Abstract
Mu desynchronization is the attenuation of EEG power in the alpha frequency range recorded over central scalp locations thought to reflect motor cortex activation. Mu desynchronization during observation of an action is believed to reflect mirroring system activation in humans. However, this notion has recently been questioned because, among other reasons, the potential contamination of mu rhythm and occipital alpha activity induced by attention processes following presentation of visual stimuli in observation conditions. This study examined the validity of mu desynchronization as a measure of mirroring system activation in infants and further investigated the pattern of functional connectivity between the central and occipital regions during execution and observation of movement. EEG was recorded while 46 9-monthold infants executed grasping actions and observed an experimenter grasping. Current source density (CSD) was applied to EEG data and, time-frequency and connectivity analyses were performed in CSD transformed data. Mu desynchronization was evident over central regions during both execution and observation of movements. Independent alpha desynchronization over occipital region was also present in both conditions. The connectivity analyses revealed that central-occipital areas were functionally more connected compared to other areas of the brain during observation of movements. Collectively, the results demonstrate the validity of mu desynchronization as an index of infant mirroring system activity and support the proposal of a functional connection between distinct mirroring and attention processes during observation of action.
Keywords: mu rhythm, occipital alpha, motor cortex, mirroring system, functional connectivity
1. Introduction
Modulation of the mu rhythm, as measured via electroencephalogram (EEG), is widely used as a marker of mirroring system activity in humans, however concerns over its validity as such have recently been raised. Specifically, the contamination of mu rhythm and visual alpha has been a major concern in mu rhythm investigation (Hobson and Bishop, 2016). This issue may be particularly problematic in studies utilizing the mu rhythm as the measure of mirroring activity in developmental populations, as children and infants are likely to exhibit greater attention to others’ actions as they learn about objects, actions, and the goals and intentions driving actions (Bowman et al., 2017). Since mu desynchronization is extensively used as an index of mirroring activity in developmental populations, the issue of its validity as a measure becomes particularly important. The goal of the present study was to investigate the validity of mu desynchronization as the measure of mirroring activity and examine the proposal that the mu rhythm over central cortical regions is distinct from yet functionally connected to alpha activity over the occipital region during observation of actions in infancy.
Mirror neurons are a class of neurons that fire when an action is performed and also when an action is passively observed (di Pellegrino et al., 1992; Gallese et al., 1996). These neurons were first discovered in the inferior premotor cortex (area F5) of the macaque monkey (di Pellegrino et al., 1992; Gallese et al., 1996) and posited to form a system for matching the execution and observation of actions in the macaque’s brain, which was suggested to be the basis of understanding actions (Gallese et al., 1996). Following the early findings in macaques, several studies have reported evidence of neural mirroring activity and an observation–execution matching system in humans (Hari et al., 1998; Iacoboni et al., 1999; Lepage and Théoret, 2006). In humans, the mirroring system is thought to play a role in understanding others’ actions (Rizzolatti et al., 2001) and in a range of social cognitive processes, such as action anticipation (Csibra and Gergely, 2007; Kilner et al., 2007), understanding intention (Rizzolatti and FabbriDestro, 2008; Rizzolatti and Sinigaglia, 2010), imitation (Iacoboni, 2009b; Iacoboni et al., 1999), empathy (Carr et al., 2003; Gallese, 2001; Iacoboni, 2009a), and language (Rizzolatti and Arbib, 1998; Théoret and Pascual-Leone, 2002).
In humans, mu rhythm desynchronization, measured via EEG, is a widely used measure of motor cortex activation (Pfurtscheller and Neuper, 1994; Pfurtscheller et al., 2000), and has more recently been identified as a possible measure of the mirroring system (Fox et al., 2016). Mu rhythm is an EEG oscillation in the alpha frequency range (i.e., ~8–13Hz in adults and ~6–9Hz in infants and young children; Marshall et al., 2002) recorded over central scalp areas. The mu rhythm is generated most prominently in resting state and is attenuated or reduced immediately prior to or during motor events, which is often called mu desynchronization or mu suppression (Frenkel-Toledo et al., 2013; Llanos et al., 2013; Muthukumaraswamy et al., 2004). This pattern of mu desynchronization is regarded as a reliable indicator and electrophysiological correlate of motor cortex activation in planning and execution of movement (Llanos et al., 2013; Pfurtscheller and Aranibar, 1979), and more recently the mu rhythm has also been shown to be desynchronized during observation of movements performed by another individual, suggesting that this pattern of activity may reflect activation of the mirroring system in humans (Muthukumaraswamy et al., 2004; Oberman et al., 2005; Oberman et al., 2007). To address the question as to whether mu desynchronization during action observation is consistently present and similar to mu desynchronization during action execution and thus an appropriate measure of the mirroring system, Fox et al. (2016) conducted a meta-analysis of 85 studies in which mu was used to infer mirroring system activity. Their results were consistent with the interpretation that mu rhythm desynchronization is a valid means for measuring mirroring system activity.
Recently, serious concerns have been raised regarding the validity of the mu rhythm as a measure of the mirroring system (Hobson and Bishop, 2016, 2017). Hobson and Bishop (2016, 2017) argued that mu desynchronization measured in mirror neuron studies is contaminated with visual alpha desynchronization, which is associated with visual attention and occurs in response to the presentation of a visual stimulus. Alpha contamination, as claimed by Hobson and Bishop (2016, 2017), makes mu desynchronization an invalid index for examining mirroring system activity, as it may simply be capturing changes in visual attention during observation of others’ actions. Neural activity can spread across brain regions via volume conduction, which can make it difficult to establish a specific functional relation between mu rhythm desynchronization and mirroring system activity. However, it is also likely that both attention, induced by presentation of visual stimulus and mirroring processes take place simultaneously during the observation of an action (Bowman et al., 2017). Therefore, concurrent central mu and occipital alpha activation would be evident during observation of action.
Pineda (2005) proposed that the EEG signal consists of multiple independent rhythmic oscillations. Sensory inputs may cause them to become coupled and to act together in a coherent fashion. When the independent sources of alpha in different brain regions are involved in coherent function, a global surge in alpha activity may emerge. Indeed, Pineda (2005) suggested that a common underlying frequency band for both motor (mu) and sensory (alpha) cortices can act together and allow for communication across cortical regions. Research into other neural processes have found evidence for the functional coupling of independent alpha networks (e.g., during sleep: Cantenaro et al., 2000), but whether such coupling exists between occipital alpha and central mu during action observation has yet to be explored. Mirroring and attention processes may be mediated by a common functional network and a shared oscillatory frequency (mu/alpha). In fact, a large number of studies provide EEG evidence for coactivation of occipital and central regions during execution and observation of actions (Bowman et al., 2016; Cannon et al., 2016; Crone et al., 1998; Yoo et al., 2016), however few have directly explored the functional importance of this coactivation. Evidence for simultaneous, but distinct and correlated activity representing a coupling of occipital alpha and central mu would provide support for use of the mu rhythm desynchronization as a reliable index of mirroring activity, and could provide further insight into the broader network of neural processes that support mirroring activity in humans.
The possibility of simultaneous attention/mirror system activity is especially relevant in developmental studies in which participants might be particularly attentive to the actions that they are learning (Bowman et al., 2017). Over the past decade there has been a substantial increase in developmental research utilizing mu rhythm desynchronization in infants (Cuevas et al., 2014; de Klerk et al., 2015; Nyström et al., 2011; Rayson et al., 2017; St. John et al., 2016), and researchers have made broad inferences about the mirroring system in infants based on this measure. For example, the mirroring system has been proposed to reflect infant’s ability to map similarities between self and other, and thus forming the foundation for imitation and socialcognitive development (Marshall and Meltzoff, 2014). Indeed, researchers have found relations between mu rhythm desynchronization during action observation and development of infant’s motor skill (Cannon et al., 2016; Yoo et al., 2016) and social behavior (Filippi et al., 2016). Cannon and colleagues (2016) assessed infants’ reaching-grasping competence by measuring reach latency, errors, preshaping of the hand and bimanual reaches. They found that mu desynchronization during observation of grasps was associated with infants’ own grasping and reaching competence. Filippi and colleagues (2016) found that the strength of mu rhythm desynchronzation in 7-month-old infants during observation of an experimenter grasping was related to the infants’ tendency to imitate the goal of the experimenter’s action, reaching for and grasping the same object as the experimenter. However, there is a dearth of studies exploring the possibility of simultaneous attention and mirror system activity within developmental populations. During the second half of the first year infants exhibit rapid changes in their perception of object functions, as indexed by changing patterns of visual attention to relations between objects and actions (Baumgartner & Oakes, 2011; Perone & Oakes, 2006). During this same time period, infants exhibit an emerging ability to understand that actions are intentional, goal-driven, and ‘about’ specific objects (Woodward, 2003; Woodward & Guajardo, 2002). Thus, the issue of potential contamination of activity between the attention and mirroring systems during the observation of actions is particularly problematic in infant research.
To address the concern regarding contamination of mu rhythm and alpha activity, the application of surface Laplacian can be useful to minimize the effect of volume conduction (Kayser and Tenke, 2015), thus improving both spatial and functional specificity of mu and alpha activity during action execution and observation. Spatial filters, such as surface Laplacian, have been shown to minimize volume conduction by filtering out spatially broad features of the data (Cohen, 2014; Tenke and Kayser, 2012). Surface Laplacian transforms the EEG signal into a reference free current source density (CSD) waveform (Kayser and Tenke, 2006; Tenke and Kayser, 2012). CSD transformation highlights high-spatial-frequency activity – activity that is evident only at a small cluster of electrodes, whereas low-spatial-frequency activity – activity that is evident at most or all electrodes – is attenuated (Cohen, 2014). Moreover, a measure of phase-connectivity, which indicates a mechanism for functionally coupled interregional activity (Cavanagh et al., 2009), can provide key insight regarding potential functional connectivity between mirroring and attention processes.
CSD transformed data improves topographic localization, minimizes volume-conduction effects, which makes CSD transformed data suitable for connectivity analysis (Cohen, 2014; Winter et al., 2007 ). Surface Laplacian transformation methods have been used in some adult studies (Muthukumaraswamy and Johnson, 2004) to investigate neural mechanism of execution and observation of action, showing concentrated activity over the central cortical regions. However, no studies have yet looked at functional connectivity between occipital alpha and central mu rhythm activity. Further, no studies have yet applied either of these methods in developmental research. Doing so would provide insight into the two major issues in question – whether central mu rhythm and occipital alpha are in fact distinct sources of neural activity, and if so, whether they are functionally connected – at a point when there is still variability in attention to and understanding of others’ simple actions (i.e., grasping: Falck-Ytter et al., 2006; Sommerville and Woodward, 2005) and the efficiency of the mirroring system is still in development (Yoo et al., 2016). Thus, in the present study, we applied surface Laplacian and connectivity measures to investigate the characteristics of mu/alpha (6–9Hz) frequency range oscillations in 9-month old infants during execution and observation of grasping movements.
In the current study, we provide the first estimate, to our knowledge, of infant mu rhythm desynchronization using CSD. Additionally, we provide the first investigation of inter-relations between visual and motor cortex activity during infant observation of actions through a timefrequency approach and phase-connectivity analyses. We hypothesized that when infants observed others’ actions, distinct mu and alpha activity would be observed over central and occipital regions, respectively. Moreover, we predicted increased functional connectivity between the central and occipital regions as compared to the connectivity between other brain regions during action observation. Overall the present study examined the validity of mu rhythm as a measure of mirroring system activity and provides improved temporospatial estimates of this system in infancy.
2. Methods
2.1. Participants
Forty-six full-term 9-month old infants (28 females, Mage = 9.07, SD = 0.43) were recruited for the study. Nine participants were excluded from final sample because they had an insufficient number of trials (<3) in both conditions. Therefore, thirty-seven subjects were included for EEG preprocessing. We chose to focus on 9-month-olds for this study because previous research has already found evidence of mu rhythm desynchronization during observation of manual grasping actions at this age (e.g., Cannon et al., 2015; Southgate et al., 2009). All infants were typically developing with no known or suspected neurodevelopmental or medical diagnoses. Prior to an infant’s participation in the study, informed consent was obtained from the infant’s parents. The experiment was approved by the University of Maryland Institutional Review Board.
2.2. Procedure
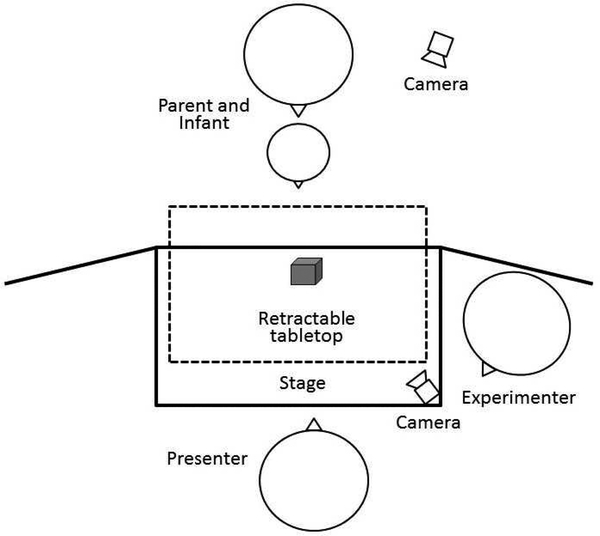
Each infant was fitted with an EEG net and seated on his or her parent’s lap in front of a black puppet stage (99 cm wide × 61 cm deep × 84 cm high) placed on a tabletop covered with a black cloth. Areas surrounding the puppet stage were covered with black panel curtains to hide two experimenters (both females) and the equipment from the infant’s view. Each testing session was video recorded with two cameras, which focused on the infant and the Presenter separately (See Figure 1 for room set up). Parents were instructed to be passive observers during the task and to not exhibit any behavior, such as pointing at the toy or at the Presenter or talking, which might shift the infant’s attention.
Figure 1.
Diagram of experimental room set-up.
The task consisted of a grasp observation and a grasp execution condition, each preceded by a 3second baseline period. Thus, each trial involved baseline, observation, baseline and execution. A taupe curtain was raised and lowered at the start and end of each of the three events – baseline, observation and execution. For the baseline period, the curtain was raised to reveal the Presenter sitting across the table with her head down rotating a flashcard with a black geometric shape on a white background attached to a wooden handle. The baseline period lasted for 3 seconds, after which the curtain was lowered. At the start of the observation condition, the curtain was raised revealing the Presenter sitting across the table with a toy placed on the table in between the Presenter and the infant, but out of reach of the infant. The Presenter first made eye contact with the infant while saying ‘Hi baby!’ to capture the infant’s attention then shifted her gaze to the toy, grasped the toy with her right hand, picked the toy up off of the table bringing it to chest height and gave the toy a brief shake, all the while looking at the toy. The curtain was then lowered to end the observation condition, which lasted approximately 4 seconds.
During the execution condition, the curtain was raised to reveal the Presenter, with her head down to avoid eye contact with the infant, and a toy on the table closer to the infant. Without looking at or saying anything to the infant, the Presenter pushed the tabletop towards the infant. The event ended once the infant grasped the toy, or after approximately 60 seconds had passed if the infant did not grasp the toy, and the Presenter retracted the tabletop and the curtain was lowered.
Infants completed up to 12 possible trials (range 5–12) in each condition. The order of execution and observation presentation was pseudo-randomized. Within a trial set the order in which the execution or observation phase was presented was randomized. Six unique toys were used, with the same toy used in a trial for both execution and observation. The random order within trial sets was fixed across participants, however the order in which toys were presented was randomized across infants. The same set of six toys was reused for the second half of the task.
2.3. Behavioral coding of EEG task
Videos of the EEG task were coded to identify and synchronize live events with the continuous EEG recording. Two independent coders viewed each video off-line, frame-by-frame, to identify the following events of interest. For grasp observation events, coders identified the frame in which the Presenter completed the grasp, defined as the frame in which the Presenter’s fingers were fully closed around the toy. Coders also identified the frame in which the Presenter started the grasping action, defined as the frame in which the Presenter’s arm moved away from her body starting to reach for the toy. For grasp execution trials, coders identified the frame in which the infant completed the grasp (if a grasp was executed). This was defined as either a) the frame prior to that in which the infant lifted the toy from the table, if the infant picked the toy up, or b) the frame in which the infant’s fingers were fully closed around the toy, if there is no pick up. Coders were within 100ms (approximately 3 frames) of each other on 97% of the trials for identifying when the Presenter started moving, 97% for identifying when the Presenter completed the grasp, and 87% for identifying when the infant completed the grasp. Baseline and observation trials in which the infant or caregiver appeared to make a reach, gesture, grasping motion, gross motor movement or leg movement were coded and excluded from analysis. In addition, execution trials in which infants were not reaching/grasping were also excluded.
2.4. EEG data acquisition and processing
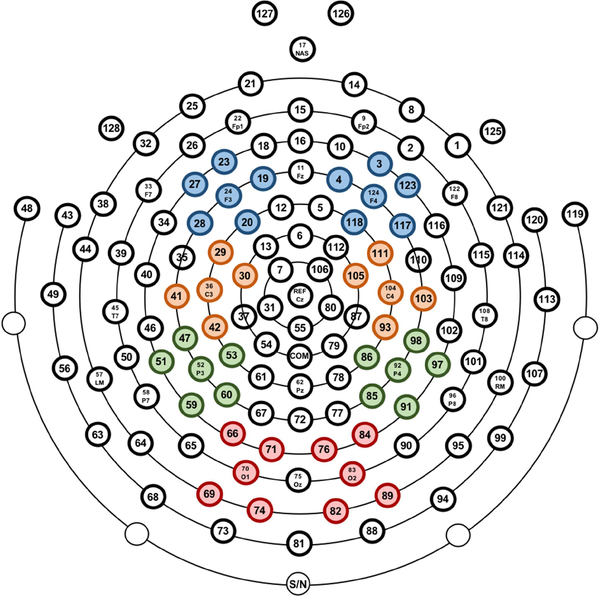
EEG was recorded throughout the task using a 128-channel HydroCel Geodesic Sensor Net (Electrical Geodesics, Inc., Eugene, OR). The vertex (Cz) electrode was used as online reference. EEG data were sampled at 500Hz using EGI’s Net Station (v4.5.4) software. Impedances were kept below 100 kΩ. After recording, EEG data were exported to a Matlab (Mathworks, Natick, MA) compatible format using Net Station software for offline processing with EEGLAB (v13.4.4b) toolbox (Delorme and Makeig, 2004) and Matlab scripts (Matlab2016b) developed by the first and fourth authors. EEG channels on the boundary of the electrode net were excluded from analyses since they were heavily susceptible to eye, face and head movements (17, 38, 43, 44, 48, 49, 113, 114, 119, 120, 121, 125, 126, 127, 128, 56, 63, 68, 73, 81, 88, 94, 99, 107; see figure 2). This step removed 24 channels, leaving 104 channels included for further analysis. Continuous data were high pass filtered at 0.3Hz and then low pass filtered at 49Hz using windowed sinc FIR filters with a Hamming window with FIRfilt plugin of EEGLAB (developed by A. Widmann: www.unileipzig.de/~biocog/content/widmann/eeglab-plugins/). Artifact-laden channels were identified and removed using the EEGLAB plug-in FASTER (Nolan et al., 2010). To classify a channel as artifactual, FASTER calculates three parameters - variance, mean correlation and Hurst exponent - for each channel. A channel whose data had a Z-score of ±3 for a parameter was deemed to be artifacted. In this process, on average 5% of the channels were discarded (M = 5.04, SD = 1.78, range = 1–11). To further remove ocular artifacts and generic noise, extended infomax independent component analysis (ICA) was performed on an identical copy of the dataset. Before ICA, this copied dataset was high pass filtered at 1Hz and segmented into 1s epochs. To achieve an improved ICA decomposition, noisy segments of the data were rejected using a combined voltage threshold of ±1000μV and spectral threshold (range −30dB to +100dB) within the 24–40Hz frequency band to remove EMG-like activity. If this artifact rejection process identified more than 20% of the epochs for a given channel as containing artifact, that channel was removed from both the ICA copied dataset and the original dataset. After ICA decomposition, independent components (ICs) were then transferred from the ICA copied dataset to the original dataset. All further analyses were performed on this original dataset.
Figure 2.
Electrode clusters, from top to bottom, for Frontal, Central, Parietal, and Occipital scalp locations.
Artifactual ICs were removed from the original dataset by a semiautomatic process that included using the ADJUST plugin (Mognon et al., 2011) of EEGLAB and also visual inspection of individual ICs. The time-frequency data were then epoched from −1.5s to 1.5s relative to the three event markers: execution grasp complete, observation movement onset and observation grasp complete. An event marker was added at the midpoint of the 3-second baseline periods for both pre-observation baseline and pre-execution baseline, and −1.5 to 1.5s epochs were created around those markers, as well. The periods in which the curtain was raised and lowered at the start and end of each trial were not included in any of the segments of interest. After segmenting data into 3-second epoch, all the epochs containing unwanted movements, as described above, were excluded. To further exclude eye movement artifacts, a voltage threshold rejection (±150μV) was applied in the six frontal channels (1, 8, 14, 21, 25, 34; see figure 2). If an epoch in these six channels exceeded the voltage threshold of ±150μV, that epoch was rejected. For all other channels, artifacted channels in each epoch were interpolated by artifact free data of the surrounding channels within that epoch. If more than 10% of channels within an epoch was interpolated, that epoch was rejected. After artifact rejection, missing channels were interpolated using spherical interpolation as implemented in EEGLAB.
After preprocessing, 8 participants were excluded in the execution condition and 15 participants were excluded in the observation condition from further time-frequency and connectivity analysis because they had an insufficient number of artifact free trials (< 3 in either observation or execution condition). This minimum trial requirement was based on that used in similar infant mu rhythm studies (Marshall et al., 2011; Monroy et al., 2017). On average, there were 5 artifact free trials (total 145, range 3–11) in the execution condition and 5.59 artifact free trials (total 123, range 3–11) in the observation condition per participant after preprocessing. All epoched data were then converted into current source density (CSD) using the CSD toolbox (Kayser and Tenke, 2006). All further analyses were performed on the CSD transformed data.
2.5. Time-frequency analysis
Time-frequency decomposition was computed using the EEGLAB newtimef function. Event related spectral perturbation (ERSP) was calculated for the epoched data. ERSP provides a twodimensional (latency by frequency) estimate of average changes in spectral power (in dB) relative to baseline (Makeig et al., 2004; Delorme and Makeig, 2004). To compute ERSP, each CSD converted epoch was convolved with Mortel wavelets, which estimated spectral power in the frequency range 5–40Hz (in 120 linearly spaced steps). To optimize the time-frequency resolution, wavelet cycles were set at 3 cycles at the lowest frequency (5Hz) increasing to 12 cycles at the highest frequency (40Hz). ERSPs were computed for all channels and separately for the three events of interests: execution grasp complete, observation movement onset and observation grasp complete. ERSPs were calculated for each epoch relative to the first 1-second of the preceding 3-second baseline period. For epochs time locked to observation movement onset and observation grasp complete, a common 1-second baseline was used because within trials the same baseline period preceded both events. ERSPs were then averaged across the clusters of electrodes overlying the frontal, central, parietal and occipital scalp sites according to the 10/20 system (F3: 19, 20, 23, 24, 27, 28; F4: 3, 4, 117, 118, 123, 124; C3: 29, 30, 36, 41, 42; C4: 93, 103, 104, 105, 111; P3: 47, 51, 52, 53, 59, 60; P4: 85, 86, 91, 92, 97, 98; O1: 66, 69, 70, 71, 74; O2: 76, 82, 83, 84, 89; see figure 2). The primary ERSP of interest was mu (6–9Hz) rhythm activity on electrodes overlying the motor cortex (C3, C4). However, ERSPs in the same frequency band as mu rhythm were analyzed for all electrode clusters.
2.6. Statistically significant time-frequency intervals
To visualize the significant time-frequency activation, we performed point-wise analysis of spectral power modulation in all channels for each event of interest. The point-wise analysis tests the significant modulation of activation against a null hypothesis of no change in power (represented by zero) during the event of interest. We computed one sample non-parametric permutation tests against zero for each time point using the “std_stat” function of the EEGLAB toolbox. We conducted 2000 permutations with false discovery rate (FDR) correction. This method allowed visualization of the statistically significant time-frequency intervals.
2.7. Connectivity analysis
Connectivity was measured by calculating interchannel phase coherence (ICPC), which estimates the consistency of phase angle difference between two channels (or clusters of channels) over time-frequency (Cohen, 2014). ICPC is calculated as follows:
where n is the number of trials for each time and each frequency band, ɸx and ɸy are the phase angles of electrodes x and y at frequency f and time t, ei is from Euler’s formula and provides complex polar representation of phase angle difference. Thus, phase angles are calculated from two electrodes and then subtracted. An ICPC value of close to 1 indicates that the phase angles from two channels are completely synchronized whereas an ICPC value close to 0 indicates random phase angle difference between two channels (Cavanagh et al., 2009).
In this study, we calculated ICPC to measure connectivity between channel clusters overlying central and frontal, parietal, and occipital regions in both the left (C3-F3, C3-P3, C3-O1) and right (C4-F4, C4-P4, C4-O2) hemisphere. We also measured connectivity between occipital and the three other brain regions in both hemispheres (left: O1-F3, O1-C3, O1-P3; right: O2-F4, O2C4, O2-P4). ICPC was calculated for each time point of a trial and then averaged across trials using the above equation at 6–9Hz frequency band around the time windows of interest in both execution and observation conditions. For convenience, we use the term ‘central-ICPC’ for ICPC between central and other brain regions and ‘occipital-ICPC’ for ICPC between occipital and other regions.
3. Results
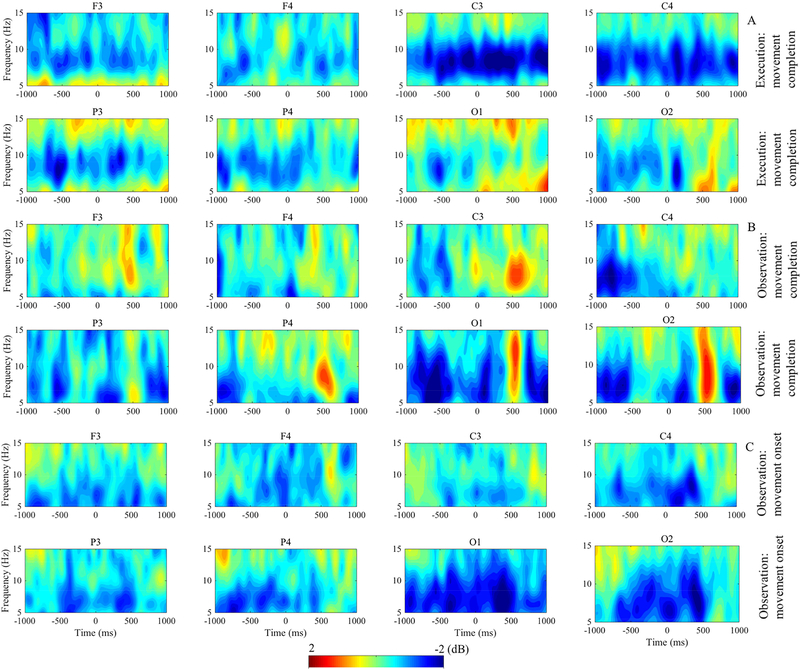
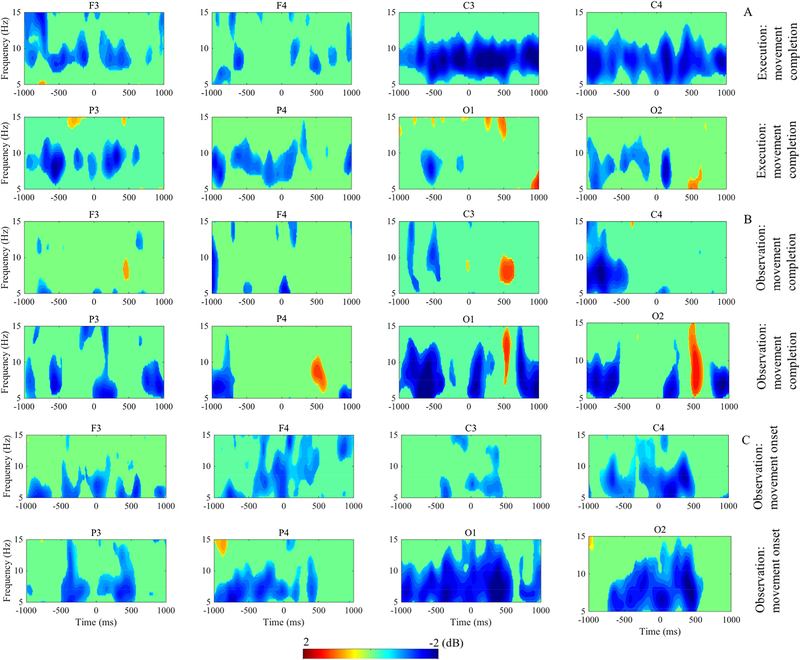
Figure 3 displays results of the time-frequency analysis and figure 4 shows the statistically significant time-frequency intervals as revealed by point-wise analysis. In figure 4, all activity shown is significantly different from zero at the p < .05 level with FDR correction for multiple comparison. Both execution and observation of the movement evoked mu/alpha desynchronization in all four of the examined electrode clusters, however there were temporal and topographic differences across the conditions. The point-wise analysis revealed that in the execution condition, there was significant mu rhythm desynchronization in the electrode clusters overlying the motor cortex (C3, C4) both prior to grasp completion (during the action) and in the post-movement time period (figure 4A, C3 and C4). Similarly, we saw significant mu rhythm desynchronization during the observation of movement (figure 4B, C3 and C4). In both conditions, the mu desynchronization was followed by a significant rebound in activity, which was most prominent in occipital regions around 500ms post-grasp completion (figure 3A, 3B, O1 and O2). Analysis of activity during the observation condition surrounding the onset of the observed movement indicated a period of significant mu rhythm desynchronization prior to the start of the Presenter’s arm movement and continuing through the movement period (figure 4C, C3 and C4). However, desynchronization appeared to be greatest over the occipital region during the observation condition (figure 4C, O1 and O2). The point-wise analysis showed that the modulation of spectral power was significant predominantly in the mu/alpha frequency band (6–9 Hz) across electrodes in all three time-frequency events of interest.
Figure 3.
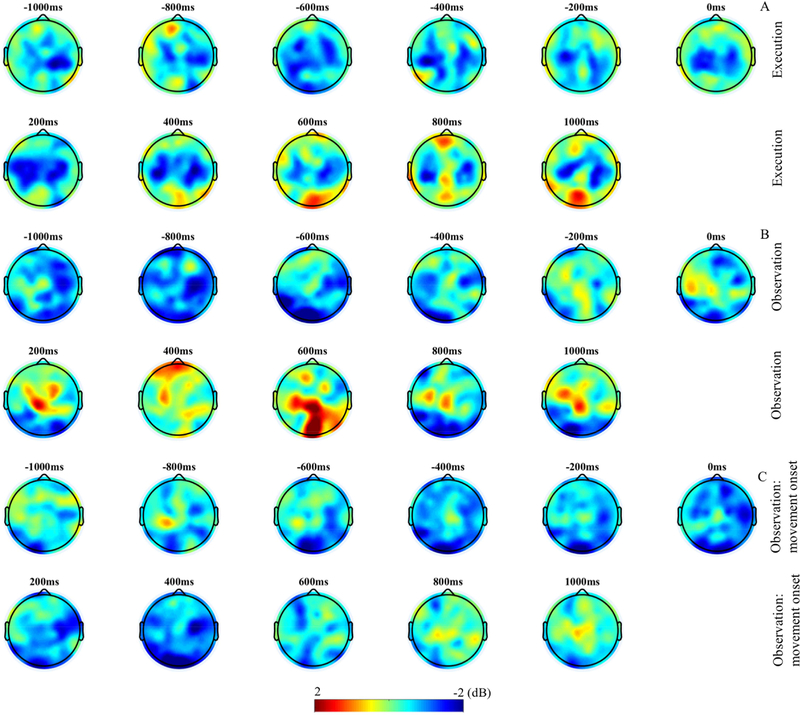
Grand average ERSP. (A-C) The ERSP images show the time-frequency results during the execution and observation of movement for electrode clusters overlying four cortical locations. The top two panels (A) display activity for the execution of movement condition time locked to movement completion. The middle two panels (B) show activity for the observation of movement condition time locked to movement completion. (A-B) Time 0 indicates completion of movement; −1000 to 0 is the time period of performed or observed movement and 0 to 1000 is the post-movement time period. The bottom two panels (C) display time-frequency results for the observation condition time locked to movement onset. (C) Time 0 indicates onset of observed movement; −1000 to 0 is the time window of pre-movement and 0 to 1000 is the movement time window. Power (decibels, dB) of ERSP activity is shown by the color bar.
Figure 4.
Time-frequency interval with masked non-significant activation. Execution, observation and observation time locked to movement onset are presented in top (A), middle (B) and bottom (C) panels, respectively. The blue area in the images indicates significant decrease and red area indicates significant increase of ERSP power. Green in images depicts nonsignificant (p > 0.05) ERSP activity. Power (decibels, dB) of ERSP activity is shown by the color bar.
We performed further statistical analysis in the 6–9Hz frequency band. Mean power at 6–9Hz frequency band was analyzed at 200ms time windows ranging from −1000ms to 1000ms (which created 10 time windows). To compare the mu/alpha rhythm activation across conditions, we performed a Condition (observation, execution) x Hemisphere (right, left) x Region (frontal, central, parietal, occipital) x Time (10 time windows) repeated-measures analysis of variance (ANOVA). We included only those subjects (N = 19) who had data in both conditions into the ANOVA. Throughout the statistical analysis Greenhouse-Geisser corrected degrees of freedom and p values were used for violations of sphericity. Bonferroni correction was applied for follow up pairwise comparisons and all post hoc analyses. There was no main effect of condition suggesting that mu/alpha activation was not significantly different between the execution and observation conditions (F(1, 18) = .393, p = .538). Likewise, there was no significant main effect of Hemisphere (F(51, 18) =.006, p = .941) and Region (F(2.040, 36.723) = 1.358, p = .270). However, there was a significant main effect of Time (F(4.648, 83.662) = 5.449, p < .001). Follow up analysis showed that across conditions, mu/alpha (6–9Hz) activation was desynchronized in all 200ms time windows except 400–600ms post-movement, which showed synchronization of power (M = .005, SE = .156). Pair wise comparison between time windows that showed desynchronization did not reveal any time window with significantly higher activation. The ANOVA revealed a significant Condition x Region (F(3, 54) = 9.523, p < .001) and Condition x Region x Time (F(27, 486) = 3.093, p < .001) interaction effect. Further analyses were performed within each condition to explore these interactions. For within group analysis, we included all the subjects within that condition (execution: N= 29; observation: N=22).
3.1. Execution condition
To assess mu/alpha activation in the execution condition, a Hemisphere (right, left) x Region (frontal, central, parietal, occipital) x Time (10 time windows) repeated-measures ANOVA was performed. The result showed a significant main effect of region (F(2.74, 63.685) = 7. 451, p = .001) and an interaction effect of region x time (F(11.981, 431.317) = 6.495, p < .001). Follow up pairwise comparisons after Bonferroni correction showed that mu/alpha (6–9Hz) rhythm was significantly more desynchronized over the central (M = −1.301, SE = .215) compared to frontal (M = −.578, SE = .233, p = .004), parietal (M = −.653, SE = .161, p = .001) and occipital regions (M = −.291, SE = .166, p = .004). There were no differences in activation between the left (M = −.677, SE = .158) and right (M = −.734, SE = .169) hemispheres and there was no Hemisphere x Region interaction effect. These results are in line with previous findings that execution of a movement significantly increases bilateral motor cortex activity and that this motor cortex activation is greater than that over all other regions.
3.2. Observation condition
Activity time-locked to grasp completion.
We first examined activity during the observation condition surrounding the completion of the grasp and performed the same repeated-measures ANOVA as described above for the execution condition: Hemisphere (right, left) x Region (frontal, central, parietal, occipital) x Time (10 time windows). Unlike the execution condition, there was no main effect of Region (F(3,63) = 2.740, p = .051). There was a significant Region x Hemisphere interaction effect (F(2.601,54.630) = 3.353, p = .031); however, follow up paired sample t-tests for each region between two hemispheres did not show any differences. Results of the ANOVA also showed a significant main effect of Time (F(4.556, 95.667) = 10.073, p < .001) and an interaction effect of Region x Time (F(27, 567) = 3.189 p < .001). Follow up pairwise comparisons for the main effect of time revealed that during the movement time period (−1000 to 0ms) activation was maximum in the −800 to −600ms time window (M = −1.032, SE = .229); which differed significantly from the activation in post-movement time interval 400–600ms (M = .514, SE = .260, p < .001) that showed synchronization of power. The post-movement synchronization of power around 400–600ms was present across all four regions and both hemispheres. Follow up paired sample t-tests for the interaction effect of Region x Time after Bonferroni correction revealed that during the movement time window (−1000 to 0ms) activation between central and occipital regions did not differ in any of the 200ms time interval. However, alpha desynchronization over the occipital area was significantly higher than mu desynchronization towards the end of post-movement time window 8000–10000ms (t(1,21) = 3.817, p = .001). As in the execution condition, activity between left (M = −.479, SE = .229) and right (M = −.506, SE = .234) hemispheres did not differ in the observation condition.
Activity time-locked to movement onset.
Southgate et al. (2009) found that observing a grasping action resulted in mu desynchronization that began prior to onset of the action. To examine the activity prior the onset of the observed movement, we analyzed the activity surrounding the onset of the Presenter’s movement (figures 3C and 4C). The activity within two time periods time-locked to the onset of the observed grasp: pre-movement (−1000 to 0ms) and movement (0 to 1000ms), was analyzed at 200ms time intervals with a Hemisphere (right, left) x Region (frontal, central, parietal, occipital) x Time (10 time windows) repeated measure
ANOVA. The results revealed only a main effect of time (F(4.522, 122.097) = 4.766, p = .001). Follow up pair-wise comparisons showed that mu/alpha activation was desynchronized in all pre-movement and movement time intervals. In the pre-movement time period, the activation was maximum in the −400ms to −200ms time window (M = −1.053, SE = .206), but did not differ significantly from other pre-movement time intervals. In the movement period, the 200–400ms time window showed maximum activation (M = −1.090, SE = .206) and was significantly stronger than activation in 800–100ms time interval (M = −.382, SE = .227, p = .037). The results did not show any other main or interaction effects. These results suggest that observation of a movement evokes activity over the motor cortex, which is a signature of the mirroring system activation in human, in addition to activity over the occipital region. The cortical activation prior to an observed movement suggests that the infants were able to anticipate the forthcoming movement.
3.3. Topographic distribution of ERSP
Figure 5 displays the topographic distribution of activation during execution and observation of movements. In the execution condition (figure 5A), ERSP shows a bilateral distribution over the central-parietal area, but with overall larger amplitude in the left hemisphere. Similar bilateral activation can also be seen in the observation condition (figure 5B, 5C); however, contrasting to the execution condition, the right hemisphere shows higher activity than the left hemisphere. As hypothesized in addition to mu activation, alpha activation in the occipital region can also be seen during the observation of movement. Additional increased power can be seen in the occipital region in the post-movement time window in both conditions. In the observation condition, the topographic maps further show that prior to the movement onset cortical activity is relatively diffuse. However, over the course of movement observation (around −200ms of movement onset; figure 5C) the activity becomes more focal.
Figure 5.
(A-C) Topographic maps of mu/alpha (6–9Hz) band ERSP in 200ms interval. The top two panels (A) display activity for the execution of movement condition. The middle two panels (B) show activity for the observation of movement condition and the bottom two panels (C) display activity for the observation condition time locked to movement onset.
3.4. Connectivity results
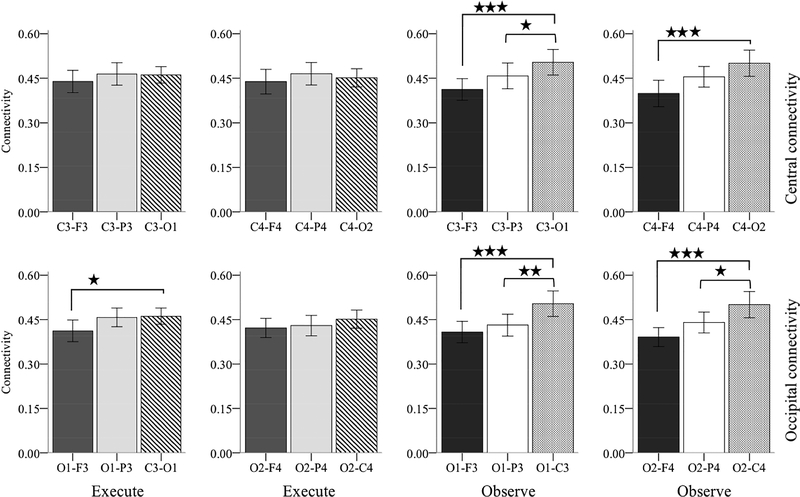
We computed ICPC during movement time periods (−1000 to 0ms time-locked to grasp completion) in both execution and observation at 6–9Hz frequency for the cluster of electrodes pairs described above (figure 6). We first analyzed the central-ICPC with Hemisphere (right, left) x Electrode-pair (central-frontal, central-parietal, central-occipital) repeated measure ANOVA separately for each condition. In the execution condition, the ANOVA did not reveal any main or interaction effect suggesting that connectivity did not differ between hemispheres and also between brain regions. In the observation condition, the analysis showed a main effect of electrode-pair (F(2, 42) = 24.778, p < .001). Follow up pair wise comparisons after Bonferroni correction showed that ICPC between central-occipital electrodes pair (M = .502, SE = .019) was significantly higher than central-parietal (M = .456, SE = .015, p = .029) and central-frontal (M = .405, SE = .018, p < .001). Even though there was no Hemisphere X Electrode-pair interaction, we further explored the nature of this higher central-occipital connectivity within each hemisphere with paired-sample t-tests. The comparison between electrode pairs overlying the left hemisphere revealed that the C3-O1 (M = .504, SE = .021) connectivity was significantly higher than both C3-F3 (M = .412, SE = .018, T(1, 21) = 5.305, p < .001) and C3-P3 (M = .458, SE = .122, T(1, 21) = 2.130, p = .045) connectivity. The analysis for electrode pairs overlying the right hemisphere showed that the C4-O2 (M = .501, SE = .022) connectivity was highly significant than C4-F4 (M = .398, SE = .022, T(1, 21) = 5.060, p < .001) and marginally significant than C4-P4 (M = .455, SE = .017, T(1, 21) = 1.794, p = .087) connectivity. Paired-sample t-tests in the execution condition did not show any significant difference.
Figure 6.
Grand average interchannel phase coherence (ICPC) between channels pairs overlying frontal, central, parietal and occipital areas in 6–9Hz frequency band. The time window includes −1000 to 0ms before grasp completion. Error bar indicates +/− 2 SE. Upper two panels show Central-ICPC and lower two panels show Occipital-ICPC. * p < .05, ** p < .005, *** p < .001.
We performed similar Hemisphere (right, left) x Electrode-pair (central-frontal, central-parietal, central-occipital) repeated measure ANOVA for occipital-ICPC in each condition. In the execution condition, the ANOVA revealed a main effect of electrode-pair (F(1.585, 44.317) = 6.322, p = .007). Follow up pair-wise comparisons showed that occipital-central ICPC (M = .456, SE = .013) was significantly higher than occipital-frontal ICPC (M = .417, SE = .016, p = .022); but did not differ from occipital-parietal ICPC (M = .444, SE = .015). In the observation condition, the analysis also revealed a main effect of electrode-pair (F(2, 42) = 27.259, p < .001). Pair-wise comparison showed that occipital-central ICPC (M = .502, SE = .019) was significantly greater than both occipital-frontal (M = .400, SE = .015, p < .001) and occipitalparietal (M = .436, SE = .017, p = .001) ICPC. As in central-ICPC, we further explored the higher occipital-central connectivity within each hemisphere with paired sample t-tests. In the observation condition, the analysis revealed that in the left hemisphere the O1-C3 (M = .504, SE = .021) connectivity was significantly greater than O1-P3 (M = .431, SE = .018, T(1, 21) = 3.498, p = .002) and O1-F3 (M = .408, SE = .018, T(1, 21) = 4.426, p < .001) connectivity. The comparison for electrodes pairs in the right hemisphere also showed significantly higher O2-C4 (M = .501, SE = .022) connectivity than both O2-P4 (M = .440, SE = .017, T(1,21) = 2.894, p = .009) and O2-F4 (M = .390, SE = .016, T(1, 21) = 5.784, p < .001) connectivity. Similar comparison in execution condition showed that O1-C3 (M = .461, SE = .014) connectivity was significantly greater than O1-F3 (M = .412, SE = .018, T(1,28) = 2.772, p = .010) connectivity; but comparison between all other electrode pairs did not show any significant difference.
Finally, we compared central-occipital connectivity between execution and observation conditions. The result showed that the central-occipital connectivity in observation condition was significantly greater compared to the execution condition (T(1,21) = 2.135, p = .045).
Furthermore, in the observation condition, we computed connectivity within the beta and gamma frequency bands (see supplementary document). Connectivity in the beta and gamma frequency bands did not show the pattern found for the mu/alpha frequency band. Specifically, the significantly higher central-occipital connectivity compared to the short distance central-parietal and occipital-parietal connectivity was not evident within the beta and gamma frequency bands. These findings suggest that during observation of other’s action the functional coupling between central and occipital regions is specific to mu/alpha frequency band. Overall the connectivity results showed that during the observation of movement the central-occipital ICPC was strongest compared to the ICPC between other brain regions, suggesting that the cognitive processes mediated by central (mirroring) and occipital (attention) areas during the observation of movements were functionally coupled and temporally synchronized.
4. Discussion
Recently concerns have been raised regarding whether the EEG mu rhythm desynchronization is a useful and valid measure of the mirroring system in humans (Hobson and Bishop, 2016, 2017). One specific concern is that mu rhythm activity is contaminated by occipital alpha activity associated with visual attention. This may be particularly problematic in developmental studies in which children and infants pay particularly close attention to actions and objects they are learning about (Bowman et al., 2017). In the present study, we investigated the validity of mu rhythm desynchronization as an index of mirroring in infants by examining mu/alpha (6–9Hz) frequency band EEG oscillations during execution and observation of grasp movements. More specifically, we examined whether central mu and occipital alpha activity can be identified as distinct sources of cortical activity, and whether these two sources are functionally connected. We employed high density EEG recording and performed a comprehensive analysis of the EEG data, which included: time-frequency decomposition in mu/alpha (6–9Hz) frequency range, scalp topographies and functional connectivity between brain areas in 6–9Hz frequency range. As expected based on previous research we found evidence of mu desynchronization during both execution and observation of movements. In the observation condition, mu desynchronization was present even before the onset of the observed movement. The topographic maps complement the specific pattern of mu/alpha rhythm activation in both conditions, showing two distinct clusters of activity over central and occipital cortical regions, thus providing further evidence supporting the mu rhythm desynchronization as an index of mirroring system activation in human infants. The connectivity analysis revealed that during observation of movements, central and occipital areas were functionally more connected compared to their connections with other brain regions, suggesting that while these two areas of activity are distinct they may also work in concert. Overall these findings provide significant information about the mu rhythm desynchronization and its validity as a measure of mirroring system activity in human.
The mu rhythm desynchronization is extensively used as an index of mirroring activity in humans (Muthukumaraswamy et al., 2004; Nyström et al., 2011; Oberman et al., 2007) and particularly it has been used to study a wide range of cognitive and social functions in developmental populations (Cannon et al., 2016; Filippi et al., 2016; Yoo et al., 2016). While some argue that mu desynchronization provides a reliable and valid measure of mirroring activity in humans (Bowman et al., 2017; Fox et al., 2016), others are critical about the mu desynchronization as a measure of mirroring activity (Hobson and Bishop, 2016, 2017). It was therefore necessary to perform a comprehensive analysis of mu rhythm desynchronization to evaluate its validity as an index of the mirroring system. We replicated previous findings (Cannon et al., 2016; Marshall et al., 2011; Yoo et al., 2016) showing that both execution and observation of movements induced mu/alpha desynchronization at electrodes sites overlying frontal, central, parietal and occipital sites. The broad distribution is a typical pattern of the infant mu rhythm, which peaks at lower frequency (6–9) and has a diffuse scalp distribution (Marshall et al., 2011; Thorpe et al., 2016). Cannon et al. (2016) offered two explanations for the broad distribution of mu/alpha desynchronization. First, desynchronization of activity across multiple brain areas suggests that in addition to the mirroring process, an attention process or higher cognitive load might be involved in action execution and observation. Second, the mirroring system in humans might not be confined to the motor areas. Although initial human studies reported existence of mirror neurons in motor cortex (Fadiga et al., 2005; Hari et al., 1998), subsequent studies found characteristics of mirror neurons in frontal and parietal areas (Iacoboni et al., 1999; Molenberghs et al., 2012). Some authors suggest that the mu desynchronization underlies a broad neural network capable of supporting multiple functions under different circumstances (Crone et al., 1998). Because neurons consisting of this broad network are interconnected, activation of a subset of the network may result in a widespread desynchronization. It is therefore plausible, as suggested by Pineda (2005), that the diffuse and distributed mu/alpha activation arises when different brain regions are engaged in coherent function.
Our data revealed a specific pattern of mu and alpha oscillations. In the execution condition, mu desynchronization over central scalp locations was significantly higher compared to the alpha desynchronization at frontal, parietal and occipital electrodes sites. In the observation condition, the activation did not significantly differ among electrode sites. This pattern of mu and alpha activation was also found in previous studies (Cannon et al., 2016; Hobson and Bishop, 2016; Yoo et al., 2016), and has been identified as a concern as it suggests lack of specificity of mu/alpha to central electrode locations. However, the fact that these regions exhibited similar strength of activity does not necessarily mean that the mu rhythm is simply reflecting spread of alpha from the occipital cortex. In fact, the current results suggest that central mu and occipital alpha are distinct yet correlated foci of activity.
The neural activity spreads from one brain region to another physically distinct brain region primarily due to volume conduction. Volume conduction reduces spatial precision of EEG by contaminating sources of activity (Cohen, 2014; He et al., 2011). Volume conduction, if not addressed, can lead to spurious conclusions. Surface Laplacian has been shown to be successful in addressing the volume conduction problem by transforming the EEG signal into a reference free CSD waveform (Kayser and Tenke, 2006; Tenke and Kayser, 2012). CSD transformation of EEG data reduces the effect of volume conduction, increases topographical localization and highlights local spatial features (Cohen, 2014). In this study, we transformed our EEG data into CSD format, which would minimize the spread of activation. Therefore, the mu desynchronization found in this study is unlikely to be contaminated by visual alpha from occipital cortex. The connectivity results of this study provide additional evidence refuting the contamination argument. In general, volume conduction results in stronger connectivity between electrodes that are close to each other (Cohen, 2014). We would therefore expect higher connectivity between occipital-parietal and central-parietal electrode pairs than central-occipital electrode pairs, which have longer spatial distance compared to the distance between occipitalparietal and central-parietal electrode pairs. However, our results showed significantly higher connectivity between central-occipital electrodes than neighboring occipital-parietal and centralparietal electrodes (see figure 6) in the observation condition. These findings suggest that the mu desynchronization evident during the observation of actions in this study is very unlikely to be the spread of occipital alpha rather mu and visual alpha exhibit distinct cortical activation. The distinct mu and visual alpha activation might reflect, as Fox et al. (2016) suggested, a close coordination between mirroring and attention.
4.1. Mechanism for interaction between mirroring and attention process
Phase coherence or connectivity is thought to indicate a mechanism for synchronized interregional brain activity (Cavanagh et al., 2009). Phase coherence relies on consistency of oscillatory phase angles between two electrodes placed over different brain regions. It is believed that when two brain regions are functionally connected, the timing of their oscillatory process, as measured by phase, become synchronized (Cohen, 2014; Cohen, 2015). Phase synchrony over distributed brain regions has been found in different frequency bands and for different cognitive functions (Bhattacharya and Petsche, 2005; Cavanagh et al., 2009; Cohen, 2015). In this study, electrode cluster pairs overlying central and occipital regions showed higher phase synchrony than the electrode pairs overlying any other brain regions during observation of movements. In the observation condition, occipital-central phase synchronization was higher than the synchronization between relatively short distance occipital-parietal and long distance occipitalfrontal electrode pairs in both hemispheres. The higher phase synchrony between occipital and central regions suggests that these two regions became functionally connected during the observation of movement. Moreover, the increased connectivity between occipital-central regions than the short distance occipital-parietal connectivity suggests that it is not volume conduction of activity but rather specific functional connection between these two brain regions. The oscillatory phase synchrony might be a mechanism by which the attention process mediated by the visual system and mirroring function communicate. Overall our findings support the proposal of concurrent attention and mirroring processes, which are distinct but become functionally coupled during observation (Bowman et al., 2017; Pineda, 2005). Functional connectivity is a novel measure in developmental research and we adopted a somewhat exploratory approach in this study. However, connectivity measures can be valuable to understand the network dynamics of developing brain and future research should continue to utilize such measures for systematic investigation of brain network in developmental population.
4.2. Time course of mu/alpha desynchronization
Although not a central question of the current study, the time frequency decomposition of the EEG activity revealed temporal nuances of the mu/alpha activity. We examined activity in the observation condition surrounding the onset of the Presenter’s movement. Results revealed that the desynchronization of mu/alpha activity began before initiation of the Presenter’s movements, a finding that is consonant with previous research (Southgate et al., 2009) reporting mu desynchronization prior to the observation of action in 9-month old infants. Motor activation before observation of the actual movement has also been demonstrated in adult studies (Caetano et al., 2007; Kilner et al., 2004). Our finding that mu desynchronization starts prior to the observed movement fits well with the proposal that knowledge of an impending motor event is sufficient to excite one’s own motor system (Kilner et al., 2004), and that this early activation may be an index of action prediction or anticipation.
Neural activity of the developmental brain has been widely studied within mu/alpha frequency band in the time window when a motor event occurs and to a less extent in the time period immediately prior to a motor event, as mu has been shown to be modulated prior to and during execution and observation of an action (Fox et al., 2016; Marshall et al., 2011; Nyström et al., 2011; Southgate et al., 2009). Here we report modulation of EEG oscillation in the postmovement time period for the first time, to our knowledge, in infants. We found that following the completion of the movement mu/alpha activation began to synchronize at about 400ms. The synchronization of activation was most prominent in 400–600ms post-movement time interval in occipital areas in the both execution and observation conditions. In the observation condition, the synchronization of activation was also present in frontal, central and parietal areas in that time window. Post-movement synchronization of alpha activation was studied in adults and is thought to reflect a deactivated or actively inhibited neural process (Pfurtscheller, Neuper, Andrew, & Edlinger, 1997). We speculate that in infants, post-movement synchronization of activation may be related to the similar process of active inhibition or termination of motor and attention process, however further evidence is required to demonstrate the post-movement deactivation. It will be of particular interest for future research to further study the post-movement modulation of neural oscillation across development.
4.6. Limitations
Although this study was carefully designed and performed, there are limitations to consider in interpreting these results. This study included only 9-month-old infants. To gain a more full perspective on the functional connectivity between occipital and central regions and whether the human mirroring system indeed encompasses an integration of both visual attention and mirroring processes, it will be important to expand these methods to populations across the lifespan.
5. Conclusion
In summary the current study contributes to the existing knowledge of and also provides novel insights about mu/alpha rhythm oscillation in infants. Our findings demonstrate that observation of movement induces activation of the mirroring system, which is not mere spread of alpha activation from the occipital cortex. Our results also replicate a previous finding demonstrating mu desynchronization prior to the actual observed movement in 9-month old infants. In addition, this study reveals new information about the post-movement rebound of mu/alpha activation, which future research should investigate in more detail. Finally, our findings suggest that oscillatory phase synchrony between motor and visual areas might be an underlying mechanism by which distinct mirroring and attention processes become functionally connected during observation of movements.
Supplementary Material
6. Acknowledgments
This research was supported by NIH grant (P01 HD064653) to NAF. We wish to thank undergraduate research assistants at the UMD Child Development Lab for their help in the collection and the coding of these data. We are especially thankful to the families who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Arbib MA, Mundhenk TN, 2005. Schizophrenia and the mirror system: an essay. Neuropsychologia 43, 268–280. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Petsche H, 2005. Phase synchrony analysis of EEG during music perception reveals changes in functional connectivity due to musical expertise. Signal Processing 85, 21612177. [Google Scholar]
- Bowman LC, Bakermans-Kranenburg MJ, Yoo KH, Cannon EN, Vanderwert RE, Ferrari PF, van Ijzendoorn MH, Fox NA, 2017. The mu-rhythm can mirror: Insights from experimental design, and looking past the controversy. Cortex. In press. [DOI] [PubMed] [Google Scholar]
- Bowman LC, Thorpe SG, Cannon EN, Fox NA, 2016. Action mechanisms for social cognition: behavioral and neural correlates of developing Theory of Mind. Dev. Sci 20, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano G, Jousmäki V, Hari R, 2007. Actor’s and observer’s primary motor cortices stabilize similarly after seen or heard motor actions. Proc. Natl. Acad. Sci 104, 9058–9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon EN, Simpson EA, Fox NA, Vanderwert RE, Woodward AL, Ferrari PF, 2016. Relations between infants’ emerging reach-grasp competence and event-related desynchronization in EEG. Dev. Sci 19, 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Salas RM, 2000. State-modulation of cortico-cortical connections underlying normal EEG alpha variants. Physiol. Behav 71, 107–115. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL, 2003. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci 100, 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB, 2009. Prelude to and Resolution of an Error: EEG Phase Synchrony Reveals Cognitive Control Dynamics during Action Monitoring. J. Neurosci 29, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, 2014. Analyzing neural time series data: theory and practice. MIT Press. [Google Scholar]
- Cohen MX, 2015. Effects of time lag and frequency matching on phase-based connectivity. J. Neurosci. Methods 250, 137–146. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP, 1998. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain 121, 2271–2299. [DOI] [PubMed] [Google Scholar]
- Csibra G, Gergely G, 2007. ‘Obsessed with goals’: Functions and mechanisms of teleological interpretation of actions in humans. Acta. Psychol 124, 60–78. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Cannon EN, Yoo K, Fox NA, 2014. The infant EEG mu rhythm: Methodological considerations and best practices. Dev. Rev. 34, 26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klerk CCJM, Johnson MH, Southgate V, 2015. An EEG study on the somatotopic organisation of sensorimotor cortex activation during action execution and observation in infancy. Dev. Cog. Neurosci 15, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G, 1992. Understanding motor events: a neurophysiological study. Exp. Brain. Res 91, 176–180. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Behrmann M, Heeger DJ, 2008. A mirror up to nature. Curr. Biol 18, R13–R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Olivier E, 2005. Human motor cortex excitability during the perception of others’ action. Curr. Opin. Neurobiol 15, 213–218. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter T, Gredebäck G, von Hofsten C, 2006. Infants predict other people’s action goals. Nat. Neurosci 9, 878. [DOI] [PubMed] [Google Scholar]
- Filippi CA, Cannon EN, Fox NA, Thorpe SG, Ferrari PF, Woodward AL, 2016. Motor System Activation Predicts Goal Imitation in 7-Month-Old Infants. Psychol. Sci 27, 675684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Bakermans-Kranenburg MJ, Yoo KH, Bowman LC, Cannon EN, Vanderwert RE, Ferrari PF, van Ijzendoorn MH, 2016. Assessing human mirror activity with EEG mu rhythm: A meta-analysis. Psychol. Bull 142, 291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel-Toledo S, Bentin S, Perry A, Liebermann DG, Soroker N, 2013. Dynamics of the EEG power in the frequency and spatial domains during observation and execution of manual movements. Brain. Res 1509, 43–57. [DOI] [PubMed] [Google Scholar]
- Gallese V, 2001. The ‘shared manifold’ hypothesis. From mirror neurons to empathy. J. Conscious. Stud 8, 33–50. [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G, 1996. Action recognition in the premotor cortex. Brain 119, 593–609. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G, 1998. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc. Natl. Acad. Sci. 95, 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Yang L, Wilke C, Yuan H, 2011. Electrophysiological Imaging of Brain Activity and Connectivity—Challenges and Opportunities. IEEE Trans. Biomed. Eng 58, 1918–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, 2009. Eight Problems for the Mirror Neuron Theory of Action Understanding in Monkeys and Humans. J. Cogn. Neurosci 21, 1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson HM, Bishop DVM, 2016. Mu suppression – A good measure of the human mirror neuron system? Cortex 82, 290–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson HM, Bishop DVM, 2017. Reply to Bowman et al: Building the foundations for moving mu suppression research forward. Cortex. In press. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, 2009a. Imitation, Empathy, and Mirror Neurons. Annu. Rev. Psychol 60, 653–670. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, 2009b. Neurobiology of imitation. Curr. Opin. Neurobiol 19, 661–665. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G, 1999. Cortical Mechanisms of Human Imitation. Science 286, 2526–2528. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, 2006. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin. Neurophysiol 117, 348–368. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, 2006. Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: A tutorial review. Int. J. Psychophysiol 97, 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD, 2007. Predictive coding: an account of the mirror neuron system. Cogn. Process 8, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Vargas C, Duval S, Sarah-Jayne B, Sirigu A, 2004. Motor activation prior to observation of a predicted movement. Nat. Neurosci 7, 1299–1301. [DOI] [PubMed] [Google Scholar]
- Lepage J-F, Théoret H, 2006. EEG evidence for the presence of an action observation– execution matching system in children. Eur. J. Neurosci 23, 2505–2510. [DOI] [PubMed] [Google Scholar]
- Lingnau A, Gesierich B, Caramazza A, 2009. Asymmetric fMRI adaptation reveals no evidence for mirror neurons in humans. Proc. Natl. Acad. Sci 106, 9925–9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos C, Rodriguez M, Rodriguez-Sabate C, Morales I, Sabate M, 2013. Mu-rhythm changes during the planning of motor and motor imagery actions. Neuropsychologia 51, 10191026. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A, 2004. Mining event-related brain dynamics. Trends in Cognitive Sciences 8, 204–210. [DOI] [PubMed] [Google Scholar]
- Makuuchi M, 2005. Is Broca’s Area Crucial for Imitation? Cereb. Cortex 15, 563–570. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA, 2002. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol 113, 1199–1208. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN, 2014. Neural mirroring mechanisms and imitation in human infants. Philos. Trans. R. Soc. Lond. B. Biol. Sci 369, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Young T, Meltzoff AN, 2011. Neural correlates of action observation and execution in 14-month-old infants: an event-related EEG desynchronization study. Dev. Sci 14, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, Buiatti M, 2011. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 48, 229–240. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB, 2009. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neurosci. Biobehav. Rev 33, 975–980. [DOI] [PubMed] [Google Scholar]
- Monroy CD, Meyer M, Schröer L, Gerson SA, Hunnius S, 2017. The infant motor system predicts actions based on visual statistical learning. NeuroImage. In press. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB, 2012. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev 36, 341–349. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair NA, 2004. Mu rhythm modulation during observation of an object-directed grasp. Cogn. Brain Res 19, 195–201. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, 2004. Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology 41, 152–156. [DOI] [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB, 2010. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J. Neurosci. Methods 192, 152–162. [DOI] [PubMed] [Google Scholar]
- Nyström P, Ljunghammar T, Rosander K, von Hofsten C, 2011. Using mu rhythm desynchronization to measure mirror neuron activity in infants. Dev. Sci 14, 327–335. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA, 2005. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res 24, 190–198. [DOI] [PubMed] [Google Scholar]
- Oberman LM, McCleery JP, Ramachandran VS, Pineda JA, 2007. EEG evidence for mirror neuron activity during the observation of human and robot actions: Toward an analysis of the human qualities of interactive robots. Neurocomputing 70, 2194–2203. [Google Scholar]
- Pfurtscheller G, Aranibar A, 1979. Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalogr. Clin. Neurophysiol 46, 138–146. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G, 1997. Foot and hand area mu rhythms. Int J Psychophysiol. 26, 121–135. [DOI] [PubMed] [Google Scholar]
- Pineda JA, 2005. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Res. Rev 50, 57–68. [DOI] [PubMed] [Google Scholar]
- Rayson H, Bonaiuto JJ, Ferrari PF, Murray L, 2017. Early maternal mirroring predicts infant motor system activation during facial expression observation. Sci. Rep 7, 11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA, 1998. Language within our grasp. Trends. Neurosci 21, 188–194. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M, 2008. The mirror system and its role in social cognition. Curr. Opin. Neurobiol 18, 179–184. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M, 2010. Mirror neurons: from discovery to autism. Exp. Brain Res 200, 223–237. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V, 2001. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci 2, 661–670. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C, 2010. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat. Rev. Neurosci 11, 264–274. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hámáaláinen M, Kajola M, Hari R, 1995. Functional Segregation of Movement-Related Rhythmic Activity in the Human Brain. NeuroImage 2, 237–243. [DOI] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, 2005. Pulling out the intentional structure of action: the relation between action processing and action production in infancy. Cognition 95, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Osborne T, Csibra G, 2009. Predictive motor activation during action observation in human infants. Biol. Lett 5, 769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John AM, Kao K, Choksi M, Liederman J, Grieve PG, Tarullo AR, 2016. Variation in infant EEG power across social and nonsocial contexts. J. Exp. Child. Psychol 152, 106–122. [DOI] [PubMed] [Google Scholar]
- Stancák A, Pfurtscheller G, 1995. Desynchronization and recovery of β rhythms during brisk and slow self-paced finger movements in man. Neurosci Lett. 196, 21–24. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, 2012. Generator localization by current source density (CSD): Implications of volume conduction and field closure at intracranial and scalp resolutions. Clin. Neurophysiol 123, 2328–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théoret H, Pascual-Leone A, 2002. Language Acquisition: Do as You Hear. Curr. Biol 12, R736–R737. [DOI] [PubMed] [Google Scholar]
- Thorpe SG, Cannon EN, Fox NA, 2016. Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clin. Neurophysiol 127, 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turella L, Pierno AC, Tubaldi F, Castiello U, 2009. Mirror neurons in humans: Consisting or confounding evidence? Brain. Lang 108, 10–21. [DOI] [PubMed] [Google Scholar]
- Winter WR, Nunez PL, Ding J, Srinivasan R, 2007. Comparison of the effect of volume conduction on EEG coherence with the effect of field spread on MEG coherence. Stat. Med 26, 3946–3957. [DOI] [PubMed] [Google Scholar]
- Yoo KH, Cannon EN, Thorpe SG, Fox NA, 2016. Desynchronization in EEG during perception of means-end actions and relations with infants’ grasping skill. Br. J. Dev. Psychol 34, 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.