-associated molecular patterns (PAMPs). Bone marrow derived dendritic cells (BMDCs) from C57BL/6 mice were treated with soluble (Sol.) murabutide (10 μg/mL), MPL (1 μg/mL), poly(dA:dT) (10 μg/mL), CpG (1 μg/mL), poly(I:C) (10 μg/mL), or R848 (0.01 μg/mL) alone, or in combination with 1 μg/mL cGAMP MPs or an equivalent amount of blank MPs. The left-most data group displayed in each panel includes a PBS control (checkered bar), an equivalent amount of blank MPs to the cGAMP MPs (black bar), and 1 μg/mL cGAMP MPs only (gray bar). Cell supernatants were collected after 22 h and were analyzed for (A) IL-6, (B) TNF, (C) IL-1β, (D) IL-12p70, and (E) IFN-β (n = 4 ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p <0.0001).
MPL in combination with cGAMP MPs demonstrated significant increases in cytokine production;, however, R848 was overall more potent than MPL. R848 is an imidazoquino-line agonist of TLR7/8 with potent myeloid differentiation primary response gene 88 (MyD88)-dependent transcriptional activation of NF-κB pathways. The use of R848 in a microparticulate vaccine is relevant to humans, as TLR7 and TLR8 are predominantly expressed in human APCs such as plasmacytoid dendritic cells, myeloid dendritic cells, and monocytes.49 R848 has previously been shown to have additive effects in vivo with a MyD88-independent TLR3 agonist,50 which suggests that R848 and cGAMP would have significant activity in vivo.
In addition to R848 eliciting the most robust responses, it was chosen for further study for several other reasons. First, very low doses were required (~100-fold lower than MPL). Second, we have previously demonstrated that the acid-sensitive Ace-DEX MPs are highly effective for delivering R848.29,30,51,52 Finally, while other groups have previously demonstrated that combinations of cGAMP with the TLR9 agonist CpG result in IL-12 production and improved in vivo vaccine outcomes,53,54 and liposomal c-di-GMP (another CDN) with MPL led to improved humoral and cellular immunity,10 combinations of CDNs with R848 remained unexplored.
To formulate cGAMP and R848 into Ace-DEX MPs, we took several approaches including emulsion and electrospray processes. Emulsion-based, solvent evaporation MPs were formulated to determine if encapsulation efficiencies (EEs) of cGAMP and R848 would remain constant when combining the two molecules within the same formulation. We also examined the clinically relevant biodegradable polymer, PLGA. Because we have previously formulated various PAMPs into PLGA (85:15) MPs,22,26 for comparative purposes we chose to use this polymer grade again. The MPs demonstrated a poor 3% encapsulation efficiency (EE) of cGAMP and were unable to encapsulate R848 (Table 1). Conversely, the acid-sensitive biopolymer, Ace-DEX, demonstrated modestly higher EEs of cGAMP (52%) and R848 (8%) in singularly encapsulated particles. These EEs for R848 were consistent with previous work reported by our lab29,30,52 and are likely driven by loss of the cargo to the external aqueous phase of the emulsion.55
Table 1.
Encapsulation Efficiencies (EE) of cGAMP, Resiquimod (R848), or a Combination of Both (cGAMP/R848) within Acetalated Dextran (Ace-DEX) or Poly Lactic-co-glycolic Acid (PLGA) Microparticles by Emulsion and Electrospray
| fabrication technique |
microparticles | cGAMP EE (%) |
R848 EE (%) |
|---|---|---|---|
| emulsion | cGAMP Ace-DEX | 52 | -- |
| cGAMP PLGA | 3 | -- | |
| R848 Ace-DEX | -- | 8 | |
| R848 PLGA | -- | 0 | |
| cGAMP/R848 Ace-DEX | 25 | 8 | |
| electrospray | cGAMP Ace-DEX | 94 | -- |
| R848 Ace-DEX | -- | 86 | |
| cGAMP/R848 Ace-DEX | 83 | 75 | |
| cGAMP/R848 PLGA | 74 | 67 |
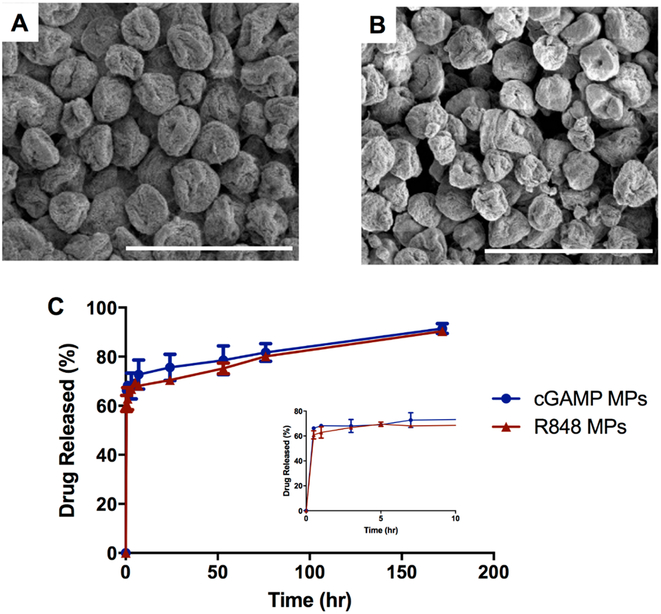
Formulating cGAMP MPs and R848 MPs through coaxial electrospray yielded significantly higher EEs compared to MPs formulated through the emulsion-based method. Scanning electron micrographs demonstrated spherical and fairly monodispersed electrospray MPs with EEs of 94 and 86% for cGAMP and R848, respectively (Figure 2). The release kinetics of cGAMP and R848 MPs were very similar, with a large burst release due to drug diffusion occurring within the first 30 min of incubation, followed by a sustained release for the duration of the 1 week experiment (Figure 2C). This is similar to previously reported cGAMP and R848-loaded Ace-DEX MP formulations.22,27
Figure 2.
Morphology and release kinetics of cGAMP and R848 individually encapsulated in acetalated dextran microparticles (Ace-DEX MPs) made by electrospray. Scanning electron micrographs of (A) cGAMP Ace-DEX MPs and (B) R848 Ace-DEX MPs. Scale bars = 4 μm. (C) Release profiles of cGAMP and R848 individually encapsulated into acetalated dextran MPs were conducted at pH 7.4. Data are presented as mean ± SEM (n = 3).
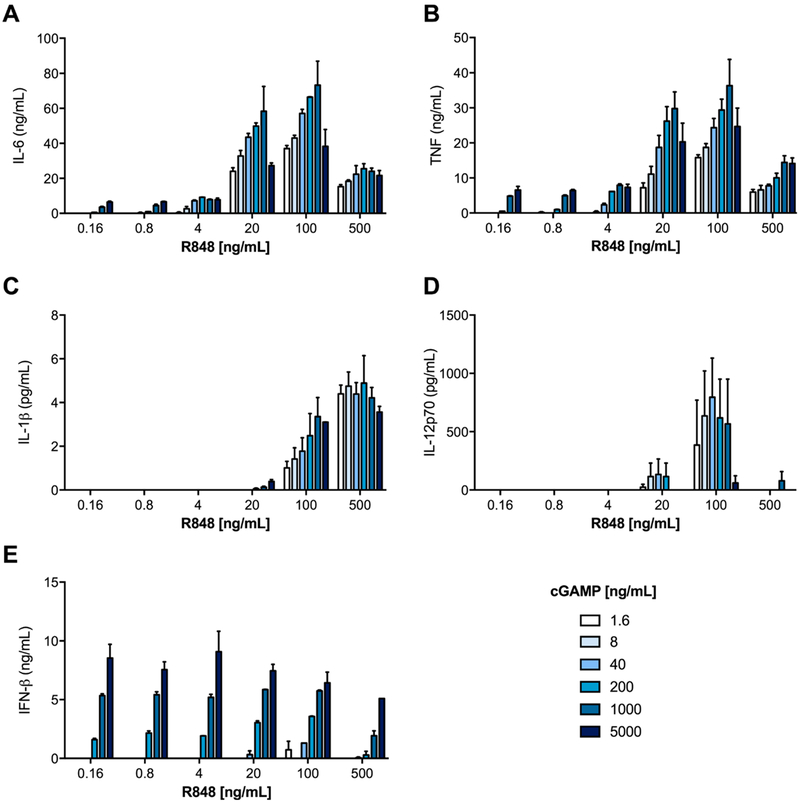
We next set out to identify the ratio of cGAMP and R848 that yielded optimal biological activity, in order to inform target weight loadings for coencapsulated particles. BMDCs were treated for 24 h with the indicated doses of individually encapsulated cGAMP and R848 Ace-DEX MPs, and super-natants were then assayed for cytokine production (Figure 3, heat mapped in Supplementary Figure S1). The trends in the IL-6, TNF, IL-1β, and IL-12p70 data suggest that the optimal responses occurred at a 10:1 weight-to-weight (w/w) ratio of cGAMP to R848, which was therefore used in subsequent particle formulations. Decreased cytokine production at higher cGAMP concentrations was also illustrated in our previous work using cGAMP MPs alone.22
Figure 3.
Optimal ratio determination for cGAMP and resiquimod (R848) microparticles. Bone marrow derived dendritic cells from C57BL/6 mice were treated for 22 h with indicated concentrations of resiquimod (R848) and cGAMP, both of which were individually encapsulated within acetalated dextran (Ace-DEX) microparticles. Supernatants were analyzed for (A) IL-6, (B) TNF, (C) IL-1β, (D) IL-12p70, and (E) IFN-β. Data are presented as mean ± SEM (n = 4).
In our previous work, multiple analytes have been loaded into Ace-DEX MPs via double-emulsion and coaxial electro-spray,23,52,56 and those methods were also employed to coformulate cGAMP and R848. The emulsion-based combination MPs of cGAMP and R848 resulted in a drop from 52 to 25% EE of cGAMP (Table 1), essentially encapsulating half the drug of the individually encapsulated formulation. The EE of R848 persisted at a low 8%. Although certain parameters can be modified to enhance the EEs of polymeric MPs formed through solvent evaporation, a target ratio is extremely difficult to repeatedly formulate.57 Furthermore, emulsion-based solvent evaporation methods are a batch process, which can result in inconsistencies in loading, a high cost for scale-up, and be difficult in aseptic environments, thus limiting the potential for clinical applications.58 More recently, a dual impinger technology has become increasingly popular, in which two phases are pumped together simultaneously with turbulent flow to generate particles; however, an external aqueous phase is still a driving force for poor EE.55 To increase the EE of cGAMP and R848 within that of Ace-DEX MPs and generate particles using a more scalable process, electrospray was utilized to formulate particles used in all subsequent experiments.
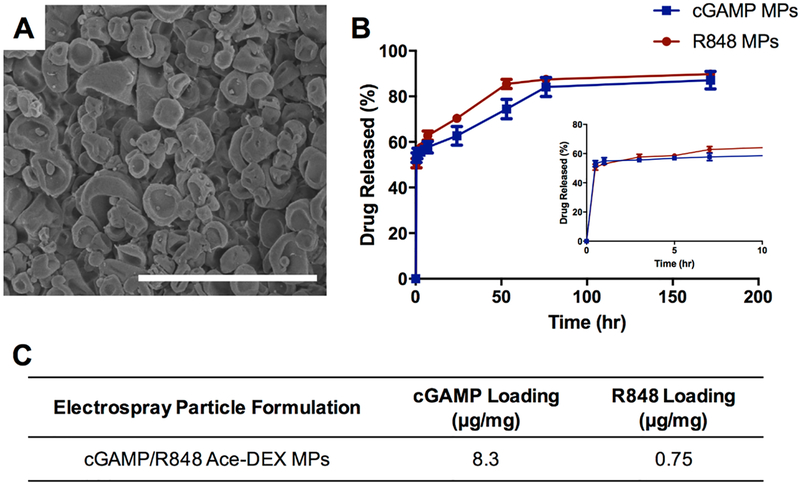
Combination MPs containing cGAMP and R848 (10:1 ratio) in the same particle were coaxially electrosprayed and displayed an erythrocyte-like morphology (Figure 4). This differed from the more spherical singularly encapsulated cGAMP or R848 MPs (Figure 2). The release profile of each adjuvant was very similar to the release of the individually encapsulated particles with a large burst occurring within the first 30 min of incubation, followed by a sustained release for the duration of the experiment. The release kinetics following the burst release of the combination Ace-DEX MPs was faster in a pH 7.4 solution than that of the singularly encapsulated particles (Figure 2C). The EE decreased for both PAMPs when coencapsulated via electrospray, but overall, the EEs were significantly greater than for coencapsulation MPs formed through emulsion or either method with PLGA (Table 1). Drug loading of the combination MPs was measured at 8.3 μg/mg of cGAMP and 0.75 μg/mg of R848, very close to the target ratio of 10:1 (Figure 4C). To ensure that both compounds could be encapsulated into a single MP, multiple fluorophores were sprayed and analyzed using flow cytometry (Supplemental Figure S.2). This analysis demonstrated that all three fluorescent small molecules were distributed throughout the entire particle population at high levels.
Figure 4.
Morphology and release kinetics of combination microparticles made by electrospray. (A) Scanning electron micrographs of acetalated dextran microparticles encapsulating a combination of cGAMP and resiquimod (cGAMP/R848 Ace-DEX MPs). Scale bar indicates 4 μm. (B) Release profiles of cGAMP and R848 within combination MPs conducted at pH 7.4. (C) Drug loading of cGAMP and R848 (μg/mg). Data are presented as mean ± SEM (n = 3).
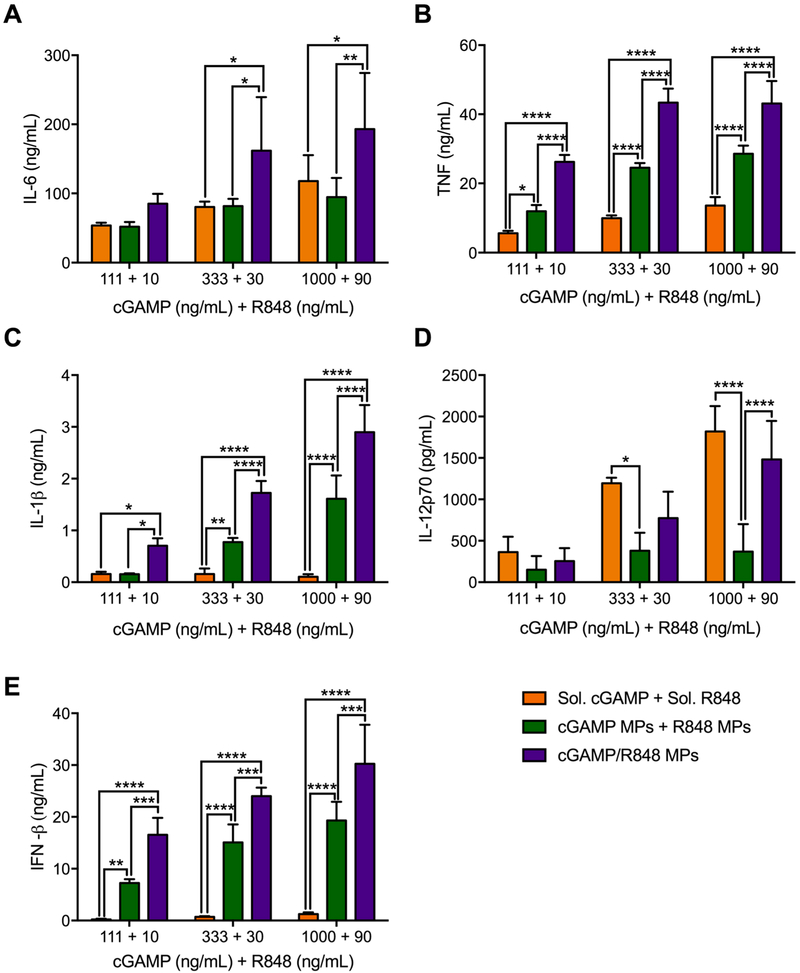
The ability to target a specific drug ratio and create a particle population with both drugs at these ratios with minimal drug loss is a highly advantageous formulation strategy. Once the ratio was selected and MPs made, the combination MPs were analyzed in comparison to singularly encapsulated particles delivered together in vitro (Figure 5). Comparing the separate and combination particles, our data indicated that there is an advantage to having the adjuvants within the same particle.
Figure 5.
Coencapsulation of cGAMP and R848 in acetalated dextran microparticles (Ace-DEX MPs) compared to individual encapsulation. Bone marrow derived dendritic cells from C57BL/6 mice were treated with indicated concentrations of cGAMP and R848, respectively, delivered as soluble (Sol.) drugs, encapsulated in separate Ace-DEX MPs (cGAMP MPs + R848 MPs) or coencapsulated within the same Ace-DEX MPs (cGAMP/R848 MPs). After 22 h, supernatants were harvested and analyzed for (A) IL-6, (B) TNF, (C) IL-1β, (D) IL-12p70, and (E) IFN-β (n = 4 ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
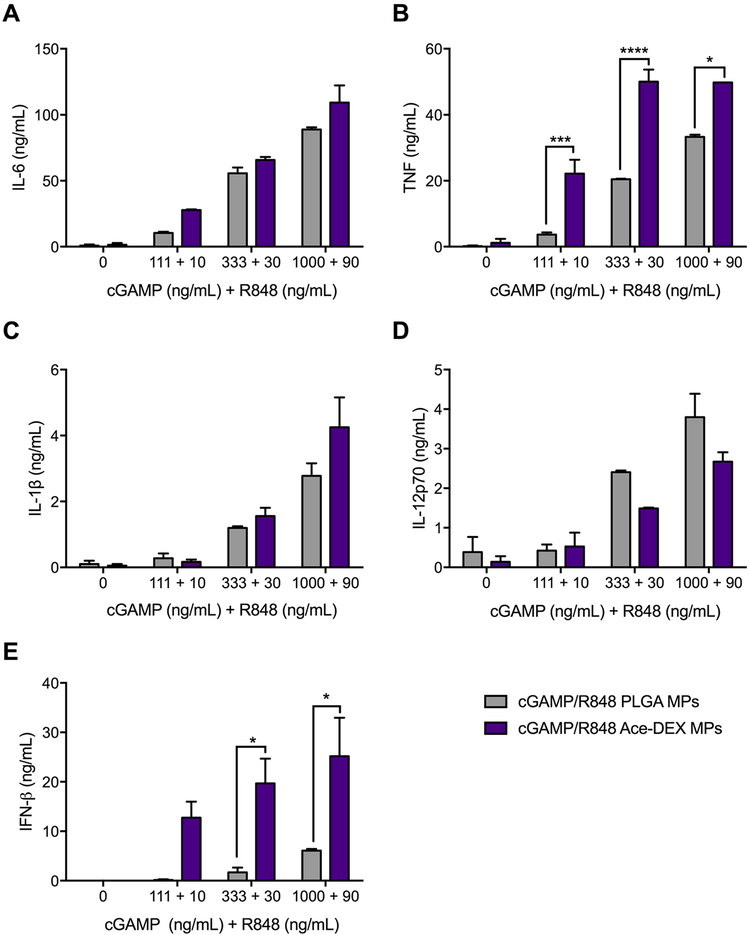
We wanted to then evaluate if the differences were dependent on the polymer chosen, so we compared Ace-DEX versus PLGA MPs. Ace-DEX is an ideal delivery vehicle for adjuvants with intracellular targets such as STING and TLR7/8 because of its acid-sensitive degradation kinetics.19 Once an Ace-DEX particle is phagocytosed by an phagocytic cell,23 the particle will rapidly degrade within the low pH environment of the lysosome.38 According to the release profiles in Figures 2 and 4, both water-soluble PAMPs take over 7 days to achieve maximum release at pH 7.4; however, in the lower pH environment of the lysosome, maximum release should occur in only a few hours or less.22 While the burst release observed for R848 and cGAMP is not ideal, these are both water-soluble molecules that may be diffusing rapidly through the hydrophobic polymeric formulation to reach the external aqueous environment. Indeed, burst release is not uncommon with water-soluble molecules encapsulated within hydrophobic microparticle formulations.59 Since PLGA is used in approximately 15 FDA-approved drug delivery formulations,60 we evaluated the innate immune response of Ace-DEX or PLGA MPs encapsulating cGAMP and R848 (cGAMP/R848 PLGA MPs; Table 1, Figure 6). cGAMP/R848 Ace-DEX MPs elicit robust IL-6, TNF, IL-1β, IL-12p70, and IFN-β responses, indicating that Ace-DEX is an ideal drug carrier for vaccine adjuvant systems. Additionally, cGAMP/R848 Ace-DEX MPs result in significantly greater induction of TNF and IFN-β compared to cGAMP/R848 PLGA MPs, which aligns with our previous work where we demonstrated that cGAMP MPs consisting of Ace-DEX elicited more potent immune responses than PLGA as well as liposomes.22 It is theorized that the slow degradation kinetics of PLGA and minimal pH sensitivity of the polymer prohibited cGAMP from releasing quick enough within the lysosome to induce potent cytokine production.
Figure 6.
Cytokine profiles of combination cGAMP/R848 acetalated dextran (Ace-DEX) or poly(lactic-co-glycolic acid) (PLGA) MPs. Bone marrow derived dendritic cells from C57BL/6 mice were treated with indicated concentrations of cGAMP and R848, respectively, coencapsulated within Ace-DEX or PLGA MPs. After 22 h, supernatants were harvested and analyzed for (A) IL-6, (B) TNF, (C) IL-1β, (D) IL-12p70, and (E) IFN-β (n = 4 ± SEM *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
To better characterize the promising results we observed in vitro, we next evaluated the formulation in vivo. Initially we evaluated the acute toxicity of the combination MPs. cGAMP/R848 Ace-DEX MPs (200 ng of cGAMP and 18 ng of R848) were injected intramuscularly (i.m), and mice were monitored for 7 days to assess toxicological outcomes including body weight, body temperature, body condition (a composite of activity, weight loss, body temperature, hydration, physical appearance, and appetite), and survival (Supplementary Figure S3). No acute toxicity, adverse events, or injection site reactogenicity were observed.
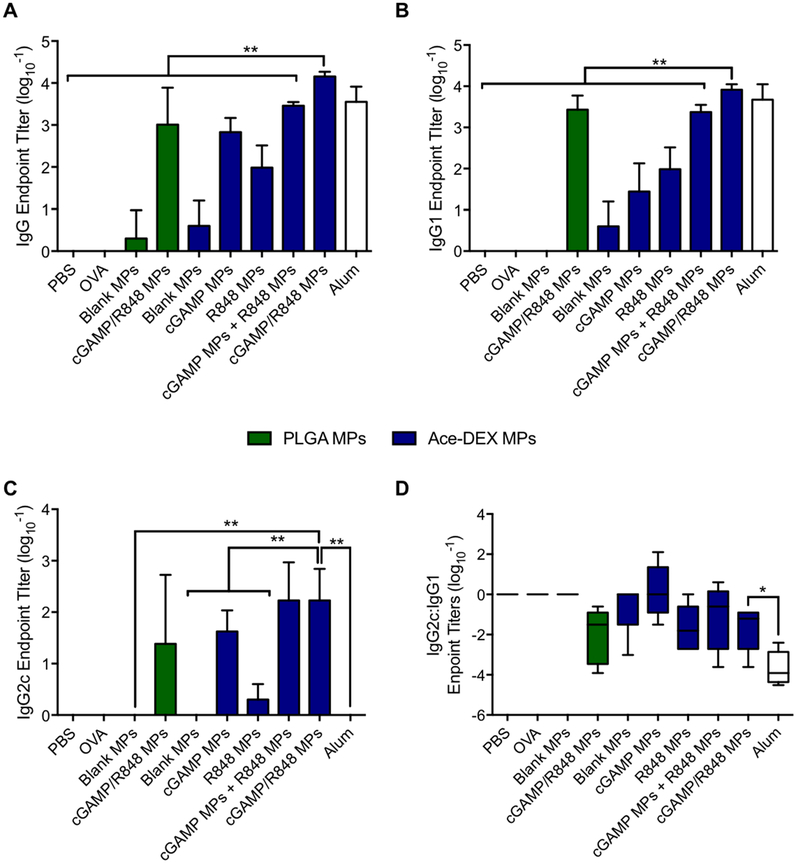
After acute toxicity of the formulation was evaluated, mice were vaccinated against the model antigen OVA with various formulations (Table S1). Blank Ace-DEX and PLGA MP controls were included, as well as alum, a clinically relevant adjuvant which served as a positive control. On day 28, serum was collected and analyzed for antibody titers against OVA (Figure 7). In comparing the formulations, no other MP group resulted in a greater than 2.5-fold increase in total IgG with respect to cGAMP MPs except for cGAMP/R848 Ace-DEX MPs, which resulted in over a 7.5-fold increase. Strikingly, delivery of cGAMP/R848 Ace-DEX MPs resulted in antibody titers 10-fold higher than those obtained using combination cGAMP/R848 PLGA MPs, supporting the in vitro bioactivity results using the Ace-DEX MP formulation. Combination cGAMP/R848 Ace-DEX MPs also induced higher total IgG titers compared to individually encapsulated cGAMP and R848 Ace-DEX MPs delivered either alone or together. This complements results observed by Kasturi et al.57 and demonstrates the importance of having multiple adjuvants within the same particle for humoral responses. These results suggest that the antibody titers produced from the combination MPs are not simply an additive effect between the two adjuvants but rather a synergistic effect.61
Figure 7.
Comparison of antibody responses induced by cGAMP and R848 delivered via acetalated dextran (Ace-DEX) or poly(lactic-co-glycolic acid) (PLGA) microparticles (MPs). C57BL/6 mice were immunized intramuscularly on days 0 and 21. All groups received 10 μg of OVA, except the PBS group. OVA was given alone or in combination with cGAMP (200 ng) and R848 (18 ng) delivered in single-loaded or dual-loaded Ace-DEX or PLGA MPs, blank Ace-DEX or PLGA MPs, or alum. On day 28, serum was collected and analyzed for ovalbumin (OVA)-specific (A) total IgG, (B) IgG1, and (C) IgG2c as well as (D) the ratio between IgG2c and IgG1 isotypes (n = 4–5 ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Data are presented as n = 5 ± SD.
While total IgG generation reveals the humoral response to vaccination, IgG1 and IgG2c subtypes are indicative of the strength of the inflammatory process by Th2- and Th1-skewed responses, respectively.62 For certain pathogens, it is important to achieve more balanced Th1/Th2 humoral immunity, because both responses are critical for successful clearance. As with total IgG, combination cGAMP/R848 Ace-DEX MPs induced the highest overall IgG1 and IgG2c titers. Consistent with our previously reported findings, all formulations including cGAMP induced significant levels of IgG2c and resulted in a near even ratio of IgG2c/IgG1, indicating a balanced Th1/Th2 humoral response.22 This is in stark contrast to alum, which showed a profound bias toward IgG1, indicating potent Th2 polarization. The IgG1 responses in groups with combination adjuvant systems (coencapsulated and singularly encapsulated combination) were larger than the responses seen with cGAMP alone, demonstrating that Th2-type responses can be influenced by dual activation of the immune system (Figure 7). Vaccination with cGAMP/R848 Ace-DEX MPs elicited the largest production of IgG1 compared to all other formulations, indicating that Th2 skewing could benefit from coencapsulation and Ace-DEX degradation kinetics.24 Th1 skewing has been implicated in long-lasting immunity, which is pivotal for a successful vaccine.63 IgG2c production seemed to be independent of coencapsulation, as vaccination with cGAMP/R848 Ace-DEX MPs or the combination of cGAMP MPs and R848 MPs resulted in similar titers. These results shed some light on how adjuvant systems can induce more balanced humoral immune skewing, which is critical for broad acting vaccines.
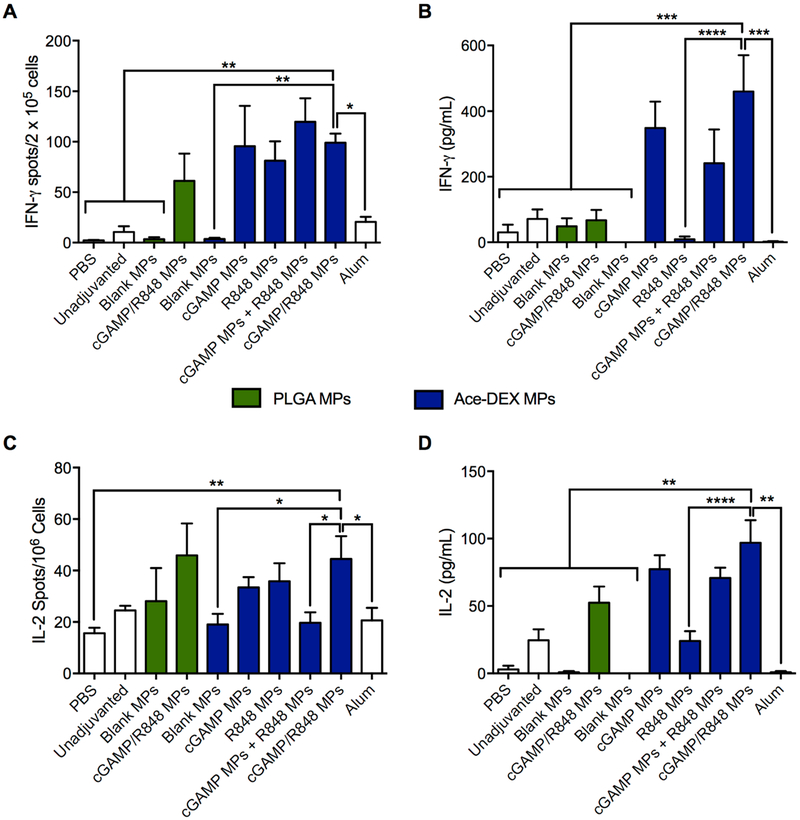
Th1 and Th2 skewing via IgG subtypes is not fully indicative of cellular and humoral recognition of antigens, and thus, CD8+ and CD4+ T cell activities were assessed. Splenocytes were collected on day 42 and restimulated with the CD8+ restricted OVA T cell epitope SIINFEKL for the assessment of antigen-specific IFN-γ or IL-2 producing T cells by ELISpot (Figure 8A,C) or whole OVA protein, for the total production of IFN-γ and IL-2 in culture supernatants as assessed by ELISAs (Figure 8B,D). The combination cGAMP/R848 Ace-DEX MPs induced significant IFN-γ and IL-2 production compared to controls containing OVA alone, OVA plus either R848 MPs, or alum (Figure 8B,D). Interestingly, while the cGAMP/R848 PLGA MPs had comparable OVA-specific T-cell responses compared to cGAMP/R848 Ace-DEX MPs by ELISpot (not statistically different), the Ace-DEX MP formulation induced significantly greater IFN-γ and IL-2 responses (as observed by ELISA), indicating a more potent antigen-specific T cell activation (Figure 8B,D). These differential responses indicate that perhaps the CD8+ activity of the vaccine is not solely dependent upon coencapsulation of adjuvants but also dependent on the timing of adjuvant delivery, which agrees with our previous work.20,24 Also, IFN-γ production was measured following whole OVA stimulation of splenocytes, indicating that the CD4+ epitope was successfully recognized. Together, these results indicate that both coencapsulation of adjuvants and degradation kinetics of the vehicle can enhance antigen-specific T cell responses, similar to results documented by Savelkoul et al.64 and Chen et al.,24 respectively.
Figure 8.
Comparison of T cell responses induced by cGAMP and R848 delivered via acetalated dextran (Ace-DEX) or poly(lactic-co-glycolic acid) (PLGA) microparticles (MPs). C57BL/6 mice were immunized intramuscularly on days 0, 21, and 35. All groups received 10 μg of OVA, except the PBS group. OVA was given alone or in combination with cGAMP (200 ng) and R848 (18 ng) delivered in single-loaded or dual-loaded Ace-DEX or PLGA MPs, blank Ace-DEX or PLGA MPs, or alum. On day 42, mice were sacrificed and (A) IFN-γ and (C) IL-2 ELISpots were performed on splenocytes restimulated with SIINFEKL peptide (10 μg/mL) for 36 h. Alternatively, splenocytes were stimulated with 10 μg/mL ovalbumin (OVA) protein for 36 h. Supernatants were analyzed for (B) IFN-γ and (D) IL-2 by ELISA (n = 4–5 ± SD, *p < 0.05, **p ← 0.01, ***p < 0.001, ****p < 0.0001).
Several previous reports have examined codelivery of multiple TLR agonist adjuvants in PLGA particles fabricated through single- or double-emulsion procedures.57,65–71 These reports agree with our observations using cGAMP/R848 Ace-DEX MPs, in that coencapsulation of TLR agonists provided either dose sparing66 and/or enhancement of biological responses65–67,69 compared to soluble TLR agonist combinations.
We also determined experimentally that delivery of PAMPs in the same MPs induced superior responses both in vitro and in vivo when compared to the same dose of PAMPs delivered in separate MPs. One likely explanation for this observation is that coencapsulated agonists will always engage their respective PRRs in the same cell (cis-engagement), allowing for PRR cross-talk and enhanced immune activation. Conversely, when PAMPs are delivered in separate particles, both particle types need to be delivered intracellularly in order for PRR crosstalk to occur (trans-engagement). The likelihood of this happening is dependent upon both particle location and the phagocytic capacity of the cell, leading to a more variable response. This cis- vs trans-engagement of receptors is poorly studied in the context of innate immunity but may be important for optimal biological responses, as PRR cross-talk plays a central role in innate immunity and allows the generation of a pathogen-specific immune response.35 In addition, others have shown that the combination of coencapsulated PAMPs had a stronger adjuvant effect than the delivery of individually encapsulated PAMPs.57,71 Therefore, our work and the work of others strongly supports that coengagement of multiple PRRs is able to generate potent humoral and cellular responses.57,67,70,71
Taken together, Ace-DEX MPs containing cGAMP and R848 stand as a novel and efficacious formulation for vaccine development. Novel and potent adjuvant systems are needed to develop efficacious vaccines that generate balanced immune responses. While the MPL and alum adjuvant system (AS04) provides a balanced immune response, cGAMP/R848 Ace-DEX MPs offer a unique delivery vehicle that can passively target APCs and deliver adjuvants extremely quickly once internalized. The doses used within these experiments were on the nanogram scale, also offering a dose sparing alternative to traditional adjuvant vaccine systems. cGAMP/R848 Ace-DEX MPs represent a potent adjuvant system that can be administered at extremely low doses with strong immune responses for vaccine applications.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the National Institutes of Health U19AI109784 and U54CA198999. The authors would like to acknowledge the assistance from the UNC Chapel Hill Analytical and Nanofabrication Laboratory (CHANL) in acquiring SEM images and would also like to thank the UNC CFAR Virology, Immunology, and Micro-biology Core for use of their ELISpot reader. Cell lines (Madin–Darby canine kidney (MDCK) cells, London Line, and FR-58) were obtained through the Influenza Reagent Resource, Influenza Division, WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza (Centers for Disease Control and Prevention, Atlanta, GA, USA). Endpoint ELISA assays were performed in the Immunology Unit of the Duke Regional Biocontainment Laboratory, which received partial support for construction from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (UC6-AI058607). The authors would also like to acknowledge the valuable technical assistance of: Dr. Willie June Brickey, Kimberly Parks, Kristina Riebe, Melissa Samo, and Christopher Sample.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.molpharmaceut.8b00579.
Supplementary Table 1: vaccine formulations for in vivo studies; Supplementary Figure 1: heat map of cytokines induced by treatment with combination cGAMP and R848 acetalated dextran microparticles (Ace-DEX MPs); Supplementary Figure 2: flow cytometry analysis of acetalated dextran microparticles (Ace-DEX MPs) encapsulating various fluorophores; Supplemental Figure 3: characterization of toxicity outcomes in vivo following acetalated dextran microparticle (Ace-DEX MP) admin-istration (PDF)
The authors declare the following competing financial interest(s): Drs. Ainslie, Ting, and Bachelder serve on the advisory board for IMMvention Therapeutix, Inc. Although a financial conflict of interest was identified for management based on the overall scope of the project and its potential benefit to IMMvention Therapeutix, Inc., the research findings included in the publication may not necessarily relate to the interests of IMMvention Therapeutix, Inc. The terms of this arrangement have been reviewed by the University of North Carolina at Chapel Hill in accordance with its policy on objectivity in research.
REFERENCES
- (1).Pliaka V; Kyriakopoulou Z; Markoulatos P Risks associated with the use of live-attenuated vaccine poliovirus strains and the strategies for control and eradication of paralytic poliomyelitis. Expert Rev. Vaccines 2012, 11 (5), 609–28. [DOI] [PubMed] [Google Scholar]
- (2).Minor PD Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–92. [DOI] [PubMed] [Google Scholar]
- (3).Riese P; Schulze K; Ebensen T; Prochnow B; Guzman CA Vaccine adjuvants: key tools for innovative vaccine design. Curr. Top. Med. Chem 2013, 13 (20), 2562–80. [DOI] [PubMed] [Google Scholar]
- (4).O’Hagan DT; Ott GS; De Gregorio E; Seubert A The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine 2012, 30 (29), 4341–8. [DOI] [PubMed] [Google Scholar]
- (5).Awate S; Babiuk LA; Mutwiri G Mechanisms of action of adjuvants. Front. Immunol 2013, 4, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Melchjorsen J Learning from the messengers: innate sensing of viruses and cytokine regulation of immunity - clues for treatments and vaccines. Viruses 2013, 5 (2), 470–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zhang X; Shi H; Wu J; Zhang X; Sun L; Chen C; Chen ZJ Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol. Cell 2013, 51 (2), 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Diner EJ; Burdette DL; Wilson SC; Monroe KM; Kellenberger CA; Hyodo M; Hayakawa Y; Hammond MC; Vance RE The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Rep. 2013, 3 (5), 1355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Miyabe H; Hyodo M; Nakamura T; Sato Y; Hayakawa Y; Harashima H A new adjuvant delivery system ‘cyclic di-GMP/YSK05 liposome’ for cancer immunotherapy. J. Controlled Release 2014, 184, 20–7. [DOI] [PubMed] [Google Scholar]
- (10).Hanson MC; Crespo MP; Abraham W; Moynihan KD; Szeto GL; Chen SH; Melo MB; Mueller S; Irvine DJ Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Invest 2015, 125 (6), 2532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Nakamura T; Miyabe H; Hyodo M; Sato Y; Hayakawa Y; Harashima H Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J. Controlled Release 2015, 216, 149–57. [DOI] [PubMed] [Google Scholar]
- (12).Goodwin TJ; Huang L Investigation of phosphorylated adjuvants co-encapsulated with a model cancer peptide antigen for the treatment of colorectal cancer and liver metastasis. Vaccine 2017, 35 (19), 2550–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lee E; Jang HE; Kang YY; Kim J; Ahn JH; Mok H Submicron-sized hydrogels incorporating cyclic dinucleotides for selective delivery and elevated cytokine release in macrophages. Acta Biomater. 2016, 29, 271–281. [DOI] [PubMed] [Google Scholar]
- (14).Wilson DR; Sen R; Sunshine JC; Pardoll DM; Green JJ; Kim YJ Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine 2018, 14 (2), 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Irvine DJ; Hanson MC; Rakhra K; Tokatlian T Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem. Rev 2015, 115 (19), 11109–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wang C; Ge Q; Ting D; Nguyen D; Shen HR; Chen J; Eisen HN; Heller J; Langer R; Putnam D Molecularly engineered poly(ortho ester) microspheres for enhanced delivery of DNA vaccines. Nat. Mater 2004, 3 (3), 190–6. [DOI] [PubMed] [Google Scholar]
- (17).Jiang W; Gupta RK; Deshpande MC; Schwendeman SP Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv. Drug Delivery Rev. 2005, 57 (3), 391–410. [DOI] [PubMed] [Google Scholar]
- (18).Bachelder EM; Beaudette TT; Broaders KE; Dashe J; Frechet JM Acetal-derivatized dextran: an acid-responsive biodegradable material for therapeutic applications. J. Am. Chem. Soc 2008, 130 (32), 10494–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Broaders KE; Cohen JA; Beaudette TT; Bachelder EM; Frechet JM Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (14), 5497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bachelder EM; Pino EN; Ainslie KM Acetalated Dextran: A Tunable and Acid-Labile Biopolymer with Facile Synthesis and a Range of Applications. Chem. Rev 2017, 117 (3), 1915–1926. [DOI] [PubMed] [Google Scholar]
- (21).Kohane DS Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng 2007, 96 (2), 203–9. [DOI] [PubMed] [Google Scholar]
- (22).Junkins RD; Gallovic MD; Johnson BM; Collier MA; Watkins-Schulz R; Cheng N; David CN; McGee CE; Sempowski GD; Shterev I; McKinnon K; Bachelder EM; Ainslie KM; Ting JP A robust microparticle platform for a STING-targeted adjuvant that enhances both humoral and cellular immunity during vaccination. J. Controlled Release 2018, 270, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chen N; Peine K; Collier M; Gautam S; Jablonski K; Guerau-de-Arellano M; Ainslie K; Bachelder E Co-Delivery of Disease Associated Peptide and Rapamycin via Acetalated Dextran Microparticles for Treatment of Multiple Sclerosis. Advanced Biosystems 2017, 1, 1700022. [Google Scholar]
- (24).Chen N; Johnson MM; Collier MA; Gallovic MD; Bachelder EM; Ainslie KM Tunable degradation of acetalated dextran microparticles enables controlled vaccine adjuvant and antigen delivery to modulate adaptive immune responses. J. Controlled Release 2018, 273, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Bachelder EM; Beaudette TT; Broaders KE; Fréchet JM; Albrecht MT; Mateczun AJ; Ainslie KM; Pesce JT; Keane-Myers AM In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol. Pharmaceutics 2010, 7 (3), 826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Peine KJ; Bachelder EM; Vangundy Z; Papenfuss T; Brackman DJ; Gallovic MD; Schully K; Pesce J; Keane-Myers A; Ainslie KM Efficient delivery of the toll-like receptor agonists polyinosinic:polycytidylic acid and CpG to macrophages by acetalated dextran microparticles. Mol. Pharmaceutics 2013, 10 (8), 2849–57. [DOI] [PubMed] [Google Scholar]
- (27).Chen N; Collier MA; Gallovic MD; Collins GC; Sanchez CC; Fernandes EQ; Bachelder EM; Ainslie KM Degradation of acetalated dextran can be broadly tuned based on cyclic acetal coverage and molecular weight. Int. J. Pharm 2016, 512 (1), 147–157. [DOI] [PubMed] [Google Scholar]
- (28).Duong AD; Sharma S; Peine KJ; Gupta G; Satoskar AR; Bachelder EM; Wyslouzil BE; Ainslie KM Electrospray encapsulation of toll-like receptor agonist resiquimod in polymer microparticles for the treatment of visceral leishmaniasis. Mol. Pharmaceutics 2013, 10 (3), 1045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Schully KL; Bell MG; Prouty AM; Gallovic MD; Gautam S; Peine KJ; Sharma S; Bachelder EM; Pesce JT; Elberson MA; Ainslie KM; Keane-Myers A Evaluation of a biodegradable microparticulate polymer as a carrier for Burkholderia pseudomallei subunit vaccines in a mouse model of melioidosis. Int. J. Pharm 2015, 495 (2), 849–61. [DOI] [PubMed] [Google Scholar]
- (30).Schully KL; Sharma S; Peine KJ; Pesce J; Elberson MA; Fonseca ME; Prouty AM; Bell MG; Borteh H; Gallovic M; Bachelder EM; Keane-Myers A; Ainslie KM Rapid vaccination using an acetalated dextran microparticulate subunit vaccine confers protection against triplicate challenge by bacillus anthracis. Pharm. Res 2013, 30 (5), 1349–61. [DOI] [PubMed] [Google Scholar]
- (31).Kayamuro H; Yoshioka Y; Abe Y; Arita S; Katayama K; Nomura T; Yoshikawa T; Kubota-Koketsu R; Ikuta K; Okamoto S; Mori Y; Kunisawa J; Kiyono H; Itoh N; Nagano K; Kamada H; Tsutsumi Y; Tsunoda S Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J. Virol 2010, 84 (24), 12703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ichinohe T; Lee HK; Ogura Y; Flavell R; Iwasaki A Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med 2009, 206 (1), 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Arulanandam BP; Mittler JN; Lee WT; O’Toole M; Metzger DW Neonatal administration of IL-12 enhances the protective efficacy of antiviral vaccines. J. Immunol 2000, 164 (7), 3698–704. [DOI] [PubMed] [Google Scholar]
- (34).Arulanandam BP; O’Toole M; Metzger DW Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis 1999, 180 (4), 940–9. [DOI] [PubMed] [Google Scholar]
- (35).Tan RS; Ho B; Leung BP; Ding JL TLR cross-talk confers specificity to innate immunity. Int. Rev. Immunol 2014, 33 (6), 443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Querec T; Bennouna S; Alkan S; Laouar Y; Gorden K; Flavell R; Akira S; Ahmed R; Pulendran B Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med 2006, 203 (2), 413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kawai T; Akira S Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34 (5), 637–50. [DOI] [PubMed] [Google Scholar]
- (38).Kauffman KJ; Do C; Sharma S; Gallovic MD; Bachelder EM; Ainslie KM Synthesis and characterization of acetalated dextran polymer and microparticles with ethanol as a degradation product. ACS Appl. Mater. Interfaces 2012, 4 (8), 4149–55. [DOI] [PubMed] [Google Scholar]
- (39).Gallovic MD; Montjoy DG; Collier MA; Do C; Wyslouzil BE; Bachelder EM; Ainslie KM Chemically modified inulin microparticles serving dual function as a protein antigen delivery vehicle and immunostimulatory adjuvant. Biomater. Sci 2016, 4 (3), 483–93. [DOI] [PubMed] [Google Scholar]
- (40).Brito LA; Singh M Acceptable levels of endotoxin in vaccine formulations during preclinical research. J. Pharm. Sci 2011, 100 (1), 34–7. [DOI] [PubMed] [Google Scholar]
- (41).Lutz MB; Kukutsch N; Ogilvie AL; Rossner S; Koch F; Romani N; Schuler G An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223 (1), 77–92. [DOI] [PubMed] [Google Scholar]
- (42).Samo M; Choudhary NR; Riebe KJ; Shterev I; Staats HF; Sempowski GD; Leduc I Immunization with the Haemophilus ducreyi trimeric autotransporter adhesin DsrA with alum, CpG or imiquimod generates a persistent humoral immune response that recognizes the bacterial surface. Vaccine 2016, 34 (9), 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Guglani L; Khader SA Th17 cytokines in mucosal immunity and inflammation. Curr. Opin. HIV AIDS 2010, 5 (2), 120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Schmitz N; Kurrer M; Bachmann MF; Kopf M Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol 2005, 79 (10), 6441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Huang T; Zhao K; Zhang Z; Tang C; Zhang X; Yue B DNA vaccination based on pyolysin co-immunized with IL-1beta enhances host antibacterial immunity against Trueperella pyogenes infection. Vaccine 2016, 34 (30), 3469–77. [DOI] [PubMed] [Google Scholar]
- (46).Staats HF; Ennis FA Jr. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol 1999, 162 (10), 6141–6147. [PubMed] [Google Scholar]
- (47).Metzger DW Interleukin-12 as an adjuvant for induction of protective antibody responses. Cytokine+ 2010, 52 (1–2), 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Stevceva L; Moniuszko M; Grazia Ferrari M Utilizing IL-12, IL-15 and IL-7 as mucosal vaccine adjuvants. Letters in drug design & discovery 2006, 3 (8), 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Tomai MA; Miller RL; Lipson KE; Kieper WC; Zarraga IE; Vasilakos JP Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev. Vaccines 2007, 6 (5), 835–47. [DOI] [PubMed] [Google Scholar]
- (50).Makela SM; Osterlund P; Julkunen I TLR ligands induce synergistic interferon-beta and interferon-lambda1 gene expression in human monocyte-derived dendritic cells. Mol. Immunol 2011, 48 (4), 505–15. [DOI] [PubMed] [Google Scholar]
- (51).Duong AD; Sharma S; Peine KJ; Gupta G; Satoskar AR; Bachelder EM; Wyslouzil BE; Ainslie KM Electrospray encapsulation of toll-like receptor agonist resiquimod in polymer microparticles for the treatment of visceral leishmaniasis. Mol. Pharmaceutics 2013, 10 (3), 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Gallovic MD; Schully KL; Bell MG; Elberson MA; Palmer JR; Darko CA; Bachelder EM; Wyslouzil BE; Keane-Myers AM; Ainslie KM Acetalated Dextran Microparticulate Vaccine Formulated via Coaxial Electrospray Preserves Toxin Neutralization and Enhances Murine Survival Following Inhalational Bacillus Anthracis Exposure. Adv. Healthcare Mater. 2016, 5 (20), 2617–2627. [DOI] [PubMed] [Google Scholar]
- (53).Temizoz B; Kuroda E; Ohata K; Jounai N; Ozasa K; Kobiyama K; Aoshi T; Ishii KJ TLR9 and STING agonists synergistically induce innate and adaptive type-II IFN. Eur. J. Immunol 2015, 45 (4), 1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Yildiz S; Alpdundar E; Gungor B; Kahraman T; Bayyurt B; Gursel I; Gursel M Enhanced immunostimulatory activity of cyclic dinucleotides on mouse cells when complexed with a cell-penetrating peptide or combined with CpG. Eur. J. Immunol 2015, 45(4), 1170–9. [DOI] [PubMed] [Google Scholar]
- (55).Kim Y; Lee Chung B; Ma M; Mulder WJ; Fayad ZA; Farokhzad OC; Langer R Mass production and size control of lipid-polymer hybrid nanoparticles through controlled microvortices. Nano Lett. 2012, 12 (7), 3587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Peine KJ; Guerau-de-Arellano M; Lee P; Kanthamneni N; Severin M; Probst GD; Peng H; Yang Y; Vangundy Z; Papenfuss TL; Lovett-Racke AE; Bachelder EM; Ainslie KM Treatment of experimental autoimmune encephalomyelitis by codelivery of disease associated Peptide and dexamethasone in acetalated dextran microparticles. Mol. Pharmaceutics 2014, 11 (3), 828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Kasturi SP; Skountzou I; Albrecht RA; Koutsonanos D; Hua T; Nakaya HI; Ravindran R; Stewart S; Alam M; Kwissa M; Villinger F; Murthy N; Steel J; Jacob J; Hogan RJ; Garcia-Sastre A; Compans R; Pulendran B Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470 (7335), 543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Johansen P; Martinez Gomez JM; Gander B Development of synthetic biodegradable microparticulate vaccines: a roller coaster story. Expert Rev. Vaccines 2007, 6 (4), 471–4. [DOI] [PubMed] [Google Scholar]
- (59).Joshi VB; Geary SM; Salem AK Biodegradable particles as vaccine delivery systems: size matters. AAPS J. 2013, 15 (1), 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wang Y; Qu W; Choi SH FDA’s Regulatory Science Program for Generic PLA/PLGA-Based Drug Products. American Pharmaceutical Review [Online] 2016. [Google Scholar]
- (61).Foucquier J; Guedj M Analysis of drug combinations: current methodological landscape. Pharmacol. Res. Perspect 2015, 3 (3), e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Vidarsson G; Dekkers G; Rispens T IgG subclasses and allotypes: from structure to effector functions. Front. Immunol 2014, 5, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Shibaki A; Katz SI Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund’s adjuvant. Exp. Dermatol 2002, 11 (2), 126–34. [DOI] [PubMed] [Google Scholar]
- 64).Savelkoul HF; Ferro VA; Strioga MM; Schijns VE Choice and Design of Adjuvants for Parenteral and Mucosal Vaccines. Vaccines 2015, 3 (1), 148–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Tel J; Lambeck AJ; Cruz LJ; Tacken PJ; de Vries IJ; Figdor CG Human plasmacytoid dendritic cells phagocytose, process, and present exogenous particulate antigen. J. Immunol 2010, 184 (8), 4276–83. [DOI] [PubMed] [Google Scholar]
- (66).Tacken PJ; Zeelenberg IS; Cruz LJ; van Hout-Kuijer MA; van de Glind G; Fokkink RG; Lambeck AJ; Figdor CG Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 2011, 118 (26), 6836–44. [DOI] [PubMed] [Google Scholar]
- (67).Cruz LJ; Tacken PJ; Pots JM; Torensma R; Buschow SI; Figdor CG Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via DC-SIGN. Biomaterials 2012, 33 (16), 4229–39. [DOI] [PubMed] [Google Scholar]
- (68).Zhu Q; Talton J; Zhang G; Cunningham T; Wang Z; Waters RC; Kirk J; Eppler B; Klinman DM; Sui Y; Gagnon S; Belyakov IM; Mumper RJ; Berzofsky JA Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med 2012, 18 (8), 1291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Silva JM; Zupancic E; Vandermeulen G; Oliveira VG; Salgado A; Videira M; Gaspar M; Graca L; Preat V; Florindo HF In vivo delivery of peptides and Toll-like receptor ligands by mannose-functionalized polymeric nanoparticles induces prophylactic and therapeutic anti-tumor immune responses in a melanoma model.J. Controlled Release 2015, 198, 91–103. [DOI] [PubMed] [Google Scholar]
- (70).Siefert AL; Caplan MJ; Fahmy TM Artificial bacterial biomimetic nanoparticles synergize pathogen-associated molecular patterns for vaccine efficacy. Biomaterials 2016, 97, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Madan-Lala R; Pradhan P; Roy K Combinatorial Delivery of Dual and Triple TLR Agonists via Polymeric Pathogen-like Particles Synergistically Enhances Innate and Adaptive Immune Responses. Sci. Rep 2017, 7 (1), 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.