Abstract
Purpose of review
This chapter will summarize the most recent literature regarding the current state of medical treatment for vascular anomalies.
Recent findings
Research into the biology of these anomalies has strengthened our understanding of each anomaly and has helped to pave the way for more tailored treatment options involving molecular and/or genetic targets.
Summary
While there is still a role for surgical intervention, medical therapies that target the etiology of vascular anomalies may represent an alternative or adjunctive approach in the management of these lesions.
Keywords: Infantile hemangioma, propranolol, genetics, rapamycin, lymphatic malformation, vascular anomalies
Introduction
The study of vascular anomalies has progressed significantly through the last three decades. With more advanced diagnostic testing modalities, the characteristics that make each anomaly unique can be better described. Defining the specific features of each type of anomaly has allowed in-depth research to better elucidate genetic etiologies of these lesions. The human genome project was completed in 2003 and that information has become a valuable resource in guiding this research.1 The importance of genomic medicine is incorporated into the vascular anomaly classification of the International Society for the Study of Vascular Anomalies' (ISSVA) (Table 1). This comprehensive list divides the larger group of vascular anomalies into specific variations and, when known, correlates each to the causative gene. These known genetic causes have led to the discovery of potential molecular targets for new medical therapies as well as an explanation for the efficacy of other treatments.
Table 1. ISSVA 2014 Vascular Anomalies Classification Scheme and Associated Genetic Basis.
Abbreviations: CM, capillary malformations; AVM, arteriovenous malformations; CNS, central nervous system; HHT, hereditary hemorrhagic telangiectasia; CMTC, cutis marmorata telangiectatica congenita; VM, venous malformation; CCM, cerebral cavernous malformations; JPHT, juvenile polyposis/hereditary hemorrhagic telangiectasia syndrome; MCAP, macrocephaly-capillary malformation; MICCAP, microcephaly-capillary malformations; PIK3CA, phosphatyidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha
| Capillary Malformations | |
|---|---|
| Cutaneous and/or mucosal CM (port wine stain) | GNAQ |
| CM with bone and/or soft tissue hyperplasia | - |
| CM with CNS and/or eye anomalies (Surge-Weber) | GNAQ |
| CM of CM-AVM | RASA1 |
| Telangiectasia | - |
| HHT | - |
| HHT1 | ENG |
| HHT2 | ACVRL1 |
| HHT3 | - |
| Others | - |
| CMTC | - |
| Nevus simplex/salmon patch | - |
| LM | |
| Primary lymphedema | - |
| Nonne-Milroy syndrome | fLT4/VEGRFR3 |
| Primary hereditary lymphedema | VEGFC |
| Primary hereditary lymphedema | GJC2/connexin 47 |
| Lymphedema-distichiasis | FOXC2 |
| Hypotrichosis-lymphedema-telangiectasia | SOX18 |
| Primary lymphedema with myelodysplasia | GATA2 |
| Primary generalized lymphatic anomaly | CCBE1 |
| Microcephaly with/without chorioretinopathy | KIF11 |
| Lymphedema or mental retardation syndrome | - |
| Lymphedema-choanal atresia | PTEN14 |
| VMs | |
| Common VM | TIE2 somatic |
| VMCM | TIE2 |
| Blue rubber bleb nevus (Bean) syndrome VM | - |
| Glomuvenous malformation (VM with glomus cells) | Glomulin |
| CCM | - |
| CCM1 | KRIT1 |
| CCM2 | Malcavernin |
| CCM3 | PDCD10 |
| AVMs | |
| Sporadic in HHT | - |
| HHT1 | ENG |
| HHT2 | ACVRL1 |
| JPHT | SMADA4 |
| CM-AVM | RASA1 |
| AVFs | |
| VMs associated with other anomalies | |
| Klippel-Trenaunay syndrome | - |
| Parkes Weber syndrome | RASA1 |
| Servelle-Martorell syndrome | - |
| Sturge-Weber syndrome | GNAQ |
| Limb CM + congenital nonprogressive limb overgrowth | - |
| Maffucci syndrome | - |
| MCAP | PIK3CA |
| MICCAP | STAMBP |
| CLOVES syndrome | PIK3CA |
| Proteus syndrome | AKT1 |
| Bannayan-Riley-Ruvalcaba syndrome | PTEN |
| Provisionally unclassified Vascular Anomalies | |
| Verrucous hemangioma | - |
| Multifocal lymphangioendotheliomatosis with thrombocytopenia/cutaneovisceral angiomatosis with thrombocytopenia | - |
| Kaposiform lymphangiomatosis | - |
| PTEN (type) hamartoma of soft tissue/angiomatosis of soft tissue | PTEN |
The most recent research exploring new medical therapeutic options for patients with infantile hemangiomas (IH), PHACES (posterior fossa malformations, hemangiomas, arterial anomalies, cardiac defects, eye abnormalities, sternal cleft and supraumbilical raphe) syndrome, lymphatic malformations (LM), venous malformations (VM) and arteriovenous malformations (AVM) is described.
Infantile Hemangiomas
Description of Disease and Complications
The most common benign tumor of childhood is infantile hemangioma (IH) and occurs in 4-10% of infants.2,3 IH are more common in Caucasian females, prematurity and low birth weight.4 IH are absent at birth, typically occur during infancy, rapidly proliferate after the first few months of life, and then slowly involute over the course of years. Most IH (80-85%) involute spontaneously with the loss of IH endothelium and replacement with fibrofatty tissue.5,6
If left untreated, IH can lead to complications based on its location and size. Growing lesions in a small infant airway can cause airway compromise. Large IH can lead to cardiac failure during rapid lesion proliferation from intralesional blood shunting directly from the arterial to venous circulation. Those involving the face can cause psychosocial developmental abnormalities, significant disfigurement depending on the location, and vision loss if there is periorbital involvement. Patients with periorbital IH should have an ophthalmologic evaluation to rule out amblyopia.7,8 Ulceration and bleeding can also be complications to warrant prompt treatment.8-11
Etiology of Disease
IH endothelium are unique as compared to other endothelium as they have high expression of the glucose transport protein, GLUT-1 and a cluster of microRNA from chromosome 19 (C19MC), all of which are highly expressed in specific placental cells.12,13 In addition to the possible placental origins of IH is the idea that IH arise from CD 133+ stem-cells.6 One of the problems in determining stem cell origins of IH endothelium is that immunostaining IH tissue for CD 133 cells has not been reliable, so it has not been possible to delineate histologically whether GLUT-1+ IH endothelium and/or placental cells are also stem cells. Certainly, these complex cellular interactions distinguish IH clinically and biologically from other vascular anomalies even though the exact molecular mechanisms giving rise to IH are being determined.
Historic Medical Treatments
When IH were thought to result from disordered angiogenesis, interferon-alpha, the first known naturally occurring angiogenesis inhibitor, was used systemically in complicated IH cases. It was shown to be effective in 45% of cases; however, the undesirable side effect of spastic diplegia made it an unattractive first-line therapy in young children.14 Until 2008, corticosteroids, also angiogenesis inhibitors, were the mainstay of IH treatment. These drugs were used systemically or via intralesional injection and had variable efficacy. Long-term, high dose corticosteroids needed to treat large IH have side effects such as adrenal insufficiency, insomnia, irritability, glucose intolerance and osteoporosis.15 Vincristine is a chemotherapeutic drug that inhibits microtubule function, arresting cell mitosis. Vincristine inconsistently improved life-threatening IH.16-19 This inconsistent treatment response and poor safety profile makes vincristine a less popular IH therapy. The mention of these medications is of historic reference and they are no longer routinely used.
Propranolol
Discovery
Since 2008, propranolol has become the medical therapy of choice for IH requiring treatment. The serendipitous discovery that propranolol reduced IH size and redness occurred in 2008, and due to its effectiveness and excellent safety profile, it is now the first-line IH treatment.20 Previously, propranolol was used in the pediatric population for hypertension, supraventricular tachycardia, prolonged QT syndrome and thyrotoxicosis.21 Following 2008 in the United States, the generic liquid preparation of propranolol was used as an off-label therapy for IH. In Europe, where liquid propranolol was unavailable, Hemangeol™ was created and since 2014, it has been FDA approved for pediatric IH therapy.
Pharmacology
Propranolol is a non-selective beta-adrenergic receptor antagonist. The liver metabolizes the medication and 25% of oral propranolol reaches systemic absorption. The duration of action is 6-12 hours depending on dose and the peak effect occurs 2 hours after administration.9,21
Propranolol is contraindicated for patients who have cardiogenic shock, 1st degree AV block, heart failure, bronchial asthma, hypersensitivity to propranolol, resting heart rate and/or blood pressure 2 standard deviations below normal, unexplained syncope, impaired renal or liver function, <1 week of age and in patients who have to endure long periods of fasting.9
Propranolol for PHACES syndrome
PHACES syndrome consists of posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of aorta and cardiac defects, eye abnormalities and sternal defects.22 Recognizing cerebrovascular abnormalities in these patients is important because of the possible increased risk of ischemic stroke.22,23 It has been suggested that steroids and propranolol in these patients could increase the risk of stroke, as steroids can cause hypertension and propranolol can cause hypotension.17 Patients with suspected PHACES syndrome need evaluation before propranolol initiation to help prevent these complications.
Mechanism of Action
The mechanism of action by which propranolol disassembles IH vasculature, reducing intralesional vessel density, is unknown; while there are many theories seeking to explain this, there is no supporting evidence to fully adopt any of these theories.24 It is unclear if propranolol inhibits angiogenesis through the beta receptor blockade, as these receptors are on all IH cells and are present in other vascular anomalies that are non-responsive to propranolol. Other studies have explored the role of the beta receptors in endothelial cells. They found that if the gene encoding the adrenergic receptor is lost, angiogenesis is inhibited; however, how this relates to IH regression with propranolol therapy remains unclear.25,26 As discussed previously, certain miRNAs have been shown to be uniquely present in IH as compared to other vascular anomalies and one study focused on propanolol's ability to downregulate the miRNA, miR-382, that is also present in IH.27 Propranolol may increase in the pro-apoptotic protein Bax and the decrease in the anti-apoptotic proteins Bcl-2 and Caspase3 in IH tissue.28 Further studies are necessary to understand propranolol's molecular mechanism that stops IH growth.
Monitoring of Efficacy
Propranolol is efficacious in 50-88% of cases.3,29 Studies reporting efficacy rates have used various measures to define success, such as decrease in astigmatism for periorbital IH, time to ulceration resolution, complete resolution in IH, reduction in size by 50%, and improvement in color.8,11,30-32 Periocular hemangiomas showed a reduction in size by 40-47% with the use of propranolol.30,33 Nasal hemangiomas are disfiguring and surgical excision is challenging, but propranolol therapy decreased surgical intervention for nasal hemangiomas by 56%.34 Similarly airway hemangiomas treated with propranolol have reduced surgical interventions.15
Ultrasound is a non-invasive way of monitoring response to treatment more objectively and for deeper lesions.24 As discussed above, C19MC is a microRNA cluster found to be upregulated in IH tumor endothelial cells and present in IH patient plasma. Plasma levels decreased in IH that were responsive to propranolol, but not in those that did not respond to propranolol. This could be a future tool to diagnose and monitor treatment.13
It is prudent to briefly mention that congenital hemangiomas [non-involuting congenital hemangiomas (NICH), rapidly-involuting congenital hemangiomas (RICH)|] are different entities compared to IH due to their presence at birth. NICH's do not express the GLUT-1 protein, express beta-adrenergic receptors, and persist as a patient grows.35,36 RICH's similarly do not express the GLUT-1 protein; however, typically regress by 14 months of age.37,38 Due to the rarity of these lesions, the true response rates to propranolol is unknown, but have anecdotally been less than that for IH.
Propranolol Initiation
Since the introduction of propranolol for IH treatment, its initiation is not standardized.39 Previously, most institutions had IH patients evaluated by cardiology preinitiation to rule out any cardiac abnormalities that were treatment contraindications. Now after experience with propranolol for IH therapy, there is question of the utility of routine cardiology evaluation in patients without risk factors.40 Most patients are being treated by a multidisciplinary vascular anomalies clinic and the first dose is administered in clinic.39 If the patient's heart rate or blood pressure is below 2 standard deviations of normal, if they are <6 weeks corrected gestational age, or if they have airway involvement, inpatient monitoring is recommended.21
Though not standardized, the recommended starting dose is 0.66 mg/kg/dose every 8 hours to scale to a final dose of 2 mg/kg/day.41 Patients are treated for variable amounts of time depending on how well the lesion responds; however treatment for 6 months has been shown to be more effective than a shorter course.30
At Seattle Children's Hospital (SCH), patients 6 weeks of gestationally corrected age or older may start propranolol as an outpatient. The patient and family attend a visit to go through a cardiology screening checklist and teaching sessions regarding propranolol administration. The checklist was created with cardiology assistance to identify patients who need cardiology clearance before starting the propranolol. If the patient has any of the following, they are referred to cardiology: persistent poor oral feeding; persistent poor weight gain; baseline/persistent dyspnea, diaphoresis, tachypnea, tachycardia; syncope; congenital heart disease; brain malformations; family history of congenital heart disease, arrhythmia, sudden death before age 50, or connective tissue disorder; vital signs outside of the approved parameters by age; irregular heart rate or rhythm; pathologic sounding heart murmur; increased work and rate of breathing; hepatomegaly; cool extremities; delayed capillary refill; or probable or definite PHACES syndrome. At the medication initiation visit, the family is taught how to administer the medication. The patient is started at a dose of 1-2 mg/kg/day divided into 3 doses. The blood pressure and heart rate are checked 1, 2 and 3 hours after administration. The family is given a stethoscope and taught how to listen and calculate the heart rate at home for the first two weeks after propranolol initiation. The side effects and symptoms to prompt medical attention are reviewed.
Side Effects
Side effects may include: hypotension, bradycardia and hypoglycemia. Patients are observed at SCH for 3 hours after the first dose of propranolol to monitor for hypotension and bradycardia. Rarely, the hypotension and bradycardia become clinically relevant; however, parents are advised to check the patient's heart rate twice daily, 1-2 hours after the propranolol dose, for 2 weeks after initiating propranolol or if there are acute illnesses that could worsen hypotension (e.g. diarrhea).42 Hypoglycemia is thought to be due to the inhibition of catecholamine-induced glycogenolysis and gluconeogenesis that can occur with fasting. The incidence of hypoglycemia is low (0.3%).43 To avoid hypoglycemia, at the clinic visit, the family is educated about holding propranolol should the patient be fasting related to illness or other reasons. They may resume propranolol when on a normal diet. Pedialyte® can be helpful in these situations. If a patient has known asthma or an overlying viral illness, families are educated on observing for wheezing or symptoms of bronchospasms. This is also an infrequently encountered side effect (0.4%).43 Even rarer side effects include hyperkalemia potentially from tumor lysis, and dental caries. Minor, more common side effects include acrocyanosis and gastrointestinal and sleep disturbances.9,44
Propranolol is the first-line therapy for IH needing treatment. Further research into IH etiology will clarify propranolol's mechanism of action to help predict IH responsiveness and determine which patients will benefit most from therapy.
Vascular Malformations
Congenital vascular malformations may be composed of abnormal vessels of lymphatic, venous, or arterial origin. In many cases, a mixture of vessel types is present. The genetic etiology of some malformations has been described and is resulting in new medical therapeutic options. In the following sections, novel medical therapy for lymphatic, venous and arteriovenous malformations will be discussed.
Lymphatic Malformation
Description of Disease and Current Treatment
Lymphatic malformations (LM) are congenital lesions that occur between 1-3 per 6000 births.45,46 It is estimated that 75% occur in the head and neck.47,48 Radiologically, LM can be broadly categorized as microcystic (cysts <2 cm) or macrocystic (cysts ≥2 cm).49 deSerres proposed a clinical LM staging system based on malformation laterality and anatomic relationship to the hyoid bone, to help predict prognosis and surgical complications (Table 2).50
Table 2. deSerres Classification Scheme for Lymphatic Malformations.
| Stage | Location |
|---|---|
| I | Unilateral infrahyoid |
| II | Unilateral suprahyoid |
| III | Unilateral suprahyoid and infrahyoid |
| IV | Bilateral suprahyoid |
| V | Bilateral suprahyoid and infrahyoid |
Until recently, LM-induced overgrowth, dysfunction and/or disfigurement have been treated with excision and/or sclerotherapy.51 Clinical behavior of LM varies and a careful retrospective study has found that some stage 1 and stage 2 LM can spontaneously regress, whereas stage 3 and 4 LM can cause aerodigestive tract dysfunction and resist cure.52 When comparing sclerotherapy to surgical excision in large series, it has been found that low stage 1-3 LM represented 85% of all head and neck lesions and that both treatment modalities appeared to be equivalent in efficacy and resource utilization.46,47,51,53 Even with this type of invasive treatment, large, high stage LM are associated with often unexplained systemic symptoms and complications that can consist of coagulopathy, chronic pain, cellulitis, ulceration, and visceral/bone involvement, that are unchanged by standard invasive therapy.54
Etiology of LM
A better understanding of the etiology of LM could lead to new treatment options. Recently DNA sequencing studies in LM tissue has led to the discovery that de-novo post-zygotic somatic mutations are the cause of LM. New approaches to DNA sequencing has enabled detection of de-novo post-zygotic somatic mutations in LM tissue and cells, revealing the cause of LM. Somatic mutations differ from germline mutations, present in all cells, as somatic mutations are only in affected cells that are located in varied anatomic locations, often causing unique phenotypic mosaicism.55 Gain of function somatic mutations in PIK3CA, a kinase in the PI3K/AKT pathway, are present in LM tissue. When activated, PIK3CA promotes cell proliferation, growth, angiogenesis and protein synthesis. This gene is in the PI3K/AKT pathway that when activated, increases cell proliferation, growth, angiogenesis and protein synthesis (Figure 1).56 Overactivation of this molecular pathway correlates with the tissue overgrowth and persistence seen in high stage LMs.57,58 Understanding the role of this pathway in the development of LM has opened the possibility of molecular based therapy and personalized medicine for LM patients.
Figure 1.
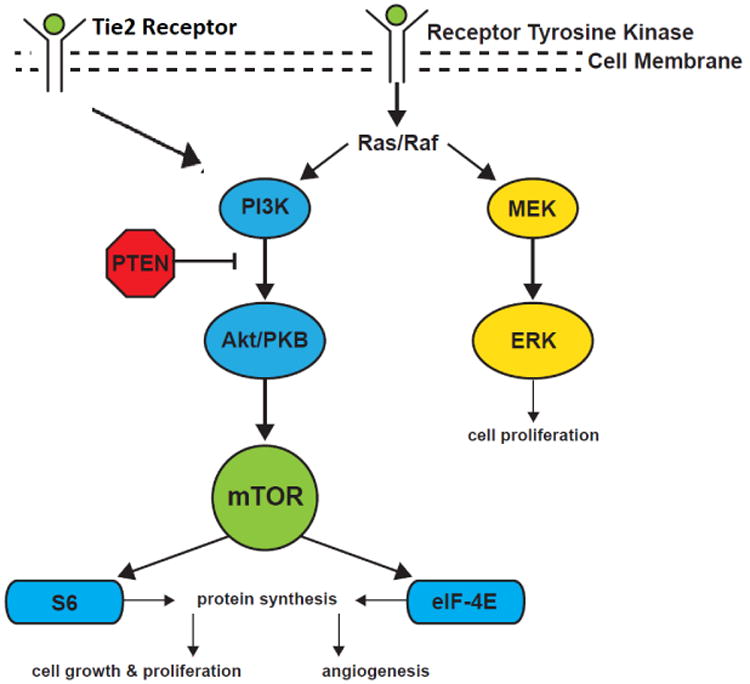
PI3K/AKT pathway and MEK pathway leading to cell growth and proliferation.
Current Medical Practice
Identification of a disordered genetic pathway in LM tissue has encouraged investigators to apply treatment of other conditions with PIK3CA mutations and tissue overgrowth (i.e. tumors) to LM. Rapamycin inhibits a component of the PI3K/AKT1 pathway called mTOR, or mammalian target of rapamycin (Figure 1). Rapamycin is a macrolide that was first discovered from soil on Easter Island.59 It has shown to be efficacious in other disease processes related to mTOR activation or when immune suppression is desired with solid organ transplants or autoimmune lymphoproliferative syndrome.60,61 Recently, a phase 2 trial showed that empiric use of rapamycin in patients with complicated lymphatic malformations was safe and led to a decreased incidence of cellulitis, hospitalized days and frequency of infections. Side effects included gastrointestinal disturbances, metabolic toxicity such as lipid abnormalities, and blood/bone marrow abnormalities.54 Response to treatment included reduction in lesional pain and bleeding, though complete responses were not common.62 Lesional pain and mucosal bleeding in treated LM was diminished. Reasons for the lack of complete lesion response are unclear and future investigations will be necessary to determine optimal LM patient selection for this therapy.
Since head and neck LM are associated with significant morbidity and mortality, such as oral bleeding and pain, empiric supportive medical therapy directed at these symptoms is necessary, particularly in high stage LM. Corticosteroids can help decrease the inflammation associated with LM.63,64 These drugs are most frequently necessary in adolescent patients with intraoral suprahyoid LM. Frequent intraoral trauma to a large infiltrating tongue lesion can precipitate inflammation, swelling, bleeding and pain. Antibiotics, along with corticosteroids, are used to treat these episodes and prolonged courses of antibiotics have been recommended to help prevent chronic infections.65 A subset of patients with large, high stage LM may have lymphocytopenia.66 These patients may have persistent and recurrent infections that may respond to a rotating schedule of prophylactic antibiotics. Occasionally, young patients with LM can present with acute episodes of enlargement of their malformation from intralesional hemorrhage. This intralesional bleeding can cause LM inflammation and even post hemorrhage anemia leading to inpatient admissions.
Clinical and base science research is ongoing that will ultimately lead to more reliable, biologically-based medical LM therapy. As this develops, methods to aid in standardizing outcome measures will be important to identify.67
Venous Malformations
Description of Disease and Treatment
Venous malformations (VM) are anomalies that are common in the head and neck region (40%). They are present at birth and continue to increase in size with the growth of the child.68 The incidence is 2-5 in 10,000 births and they arise due to a discontinuous smooth muscle layer of developing venous channels. These malformed, dysfunctional venous channels expand erratically and infiltrate normal surrounding structures.69 They typically present with a bluish hue in involved skin or mucosa and are differentiated from deep hemangiomas by the presence of intermittent pain and dependent swelling.
The venous channels can also develop thrombi or phleboliths due to the venous stasis, inducing persistent VM enlargement. Enlargement can also occur during times of puberty and pregnancy with increased symptoms at those times.70 Patients at risk for localized intravascular coagulopathy (LIC) are patients who have: 1). a large volume malformation (>10 mL), 2). presence of a phlebolith, 3). multifocal disease, or 4). history of Klippel-Trenaunay syndrome. These patients should have their pro-thrombin time, activated partial thromboplastin time, fibrinogen levels, D-dimer, and complete blood count tested.71
VM tend to grow with time and do not regress on their own. Much like LM treatment, VM treatment has primarily been invasive with either sclerotherapy or surgery. Observation is preferred if the lesion is in a location that does not have any functional compromise or symptoms. Sclerotherapy can be offered to help reduce pain and some of lesional volume by creating endothelial damage and channel fibrosis in symptomatic patients.72 Surgery is also an option for localized malformations.73 Surgery and sclerotherapy for VM, however, are associated with reliably high recurrent rates. As such, at SCH, our preferred treatment is a combination glue embolization surgery (GES) with the interventional radiology department. Intralesional glue embolization is done prior to surgical resection allowing more complete VM removal with better tissue planes, less intraoperative bleeding, and better identification of nerves.74
Etiology of VM
Most VM are sporadic.75 However, there are familial cases of VM that have been linked to germline mutations in the TIE2 tyrosine kinase receptor (Figure 1).76 This mutation has also been found in 50% of VM.77 Since the vast majority of VM are sporadic and not familial, more research is being done to identify possible somatic mutations associated with the TIE2 pathway in patients with sporadic VM.78 This investigation has shown that up to 27% of VM have associated PIK3A somatic mutations.79
Current Medical Treatment
In patients with more extensive lesions that are less amenable to invasive procedures, medical therapy can play a role. Low-dose aspirin, non-steroidal anti-inflammatory medications, or anticoagulation with enoxaparin is used as a prophylactic method to prevent thrombosis within VM as a way to reduce pain, especially in patients at risk for LIC.80 In patients with lesions on the extremities, compression garments can provide enough pressure to these valveless lesions to prevent dilation and the pain that accompanies growth of the VM.81
As discussed previously, the TIE2 receptor plays a role in the development of some VM. The downstream pathway of TIE2 leads to the mTOR which then signals downstream to increase cellular growth and proliferation, similar to LM (Figure 1).79 Additionally, the discovery that some VM are caused by PIK3CA has allowed rapamycin, an mTOR inhibitor, to provide an alternative treatment for painful, swollen VM in a small patient series.79
Arteriovenous Malformations
Description of Disease and Treatment
Arterio-venous malformations (AVMs) are high-flow, congenital vascular anomalies that are due to localized shunts (i.e. nidus) between the arterial and venous systems. AVMs present as warm, palpable, pulsatile lesions that can hemorrhage if epithelial or mucosal coverings are disrupted.82,83
The high vascular flow and growth causes surrounding tissue destruction, pain, ulceration, bleeding and cardiac overload.84
Treatment of AVM centers on nidus removal or ablation. Surgical resection can be offered in conjunction with embolization; however, disfigurement and effect on function can occur due to the invasive nature of the lesion into the surrounding tissues and need for complete removal.85 Embolization alone is associated with high AVM recurrence compared to surgery, making it a suboptimal treatment. Ethanol sclerotherapy has shown some success; however, there is a need for multiple treatments and risk of cranial neuropathy if the lesion is in close proximity to the skull base or other critical structures.86
Etiology of AVM
Sequencing DNA from head and neck AVM tissue has shown somatic mutations of KRAS that adversely impacts vascular assembly by increasing downstream ERK signaling.87 Other variants were noted in NRAS, BRAF and MAP2K1 genes that similarly are associated with vascular assembly.88 While this is very exciting, it is too early to tell whether this will result in a biologically based medical AVM treatment.
Current Medical Treatments
Surgery and embolization are imperfect methods of AVM treatment. There is a need for novel, more effective therapies. Molecular pathways recently identified in the development of these lesions could potentially be inhibited improving treatment. A proposed theory is that the endothelial dysfunction that causes AVMs is due to the increased activity of the mitogen-activated protein kinase kinase (MAP2K1), also known as MEK1 (Figure 1). MEK1 inhibitors are used in cancers and can have a potential application in AVM.89
Conclusion
Medical therapy for vascular anomalies has become more prominent as a treatment modality. As genetic and molecular testing becomes more sophisticated, precision medicine may direct medical and surgical therapy of these complex lesions. Medical and surgical therapy that can remove or destroy diseased cells may have advantages over other treatment modalities. Careful evaluation of all patients with vascular anomalies with a multidisciplinary team, including surgical specialists, geneticists, interventional radiologists, dermatologists, and hematologists/oncologists, is needed to help make the most efficient and effective treatment decisions.
General Comments.
Not all vascular lesions are infantile hemangiomas. Careful attention to the history and time course can help distinguish between vascular anomalies.
Patients who have lesions that require surgical or medical therapy that is beyond the comfort level of the pediatrician, lesions unresponsive to first-line therapy, or complicated vascular lesions (e.g. ulceration, chronic pain, airway or intracranial involvement, bleeding, coagulopathy, etc.) would benefit from a consultation to a vascular anomalies center. Vascular anomalies centers offer multidisciplinary care and up to date treatment methods.
Molecular genetics has furthered our understanding of the etiology of vascular anomalies and has aided in the introduction of novel medical therapies.
Acknowledgments
The authors thank Eden Palmer for figure creation and preparation for publication.
Funding: Seattle Children's Guild Association Funding Focus Award, NIH RO1 NS092772 and NIHMS 905693
Footnotes
Conflict of Interest: Reema Padia, Catherine Bull, Randall Bly, Amy E. Geddis, and Jonathan Perkins declare no conflicts of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Carrasco-Ramiro F, Peiro-Pastor R, Aguado B. Human genomics projects and precision medicine. Gene Ther. 2017;24:551–561. doi: 10.1038/gt.2017.77. [DOI] [PubMed] [Google Scholar]
- 2.Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567–1576. doi: 10.1001/archderm.138.12.1567. [DOI] [PubMed] [Google Scholar]
- 3.Buckmiller LM, Munson PD, Dyamenahalli U, Dai Y, Richter GT. Propranolol for infantile hemangiomas: early experience at a tertiary vascular anomalies center. Laryngoscope. 2010;120:676–681. doi: 10.1002/lary.20807. [DOI] [PubMed] [Google Scholar]
- 4.Fuchsmann C, Quintal MC, Giguere C, et al. Propranolol as first-line treatment of head and neck hemangiomas. Arch Otolaryngol Head Neck Surg. 2011;137:471–478. doi: 10.1001/archoto.2011.55. [DOI] [PubMed] [Google Scholar]
- 5.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–274. doi: 10.1111/j.1365-2133.2010.09848.x. [DOI] [PubMed] [Google Scholar]
- 6.Dadras SS, North PE, Bertoncini J, Mihm MC, Detmar M. Infantile hemangiomas are arrested in an early developmental vascular differentiation state. Mod Pathol. 2004;17:1068–1079. doi: 10.1038/modpathol.3800153. [DOI] [PubMed] [Google Scholar]
- 7.Costa VA, Haimowitz R, Cheng YI, Wang J, Silverman RA, Bauman NM. Social Impact of Facial Infantile Hemangiomas in Preteen Children. JAMA Otolaryngol Head Neck Surg. 2016;142:13–19. doi: 10.1001/jamaoto.2015.2597. [DOI] [PubMed] [Google Scholar]
- 8.Herlihy EP, Kelly JP, Sidbury R, Perkins JA, Weiss AH. Visual acuity and astigmatism in periocular infantile hemangiomas treated with oral beta-blocker versus intralesional corticosteroid injection. J AAPOS. 2016;20:30–33. doi: 10.1016/j.jaapos.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128–140. doi: 10.1542/peds.2012-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawley LP, Siegfried E, Todd JL. Propranolol treatment for hemangioma of infancy: risks and recommendations. Pediatr Dermatol. 2009;26:610–614. doi: 10.1111/j.1525-1470.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- 11.Hermans DJ, van Beynum IM, Schultze Kool LJ, van de Kerkhof PC, Wijnen MH, van der Vleuten CJ. Propranolol, a very promising treatment for ulceration in infantile hemangiomas: a study of 20 cases with matched historical controls. J Am Acad Dermatol. 2011;64:833–838. doi: 10.1016/j.jaad.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 12.North PE, Waner M, Mizeracki A, Mihm MC., Jr GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11–22. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- 13.Strub GM, Kirsh AL, Whipple ME, et al. Endothelial and circulating C19MC microRNAs are biomarkers of infantile hemangioma. JCI Insight. 2016;1:e88856. doi: 10.1172/jci.insight.88856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaud AP, Bauman NM, Burke DK, Manaligod JM, Smith RJ. Spastic diplegia and other motor disturbances in infants receiving interferon-alpha. Laryngoscope. 2004;114:1231–1236. doi: 10.1097/00005537-200407000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Elluru RG, Friess MR, Richter GT, et al. Multicenter Evaluation of the Effectiveness of Systemic Propranolol in the Treatment of Airway Hemangiomas. Otolaryngol Head Neck Surg. 2015;153:452–460. doi: 10.1177/0194599815591809. [DOI] [PubMed] [Google Scholar]
- 16.Hara K, Yoshida T, Kajiume T, Ohno N, Kawaguchi H, Kobayashi M. Successful treatment of Kasabach-Merritt syndrome with vincristine and diagnosis of the hemangioma using three-dimensional imaging. Pediatr Hematol Oncol. 2009;26:375–380. doi: 10.1080/08880010902976643. [DOI] [PubMed] [Google Scholar]
- 17.Haggstrom AN, Skillman S, Garzon MC, et al. Clinical spectrum and risk of PHACE syndrome in cutaneous and airway hemangiomas. Arch Otolaryngol Head Neck Surg. 2011;137:680–687. doi: 10.1001/archoto.2011.113. [DOI] [PubMed] [Google Scholar]
- 18.Enjolras O, Breviere GM, Roger G, et al. Vincristine treatment for function- and life-threatening infantile hemangioma. Arch Pediatr. 2004;11:99–107. doi: 10.1016/j.arcped.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Kothari A, Hittelman WN, Chambers TC. Cell Cycle-Dependent Mechanisms Underlie Vincristine-Induced Death of Primary Acute Lymphoblastic Leukemia Cells. Cancer Res. 2016;76:3553–3561. doi: 10.1158/0008-5472.CAN-15-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leaute-Labreze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 21.Cushing SL, Boucek RJ, Manning SC, Sidbury R, Perkins JA. Initial experience with a multidisciplinary strategy for initiation of propranolol therapy for infantile hemangiomas. Otolaryngol Head Neck Surg. 2011;144:78–84. doi: 10.1177/0194599810390445. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Castroviejo I. Vascular and nonvascular intracranial malformation associated with external capillary hemangiomas. Neuroradiology. 1978;16:82–84. doi: 10.1007/BF00395211. [DOI] [PubMed] [Google Scholar]
- 23.Siegel DH, Tefft KA, Kelly T, et al. Stroke in children with posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities (PHACE) syndrome: a systematic review of the literature. Stroke. 2012;43:1672–1674. doi: 10.1161/STROKEAHA.112.650952. [DOI] [PubMed] [Google Scholar]
- 24.Bingham MM, Saltzman B, Vo NJ, Perkins JA. Propranolol reduces infantile hemangioma volume and vessel density. Otolaryngol Head Neck Surg. 2012;147:338–344. doi: 10.1177/0194599812451570. [DOI] [PubMed] [Google Scholar]
- 25.Zahalka AH, Arnal-Estape A, Maryanovich M, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321–326. doi: 10.1126/science.aah5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa Y, Wang TC. Nerves switch on angiogenic metabolism. Science. 2017;358:305–306. doi: 10.1126/science.aaq0365. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Li P, Guo Z, Wang H, Pan W. Downregulation of miR-382 by propranolol inhibits the progression of infantile hemangioma via the PTEN-mediated AKT/mTOR pathway. Int J Mol Med. 2017;39:757–763. doi: 10.3892/ijmm.2017.2863. [DOI] [PubMed] [Google Scholar]
- 28.Wnek A, Andrzejewska E, Kobos J, Taran K, Przewratil P. Molecular and immunohistochemical expression of apoptotic proteins Bax, Bcl-2 and Caspase 3 in infantile hemangioma tissues as an effect of propranolol treatment. Immunol Lett. 2017;185:27–31. doi: 10.1016/j.imlet.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Bernabeu-Wittel J, Pereyra-Rodriguez JJ, Mantrana-Bermejo ME, Fernandez-Pineda I, de Agustin JC, Conejo-Mir J. Propranolol for the treatment of severe hemangiomas of infancy: results from a series of 28 patients. Actas Dermosifiliogr. 2011;102:510–516. doi: 10.1016/j.ad.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 30*.Leaute-Labreze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735–746. doi: 10.1056/NEJMoa1404710. This is a randomized, controlled trial showing the effectiveness of propranolol for infantile hemangiomas. This article follows the landmark paper in 2008 by the same author (Reference 20) [DOI] [PubMed] [Google Scholar]
- 31.Betlloch-Mas I, Martinez-Miravete MT, Lucas-Costa A, Martin de Lara AI, Selva-Otalaurruchi J. Outpatient treatment of infantile hemangiomas with propranolol: a prospective study. Actas Dermosifiliogr. 2012;103:806–815. doi: 10.1016/j.ad.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Zaher H, Rasheed H, Hegazy RA, Hegazy RA, Abdelhalim DM, Gawdat HI. Oral propranolol: an effective, safe treatment for infantile hemangiomas. Eur J Dermatol. 2011;21:558–563. doi: 10.1684/ejd.2011.1372. [DOI] [PubMed] [Google Scholar]
- 33.Snir M, Reich U, Siegel R, et al. Refractive and structural changes in infantile periocular capillary haemangioma treated with propranolol. Eye (Lond) 2011;25:1627–1634. doi: 10.1038/eye.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perkins JA, Chen BS, Saltzman B, Manning SC, Parikh SR. Propranolol therapy for reducing the number of nasal infantile hemangioma invasive procedures. JAMA Otolaryngol Head Neck Surg. 2014;140:220–227. doi: 10.1001/jamaoto.2013.6524. [DOI] [PubMed] [Google Scholar]
- 35.Boucek RJ, Jr, Kirsh AL, Majesky MW, Perkins JA. Propranolol responsiveness in vascular tumors is not determined by qualitative differences in adrenergic receptors. Otolaryngol Head Neck Surg. 2013;149:772–776. doi: 10.1177/0194599813503445. [DOI] [PubMed] [Google Scholar]
- 36.Song J, Ouyang TX, Huang YY, et al. Comparative study on pathology of non involuting congenital hemangioma and infantile hemangioma. Zhonghua Zheng Xing Wai Ke Za Zhi. 2011;27:178–181. [PubMed] [Google Scholar]
- 37.Rogers M, Lam A, Fischer G. Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH) Pediatr Dermatol. 2002;19:5–11. doi: 10.1046/j.1525-1470.2002.00011.x. [DOI] [PubMed] [Google Scholar]
- 38.Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495–510. doi: 10.1007/s10024-003-2134-6. [DOI] [PubMed] [Google Scholar]
- 39.Parikh SR, Darrow DH, Grimmer JF, Manning SC, Richter GT, Perkins JA. Propranolol use for infantile hemangiomas: American Society of Pediatric Otolaryngology Vascular Anomalies Task Force practice patterns. JAMA Otolaryngol Head Neck Surg. 2013;139:153–156. doi: 10.1001/jamaoto.2013.1218. [DOI] [PubMed] [Google Scholar]
- 40.Tang LY, Hing JW, Tang JY, et al. Predicting complications with pretreatment testing in infantile haemangioma treated with oral propranolol. Br J Ophthalmol. 2016;100:902–906. doi: 10.1136/bjophthalmol-2015-307284. [DOI] [PubMed] [Google Scholar]
- 41.Tan ST, Itinteang T, Leadbitter P. Low-dose propranolol for infantile haemangioma. J Plast Reconstr Aesthet Surg. 2011;64:292–299. doi: 10.1016/j.bjps.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Salice P, Giovanni Bianchetti M, Giavarini A, et al. Cardiovascular Profile of Propranolol after Multiple Dosing in Infantile Hemangioma. Pharmacology. 2017;99:75–78. doi: 10.1159/000450755. [DOI] [PubMed] [Google Scholar]
- 43.Puttgen KB, Summerer B, Schneider J, Cohen BA, Boss EF, Bauman NM. Cardiovascular and blood glucose parameters in infants during propranolol initiation for treatment of symptomatic infantile hemangiomas. Ann Otol Rhinol Laryngol. 2013;122:550–554. doi: 10.1177/000348941312200903. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen HP, Pickrell BB, Wright TS. Beta-blockers as therapy for infantile hemangiomas. Semin Plast Surg. 2014;28:87–90. doi: 10.1055/s-0034-1376259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy TL, Whitaker M, Pellitteri P, Wood WE. Cystic hygroma/lymphangioma: a rational approach to management. Laryngoscope. 2001;111:1929–1937. doi: 10.1097/00005537-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Churchill P, Otal D, Pemberton J, Ali A, Flageole H, Walton JM. Sclerotherapy for lymphatic malformations in children: a scoping review. J Pediatr Surg. 2011;46:912–922. doi: 10.1016/j.jpedsurg.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 47.Balakrishnan K, Menezes MD, Chen BS, Magit AE, Perkins JA. Primary surgery vs primary sclerotherapy for head and neck lymphatic malformations. JAMA Otolaryngol Head Neck Surg. 2014;140:41–45. doi: 10.1001/jamaoto.2013.5849. [DOI] [PubMed] [Google Scholar]
- 48.Ma J, Biao R, Lou F, et al. Diagnosis and surgical treatment of cervical macrocystic lymphatic malformations in infants. Exp Ther Med. 2017;14:1293–1298. doi: 10.3892/etm.2017.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malic CC, Guilfoyle R, Courtemanche RJM, Arneja JS, Heran MKS, Courtemanche DJ. Lymphatic Malformation Architecture: Implications for Treatment With OK-432. J Craniofac Surg. 2017;28:1721–1724. doi: 10.1097/SCS.0000000000003789. [DOI] [PubMed] [Google Scholar]
- 50.de Serres LM, Sie KC, Richardson MA. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck Surg. 1995;121:577–582. doi: 10.1001/archotol.1995.01890050065012. [DOI] [PubMed] [Google Scholar]
- 51.Adams MT, Saltzman B, Perkins JA. Head and neck lymphatic malformation treatment: a systematic review. Otolaryngol Head Neck Surg. 2012;147:627–639. doi: 10.1177/0194599812453552. [DOI] [PubMed] [Google Scholar]
- 52.Perkins JA, Maniglia C, Magit A, Sidhu M, Manning SC, Chen EY. Clinical and radiographic findings in children with spontaneous lymphatic malformation regression. Otolaryngol Head Neck Surg. 2008;138:772–777. doi: 10.1016/j.otohns.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Smith MC, Zimmerman MB, Burke DK, et al. Efficacy and safety of OK-432 immunotherapy of lymphatic malformations. Laryngoscope. 2009;119:107–115. doi: 10.1002/lary.20041. [DOI] [PubMed] [Google Scholar]
- 54.Adams DM, Trenor CC, 3rd, Hammill AM, et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics. 2016;137:e20153257. doi: 10.1542/peds.2015-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karki R, Pandya D, Elston RC, Ferlini C. Defining “mutation” and “polymorphism” in the era of personal genomics. BMC Med Genomics. 2015;8:37. doi: 10.1186/s12920-015-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glaser K, Dickie P, Neilson D, Osborn A, Dickie BH. Linkage of Metabolic Defects to Activated PIK3CA Alleles in Endothelial Cells Derived from Lymphatic Malformation. Lymphat Res Biol. 2018 doi: 10.1089/lrb.2017.0033. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Garijo A, Steller H. Spreading the word: non-autonomous effects of apoptosis during development, regeneration and disease. Development. 2015;142:3253–3262. doi: 10.1242/dev.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Perkins JA. New Frontiers in Our Understanding of Lymphatic Malformations of the Head and Neck: Natural History and Basic Research. Otolaryngol Clin North Am. 2018;51:147–158. doi: 10.1016/j.otc.2017.09.002. This is a review of lymphatic malformations. The article highlights the clinical characteristics of lymphatic malformations and discusses the molecular contributions to the development of the disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arriola Apelo SI, Lamming DW. Rapamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island. J Gerontol A Biol Sci Med Sci. 2016;71:841–849. doi: 10.1093/gerona/glw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasongko TH, Ismail NF, Zabidi-Hussin Z. Rapamycin and rapalogs for tuberous sclerosis complex. Cochrane Database Syst Rev. 2016;7:CD011272. doi: 10.1002/14651858.CD011272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teachey DT, Greiner R, Seif A, et al. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. 2009;145:101–106. doi: 10.1111/j.1365-2141.2009.07595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strychowsky JE, Rahbar R, O'Hare MJ, Irace AL, Padua H, Trenor CC., 3rd Sirolimus as treatment for 19 patients with refractory cervicofacial lymphatic malformation. Laryngoscope. 2018;128:269–276. doi: 10.1002/lary.26780. [DOI] [PubMed] [Google Scholar]
- 63.Kim D, Benjamin L, Wysong A, Hovsepian D, Teng J. Treatment of complex periorbital venolymphatic malformation in a neonate with a combination therapy of sirolimus and prednisolone. Dermatol Ther. 2015;28:218–221. doi: 10.1111/dth.12208. [DOI] [PubMed] [Google Scholar]
- 64.Harsha WJ, Crawford JV, Sorensen DM. An unusual case of adult airway obstruction from a lymphovenous malformation. Ear Nose Throat J. 2008;87:402–404. [PubMed] [Google Scholar]
- 65.Wagner KM, Lokmic Z, Penington AJ. Prolonged antibiotic treatment for infected low flow vascular malformations. J Pediatr Surg. 2017 doi: 10.1016/j.jpedsurg.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 66.Tempero RM, Hannibal M, Finn LS, Manning SC, Cunningham ML, Perkins JA. Lymphocytopenia in children with lymphatic malformation. Arch Otolaryngol Head Neck Surg. 2006;132:93–97. doi: 10.1001/archotol.132.1.93. [DOI] [PubMed] [Google Scholar]
- 67.Balakrishnan K, Bauman N, Chun RH, et al. Standardized outcome and reporting measures in pediatric head and neck lymphatic malformations. Otolaryngol Head Neck Surg. 2015;152:948–953. doi: 10.1177/0194599815577602. [DOI] [PubMed] [Google Scholar]
- 68.Benoiton LA, Chan K, Steiner F, FitzJohn T, Tan ST. Management of Orbital and Periorbital Venous Malformation. Front Surg. 2017;4:27. doi: 10.3389/fsurg.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Seront E, Vikkula M, Boon LM. Venous Malformations of the Head and Neck. Otolaryngol Clin North Am. 2018;51:173–184. doi: 10.1016/j.otc.2017.09.003. This review provides details about venous malformations and various treatment options. [DOI] [PubMed] [Google Scholar]
- 70.Dasgupta R, Patel M. Venous malformations. Semin Pediatr Surg. 2014;23:198–202. doi: 10.1053/j.sempedsurg.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 71.Hung JW, Leung MW, Liu CS, et al. Venous Malformation and Localized Intravascular Coagulopathy in Children. Eur J Pediatr Surg. 2017;27:181–184. doi: 10.1055/s-0036-1582241. [DOI] [PubMed] [Google Scholar]
- 72.Dompmartin A, Vikkula M, Boon LM. Venous malformation: update on aetiopathogenesis, diagnosis and management. Phlebology. 2010;25:224–235. doi: 10.1258/phleb.2009.009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steiner F, FitzJohn T, Tan ST. Surgical treatment for venous malformation. J Plast Reconstr Aesthet Surg. 2013;66:1741–1749. doi: 10.1016/j.bjps.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 74.Tieu DD, Ghodke BV, Vo NJ, Perkins JA. Single-stage excision of localized head and neck venous malformations using preoperative glue embolization. Otolaryngol Head Neck Surg. 2013;148:678–684. doi: 10.1177/0194599813475586. [DOI] [PubMed] [Google Scholar]
- 75.Brouillard P, Vikkula M. Genetic causes of vascular malformations. Hum Mol Genet. 2007;16 Spec No. 2:R140–149. doi: 10.1093/hmg/ddm211. [DOI] [PubMed] [Google Scholar]
- 76.Redondo P. The hidden face of venous malformations: a multidisciplinary therapeutic approach. Arch Dermatol. 2008;144:922–926. doi: 10.1001/archderm.144.7.922. [DOI] [PubMed] [Google Scholar]
- 77.Soblet J, Limaye N, Uebelhoer M, Boon LM, Vikkula M. Variable Somatic TIE2 Mutations in Half of Sporadic Venous Malformations. Mol Syndromol. 2013;4:179–183. doi: 10.1159/000348327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Limaye N, Wouters V, Uebelhoer M, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009;41:118–124. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boscolo E, Limaye N, Huang L, et al. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015;125:3491–3504. doi: 10.1172/JCI76004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wieck MM, Nowicki D, Schall KA, Zeinati C, Howell LK, Anselmo DM. Management of pediatric intramuscular venous malformations. J Pediatr Surg. 2017;52:598–601. doi: 10.1016/j.jpedsurg.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 81.Rivas S, Lopez-Gutierrez JC, Diaz M, Andres AM, Ros Z. Venous malformations. Diagnosis and treatment during the childhood. Cir Pediatr. 2006;19:77–80. [PubMed] [Google Scholar]
- 82.Rosenberg TL, Suen JY, Richter GT. Arteriovenous Malformations of the Head and Neck. Otolaryngol Clin North Am. 2018;51:185–195. doi: 10.1016/j.otc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 83.Han HH, Choi JS, Seo BF, et al. Successful treatment of posttraumatic arteriovenous malformation of the lower lip. J Craniofac Surg. 2015;26:e199–201. doi: 10.1097/SCS.0000000000001282. [DOI] [PubMed] [Google Scholar]
- 84.Liu AS, Mulliken JB, Zurakowski D, Fishman SJ, Greene AK. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185–1194. doi: 10.1097/PRS.0b013e3181d18070. [DOI] [PubMed] [Google Scholar]
- 85.Bradley JP, Zide BM, Berenstein A, Longaker MT. Large arteriovenous malformations of the face: aesthetic results with recurrence control. Plast Reconstr Surg. 1999;103:351–361. doi: 10.1097/00006534-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Pekkola J, Lappalainen K, Vuola P, Klockars T, Salminen P, Pitkaranta A. Head and neck arteriovenous malformations: results of ethanol sclerotherapy. AJNR Am J Neuroradiol. 2013;34:198–204. doi: 10.3174/ajnr.A3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nikolaev SI, Vetiska S, Bonilla X, et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N Engl J Med. 2018;378:250–261. doi: 10.1056/NEJMoa1709449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Olabi L, Polubothu S, Dowsett K, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest. 2018 doi: 10.1172/JCI124649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Couto JA, Huang AY, Konczyk DJ, et al. Somatic MAP2K1 Mutations Are Associated with Extracranial Arteriovenous Malformation. Am J Hum Genet. 2017;100:546–554. doi: 10.1016/j.ajhg.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


