Abstract
The impact that microplastics (<5 mm) have on scleractinian coral is largely unknown. This study investigated calcification effects, size limits, and retention times of microbeads and microfibers in two Caribbean species, Montastraea cavernosa and Orbicella faveolata, in a series of three experiments. No calcification effects were seen in the two-day exposure to a microbead concentration of 30 mg L−1. M. cavernosa and O. faveolata actively ingested microbeads ranging in size from 425 μm–2.8 mm, however, a 212–250 μm size class did not elicit a feeding response. The majority of microbeads were expelled within 48 hours of ingestion. There was no difference in ingestion or retention times of 425–500 μm microbeads versus 3–5 mm long microfibers. M. cavernosa and O. faveolata have the ability to recognize and reject indigestible material, yet, there is still a need to study effects of energetics and microplastic contamination as a result of ingestion and egestion.
Keywords: microplastic, coral, retention, egestion, calcification
1.0. Introduction
Ingestion of plastic pollution in the marine environment first drew attention in the 1970s with numerous reports of plastics found in seabirds (Parslow and Jefferies 1972, Rothstein 1973, Hays and Cormons 1974, Baltz and Morejohn 1976, Ohlendorf et al. 1978). Given the persistent nature of plastic, it is alarming that total global estimates of plastics produced are over 8300 million metric tons (Geyer et al. 2017), with upwards of 12.8 million metric tons entering the ocean in 2010 (Jambeck et al. 2015). The widespread occurrence of plastics in the marine environment has led to a surge of research on the potential impacts that the smaller pieces of plastics, termed microplastics (<5mm), may have on wildlife.
Microplastics can originate from various sources including degradation of macroplastic, industrial processes, synthetic clothing, tire fibers, and personal care products (GESAMP 2015, Thompson 2015, Boucher and Friot 2017). Once in the marine environment, microplastics tend to serve as a surface for microbial community growth as well as attracting pollutants (Teuten et al. 2007, Andrady 2011, Zettler et al. 2013). As a result, their density increases and they become more bioavailable to organisms in the environment (Ye and Andrady 1991, Teuten et al. 2007), including benthic organisms such as scleractinian coral.
Scleractinian corals are both phototrophic and heterotrophic feeders. Corals receive nutrition via translocation of photosynthetic products produced by zooxanthellae. However, corals also rely on exogenous food sources to meet their nutritional needs which can account for 15–35% of their daily energetic demand (Houlbrèque and Ferrier-Pages 2009). Coral are passive suspension feeders that feed on plankton passing over their tentacles. Small debris, such as microplastics, may inadvertently be captured and ingested by the coral. Hall et al. (2015) demonstrated that corals ingest microplastic and did so at rates similar to plankton uptake, which was supported in subsequent studies (Allen et al. 2017, Reichert et al. 2018). Ingestion of plastics by other invertebrates has been shown to reduce energy budgets (Wright et al. 2013, Watts et al. 2015, Sussarellu et al. 2016), which could have significant impacts on health and reproduction. Although coral calcification relies heavily on photosynthesis from zooxanthellae (Porter et al. 1989), heterotrophic feeding becomes important when light is restricted or during bleaching (Palardy et al. 2008, Grottoli et al. 2006, Anthony et al. 2009). Ingested microplastics by coral could potentially reduce the energetic demands needed for the calcification process by inhibiting digestion of exogenous food sources, especially in times of stress. The evidence of ingested microplastics by corals confirms the need for further investigations looking at physiological interactions and potential threats. The objective of this study is to evaluate potential effects and interactions of coral responses to ingested microplastics for two Caribbean, scleractinian corals, Montastraea cavernosa and Orbicella faveolata. In a series of three experiments, this study (1) investigated effects ingested microbeads on calcification, (2) determined ingested size ranges (425 μm – 2.8 mm) and retention times of microplastics by coral, and (3) compared ingestion and retention of microbeads versus microfibers by coral.
2. Methods
Three laboratory experiments were conducted using the Caribbean, scleractinian coral species, Montastraea cavernosa (large polyp coral) and Orbicella faveolata (small polyp coral). Coral were collected from Florida Keys National Marine Sanctuary Coral Nursery Program (Permit Numbers FKNMS-2005–057 & FKNMS-2011–036). Species were chosen to represent large and small polyp coral species. Coral specimens were maintained for at least three months in an indoor coral research facility at the U.S. Environmental Protection Agency’s Gulf Ecology Division in Gulf Breeze, Florida. Corals were maintained in recirculating culture systems (~1000 L) prior to experimentation. Experiments were conducted in a separate recirculating system (~820 L). All systems were kept at a temperature of 26.0 ± 1.0°C and salinity of 35.0 ± 0.3 ppt with metal halide lights on a 9.5:14.5 light:dark cycle. Other water quality parameters such as calcium, pH, alkalinity, magnesium, ammonia, and nitrate were all measured prior to experimentation and were within culture condition parameters (Borneman 2001, Delbeek and Sprung 2005, Holmes-Farley 2010). Light intensity in culture and experimental systems ranged from 25.0–40.0 W m−2 depending on age of the bulbs and placement within the system. For each experiment, at least three parent colonies of each species were used to cut 4 cm2 fragments using a Gryphon Corp. Aquasaw XL (Model C-40). Fragments acclimated in the experimental system five days prior to the start of the experiment. Coral fragments in experiment 1 were glued to acrylic pegs to sit securely on egg crate. Fragments in experiments 2 and 3 sat freely on egg crate within the chambers. The number of replicates varied between experiments based on experimental design. During all experiments 100% cotton clothing was worn. Exposure to microplastics had to be assumed as corals originated from a natural environment. Additionally, culture conditions were not strictly monitored in the years leading to the study. Microplastics used in experiments needed to be clearly identified, therefore, fluorescent microplastics were used in the smaller size classes.
2.1. Experiment 1: Effects of ingested microbeads on calcification
The first experiment determined if microplastic ingestion impacted calcification in the two coral species. Three different size classes of fluorescent, polyethylene microplastic beads consisting of mostly smooth surfaces (Cospheric®) were used in this experiment: 90–106, 425–500, and 850–1000 μm. Polyethylene is a common polymer found in marine sediment and surface waters (Teuten et al. 2007, Erni-Cassola et al. 2017). The density of each of the three size classes was 1.002±0.006 g cc−1. The approximate density of 26.0°C, 35.0 ppt sea water is 1.025 g cc−1, denser than the microbeads. Prior to initiating the experiment, microbeads of each size class were placed in separate 80 μm mesh containers and placed in culture for curing (i.e. growing biofilm on surface) for six weeks to decrease their buoyancy.
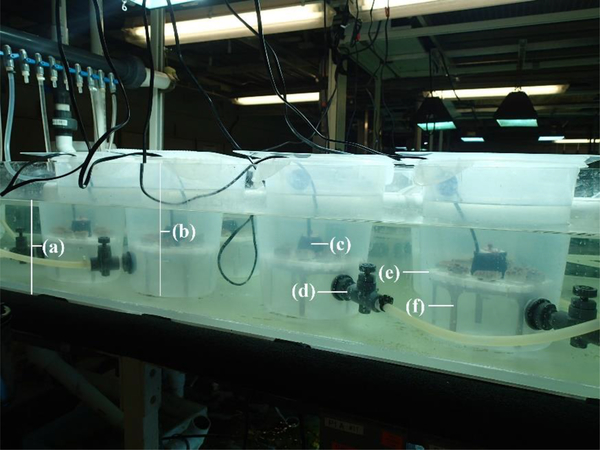
Ten 8 L plastic, circular chambers were used as experimental chambers and contained in a water bath to maintain temperature (26.0 ± 0.5 °C; Figure 1). Each chamber had a 3/8” needle valve near the bottom for chamber filling. Within each chamber, four fragments of each M. cavernosa and O. faveolata rested on egg crate (i.e. louvered ceiling panels) which sat atop an 8.5 cm tall stand. A Hydor Koralia 240 pump was used for circulation and was positioned in a hole made in the center of the egg crate, and pointed in a downward direction (Figure 1). Curing the microbeads prior to the experiment seemed to decrease their buoyancy. Although the density of the microbeads was not determined after the curing process, the microbeads did not float at the water’s surface and the circulation within the chamber was sufficient to keep microbeads in suspension. The 90–106 μm size class required the surface of the water to be agitated in order to get the beads in suspension. After agitation, all size classes generally remained in suspension as few were detected by means of ultraviolet light on chamber walls or at the water’s surface.
Figure 1.
Diagram of experimental system set-up including 8L experimental chambers used in Experiment 1: (a) water bath, (b) experimental chamber with acrylic lid, (c) circulation pump, (d) needle valve, (e) egg crate, and (f) stand.
The two treatments for the experiment consisted of a control group (not exposed to microbeads) and an exposed group, with each group consisting of five replicates. Each chamber of the exposed group contained all three microbead size classes at a dose of 80 mg microbeads per size class, resulting in a final microbead concentration of 30 mg L−1 (10 mg L−1 per size class). Number of particles per liter was approximately 24 for the 850–1000 μm size class, approximately 215 particles for the 425–500 μm size class, and undetermined for the 90–106 μm size class. Before the addition of microbeads, water flow from the recirculating system was shut off to each chamber and remained off for the two-day exposure; water inside the chamber remained circulating via the pump. Each chamber was fed 5 mL from a mixture of 0.156 g Golden Pearls® (Brine Shrimp Direct, Ogden, UT) 5–50 μm coral food in 100 mL seawater to elicit a feeding response in conjunction with microplastic application. The recirculating system is equipped with a protein skimmer, phosphate reactor, as well as a 1 μm filter to eliminate all sources of nutrients and debris that may otherwise be mistaken as food. The addition of food was to simulate the presence of zooplankton on a reef to mimic natural conditions whereby microplastics may mix with plankton (Boerger et al. 2010). Salinity was not regulated during the static, two-day exposure period to eliminate the risk of losing any microplastics that might have adhered to the sensor. In preliminary tests, the maximum salinity increase within the chambers was 0.3 ppt over two days without de-ionized (DI) water adjustments.
Following the two-day exposure, coral tissue was processed to recapture microbeads. Processing occurred under UV light. All rinsing steps were performed using DI water, unless otherwise specified. Each coral fragment was removed from the chamber and placed into individual glass culture bowls. Corals were vigorously rinsed to remove microbeads that may have been stuck to the outside of the polyp or acrylic base with filtered (1 μm) seawater. The seawater used for rinsing was placed back into its respective chamber. Coral tissue was removed from its skeleton using the airbrush method first described by Johannes and Wiebe (1970). A metal funnel was placed into a 50 mL Falcon tube to collect all coral tissue. Each Falcon tube was then exposed to an ultrasonic cell disruptor (Tekmar sonic disrupter Model TM 600 with a Branson ultrasonic converter Model CV17) for 15 s at 30% amplitude to emulsify organic matter, a method based from Wagner et al. (2017). A rubber stopper was placed on the converter wand to ensure a secure fit inside the Falcon tube. For tissue sample filtration, a series of metal sieves (Gilson Company, Inc.) were used: 63 μm, 250 μm, 500 μm, and 1 mm. Each sieve corresponded with a size class of microbeads: 63 μm to collect 90–106 μm, 250 μm for 425–500 μm and 500 μm for 850–1000 μm. The 1 mm sieve was used to remove large, miscellaneous debris that may have been present. Each coral sample was gravity filtered through the sieves to separate out the size classes of ingested microbeads. The contents of the sieves were rinsed using DI water into individual 50 mL Falcon tubes for each size class. Each size class sample was vacuum filtered through a ceramic, Buchner funnel onto a pre-weighed Millipore glass filter (APFD09050, 2 μm pore size). Falcon tubes were rinsed with DI water to ensure all potential microplastic beads were removed as well as to rinse any residual salt remaining from coral tissue. Samples were placed in a dehumidifier for 72 hours until dry. Samples were then individually weighed on an analytical balance and the weight of each size class of microbead was determined. Additionally, the number of microbead particles was counted for the 425–500 and the 850–1000 μm size classes. Filtration methods were validated by adding doses of each size class to a chamber (n=3) containing egg crate, stand, and pump. The percent average (SD) recovered for each size class was 99.37% (SD=0.88), 99.92% (SD=0.50), and 88.8% (SD=16.7) for 850–1000, 425–500, and 90–106 μm, respectively.
Microbead data was normalized to surface area of the coral fragment. Following the acclimation period, and prior to exposure, the top-side of each coral fragment was photographed (Fournie et al. 2012, Enzor et al. 2018). A mask of tissue area was generated in image processing software (Adobe® Photoshop® CS3 version 10.0, ©1990–2007 Adobe® Systems Inc.). The mask was then exported to ImageJ software (ImageJ 1.38×, Wayne Rasband, National Institutes of Health, USA; http://rsb.info.nih.gov/ij/; public domain) to determine surface area values.
Total alkalinity was measured before and after exposure using open celled titration (Metrohm Titrando 905) and used to measure calcification based off of the alkalinity anomaly principle, which assumes that for every one mole of calcium carbonate precipitated, total alkalinity decreases by two moles (Kinsey 1978). This method is ideal as a non-destructive protocol to measure short term calcification (Smith and Kinsey 1978, Chisholm and Gattuso 1991). Alkalinity was measured at each time point to determine calcification by the following equation:
| AT2 – AT1 = ΔAT/2 = G |
where, AT is total alkalinity (μmol) and G is calcification, i.e. calcium carbonate precipitated (μmol). Calcification data were analyzed using a one-way analysis of variance (ANOVA) (Minitab 16, Inc.).
2.2. Experiment 2: Determination of ingestion size ranges (425 μm-2.8 mm) and retention times
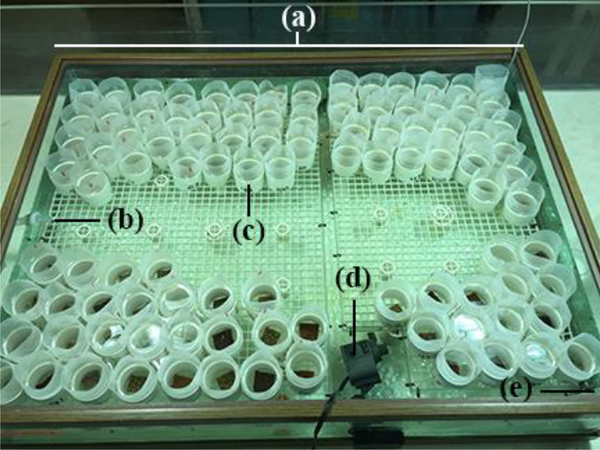
The second experiment measured the retention time of different size microbeads in coral polyps following ingestion. The experimental system was composed of a 170 L tank containing 120 experimental chambers, made from 2”-diameter polyvinyl chloride (PVC) pipe cut into ~5 cm lengths (Figure 2). The bottom of the experimental chamber consisted of 118 μm Nitex® mesh. The top of the chambers contained the same size mesh around the circumference of the top. Experimental chambers contained one coral fragment each and were placed on egg crate which sat ~9 cm off the bottom of the tank. Water flow from the recirculating system remained on during exposure and two pumps were placed in the tank for additional water circulation.
Figure 2.
Experimental setup for Experiments 2 and 3: (a) 170L experimental system, (b) drain, (c) meshed experimental PVC chambers, (d) circulation pump, and (e) water source (not pictured).
Coral fragments (n=10 per species for each of five treatments) were exposed to three microbeads in each of four size classes of uncured, polyethylene microbeads (Cospheric®) of the following treatments: (1) 212–250 μm, (2) 425–500 μm, (3) 850–1000 μm, (4) 1.7–2.0 mm, (5) 2.4–2.8 mm, and (6) a control group not exposed to microbeads. The 212–250 μm size class was attempted, however, neither M. cavernosa nor O. faveolata actively ingested this size microbead, i.e., they did not retract tentacles after capture as they did with other size classes, therefore, samples were not furthered observed. Microbeads were not cured in this experiment to ensure that they floated to the surface if egested. Each fragment was fed 1 mL mixture of 0.156 g Golden Pearls® (5–50 μm) (in 100 mL seawater) with microplastic application to elicit a feeding response. While in a PVC chamber, three microbeads of one size class were applied directly to coral polyp tentacles (different polyps of a single fragment) using a 1 mL transfer pipette, using surrounding seawater if needed. Each fragment was visually monitored to ensure ingestion. Corals had a 20-minute time limit to ingest the microbeads; microbeads not ingested were removed from the chamber. The control group was fed in the same manner as exposed fragments but without microbead application. Video recordings documented ingestion of microbeads except for 425–500 μm size class in M. cavernosa, as ingestion occurred too rapidly to record after application of the three microbeads. Additionally, the time it took O. faveolata to ingest the 2.4–2.8 mm size class exceeded the memory capacity of the camera (18 minutes) for all fragments. The number of microbeads in the water at the end of the study were observed to determine if the coral polyp had egested the microbeads. These observations were made 48 hours after application. Data were recorded as the percentage of microbeads egested per fragment. A one-way ANOVA was performed (Minitab 16, Inc.) along with Tukey’s post-hoc pairwise comparison.
2.3. Experiment 3: Comparison of ingestion and retention of microbeads versus microfiber
The experimental tank, egg crate, and PVC chamber setup was similar to Experiment 2 (Figure 2). However, only 60 PVC chambers were used and PVC chambers were meshed with 80 μm Nitex®. Preliminary studies showed that the 80 μm Nitex® retained the 3–5 mm length microfibers for this experimental design. Ten M. cavernosa and ten O. faveolata fragments were used per treatment group: (1) exposed to three uncured, fluorescent, polyethylene 425–500 μm microbeads (Cospheric®), (2) exposed to three uncured, fluorescent 3–5 mm long polyester microfibers (J & P Coats® Model No. C11V24.0009), and (3) control group (no microplastic exposure). Polyester has been found to be the most prominent type of fiber found in beach sediment (Browne et al. 2011). Microfibers were obtained by disassembling polyester thread into single fibers using forceps under a dissecting scope. Application of treatments occurred with the same feeding technique as Experiment 2, initiating a feeding response with a 1 mL mixture of Golden Pearls® and seawater and placing the microbead or fiber on the polyps with a plastic transfer pipette. Each fragment was visually monitored to ensure ingestion; microplastics not ingested were removed from chamber. All PVC chambers were observed to quantify microbeads in the water indicating that coral polyps had egested microbeads. Observations were made 48 hours after application.
Data were recorded as percentage of microbeads and microfibers egested per fragment and a one-way ANOVA was performed (Minitab 16, Inc.) along with Tukey’s post-hoc pairwise comparison.
3. Results
3.1. Experiment 1: Effects of ingested microbeads on calcification
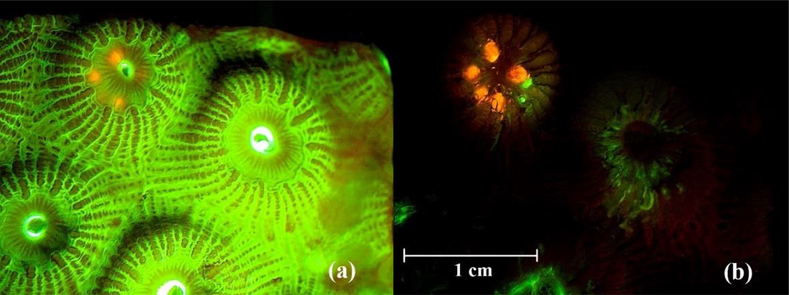
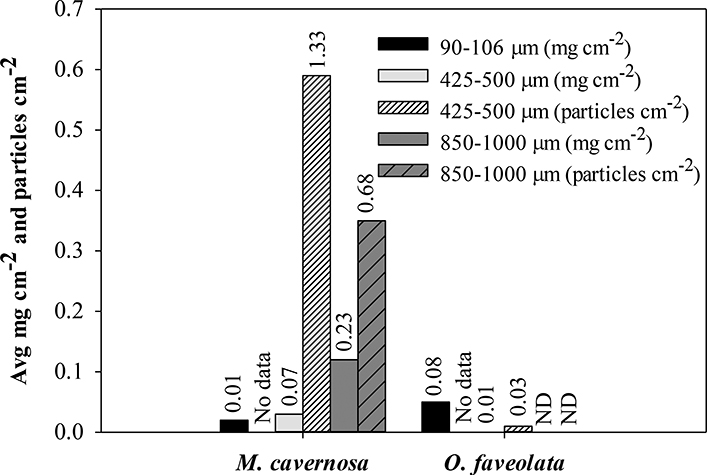
Calcification in the control group (M=42.55, SD=20.74 μmol) was slighter higher than the exposed treatment (M=39.34, SD=26.97 μmol), however, there was no significant difference between the two groups (ANOVA df=1, F=0.56, p>0.05). Of the 40 coral fragments in the exposed group, seven fragments contained microplastics from all size classes at the end of exposure. Of the microbeads that could be seen through tissue, it appeared that multiple microbeads were ingested by a single polyp (Figure 3). As ingestion was haphazard due to the chance encounter of microbead and coral polyp, the sum of the weight of each microbead size class was calculated for each species (Figure 4). Microbeads from the 90–106 μm size class were recovered in 11 coral fragments across all five control chambers, nine of which contained 1–5 microbeads and two of which contained an estimated 40–70 microbeads with weights ranging 0.0–0.8 mg. The consistency of the 90–106 μm microbeads was powder-like when dry which made them easily disperse and we speculate that contamination of control fragments was from air-borne microbeads.
Figure 3.
Photograph of Montastraea cavernosa under fluorescent stereomicroscope. (a) before removing coral tissue and (b) coral tissue partially removed showing ingested microbeads, five 850–1000 μm (orange) and two 425–500 μm (green) microbeads.
Figure 4.
Average weight (mg) and number of microbead particles per unit of surface area (cm2) ingested and recovered from coral fragments at time of tissue processing. No data for number of particles in the 90–106 μm size range. Numbers above bars indicated standard deviation. ND = not detected.
3.2. Experiment 2: Determination of ingestion size ranges (425 μm-2.8 mm) and retention times
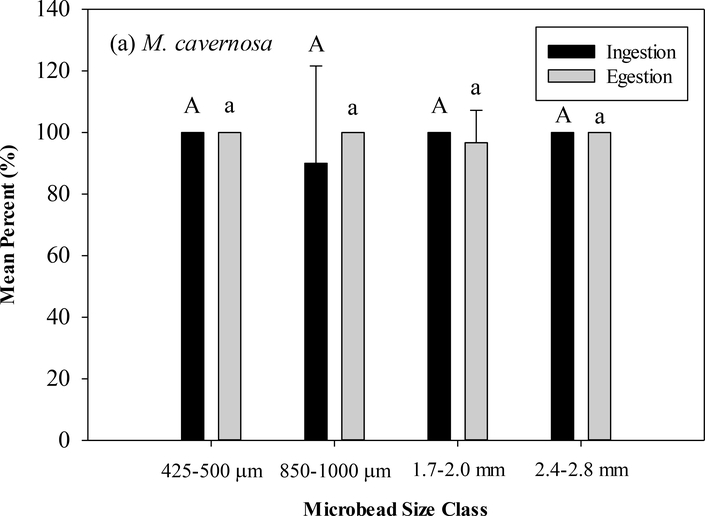
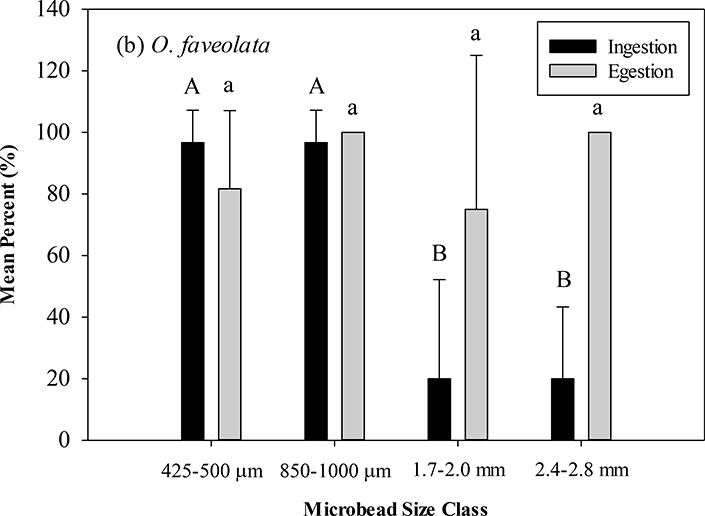
M. cavernosa ingested 100% of all microbeads offered in the 425–500 μm, 1.7–2.0 mm, and 2.4–2.8 mm size classes and a mean of 90.0% (SD=31.6) in the 850–1000 μm size class (Figure 5), with no significant differences between size classes (ANOVA, df=4, F=1.0, p>0.05) Significant differences (Tukey’s post-hoc p<0.05) ingestion were seen between the two smaller size classes and the two larger size classes with O. faveolata ingesting a mean of 96.7% for both the 425–500 (SD=10.5) and 850–1000 μm (SD=10.5) size classes and a mean of 20.0% for the 1.7–2.0 mm (SD=32.2) and 2.4–2.8 mm (SD=23.3) size classes (ANOVA, df=3, F=43.48, p<0.05) (Table 1). At 48 hours, M. cavernosa egested 100% of ingested microbeads from 425–500 μm, 850–1000 μm, and 2.4–2.8 mm size classes and a mean of 96.7% (SD=10.5) from the 1.7–2.0 mm size class. O. faveolata egested 100% of ingested microbeads from the 850–1000 μm and 2.4–2.8 mm size classes and means of 81.7% and 75.0% of the 425–500 μm (SD=24.4) and 1.7–2.0 mm (SD=50.0) size classes, respectively. No significant differences in egestion were detected in either species in any size class (M. cavernosa: ANOVA, df=3, F=0.96, p>0.05, O. faveolata: ANOVA, df=3, F=1.94, p>0.05).
Figure 5.
(a) Mean percent with standard deviation of ingested and egested microbeads in (a) M. cavernosa. (b) O. faveolata. Capital letters indicate significant difference between ingested size classes (Tukey’s post-hoc). Lower case letters indicate significant difference between egested size classes (Tukey’s post-hoc).
Table 1.
Mean percent average with standard deviation in ingested and egested microbeads and microfibers in M. cavernosa and O. faveolata.
| Microbead | Microfiber | |
|---|---|---|
| Ingestion | ||
| M. cavernosa | 100% (SD=0.0) | 100% (SD=0.0) |
| O. faveolata | 100% (SD=0.0) | 97.0% (SD=10.5) |
| Egestion | ||
| M. cavernosa | 100% (SD=0.0) | 100% (SD=0.0) |
| O. faveolata | 80.0% (SD=23.3) | 76.7% (SD=35.3) |
3.3. Experiment 3: Comparison of ingestion and retention of microbeads versus microfiber
M. cavernosa ingested 100% of the microbeads and microfibers (Table 1). O. faveolata ingested 100% of the microbeads and a mean of 96.7% (SD=10.5) of the microfibers, with no differences of ingestion (ANOVA, df=1, F=1.00, p>0.05). At 48 hours, M. cavernosa egested 100% of the microbeads and microfibers; O. faveolata egested means of 80.0% (SD=23.3) and 76.7% (SD=35.3) of ingested microbeads and microfibers, respectively, with no differences of egestion (ANOVA, df=1, F=0.06, p>0.05).
4. Discussion
The results of this study validate other studies showing that scleractinian coral ingest microplastics (Hall et al. 2015, Allen et al. 2017, Riechert et al. 2018) and identify several areas in need of further research. In this study, we did not find that ingestion significantly impacted calcification in two-day exposures. We observed that both the large polyp coral, M. cavernosa, and the small polyp coral, O. faveolata, ingested the largest size class (2.4–2.8 mm) offered, with both species egesting most microbeads within 48 hours. Additionally, we did not find a difference in retention times in the 425–500 μm microbead size class compared to the 3–5 mm long microfibers. While the largest microbeads offered to the coral were ingested, the smallest size microbead originally obtained for this work (212–250 μm) did not illicit a feeding response from coral, providing a lower bound of a size range that M. cavernosa and O. faveolata will actively ingest.
In the first experiment, microplastics did not impact overall coral calcification. However, this may be because only seven of 40 exposed fragments contained microbeads of all size classes upon the conclusion of the experiment. Conversely, preliminary studies leading up to these investigations showed that the coral fragments ingested every piece of microplastic that was hand-fed to them, upwards of five microbeads per fragment within a minute (unpublished data). Although corals were not directly fed in experiment 1, the amount of recaptured microbeads from coral tissue seemed relatively low even though microbeads were in suspension in the experimental chamber. Hall et al. (2015) observed similar responses with only a small percentage of polyps ingesting microplastics also for a two-day exposure. We hypothesized that the low number of recaptured microbeads in tissue was because of the ability of corals to egest indigestible items (Muscatine 1973, personal observations). Based on observations from experiment 1, experiment 2 was designed to determine if ingested microplastics are retained or egested by the coral polyp.
Montastraea cavernosa and Orbicella faveolata actively ingested microbeads ranging in size from 425 μm −2.8 mm, providing a basis to identify a size range for microplastic ingestion by coral. Feeding responses (e.g. active ingestion) in our experiments were defined by tentacle capture of microplastic, delivery to the mouth, and enveloping the particle. The 212–250 μm size class did not elicit a feeding response in either species, notwithstanding, that does not mean that corals will not ingest small microplastics. Small or light particles may inadvertently be ingested with larger particles as may have been the case in experiment 1 with the 90–106 μm size class. Since the largest microplastic offered (2.8 mm) was ingested, a maximum ingestion size for either species was not determined in this study. Ingestion of the two largest size classes was less in O. faveolata compared to M. cavernosa. Retention varied by species with O. faveolata generally retaining more microplastics than M. cavernosa. Allen et al. (2017) also showed retention of microplastic in the scleractinian coral, Astrangia poculata, with 8% retention of 500–1000 μm microplastics. Although microbeads are of concern, they represent only one style of plastic.
Microfibers, or plastic threads from synthetic textiles, are another plastic of concern in the aquatic environment due to contamination from sewage and wastewater discharge (Browne et al. 2011). Microfibers have been found in surface waters, throughout the water column, and in marine sediments (Brown et al. 2011, Cincinelli et al. 2017). Results of experiment 3 show that microfibers 3–5 mm in length can be just as easily ingested as microbeads less than 1 mm. Additionally, egestion within each species was roughly the same for microfibers and microbeads. By these comparisons, microfibers pose no greater potential harm than microbeads, however, this is a cautionary statement as ingestion rates, are likely not only species-specific, but size specific as well, as seen in the different rates of 1.7–2.0 mm and 2.4–2.7 mm microbead size classes in M. cavernosa and O. faveolata.
Although the results of this study do not identify direct acute effects of microplastic ingestion by corals, the impact of cumulative consumption remains a concern. Of the microplastics that are not expelled, a coral polyp could retain enough microplastic material to block their digestive tract. Coral polyps are interconnected by gastrovascular canals within the coenechyme, allowing for the transport of organic compounds throughout the colony (Gateño et al. 1998). Presuming that retained microplastics could be transported by this mechanism, ingestion of microplastics by even a few polyps may diminish nutrition throughout the entire colony. Additionally, repeated egestion of microplastics by coral polyps comes with an assumed energetic cost which could eventually have adverse effects on the coral colony. We found that M. cavernosa and O. faveolata egest at least 75% of all microplastics. While our two-day exposure did not demonstrate a calcification effect, a longer exposure may elicit a difference response.
Another emerging issue of ingested microplastics is pollutant toxicity. In addition to the polymer composition and potential additives, plastic debris will also adsorb contaminants, including metals (often referred to as “cocktail of contaminants” or “plastisphere”), from the water column (Mato et al. 2001, Ogata et al. 2009, Van et al. 2012, Rochman et al. 2013a, Ashton et al. 2010, Holmes et al. 2012, Rochman et al. 2013a). These cocktails have been shown to be bioavailable (via ingestion) across many genera in the food web (Fossi et al. 2012, Teuten et al. 2009, Besseling et al. 2013, Rochman et al. 2013b, Chua et al. 2014). Contaminant uptake by coral could have adverse effects that include reduced fertilization, metabolism, and photosynthesis (Pait et al. 2007). Additionally, pathogens have been found on microplastics (Zettler et al. 2013, Kirstein et al. 2016), potentially making them vectors for infectious diseases, such as those caused by Vibrio coralliilyticus (Ben-Haim et al. 2003).
This study observed the ingestion and egestion rates of two scleractinian coral species and shows that corals egest most of the microplastic that they ingest. The results advise that sampling coral may not provide the true number or amount of microplastics that they have previously ingested, only what remains at the time of sampling. Additionally, results of this study suggest small polyp corals, rather than large polyp corals, may be affected more if the retained microplastics inhibits consumption and/or digestion of exogenous food sources. The additional stress of plastics on coral is an area of increasing concern and thus far is demonstrating negative consequences. Marine debris containing plastic, aluminum, glass, and metal has been shown to reduce coral cover (Richards and Beger 2011). Additionally, corals are 4–89% more likely to become diseased when exposed to macroplastics (Lamb et al. 2018). Lastly, microplastics on the surface of coral can cause tissue necrosis (Reichert et al. 2018).
Global stressors such as elevated temperature and increased atmospheric carbon dioxide are impacting coral reef habitats worldwide (Hughes et al. 2003, Hoegh-Guldberg et al. 2007). A better understanding of the interactions between coral and microplastics can help inform local policy development and mitigation strategies. Based on our work, future research with longer exposure, different size classes of microbeads and microfibers, as well as introduction of pollutants via microplastics in a controlled laboratory setting would help guide protection on a local level and improve the resiliency of coral, making them less susceptible to global environmental threats.
Acknowledgements.
We thank Kay Ho, Sandy Raimondo, and Charles LoBue, for providing reviewer assistance and for the support from U.S. Environmental Protection Agency’s (EPA) Region 2. We would also like to thank NOAA’s Florida Keys National Marine Sanctuary and Mote Marine Laboratory (Summerland Key, FL) for their assistance with coral collection. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA.
REFERENCES
- Allen AS, Seymore A, Rittschof D, 2017. Chemoreception drives plastic consumption in hard coral. Marine Pollution Bulletin 124:198–205. [DOI] [PubMed] [Google Scholar]
- Andrady AL, 2011. Microplastics in the marine environment. Marine Pollution Bulletin 62:1596–1605. [DOI] [PubMed] [Google Scholar]
- Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R, 2009. Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Functional Ecology 23(3):539–550. [Google Scholar]
- Ashton K, Holmes L, Turner A, 2010. Association of metals with plastic production pellets in the marine environment. Marine Pollution Bulletin 60:2050–2055. [DOI] [PubMed] [Google Scholar]
- Baltz DM, Morejohn GV, 1976. Evidence from seabirds of plastic particle pollution off central California. Western Birds 7:111–112. [Google Scholar]
- Ben-Haim Y, Zicherman-Keren M, Rosenburg E, 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Applied and Environmental Microbiology 69(7):4326–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseling E, Wegner A, Foekema E, Van Den Heuvel-Greve M, Koelmans AA, 2013. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environmental Science and Technology 47:593–600. [DOI] [PubMed] [Google Scholar]
- Boerger CM, Lattin GL, Moore SL, Moore CJ, 2010. Plastic ingestion by planktivorous fishes in North Pacific Central Gyre. Marine Pollution Bulletin 60(12):2275–2278. [DOI] [PubMed] [Google Scholar]
- Borneman EH, 2001. Water chemistry: parameters to know and maintain for success with coral T.H.F. Publications, Inc., Aquarium corals: Selection, Husbandry, and Natural History, Neptune City, NJ: pp 343–365. [Google Scholar]
- Boucher J, Friot D, 2017. Primary microplastics in the oceans: a global evaluation of sources. Gland, Switzerland: IUCN; 43 pp. [Google Scholar]
- Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R, 2011. Accumulation of microplastic on shorelines worldwide: sources and sinks. Environmental Science and Technology 45:9175–9179. [DOI] [PubMed] [Google Scholar]
- Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO, 2014. Assimilation of Polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environmental Science and Technology 48(14):8127–8134. [DOI] [PubMed] [Google Scholar]
- Chisholm JRM, Gattuso JP, 1991. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnology and Oceanography 36(6):1232–1239. [Google Scholar]
- Cincinelli A, Scopetani C, Chelazzi D, Lombardini E, Martellini T, Katsoyiannis A, Fossi MC, Corsolini S, 2017. Microplastic in the surface waters of the Ross Sea (Antarctica): occurrence, distribution and characterization by FTIR. Chemosphere 175:391–400. [DOI] [PubMed] [Google Scholar]
- Delbeek CJ, Sprung J, 2005. Physical and chemical parameters of reef aquarium water The Reef Aquarium: Science, Art, and Technology Volume 3 Two Little Fishies, Inc. d.b.a. Ricordea Publishing, Coconut Grove, FL: pp 132–197. [Google Scholar]
- Enzor LA, Hankins C, Vivian DN, Fisher WS, Barron MG, 2018. Calcification in Caribbean reef-building corals at high pCO2 levels. Journal of Experimental Marine Biology and Ecology 499:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA, 2017. Lost, but found with Nile Red: a novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples. Environmental Science and Technology 51(23):13641–13648. [DOI] [PubMed] [Google Scholar]
- Fournie JW, Vivian DN, Yee SH, Courtney LA, Barron MG, 2012. Comparative sensitivity of six scleractinian corals to temperature and solar radiation. Diseases of Aquatic Organisms 99:85–93. [DOI] [PubMed] [Google Scholar]
- Fossi MC, Panti C, Guerranti C, Coppola D, Giannetti M, Marsili L, Minutoli R, 2012. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Marine Pollution Bulletin 64:2374–2379. [DOI] [PubMed] [Google Scholar]
- Gateño D, Israel A, Barki Y, Rinkevich B, 1998. Gastrovascular circulation in a octocoral: evidence of significant transport of coal and symbiont cells. Biological Bulletin 194(2):178–186. [DOI] [PubMed] [Google Scholar]
- GESAMP 2015. Sources, fate and effects of microplastics in the marine environment: a global assessment, Kershaw PJ ed. IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection; Rep. Stud. GESAMP no. 90, 96 pp. [Google Scholar]
- Geyer R, Jambeck JR, Law KL, 2017. Production, use, and fate of all plastics ever made. Science Advances 3:e1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottoli AG, Rogridues LJ, Palardy JE, 2006. Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189. [DOI] [PubMed] [Google Scholar]
- Hall NM, Berry KLE, Rintoul L, Hoogenboom MO, 2015. Microplastic ingestion by scleractinian corals. Marine Biology 162:725–732. [Google Scholar]
- Hays H, Cormons G, 1974. Plastic particles found in tern pellets, on coastal beaches and at factory sites. Marine Pollution Bulletin 5(3):44–46. [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards J, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME, 2007. Coral reefs under rapid climate change and ocean acidification. Science 318(5857):1737–1742. [DOI] [PubMed] [Google Scholar]
- Holmes L, Turner A, Thompson RC, 2012. Adsorption of trace metals to plastic resin pellets in the marine environment. Environmental Pollution 160:42–48. [DOI] [PubMed] [Google Scholar]
- Holmes-Farley R, 2004. Reef aquarium water parameters. Reefkeeping Online 3(4): retrieved from http://reefkeeping.com/issues/2004-05/rhf/index.php. [Google Scholar]
- Houlbrèque F, Ferrier-Pages C, 2009. Heterotrophy in tropical scleractinian corals. Biological Reviews 84:1–17. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson C, Kleypas J, Lough JM, Marshall P, Nyström M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J, 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301(5635):929–933. [DOI] [PubMed] [Google Scholar]
- Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL, 2015. Plastic waste inputs from land into the ocean. Science 347(6223):768–771. [DOI] [PubMed] [Google Scholar]
- Johannes RE, Wiebe WJ, 1970. Method for determination of coral tissue biomass and composition. Limnology and Oceanography 15(5):822–824. [Google Scholar]
- Kinsey DW, 1978. Alkalinity changes and coral reef calcification. Limnology and Oceanography 23:989–991. [Google Scholar]
- Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G, 2016. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Marine Environmental Research 120:1–8. [DOI] [PubMed] [Google Scholar]
- Lamb JB, Willis BL, Fiorenza EA, Couch CS, Howard R, Rader DN, True JD, Kelly LA, Ahmad A, Jompa J, Harvell CD, 2018. Plastic waste associated with disease on coral reefs. Science 359:460–462. [DOI] [PubMed] [Google Scholar]
- Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T, 2001. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environmental Science and Technology 35(2):318–324. [DOI] [PubMed] [Google Scholar]
- Muscatine L, 1973. Nutrition of corals Jones OA, Endean R eds. Biology and Geology of Coral Reefs Vol 2 Biology I, Academic Press, Inc; New York, pp 77–115 [Google Scholar]
- Ogata Y, Takada H, Mizukawa K, Hirai H, Iwasa S, Endo S, Mato Y, Saha M, Okuda K, Nakashima A, Murakami M, Zurcher N, Booyatumanondo R, Zakaria MP, Dung LQ, Gordon M, Miguez C, Suzuki S, Moore C, Karapanagioti HK, Weerts S, McClurg T, Burres E, Smith W, Van Velkenburg M, Lang JS, Lang RC, Laursen D, Danner B, Stewardson N, Thompson RC, 2009. International pellet watch: global monitoring of persistent organic pollutants (POPs) in coastal water. 1. Initial phase data on PCBs, DDTs, and HCHs. Marine Pollution Bulletin 58(10):1437–1446. [DOI] [PubMed] [Google Scholar]
- Ohlendorf HM, Risebrough RW, Vermeer K, 1978. Exposure of marine birds to environmental pollutants. U.S. Fish and Wildlife Service Research Report 9, Washington D.C. [Google Scholar]
- Pait AS, Whitall DR, Jeffrey CFG, Caldow C, Mason AL, Christensen JD, Monaco ME, Ramirez J, 2007. An assessment of chemical contaminants in the marine sediments of southwest Puerto Rico. Silver Spring, MD, NOAA/National Centers for Coastal Ocean Science, NOAA Technical Memorandum NOS NCCOS, 52. [Google Scholar]
- Palardy JE, Rodrigues LJ, Grottoli AG, 2008. The importance of zooplankton to the daily metabolic carbon requirements of health and bleached corals at two depths. Journal of Experimental Marine Biology and Ecology 367(2):180–188. [Google Scholar]
- Parslow JLF, Jefferies DJ, 1972. Elastic thread pollution of puffins. Marine Pollution Bulletin 3(3):43–45. [Google Scholar]
- Porter JW, Fitt W, Spero H, Rogers CS, White MW, 1989. Bleaching in reef corals: physiological and stable isotopic responses. Proceedings of the National Academy of Science 86:9342–9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards ZT, Beger M, 2011. A quantification of the standing stock of macro-debris in Majuro lagoon and its effect on hard coral communities. Marine Pollution Bulletin 62:1693–1701. [DOI] [PubMed] [Google Scholar]
- Riechert J, Schellenberg J, Schubert P, Wilke T, 2018. Responses of reef building corals to microplastic exposure. Environmental Pollution 237:955–960. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Hoh E, Hentschel BT, Kaye S, 2013a. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environmental Science and Technology 47:1646–1654. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Hoh E, Kurobe T, Teh SJ, 2013b. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Scientific Reports 3:3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein SI, 1973. Plastic particle pollution of the surface of the Atlantic Ocean: evidence from a seabird. The Condor 75:344–366. [Google Scholar]
- Smith SV, Kinsey DW, 1978. Calcification and organic carbon metabolism as indicated by carbon dioxide Stoddard DR Johannes RE eds. Coral Reefs: Research Methods. United Nations Educational, Scientific, and Cultural Organization, Paris, France: pp 469–484. [Google Scholar]
- Sussarellu R, Suquet M, Thomas Y, Lambert Y, Lambert C, Fabioux C, Pernet MEJ, Le Goïc N, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A, 2016. Oyster reproduction is affected by exposure to polystyrene microplastics. Proceedings of the National Academy of Sciences of USA 113(9):2430–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuten EL, Rowland SJ, Galloway TS, Thompson RC, 2007. Potential for plastics to transport hydrophobic contaminants. Environmental Science and Technology 41:7759–7764. [DOI] [PubMed] [Google Scholar]
- Teuten EL, Saquing JM, Knappe DRU, Barlaz MA, Jonsson S, Björn A, Rowland SJ, Thompson RC, Galloway TS, Yamashita R, Ochi D, Watanuki Y, Moore C, Viet PH, Tana TS, Prudente M, Boonyatumanond R, Zakaria MP, Akkhavong K, Ogata Y, Hirai H, Iwasa S, Mizukawa K, Hagino Y, Imamura A, Saha M, Takada H, 2009. Transport and release of chemicals from plastics to the environment and to wildlife. Philosophical Transactions of the Royal Society B 365:2027–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RC, 2015. Microplastics in the marine environment: sources, consequences and solutions Bergmann M, Gutow L and Klages M eds. Marine Anthropogenic Litter. Springer, Berlin, Germany, pp 185–200. [Google Scholar]
- Van A, Rochman CM, Flores EM, Hill KL, Vargas E, Vargas SA, Hoh E, 2012. Persistent organic pollutants in plastic marine debris found on beaches in San Diego, California. Chemosphere 86(3): 258–263. [DOI] [PubMed] [Google Scholar]
- Wagner J, Wang Z, Ghosal S, Rochman C, Gassel M, Wall S, 2017. Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Analytical Methods 9:1479–1490. [Google Scholar]
- Watts AJ, Urbina MA, Corr S, Lewis C, Galloway TS, 2015. Ingestion of plastic microfibers by the crab Carcinus maenas and its effect on food consumption and energy balance. Environmental Science and Technology 49(24):14597–14604. [DOI] [PubMed] [Google Scholar]
- Wright SL, Rowe D, Thompson RC, Galloway TS, 2013. Microplastic ingestion decreases energy reserves in marine worms. Current Biology 23:R1031–R1033. [DOI] [PubMed] [Google Scholar]
- Ye S, Andrady AL, 1991. Fouling of floating plastic debris under Biscayne Bay exposure conditions. Marine Pollution Bulletin 22(12): 608–613. [Google Scholar]
- Zettler ER, Mincer TJ, Amaral-Zettler LA, 2013. Life in the “plastisphere”: microbial communities on plastic marine debris. Environmental Science and Technology 47:7137–7146. [DOI] [PubMed] [Google Scholar]