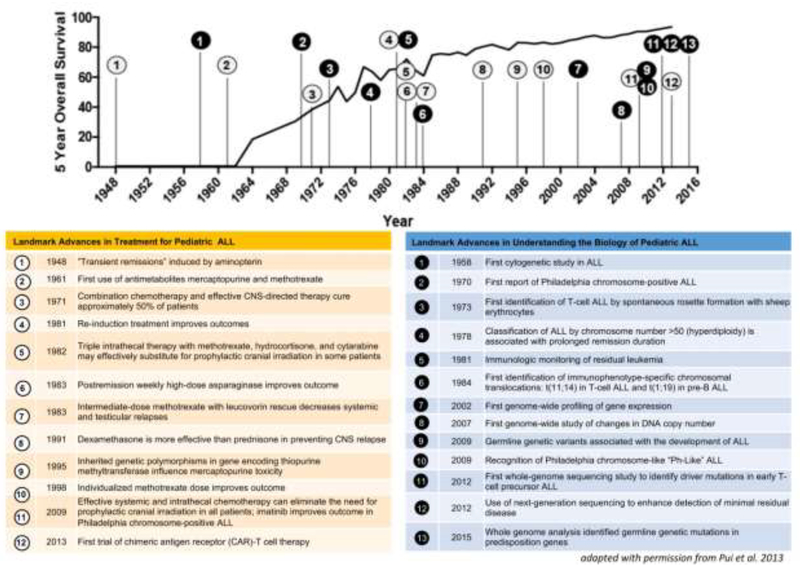
Advances in the diagnosis and treatment of pediatric acute lymphoblastic leukemia (ALL) are one of the greatest successes of modern medicine. Over the past 50 years, there has been a rapid increase in overall survival for pediatric ALL (Figure 1). Several factors have led to these remarkable improvements. First is the development of risk-adapted therapy based on both clinical and biologic presenting features, as well as early response to treatment1. Second, the effectiveness of molecularly targeted agents for specific genetic abnormalities has boosted outcomes for some high-risk groups1. Third, international collaboration among clinical trial networks has led to standardization of definitions and reporting of results that allows comparison of data across multiple national study groups to identify optimal treatment (Table; available at www.jpeds.com)1–3. Now, the long-term survival rate for pediatric ALL is approaching 90% in many high income countries, the highest of any type of leukemia in either children or adults, 4. These remarkable achievements notwithstanding, there remain a number of challenges in ALL pathology and treatment that need to be addressed. Relapse still occurs in 10–15% of patients, and death due to relapsed ALL remains one of the leading causes of cancer mortality in children. Conventional cytotoxic chemotherapy continues to be associated with both short- and long-term toxic effects and is unlikely to be modified substantially in the near future. Thus, it will be important to take advantage of emerging molecular and immunologic insights to improve risk stratification and to devise targeted therapies to avoid over- or under-treatment.
Figure 1: Landmark advances in pediatric Acute Lymphoblastic Leukemia (ALL).
5-year overall survival data for pediatric ALL from the Surveillance, Epidemiology, and End Results (SEER) Program120 is overlaid with landmark advances in the treatment (white; left table) and in understanding the biology of pediatric ALL (black; right table).
In this review, we discuss advances in the diagnosis and treatment of pediatric ALL that are reshaping the landscape of this disease. In the future, all patients may undergo genetic sequencing of both cancer and germline genomes to increase the precision of risk stratification and hence the specificity of treatment. Patients with high-risk genetic subtypes or poor response to treatment, as measured by minimal residual disease (MRD), may benefit from molecularly targeted therapy or immunotherapy. With advances in identifying molecular lesions that are amenable to targeted therapy and in developing risk-adapted therapy for ALL, we believe that precision medicine will drive ALL treatment in the future.
Evaluation and Risk Stratification
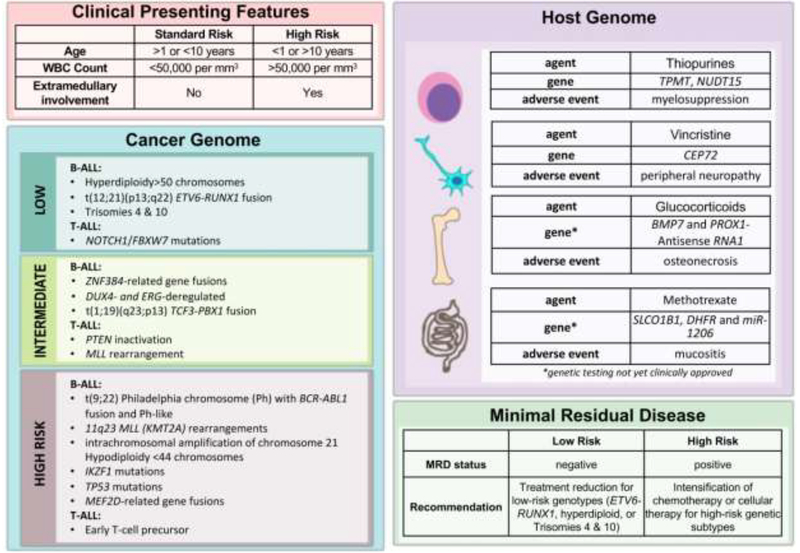
Risk stratification of patients with pediatric ALL is used to determine the optimal type, intensity and duration of treatment. We will discuss risk stratification based on the following factors: (1) clinical presenting features, (2) leukemia genetic subtype (3) germline cancer predisposition, and (4) initial response to treatment as measured by MRD (Figure 2).
Figure 2: Factors influencing risk stratification and outcome for patients with ALL.
Clinical presenting features, leukemia cell genetics (cancer genome), host germline genome and minimal residual disease should be considered when determining risk-adapted therapy. Advances in whole genome sequencing of both host (germline) and cancer (leukemia) genomes has deepened our understanding of genetic factors that determine risk (high-, intermediate-, and low-risk) and influence treatment response and toxicity of cytotoxic chemotherapy agents.
Clinical presenting features.
Age and white blood cell (WBC) count at presentation are used to risk stratify patients with pediatric ALL, according to National Cancer Institute (NCI) consensus criteria5. High-risk features include age less than 1 year or older than 10 years, and WBC>50,000 cells per cubic millimeter. Extramedullary involvement at sanctuary sites such as the central nervous system and testes and prolonged pre-treatment with corticosteroids are also considered high-risk features.
In addition to presenting features, genetic evaluation has important prognostic and therapeutic implications. Increasingly, genome sequencing technologies are being used to diagnose and guide therapy for pediatric ALL.
Next-generation sequencing (NGS).
Next-generation sequencing (NGS) is the latest genomic sequencing technology that enables high-throughput, massively parallel DNA sequencing6. In pediatric ALL, NGS is increasingly being used to comprehensively define the somatic genetic alterations and the role of inherited genetic variants in leukemogenesis, to monitor drug response and treatment toxicity, and to enhance the sensitivity of MRD detection compared with current methods7–15. Indeed, NGS of both host and cancer genomes is required to develop truly personalized risk-adapted therapy (Figure 2).
Genetic subtypes of ALL.
Pediatric ALL is a genetically heterogeneous disease, arising from the malignant transformation of hematopoietic cells at various stages of lymphoid development. Efforts to define the mutational landscape of pediatric ALL have revealed three major categories of genetic alterations: (1) chromosomal translocations, (2) duplications or deletions of large segments of DNA, and (3) point mutations in oncogenes or tumor suppressors16. Based on genetic alterations, patients with B- and T-ALL may be stratified into high-, intermediate-, or low-risk categories (Figure 2).
High-risk subtypes.
Patients with high-risk disease should be identified early in therapy because they benefit from intensified induction and consolidative treatment. Among patients with B-ALL, a high-risk group harbor the “Philadelphia” chromosome (Ph), the product of a t(9;22) translocation that results in BCR-ABL1 fusion, which can be treated with ABL1 tyrosine kinase inhibitors (TKI). Ph+ ALL generally occurs in older children and has a poor prognosis overall17.
‘Ph-like ALL’ is another high-risk subtype of B-ALL characterized by a gene expression profile and a high frequency of IKZF1 alterations similar to Ph+ ALL but lacking BCR ABL1 fusion14. Among this clinically and biologically heterogeneous group, a wide range of genetic alterations in Ph-like ALL results in dysregulation of several cytokine receptors and kinase signaling pathways12, 18. Alterations in IKZF1 are an independent risk factor associated with poor prognosis18–22. Additional treatment strategies are needed for this high-risk group, particularly for a subset of patients termed IKZFplus with certain co-occurring mutations that confer the worst prognosis among patients with Ph-like ALL23. Notably, a subset of these IKZF1plus patients with rapid early response had excellent outcomes, emphasizing the utility of MRD assessment in assigning risk of relapse, even among high-risk ALL.
Older children are also more likely to harbor rearrangements involving the MEF2D gene, a recently identified fusion partner that carries a high risk of relapse24, 25. For some high-risk groups, such as those with intrachromosomal amplification of chromosome 21 (iAMP21), intensification of conventional chemotherapy has led to a reduced risk of relapse26–29. Treatment outcome remains poor for infant ALL. Among infants with B-ALL, chromosomal translocations involving the mixed lineage leukemia (MLL/KMT2A) gene are common and are associated with high rates of induction failure and relapse30.
Hypodiploid ALL with less than 44 chromosomes is another high-risk subtype of ALL8, 31. Of interest, low-hypodiploid ALL with 32 to 39 chromosomes is characterized by a high frequency of genetic alterations of TP53 that are often inherited31. A recent study suggested that response-adapted treatment can improve outcomes in patients with lowhypodiploid ALL who attain MRD-negative status after remission induction, as these patients have a high cure rate with intensive chemotherapy31.
Among patients with T-ALL, those with early T-cell precursors (ETP)-ALL have an especially poor response to induction therapy32–34. Patients in this high-risk group typically lack specific chromosomal rearrangements, but they share a distinct gene expression profile and immunophenotype of a subset of thymocytes that retain stem cell-like features32. Recent studies suggest that ETP-ALL patients may benefit from intensive consolidative treatment with cyclophosphamide, mercaptopurine and cytarabine and may not require HSCT35, 36.
Intermediate-risk subtypes.
Patients with intermediate-risk disease require intensive chemotherapy to prevent relapse and are not candidates for treatment reduction. Approximately half of all cases of B-ALL would be classified as intermediate-risk, including those recently found to have translocations involving the ZNF384 gene24, 37. T-ALL patients with abnormalities in the tumor suppressor gene PTEN have intermediate outcomes, though prognosis depends on the mechanism of PTEN inactivation38–40. MLL rearrangements have also been observed in T-ALL, but these patients have better outcomes than those with B-ALL and MLL rearrangements41, 42.
Low-risk subtypes.
Patients with low-risk subtypes of pediatric ALL may be considered excellent candidates for treatment reduction to decrease the toxicity associated with intensive chemotherapy. However, such reduction should be applied judiciously to ‘rapid early responders’ with negative MRD and favorable leukemia genetic subtype so that the overall cure rate is not compromised. In a recent study, ‘standard-risk’ patients (defined by favorable age 1 to 10 years and WBC <50,000 cells per cubic millimeter) with unfavorable leukemia genetics who were ‘rapid early responders’ had worse outcomes when delayed intensification treatment was reduced43. We contend that only B-ALL patients with low-risk genetic features such as ETV6-RUNX1 fusion (previously known as TEL-AML1), trisomies 4 and 10, or hyperdiploid ALL and rapid early response to treatment following 1–2 weeks of 3-drug remission induction are good candidates for treatment reduction43. In patients with T-ALL, those with NOTCH/FBXW7 mutations have favorable outcomes44–46.
Detecting minimal residual disease (MRD).
Risk-adapted therapy guided by MRD level measured during remission induction and consolidation treatment has contributed greatly to the improved outcome in pediatric ALL47, 48. Because it accounts for leukemic cell genetics, host pharmacogenetics, leukemia cell environment, and treatment efficacy, the MRD level has become an important prognostic factor in ALL49. High levels of MRD at the end of remission induction or persistent MRD after consolidative treatment are an indication for intensification of treatment or even HSCT.
Traditionally, MRD has been measured with multicolor flow cytometry or quantitative PCR (qPCR). Recent studies based on NGS showed enhanced sensitivity and specificity compared with standard methods of MRD detection in both B- and T-ALL50, 51. These results suggest that NGS may detect as few as 1 leukemic blast out of 1 million normal cells, corresponding to a 10-times greater sensitivity than qPCR and a 100-times greater sensitivity than flow cytometry52, 53. In this regard, negative MRD findings by NGS can identify a subgroup of ‘rapid early responders’ who appear to be at even lower risk of relapse with chemotherapy or after HSCT compared with those with negative MRD defined by flow cytometry or qPCR (with limits of <1 leukemic cell among 10,000 normal cells)54–56.
Predicting treatment failure and toxicity.
More recently, NGS has revealed genetic variations in the host and cancer genome that may predict the risk of treatment failure and toxicity associated with chemotherapy (Figure 2). For example, patients with B-ALL harboring activating mutations in the CREB binding protein (CREBBP) gene have high rates of relapse associated with resistance to glucocorticoids57, and those with activating mutations in NT5C2 or loss of function mutations in PRPS1 are resistant to thiopurines such as mercaptopurine58, 59. Understanding how these mutations confer drug resistance may inform the design of optimal frontline or salvage therapy.
Genome-wide analyses have also uncovered genetic variations in the host germline genome that are associated with particular adverse outcomes of chemotherapy (Figure 2). Both thiopurine methyltransferase (TPMT) and nudix hydrolase 15 (NUDT15) genetic polymorphisms, for example, have been associated with intolerance to thiopurine treatment; that is, patients with homozygous polymorphisms are at increased risk of life-threatening myelosuppression when exposed to conventional doses of thiopurines such as mercaptopurine60, 61. Similarly, patients with polymorphisms in the promoter region of the centrosomal protein 72 (CEP72) gene, which encodes a protein involved in microtubule assembly, have an increased risk of severe peripheral neuropathy when treated with vincristine62. More recent discoveries include genetic polymorphisms that are associated with increased risk of steroid-induced osteonecrosis and methotrexate-related mucositis63–65. As genome-wide analyses such as NGS become widely available, future treatment plans could utilize genomic technology to prospectively identify patients at high risk of treatment intolerance, in order to dose-adjust chemotherapy and minimize morbidity.
Cancer predisposition genes in pediatric ALL.
Epidemiologic studies have demonstrated different frequencies of pediatric ALL among various ethnic groups. Children of Hispanic or Native American ancestry have the highest incidence of ALL, followed by those of European descent, and finally by those of African descent66. Population-based genome-wide association studies have revealed ethnicity-specific polymorphisms that may account for these patterns. For example, the highest frequencies of polymorphisms in the ARID5B, BMI1-PIP4K2A, and GATA3 genes were found among Hispanics67–69. These germline risk alleles can also influence treatment outcome, as illustrated by GATA3 variants associated with Ph-like ALL and a poor outcome69.
Recent studies revealed germline mutations in cancer predisposition genes associated with a high risk of developing ALL, such as TP53, PAX5, ETV6, and IKZF1 in up to 5% of pediatric patients with ALL70–75. Such results have implications not only for the patients but also for their families, as close relatives may benefit from genetic testing, counseling and surveillance. Notably, patients with TP53 germline variations also have increased risk of relapse and development of second cancers76.
Personalized Treatment
Pediatric ALL is a highly disseminated and heterogeneous disease, warranting the current focus on personalized treatment. At most centers, therapy for this disease is tailored to the patient’s features: (1) specific genetic subtype, (2) extent of disease, and (3) drug sensitivity as assessed by MRD after remission induction or consolidative treatment. Patients with high-risk genetic subtypes, disseminated disease involving sanctuary sites such as the central nervous system or testes, or a positive MRD finding after remission induction, typically require more intensive chemotherapy to prevent recurrence. Although uniform treatment of large groups of patients has become a relic of the past, there is still a need for standardized, protocol-directed therapy, which currently proceeds in 3 phases: induction, consolidative therapy, and maintenance.
Conventional cytotoxic chemotherapy.
Initial treatment with induction chemotherapy eliminates 99.99% or more of leukemia cells. This phase of treatment most often begins with 4 weeks of vincristine, a corticosteroid (prednisone or dexamethasone), and asparaginase, with the addition of an anthracycline (doxorubicin or daunorubicin) for patients with higher-risk leukemia77. The next phase of treatment aims to further reduce submicroscopic leukemia, and is therefore termed consolidative therapy. The duration, intensity, choice of agent, and number of treatment courses used during this phase varies according to risk group and protocol, but consolidative therapy typically involves high-dose methotrexate, mercaptopurine, asparaginase, dexamethasone, and vincristine, with or without an anthracycline. For high-risk patients, cytarabine and cyclophosphamide may be added to post-induction consolidative therapy; however, these agents may not be necessary for low- or standard-risk patients, especially in light of their potential effects on future fertility78. The final phase of treatment, the so-called maintenance or continuation component, aims to eradicate any remaining leukemic or pre-leukemic cells and consists of antimetabolites (daily mercaptopurine and weekly methotrexate) with or without pulses of a corticosteroid plus vincristine. Dexamethasone improves survival in patients <10 years old; however, prednisone is used in lieu of dexamethasone for patients >10 years old in some protocols out of concern for increased risk of osteonecrosis associated with dexamethasone treatment in this age group79. Although one study showed that two-thirds of patients (including 16 of the 18 patients with ETV6-RUNX1 B-ALL) could be cured with 1 year of maintenance treatment80, this phase typically lasts for 2 to 2.5 years in virtually all contemporary protocols, as there are no reliable markers to identify patients who may be cured with abbreviated treatment.
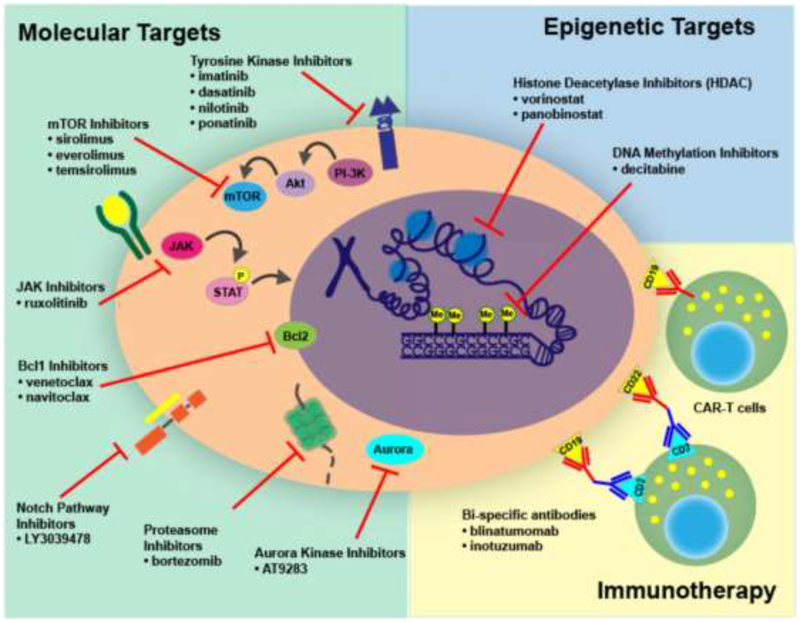
It is quite possible that cytotoxic chemotherapy may one day be replaced by shorter treatments with targeted agents (Figure 3), but until the genes and pathways essential for leukemia cell survival can be targeted with certainty, the need for distinct phases of chemotherapy will likely persist.
Figure 3: Mechanisms of therapy (molecular, epigenetic, and immunologic) for pediatric ALL.
Precision medicine has revolutionized treatment of pediatric ALL by providing novel molecular and epigenetic targets, in addition to immunotherapeutic approaches to cure patients with relapsed/refractory disease.
Targeted therapy.
Next-generation sequencing has revolutionized the treatment of pediatric ALL by revealing genetic alterations that are amenable to targeted therapy. Indeed, initiatives such as the St. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome Project (PCGP) and the Therapeutically Applicable Research to Generate Effective Therapies (TARGET) Project have uncovered a number of novel genetic alterations in pediatric ALL81, 82. Thus far, trials of promising targeted therapies have significantly improved outcomes for some high-risk groups, such as patients with Ph+ ALL and subsets of those with Ph-like ALL with ABL-class fusion transcripts. Novel immunotherapeutic approaches, including chimeric antigen receptor-based cellular therapies have also cured a substantial portion of patients with highly refractory leukemia, some of whom had relapsed after HSCT (Figure 3). A representative selection of immunologic and genetically based treatment strategies are described below.
ABL1 tyrosine kinase inhibitors in Ph+ ALL.
The first successful use of precision medicine in childhood ALL began with the discovery that Ph+ B-ALL with the BCR-ABL1 fusion were sensitive to the ABL1 tyrosine kinase inhibitors (TKIs)83–86. Once associated with dismal prognosis despite HSCT17, the outcome for Ph+ ALL has significantly improved since the ABL1 TKI imatinib was incorporated into an intensive chemotherapy regimen83–86. Dasatinib, a second-generation ABL1 TKI targeting multiple kinases, has comparable safety and efficacy to imatinib, and reduced the need for HSCT in a recently completed phase II clinical trial (NCT01460160)87, 88. Ponatinib, a third-generation ABL1 TKI, is more effective than earlier generations of ABL1 TKIs in adults; however, due to the associated toxicities,89 it should be used and dosed judiciously in children.
ABL1 TKIs and Janus kinase (JAK) inhibitors in Ph-like ALL.
Ph-like ALL is characterized by a wide range of genetic alterations that dysregulate several cytokine receptor and kinase signaling pathways, including CRLF2 rearrangement in half of cases and translocation of non-receptor tyrosine kinases (predominantly ABL-class and JAK)90. Patients with ABL-class fusions (including ABL1, ABL2, CSF1R, PDGFRB and PDGFRA) respond clinically to ABL1 TKIs, whereas in preclinical models mutations activating the JAK-STAT pathway have been amenable to treatment with JAK inhibitors (e.g., ruxolitinib)12. Ongoing prospective studies are testing whether incorporating a TKI targeting kinase alterations into intensive chemotherapy regimens will improve outcome in patients with Ph-like ALL (NCT02723994, NCT03117751)91, 92.
BCL2 inhibitors.
Preclinical studies have shown that a new class of drugs targeting the transcription factor BCL2 holds promise for MLL (KMT2A)-rearranged ALL and Ph+ALL93, 94. The BCL2 inhibitors venetoclax and navitoclax work by forcing leukemic cells to undergo apoptotic programmed cell death93. A phase II clinical trial of venetoclax in adults with relapsed or refractory chronic lymphocytic leukemia demonstrated a response rate of approximately 80%95. Pediatric clinical trials of venetoclax are currently underway for children with relapsed or refractory ALL (NCT03236857)96.
FMS-like tyrosine kinase 3 (FLT3) inhibitors.
MLL-rearranged ALL displays constitutive activation of FMS-like tyrosine kinase (FLT3), and a subset harbor genetic alterations in this gene97, 98. In MLL-rearranged ALL, FLT3 alterations are associated with a poor prognosis99, 100. A phase III trial of the FLT3 inhibitor lestaurtinib (CEP-701) in combination with cytotoxic chemotherapy showed no benefit over chemotherapy alone (NCT00557193)101, 102. Phase I/II trials have been conducted for the FLT3 inhibitors sorafenib103, midostaurin104, and quizartinib (AC220)105 demonstrating safety and tolerability, but further trials are needed to determine efficacy.
Nucleoside analogs.
Nucleoside analogs are currently in clinical trials for B-ALL and TALL. A phase II trial of the purine analog clofarabine in combination with cyclophosphamide and etoposide for relapsed B-ALL demonstrated an overall response rate of 44%106. For newly-diagnosed T-ALL, a recent phase III trial of the purine analog nelarabine in combination with intensive chemotherapy demonstrated improved disease-free survival without excessive toxicity107. In the future, incorporating nelarabine into upfront therapy for T-ALL may become standard of care.
Immunotherapy.
Chimeric antigen receptor T cells (CAR-T cells).
For patients with relapsed B-ALL, the most significant advance in the past decade has been the development of immunotherapies using chimeric antigen receptor-T cells (CAR-T cells). These genetically engineered autologous T cells are able to recognize and kill leukemic B cells bearing their target antigen108. The most commonly used CAR consists of an extracellular immunoglobulin domain that recognizes CD19 and an intracellular T-cell signaling domain that activates T cells to kill CD19+ leukemia cells (Figure 3). Because normal B-cells also express CD19, these patients also develop B-cell aplasia and require monthly intravenous immunoglobulin replacement therapy109. Unlike chemotherapy, where the therapeutic window is limited by the pharmacokinetics of drug clearance, CAR-T cells can persist for months or years in vivo, depending on the costimulatory molecule used, to provide long-term immune surveillance against leukemia cells110.
The first pediatric phase I trial of CD19-directed CAR-T cell therapy (CTL019) demonstrated complete remissions in 93% of multiply relapsed B-ALL patients, two-thirds of whom had prior HSCT111, 112. Remarkably, relapse-free survival rates at 6 and 12 months were 76% and 55%, respectively111, 112. In patients who relapsed, the leukemic cells evaded immunotherapy by two mechanisms: (1) CAR-T cells did not persist, and (2) the leukemia re-emerged as a CD19 negative clone111, 113. To circumvent the problem of CD19 escape, CAR-T cells targeting another B-ALL associated antigen (CD22) have been developed114. In addition, tandem CARs recognizing both CD19 and CD22 on leukemic B cells is another strategy currently in preclinical development115. Phase II trials of CAR-T cell therapy are currently underway.
In 2017, the FDA designated CD19-directed CAR-T cells a “Breakthrough Therapy,” and approved CTL019 for relapsed pediatric and adult B-ALL. Given the therapeutic results reported thus far, it seems reasonable to predict that CAR-T cell-based immunotherapy will eventually be incorporated into frontline treatments for high-risk pediatric ALL, and possibly could replace HSCT for patients with relapsed or refractory ALL.
Bi-specific T cell engagers (BiTEs).
Currently, autologous CAR-T cell therapy has several limitations, including a lengthy and costly manufacturing process that sometimes results in failure to produce these genetically engineered cells. Therefore, some patients with rapidly progressive leukemia are unable to receive CAR-T cell therapy116. An alternative immunotherapeutic class of drugs called bi-specific T-cell engagers (BiTEs) can eliminate these obstacles. BiTEs contain 2 domains: (1) a CD19 or CD20 recognition domain that binds to leukemic B cells, and (2) a T cell-receptor recognition domain that binds and activates T cells to kill leukemic B cells116, 117. These bi-specific antibodies can be used in patients for whom no T cells are available, and are available immediately as “off the shelf” products.
The most potent drug of this class — blinatumomab — had a response rate of 39% in a phase I/II trial of patients with relapsed or refractory B-ALL117. Another study demonstrated durable remissions in pediatric patients who had undergone HSCT118. However, in patients with overt or multiple relapses, blinatumomab by itself did not induce durable remissions, and instead was used as a bridge to HSCT117. Ongoing clinical trials are testing whether blinatumomab in combination with intensive chemotherapy can improve outcomes in patients with newly diagnosed B-ALL and persistent MRD after remission and consolidative therapy, in the absence of HSCT.
Conclusions
Future progress in pediatric ALL research will be driven not only by advances in science and technology, but also by greater international collaboration among investigators to standardize risk group classification, definition of treatment response, and toxicity criteria. Innovations in genomic sequencing can be expected to aid in diagnosis, identify targetable lesions, and guide risk stratification to optimize therapy, and novel therapies will likely become available for various disease subtypes. At the same time, efforts to optimize immunotherapy for relapsed and refractory disease should yield clear breakthroughs in this challenging research area. Whether immunotherapy and molecular targeting strategies will ultimately replace cytotoxic chemotherapy for ALL remains unclear, although recent observations of durable complete remissions among patients responding to targeted therapies after failing chemotherapy or hematopoietic stem cell transplantation (HSCT) suggest a major impact for this approach in the future. Finally, as more attention is directed toward smaller subsets of patients with drug-resistant leukemia, the importance of collaborative international research will grow considerably so that therapeutic gains in high income countries can be translated to patients in middle or low income countries119. Finally, increasing our understanding of the biology of ALL and factors that predispose patients to leukemia may lead to preventive measures to decrease the risk of ALL in the future.
Table I;
online: Results of Recent Major Clinical Trials of Pediatric ALL
| Study group | Trial design | Number of patients | 5-Year EFS | 5-Year OS | Main finding of the study |
| Induction Chemotherapy | |||||
| AIEOP-BFM ALL 2000121 | Randomized trial | 3727 | 83.9% (Dexamethasone) 80.8% (Prednisone) |
90.3% (Dexamethasone) 90.5% (Prednisone) |
Dexamethasone was superior to prednisone, and led to a significant reduction in the rate of relapse, but increased treatment-related mortality. |
| COG AALL023279 | Randomized trial | 3,154 | 91.2 ± 2.8% (DH) 83.2 ± 3.4% (DC) 82.1 ± 3.5% (PC) 80.8 ± 3.7% (PH) 75.2 ± 1.1% (overall) |
96.3 6 1.9% (DH) 92.3 6 2.4% (DC) 92.3 6 2.5% (PC) 92.7 6 2.4% (PH) 85.0 ± 0.9% (overall) |
Among high-risk patients, high-dose methotrexate (H) led to superior EFS and OS compared to Capizzi escalating-dose methotrexate (C), with no increase in toxicity. Dexamethasone (D) was more effective than prednisone (P) and led to improved EFS and OS in younger children, but led to higher risk of osteonecrosis in children >10 years old without improvement in EFS or OS. |
| DFCI 05–001122 | Randomized trial | 551 | 90% (PEG-asparaginase) 89% (E. coli asparaginase) 85 ± 1.5% (overall) |
96% (PEG-asparaginase) 94% (E. coli l asparaginase) 91 ± 1% (overall) |
PEG-asparaginase had similar toxicity and efficacy as native E coli l-asparaginase. |
| EORTC-CLG 58951123 | Randomized trial | 1947 | 81.5% (Dexamethasone) 81.2% (Prednisone) 82.6 ± 0.9% (overall) |
87.2% (Dexamethasone) 89.0% (Prednisone) 89.7± 0.7% (overall) |
Given during remission induction, dexamethasone led to a significant reduction in the rate of relapse, and had similar toxicity compared to prednisone. |
| Delayed Intensification/Consolidation Chemotherapy | |||||
| IC-BFM 2002124 | Randomized trial among 15 countries on 3 continents | 5197 | 74 ± 1% (overall) | 82 ± 1% (overall) | International collaborative clinical trials among middle income countries are feasible, but improved supportive care is necessary to prevent excessive mortality from intensive chemotherapy. |
| NOPHO ALL2008125 | Nonrandomized trial | 1908 | 89%±1% (1–9 years old) 80±3% (10–17 years old) 74±4% (18–45 years old) |
94±1% (1–9 years old) 87±2% (10–17 years old) 78±3% (18–45 years old) |
Pediatric-based treatment protocols are tolerable and effective for young adults. There was no benefit to EFS for individualized 6-mercaptopurine dosing during consolidation therapy. |
| Risk stratification | |||||
| CoALL 97126 | Nonrandomized trial | 667 | 80 ± 3% (PVA score 3+4) 73 ± 3% (PVA score 5–7) 63 ± 8% (PVA score 8+9) 76.7 ± 1.7% (overall) |
85.4 ± 1.4% (overall) | MRD was a superior prognostic indicator when compared with in vitro drug sensitivity testing based on PVA score (sensitivity to prednisolone, vincristine and asparaginase) for risk-stratification of patients. |
| DCOG ALL10127 | Nonrandomized trial | 865 | 93 ± 2% (standard-risk) 88 ± 2% (intermediate-risk) 78 ± 8% (high-risk) 87 ± 1.2% (overall) |
99 ± 1% (standard-risk) 92.3 ± 4% (intermediate-risk) 82.1 ± 12% (high-risk) 91.9 ± 1.0% (overall) |
Based on MRD risk stratification, chemotherapy was safely reduced in low-risk groups without compromising survival, and intensification of therapy improved EFS in intermediate- and high-risk groups. |
| JCCLSG ALL2000128 | Nonrandomized trial | 321 | 82.5 ± 2.6% (MRD) 74.7 ±5.7% (clinical) 79.7± 2.4% (overall) |
91.4 ± 1.9% (MRD) 85.3 ± 4.5% (clinical) 89.2 ± 1.8% (overall) |
MRD was a superior prognostic indicator when compared to clinical presenting features for risk-stratification of patients. |
| Ma-Spore 2003129 | Nonrandomized trial | 556 | 93.2 ± 4.1 %∇ (standard-risk) 91.2 ± 4.9%∇ (intermediate-risk) 51.8 ± 10.0%∇ (high-risk) 83.2 ± 3.5%∇ (overall) |
82.1 ± 3.3%∇ (standard-risk) 92.1 ± 3.3%∇ (intermediate-risk) 67.7 ± 11%∇ (high-risk) 75.2 ± 3.1%∇ (overall) |
Using risk stratification based on MRD as well as clinical and genetic features, chemotherapy was safely reduced in low-risk groups without compromising survival. |
| MRC UKALL 2003130 | Randomized trial | 3126 | 82.8± 4.7% (standard) 89.6 ± 3.7% (intensified) 87.3 ± 1.4% (overall) |
88.9± 3.9% (standard) 92.9± 3.1% (intensified) 91.6 ± 1.2% (overall) |
Intensification of therapy for MRD positive high-risk patients leads to better EFS but not OS, compared to standard therapy. Intensified therapy was also associated with more adverse events. |
| Adjuvant therapy to prevent CNS relapse | |||||
| Study group | Trial design | Number of patients | 5-Year EFS | 5-Year OS | Main finding of the study |
| SJCRH Total Therapy XV48,131 | Nonrandomized trial | 498 | 96.3 ± 2.6% (low-risk) 92.3 ± 4.5%(standard-risk) 74.7 ± 15.3% (high-risk) 79.7 ± 2.9% (overall) |
98.7 ± 1.3% (low-risk) 92.5 ± 3.0% (standard-risk) 67.9 ± 12.8% (high-risk) 93.5 ± 1.9% (overall) |
With effective MRD-guided systemic chemotherapy, optimal triple intrathecal therapy, and risk adjusted chemotherapy, prophylactic cranial irradiation can be safely omitted from the treatment all patients with pediatric ALL. |
| TPOG ALL 2002132 | Randomized trial | 1366 | 85.2 ±2.7% (one course) 89.8 ± 2.3% (two courses) 74.3 ± 1.2% (overall) |
91.6 ± 2.1% (one course) 93.7 ± 1.8% (two courses) 81.6 ± 1.1% (overall) |
There was no difference in EFS or OS in standard risk patients who received one versus two course of re-induction chemotherapy |
Abbreviations: AIEOP, Associazione Italiana di Ematologia Pediatrica; ALL, acute lymphoblastic leukemia; BFM, Berlin-Frankfurt-Münster; CoALL, Cooperative ALL (study group); COG, Children’s Oncology Group; DCOG, Dutch Children’s Oncology Group; DFCI, Dana-Farber Cancer Institute (consortium); DFS, disease-free survival; EFS, event-free survival; EORTC-CLG, European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group; IC-BFM, Intercontinental BFM; i.m., intramuscular; i.v., intravenous; IR, JCCLSG, Japanese Children’s Cancer and Leukemia Study Group; Ma-Spore, Malaysia-Singapore; MRC UKALL, Medical Research Council United Kingdom Acute Lymphoblastic Leukemia; MRD, minimal residual disease: N/A, not applicable; NOPHO, Nordic Society of Pediatric Hematology and Oncology; OS, overall survival; SJCRH, St Jude Children’s Research Hospital; TPOG, Taiwan Pediatric Oncology Group.
Results for 6-year EFS and OS are shown.
Acknowledgments
Supported in part by U.S. National Institutes of Health (CA21765, CA36401, and GM115279), and American Lebanese Syrian Associated Charities of St Jude Children’s Research Hospital.
List of abbreviations
- ALL
acute lymphoblastic leukemia
- B-ALL
B-cell acute lymphoblastic leukemia
- BiTEs
bi-specific T cell engagers
- CAR-T
chimeric antigen receptor T cells
- ETP-ALL
early T cell precursor acute lymphoblastic leukemia
- FLT3
FMS-like tyrosine kinase 3
- HSCT
hematopoietic stem cell transplantation
- JAK
Janus kinase
- NGS
next-generation sequencing
- MRD
minimal residual disease
- MLL
mixed lineage leukemia
- Ph+ ALL
Philadelphia chromosome positive-acute lymphoblastic l6eukemia
- Ph-like ALL
Philadelphia chromosome-like acute lymphoblastic leukemia
- T-ALL
T-cell acute lymphoblastic leukemia
- TKI
tyrosine kinase Inhibitor
- WBC
White blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- [1].Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J Clin Oncol. 2015;33:2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schrappe M, Camitta B, Pui CH, Eden T, Gaynon P, Gustafsson G, et al. Long-term results of large prospective trials in childhood acute lymphoblastic leukemia. Leukemia. 2000;14:2193–4. [DOI] [PubMed] [Google Scholar]
- [3].Schrappe M, Nachman J, Hunger S, Schmiegelow K, Conter V, Masera G, et al. ‘Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985–2000)’. Leukemia. 2010;24:253–4. [DOI] [PubMed] [Google Scholar]
- [4].Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50:185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. [DOI] [PubMed] [Google Scholar]
- [6].Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11:685–96. [DOI] [PubMed] [Google Scholar]
- [7].Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–64. [DOI] [PubMed] [Google Scholar]
- [8].Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L, et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet. 2013;45:242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu Y, Easton J, Shao Y, Maciaszek J, Wang Z, Wilkinson MR, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49:1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481:157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22:153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Andersson AK, Ma J, Wang J, Chen X, Gedman AL, Dang J, et al. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat Genet. 2015;47:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lindqvist CM, Lundmark A, Nordlund J, Freyhult E, Ekman D, Carlsson Almlof J, et al. Deep targeted sequencing in pediatric acute lymphoblastic leukemia unveils distinct mutational patterns between genetic subtypes and novel relapse-associated genes. Oncotarget. 2016;7:64071–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roberts KG, Mullighan CG. Genomics in acute lymphoblastic leukaemia: insights and treatment implications. Nat Rev Clin Oncol. 2015;12:344–57. [DOI] [PubMed] [Google Scholar]
- [17].Arico M, Valsecchi MG, Camitta B, Schrappe M, Chessells J, Baruchel A, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342:998–1006. [DOI] [PubMed] [Google Scholar]
- [18].Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kuiper RP, Waanders E, van der Velden VH, van Reijmersdal SV, Venkatachalam R, Scheijen B, et al. IKZF1 deletions predict relapse in uniformly treated pediatric precursor B-ALL. Leukemia. 2010;24:1258–64. [DOI] [PubMed] [Google Scholar]
- [20].Dorge P, Meissner B, Zimmermann M, Moricke A, Schrauder A, Bouquin JP, et al. IKZF1 deletion is an independent predictor of outcome in pediatric acute lymphoblastic leukemia treated according to the ALL-BFM 2000 protocol. Haematologica. 2013;98:428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Olsson L, Ivanov Ofverholm I, Noren-Nystrom U, Zachariadis V, Nordlund J, Sjogren H, et al. The clinical impact of IKZF1 deletions in paediatric B-cell precursor acute lymphoblastic leukaemia is independent of minimal residual disease stratification in Nordic Society for Paediatric Haematology and Oncology treatment protocols used between 1992 and 2013. Br J Haematol. 2015;170:847–58. [DOI] [PubMed] [Google Scholar]
- [22].van der Veer A, Waanders E, Pieters R, Willemse ME, Van Reijmersdal SV, Russell LJ, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122:2622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stanulla M, Dagdan E, Zaliova M, Moricke A, Palmi C, Cazzaniga G, et al. IKZF1(plus) Defines a New Minimal Residual Disease-Dependent Very-Poor Prognostic Profile in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. J Clin Oncol. 2018;36:1240–9. [DOI] [PubMed] [Google Scholar]
- [24].Liu YF, Wang BY, Zhang WN, Huang JY, Li BS, Zhang M, et al. Genomic Profiling of Adult and Pediatric B-cell Acute Lymphoblastic Leukemia. EBioMedicine. 2016;8:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Suzuki K, Okuno Y, Kawashima N, Muramatsu H, Okuno T, Wang X, et al. MEF2DBCL9 Fusion Gene Is Associated With High-Risk Acute B-Cell Precursor Lymphoblastic Leukemia in Adolescents. J Clin Oncol. 2016;34:3451–9. [DOI] [PubMed] [Google Scholar]
- [26].Harrison CJ, Moorman AV, Schwab C, Carroll AJ, Raetz EA, Devidas M, et al. An international study of intrachromosomal amplification of chromosome 21 (iAMP21): cytogenetic characterization and outcome. Leukemia. 2014;28:1015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heerema NA, Carroll AJ, Devidas M, Loh ML, Borowitz MJ, Gastier-Foster JM, et al. Intrachromosomal amplification of chromosome 21 is associated with inferior outcomes in children with acute lymphoblastic leukemia treated in contemporary standard-risk children’s oncology group studies: a report from the children’s oncology group. J Clin Oncol. 2013;31:3397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moorman AV, Richards SM, Robinson HM, Strefford JC, Gibson BE, Kinsey SE, et al. Prognosis of children with acute lymphoblastic leukemia (ALL) and intrachromosomal amplification of chromosome 21 (iAMP21). Blood. 2007;109:2327–30. [DOI] [PubMed] [Google Scholar]
- [29].Moorman AV, Robinson H, Schwab C, Richards SM, Hancock J, Mitchell CD, et al. Risk-directed treatment intensification significantly reduces the risk of relapse among children and adolescents with acute lymphoblastic leukemia and intrachromosomal amplification of chromosome 21: a comparison of the MRC ALL97/99 and UKALL2003 trials. J Clin Oncol. 2013;31:3389–96. [DOI] [PubMed] [Google Scholar]
- [30].Pui CH, Behm FG, Downing JR, Hancock ML, Shurtleff SA, Ribeiro RC, et al. 11q23/MLL rearrangement confers a poor prognosis in infants with acute lymphoblastic leukemia. J Clin Oncol. 1994;12:909–15. [DOI] [PubMed] [Google Scholar]
- [31].Mullighan CG, Jeha S, Pei D, Payne-Turner D, Coustan-Smith E, Roberts KG, et al. Outcome of children with hypodiploid ALL treated with risk-directed therapy based on MRD levels. Blood. 2015;126:2896–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Neumann M, Heesch S, Gokbuget N, Schwartz S, Schlee C, Benlasfer O, et al. Clinical and molecular characterization of early T-cell precursor leukemia: a high-risk subgroup in adult T-ALL with a high frequency of FLT3 mutations. Blood Cancer J. 2012;2:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Inukai T, Kiyokawa N, Campana D, Coustan-Smith E, Kikuchi A, Kobayashi M, et al. Clinical significance of early T-cell precursor acute lymphoblastic leukaemia: results of the Tokyo Children’s Cancer Study Group Study L99–15. Br J Haematol. 2012;156:358–65. [DOI] [PubMed] [Google Scholar]
- [35].Patrick K, Wade R, Goulden N, Mitchell C, Moorman AV, Rowntree C, et al. Outcome for children and young people with Early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2014;166:421–4. [DOI] [PubMed] [Google Scholar]
- [36].Conter V, Valsecchi MG, Buldini B, Parasole R, Locatelli F, Colombini A, et al. Early T-cell precursor acute lymphoblastic leukaemia in children treated in AIEOP centres with AIEOP-BFM protocols: a retrospective analysis. The Lancet Haematology. 2016;3:e80–e6. [DOI] [PubMed] [Google Scholar]
- [37].Gocho Y, Kiyokawa N, Ichikawa H, Nakabayashi K, Osumi T, Ishibashi T, et al. A novel recurrent EP300-ZNF384 gene fusion in B-cell precursor acute lymphoblastic leukemia. Leukemia. 2015;29:2445–8. [DOI] [PubMed] [Google Scholar]
- [38].Petit A, Trinquand A, Chevret S, Ballerini P, Cayuela JM, Grardel N, et al. Oncogenetic mutations combined with MRD improve outcome prediction in pediatric T-Cell Acute Lymphoblastic Leukemia. Blood. 2017. [DOI] [PubMed] [Google Scholar]
- [39].Tesio M, Trinquand A, Ballerini P, Hypolite G, Lhermitte L, Petit A, et al. Age-related clinical and biological features of PTEN abnormalities in T-cell acute lymphoblastic leukaemia. Leukemia. 2017. [DOI] [PubMed] [Google Scholar]
- [40].Jenkinson S, Kirkwood AA, Goulden N, Vora A, Linch DC, Gale RE. Impact of PTEN abnormalities on outcome in pediatric patients with T-cell acute lymphoblastic leukemia treated on the MRC UKALL2003 trial. Leukemia. 2016;30:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lo Nigro L, Mirabile E, Tumino M, Caserta C, Cazzaniga G, Rizzari C, et al. Detection of PICALM-MLLT10 (CALM-AF10) and outcome in children with T-lineage acute lymphoblastic leukemia. Leukemia. 2013;27:2419–21. [DOI] [PubMed] [Google Scholar]
- [42].Matlawska-Wasowska K, Kang H, Devidas M, Wen J, Harvey RC, Nickl CK, et al. MLL rearrangements impact outcome in HOXA-deregulated T-lineage acute lymphoblastic leukemia: a Children’s Oncology Group Study. Leukemia. 2016;30:1909–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schrappe M, Bleckmann K, Zimmermann M, Biondi A, Moricke A, Locatelli F, et al. Reduced-Intensity Delayed Intensification in Standard-Risk Pediatric Acute Lymphoblastic Leukemia Defined by Undetectable Minimal Residual Disease: Results of an International Randomized Trial (AIEOP-BFM ALL 2000). J Clin Oncol. 2018;36:244–53. [DOI] [PubMed] [Google Scholar]
- [44].Kox C, Zimmermann M, Stanulla M, Leible S, Schrappe M, Ludwig WD, et al. The favorable effect of activating NOTCH1 receptor mutations on long-term outcome in TALL patients treated on the ALL-BFM 2000 protocol can be separated from FBXW7 loss of function. Leukemia. 2010;24:2005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–71. [DOI] [PubMed] [Google Scholar]
- [46].Zuurbier L, Homminga I, Calvert V, te Winkel ML, Buijs-Gladdines JG, Kooi C, et al. NOTCH1 and/or FBXW7 mutations predict for initial good prednisone response but not for improved outcome in pediatric T-cell acute lymphoblastic leukemia patients treated on DCOG or COALL protocols. Leukemia. 2010;24:2014–22. [DOI] [PubMed] [Google Scholar]
- [47].Pui CH, Pei D, Raimondi SC, Coustan-Smith E, Jeha S, Cheng C, et al. Clinical impact of minimal residual disease in children with different subtypes of acute lymphoblastic leukemia treated with Response-Adapted therapy. Leukemia. 2017;31:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol. 2015;16:465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017;129:1913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E, et al. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120:5173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu D, Sherwood A, Fromm JR, Winter SS, Dunsmore KP, Loh ML, et al. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012;4:134ra63. [DOI] [PubMed] [Google Scholar]
- [52].van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–11. [DOI] [PubMed] [Google Scholar]
- [53].Coustan-Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M, et al. New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2011;117:6267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kotrova M, van der Velden VHJ, van Dongen JJM, Formankova R, Sedlacek P, Bruggemann M, et al. Next-generation sequencing indicates false-positive MRD results and better predicts prognosis after SCT in patients with childhood ALL. Bone Marrow Transplant. 2017;52:962–8. [DOI] [PubMed] [Google Scholar]
- [55].Pulsipher MA, Carlson C, Langholz B, Wall DA, Schultz KR, Bunin N, et al. IgHV(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood. 2015;125:3501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wood B, Wu D, Crossley B, Dai Y, Williamson D, Gawad C, et al. Measurable residual disease detection by high throughput sequencing improves risk stratification for pediatric B-ALL. Blood. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mullighan CG, Zhang J, Kasper LH, Lerach S, Payne-Turner D, Phillips LA, et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature. 2011;471:235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Meyer JA, Wang J, Hogan LE, Yang JJ, Dandekar S, Patel JP, et al. Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat Genet. 2013;45:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Li B, Li H, Bai Y, Kirschner-Schwabe R, Yang JJ, Chen Y, et al. Negative feedback-defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat Med. 2015;21:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bhatia S, Landier W, Hageman L, Chen Y, Kim H, Sun CL, et al. Systemic Exposure to Thiopurines and Risk of Relapse in Children With Acute Lymphoblastic Leukemia: A Children’s Oncology Group Study. JAMA Oncol. 2015;1:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Diouf B, Crews KR, Lew G, Pei D, Cheng C, Bao J, et al. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313:815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gervasini G, de Murillo SG, Jimenez M, de la Maya MD, Vagace JM. Dihydrofolate Reductase Genetic Polymorphisms Affect Methotrexate Dose Requirements in Pediatric Patients With Acute Lymphoblastic Leukemia on Maintenance Therapy. J Pediatr Hematol Oncol. 2017. [DOI] [PubMed] [Google Scholar]
- [64].Karol SE, Larsen E, Cheng C, Cao X, Yang W, Ramsey LB, et al. Genetics of ancestry-specific risk for relapse in acute lymphoblastic leukemia. Leukemia. 2017;31:1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ramsey LB, Panetta JC, Smith C, Yang W, Fan Y, Winick NJ, et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100:1957–64. [DOI] [PubMed] [Google Scholar]
- [67].Xu H, Cheng C, Devidas M, Pei D, Fan Y, Yang W, et al. ARID5B genetic polymorphisms contribute to racial disparities in the incidence and treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2012;30:751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Xu H, Yang W, Perez-Andreu V, Devidas M, Fan Y, Cheng C, et al. Novel susceptibility variants at 10p12.31–12.2 for childhood acute lymphoblastic leukemia in ethnically diverse populations. J Natl Cancer Inst. 2013;105:733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Perez-Andreu V, Roberts KG, Harvey RC, Yang W, Cheng C, Pei D, et al. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat Genet. 2013;45:1494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Moriyama T, Metzger ML, Wu G, Nishii R, Qian M, Devidas M, et al. Germline genetic variation in ETV6 and risk of childhood acute lymphoblastic leukaemia: a systematic genetic study. The Lancet Oncology. 2015;16:1659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Noetzli L, Lo RW, Lee-Sherick AB, Callaghan M, Noris P, Savoia A, et al. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat Genet. 2015;47:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shah S, Schrader KA, Waanders E, Timms AE, Vijai J, Miething C, et al. A recurrent germline PAX5 mutation confers susceptibility to pre-B cell acute lymphoblastic leukemia. Nat Genet. 2013;45:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Topka S, Vijai J, Walsh MF, Jacobs L, Maria A, Villano D, et al. Germline ETV6 Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia. PLoS Genet. 2015;11:e1005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med. 2015;373:2336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Churchman ML, Qian M, Te Kronnie G, Zhang R, Yang W, Zhang H, et al. Germline Genetic IKZF1 Variation and Predisposition to Childhood Acute Lymphoblastic Leukemia. Cancer Cell. 2018;33:937–48e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Qian M, Cao X, Devidas M, Yang W, Cheng C, Dai Y, et al. TP53 Germline Variations Influence the Predisposition and Prognosis of B-Cell Acute Lymphoblastic Leukemia in Children. J Clin Oncol. 2018;36:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78. [DOI] [PubMed] [Google Scholar]
- [78].Maloney KW, Devidas M, Mattano LA, Friedmann AM, Buckley P, Borowitz MJ, Carroll AJ, Gastier-Foster JM, Heerema NA, Kadan N, Loh ML, Matloub Y, Marshall DT, Stork LC, Raetz EA, Wood BL, Winick NJ, Hunger SP, and Carroll WL Excellent Event Free (EFS) and Overall Survival (OS) For Children With Standard Risk Acute Lymphoblastic Leukemia (SR ALL) Despite The Absence Of a Significant Impact On Outcome With The Addition Of An Intensified Consolidation:Results Of Children’s Oncology Group (COG) AALL033. Blood. 2013;122:837.23719300 [Google Scholar]
- [79].Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34:2380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kato M, Ishimaru S, Seki M, Yoshida K, Shiraishi Y, Chiba K, et al. Long-term outcome of 6-month maintenance chemotherapy for acute lymphoblastic leukemia in children. Leukemia. 2017;31:580–4. [DOI] [PubMed] [Google Scholar]
- [81].Downing JR, Wilson RK, Zhang J, Mardis ER, Pui CH, Ding L, et al. The Pediatric Cancer Genome Project. Nat Genet. 2012;44:619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mody RJ, Wu YM, Lonigro RJ, Cao X, Roychowdhury S, Vats P, et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA. 2015;314:913–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–42. [DOI] [PubMed] [Google Scholar]
- [84].Biondi A, Schrappe M, De Lorenzo P, Castor A, Lucchini G, Gandemer V, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13:936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27:5175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia. 2014;28:1467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hunger SP, Saha V, Devidas M, Valsecchi MG, Gastier Foster J, Cazzaniga G, Reshmi SC, Borowitz M, Moorman A, Heerema NA, Carroll AJ, Barnette P, Gramatges M, Maloney K, Sun W, Swanink R, Termuhlen A, Loh ML, Raetz AR, Silverman LB, Schrappe M, Schultz KR, Slayton W, Healey D, and Biondi A CA180–372: An International Collaborative Phase 2 Trial of Dasatinib and Chemotherapy in Pediatric Patients with Newly Diagnosed Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia (Ph+ ALL). Blood. 2017;130:98.28705853 [Google Scholar]
- [88]. NCT01460160: https://clinicaltrials.gov/ct2/show/NCT01460160.
- [89].Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis. Cancer. 2016;122:3650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Pui CH, Roberts KG, Yang JJ, Mullighan CG. Philadelphia Chromosome-like Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2017;17:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. NCT02723994: https://clinicaltrials.gov/ct2/show/NCT02723994.
- [92]. NCT03117751: https://clinicaltrials.gov/ct2/show/NCT03117751.
- [93].Khaw SL, Suryani S, Evans K, Richmond J, Robbins A, Kurmasheva RT, et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood. 2016;128:1382–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Leonard JT, Rowley JS, Eide CA, Traer E, Hayes-Lattin B, Loriaux M, et al. Targeting BCL-2 and ABL/LYN in Philadelphia chromosome-positive acute lymphoblastic leukemia. Sci Transl Med. 2016;8:354ra114. [DOI] [PubMed] [Google Scholar]
- [95].Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–78. [DOI] [PubMed] [Google Scholar]
- [96]. NCT03236857: https://clinicaltrials.gov/ct2/show/NCT03236857.
- [97].Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–7. [DOI] [PubMed] [Google Scholar]
- [98].Taketani T, Taki T, Sugita K, Furuichi Y, Ishii E, Hanada R, et al. FLT3 mutations in the activation loop of tyrosine kinase domain are frequently found in infant ALL with MLL rearrangements and pediatric ALL with hyperdiploidy. Blood. 2004;103:1085–8. [DOI] [PubMed] [Google Scholar]
- [99].Chillon MC, Gomez-Casares MT, Lopez-Jorge CE, Rodriguez-Medina C, Molines A, Sarasquete ME, et al. Prognostic significance of FLT3 mutational status and expression levels in MLL-AF4+ and MLL-germline acute lymphoblastic leukemia. Leukemia. 2012;26:2360–6. [DOI] [PubMed] [Google Scholar]
- [100].Stam RW, Schneider P, de Lorenzo P, Valsecchi MG, den Boer ML, Pieters R. Prognostic significance of high-level FLT3 expression in MLL-rearranged infant acute lymphoblastic leukemia. Blood. 2007;110:2774–5. [DOI] [PubMed] [Google Scholar]
- [101].Brown P, Kairalla J, Wang C, Dreyer Z, Salzer W, Sorenson M, Borowitz M, Carroll A, Heerema N, Rao K, Gore L, Devidas M, Carroll W, Winick N, Raetz E, Loh M, Hunger S, and Hilden J Addition of FLT3 Inhibitor Lestaurtinib to Post-Induction Chemotherapy does not Improve Outcomes in Mll-Rearranged Infant Acute Lymphoblastic Leukemia (ALL): AALL0631, A Children’s Oncology Group Study. Pediatric Blood & Cancer. 2016;63:S7.27077670 [Google Scholar]
- [102]. NCT00557193: https://clinicaltrials.gov/ct2/show/NCT00557193.
- [103].Widemann BC, Kim A, Fox E, Baruchel S, Adamson PC, Ingle AM, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children’s Oncology Group Phase I Consortium report. Clin Cancer Res. 2012;18:6011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zwaan MC, Söderhäll S, Brethon B, Luciani M, Rizzari C, Sternberg D, Besse E, Dutreix C, Fagioli F, Ho P, Dufour C, and Pieters R A Phase 1/2, Open-Label, Dose-Escalation Study of Midostaurin in Pediatric Patients (Pts) with Relapsed or Refractory (R/R) Acute Leukemia: Final Results of Study ITCC-024 (CPKC412A2114). 2015. [Google Scholar]
- [105].Cooper TM, Cassar J, Eckroth E, Malvar J, Sposto R, Gaynon P, et al. A Phase I Study of Quizartinib Combined with Chemotherapy in Relapsed Childhood Leukemia: A Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Clin Cancer Res. 2016;22:4014–22. [DOI] [PubMed] [Google Scholar]
- [106].Hijiya N, Thomson B, Isakoff MS, Silverman LB, Steinherz PG, Borowitz MJ, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118:6043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Dunsmore KP WS, Devidas M, Wood BL, Esiashvili N, Eisenberg N, Briegel N, Hayashi RJ, Gastier-Foster JM, Carroll AJ, Heerema NA, Asselin B, Rabin KR, Zweideler-McKay P, Raerz EA, Loh ML, Winick NJ, Carroll WL, Hunger S. COG AALL0434: A randomized trial testing nelarabine in newly diagnosed t-cell malignancy. J Clin Oncol. 2018;36 (suppl; abstr 10500). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell. 2017;168:724–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sabatino M, Hu J, Sommariva M, Gautam S, Fellowes V, Hocker JD, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood. 2016;128:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Maude SL. Future directions in chimeric antigen receptor T cell therapy. Curr Opin Pediatr. 2017;29:27–33. [DOI] [PubMed] [Google Scholar]
- [113].Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Schneider D, Xiong Y, Wu D, Nlle V, Schmitz S, Haso W, et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. 2017;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Velasquez MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2016;34:4381–9. [DOI] [PubMed] [Google Scholar]
- [118].Schlegel P, Lang P, Zugmaier G, Ebinger M, Kreyenberg H, Witte KE, et al. Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica. 2014;99:1212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Pui CH, Yang JJ, Bhakta N, and Rodriguez-Galindo C Global efforts toward the cure of childhood acute lymphoblastic leukaemia. The Lancet Child & Adolescent Health. 2018;2:440–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Surveillance, Epidemiology, and End Results (SEER) Program (http://www.seer.cancer.gov/) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (2000–2014) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.
- [121].Moricke A, Zimmermann M, Valsecchi MG, Stanulla M, Biondi A, Mann G, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127:2101–12. [DOI] [PubMed] [Google Scholar]
- [122].Place AE, Stevenson KE, Vrooman LM, Harris MH, Hunt SK, O’Brien JE, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16:1677–90. [DOI] [PubMed] [Google Scholar]
- [123].Domenech C, Suciu S, De Moerloose B, Mazingue F, Plat G, Ferster A, et al. Dexamethasone (6 mg/m2/day) and prednisolone (60 mg/m2/day) were equally effective as induction therapy for childhood acute lymphoblastic leukemia in the EORTC CLG 58951 randomized trial. Haematologica. 2014;99:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Stary J, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S, et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol. 2014;32:174–84. [DOI] [PubMed] [Google Scholar]
- [125].Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2017. [DOI] [PubMed] [Google Scholar]
- [126].Escherich G, Horstmann MA, Zimmermann M, Janka-Schaub GE, group Cs. Cooperative study group for childhood acute lymphoblastic leukaemia (COALL): long-term results of trials 82,85,89,92 and 97. Leukemia. 2010;24:298–308. [DOI] [PubMed] [Google Scholar]
- [127].Pieters R, de Groot-Kruseman H, Van der Velden V, Fiocco M, van den Berg H, de Bont E, et al. Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. J Clin Oncol. 2016;34:2591–601. [DOI] [PubMed] [Google Scholar]
- [128].Yamaji K, Okamoto T, Yokota S, Watanabe A, Horikoshi Y, Asami K, et al. Minimal residual disease-based augmented therapy in childhood acute lymphoblastic leukemia: a report from the Japanese Childhood Cancer and Leukemia Study Group. Pediatr Blood Cancer. 2010;55:1287–95. [DOI] [PubMed] [Google Scholar]
- [129].Yeoh AE, Ariffin H, Chai EL, Kwok CS, Chan YH, Ponnudurai K, et al. Minimal residual disease-guided treatment deintensification for children with acute lymphoblastic leukemia: results from the Malaysia-Singapore acute lymphoblastic leukemia 2003 study. J Clin Oncol. 2012;30:2384–92. [DOI] [PubMed] [Google Scholar]
- [130].Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15:809–18. [DOI] [PubMed] [Google Scholar]
- [131].Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Li MJ, Liu HC, Yen HJ, Jaing TH, Lin DT, Yang CP, et al. Treatment for childhood acute lymphoblastic leukemia in Taiwan: Taiwan Pediatric Oncology Group ALL-2002 study emphasizing optimal reinduction therapy and central nervous system preventive therapy without cranial radiation. Pediatr Blood Cancer. 2017;64:234–41. [DOI] [PubMed] [Google Scholar]