Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease with a strong genetic component and etiology characterized by chronic inflammation and autoantibody production. The purpose of this study was to ascertain copy number variation (CNV) in SLE using a case-control design in an admixed Brazilian population. The whole-genome detection of CNV was performed using Cytoscan HD array in SLE patients and healthy controls. The best CNV candidates were then evaluated by quantitative real-time PCR in a larger cohort or validated using droplet digital PCR. Logistic regression models adjusted for sex and ancestry covariates was applied to evaluate the association between CNV with SLE susceptibility. The data showed a synergistic effect between the FCGR3B and ADAM3A loci with the presence of deletions in both loci significantly increasing the risk to SLE (5.9-fold) compared to the deletion in the single FCGR3B locus (3.6-fold). In addition, duplications in these genes were indeed more frequent in healthy subjects, suggesting that high FCGR3B/ADAM3A gene copy numbers are protective factors against to disease development. Overall, 21 rare CNVs were identified in SLE patients using a four-step pipeline created for identification of rare variants. Furthermore, heterozygous deletions overlapping the CFHR4, CFHR5 and HLA-DPB2 genes were described for the first time in SLE patients. Here we present the first genome-wide CNV study of SLE patients in a tri-hybrid population. The results show that novel susceptibility loci to SLE can be found once the distribution of structural variants is analyzed throughout the whole genome.
Introduction
Systemic lupus erythematosus (SLE, MIM 152700) is an autoimmune polygenic disease characterized by local or systemic inflammation from the production of autoantibodies and immune complex deposition in several tissues [1]. SLE has a wide range of clinical manifestations such as malar rash, discoid lesions, nephritis, and arthritis [2]. SLE is more prevalent in women than in men at a rate of 9:1, and onset predominantly during childbearing age [3]. In general, it is less prevalent in European ancestry populations than in African-Americans, African-Caribbean, Asians, and Hispanics [4].
In addition to the genetic component; hormonal, environmental, epigenetic and immunological factors contribute to the complex etiology of SLE [5]. High heritability and increased concordance rates were identified in monozygotic twins (24–57%) compared with dizygotic twins or full siblings (2–5%), suggesting that SLE has a complex genetic basis with sizable genetic and environmental components [6, 7]. Linkage and genome-wide association studies conducted in cohorts of patients with SLE have confirmed that HLA and other loci are associated with SLE [8]. This method has also been useful in identifying new candidate single nucleotide polymorphisms (SNPs) correlated with the disease [9, 10].
In addition to SNPs, genomic segments that vary in copy number in relation to a reference genome (denoted as copy number variations, or CNVs, and typically greater than 50 bp [11]) have been associated with susceptibility to autoimmune diseases, including SLE [2]. Well-documented CNVs that increase risk for SLE include deletions in C4 [12] and FCGR3B [13] genes, while CNVs in other genes were associated with SLE in single-population studied, e.g., TLR7 [14], DEFB4 [15], RABGAP1L [16] and HLA-DRB5 [17].
The small number of large-scale studies relating CNVs to SLE remains a significant gap in the genetic analysis of the disease [16, 18]. Additionally, the ancestral composition of populations can often modify the results of association tests, such that the study of admixed populations can produce different or even conflicting results compared with those reported in the literature [19, 20].
Based on the hypothesis that new SLE-related loci remain to be discovered using CNV approach, this study has evaluated the role of structural variation in SLE through genome-wide screening in Brazilian SLE patients.
Materials and methods
DNA samples
The total case group comprised 135 unrelated SLE patients treated at the Collagen Disease Outpatient Clinic of the University Hospital at the Ribeirão Preto Medical School (HCFMRP, USP) in Brazil. All patients fulfilled the American College of Rheumatology revised criteria for SLE diagnosis [21]. The healthy control (HC) cohort includes 200 healthy unrelated subjects resident in São Paulo state, Brazil. DNA was extracted from the blood samples of SLE patients and control subjects using salting-out method [22] and QIAamp DNA Blood Maxi Kit (QIAGEN, Hilden, Germany), respectively.
For the array analysis, we selected a subgroup of 23 lupus nephritis patients (18 female, mean age at diagnosis: 31 ± 13 years) from the total of 135 SLE patients (125 female, mean age at diagnosis: 32 ± 12 years). The frequency of clinical characteristics for the group and subgroup of SLE patients was, respectively: (1) nephritis: 61%/100%, (2) arthritis: 61%/74%, (3) malar rash: 38%/48%, (4) oral ulcers: 13%/13%, (5) photosensitivity: 40%/52%, (6) convulsions and/or psychoses: 21%/30%, (7) hematologic disorder (hemolytic anemia or leukopenia < 4.000/mm3, lymphopenia < 1.500/mm3, and/or thrombocytopenia < 150.000/mm3): 57%/52%, (8) immunologic disorder (anti-dsDNA, anti-Sm, and/or anti-phospholipid): 73%/78%.
Ethics
The study design was approved by the Research Ethics Committee of FMRP/USP (CAAE: 03199712.0.0000.5440) and FCM/UNICAMP (CAAE: 03199712.0.3001.5404). All subjects enrolled in this research have signed the consent form approved by the ethics committees.
Cytoscan HD array
The genome-wide human Cytoscan HD array (Affymetrix, CA, USA) was used to detect CNVs in SLE patients (n = 23) and healthy controls (n = 110) according to the manufacturer's protocol. Scanned data files were generated using Affymetrix GeneChip Command Console software v. 1.2 and analyzed by Affymetrix Chromosome Analysis Suite (ChAS) software v. 3.0.
CNV detection
To calculate copy number regions throughout the genome the data were normalized to baseline intensities according to an internal reference model of ChAS software that comprised 270 HapMap samples and 96 other healthy subjects from BioServe Biotechnologies (BioServe, Beltsville, USA). CNVs regions were mapped according to the human reference sequence version GRCh37/hg19. The number of consecutive probes required defining each deletion or duplication and was limited to a minimum of 25/ 50 consecutive probes, respectively. After variant detection by ChAS, CNV distribution per subjects and chromosomes was analyzed using Plink v.1.07 [23].
Determination of CNV regions
Plink was used to evaluate the recurrence of common CNVs, CNV regions (CNVRs), which are the union of overlapping CNVs among subjects [24]. Duplications and deletions were analyzed separately and classified as gain- or loss-type CNVRs.
Rare CNVs
A four-step pipeline was created for the identification of rare variants (population frequency < 1%) based on the Brazilian/HapMap population frequencies, public databases of genomic variants and CNV detection by two different algorithms: ChAS and Nexus Copy Number 8.0 (BioDiscovery, CA, USA) (S1 Fig).
CNVs located in genes with functional relevance to SLE
Using the cytoregion tool from ChAS software, three gene lists were created: (1) genes overlapping deletion or duplication-type CNVs described in association with SLE, (2) genes previously associated to SLE in linkage analysis and/or in genome-wide association studies, (3) genes related to autoimmunity. For this analysis, the number of consecutive markers for detecting deletions and duplications that overlap the genes included in the three lists was reduced from 25/50 to 15/15.
Validation of CNVs using target-specific methodology
For all copy number genes selected for validation by target-specific methodology, we designed primers using Primer3Plus [25], verified their specificity with in silico PCR tool available from UCSC Genome Browser [26], and purchased them from Eurofins Genomics (Louisville, KY, USA) or Sigma-Aldrich (St Louis, MO, USA). Primer sequences are listed in S1 Table. FOXP2 or PAX6 were used as reference genes for diploid copy number.
The CNVR encompassing the ADAM3A gene was selected for validation using quantitative real-time PCR (qPCR) in a larger case-control cohort. In addition to ADAM3A target, an individual CNV assay was conducted for FCGR3B gene since its coverage is very low in the Cytoscan HD chip (S3 Table). The ADAM3A and FCGR3B gene copy number genotyping was performed by SYBR Green-based genomic qPCR in cases (n = 135) and controls (n = 200) using the StepOne Plus Real-Time PCR System according to the manufacturer's protocol (Applied Biosystems, CA, USA). All experiments were designed using technical triplicates for each sample. The reference and target genes for each sample were ran in the same 96-well plate to avoid introducing experimental bias. The copy number of the target gene in each test sample was determined by the ΔΔCT-based relative quantification method [27].
Droplet digital PCR (ddPCR) was used as a target-specific methodology to validate three heterozygous deletions (CN = 1) overlapping the CFHR4, CFHR5, HLA-DPB2 genes, and a heterozygous duplication (CN = 3) encompassing the LDHB, KCNJ8, ABCC9, CMAS and ST8SIA1I genes (S4 Table). The ddPCR experiments were performed according to the manufacturer's protocol (Bio-Rad Laboratories, CA, USA). As the initial step, we treated all genomic DNA samples with HindIII restriction enzyme for 2 h at 37°C and then proceed to EvaGreen ddPCR assay. We calculated copy number using the QuantaSoft Pro software (Bio-Rad Laboratories, CA, USA). The error reported for a single well was the Poisson 95% confidence interval (95% CI). We used the automated clustering analysis for both target and reference and then calculated the final copy number as two times the ratio of target concentration versus reference concentration. A reference sample, expected to be in a diploid status in both target and reference genes was used as an internal control of the reactions.
Ancestry inference
Due to tri-hybrid composition of the Brazilian population, a panel of 345 ancestry informative markers based on SNP data from the array was used to infer the proportion of European, African and Amerindian ancestries of each SLE and control subjects [28]. These estimates were used as covariates in logistic regression models for the association between CNV with SLE susceptibility.
Statistical analysis
Statistical analyses were performed using the computing environment R version 3.1.1 (http://www.r-project.org/). The call and size of CNV was compared between SLE patients and controls using logistic regression models adjusted for sex and ancestry covariates, while Student's t-test was performed to compare the call and size of CNV per chromosome between case and control groups.
Results
Characteristics of CNVs
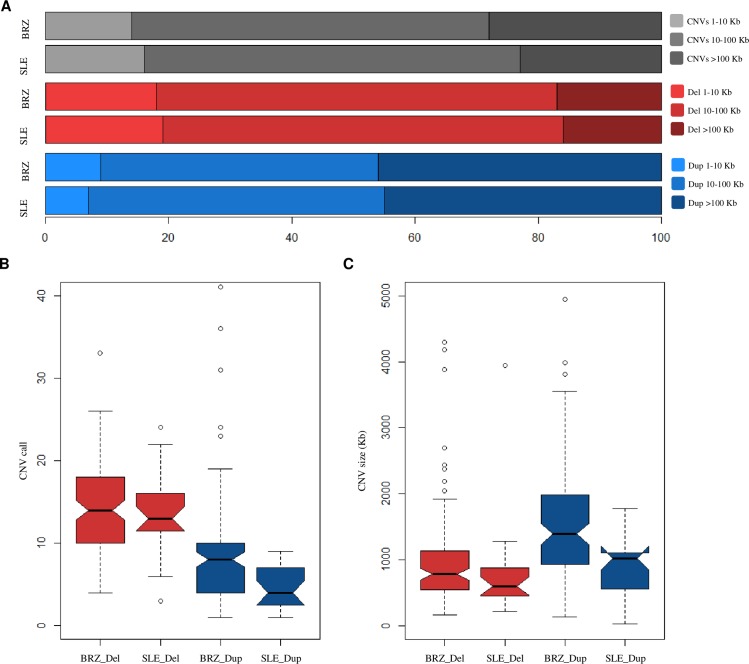
Genomic screening using the Cytoscan HD array found 447 CNVs in 23 patients and 2652 CNVs in 110 controls. An average of 19 CNVs were identified per patient (SD = 10, range 5–55 CNVs), while in controls the average was 24 CNVs (SD = 11, range 7–74 CNVs) (S2 Fig). Deletions were 1.6–3.2 times more frequent than duplications, corresponding to 62% of CNVs identified in controls and 76% in SLE patients; however the average deletion size was 3.2–4.0 smaller than the average duplication size both control and case groups, respectively (Fig 1A).
Fig 1. Deletion and duplication profiles in the systemic lupus erythematosus (SLE) patients and Brazilian controls.
Distribution of copy number variation (CNVs), deletions (del) and duplications (dup) according to the size ranges in SLE patients and Brazilian healthy individuals (BRZ) (A). Notched box plot comparing the distribution of call (B) and size (C) of deletions and duplications in SLE patients and controls. The boxes represent the interquartile range with black lines indicating the median of the data and the leaked circles symbolizing outliers. The notches exhibit a 95% confidence interval around the median. According to Chambers (1983), if there is no overlap between the notches, there is evidence that the differences between the groups evaluated are significant, as we observed in the call and size of duplications between cases and controls.
Case-control comparisons with logistic regression models adjusted for sex and ancestry showed that duplications were present in smaller number (p = 0.001, OR = 0.7 [95% CI, 0.6–0.9]) in SLE patients than in healthy subjects. No evidence for differences in the total number (p = 0.552, OR = 1.0 [95% CI, 0.9–1.0]) and size (p = 0.341, OR = 1.0 [95% CI, 1.0–1.0]) of deletions were observed between case and control groups (Fig 1B).
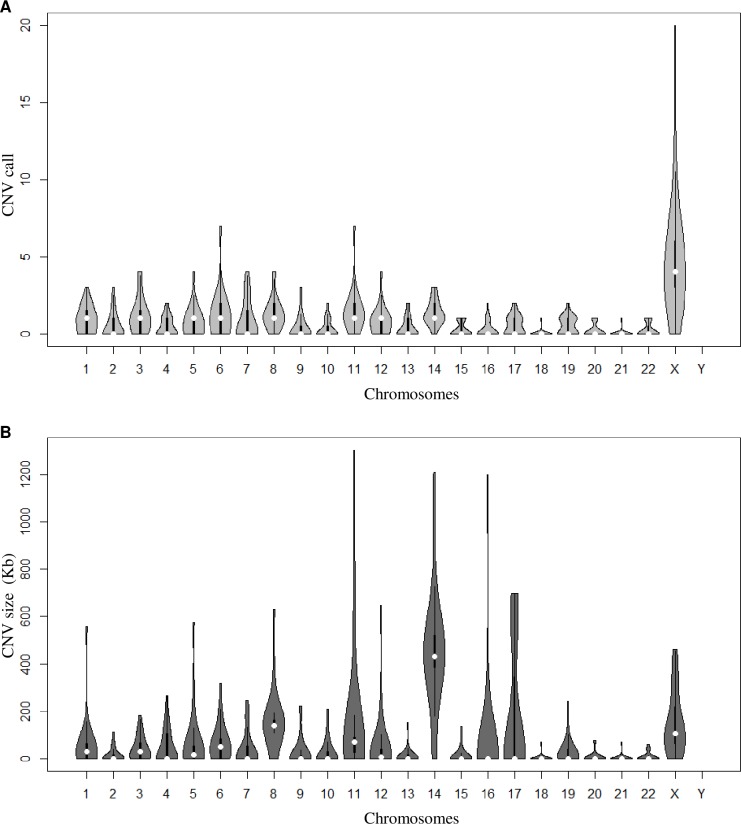
CNVs were detected in all autosomes and in the X chromosome. In both groups, the X chromosome showed the highest number of CNVs, representing an average of 10 X-linked CNVs per SLE patient (SD = 4, range 0–20 CNVs) and 6 CNVs per control (SD = 5, range 0–29 CNVs). On the other hand, the larger CNVs were concentrated on chromosome 14, and showed more CNVs (p = 0.020) of larger size (p = 0.004) in SLE patients compared to controls. Chromosome 1 also showed a higher number of CNV calls per patient compared to controls (p = 0.041) (Fig 2).
Fig 2. Chromosome distribution of copy number variation (CNV) in systemic lupus erythematosus (SLE) patients.
Violin plot (R package: vioplot v. 0.2) illustrating the distribution of the data and its probability density for call (A) and size (B) of the total number of CNVs per chromosome. The thicker black bars in the center represent the interquartile range, while the thin black line extended from it represents the 95% confidence intervals around the median (white dot).
Synergic effect of deletion in the FCGR3B and ADAM3A genes
In the CNVRs analysis performed from the Cytoscan HD array data (23 SLE and 110 controls), a deletion (CN < 2) partially encompassing the ADAM5 gene and entirely overlapping the ADAM3A gene was identified as a potential candidate for increased susceptibility to SLE (p = 0.0352; OR = 3.3 [95% CI, 1.0–14.1]) (S3 Fig and S3 Table). Based on this result, we designed a qPCR assay using the ADAM3A as a target gene in order to validate the association in a larger population of SLE patients (n = 135) and controls (n = 200). The association of the deletion in the ADAM3A gene with SLE was not replicated using qPCR in the larger sample population (p = 0.99, OR = 1.0 [95% CI, 0.6–1.8]). However, in agreement with our expectations we observed that ADAM3A duplication was statistically lower in SLE patients than in controls (p = 1.23 x 10−2, OR = 0.2 [95% IC, 0.1–0.7]) (S4 Fig), suggesting that gains in the ADAM3A gene are a protective factor for the development of SLE.
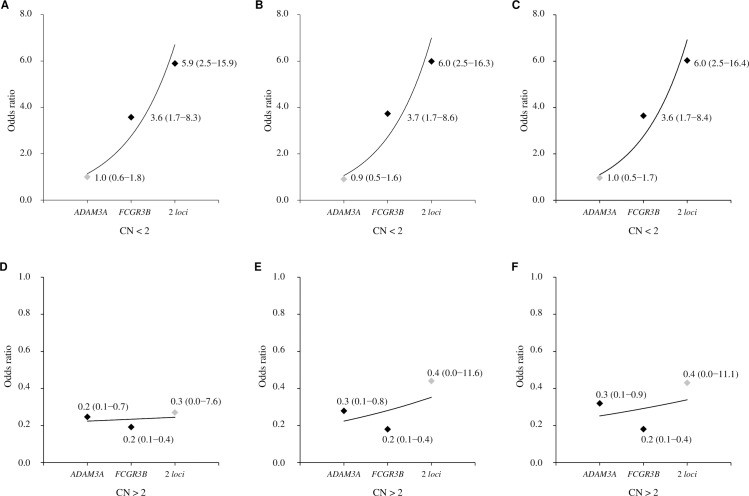
In an independent CNV assay, we showed that deletion in the FCGR3B gene is associated with increased susceptibility to SLE (p = 1.66 x 10−3, OR = 3.6 [95% CI, 1.7–8.3]), while duplication in this gene is a protective factor for the development of SLE (p = 1.55 x 10−4, OR = 0.2 [95% CI, 0.1–0.4]) (S4 Fig). Evaluating the simultaneous presence of the deletion in both FCGR3B and ADAM3A loci, we observed that their joint presence contributes to the increase of susceptibility to SLE (p = 1.4 x 10−4, OR = 5.9 [95% CI, 2.5–15.9]) when compared to a deletion only in the FCGR3B gene. This suggests a genetic interaction between these loci in SLE. The values presented above correspond to the logistic regression models adjusted for sex and African component. The adjusted models for the European and Amerindian components show similar results to the African one (Fig 3).
Fig 3. Copy number variation in ADAM3A and FCGR3B genes in systemic lupus erythematosus (SLE).
The risk of SLE in relation to the copy number (CN) < 2 (deletions at ADAM3A, FCGR3B, or 2 loci) and CN > 2 (duplications at ADAM3A, FCGR3B, or 2 loci), was estimated relative to subjects without any variation in both loci (diploid status at 2 loci) as a reference. The point estimates values represent odds ratios with 95% confidence interval according to adjusted logistic regression models for sex and African (A, D), European (B, E) and Amerindian (C, F) ancestries.
Rare CNVs and CNVs overlapping genes with functional relevance to SLE
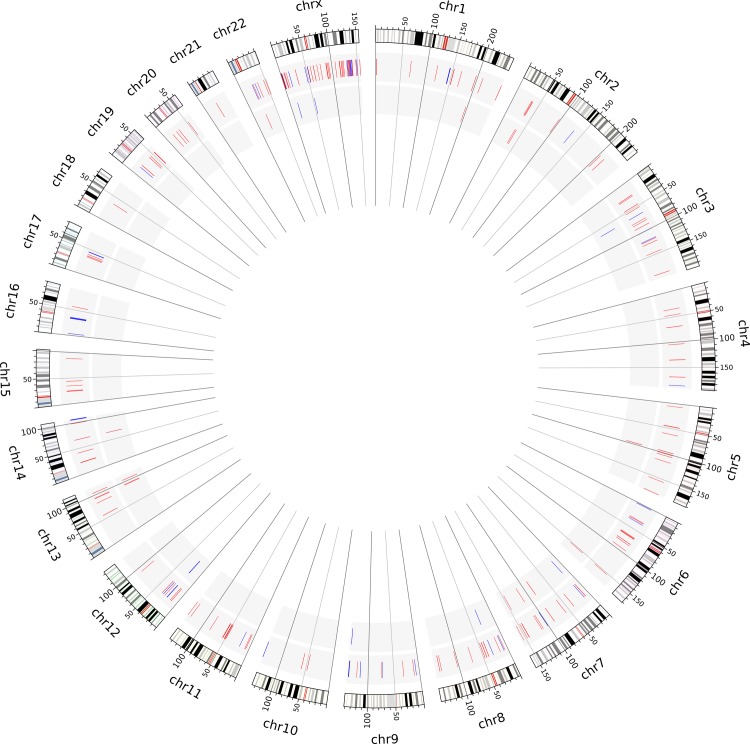
We applied the four-step pipeline to identify rare CNVs in the set of 447 CNVs detected in the 23 SLE patients. Comparing all CNVs identified in SLE patients with the 2652 CNVs reported for the control group, we identified 88/447 variants not present in the control subjects, i.e. exclusive CNVs of SLE. After filtering using population data of healthy subjects from the HapMap project and then the Database of Genomic Variants, we identified 67/88 CNVs and 49/67 CNVs as exclusive to our SLE sample. As a final step, we used an alternative algorithm (Nexus Copy Number) to detect CNVs in SLE patients. Considering only variants identified using both ChAS and Nexus Copy Number software, our four-step pipeline resulted in the detection of 21 rare CNVs that are exclusive to our SLE sample (Fig 4 and Table 1).
Fig 4. Circo plot showing the distribution of copy number variation (CNV) in the genome of systemic lupus erythematosus (SLE) patients.
The largest circos represent the 22 autosomal chromosomes and the X chromosome according to the GRCh37/hg19 version of the human genome, followed by the set of CNVs identified in SLE patients and the rare CNVs. Deletions and duplications are represented in red bars and blue bars.
Table 1. Description of rare copy number variation identified in systemic lupus erythematosus (SLE) patients from the four-step pipeline application.
| Genomic location (GRCh37/hg19) | Genes | Type | Size (Kb) | SLE ID |
Pop freq |
|---|---|---|---|---|---|
| chr1:196964969–196987806 | CFHR5 | Del | 23 | SLE005 | – |
| chr2:34219274–34313413 | – | Del | 94 | SLE009 | < 1% |
| chr2:36932842–36964864 | VIT | Del | 32 | SLE010 | – |
| chr3:65517399–65595008 | MAGI1 | Dup | 78 | SLE015 | < 1% |
| chr5:96625630–96865984 | – | Del | 240 | SLE014 | – |
| chr6:152303218–152311895 | ESR1 | Del | 9 | SLE003 | < 1% |
| chr7:11983316–12078163 | – | Dup | 95 | SLE005 | < 1% |
| chr7:85522989–85709099 | – | Del | 186 | SLE003 | < 1% |
| chr7:114209440–114215739 | FOXP2 | Del | 6 | SLE023 | – |
| chr7:146859536–146868357 | CNTNAP2 | Del | 9 | SLE003 | – |
| chr8:30079990–30202711 | MIR548O2 | Dup | 123 | SLE018 | – |
| chr9:137354612–137529641 | – | Dup | 175 | SLE023 | < 1% |
| chr12:21796951–22445614 | LDHB, KCNJ8, ABCC9, CMAS, ST8SIA1 | Dup | 649 | SLE018 | < 1% |
| chr13:93378273–93386333 | GPC5 | Del | 8 | SLE023 | – |
| chr13:94506273–94515199 | GPC6, GPC6-AS2 | Del | 9 | SLE023 | < 1% |
| chr13:104521177–104647267 | – | Del | 126 | SLE013 | – |
| chr14:67111441–67148873 | GPHN | Dup | 37 | SLE011 | – |
| chr14:67193022–67217571 | GPHN | Del | 25 | SLE016 | < 1% |
| chr22:25066486–25112325 | – | Del | 46 | SLE006 | < 1% |
| chrX:15723836–15831401 | CA5B, INE2, ZRSR2 | Dup | 108 | SLE018 | – |
| chrX:52833688–52904643 | XAGE5, XAGE3 | Dup | 71 | SLE017 | – |
chr = chromosome; Del = deletion; Dup = duplication; ID = identification; Pop freq = population frequency.
Gene list analysis was used to evaluate the presence of genes with functional relevance to SLE within the interval of CNV, and revealed CNVs overlapping 8/153 genes in SLE group (S2 Table).
Based on population frequency of CNV, deletions in CFHR4, CFHR5, STAT4 and HLA-DPB2 genes identified using Cytoscan HD array in the rare and functional relevant CNVs were selected for validation by ddPCR. In addition to these selected CNVs, a rare duplication of 649 Kb in size encompassing five genes (LDHB, KCNJ8, ABCC9, CMAS and ST8SIA1I) was also selected for target-specific validation (S3 Fig). The genotypes were confirmed for 4/5 CNVs selected for ddPCR validation. Therefore, we described heterozygous deletions (CN = 1) in CFHR4, CFHR5, HLA-DPB2 genes, and a heterozygous duplication (CN = 3) encompassing the LDHB, KCNJ8, ABCC9, CMAS and ST8SIA1I genes (S5 Fig). Heterozygous deletion in the STAT4 gene was not confirmed by ddPCR.
Discussion
Despite remarkable progress in the identification of loci-specific CNVs associated with SLE, questions regarding the role of structural variation in genetic variability and SLE susceptibility have remained [13, 15, 16, 29]. Here we present the first genome-wide CNV study of SLE patients in a tri-hybrid population. As crucial step to obtain reliable association results for admixed cohorts [20, 28], we performed adjustment for population stratification for all CNV statistical comparisons between case and control groups. We reported the synergistic effect of common multi-allelic CNVs increasing the risk for SLE compared to the variation in a single locus, further supporting the involvement of multi-loci deletions in the etiology of the disease. Our study incorporate two CNV calling algorithms and added data of other healthy subjects in external populations and databases with the aim of increasing stringency and improving the sensitivity and specificity of detecting rare CNVs throughout the genome.
The higher total number of deletions in whole-genome in relation to duplications and the smaller average size of losses compared to gains found in this study for both case and control groups, were also reported for Korean women SLE patients and their respective controls [16], as well as for healthy subjects from the Database of Genomic Variants [11]. The excess of X-linked CNVs observed here also corroborates methodologically similar reports, suggesting that there is predominance of variants in the X chromosome over the other chromosomes for both disease and control groups [18, 30]. These findings that found no evidence of differences in CNV distribution in disease and non-disease cohorts underscores the need to search for loci-specific CNVs as causal variants in the susceptibility and severity of complex diseases for CNV-phenotype associations, instead of focusing in the total burden of structural variants.
The putative association of low copy number of FCGR3B gene with an increased risk for SLE previously identified in African, Chinese and European ancestry populations [31] was replicated in our Brazilian cohort. The data corroborates the association of this gene with the disease even in an admixed population such as the one presented here. As FCGR3B is involved in the recruitment of polymorphonuclear neutrophils to sites of inflammation and clearance of immune complexes [31], losses in this gene could result in the reduction of neutrophil trafficking to inflammatory lesions and in a decrease in the ability to control immune response [32]. In addition to the 3.6-fold increase in the susceptibility of developing SLE observed in the single FCGR3B deletion, an additive effect was observed when there was in a deletion of both FCGR3B and ADAM3A genes, leading to a 5.9-fold increase risk for SLE. Similar synergistic effect of losses in three other loci encompassing the RABGAP1L, C4, and a region on chromosome 10q21 with no genes in the interval, which resulted in a 5.5-fold increase in the risk for developing SLE compared to deletions in any loci [16]. The observations are in agreement with the hypothesis that the combined effect of multiple dosage-sensitive genomic regions may lead to the predisposition to the disease.
Previous reports associated SLE with SNPs [8] and CNVs mapped in the major histocompatibility complex region, i.e., duplications in HLA-DRB5 [17] and deletions in complement 4 (C4) gene [29, 33]. Here we report for the first time identified in SLE patients three heterozygous deletions overlapping HLA (HLA-DPB2) and complement-related genes (CFHR4 and CFHR5). The deletions were identified using microarray screening followed by target-specific validation. Although the ability to attribute pathogenicity to a particular CNV remains limited, deep analysis of candidate risk loci harboring losses in the HLA-DRB2 and other HLA genes could provide one possible update for the unexplained genetic disease susceptibility. SNPs in complement factor H (CFH) genes, a key regulator of the alternative complement pathway, have also been associated with SLE [34]. Our findings suggest new insights into the pathogenic mechanisms of complement factor H in SLE involving changes in copy number in the factor H-related genes, highlighting the homeostatic balance between CFH-CFHR genes as critical to maintain regulation in complement activation [34]. Since CFHR4 gene plays a key role in regulating complement activation and opsonization on biological surfaces by interacting with C-reactive protein [35], deletion in this gene could lead to reduced protein binding and thus would limit its ability to inhibit inflammation, facilitating SLE development. The involvement of CFHR5 in renal diseases, e.g. CFHR5 nephropathy [36], has particular interest in view of the SLE patient who present the rare deletion in CFHR5 here identified has lupus nephritis, suggesting that losses in this gene may compromise renal functioning and indicating that this variation may be related to lupus nephritis development.
Conclusions
Here we show that novel susceptibility loci for SLE can be identified using large-scale evaluation of CNVs. This was the first-time identification of three heterozygous deletions encompassing the CFHR4, CFHR5 and HLA-DPB2 genes related to this disease. Additionally, a set of other rare CNVs of SLE patients were reported. We also showed the synergistic effect of deletion in both FCGR3B and ADAM3A genes increasing the risk to SLE. The detection of rare and common CNVs in functionally relevant genes may elucidate how lupus is triggered and clarify the relationship between clinical manifestations and biological pathways that underlie disease progression. The evaluation of the fine-scale architecture of CNV regions, as well as the prediction of pathogenicity of long segments encompassing several variants found in homozygosity, would contribute to understanding how risk loci harboring CNVs segments acts on the etiology of SLE.
Supporting information
(TIF)
Histogram showing the frequency of each class of CNV call in systemic lupus erythematosus (SLE) patients (A) and in Brazilian controls (B). Kernel density plot showing the non-parametric distribution of probability density curve of CNVs in SLE patients (C) and in Brazilian controls (D).
(TIF)
Genes in bold highlight those selected for further validation of the copy number status by target-specific methodology.
(TIF)
Frequency of copy number (CN), deletions (del) and duplications (dup) in ADAM3A (A, B) and FCGR3B (C, D) genes in systemic lupus erythematosus (SLE) and Brazilian control (BRZ) groups.
(TIF)
Charts showing the copy number (CN) for each SLE patient, confirming heterozygous deletions of CFHR4 in SLE002 patient (A), CFHR5 in SLE005 patient (B), HLA-DPB2 in SLE019 patient (C), and heterozygous duplication involving the LDHB, KCNJ8, ABCC9, CMAS and ST8SIA1 genes in the SLE018 patient (D). In all cases, the reference gene (FOXP2) shows invariable diploid status in the subjects analyzed. The reference sample confirmed the diploid copy number for both target and reference genes.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to thank all of the participants who donated their blood for this study.
We are grateful to Dr Íscia L Cendes (Faculty of Medical Sciences, State University of Campinas) who provided the healthy samples to Cytoscan HD array and Brazilian Biosciences National Laboratory (Campinas, SP) for technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files. Microarray data are available from BIPMed (http://bipmed.iqm.unicamp.br/lupus/).
Funding Statement
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant number 2011/23794-7; http://www.fapesp.br/), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (http://www.capes.gov.br/), Fundação de Apoio ao Ensino, Pesquisa e Assistência do HCFMRP-USP (http://www.faepa.br/), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq;http://cnpq.br/). FBB (grant numbers 2013/17062-9, 2016/10306-8) is currently supported by a research fellowship from FAPESP, while ALS (grant number 312547/2009-9), EAD (grant number 304931/2014-4) and VLGSL (grant number 304455/2012-1) are supported by CNPq/Brazil.
References
- 1.Cui Y, Sheng Y, Zhang X. Genetic susceptibility to SLE: recent progress from GWAS. Journal of autoimmunity. 2013;41:25–33. 10.1016/j.jaut.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 2.Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nature reviews Nephrology. 2015;11(6):329–41. 10.1038/nrneph.2015.33 . [DOI] [PubMed] [Google Scholar]
- 3.Margery-Muir AA, Bundell C, Nelson D, Groth DM, Wetherall JD. Gender balance in patients with systemic lupus erythematosus. Autoimmun Rev. 2017;16(3):258–68. 10.1016/j.autrev.2017.01.007 . [DOI] [PubMed] [Google Scholar]
- 4.Pons-Estel GJ, Alarcón GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Seminars in arthritis and rheumatism. 2010;39(4):257–68. 10.1016/j.semarthrit.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costenbader KH, Gay S, Alarcón-Riquelme ME, Iaccarino L, Doria A. Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmunity reviews. 2012;11(8):604–9. 10.1016/j.autrev.2011.10.022 [DOI] [PubMed] [Google Scholar]
- 6.Amur S, Parekh A, Mummaneni P. Sex differences and genomics in autoimmune diseases. Journal of autoimmunity. 2012. [DOI] [PubMed] [Google Scholar]
- 7.Alarcón-Segovia D, Alarcón Riquelme ME, Cardiel MH, Caeiro F, Massardo L, Villa AR, et al. Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis & Rheumatism. 2005;52(4):1138–47. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong D, Zidovetzki R, Alarcón-Riquelme M, Tsao B, Criswell L, Kimberly R, et al. GWAS identifies novel SLE susceptibility genes and explains the association of the HLA region. Genes and immunity. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun C, Molineros JE, Looger LL, Zhou XJ, Kim K, Okada Y, et al. High-density genotyping of immune-related loci identifies new SLE risk variants in individuals with Asian ancestry. Nat Genet. 2016;48(3):323–30. 10.1038/ng.3496 ; PubMed Central PMCID: PMC4767573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alarcón-Riquelme ME, Ziegler JT, Molineros J, Howard TD, Moreno-Estrada A, Sanchez-Rodriguez E, et al. Genome-Wide Association Study in an Amerindian Ancestry Population Reveals Novel Systemic Lupus Erythematosus Risk Loci and the Role of European Admixture. Arthritis & rheumatology (Hoboken, NJ). 2016;68(4):932–43. 10.1002/art.39504 ; PubMed Central PMCID: PMC4829354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarrei M, MacDonald JR, Merico D, Scherer SW. A copy number variation map of the human genome. Nature reviews genetics. 2015;16(3):172–83. 10.1038/nrg3871 [DOI] [PubMed] [Google Scholar]
- 12.Yih Chen J, Ling Wu Y, Yin Mok M, Jan Wu YJ, Lintner KE, Wang CM, et al. Effects of complement C4 gene copy number variations, size dichotomy, and C4A deficiency on genetic risk and clinical presentation of systemic lupus erythematosus in east asian populations. Arthritis & Rheumatology. 2016;68(6):1442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YH, Bae SC, Seo YH, Kim JH, Choi SJ, Ji JD, et al. Association between FCGR3B copy number variations and susceptibility to autoimmune diseases: a meta-analysis. Inflammation research: official journal of the European Histamine Research Society [et al. ]. 2015;64(12):983–91. 10.1007/s00011-015-0882-1 . [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Ortiz H, Velazquez-Cruz R, Espinosa-Rosales F, Jimenez-Morales S, Baca V, Orozco L. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis. 2010;69(10):1861–5. 10.1136/ard.2009.124313 . [DOI] [PubMed] [Google Scholar]
- 15.Zhou XJ, Cheng F-J, Lv JC, Luo H, Yu F, Chen M, et al. Higher DEFB4 genomic copy number in SLE and ANCA-associated small vasculitis. Rheumatology. 2012;51(6):992–5. 10.1093/rheumatology/ker419 [DOI] [PubMed] [Google Scholar]
- 16.Kim J-H, Jung S-H, Bae JS, Lee H-S, Yim S-H, Park S-Y, et al. Deletion variants of RABGAP1L, 10q21. 3, and C4 are associated with the risk of systemic lupus erythematosus in Korean women. Arthritis & rheumatism. 2013;65(4):1055–63. [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Guo S, Yang D, Ma Y, Ji H, Chen Y, et al. Copy number variations of HLA-DRB5 is associated with systemic lupus erythematosus risk in Chinese Han population. Acta biochimica et biophysica Sinica. 2014;46(2):155–60. 10.1093/abbs/gmt137 [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Traisak P, Martin KA, Kaplan MJ, Cohen PL, Denny MF. Genomic alterations in abnormal neutrophils isolated from adult patients with systemic lupus erythematosus. Arthritis research & therapy. 2014;16(4):R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Lewinger JP, Gilliland FD, Gauderman WJ, Conti DV. Confounding and heterogeneity in genetic association studies with admixed populations. American journal of epidemiology. 2013;177(4):351–60. 10.1093/aje/kws234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin LS, Eskin E. Population Structure in Genetic Studies: Confounding Factors and Mixed Models. bioRxiv. 2016:092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatology. 1997;40(9):1725–. [DOI] [PubMed] [Google Scholar]
- 22.Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids research. 1988;16(3):1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. The American journal of human genetics. 2007;81(3):559–75. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, et al. Global variation in copy number in the human genome. Nature. 2006;444(7118):444–54. 10.1038/nature05329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic acids research. 2007;35(suppl 2):W71–W4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyner C, Barber GP, Casper J, Clawson H, Diekhans M, Eisenhart C, et al. The UCSC Genome Browser database: 2017 update. Nucleic acids research. 2016;45(D1):D626–D34. 10.1093/nar/gkw1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 28.Barbosa FB, Cagnin NF, Simioni M, Farias AA, Torres FR, Molck MC, et al. Ancestry informative marker panel to estimate population stratification using genome-wide human array. Annals of human genetics. 2017;81(6):225–33. 10.1111/ahg.12208 [DOI] [PubMed] [Google Scholar]
- 29.Li N, Zhang J, Liao D, Yang L, Wang Y, Hou S. Association between C4, C4A, and C4B copy number variations and susceptibility to autoimmune diseases: a meta-analysis. Sci Rep. 2017;7:42628 10.1038/srep42628 ; PubMed Central PMCID: PMC5311832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidd JM, Cooper GM, Donahue WF, Hayden HS, Sampas N, Graves T, et al. Mapping and sequencing of structural variation from eight human genomes. Nature. 2008;453(7191):56–64. 10.1038/nature06862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan J, Zhao D, Wu L, Xu X, Pang Y, Zhang J, et al. FCGR3B copy number loss rather than gain is a risk factor for systemic lupus erythematous and lupus nephritis: a meta-analysis. International journal of rheumatic diseases. 2015. [DOI] [PubMed] [Google Scholar]
- 32.Rahbari R, Zuccherato LW, Tischler G, Chihota B, Ozturk H, Saleem S, et al. Understanding the genomic structure of copy‐number variation of the low‐affinity Fcγ receptor region allows confirmation of the association of FCGR3B deletion with rheumatoid arthritis. Human mutation. 2017;38(4):390–9. 10.1002/humu.23159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira KM, Faria AG, Liphaus BL, Jesus AA, Silva CA, Carneiro-Sampaio M, et al. Low C4, C4A and C4B gene copy numbers are stronger risk factors for juvenile-onset than for adult-onset systemic lupus erythematosus. Rheumatology. 2016;55(5):869–73. 10.1093/rheumatology/kev436 [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Wu H, Khosravi M, Cui H, Qian X, Kelly JA, et al. Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS genetics. 2011;7(5):e1002079 10.1371/journal.pgen.1002079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantsilieris S, White SJ, Richardson AJ, Guymer RH, Baird PN. Comprehensive analysis of copy number variation of genes at chromosome 1 and 10 loci associated with late age related macular degeneration. PloS one. 2012;7(4):e35255 10.1371/journal.pone.0035255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gale DP, Pickering MC. Regulating complement in the kidney: insights from CFHR5 nephropathy. Disease models & mechanisms. 2011;4(6):721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Histogram showing the frequency of each class of CNV call in systemic lupus erythematosus (SLE) patients (A) and in Brazilian controls (B). Kernel density plot showing the non-parametric distribution of probability density curve of CNVs in SLE patients (C) and in Brazilian controls (D).
(TIF)
Genes in bold highlight those selected for further validation of the copy number status by target-specific methodology.
(TIF)
Frequency of copy number (CN), deletions (del) and duplications (dup) in ADAM3A (A, B) and FCGR3B (C, D) genes in systemic lupus erythematosus (SLE) and Brazilian control (BRZ) groups.
(TIF)
Charts showing the copy number (CN) for each SLE patient, confirming heterozygous deletions of CFHR4 in SLE002 patient (A), CFHR5 in SLE005 patient (B), HLA-DPB2 in SLE019 patient (C), and heterozygous duplication involving the LDHB, KCNJ8, ABCC9, CMAS and ST8SIA1 genes in the SLE018 patient (D). In all cases, the reference gene (FOXP2) shows invariable diploid status in the subjects analyzed. The reference sample confirmed the diploid copy number for both target and reference genes.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Microarray data are available from BIPMed (http://bipmed.iqm.unicamp.br/lupus/).