Abstract
Greater aortic stiffness and pulse pressure are associated with cerebrovascular remodeling, reduced white matter microstructure and cognitive performance with aging in humans. However, it is unclear whether aortic stiffness and pulse pressure are associated with reduced basal global cerebral blood flow and cerebrovascular reserve among older adults. Global cerebral blood flow was quantified in 205 adults (range 19-87 yrs; mean ± SE: 30.6 ± 1.3 yrs) using quantitative [15O] water brain PET imaging. In a subset of older adults (n=24, 70.0 ± 2.0 yrs), aortic stiffness (carotid-femoral pulse wave velocity, PWV) and cerebrovascular reserve (change in global cerebral blood flow following intravenous infusion of acetazolamide) were assessed. In the entire cohort, global cerebral blood flow was lower in older compared with young adults (36.5 ± 1.1 vs. 50.5 ± 0.7 mL/min/100mL, p<0.001). Global cerebral blood flow was higher in young women compared with young men (51.0 ± 0.30 vs. 47.4 ± 0.03 mL/min/100mL, p<0.001) but did not differ between older women and men (p= 0.63). In older adults, greater carotid-femoral PWV was associated with lower cerebrovascular reserve (r= −0.68, p= 0.001 adjusted for age, sex and MAP) but not global cerebral blood flow (r= 0.13, p= 0.60). Brachial pulse pressure was not associated with lower cerebrovascular reserve (r= −0.37, p= 0.159) when adjusted for age and sex. These data indicate that the age-related increases in aortic stiffness may contribute in part to the brain’s impaired ability to augment blood flow in response to a stimulus with aging in humans.
Keywords: Arterial stiffness, cerebrovascular reactivity, cerebral blood flow, aging, pulse wave velocity
Keywords: Cognitive impairment, Clinical studies, Aging, CVD, Cerebrovascular disease
Summary:
Age-related increases in aortic stiffness is associated with reduced CVR, but not basal gCBF, in older adults independent of age, sex, and blood pressure.
Introduction
Heart disease, stroke and dementia continue to be the number one, five and six leading causes of death, respectively, in individuals over 65 years of age in the United States (CDC). Dementia, characterized by a decline in cognitive ability sufficient to interfere with daily life, results in substantial emotional and financial burdens to the patient, caregiver and community. Healthcare costs associated with dementia are estimated to be nearly 60% higher than costs associated with heart disease or cancer and are expected to climb as the prevalence of dementia surges from 5 million to more than 14 million by 2050 (NIH, Alzheimer’s Association). While the mechanisms that contribute to dementia remain unclear, attention has shifted from the solitary ‘amyloid hypothesis’ towards a complex etiology that acknowledges the vascular contribution in the pathogenesis of mild cognitive impairment (MCI) and dementia, including Alzheimer’s disease.1–3 In support of this paradigm shift, there is extensive overlap between traditional cardiovascular disease (CVD) and cerebrovascular disease risk factors such as older age, hypertension, diabetes, higher body mass index (BMI) and increased risk of cognitive impairment and dementia. More recently, elevated aortic stiffness has been identified as a novel risk factor associated with cognitive impairment among older adults with and without hypertension independent of other CVD risk factors.4–6 However, the mechanisms by which aortic stiffness contributes to cognitive decline with advancing age remains poorly understood.
Aortic stiffness, determined by carotid-femoral pulse wave velocity (cfPWV), is an independent predictor of CVD events in older adults without CVD at baseline.7 Aortic stiffness and related central hemodynamics are associated with reductions in cognitive performance on memory, processing speed and executive function tasks.8–12 Importantly, longitudinal increases in aortic stiffness are associated with declines in cognitive performance over the lifespan.5 Stiffening of the aorta reduces the ability of aorta to buffer pulsatile blood flow from the left ventricle during systole, resulting in elevated central systolic blood pressure (BP) and pulse pressure (PP). Furthermore, augmented aortic stiffness reduces the mismatch between the low impedance (central elastic arteries) and high impedance (peripheral muscular) arteries, thereby increasing transmission of pulsatile energy to the low resistance cerebrovasculature of high flow organs such as the brain.4 In this regard, elevated aortic stiffness and pulsatility are hypothesized to contribute in part to declines in cognitive performance with aging by promoting the development of subcortical white matter hyperintensities via increased cerebrovascular remodeling and resistance.13,14
In addition, the link between age-related aortic stiffness and pulsatility with cerebral blood flow (CBF) and cerebrovascular reactivity, defined as the ability to augment brain blood flow in response to a vasodilatory stimulus, remains relatively unexplored. Experimentally, cerebrovascular reactivity is quantified by measuring cerebrovascular reserve (CVR) in response to a physiological or pharmacological stimuli such as CO2 or acetazolamide (ACZ), a carbonic anhydrase inhibitor. Previous studies demonstrate that nonlinear increases in central PP with advancing age were associated with increases in systolic and pulsatile CBF velocity, independent of age, age2, and sex in older adults.14 In contrast, the relation between cfPWV and CBF remains unclear with some studies demonstrating associations between cfPWV with selective regional reductions in CBF15,16 but not with global CBF (gCBF).14 Additionally, brachial-ankle PWV, a composite measure of peripheral and central arterial stiffness, was associated with lower CVR in response to a physiological CO2 stimulus but not basal CBF estimated by Transcranial Doppler.17 However, the degree to which age-related increases in aortic stiffness are associated with reduced gCBF or CVR using ‘gold-standard’ measurements of CBF, specifically, [15O]water Positron Emission Tomography (PET) imaging, is unknown. Thus, properly characterizing the nature of the relations between CVR and aortic stiffness and PP with quantitative assessments of CBF is clinically important because reductions in CVR predicts cognitive decline including the conversion from MCI to dementia.18,19 Therefore, we hypothesized that higher aortic stiffness and PP would be independently associated with lower gCBF and reduced CVR among older adults.
Methods
Data, Analytic Methods (Code) and Research Materials Transparency
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
One hundred and sixty-five young (age: 19-37 years) and 40 older adults (age 55-87 years) were recruited between the years 2006- 2016 from the Iowa City, Iowa community and the University of Iowa Hospital and Clinics through flyers and email advertisements to undergo neuroimaging testing (Figure S1). All participants that were either controls in specific neuroimaging investigations or recruited specifically for a study designed to investigate relations between vascular function and cognitive performance, gCBF and CVR in atherosclerotic vascular disease (AVD) and aging20 This latter study involved a subset of 24 older adults (age range: 55-87 years) recruited to complete measurements of aortic stiffness. These individuals were free of major psychiatric illness and neurological diseases except for MCI. Two participants were categorized as MCI based on published criterion from the Alzheimer’s Disease Neuroimaging Initiative-2 (ADNI-2) at the time of PET imaging.
Participants abstained from taking vasoactive medication and antioxidant therapy on the morning of their vascular visit. Female participants in this subset were postmenopausal and not on hormone replacement therapy. The study conformed to the standards sets by the Declaration of Helsinki and all participants provided written consent to the study and the protocol was approved by the Institutional Review Board and the Medical Radiation Protection Committee at the University of Iowa.
Measurements:
Aortic stiffness and hemodynamic parameters.
Aortic stiffness was determined using non-invasive applanation tonometry via pulse wave analysis (NIHem workstation, Cardiovascular Engineering, Inc., Norwood, MA) as previously described.21 Briefly, femoral and carotid pressure waveforms were collected sequentially using a non-invasive tonometer. The pressure waveform was gated to the R-wave of the ECG to determine the time difference between the foot of the carotid and femoral diastolic pressure waveforms. The distance between the carotid and femoral pulse sites was measured as the distance between the suprasternal notch (SSN) and the carotid waveform minus the distance from the SSN to the femoral pulse site to account for parallel transmission. Corrected distances were divided by the carotid and femoral foot-to-foot time delay to calculate cfPWV.
PET imaging.
gCBF and CVR were assessed using quantitative [15O]water PET imaging with arterial blood sampling using methods previously described.20,22–24 Briefly, all imaging was performed on an ECAT EXACT HR+ Scanner (Siemens Medical Solutions USA, Inc. Knoxville, TN). Dynamic imaging (5 seconds/frame x 20 frames) and arterial blood sampling commenced at tracer injection (45-50 mCi per injection) and continued for 100 seconds. Arterial blood was sampled using an online sampler25 (binned in 1 second intervals). Parametric images were calculated from the activity images (40 second summed images starting immediately post-bolus transit) and the arterial blood curve using the autoradiographic model as implemented by the pixelwise modeling tool of the PMOD suite of tools (PXMOD, PMOD Technologies, Ltd., Zurich, v. 3.7, http:pmod.com/technologies/index.html). Consistent with the brain work, the partition coefficient was assumed to be equal to 0.9. For each injection, the gCBF was quantified by calculating a volume-weighted average of all intracerebral pixels derived from regions-of-interest (ROIs) manually drawn on each image slice of the parametric image. In the entire cohort (n= 205), gCBF was calculated as the average of all available gCBF measures (3 to 8 [15O]water injections of 45 - 50 mCi per injection per participant) from all studies because gCBF did not statistically differ by task behavior (F=0.08, p=0.922).
In the subset of older adults (n=24), gCBF and CVR was measured while the participants performed a simple counting task. For this task, participants were asked to count from 1 to 3 repetitively for the duration of the 100 second PET scan at approximately a rate of one word per second as previously described.20 Counting is an overlearned, simple cognitive task used to control variability in resting state CBF as a result of extraneous brain processes not of interest and does not augment gCBF. For this subset, anatomical T1-weighted MR images were also available. In these participants, anatomically-based volumes-of-interest (VOIs) were derived from the brain parcellation routine of the PMOD Neuro tool (PNeuro, PMOD Technologies, Ltd, Zurich, v. 3.8) on the T1-weighted images and transferred to the co-registered parametric blood flow images. gCBF values were calculated as the volume-weighted average of all VOIs. Cerebrovascular reactivity was quantified by calculating CVR by comparing gCBF after administration of ACZ (at ~15 minutes post 1 g IV), a carbonic anhydrase inhibitor to induce maximal vasodilation, to the gCBF determined during the baseline counting task.23 CVR was calculated as the absolute (absCVR= ACZ flow- basal gCBF) and relative percent change (relCVR(%)= ACZ – basal gCBF/ basal gCBF) in all participants. In 19 of the 24 participants, supine brachial blood pressure was recorded during the counting and ACZ [15O] injections using an automated BP cuff (Figure S1).
Statistical analysis.
In the entire cohort (n=205 adults), two-tailed independent t-tests were used to test differences for age (young vs. older adults) and sex differences (women vs. men; young women vs. young men; older women vs. older men) using IBM SPSS 23.0 software (IBM, Inc., Armonk, NY). One-way analysis of covariance (ANCOVA) was used to test the interaction between age and sex on gCBF in young adults. In the subset of older adults (n=24), bivariate partial correlations were used to determine relations between vascular variables and gCBF, relCVR (%) and absCVR, respectively. cfPWV was adjusted for mean arterial pressure in all partial correlational analyses. Older adults in the subset participants (n=24) were recruited to undergo neuroimaging following completion of a separate study investigating vascular function and cognitive performance.26 Therefore, measurements of arterial stiffness were completed within a median of 24 ± 3.0 months prior to completion of [15O]water PET imaging. In a separate analysis, we adjusted cfPWV values to account for this time delay between vascular and neuroimaging visits using previously published longitudinal data for predicted yearly change in cfPWV of 0.05 m/sec per year.27 All data were normally distributed and mean data are presented as mean ± SE. Finally, statistical significance was defined as a two-tailed alpha level of <0.05.
Results
Participant characteristics.
One hundred and sixty-five young and 40 older participants completed measures of gCBF. Participant characteristics are described in Table 1. Older adults had a higher BMI (26.5 ± 0.7 vs. 27.8 ± 0.7 kg/m2, p= 0.003) compared with young adults, but did not differ by any other characteristic besides age.
Table 1:
Participants characteristics
| Variables | Young adults (n=165) | Older adults (n=40) | Older adults subset with PWV (n=24) | p-value (young vs. older, n=40) | p-value (young vs. older subset, n=24) |
|---|---|---|---|---|---|
| Males/Females, n/n | 99/66 | 29/11 | 16/8 | 0.128 | 0.361 |
| Age (years) | 22.6 ± 0.3 | 68.0 ± 1.6* | 70.0 ± 2.0ψ | <0.001 | <0.001 |
| BMI (kg/m2) | 25.7 ± 0.3 | 27.8 ± 0.7* | 26.5 ± 0.7 | 0.003 | 0.359 |
| Education (years) | - | - | 15.9 ± 0.5 | - | - |
| Atherosclerotic Vascular Disease, n (%) | - | - | 5 (21.7) | - | - |
| Mild Cognitive Impairment, n (%) | - | - | 2 (8.3) | - | |
| Anti-hypertensive medication, n (%) | - | - | 7 (30.4) | - | - |
| Aspirin therapy, n (%) | - | - | 9 (39.1) | - | - |
| Statin, n (%) | - | - | 6 (26.1) | - | - |
| Blood Pressure and Vascular Outcomes | |||||
| Brachial systolic BP (mmHg) | 117.1 ± 2.5 | - | - | ||
| Brachial diastolic BP (mmHg) | 62.8 ± 1.3 | - | - | ||
| Brachial pulse pressure (mmHg) | 54.2 ± 2.6 | - | |||
| Carotid-femoral PWV (m/sec) | - | - | 10.1 ± 0.5 | - | - |
| Carotid-femoral PWV adjusted for delay between neuroimaging and vascular visits (m/sec) | - | - | 10.2 ± 0.5 | - | - |
| Cerebral blood flow | |||||
| Basal global CBF (mL/min/100mL of tissue) | 50.5 ± 0.7 | 36.5 ± 1.1* | 35.2 ± 1.5ψ | <0.001 | <0.001 |
| ACZ flow (mL/min/100mL of tissue) | - | - | 47.2 ± 1.8 | - | - |
| AbsCVR (mL/min/100mL of tissue) | - | - | 11.6 ± 1.2 | - | - |
| RelCVR (%) | - | - | 36.1 ± 3.4 | - | - |
| Cognitive Performance | |||||
| Mini Mental Status Exam | - | - | 28.9 ± 0.4 | - | - |
Data are mean ± SE. BMI, body mass index; BP, blood pressure; PWV, pulse wave velocity; CBF, cerebral blood flow; ACZ, acetazolamide; AbsCVR, absolute cerebrovascular reserve; RelCVR, relative cerebrovascular reserve. Brachial blood pressures and cfPWV were available in n=19 and n=24 participants, respectively.
denotes p<0.05 older (n=40) vs. young;
denotes p<0.05 subset of older with PWV (n=24) vs. young.
Changes in gCBF with age.
In the entire cohort, gCBF was greater in young compared with older adults (50.5 ± 0.7 vs. 36.5 ± 1.1 mL/min/100mL, p<0.001, Figure 1). gCBF was 3.6 mL/min/100mL of tissue higher in young women compared with young men (51.0 ± 0.30 vs. 47.4 ± 0.03, p<0.001, Table 2, Figure 2A). While women tended to be younger than men in the cohort of young adults, the effect of sex on gCBF in young adults remained significant (p= 0.004) after adjustment for age and the interaction between sex and age was not significant (p= 0.84). Interestingly, gCBF did not differ between older men and women (38.3 ± 1.0 vs. 37.3 ± 2.0 mL/min/100mL, p= 0.63, Table 2, Figure 2B).
Figure 1.
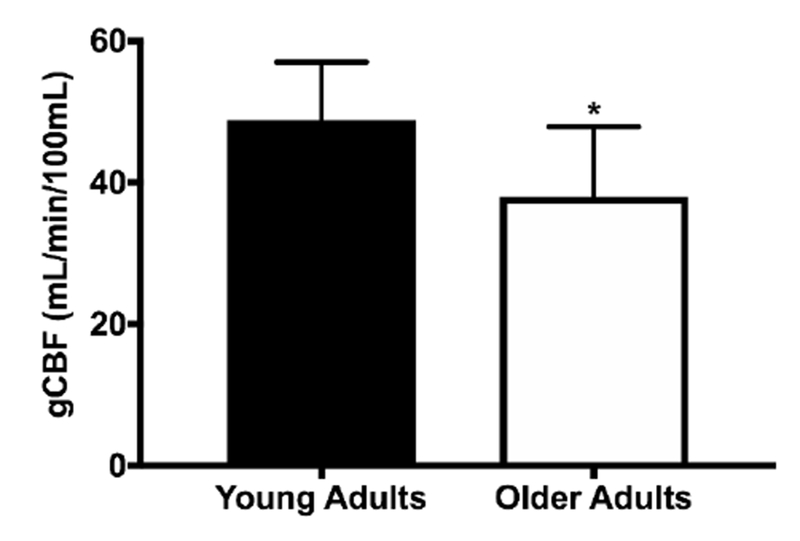
Mean global cerebral blood flow (gCBF) in young (n=165) and older (n=40) adults (n=40). *P<0.05 vs. Younger. Data are mean ± SE.
Table 2.
Basal global cerebral blood flow among males and females
| Variable | Males | Females | ||
|---|---|---|---|---|
| Younger | Older | Younger | Older | |
| Global CBF (mL/min/100mL of tissue) | 47.4 ± 0.3 | 38.3 ± 1.0Ψ | 51.0 ± 0.3* | 37.3 ± 2.0Ψ |
Data are mean ± SE;
denotes significant (p<0.05) difference between males and females;
denotes significant (p<0.05) difference between younger and older adults.
Figure 2.
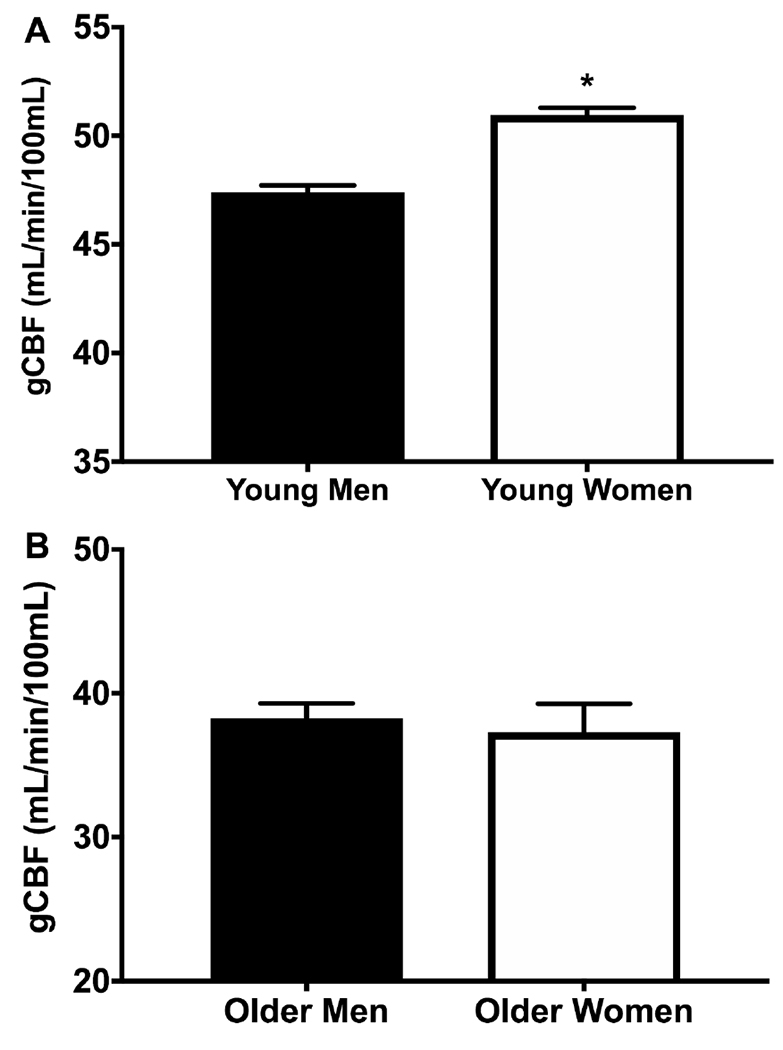
Mean global cerebral blood flow (gCBF) values between A) young men (n=99) and women (n=66) and B) older men (n=29) and women (n=11); *P<0.05 vs. Younger. Data are mean ± SE.
Participant characteristics of subset.
In the subset of 24 older adults, additional measures of gCBF, CVR and arterial stiffness. Brachial blood pressures collected during neuroimaging were available in 19 of the 24 MA/O with cfPWV as indicated in in Table 1. On average, participants were older (70.0 ± 2.0 years), highly educated (15.9 ± 0.5 years) and normotensive (brachial systolic BP 117.1 ± 2.5 mmHg). Approximately one-quarter of participants were taking anti-hypertensive, aspirin and/or statin therapy (Table 1). Importantly, older adults imaged as part of this subset are representative of the expected gCBF values based on their age and sex from the entire cohort (n=205). Finally, participants had an average cfPWV of 10.0 ± 0.5 m/s, that is within the age-expected normative values of cfPWV. 28
Relations between aortic stiffness, BP and gCBF and CVR.
Aortic stiffness, brachial systolic BP and PP were not associated with basal gCBF and remained nonsignificant after adjustment for age, sex and MAP (cfPWV only). Univariate correlations between cfPWV and all BP outcomes were not associated with absCVR or relCVR(%). However, higher cfPWV was strongly correlated with lower absCVR (r= −0.63, p=0.004) following adjustment for age, sex, and MAP. Expressing CVR as a percent change (i.e., relCVR%) did not alter the strong association between CVR and cfPWV (Table 3). Brachial systolic BP (r= −0.40, p= 0.124; r= −0.21, p= 0.443) and PP (r= −0.37, p= 0.159; r= −0.15, p= 0.585) was not associated with lower absCVR and relCVR(%), respectively (Table 3). Additional adjustment for medication use did not alter findings between cfPWV, absCVR and CVR%. Finally, associations between cfPWV, basal gCBF and absCVR were not altered using the predicted cfPWV adjusted for the delay between vascular and neuroimaging visits (Table 3).
Table 3:
Partial correlation coefficients between arterial stiffness and blood pressure variables with cerebrovascular outcomes among the subset of older adults (n=24).
| Variable | cfPWV | Adjusted cfPWV | Brachial systolic BP | Brachial diastolic BP | Brachial PP |
|---|---|---|---|---|---|
| Global CBF | 0.13 (0.60) | 0.08 (0.752) | 0.10 (0.969) | 0.28 (0.269) | −0.14 (0.587) |
| AbsCVR | −0.63 (0.004)* | −0.61 (0.026)* | −0.40 (0.124) | −0.10 (0.720) | −0.37 (0.159) |
| RelCVR(%) | −0.63 (0.004)* | −0.60 (0.030)* | −0.21 (0.443) | −0.06 (0.834) | −0.15 (0.585) |
Data are partial correlation coefficients (p values) adjusted for age and sex. Partial correlations with carotid femoral pulse wave velocity (cfPWV) were adjusted for age, sex and mean arterial pressure (MAP).
Abbreviations: cfPWV, carotid femoral pulse wave velocity; Adjusted cfPWV, cfPWV values adjusted for delay between neuroimaging and vascular study visits; BP, blood pressure; PP, pulse pressure; AbsCVR, absolute change in cerebrovascular reactivity; RelCVR(%), relative percent in change in cerebrovascular reactivity.
Discussion
The primary novel finding of the present study is that elevated aortic stiffness is strongly associated with lower CVR in older adults, independent of age, sex and MAP. In contrast, aortic stiffness is not related to basal gCBF in this older cohort. These data are consistent with the hypothesis that increased stiffening of the elastic aorta may contribute, at least in part, to the impaired ability of cerebrovasculature to dilate maximally to augment gCBF in older adults. It is possible that the concomitant rise in aortic stiffness and the loss of impedance mismatch between central and peripheral vasculature, results in chronic penetration of augmented forward pulsatile pressure waves to the vulnerable cerebral microvasculature damaging their vasodilatory response.6 Chronic exposure to pulsatile pressure results in vascular remodeling of the cortical pial and large cerebral arteries 29, at the expense of cerebral perfusion to the downstream subcortical regions. While the brain comprises only 2% of the body’s total weight, 15% of cardiac output is diverted to the brain to match the high metabolic rate and low energy stores resulting in a coupling of the metabolic demand and the blood flow supply. Interruptions in CBF or even minor decoupling of the demand-supply, such as that by reduced or impaired CVR, may produce ischemic damage to the brain parenchyma and resultant cognitive impairment. Taken together, these data provide an important mechanistic link between elevated aortic stiffness with reductions in white matter microvascular and cognitive performance.13,30
Consistent with other studies,31–34,33,34 gCBF was significantly higher in young compared with older adults. Additionally, gCBF was significantly higher in younger women compared with younger men (age <40 years) but did not differ between older men and women. However, we were not sufficiently powered to detect sex differences in gCBF with aging in the present study. Prior studies indicate the effects of age on sex-related differences in CBF may differ by region and brain matter fiber type.31,35–38 CBF in the grey matter is significantly greater in women compared with men until the 6th decade of life 34,35,39,40 when losses of vascular and neuroprotective estrogen result in reductions in CBF in post-menopausal women.41 However, interactions between age and sex on white matter CBF across the lifespan remain unknown. This is clinically significant given the link between elevations in aortic stiffness and development of white matter hyperintensities.4,42,43 Given that white matter hyperintensities are more prevalent in women compared with men,44 more studies are needed to better understand the effects of age on differences in CBF between men and women.
While the measurement of gCBF and CVR in response to acetazolamide has been performed by others previously45–48, this is the first study to investigate the quantitative measurements of gCBF and CVR in relation with aortic stiffness in the same study. Prior studies assessing the relation between vascular function and measures of CBF and CVR have used transcranial Doppler and the functional MRI method of Arterial Spin Labeling (ASL). However, there are known limitations with both methods. Transcranial Doppler used to assess CVR typically measures the change in CBF velocity in the middle cerebral artery (MCA), a surrogate marker of CBF, following a physiological stimulus (e.g., hypercapnia) under the assumption that hypercapnia does not induce a diameter change in the MCA. However, the degree to which TCD may underestimate CBF due to dilation of the large cerebral vessels (i.e., MCA) with hypercapnia remains controversial.49 Similarly, recent meta-analyses demonstrate that ASL MRI may overestimate gCBF compared with [15O]water PET imaging 50 along with regional and brain matter differences in the CBF estimates between methods. 50,51,46 Additionally, the present study induced maximal dilation using the carbonic anhydrase inhibitor, ACZ, to determine CVR. This is important because recent studies demonstrated that the reactivity to a hypercapnic stimulus may be altered in individuals with higher fitness 52 while ACZ reliably produces maximal dilation in humans.53 Taken together, to the best of our knowledge, this is the first study to assess the true relation between cfPWV and gCBF and CVR using validated measurements of CBF and arterial stiffness.
This study should be interpreted in the context of several limitations. First, our study did not recruit individuals between the ages of 39-54 years and therefore is unable to determine the change in gCBF across the entire lifespan (18-87 years) as well as detect the decade in which gCBF no longer differs between men and women. However, in two previous studies, CBF was reduced in postmenopausal compared with premenopausal women beyond anticipated age-related changes, suggesting that aging and menopause contribute synergistically towards cerebrovascular changes in post-menopausal women.41 Second, the sample size of the subset of participants is small, but cfPWV remained significantly associated with CVR even after adjustment for key covariates (age, sex, and MAP) and post-hoc power calculation revealed that 99.9% power was achieved. Third, this study did not test cognitive performance and therefore is unable to determine is stiffness-related reductions in CVR are associated with cognitive performance in the subset of older adults. Fourth, central blood pressure was not collected in the subset of 24 older adults. Central PP may more closely represent the BP in which the brain is exposed may be associated with reductions in CBF or CVR not detected by brachial BP measurements in the subset of older adults. Finally, given the cross-sectional design of the present study, we are unable to determine causality between elevated cfPWV and reduced CVR therefore, cannot rule out a possible bidirectional relation between these outcomes.
In conclusion, elevated aortic stiffness is associated with reductions in CVR among older adults independent of age, sex and MAP. Furthermore, we confirmed findings from previous smaller studies using [15O]water PET that indeed gCBF is reduced in older compared with younger adults, however, sex differences in gCBF may differ with age. In contrast to our hypothesis, aortic stiffness was not associated with basal gCBF in the present cohort, rather it was associated with the ability of the cerebral vessels to maximally augment CBF suggesting that reduced CVR may be a primary downstream hemodynamic consequence of elevated aortic stiffness. More studies are needed to explore the degree to which elevated aortic stiffness result in regional reductions in CBF and CVR as well as characterizing these relations across the lifespan.
Perspectives
This study demonstrates age-related increases in aortic stiffness impair the ability of the cerebrovasculature to dilate in response to physiological or pharmacological stimuli but does not contribute to age-related reductions in gCBF in older adults. This is clinically significant given the ability of the cerebrovasculature to dilate to physiological stimuli is important in maintaining the coupling of the neurovascular response and is predictive of cognitive decline with aging in humans. Therefore, this study suggests that lower CVR may be an important mechanistic link between elevations in aortic stiffness with alterations in white matter microstructure and cognitive impairment in older adults.
Supplementary Material
Novelty and significance
What is new?
In a large cohort using [15O]water PET imaging, basal global cerebral blood flow (gCBF) is 3.6 ml/min/100ml of tissue lower in older adults compared with young adults.
Higher aortic stiffness is associated with reduced cerebrovascular reserve (CVR) but not gCBF in older adults.
What is relevant?
Higher aortic stiffness is associated with increased cerebrovascular resistance, reduced white matter microstructure and cognitive performance. This study adds to current knowledge by suggesting that elevated aortic stiffness may contribute to the reduced ability of the cerebrovasculature to dilate in response to physiological stimuli but is not associated with age-related declines in gCBF.
Acknowledgments
Sources of Funding
Study supported by NIH grants 1R21AG043722 (GLP), P01HL014388, R01AG030417 (to DJM), 1R03AG047306 (LBP), U54TR001356 (University of Iowa), American Heart Association 13SDG143400012 (GLP) and the Biological Sciences Funding Program (LBP) from the University of Iowa Office of the Vice President for Research.
Footnotes
Disclosures: None.
References
- 1.Iadecola C Vascular and Metabolic Factors in Alzheimer’s Disease and Related Dementias: Introduction. Cellular and Molecular Neurobiology. 2016;36(2):151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s & Dementia. 2015;11(6):710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. American Journal of Physiology - Heart and Circulatory Physiology. 2017;312(1):H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility – Reykjavik Study. Brain. 2011;134(11):3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. Journal of Hypertension. 2007;25(5):1035–1040. [DOI] [PubMed] [Google Scholar]
- 6.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81(11):984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction. Journal of the American College of Cardiology. 2014;63(7):636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53(4):668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of Arterial Stiffness and Blood Pressure in Hypertension-Associated Cognitive Decline in Healthy Adults. Hypertension. 2016;67(1):171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanon O, Haulon S, Lenoir H, Seux M-L, Rigaud A-S, Safar M, Girerd X, Forette F. Relationship Between Arterial Stiffness and Cognitive Function in Elderly Subjects With Complaints of Memory Loss. Stroke. 2005;36(10):2193–2197. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain : a journal of neurology. 2011;134(Pt 11):3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. [DOI] [PubMed] [Google Scholar]
- 13.Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF. Cerebrovascular Damage Mediates Relations Between Aortic Stiffness and Memory. Hypertension. 2016;67(1):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarumi T, Khan MA, Liu J, Tseng BM, Parker R, Riley J, Tinajero C, Zhang R. Cerebral Hemodynamics in Normal Aging: Central Artery Stiffness, Wave Reflection, and Pressure Pulsatility. Journal of Cerebral Blood Flow & Metabolism. 2014;34(6):971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. American journal of hypertension. 2011;24(10):1108–1113. [DOI] [PubMed] [Google Scholar]
- 16.Tarumi T, Gonzales MM, Fallow B, Nualnim N, Pyron M, Tanaka H, Haley AP. Central artery stiffness, neuropsychological function, and cerebral perfusion in sedentary and endurance-trained middle-aged adults. Journal of Hypertension. 2013;31(12):2400–2409. [DOI] [PubMed] [Google Scholar]
- 17.Jaruchart T, Suwanwela NC, Tanaka H, Suksom D. Arterial stiffness is associated with age-related differences in cerebrovascular conductance. Experimental gerontology. 2016;73:59–64. [DOI] [PubMed] [Google Scholar]
- 18.Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, Moffa F, Provinciali L, Vernieri F. Cerebrovascular Reactivity and Cognitive Decline in Patients With Alzheimer Disease. Stroke. 2006;37(4):1010–1015. [DOI] [PubMed] [Google Scholar]
- 19.Viticchi G, Falsetti L, Vernieri F, Altamura C, Bartolini M, Luzzi S, Provinciali L, Silvestrini M. Vascular predictors of cognitive decline in patients with mild cognitive impairment. Neurobiology of aging. 2012;33(6):1127 e1121–1127. e1129. [DOI] [PubMed] [Google Scholar]
- 20.Moser DJ, Boles Ponto LL, Miller IN, Schultz SK, Menda Y, Arndt S, Nopoulos PC. Cerebral blood flow and neuropsychological functioning in elderly vascular disease patients. Journal of clinical and experimental neuropsychology. 2012;34(2):220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DuBose LE, Voss MW, Weng TB, Kent JD, Dubishar KM, Lane-Cordova AD, Sigurdsson G, Schmid P, Barlow PB, Pierce GL. Carotid β-stiffness Index is Associated with Slower Processing Speed but not Working Memory or White Matter Integrity in Healthy Middle-Aged/Older Adults. Journal of applied physiology. 2017;122(4):868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponto LLB, Brashers-Krug TM, Pierson RK, Menda Y, Acion L, Watkins GL, Sunderland JJ, Koeppel JA, Jorge RE. Preliminary investigation of cerebral blood flow and amyloid burden in veterans with and without combat-related traumatic brain injury. The Journal of neuropsychiatry and clinical neurosciences. 2015;28(2):89–96. [DOI] [PubMed] [Google Scholar]
- 23.Ponto LLB, Schultz SK, Watkins GL, Hichwa RD. Technical issues in the determination of cerebrovascular reserve in elderly subjects using 15 O-water PET imaging. Neuroimage. 2004;21(1):201–210. [DOI] [PubMed] [Google Scholar]
- 24.Hurtig RR, Hichwa RD, O’Leary DS, Ponto LLB, Narayana S, Watkins GL, Andreasen NC. Effects of Timing and Duration of Cognitive Activation in [15O]Water PET Studies. Journal of Cerebral Blood Flow & Metabolism. 1994;14(3):423–430. [DOI] [PubMed] [Google Scholar]
- 25.Wollenweber SD, Hichwa RD, Ponto LLB. A simple on-line arterial time-activity curve detector for [O-15] water PET studies. Nuclear Science, IEEE Transactions on. 1997;44(4):1613–1617. [Google Scholar]
- 26.Moser DJ, Robinson RG, Hynes SM, Reese RL, Arndt S, Paulsen JS, Haynes WG. Neuropsychological Performance Is Associated With Vascular Function in Patients With Atherosclerotic Vascular Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(1):141–146. [DOI] [PubMed] [Google Scholar]
- 27.Scuteri A, Morrell CH, Orrù M, Strait JB, Tarasov KV, Ferreli LAP, Loi F, Pilia MG, Delitala A, Spurgeon H, Najjar SS, AlGhatrif M, Lakatta EG. Longitudinal Perspective on the Conundrum of Central Arterial Stiffness, Blood Pressure, and Aging Novelty and Significance. Hypertension. 2014;64(6):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Reference Values for Arterial Stiffness C. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. European Heart Journal. 2010;31(19):2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumbach GL, Siems JE, Heistad DD. Effects of local reduction in pressure on distensibility and composition of cerebral arterioles. Circulation Research. 1991;68(2):338–351. [DOI] [PubMed] [Google Scholar]
- 30.Maillard P, Mitchell GF, Himali JJ, Beiser A, Fletcher E, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S. Aortic Stiffness, Increased White Matter Free Water, and Altered Microstructural Integrity. Stroke; a journal of cerebral circulation. 2017;48(6):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponto LLB, Watkins GL, Hichwa RD, Richmond JC, Clark J, Ward CA, Clermont DA. [15O]Water Pharmacokinetics: Influence of Age and Gender in Normal Subjects. Molecular Imaging & Biology. 2002;4(2):129–137. [DOI] [PubMed] [Google Scholar]
- 32.Melamed E, Lavy S, Bentin S, Cooper G, Rinot Y. Reduction in regional cerebral blood flow during normal aging in man. Stroke. 1980;11(1):31–35. [DOI] [PubMed] [Google Scholar]
- 33.Pase MP, Grima NA, Stough C, Scholey A, Pipingas A. Association of pulsatile and mean cerebral blood flow velocity with age and neuropsychological performance. Physiology & Behavior. 2014;130:23–27. [DOI] [PubMed] [Google Scholar]
- 34.Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34(7):855–855. [DOI] [PubMed] [Google Scholar]
- 35.Aanerud J, Borghammer P, Rodell A, Jónsdottir KY, Gjedde A. Sex differences of human cortical blood flow and energy metabolism. Journal of Cerebral Blood Flow & Metabolism. 2017;37(7):2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gur RE, Gur RC. Gender differences in regional cerebral blood flow. Schizophrenia Bulletin. 1990;16(2):247. [DOI] [PubMed] [Google Scholar]
- 37.Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magnetic Resonance in Medicine. 2004;51(4):736–743. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez G, Warkentin S, Risberg J, Rosadini G. Sex Differences in Regional Cerebral Blood Flow. Journal of Cerebral Blood Flow & Metabolism. 1988;8(6):783–789. [DOI] [PubMed] [Google Scholar]
- 39.Vriens EM, Kraaier V, Musbach M, Wieneke GH, van Huffelen AC. Transcranial pulsed doppler measurements of blood velocity in the middle cerebral artery: Reference values at rest and during hyperventilation in healthy volunteers in relation to age and sex. Ultrasound in Medicine & Biology. 1989;15(1):1–8. [DOI] [PubMed] [Google Scholar]
- 40.Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. American Journal of Roentgenology. 1999;172(1):213–218. [DOI] [PubMed] [Google Scholar]
- 41.Ohkura T, Teshima Y, Isse K, Matsuda H, Inoue T, Sakai Y, Iwasaki N, Yaoi Y. Estrogen Increases Cerebral and Cerebellar Blood Flows in Postmenopausal Women. Menopause. 1995;2(1):13–18. [Google Scholar]
- 42.Rosano C, Watson N, Chang Y, Newman AB, Aizenstein HJ, Du Y, Venkatraman V, Harris TB, Barinas-Mitchell E, Sutton-Tyrrell K. Aortic Pulse Wave Velocity Predicts Focal White Matter Hyperintensities in a Biracial Cohort of Older Adults. Hypertension. 2013;61(1):160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of Aortic Stiffness With Cognition and Brain Aging in Young and Middle-Aged Adults. The Framingham Third Generation Cohort Study. 2016;67(3):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Den Heuvel D, Admiraal-Behloul F, Ten Dam V, Olofsen H, Bollen E, Murray H, Blauw G, Westendorp R, De Craen A, Van Buchem M. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63(9):1699–1701. [DOI] [PubMed] [Google Scholar]
- 45.Oku N, Kitagawa K, Imaizumi M, Takasawa M, Piao R, Kimura Y, Kajimoto K, Matsumoto M, Hori M, Hatazawa J. Hemodynamic Influences of Losartan on the Brain in Hypertensive Patients. Hypertension Research. 2005;28:43. [DOI] [PubMed] [Google Scholar]
- 46.Jennings JR, Muldoon MF, Price J, Christie IC, Meltzer CC. Cerebrovascular Support for Cognitive Processing in Hypertensive Patients Is Altered by Blood Pressure Treatment. Hypertension. 2008;52(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura Y, Kitagawa K, Oku N, Kajimoto K, Kato H, Tanaka M, Sakaguchi M, Hougaku H, Sakoda S, Hatazawa J. Hemodynamic influences of azelnidipine, a novel calcium channel blocker, on cerebral circulation in hypertensive patients with ischemic white matter lesions. Hypertension Research. 2008;31(12):2147. [DOI] [PubMed] [Google Scholar]
- 48.Kimura Y, Kitagawa K, Oku N, Kajimoto K, Kato H, Tanaka M, Sakaguchi M, Hougaku H, Sakoda S, Hatazawa J. Blood pressure lowering with valsartan is associated with maintenance of cerebral blood flow and cerebral perfusion reserve in hypertensive patients with cerebral small vessel disease. Journal of Stroke and Cerebrovascular Diseases. 2010;19(2):85–91. [DOI] [PubMed] [Google Scholar]
- 49.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. Journal of Applied Physiology. 2014;117(10):1090–1096. [DOI] [PubMed] [Google Scholar]
- 50.Fan AP, Jahanian H, Holdsworth SJ, Zaharchuk G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: A systematic review. Journal of Cerebral Blood Flow & Metabolism. 2016;36(5):842–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang K, Herzog H, Mauler J, Filss C, Okell TW, Kops ER, Tellmann L, Fischer T, Brocke B, Sturm W, Coenen HH, Shah NJ. Comparison of Cerebral Blood Flow Acquired by Simultaneous [15O]Water Positron Emission Tomography and Arterial Spin Labeling Magnetic Resonance Imaging. Journal of Cerebral Blood Flow & Metabolism. 2014;34(8):1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas BP, Yezhuvath US, Tseng BY, Liu P, Levine BD, Zhang R, Lu H. Life‐long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. Journal of Magnetic Resonance Imaging. 2013;38(5):1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahl A, Russell D, Rootwelt K, Nyberg-Hansen R, Kerty E. Cerebral Vasoreactivity Assessed With Transcranial Doppler and Regional Cerebral Blood Flow Measurements. Dose, Serum Concentration, and Time Course of the Response to Acetazolamide. 1995;26(12):2302–2306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


