Abstract
The Notch signaling pathway is involved in cell proliferation and differentiation, and has been recognized as an active pathway in regenerating tissue and cancerous cells. Notch signaling inhibition is considered a viable approach to the treatment of a variety of conditions including colorectal cancer, pancreatic cancer, breast cancer and metastatic melanoma. The discovery that the b-annulated dihydropyridine FLI-06 (1) is an inhibitor of the Notch pathway with an EC50 ≈ 2.5μM prompted us to screen a library of related analogs. After structure activity studies were conducted, racemic compound 7 was identified with an EC50 = 0.36 μM. Synthesis of individual enantiomers provided (+)-7 enantiomer with an EC50 = 0.13 μM, or about 20-fold the potency of 1
Keywords: Notch, Dihydropyridines, Notch signaling, Colorectal cancer
Graphical Abstract
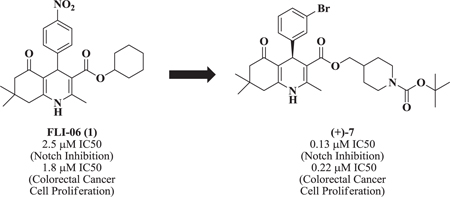
The Notch signaling pathway is involved in cell proliferation and differentiation, and has been recognized as an active pathway in regenerating tissue and cancerous cells. Notch signaling inhibition is considered a viable approach to the treatment of a variety of conditions including colorectal cancer, pancreatic cancer, breast cancer and metastatic melanoma. The discovery that the b-annulated dihydropyridine FLI-06 (1) is an inhibitor of the Notch pathway with an EC50 ≈ 2.5μM prompted us to screen a library of related analogs. After structure activity studies were conducted, racemic compound 7 was identified with an EC50 = 0.36 μM. Synthesis of individual enantiomers provided (+)-7 enantiomer with an EC50 = 0.13 μM, or about 20-fold the potency of 1.
The Notch pathway plays a role in cell fate during development by regulating processes such as proliferation, differentiation and apoptosis1,2. In the adult, the Notch pathway is limited to small populations of cells in regenerating tissues3,4. In many cancers, the Notch cascade is reactivated5, playing a significant role in metastasis and resistance to chemotherapy6,7,8,9. In mammals, Notch ligands (i.e., Jagged1, Jagged2, Delta-like 1, Delta-like 3, or Delta-like 4) interact with one of four (i.e., Notch1-Notch4) Notch receptors of an adjacent cell. The receptor-ligand interaction leads to proteolytic cleavage, including cleavage by γ-secretase in the transmembrane domain releasing the Notch intracellular domain (NICD) into the cytoplasm. Translocation of NICD into the nucleus and combination with RBP-J (a DNA binding protein also known as CSL) and coactivators induces transcription of target genes.
Because defects in Notch signaling underlie numerous pathologies, therapeutic intervention has been attempted with γ-secretase inhibitors (GSIs) that block the proteolytic activation of Notch10,11,12 . At issue with GSIs is their inherent non-selectivity and off-target effects that have led to the abandonment of the approach in a variety of studies. For example, a clinical trial for Alzheimer’s disease with the GSI Semagacestat was halted early because of undesirable but probably on-target effects of the GSI13.
The dihydropyridine 1 (i.e., FLI-0614) is a Notch inhibitor that works in the intracellular space by a mechanism different from that of secretases. FLI-06 was postulated to inhibit general secretion at a step before exit from the endoplasmic reticulum (ER), making it the first in a new class of Notch signaling inhibitors that act at an early stage of the secretory traffic. These results prompted us to evaluate a proprietary library of dihydropyridines15 in a Notch assay. Several potent Notch inhibitors emerged from this study. Further, one candidate was synthesized in enantioselective form and excellent enantioselectivity (i.e., 20-fold) of Notch inhibition was observed. Herein, we report on the first enantioselective Notch inhibitor. In addition, enantiomerically pure compound 7 (i.e., (+)-7), FLI-06 and DAPM were examined as anti-proliferative agents in a model of colon cancer. Compound (+)-7 potently inhibited proliferation of colon cancer cells, in vitro.
The b-annulated dihydropyridine FLI-06 (i.e., 1, Figure 1) was found to decrease endogenous Notch signaling in vitro and to cause unique phenotypic effects in vivo. Because we had previously identified the structurally related ITD-115 (i.e., 2, Figure 1) as an inhibitor of TGFβ signaling, a small library (59 members) of b-annulated dihydropyridines was screened in a Notch signaling assay.
Figure 1.
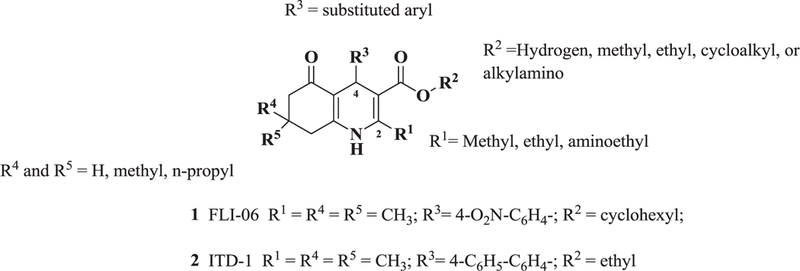
General structure of b-annulated dihydropyridines examined and chemical structures of FLI-06 (1) and ITD-1 (2)
The substitution pattern around the dihydropyridines in the library compounds can be conceptually divided into four regions as illustrated in Figure 1. At the C-2 position there were mostly methyl and ethyl substituents, as well as some (<10) amino-substituted analogs. The majority of the substituents at C-3 were relatively small alkyl groups, along with some alkylamino substituents. The R3 group present at C-4 was generally a substituted aryl. The C-7 substituents R4 and R5 were either hydrogen, methyl or propyl groups, with a large representation of gem-dimethyl groups in the library (R4 = R5 = methyl).
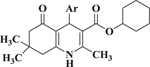
The bioactivity of these compounds was evaluated in a cell-based luciferase reporter assay, that monitors the activation of Notch1 downstream gene expression. In this assay, the intracellularly released NICD is translocated to the nucleus where it induces the expression of a luciferase reporter (see Supplementary Materials). In the structure activity studies for Notch inhibition it was consistently observed that in more potent analogs, methyl groups were present at positions R1, R4 and R5 and lipophilic groups were present at the R2 position (Figure 1). In agreement with an earlier report14, when the R2 group was a small alkyl ester such as methyl or ethyl, there was no inhibition of Notch pathway signaling. Accordingly, we synthesized another library (i.e., 20) additional dihydropyridines. We used a lipophilic ester and determined the effect of various substituents on the R3 aryl position. When a set of cyclohexyl esters was evaluated there was not a great deal of improvement in the potency for either electron rich or electron deficient substituents on the aromatic ring of R3 (Table 1). For example, compared to 1, compounds 3 – 5 showed low potency (IC50 > 10 μM). Of the group of cyclohexyl esters examined, only the 3-bromophenyl analog (i.e., compound 6, IC50 = 4.15 μM) showed potency in the range of FLI-06. Therefore, we examined dihydropyridines with 3-bromophenyl substitution where the ester group was modified.
Table 1.
Effect of modification of C-4 aryl cyclohexyl esters of 1,4-DHPs on Notch inhibition. fx
 |
| Compound Number |
R | IC50 |
|---|---|---|
|
1 (FLI-06) |
2.51 | |
| 3 |  |
12.3 |
| 4 | 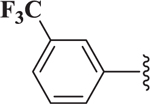 |
14.7 |
| 5 | 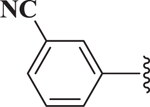 |
43.2 |
| 6 | 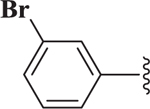 |
4.15 |
Compared to 6, when 3-bromophenyl was the 4-substituent in the 1,4-DHP ester analogs (Table 2), there was a significant improvement in potency observed for analog 7 (IC50 = 0.36μM). Modifications of the linker between the ester oxygen and the Boc-protected piperidino group led to loss of potency (i.e., 8 and 9, IC50 values of 2.95 and >10 μM, respectively). Replacement of the cyclohexyl ester group by an adamantyl group (i.e., 12) also decreased potency. Replacement of the piperidinomethyl group of 7 with 4-t- butylbenzyl (11) also led to lower potency (i.e., IC50 = 4.27 μM). The 2-piperazinomethyl compound (i.e., 10) was relatively potent (IC50 = 0.86 μM). The diastereomeric mixture of the branched benzylic derivative 13 was poorly potent as a Notch inhibitor (i.e., IC50 values of >10 μM).
Table 2.
Effect of ester modification of 4-(3-bromophenyl) 1,4-DHPs on Notch inhibition. fx
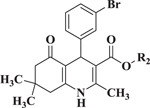 |
| Compound Number |
R2 | IC50 (μM) |
|---|---|---|
| 6 | 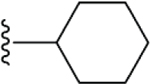 |
4.15 |
| 7 | 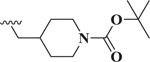 |
0.36 |
| 8 | 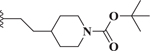 |
2.95 |
| 9 | 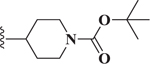 |
>10 |
| 10 | 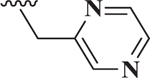 |
0.86 |
| 11 | 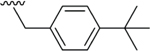 |
4.27 |
| 12 | 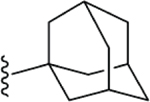 |
> 10 |
| 13 | 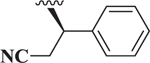 |
> 10 |
Based on the potency of (N-Boc-4-piperidinomethyl) esters of 1,4-DHPs (e.g., compound 7) the effect of the 1,4-DHP aromatic group on Notch inhibition was determined (Table 3). Preliminary studies showed that compared to 3-substitution with the protected piperidinomethyl group in place, 4-substituted 1,4-DHPs showed low Notch inhibition potency. For example, para-aryl substituted compounds 22 and 23 (i.e., IC50 > 10 μM) were not potent. In contrast, there was generally greater Notch inhibition potency with 3-substituted aryl 1,4-DHPs. For example, 3-cyanophenyl (i.e., 15, IC50 = 0.22 μM), and the 2-quinolinyl (i.e., 20, IC50 = 0.33 μM) analogs were potent Notch inhibitors with IC50 comparable to that of 7. In general, there was greater tolerance for variation on the 4-aryl substituent for N-Boc-4-piperidinomethyl esters (Table 3) than when the ester group was cyclohexyl (Table 1). Electron-deficient 3-substituted 4-aryl 1,4-DHPs (e.g., 15 and 19 with IC50 values of 0.22 and 0.76 μM, respectively) appeared to be more potent Notch inhibitors than electron-rich 3-substituted 4-aryl 1,4-DHPs (e.g., 16 and 18 with IC50 values of 1.5 and 3.3 μM, respectively).
Table 3:
Effect of C-4 aryl substituents of (N-Boc-4-piperidinomethyl) ester-substituted 1,4-DHPs on Notch inhibition. fx
| Compound Number |
Ar | IC50 (μM) |
|---|---|---|
| 7 |  |
0.36 |
| 14 | 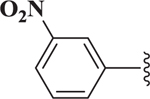 |
>10 |
| 15 | 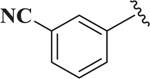 |
0.22 |
| 16 | 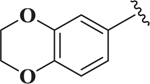 |
1.52 |
| 17 | 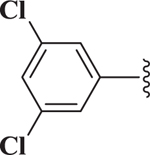 |
0.64 |
| 18 |  |
3.32 |
| 19 | 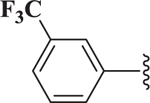 |
0.76 |
| 20 | 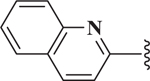 |
0.33 |
| 21 | 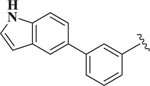 |
0.77 |
| 22 | > 10 | |
| 23 | 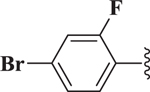 |
> 10 |
| (−)-7 | 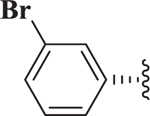 |
2.59 |
| (+)-7 | 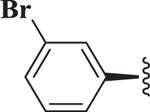 |
0.13 |
It appeared that when the ester group was a piperidinomethyl, the modifications around the C-4 aromatic ring had an effect that did not parallel the same substitution when the ester group was cyclohexyl. We speculate that the higher affinity of the substituted piperidinomethyl group forces the functional groups around the aromatic ring into positions that are not accessible when the weaker binding cyclohexyl was present.
1,4-DHPs possess a center of chirality and significant stereoselectivity was observed for Notch inhibition by 1,4-DHPs. For example, the two enantiomers of 7 were synthesized (see below) and evaluated for inhibition of Notch. Significant stereoselectivity of Notch inhibition was observed. The (R)-(+)-enantiomer (i.e., (+)-7, IC50 = 127 nM) was about 20-fold more potent than the (S)-(−)-enantiomer (i.e., (−)-7, IC50 = 2.59 μM). The data suggests the binding pocket for inhibition of Notch was quite sensitive to stereochemistry of 1,4-DHP inhibitors.
The GSI N-[N-3,5-difluorophenacetyl]-l-alanyl-S-phenylglycine methyl ester (DAPM) was reported by Miyamoto et al16 to inhibit the proliferation of colorectal cancer cells (HCT-116) by a mechanism that involved interference with the Notch pathway. To test this report and compare DAPM with compound (+)-7, the effect of (+)-7, FLI-06 and DAPM was evaluated as inhibitors of colon cancer cell proliferation. Incubation of HCT-116 cells for 72 h with either vehicle, DAPM, FLI-06, or (+)-7 resulted in dose-dependent inhibition of cell proliferation (Figure 1, Supplementary Materials). The gamma-secretase inhibitor DAPM had an IC50 of 12 μM in this assay, consistent with the results from Miyamoto et al16. The b-annulated dihydropyridines, FLI-06 and (+)-7 showed IC50 values of 1.8 and 0.22 μM, respectively (Table 4). The results show that (+)-7 enantioselectively inhibits colon cancer cell proliferation more potently than FLI-06. Accordingly, (+)-7 could be a drug candidate for treatment of conditions (i.e., colon cancer) where Notch signaling is known to be enhanced.
Table 4.
Effect of DAPM, FLI-06 and (+)-7 on HCT-116 colorectal cancer cell proliferation.
| Compound | Structure | IC50 ± SD (µM)a |
|---|---|---|
| DAPM | 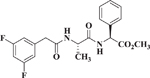 |
12 ± 4 |
| FLI-06 | 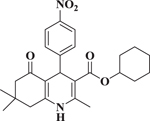 |
1.8 ± 0.3 |
| (+)-7 |  |
0.22 ± 0.03 |
The fact that FLI-06 appears to block protein secretion from the ER14 suggests a different mechanism of action from that of GSIs. Nonetheless, FLI-06 and (+)-7 are useful tool compounds that support the concept that blocking secretion from the ER in specific cancer cells can play a key role in inhibition of their proliferation. The results for (+)-7 show that a new selective Notch inhibitor or Notch ligand secretion blocker can lead to an effective anti-cancer agent.
The synthesis of b-annulated-1,4-dihydropyridines was described previously15. In the example shown for preparation of racemic 7, one equivalent of dimedone 24, one equivalent of 3-bromobenzaldehyde 25, one equivalent of β-ketoester 26, one equivalent of ammonium acetate and 0.3 equivalents of iodine were stirred overnight at room temperature in ethanol (Scheme 1).
Scheme 1:
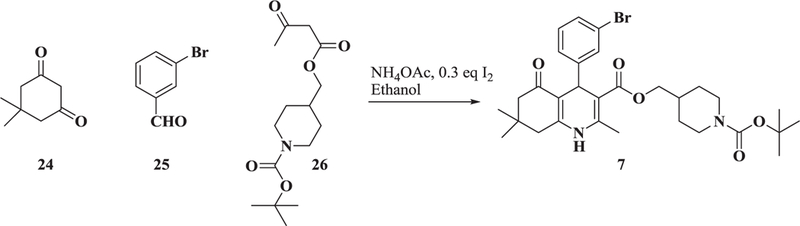
Synthesis of racemic 7.
Because 7 contains a center of chirality, it was important to prepare the (+) - and (−)- enantiomers and test them separately as Notch inhibitors. The synthesis of (+)-7 is shown in Scheme 2: the chiral carboxylic acid (+)-2715 was esterified with the precursor bromide in DMF with potassium carbonate in the presence of tetra-n-butyl ammonium iodide to yield (+)-7. Chiral HPLC analysis showed the enantiomeric excess was >99%. The (−) enantiomer was generated by the same sequence with similar results.
Scheme 2:
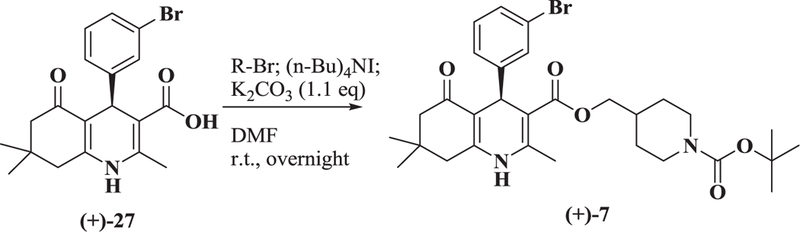
Synthesis of (+)- 7
The configuration at C-4 was assigned by analogy with the configuration of R-(+)-ITD-1 that was reported by Längle et al17.
In summary, we have shown that dihydropyridines are a viable scaffold for the identification of novel compounds that interfere with the Notch pathway. We identified the single enantiomer (+)-7 as an inhibitor of Notch signaling that is 20-fold more potent than FLI-06. (+)-7 has anti-proliferative effects in a colorectal cancer cell line that are approximately 55-fold greater than those observed with the GSI inhibitor DAPM. This shows that targeting the effect of Notch activation in colon cancer cells by a mechanism different from the use of gamma-secretase inhibitors affords a potent anti-cancer compound.
Supplementary Material
HIGHLIGHTS.
Notch trafficking plays a role in many cancer processes
Evaluated b-annulated dihydropyridines in a Notch reporter assay
Identified chiral (+)-7 as a potent inhibitor of Notch trafficking.
(+)-7 also inhibited proliferation in a colorectal cancer cell line (HCT-116)
ACKNOWLEDGEMENTS
This work was funded by NIH grant R01HL132225 (to M. Mercola) and discretionary funds from the Human BioMolecular Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTARY MATERIALS
Experimental procedures for the synthesis and characterization of 7 and (+)-7 and (−)-7. Procedures for the Notch reporter assay and for the proliferation assay on colorectal cancer cell lines can be found in the online version at.
REFERENCES
- 1.Hoppe PE, Greenspan RJ. Local function of the NOTCH gene for embryonic ectodermal pathway choice in Drosophila. Cell 1986;46:773–783. DOI:10.1016/0092-8674(86)90353-3. [DOI] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 1999;284:770–776. DOI: 10.1126/science.284.5415.770 [DOI] [PubMed] [Google Scholar]
- 3.Sander GR, Powell BC. Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem 2004;52:509–516. [DOI] [PubMed] [Google Scholar]
- 4.Stump G, Durrer A, Klein A- L, Lutolf S, Suter U, Taylor V. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev 2002;114:153–159. DOI: 10.1016/S0925-4773(02)00043-6 [DOI] [PubMed] [Google Scholar]
- 5.Ranganathan P, Weaver KL, Capobianco AJ. Notch signaling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 2011:338–351. [DOI] [PubMed] [Google Scholar]
- 6.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci 2007;64:2746–2762 DOI: 10.1007/s00018-007-7164-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer 2003;3:756–767. [DOI] [PubMed] [Google Scholar]
- 8.Abel EV, Kim EJ, Wu J, Hynes M, Bednar F, Proctor E, Wang L, Dziubinski ML, Simeone DM 2014. The Notch Pathway Is Important in Maintaining the Cancer Stem Cell Population in Pancreatic Cancer. PLoS ONE 2014: 9:91983 DOI:10.1371/journal.pone.0091983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinoza I, Pochampally R, Xing F, Watabe K, Miele L. Notch signaling: targeting cancer stem cells and epithelial-to-mesenchymal transition. Onco Targets Ther 2013;6:1249–1259. DOI: 10.2147/OTT.S36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han J, Shen Q. Targeting gamma-secretase in breast cancer. Breast cancer. 2012; 4: 83–90. DOI: 10.2147/BCTT.S26437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Wen H, Brayton C, Das P, Smithson LA, Fauq A, Fan X, Crain BJ, Price DL, Golde TE, Eberhart CG, Wong PC. Epidermal growth factor receptor and notch pathways participate in the tumor suppressor function of gamma-secretase. J Biol Chem. 2007; 282: 32264–73. DOI: 10.1074/jbc.M703649200 [DOI] [PubMed] [Google Scholar]
- 12.Lee SM, Moon J, Redman BG, Chidiac T, Flaherty LE, Zha Y, Othus M, Ribas A, Sondak VK, Gakewski TF, Margolin KA. Phase 2 study of RO49209097, a gamma-secretase inhibitor, in metastatic melanoma: SWOG 0933. Cancer 2015; 121: 432–440. DOI: 10.1002/cncr.29055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, Aisen PS. A Phase 3 Trial of Semagacestat for Treatment of Alzheimer’s Disease. N Engl J Med 2013; 369: 341–350. [DOI] [PubMed] [Google Scholar]
- 14.Kramer A, Mentrup T, Kleizen B, Rivera-Milla E, Reichembach D, Enzensperger C, Nohl R, Täuscher E, Görls H, Ploubidou A, Englert C, Werz O, Arndt H, Kaether C. Small molecules intercept Notch signaling and the early secretory pathway Nat. Chem. Biol. 2013; 9: 731–738. DOI: 10.1038/nchembio.135 [DOI] [PubMed] [Google Scholar]
- 15.Schade D, Lanier M, Willems E, Okolotowicz K, Bushway P, Wahlquist C, Gilley C, Mercola M, Cashman JR. Synthesis and SAR of b-annulated 1,4-dihydropyridines define cardiomyogenic compounds as novel inhibitors of TGFβ signaling J. Med. Chem. 2012; 55: 9946–9957. DOI: 10.1021/jm301144g\ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto S; Nakanishi M and Rosenberg DW Suppression of colon carcinogenesis by targeting Notch signaling. Carcinogenesis 2013. 34:2415–2423 DOI:10.1093/carcin/bgt191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Längle D, Marquardt V, Heider E, Vigante B, Duburs G, Luntena I, Flotgen D, Golz C, Strohmann C, Koch O, Schade D. Design, synthesis and 3D-QSAR studies of novel 1,4- dihydropyridines as TGFβ/Smad inhibitors Eur J Med Chem. 2015; 95: 249–266. DOI: 10.1016/j.ejmech.2015.03.02 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


