Abstract
Background
Yes-associated protein 1 (YAP1) is an effector of Hippo pathway, which plays a significant role in cell proliferation and tumor progression. The relationship between YAP1 and gastrointestinal cancer has been explored in many previous studies. We conducted a meta-analysis to explore the prognostic effect of YAP1 in patients with gastrointestinal cancer.
Methods
A systematic search was performed through the PubMed, Web of Science, Embase, and Cochrane library databases to collect eligible studies. The pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were used to evaluate the relationship between YAP1 expression and gastrointestinal cancer clinical outcomes.
Results
A total of 2941 patients from 18 studies were enrolled. The results showed that elevated YAP1 expression predicted a poor prognosis in gastrointestinal cancer (HR = 1.56; 95% CI: 1.29-1.89; P < 0.001). Subgroup analyses indicated significant association between YAP1 overexpression and shorter OS of patients with esophageal squamous cell carcinoma (HR = 1.85; 95% CI: 1.25-2.73; P = 0.002), gastric cancer (HR = 1.41,95% CI: 1.02-1.95; P = 0.037), and colorectal cancer (pooled HR = 1.75; 95% CI: 1.42-2.15; P < 0.001). However, YAP1 expression did not affect DFS of patients with gastrointestinal cancer (pooled HR = 1.33; 95% CI: 0.95-1.88; P = 0.101).
Conclusion
Elevated YAP1 expression in patients with gastrointestinal cancer might be related to shorter OS. YAP1 protein could serve as a potential predictor of poor prognosis in gastrointestinal cancer.
1. Introduction
Gastrointestinal cancer is one of the major malignant diseases detrimental to health. Esophageal cancer (EC), gastric cancer (GC), and colorectal cancer (CRC) are the major malignancies of gastrointestinal cancer. EC is the sixth leading cause of cancer-related death worldwide, GC is the third and CRC is the fifth [1]. Although the overall survival (OS) of patients with gastrointestinal cancer has improved due to growing early detection and more widespread implementation of radical surgery, plenty of patients with gastrointestinal cancer continue to be diagnosed at advanced stages and therefore lose the optimal opportunity for radical cure. Therefore, searching for ideal diagnostic and prognostic biomarker is significant to improve the curative effect of gastrointestinal cancer.
Recently, researches on signal transduction pathways have revealed the Hippo-YAP pathway plays a vital part in modulating tissue homeostasis, organ size, and tumor progression. Yes-associated protein 1 (YAP or YAP1), an oncoprotein encoded by the YAP1 gene located on the human chromosome 11q22, is a transcriptional effector component of the Hippo pathway to regulate cell proliferation and apoptosis [2]. YAP1 was first discovered as an intracellular binding protein and a transcriptional coactivator by Sudol in 1994 [3]. Increasing expression of YAP1 protein was detected in numerous cancers, including esophageal cancer, gastric cancer, and colorectal cancer.
Elevated YAP1 expression was reported to be associated with worse outcomes in gastrointestinal cancer in most studies, but some studies reported the dual functions of YAP1 to promote and inhibit the progression of human malignancies. Suh [4] revealed that YAP1 had a tumor suppressor function in GC. In human colon carcinoma cell line H116, YAP1 interacts with p73 and increases p73 transactivation of apoptotic genes [5]. In order to research the relationship between YAP1 protein and prognosis of gastrointestinal cancer, we performed a systematic review and meta-analysis to assess the prognostic implications of elevated YAP1 protein in patients with gastrointestinal cancer.
2. Materials and Methods
2.1. Literature Search
PubMed, Web of Science, Embase, and Cochrane library databases were searched to retrieve feasible literatures about the theme up to Jul 9, 2018. The key terms used in the search strategy were (“gastrointestinal” or “esophageal” or “gastric” or “stomach” or “colorectal” or “colon”) and (“Yes associated protein 1” or “YAP1” or “Yes associated protein” or “YAP”). The eligibility articles were limited to clinical trials. We went over the full text of identified articles. We searched manually to ensure all available studies were included in this meta-analysis. Two investigators (L. Zhang and X. Song) conducted literature collection independently. Different opinions from two investigators were resolved by consultation.
2.2. Study Selection Criteria
Literatures eligible for this meta-analysis were identified according to the following criteria: (1) all patients were diagnosed as EC or GC or CRC by pathological examination; (2) YAP1 protein expression in human tumor tissues was detected; (3) the prognostic value of YAP1 in patients with gastrointestinal cancer was investigated; (4) the hazard ratio (HR) and corresponding 95% confidence interval (CI) could be extracted for overall Survival (OS) or disease-free survival (DFS). The exclusion criteria for articles included (1) non-English language articles; (2) case reports, letters, reviews, conference abstracts, and animal experiments; (3) insufficient data available to estimate outcomes; (4) size of each study arm less than 10 participants; (5) selecting the latest or complete study if one patient cohort was researched in more than one studies.
2.3. Quality Assessment and Data Collection
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to assess the quality of the studies and a score ≥ 6 was regarded as high quality. Two researchers extracted requisite information from all the eligible studies independently, including first author's surname, year of publication, nationality, case number, detected method, outcome measure, follow-up months, the cut-off value, HR, and corresponding 95% CI. We selected multivariate result if multivariate and univariate results were both reported in a study.
2.4. Statistical Analysis
All meta-analyses were performed with STATA 12.0 Software (Stata Corporation, College Station, TX, USA), and P < 0.05 indicated statistical significance unless otherwise specified. The main outcome was OS or DFS. Pooled HRs with 95% CIs were used to quantitatively determine the association between positive YAP1 expression and clinical prognosis. If the study had reported HR and 95% CI, we extracted them directly. Otherwise, they were determined by the data extracted from Kaplan-Meier survival curves using Engauge Digitizer version 4.1 [6]. We also contacted to the corresponding authors of relevant articles if needed. A pooled HR greater than 1 indicated a shorter survival for the patients with positive YAP1 expression, while HR less than 1 suggested a longer survival. The statistical heterogeneity was measured by the I2 statistic and Chi-square test (P value) [7, 8]. If P ≤ 0.05 or I2 ≥ 50%, indicating a problem with statistically heterogeneity, we chose the random-effects model to conduct analyses. In contrast, we used the fixed-effects model. Meta-regression and subgroup analyses were performed to further explore the potential source of heterogeneity. Funnel plot, Egger's test, and Begg's test were performed to evaluate the publication bias [9].
3. Results
3.1. Study Characteristics
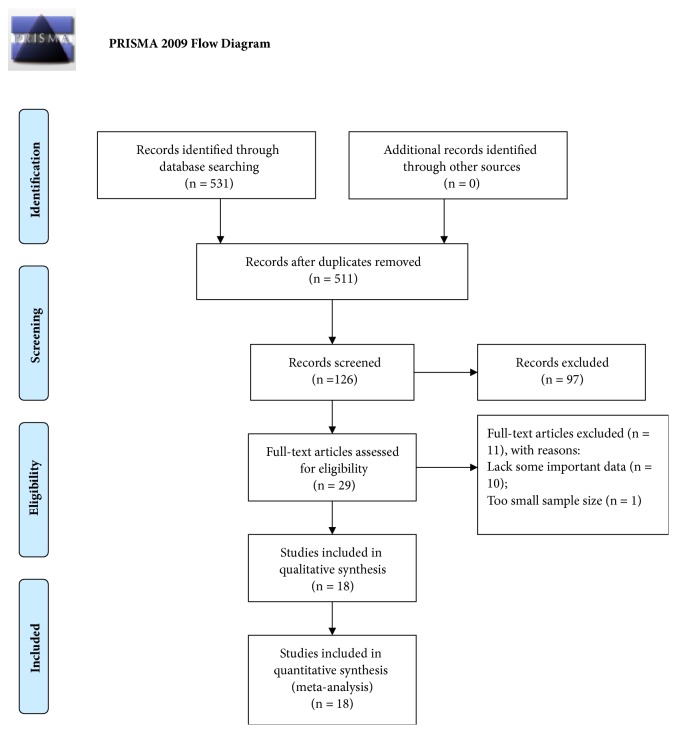
Our search strategy primarily retrieved 531 records. 385 papers were directly excluded by reading the titles and abstracts. After looking through full text of every paper, 97 articles were excluded because they were not related to YAP1 protein or did not study human gastrointestinal cancer. In the remaining 29 papers, 11 papers were excluded for the following reasons: lack some important data (n = 10) or too small sample size (n = 1). Finally, 18 articles published from 2012 to 2018 studied the effect of positive YAP1 expression on patients survival in gastrointestinal cancer were enrolled into this meta-analysis (Figure 1) [4, 10–26]. The main information of the enrolled studies is showed in Table 1. The 18 studies included 2 studies detecting YAP1 expression in esophageal squamous cell carcinoma (ESCC), 9 in GC, and 7 in CRC. A total of 2941 patients diagnosed with gastrointestinal cancer were included. 11 studies (62.50%) reported on Chinese, 7 studies (31.25%) on Koreans, and only 1 study (6.25%) on Japanese. The YAP1 protein was detected by Immunohistochemistry (IHC) in all the 18 studies, but these studies reported different cut-off values. HRs and 95% CIs for OS and/or DFS were provided directly in 12 studies and estimated from Kaplan-Meier Survival curves in the other 6 studies. According to the NOS, all included articles were of high quality (score≥7), with a mean of 7.67. Thus, all studies were eligible for this meta-analysis.
Figure 1.
Flow diagram of the studies selection process.
Table 1.
Main characteristics of all studies included in the meta-analysis.
| First author | Year | Country | tumor type | Case number | Follow-up (months) | Detected method | Cut-off value | Multivariate analysis | HRs provided from | Outcome measures | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Muramatsu | 2011 | Japan | ESCC | 120 | 1-103 | IHC | ≥30% of cells stained | yes | Report | OS | 7 |
| Yeo | 2012 | Korea | ESCC | 142 | over60 | IHC | IRS ≥4 | yes | Report | OS/DFS | 7 |
| Huang | 2017 | China | GC | 120 | longest96 | IHC | IRS ≥4 | yes | Report | OS | 9 |
| Hong | 2017 | Korea | GC | 166 | 0-119 | IHC | ≥10% of cells stained | no | SC | OS/DFS | 8 |
| Hu | 2014 | China | GC | 214 | longest65 | IHC | IRS ≥4 | yes | Report | OS | 8 |
| Li | 2016 | China | GC | 161 | median34 | IHC | IRS >2 | yes | Report | OS | 8 |
| Song | 2012 | Korea | GC | 223 | 5-75 | IHC | ≥50% of cells stained | yes | Report | OS | 7 |
| Suh | 2015 | Korea | GC | 116 | 3-51 | IHC | ≥10% of cells stained | no | SC | OS/DFS | 7 |
| Sun | 2017 | China | GC | 270 | 107 | IHC | ≥10% of cells stained | yes | Report | OS | 9 |
| Zhang | 2017 | China | GC | 178 | 80 | IHC | IRS ≥3 | no | SC | OS | 8 |
| Choi | 2018 | Korea | GC | 300 | over60 | IHC | IRS ≥6 | no | SC | OS/DFS | 8 |
| Wang | 2013 | China | CRC | 168 | 1-56 | IHC | IRS ≥4 | yes | Report | OS | 8 |
| Wang | 2018 | China | CRC | 172 | longest50 | IHC | IRS ≥4 | no | SC | OS | 7 |
| Wu | 2017 | China | CRC | 85 | 102 to 2572 days | IHC | H-score >150 | yes | Report | OS | 7 |
| Kim | 2013 | Korea | CRC | 132 | longest125 | IHC | IRS ≥7 | yes | Report | OS | 7 |
| Wang | 2013 | China | CRC | 139 | over60 | IHC | ≥10% of cells stained | yes | Report | OS | 8 |
| Ou | 2017 | China | CRC | 90 | longest60 | IHC | IRS ≥2 | no | SC | OS | 7 |
| Yang | 2018 | China | CRC | 145 | longest125 | IHC | IRS ≥2 | yes | Report | OS/DFS | 8 |
ESCC: esophageal squamous cell carcinoma; GC: gastric cancer; CRC: colorectal cancer; IHC: immunohistochemistry; IRS: immunoreactivity score; HR: hazard ratio; SC: survival curve; OS: overall survival; DFS: disease free survival; NOS:Newcastle-Ottawa Scale.
3.2. Overall Survival
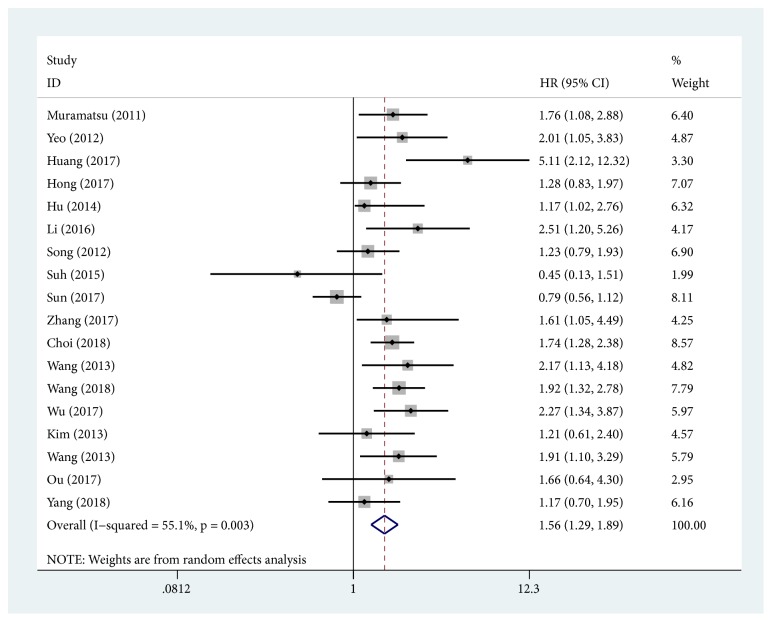
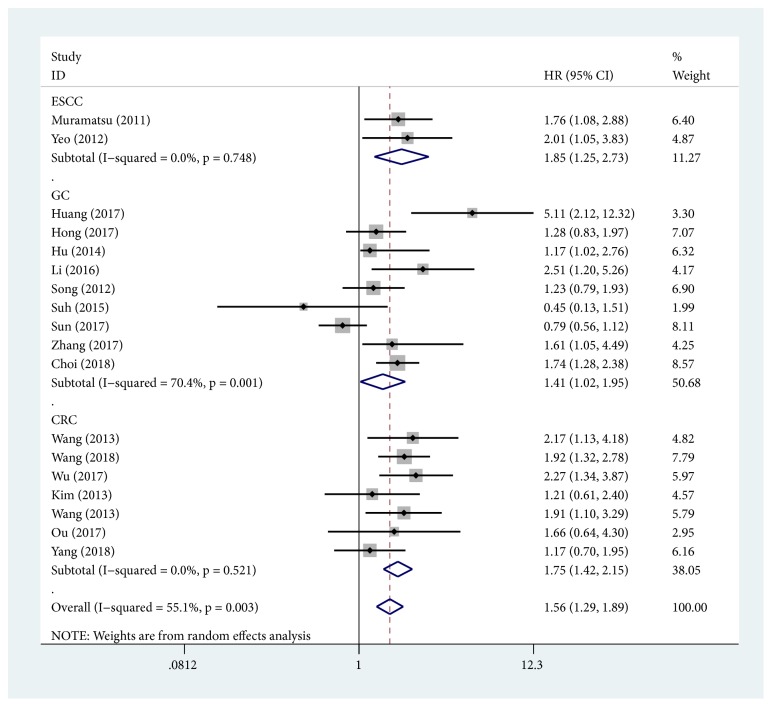
18 studies provided sufficient information for OS or DFS analysis. The key results of this meta-analysis are shown in Table 2. Because of the heterogeneity between studies in evaluating OS (I2 = 55.1%, P = 0.003), a random-effects model was used to pool the HRs. The pooled analysis indicated that higher YAP1 expression was significantly associated with shorter OS in gastrointestinal cancer patients (HR = 1.56; 95% CI: 1.29-1.89; P < 0.001) (Figure 2). The tumor type subgroup analysis demonstrated negative impact of elevated YAP1 on OS in patients with ESCC (HR = 1.85; 95% CI: 1.25-2.73; P = 0.002), GC (HR = 1.41,95% CI: 1.02-1.95; P = 0.037), and CRC (pooled HR = 1.75; 95% CI: 1.42-2.15; P < 0.001) (Figure 3). In regard to country subgroup analysis, higher expression of YAP1 was visibly associated with shorter OS in Japanese patients (pooled HR = 1.76; 95% CI: 1.08-2.88; P = 0.024), Chinese patients (pooled HR =1.70; 95% CI: 1.26-2.29; P < 0.001), and Korean patients (pooled HR = 1.41; 95% CI: 1.10-1.80; P = 0.007). For OS, pooled HR values > 1 were still calculated in subgroup meta-analyses stratified by case number, HR obtained method, and analysis type (Table 2).
Table 2.
Pooled HR for OS of patients with high expression of YAP1 according to subgroup analyses.
| Outcome subgroup | No. of patients | No. of studies | Fixed-effects model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | I 2 (%) | P | |||
| OS | 2941 | 18 | 1.56(1.29,1.89) | <0.001 | 55.1 | 0.003 |
| Tumor type | ||||||
| ESCC | 262 | 2 | 1.85(1.25,2.73) | 0.002 | 0 | 0.748 |
| GC | 1748 | 9 | 1.41(1.02,1.95) | 0.037 | 70 | 0.001 |
| CRC | 931 | 7 | 1.92(1.32,2.78) | <0.001 | 0 | 0.521 |
| Contry | ||||||
| China | 1742 | 11 | 1.70(1.26,2.29) | <0.001 | 67.1 | 0.001 |
| Korea | 1079 | 6 | 1.41(1.10,1.80) | 0.007 | 27.7 | 0.227 |
| Japan | 120 | 1 | 1.76(1.08,2.88) | 0.024 | - | - |
| Case number | ||||||
| <150 | 1089 | 9 | 1.72(1.27,2.33) | <0.001 | 47 | 0.057 |
| ≥150 | 1852 | 9 | 1.45 (1.13,1.86) | 0.003 | 60 | 0.009 |
| HR obtained method | ||||||
| Reported | 2085 | 12 | 1.61(1.24,2.10) | <0.001 | 64.2 | 0.001 |
| SC | 856 | 6 | 1.58(1.25,2.00) | <0.001 | 21.1 | 0.275 |
| Analysis type | ||||||
| Multivariate | 2085 | 12 | 1.61(1.24,2.10) | <0.001 | 64.2 | 0.001 |
| Univariate | 856 | 6 | 1.58(1.25,2.00) | <0.001 | 21.1 | 0.275 |
OS: overall survival; ESCC: esophageal squamous cell carcinoma; GC: gastric cancer; CRC: colorectal cancer; HR: hazard ratio; CI: confidence interval; SC: survival curve.
Figure 2.
Forest plots of studies evaluating hazard ratios of high YAP1 expression in gastrointestinal cancer for overall survival.
Figure 3.
Forest plot of the relationship between high YAP1 expression and overall survival in patients with a variety of cancers.
3.3. Sensitivity Analyses and Publication Bias
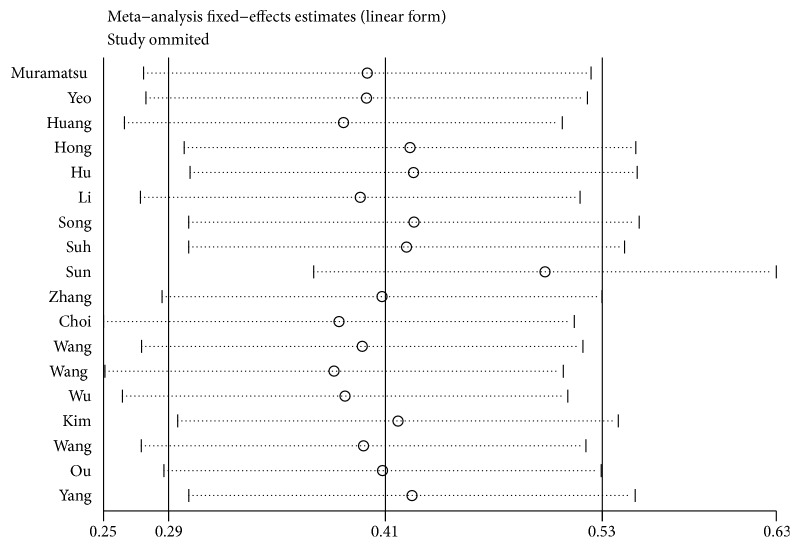
We used the random-effects model to assess sensitivity analysis by sequential omission of individual studies, and the outcome was unaffected by any single study (Figure 4). Then we performed a meta-regression to detect the potential causes for the heterogeneity. The results showed no statistically significant impact of country (P = 0.639), tumor type (P = 0.779), sample size (P = 0.405), analysis type (P = 0.830), HR obtained method (P = 0.830), or cut-off value (P = 0.326) on the combined effect size for OS. Funnel plots, Egger's test, and Begg's test were used to assess the publication bias of all enrolled studies. Funnel plot (Figure 5) suggested no publication bias existed, and Egger's test supported the same result (P = 0.37). Thus, the results of this meta-analysis were dependable.
Figure 4.
Sensitivity analysis on the relationships between YAP1 expression and overall survival in gastrointestinal cancer patients.
Figure 5.
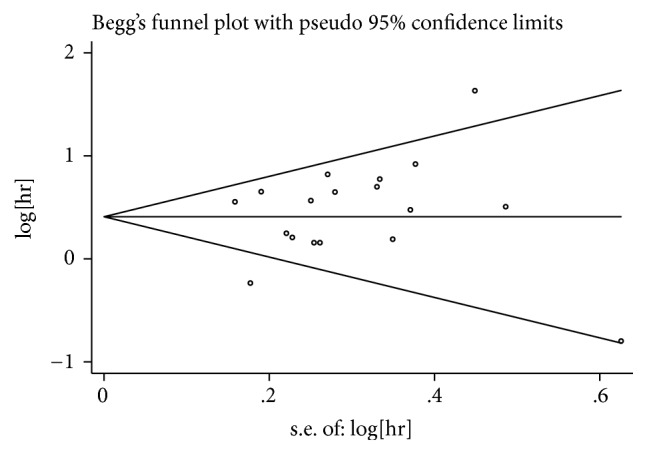
Funnel plots of publication biases on the relationships between YAP1 expression and overall survival in gastrointestinal cancer patients.
3.4. Disease-Free Survival
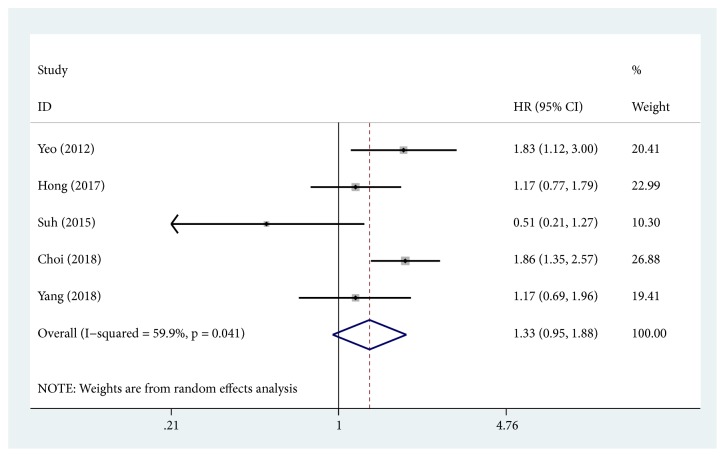
5 studies enrolled a total of 869 patients from China and Korea, providing suitable information for DFS analyses. Because of obvious statistical heterogeneity (I2 = 59.9%; P = 0.041) (Table 3), a random-effects model was used to calculate the pooled HR. The result revealed no association between higher level of YAP1 and shorter DFS (pooled HR = 1.33; 95% CI = 0.95–1.88; P = 0.101). A forest plot of study-specific HRs for DFS is presented in Figure 6. All the HRs and corresponding 95% CIs are shown in Table 3. Subgroup analysis of cancer type revealed the adverse effect of elevated YAP1 on DFS in patients with ESCC (pooled HR = 1.83; 95% CI = 1.12-3.00; P < 0.001), but there was no correlation between YAP1 expression and DFS in GC or CRC patients. Moreover, the association between YAP1 and DFS was not significant in both Chinese (pooled HR= 1.17, 95% CI = 0.69-1.97) and Korean (pooled HR = 1.35, 95% CI = 0.89-2.06) populations.
Table 3.
Pooled HR for DFS of patients with high expression of YAP1 according to subgroup analyses.
| Outcome subgroup | No. of patients | No. of studies | Fixed-effects model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | I 2 (%) | P | |||
| DFS | 869 | 5 | 1.33(0.95,1.88) | <0.001 | 59.9 | 0.041 |
| Tumor type | ||||||
| ESCC | 142 | 1 | 1.83(1.12,3.00) | 0.016 | - | - |
| GC | 582 | 3 | 1.18(0.66,2.11) | 0.584 | 76.4 | 0.014 |
| CRC | 145 | 1 | 1.17(0.69,1.88) | 0.556 | - | - |
| Contry | ||||||
| China | 145 | 1 | 1.35(0.89,2.06) | 0.556 | - | - |
| Korea | 724 | 4 | 1.17(0.95,1.88) | 0.159 | 67.4 | 0.027 |
DFS: disease-free survival; ESCC: esophageal squamous cell carcinoma; GC: gastric cancer; CRC: colorectal cancer; HR: hazard ratio; CI: confidence interval.
Figure 6.
Forest plots of studies evaluating hazard ratios of high YAP1 expression in gastrointestinal cancer for disease-free survival.
4. Discussion
The Hippo signaling pathway was first identified and named by screening for mutant tumor suppressors in flies, and it was revealed that tumor progressed due to increased cell proliferation and decreased cell apoptosis when the components of the Hippo pathway had loss-of-function mutations [27]. The Hippo pathway is strongly participated in several processes of cancer progression. Evidence shows that the Hippo pathway is interconnected with other cancer-relevant pathways, especially the transforming growth factor-β (TGF-β), G protein-coupled receptors (GPCRs), and WNT pathways [28].
YAP1 and PDZ-binding motif (TAZ) are the downstream proteins in the Hippo pathway. These two proteins are in charge of controlling cell proliferation and have important regulatory effects on stem cell self-renewal, tissue regeneration, and organ development. YAP1 contains 488 amino acids and several structural domains [29]. The most crucial domains are a TEA DNA-binding domain and two-WW domain. The former binds to the TEA Domain Transcription factor (TEAD), while the latter binds to a transcriptional coactivator, which in turn binds to the PPxY motif present on transcription factors [30]. TAZ, the YAP1 homologous protein, is identified as a 14-3-3 binding protein. It has similarity structure and biological functions [31]. Normally, phosphorylated YAP/TAZ is accumulated in the cytoplasm, maintaining a highly conservative state and without transcriptional kinase activity. Under pathological conditions, the Hippo pathway which is blocked or inactivated loses of the effect on phosphorylating YAP/TAZ. Then YAP/TAZ is combined with the transcription factor TEAD. The complex transfers to the nucleus and promotes the transcription of corresponding genes, resulting in the imbalance between cell proliferation and apoptosis; eventually these changes lead to cell over proliferation and even cause the tumor occurring [32, 33]. Although YAP1 is usually identified as an oncoprotein with functions of enhancing the tumor cells survival, migration, tumor angiogenesis, and chemotherapy resistance, some literature supports the idea that YAP1 functions as a tumor suppressor in cancers, for instance, head and neck cancers (HNC) [34], breast cancer [35], hematological malignancy [36], and CRC [5].
Gastrointestinal cancer is one of the major health care problems in the world. We performed the first meta-analysis to provide strong evidence to reveal the prognostic implications of YAP1 in gastrointestinal cancer. Elevated YAP1 protein expression was obviously correlated with shorter OS in patients with gastrointestinal cancer. In the subsequent subgroup analyses, the adverse prognostic role of elevated YAP1 expression remained stable in different country, sample size, HR obtained method, and analysis type. Nevertheless, the existing evidence from included studies was insufficient to prove a definitive correlation between YAP1 and DFS. Our findings showed YAP1 was not an independent prognostic factor for DFS in gastrointestinal cancer. Nonetheless, because of the significant heterogeneity, the results should be treated with caution. This heterogeneity might be partly due to the variation in patients' selection among the studies. For instance, the proportion of advanced stage patients was different from study to study. Stratified analyses according to clinicopathological characteristics (such as anatomic site and disease stage) were not performed in this meta-analysis for the limitation of available original studies. The analyses can be conducted in the future to further assess the relationship between YAP1 and DFS in gastrointestinal cancer with more available studies.
Furthermore, previous research shows elevated YAP1 expression is also related with shorter relapse free survival (RFS) in gastrointestinal cancer [12, 21]. In addition to YAP1 protein, elevated expression of YAP1 mRNA may also predict worse prognosis in patients with gastrointestinal cancer [37]. YAP1 also plays a role in early diagnosis of gastrointestinal cancer. Da [38] reported that detecting YAP1 and surviving together might help in early diagnosis of gastric carcinoma.
However, there are some deficiencies for this meta-analysis as well. First, there was a problem of heterogeneity in the overall analysis and in some subgroup analyses. Subgroup analyses revealed that the heterogeneity might be due to the different characteristics of the tumor types. Second, the different cut-off values of YAP1 IHC detection might impact on the precision of the prognostic role of YAP1 in gastrointestinal cancer. In order to establish the most suitable cut-off value, further well-designed studies with larger sample size are imperative to carry out. Third, it might be insufficient to provide support for all ethnic groups because all the studies included were carried out in Asian population.
5. Conclusions
In conclusion, our results suggest that YAP1 protein overexpression correlates with shorter OS in gastrointestinal cancer, especially in Asian patients. Furthermore, YAP1 has been considered to be a promising target for therapy of gastrointestinal cancer. Considering of the deficiencies of the present paper, further well-designed, prospective, and national multicenter, large sample researches are imperative to verify the clinical value of YAP1 in gastrointestinal cancer.
Acknowledgments
This work was supported by National Natural Science Foundation of China (31570877, 31570908); National Science and Technology Support Project (2015BAI12B12); Cooperative Research Foundation of National Natural Science Foundation for Hong Kong and Macao Scholars (31729001); Changzhou Science and Technology Project (CJ20160021).These funding sources primarily provided financial help for the authors' purchase paid literature.
Contributor Information
Changping Wu, Email: wcpjjt@163.com.
Jingting Jiang, Email: jiangjingting@suda.edu.cn.
Disclosure
The authors have not been paid to write this article by any pharmaceutical company or other agency.
Conflicts of Interest
All authors report no conflicts of interest.
References
- 1.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbluh J., Nijhawan D., Cox A., et al. β-Catenin-Driven Cancers Require a YAP1 Transcriptional Complex for Survival and Tumorigenesis. Cell. 2012;151(7):1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudol M. Yes-Associated Protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9(8):2145–2152. [PubMed] [Google Scholar]
- 4.Suh J. H., Won K. Y., Kim G. Y., et al. Expression of tumoral FOXP3 in gastric adenocarcinoma is associated with favorable clinicopathological variables and related with Hippo pathway. International Journal of Clinical and Experimental Pathology. 2015;8(11):14608–14618. [PMC free article] [PubMed] [Google Scholar]
- 5.Levy D., Adamovich Y., Reuven N., Shaul Y. The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death & Differentiation. 2007;14(4):743–751. doi: 10.1038/sj.cdd.4402063. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., Zeng T. Response to: Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2013;14(1):p. 391. doi: 10.1186/1745-6215-14-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins J. P. T., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 8.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L., Song X., Shao Y., Wu C., Jiang J. Prognostic value of Midkine expression in patients with solid tumors: a systematic review and meta-analysis. Oncotarget . 2018;9(37) doi: 10.18632/oncotarget.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muramatsu T., Imoto I., Matsui T., et al. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32(3):389–398. doi: 10.1093/carcin/bgq254. [DOI] [PubMed] [Google Scholar]
- 11.Yeo M.-K., Kim S.-H., Kim J. M., et al. Correlation of expression of phosphorylated and non-phosphorylated yes-associated protein with clinicopathological parameters in esophageal squamous cell carcinoma in a korean population. Anticancer Reseach. 2012;32(9):3835–3840. [PubMed] [Google Scholar]
- 12.Huang S., Zhu L., Cao Y., et al. Significant association of YAP1 and HSPC111 proteins with poor prognosis in Chinese gastric cancer patients. Oncotarget . 2017;8(46):80303–80314. doi: 10.18632/oncotarget.17932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong S. A., Son M. W., Cho J., et al. Low angiomotin-p130 with concomitant high Yes-associated protein 1 expression is associated with adverse prognosis of advanced gastric cancer. APMIS-Acta Pathologica, Microbiologica et Immunologica Scandinavica. 2017;125(11):996–1006. doi: 10.1111/apm.12750. [DOI] [PubMed] [Google Scholar]
- 14.Hu X., Xin Y., Xiao Y., Zhao J. Overexpression of YAP1 is Correlated with Progression, Metastasis and Poor Prognosis in Patients with Gastric Carcinoma. Pathology & Oncology Research. 2014;20(4):805–811. doi: 10.1007/s12253-014-9757-y. [DOI] [PubMed] [Google Scholar]
- 15.Li P., Sun D., Li X., et al. Elevated expression of Nodal and YAP1 is associated with poor prognosis of gastric adenocarcinoma. Journal of Cancer Research and Clinical Oncology. 2016;142(8):1765–1773. doi: 10.1007/s00432-016-2188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song M., Cheong J.-H., Kim H., Noh S. H., Kim H. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Reseach. 2012;32(9):3827–3834. [PubMed] [Google Scholar]
- 17.Sun D., Li X., He Y., et al. YAP1 enhances cell proliferation, migration, and invasion of gastric cancer in vitro and in vivo. Oncotarget . 2016;7(49):81062–81076. doi: 10.18632/oncotarget.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B., Gong A., Shi H. Identification of a novel YAP-14-3-3zeta negative feedback loop in gastric cancer. Oncotarget . 1894;8(42):7–7. doi: 10.18632/oncotarget.18011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Shi S., Guo Z., et al. Overexpression of YAP and TAZ Is an Independent Predictor of Prognosis in Colorectal Cancer and Related to the Proliferation and Metastasis of Colon Cancer Cells. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0065539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Wang Q., Gao X., Yu T. REGgamma controls Hippo signaling and reciprocal NF-kappaB-YAP regulation to promote colon cancer. Clinical Cancer Research. 2018 doi: 10.1158/1078-0432.CCR-17-2986. [DOI] [PubMed] [Google Scholar]
- 21.Wu D.-W., Lin P.-L., Wang L., Huang C.-C., Lee H. The YAP1/SIX2 axis is required for DDX3-mediated tumor aggressiveness and cetuximab resistance in KRAS-wild-type colorectal cancer. Theranostics. 2017;7(5):1114–1132. doi: 10.7150/thno.18175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D. H., Kim S. H., Lee O. J., et al. Differential expression of Yes-associated protein and phosphorylated Yes-associated protein is correlated with expression of Ki-67 and phospho-ERK in colorectal adenocarcinoma. Histology and Histopathology. 2013;28(11):1483–1490. doi: 10.14670/HH-28.1483. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Xie C., Li Q., Xu K., Wang E. Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumor Biology. 2013;34(4):2169–2174. doi: 10.1007/s13277-013-0751-x. [DOI] [PubMed] [Google Scholar]
- 24.Ou C., Sun Z., Li X., et al. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Letters. 2017;399:53–63. doi: 10.1016/j.canlet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Choi W., Kim J., Park J., et al. YAP/TAZ initiates gastric tumorigenesis via upregulation of MYC. Cancer Research. :p. canres.3487.2017. doi: 10.1158/0008-5472.CAN-17-3487. [DOI] [PubMed] [Google Scholar]
- 26.Yang R., Cai T., Wu X., et al. Tumour YAP1 and PTEN expression correlates with tumour-associated myeloid suppressor cell expansion and reduced survival in colorectal cancer. The Journal of Immunology. 2018;155(2):263–272. doi: 10.1111/imm.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan D. The hippo signaling pathway in development and cancer. Developmental Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriz V., Korinek V. WNT, RSPO and hippo signalling in the intestine and intestinal stem cells. Gene. 2018;9(1) doi: 10.3390/genes9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B., Li L., Lei Q., Guan K. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes & Development. 2010;24(9):862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moroishi T., Hansen C. G., Guan K.-L. The emerging roles of YAP and TAZ in cancer. Nature Reviews Cancer. 2015;15(2):73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhirajan R. K., Jain M., Walla B., et al. Cysteine S-glutathionylation promotes stability and activation of the Hippo downstream effector transcriptional Co-activator with PDZ-binding motif (TAZ) The Journal of Biological Chemistry. 2016;291(22):11596–11607. doi: 10.1074/jbc.M115.712539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saucedo L. J., Edgar B. A. Filling out the Hippo pathway. Nature Reviews Molecular Cell Biology. 2007;8(8):613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 33.Harvey K. F., Zhang X., Thomas D. M. The Hippo pathway and human cancer. Nature Reviews Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 34.Ehsanian R., Brown M., Lu H., et al. YAP dysregulation by phosphorylation or Δnp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene. 2010;29(46):6160–6171. doi: 10.1038/onc.2010.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu S. J., Hu J. Y., Kuang X. Y., et al. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clinical Cancer Research. 2013;19(6):1389–1399. doi: 10.1158/1078-0432.CCR-12-1959. [DOI] [PubMed] [Google Scholar]
- 36.Cottini F., Hideshima T., Xu C., et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nature Medicine. 2014;20(6):599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K.-W., Lee S. S., Hwang J.-E., et al. Development and validation of a six-gene recurrence risk score assay for gastric cancer. Clinical Cancer Research. 2016;22(24):6228–6235. doi: 10.1158/1078-0432.CCR-15-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Da C.-L., Xin Y., Zhao J., Luo X.-D. Significance and relationship between Yes-associated protein and survivin expression in gastric carcinoma and precancerous lesions. World Journal of Gastroenterology. 2009;15(32):4055–4061. doi: 10.3748/wjg.15.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]