Abstract
Skin produces cholesterol and a wide array of sterols and non-sterol mevalonate metabolites, including isoprenoid derivative farnesyl pyrophosphate (FPP). To characterize FPP action in epidermis, we generated transcriptional profiles of primary human keratinocytes treated with zaragozic acid (ZGA), a squalene synthase inhibitor that blocks conversion of FPP to squalene resulting in endogenous accumulation of FPP. The elevated levels of intracellular FPP resulted in regulation of epidermal differentiation and adherens junction signaling, insulin growth factor (IGF) signaling, oxidative stress response and interferon (IFN) signaling. Immunosuppressive properties of FPP were evidenced by STAT-1 downregulation and prominent suppression of its nuclear translocation by IFNγ. Furthermore, FPP profoundly downregulated genes involved in epidermal differentiation of keratinocytes in vitro and in human skin ex vivo. Elevated levels of FPP resulted in induction of cytoprotective transcriptional factor Nrf2 and its target genes. We have previously shown that FPP functions as ligand for the glucocorticoid receptor (GR), one of the major regulator of epidermal homeostasis. Comparative microarray analyses show significant but not complete overlap between FPP and glucocorticoid regulated genes, suggesting that FPP may have wider transcriptional impact. This was further supported by co-transfection and chromatin immunoprecipitation experiments where we show that upon binding to GR, FPP recruits ß-catenin and, unlike glucocorticoids, recruits co-repressor GRIP1 to suppress keratin 6 gene. These findings have many clinical implications related to epidermal lipid metabolism, response to glucocorticoid therapy as well as pleiotropic effects of cholesterol lowering therapeutics, statins.
Keywords: Farnesyl pyrophosphate, wound healing, keratinocyte migration, glucocorticoids
INTRODUCTION
Skin is a neuroendocrine and highly metabolically active organ that maintains whole-body homeostasis by providing barrier and also communicating the external signals to internal processes (Eming et al., 2014; Jozic et al., 2015; Slominski et al., 2015b). Human skin is also a very active site of de novo cholesterol biosynthesis (Bouslimani et al., 2015; Sampath et al., 2009), whereby the highest body concentration of 7-dehydrocholesterol, the precursor to cholesterol, is in the epidermis (Slominski et al., 2015a; Slominski et al., 2014; Slominski et al., 2015b). Epidermal keratinocytes have to synthesize sufficient amounts of cholesterol for export through lamellar bodies and lipid barrier functioning (Eming et al., 2014; Feingold, 2009; Pastar et al., 2008; Sampath et al., 2009). In addition cholesterol biosynthetic pathway in skin produces a rich array of metabolites, including steroid hormones and non-sterol mevalonate metabolites (Slominski et al., 2015a; Slominski et al., 2014; Vukelic et al., 2011). The mevalonate pathway is a complex pathway required for the generation of several fundamental end-products including isoprenoids, dolichol, ubiquinone, and isopentenyladenine (Goldstein and Brown, 1990) and farnesyl pyrophosphate (FPP) (Das et al., 2007; Goyanka et al., 2010). Both cholesterol and mevalonate metabolites are known for their ability to affect transcriptional and post-transcriptional events regulating various biological processes (Vukelic et al., 2011). Furthermore we have previously described FPP as a transcriptional activator of a subset of nuclear hormone receptors including glucocorticoid receptor (GR) (Das et al., 2007; Vukelic et al., 2010).
Both endogenous FPP, accumulated when conversion of FPP to squalene is blocked by the squalene synthetase inhibitor-zaragozic acid A (ZGA) (Keller, 1996; Tong et al., 2005), or exogenous FPP can cause activation of the glucocorticoid (GC) signaling pathway in primary human epidermal keratinocytes (Vukelic et al., 2010). However there is a lack of information on the role of cutaneous metabolites, including FPP in the regulation of epidermal functions and keratinocyte biology. In order to better understand skin response to this cholesterol metabolite, we obtained mRNA expression profiles of ZGA treated keratinocytes. We found that the response of keratinocytes to elevated FPP resulted in regulation of number of genes and cellular processes including interferon signaling, epithelial adherens/junction signaling, insulin growth factor (IGF) signaling and oxidative stress response. Furthermore ZGA treatment profoundly suppressed functional groups of genes involved in cell migration and keratinocyte differentiation.
We have previously shown that FPP, synthetized by epidermal keratinocytes, functions as a ligand for GR causing a conformational change, dimerization, and translocation of the receptor-ligand complex from the cytoplasm to the nucleus (Lee et al., 2005; Stojadinovic et al., 2007; Vukelic et al., 2010). In this study, we delved further into the relationship between FPP and the GR. Depending on the promoter context, the downstream effects of FPP binding to the GR could mimic the activity of glucocorticoids (GCs), show no effect, or even broaden involvement of co-regulators. Using co-transfection experiments and chromatin immunoprecipitation we show that upon activation by FPP, GR specifically recruits ß-catenin and the co-regulator GRIP1 to suppress keratin 6 (K6) and keratinocyte migration.
Taken together, these results indicate that cholesterol metabolite FPP affects a wide range of processes including anti-inflammatory and oxidative stress response, epidermal differentiation, and cell migration all of which have important clinical implications for cutaneous homeostasis and diseases. Manipulation of mevalonate pathway and, therefore, FPP synthesis in skin, may have important diagnostic and therapeutic applications, particularly in the context of statin and steroid therapies.
MATERIALS AND METHODS
Cell Culture
Primary human epidermal keratinocytes (HEK) were initially propagated in a basal phenol red-free keratinocyte medium (Gibco) custom made to exclude glucocorticoids and thyroid hormone and supplemented with epidermal growth factor (EGF), bovine pituitary extract (BPE), and antibiotic-antimycotic (LifeSci). Prior to the treatment with ZGA cells were washed several times with 1× PBS and incubated for 24hrs in the same basal phenol red-free keratinocyte medium (Gibco) without glucocorticoids, thyroid hormone, EGF and BPE. Cells were treated as follows: 50 μM ZGA (Sigma Chemical), 1 μM DEX (Sigma Chemical), or 10 μM FPP (Sigma Chemical) (Vukelic et al., 2011; Vukelic et al., 2010).
RNA isolation and RT-PCR
Total RNA from was isolated from HEK treated with ZGA and control, vehicle treated cells using RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. For real-time qPCR, 0.5 μg of total RNA was reverse transcribed using Omniscript Reverse Transcription kit (Qiagen). Real-time PCR was performed in triplicates using the CFX96 qPCR thermal cycler and detection system (BioRad) and an iQ SYBR Green Supermix (Quanta). Relative expression was normalized for levels of Actin Related Protein 2/3 Complex, Subunit 2 (ARPC2). The primer sequences used were: ARPC2, forward (5′-TCCGGGACTACCTGCACTAC-3′) and reverse (5′-GGTTCAGCACCTTGAGGAAG-3′); heme oxygenase 1 (HMOX1), forward (5′-AAGACTGCGTTCCTGCTCAAC-3′) and reverse (5′-AAAGCCCTACAGCAACTGTCG-3′); thioredoxin reductase 1 (TXNRD1), forward (5′-TAGGACAAGCCCTGCAAGACT-3′) and reverse (5′-CCCCAATTCAAAGAGCCAATGT-3′); sequestosome 1 (SQSTM1), forward (5′-AAGCCGGGTGGGAATGTTG-3′) and reverse (5′-CCTGAACAGTTATCCGACTCCAT-3′); ferritin, heavy polypeptide 1 (FTH1), forward (5′-CCCCCATTTGTGTGACTTCAT-3′) and reverse (5′-GCCCGAGGCTTAGCTTTCATT-3′); signal transducer and activator of transcription 1 (STAT1), forward (5′-ATCAGGCTCAGTCGGGGAATA-3′) and reverse (5′-TGGTCTCGTGTTCTCTGTTCT-3′); interferon-induced 15 KDa protein (ISG15), forward (5′-CGCAGATCACCCAGAAGATCG-3′) and reverse (5′-TTCGTCGCATTTGTCCACCA-3′); insulin-like growth factor binding protein 3 (IGFBP3), forward (5′-AGAGCACAGATACCCAGAACT-3′) and reverse (5′-GGTGATTCAGTGTGTCTTCCATT-3′); fibroblast growth factor binding protein 1 (FGFBP1), forward (5′-GGAAACAAGTTGCCCGGAATC-3′) and reverse (5′-AATAGAGTGGAGCTGACTAGCTT-3′); laminin, gamma 2 (LAMC2), forward (5′-TGGGAATTGCTGGAGGAACC-3′) and reverse (5′-CTGACCCCAGGCATCTAACA-3′); phosphatase and tensin homolog (PTEN), forward (5′-TTTGAAGACCATAACCCACCAC-3′) and reverse (5′-ATTACACCAGTTCGTCCCTTTC-3′); serpin peptidase inhibitor clade B member 3 (SERPINB3), forward (5′-CGCGGTCTCGTGCTATCTG-3′) and reverse (5′-ATCCGAATCCTACTACAGCGG-3′); activating transcription factor 3 (ATF3) forward (5′-TCGGGGTGTCCATCACAAAAG-3′) and reverse (5′-GGCCGATGAAGGTTGAGCA-3′); regulator of G-protein signaling 2 (Rgs2), forward (5′-AAGATTGGAAGACCCGTTTGAG-3′) and reverse (5′-GCAAGACCATATTTGCTGGCT-3′); transglutaminase 1 (TGM1) forward (5′-CATCCCTATGGCATCTCG-3′) and reverse (5′-GCAAGTGAAGACTGACTC-3′); ras homolog family member B (RhoB), forward (5′-GTGTTATTTAAGGGTGGTGATG-3′) and reverse (5′-AAGATGGTCAAGTCCTGTTC-3′); corneodesmosin (CDSN) forward (5′-GACAGATGGCTCAGTAAC-3′) and reverse (5′-GGAAGGTAGAAGAGAAACAC-3′); gap junction protein beta 3 (Gjb3) forward (5′-TGGAGATACCCAGAGGTC-3′) and reverse (5′-CTGTCTTGAGGATGTTCTATC-3′).
Microarray Analysis
5 μg of total RNA was reverse-transcribed, amplified, and labeled according to the protocol (Stojadinovic et al., 2007). Labeled cRNA was hybridized to HGU95Av2 arrays (Affymetrix), and arrays were washed and stained with anti-biotin streptavidinphycoerythin-labeled antibody using an Affymetrix fluidics station and then scanned using the Agilent Gene Array Scanner system (Hewlett-Packard). Microarray data was analyzed using Genespring 13.0. GC-RMA normalization of the raw signal intensity files was performed, and data were filtered to retain probes with normalized signal intensities above 20th percentile in at least 1 sample. Differential expression at 48 h and 72 h time points was detected using moderated t-test with Benjamini-Hochberg correction. Probes meeting corrected p-value threshold of 0.05 and displaying linear fold change>2 in ZGA vs. control groups were retained for downstream pathway analysis. Pathway enrichment was performed using Ingenuity Pathway Analysis (IPA). IPA Core Analysis was performed on differentially expressed genes at 48 and 72 h time points, and Comparison Analysis was used to detect jointly regulated pathways and biological processes in response to ZGA treatment. Enrichment p-values were calculated using Fisher’s exact test with Benjamini-Hochberg correction for multiple testing. Pathway regulation was determined via IPA-calculated absolute Z-score>2, which reflects a statistically significant pattern match of ZGA up or down-regulated genes with their known regulatory effects in other biological contexts (Kramer et al., 2014). IPA Upstream Regulator Analysis was used to identify targets of β-catenin regulated by dexamethasone (Stojadinovic et al., 2007) and ZGA, based upon categorized findings in the Ingenuity Knowledge Base.
Immunocytochemistry and Immunohistochemistry
HEK were grown on coverslips to 70% confluence. Cells were incubated for 24 h in a basal serum-free medium custom made without hydrocortisone (LifeSci) and treated as follows: 50 μM ZGA, IFNγ (100 ng/ml; Sigma Chemical), or 50 μM ZGA for 4 hrs, and then treated with IFNγ (100 ng/ml) for the next 1 hr. Cells were fixed in acetone-methanol (1:1) for 2 min, permealized with 0.1% Triton X-100 for 10 min, and incubated overnight with anti-STAT-1 antibody (Santa Cruz) and visualized using a secondary antibody AlexaFluor 488 (Invitrogen). Propidium iodide (Vector Laboratories,) was used for visualization of the nuclei. The coverslips were analyzed using a Nicon Eclipse E800 microscope (Nikon Instruments Inc.) and digital images were collected using SPOT-Camera Advanced program.
Human skin specimens were obtained from reduction surgery following an approved protocol and treated with 50 μM ZGA or vehicle for 24, 48, and 96 h as previously described (Stojadinovic et al., 2007; Vukelic et al., 2010). The incubated tissue was fixed in 10% formalin overnight and embedded in paraffin. Seven μm thick skin sections were de-waxed in xylene, re-hydrated, and washed with 1x phosphate buffered saline (PBS). For antigen retrieval, paraffin sections were heated in a 95°C water bath in Target Retrieval Solution (DAKO Corporation) and washed. Sections were blocked with 5% bovine serum albumin (BSA) (Sigma) and subsequently incubation with antibody against cytokeratin 10 (K10, Sigma), Desmoglein 1 (Dsg1; Acris Antibodies), and vinculin (Vcl, Abcam) over night at 4°C. Slides were then rinsed in PBS and visualized with either Alexa Fluor 488 anti-mouse IgG or anti-rabbit Alexa Fluor 594 conjugated goat antibody (Invitrogen). All coverslips and slides were mounted with mounting media containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories) to visualize the cell nuclei. The slides were analyzed using a Nikon Eclipse E800 microscope and digital images were collected using Nikon Camera Advanced program.
Protein Isolation and Western Blot
Extracts for immunoblotting were prepared from skin tissue and cells as previously described (Vukelic et al., 2010). Proteins were separated by on 4-20% Criterion TGX pre-cast gels (Bio-Rad) and transferred onto PVDF membranes (Bio-Rad). Membranes were blocked with I-Block (Applied Biosystems) in Phosphate-buffered saline containing 0.1% Tween-20, then incubated with anti-Nrf2 [nuclear factor (erythroid-derived 2)-like 2] antibody (Sigma-Aldrich, 1:1000). For loading control we used anti β-actin antibody (Sigma-Aldrich, 1:10,000).
Plasmids and Transient Transfections
Plasmids pK6CAT, ß-catenin, and CARM-1 have been described previously (Koh et al., 2002; Radoja et al., 2000; Stojadinovic et al., 2005). The plasmid containing GRE-CAT was a gift from Dr. P. Chambon (France) and expression plasmids GRIP-1 and GRIP-1 NRBmII/III from Dr M. Stallcup (Chen et al., 1999; Stojadinovic et al., 2005). All plasmids were isolated using Promega kit (Promega) and commercial protocols. Normal HEK were grown as described above. Cells were plated into six-well plates and grown to 60% confluence and transferred to basal serum-free medium custom made without hydrocortisone (LifeSci) 24hr prior to transfection. 5 μg of plasmid DNA were transfected using Fugene 6 reagent (Roche) following commercial protocol. Cells were incubated for 48 h after transfections in the presence or absence of 50 μM ZGA or 10 μM FPP, and 1 μM DEX. Cell extracts used for CAT assay were normalized by total protein determined by protein assay (Bio-Rad). 30 μg of protein were used for each reaction. CAT assays were performed using FastCat (Molecular Probes) following a commercial protocol. CAT assay values were quantified by Fluor Imager 575 (Molecular Dynamics). All transfection experiments were performed at least three times.
Chromatin Immunoprecipitation Assays
Chromatin immunoprecipitation (ChIP) assays were performed with HEK untreated or treated with 1 μM DEX (Sigma) and 10 μM FPP for 24h, as described (Rogatsky et al., 2001; Takayama et al., 2006; Vukelic et al., 2010). Protein/DNA complexes were immunoprecipitated using 2μg of anti-GRIP1 antibody (Bethyl Laboratories) or 2 μg of rabbit IgG. Immunoprecipitated, purified chromosomal DNA was used for PCR amplification, using the following primers:
K6-forward (5′-ATGCAGGTGTGAATCTCACTATTTGTAAAGCC-3′) and K6-reverse (5′-AGGAATCGGACTCCAGTAGCAGC-3′). One percent of the input chromatin was processed and used for PCR amplification in parallel. PCRs were carried out for 35 cycles, and products were resolved on 2% agarose gels and visualized by ethidium bromide.
RESULTS
Elevated FPP Induced Transcriptional Changes in Primary Human Keratinocytes
To analyze effects of intracellular FPP on epidermal biology, we determined full complement of ZGA-regulated genes in primary human epidermal keratinocytes (HEK). In order to elevate intracellular levels of FPP cells were treated with the squalene synthetase inhibitor – ZGA for 48 and 72 hours (h), RNA was isolated, labeled, and hybridized to Affymetrix chips. The predominant effect of FPP on gene transcription was suppression. Treatment with ZGA resulted in downregulation of 95 genes and upregulation of 50 genes at 48 h (Fig. 1 and Suppl. Material 1). After 72 h of ZGA treatment, 160 genes were downregulated and 58 genes were upregulated. In total, 96 genes were coordinately regulated at both time points. Complete list of ZGA-regulated genes is presented in Suppl. Material 1& Suppl. Material 2. Top 5 enriched pathways in keratinocytes in response to increased concentration of intracellular FPP included nuclear factor like 2 (NRF2) mediated oxidative stress response, epithelial adherens/junction signaling, ECM remodeling, interferon signaling, and insulin growth factor signaling (Fig. 1).
Figure 1. Microarray profiling of ZGA-treated keratinocytes.
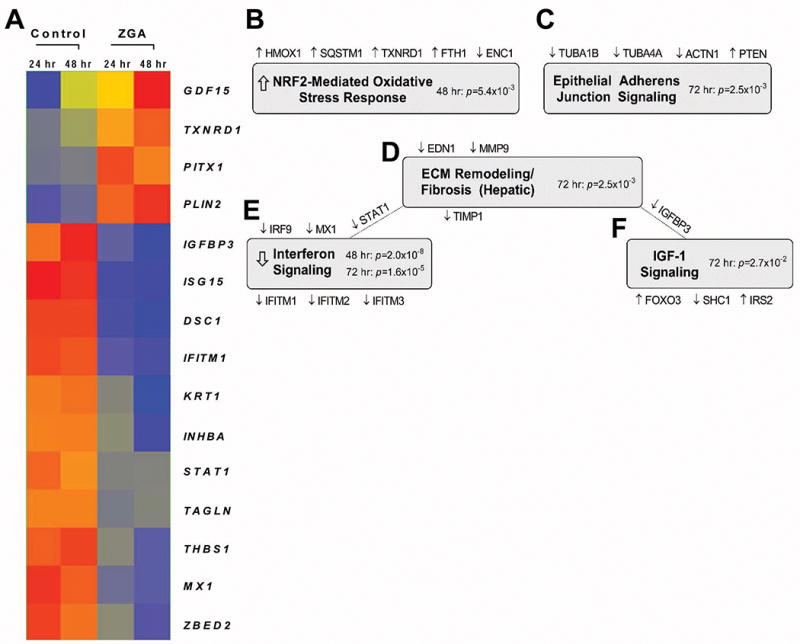
A. Expression heatmap of 15 significantly regulated genes displaying fold change >5 at 48 h and 72 h post-ZGA treatment. Red = higher expression, blue= lower expression. Expression-based gene clustering was performed using Genespring 13.0. B – F Top 5 enriched pathways in keratinocytes in response to ZGA treatment as determined by Ingenuity Pathway Analysis (IPA). Implicated genes and their corresponding regulation are shown above and below the shaded pathway titles; in some instances, genes are common to 2 pathways. Enrichment p-values were calculated using Fisher’s exact test with Benjamini-Hochberg correction; arrows represent statistically significant Z scores reflecting pathway activation/inhibition.
Elevation of FPP Induces NRF2-mediated Oxidative Stress Response Genes
Ingenuity Pathway Analysis identified Nrf2-mediated oxidative stress response pathway as enriched in our microarray dataset (Fig. 1B). To further confirm this we evaluated the effect of FPP on Nrf2 protein levels in both keratinocytes and human skin. We found that elevation of FPP leads to upregulation of Nrf2 in both primary human keratinocytes (Fig. 2A) and human skin (Fig. 2B) 24h post ZGA treatment (Fig. 2A&B). Subsequently to Nrf2 induction at 24 h, a strong increase in the expression of the well-established Nrf2 target genes heme oxygenase-1 (HMOX1), thioredoxin reductase 1 (TXNRD1), sequestosome 1 (SQSTM1), and ferritin H subunit (FTH1) was observed 48 h and 72 h post ZGA treatment (Fig. 2C).
Figure 2. Elevation of intracellular FPP induces transcriptional factor Nrf2 resulting in up-regulation of genes responsible for oxidative stress response.
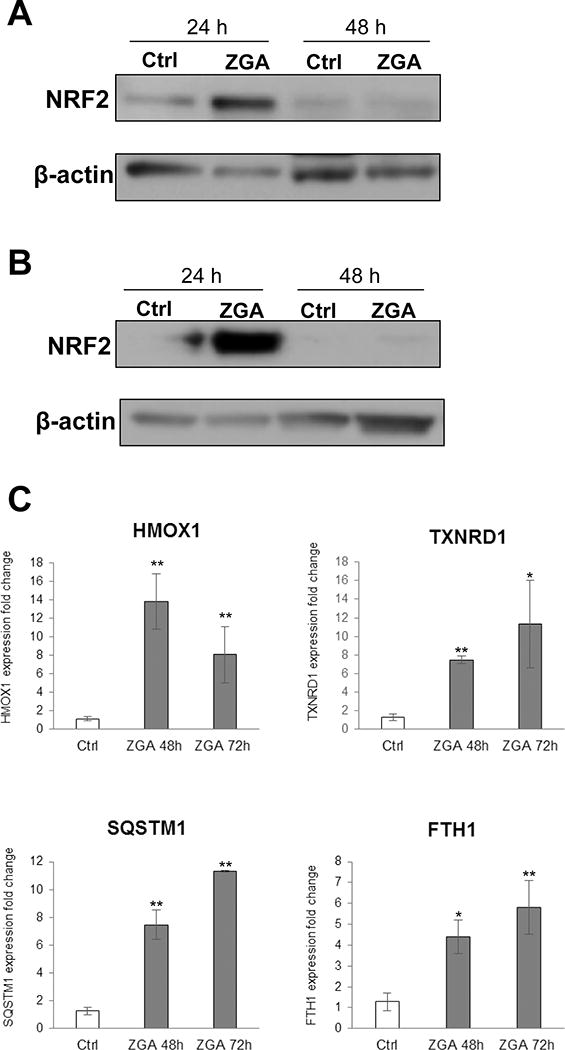
A. Western blot showing Nrf2 induction in primary human keratinocytes treated after 24 h of ZGA treatment. B. ZGA treatment induces Nrf2 protein in human skin ex vivo upon 24 h of treatment. C. Induction of Nrf2 results in subsequent up-regulation of oxidative stress response genes HMOX1, TXNRD1, SQSTM1 and FTH1. qPCR data represent the mean ± S.D. (n=3) *p≤0.05; ** p≤0.001.
Effects of FPP Elevation on Epidermal Differentiation and Epithelial Adherens Junction Signaling
Microarray analyses revealed that ZGA treatment alters expression of the genes involved in epidermal differentiation. Keratin 1 (K1) was among top ten suppressed genes at 48 h, and it was further suppressed by 72 h (Fig 1, Suppl. Material 1&2) upon ZGA treatment. Keratin 10 (K10) was also down-regulated at 72 h in ZGA-treated keratinocytes. In order to determine effects of FPP elevation on epidermal differentiation we utilized established human ex vivo skin model (Stojadinovic and Tomic-Canic, 2013; Vukelic et al., 2010) and treated human skin with ZGA. Immunohistochemistry revealed dramatic down-regulation of K10 in ZGA treated human skin (Fig. 3A). Further, cell adhesion and junctional molecules such as desmoglein (DSG1) 1 and vinculin (VCL), and were also suppressed in ZGA-treated skin confirming microarray data (Fig. 3A and Suppl. Material 1&2). We also evaluated expression of TGM1 and CDSN, a component of corneodesmosomes, by qPCR. As expected results obtained by qPCR followed the pattern of the microarray data confirming TGM1 and CDSN suppression by ZGA in cells at the 72 h time-point (Fig. 3B). These results are in contrast to our previous study showing that GR activation by dexamethasone induces genes involved in late terminal differentiation such as TGM1, CDSN, filagrin (FLG), and kruppel like factor 4 (KLF4) (Stojadinovic et al., 2007), suggesting that FPP as a ligand to nuclear receptors other than GR may result in observed suppression. Furthermore, we found down-regulation of retinoic acid (RA) binding protein (CRABP2) at both time points post ZGA treatment. CRABP2 has been also shown to contribute to RA signaling pathway regulation and epidermal differentiation (Collins and Watt, 2008; Mongan and Gudas, 2007). We conclude that FPP inhibits keratinocyte differentiation and downregulates adhesion molecules involved in desmosome and adherens junction formation and maintenance.
Figure 3. ZGA treatment suppresses epidermal differentiation.
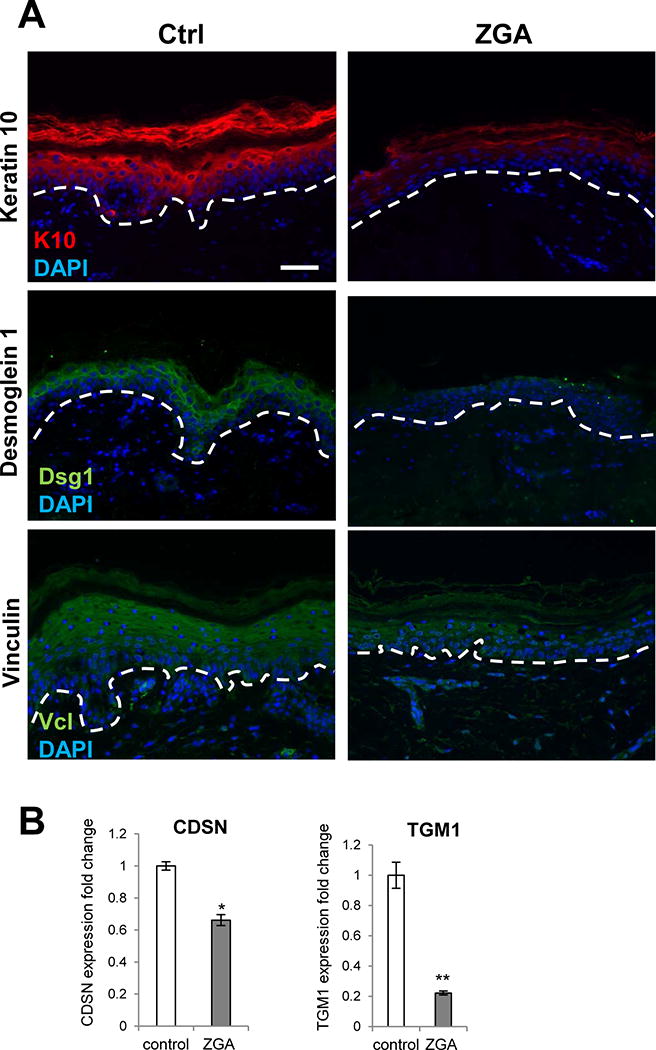
A. Immunofluorescence of keratin 10 (K10), desmoglein 1 (Dsg1) and vinculin (Vcl) in control vehicle treated skin (Ctrl) and ZGA treated ex vivo human skin for four days. Nuclei are visualized with DAPI. Dashed line demarcates epidermal-dermal boundary. Sale bar=50 μm. B. qPCR confirming down-regulation of CDSN and TGM1 genes in ZGA treated primary human keratinocytes after 72 h of ZGA treatment; data represent the mean ± S.D. (n=3) *p≤0.05; ** p≤0.001.
Elevation of FPP Suppresses Interferon Signaling
Interferon signaling was identified as a highly enriched pathway in epidermal response to ZGA treatment by Ingenuity Pathway Analysis (p=2.0 × 10−8 and p=1.6 × 10−5 at 48 and 72, respectively) (Fig. 1E). We found STAT1, ISG15, interferon induced transmembrane protein 1 (IFITM1), interferon-regulated resistance GTP-binding protein Mx (MX1) and seven additional IFN-related genes to be suppressed at both 48 and 72 h of ZGA treatment (Fig. 4A, Suppl. Material 1&Suppl. Material 2). This downregulation strongly indicates immunosuppressing properties of FPP in epidermal keratinocytes. To confirm the functional relevance of this inhibition, we evaluated the effects of ZGA in the presence of IFNγ, by testing STAT-1 activation in keratinocytes pre-treated with ZGA, and subsequently treated with IFNγ. Keratinocytes treated with IFNγ and untreated cells were used a controls (Fig. 4). We found that pretreatment with ZGA diminishes STAT-1 activation, evidenced by the prominent suppression of STAT-1 nuclear translocation by IFNγ (Fig. 4B). In contrast, treatment with IFNγ allowed full STAT-1 activation. Therefore, we conclude that elevated intracellular FPP blocks the IFNγ pathway by suppressing the expression of STAT-1 as well as by blocking its activation. As we have previously shown that GCs inhibit STAT-1 expression (Stojadinovic et al., 2007), we propose that FPP, similar to DEX, inhibits interferon signaling by acting as a GR ligand.
Figure 4. Elevation of FPP inhibits IFN signaling.
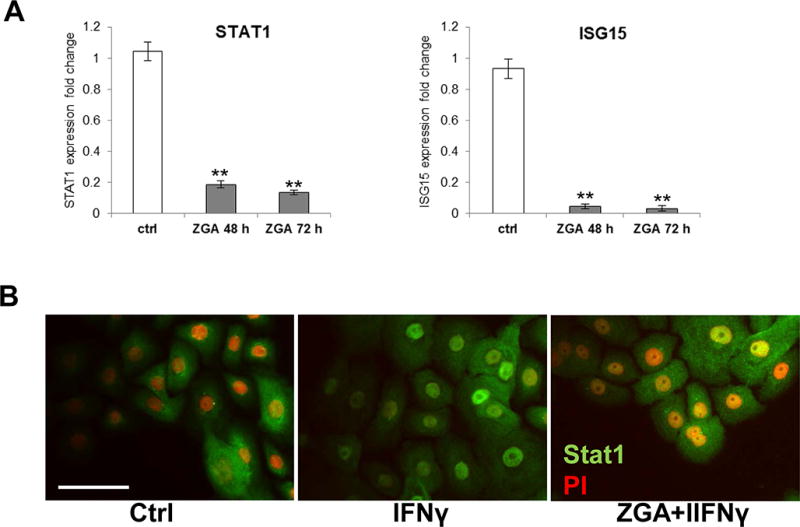
A. qPCR confirming suppression of STAT1 and ISG15 genes involved in IFNγ signaling; data represent the mean ± S.D. (n=3) *p≤0.05; ** p≤0.001. B. Primary human keratinocytes stained with anti-STAT-1 specific antibody. IFNγ treated keratinocytes show activation and nuclearization of STAT-1. Pretreatment with ZGA partially abolishes STAT-1 activation by subsequent IFNγ treatment as evidenced by less prominent STAT-1 nuclear translocation. Nuclei are visualized by counterstaining with propidium iodine (PI). Scale bar=50 μm.
Effects of FPP on IGF-1 Signaling and Genes Involved in Cell Migration
The IGF-1 signaling pathway has been shown to promote keratinocyte migration and wound healing through impacting several signaling cascades that influence keratinocyte shape and facilitate cell spreading and migration (Ando and Jensen, 1993; Emmerson et al., 2012; Haase et al., 2003). IGF-1 can signal through either the phosphatidylinositol-3 kinase pathway or the MAPK pathway resulting in reorganization of the actin cytoskeleton to promote lamellipodia formation (Haase et al., 2003; Siddle, 2011). Enrichment analysis of our profiles of ZGA-treated keratinocytes revealed 10 genes of the IGF-1 signaling pathway and actin cytoskeleton components to be deregulated at both time points (Fig. 5). Of the ten differentially expressed genes, seven genes were found to be down-regulated by intracellular FPP including IGFBP3, Src homology 2 domain containing transforming protein 1 (SHC1), myosin light chain kinase (MYLK), actin-related protein 1 homolog A (ACTR1A), tropomyosin 1 alpha (TPM1), actinin, alpha 1 (ACTN1) and myosin light chain 9 (MYL9) (Fig. 1F and 5A). These genes participate in regulating IGF-1 binding to its receptor, substrate adaptor proteins, and actin cytoskeleton organization (Campbell et al., 1999; Haase et al., 2003; Hamill et al., 2015; Siddle, 2011). The remaining genes found to be up-regulated include PTEN, insulin receptor substrate 2 (IRS2), and forkhead box O3 (FOXO3). PTEN, IRS2 and FOXO3 participate in negative feedback inhibition of IGF-1 signaling, substrate adaptor proteins, and keratinocyte migration (Fig. 5A&B) (Roupe et al., 2014; Siddle, 2011). QPCR conformed microarray data suggesting that ZGA deregulates the IGF-1 signaling pathway in a way that may affect actin cytoskeleton reorganization perhaps by preventing formation of lamellipodia protrusion thus averting keratinocytes to migrate properly (Fig. 5).
Figure 5. FPP elevation by ZGA treatment inhibits migration-related processes.
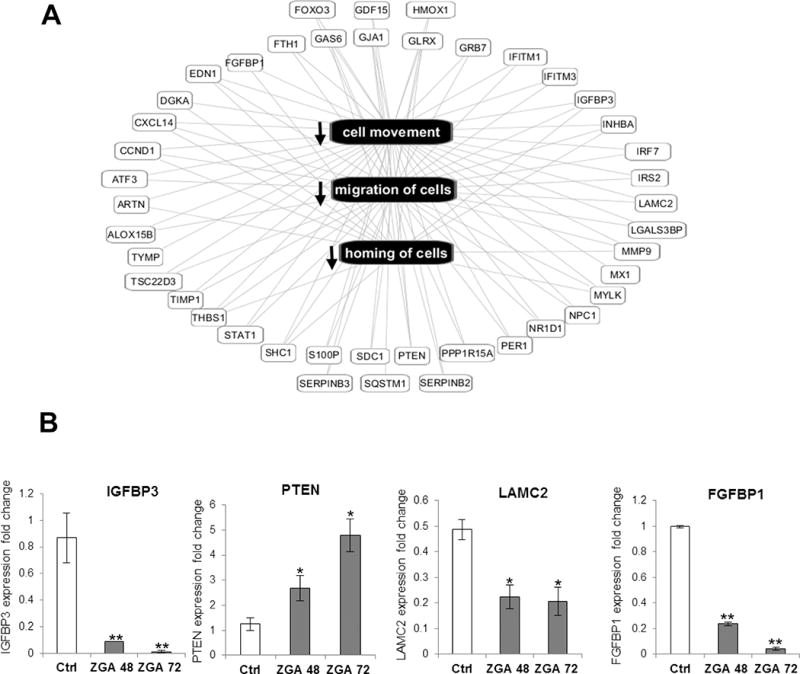
A. Enrichment and predicted inhibition of migration-related processes in ZGA-treated keratinocytes. Network was generated using IPA; lines represent literature-supported relationships. Pattern matching of ZGA-regulated genes with their known biological effects significantly supports inhibition of cellular migration. B. qPCR confirmation of regulated genes involved in suppression of cell migration at 48 h and 72 h post ZGA treatment; Ctrl-untreated keratinocytes. Data represent the mean ± S.D. (n=3) *p≤0.05; ** p≤0.001.
Microarray data also revealed suppression of the tubulin family of genes including TUBA1A and TUBA4A by ZGA (Fig. 1C). Additional suppression of TUBB2A, TUBB3, TUBB4B was observed at 72 h respectively post ZGA treatment (Suppl. Material 2). These genes are important in microtubule formation and assembly and are essential components of the cytoskeleton participating in many cellular functions including directional migration (de Forges et al., 2012). The gene for the transmembrane heparan sulfate proteoglycan syndecan 1 (SDC1), which participates in cell migration and cell-matrix interactions (Stepp et al., 2015), and LAMC2 were both down-regulated at 48 h and 72 h time-points. Thrombospondin 1 (TS1), fibronectin 1 (FN1) and matrix metalloproteinase 9 (MMP9), important for cell-matrix interactions and tissue remodeling during wound healing (Stojadinovic et al., 2007) were also found to be suppressed by FPP (Fig. 1D, Suppl. Material 1&2). Taken together, these data further support our initial findings regarding ZGA-mediated inhibition of keratinocyte migration (Vukelic et al., 2010) and provide new insights into multiple mechanisms by which FPP regulates this process.
FPP Elevation Targets the Early-Wound Healing Marker Keratin 6 through Recruitment of Repressor Complex Involving ß-catenin and CARM-1
In addition to genes described above, microarray data revealed that ZGA treatment resulted in suppression of keratin 6 (K6), the early-wound healing gene (Suppl. Material 3A). We have previously shown that FPP inhibits the K6 promoter acting as a ligand for GR in primary human keratinocytes causing profound inhibition of keratinocyte migration (Das et al., 2007; Vukelic et al., 2010). Moreover, this effect was independent of inhibition of the farnesyl isoprenylation (Vukelic et al., 2010). Nuclear receptors regulate transcription by recruiting multiple co-regulators to the promoters of specific target genes. For the GR, it has been shown that the most important predictors of transcriptional outcome are DNA context and structure of the ligand. Here, we delved further into function of FPP-activated GR. To determine the mechanism of K6 suppression, we checked whether the FPP activated GR recruits co-repressors in a similar manner to the dexamethasone (DEX) activated GR. We have previously shown that in the presence of DEX, ß-catenin can bind to and act as a co-repressor of the GR (Stojadinovic et al., 2007), leading to further suppression of the K6 promoter. To test whether FPP activation of GR leads to recruitment of ß-catenin as a co-repressor, we performed co-transfection experiments in primary human keratinocytes. As expected, ß-catenin by itself did not significantly affect K6 expression (Fig. 6). However, in the presence of elevated FPP ß-catenin acted as a co-repressor of the GR, similar to DEX. Moreover, the protein arginine methyltransferase CARM-1 further enhanced this co-repression (Fig. 6A). We have previously shown that CARM1 (Lee et al., 2005) can bind to ß-catenin and function in synergy as a co-repressor for GR in the context of epidermal specific genes (Stojadinovic et al., 2005). By participating in the GC-mediated repression of K6 as a co-repressor with CARM1, β-catenin contributes to the inhibition of keratinocyte migration through altering the cytoskeletal network (Stojadinovic et al., 2005) (Fig. 6). However, in control co-transfection experiment with consensus GRE, opposite to DEX, FPP activation of GR did not lead to recruitment of either ß-catenin or CARM1 as a co-activators (Fig. 6B). In the line of the transfection data our gene expression analyses have indicated that DEX and ZGA regulate both common and distinct β-catenin transcriptional targets, which are predicted to collectively affect cellular movement (Fig. 6C). Subtle differences between molecular mechanisms by which DEX and FPP target transcription of the same promoter, such as K6, may explain partial overlap of ß-catenin transcriptional targets between FPP and DEX (Fig. 6C).
Figure 6. FPP elevated by ZGA treatment suppresses the expression of K6 promoter through co-repressor complex involving β-catenin.
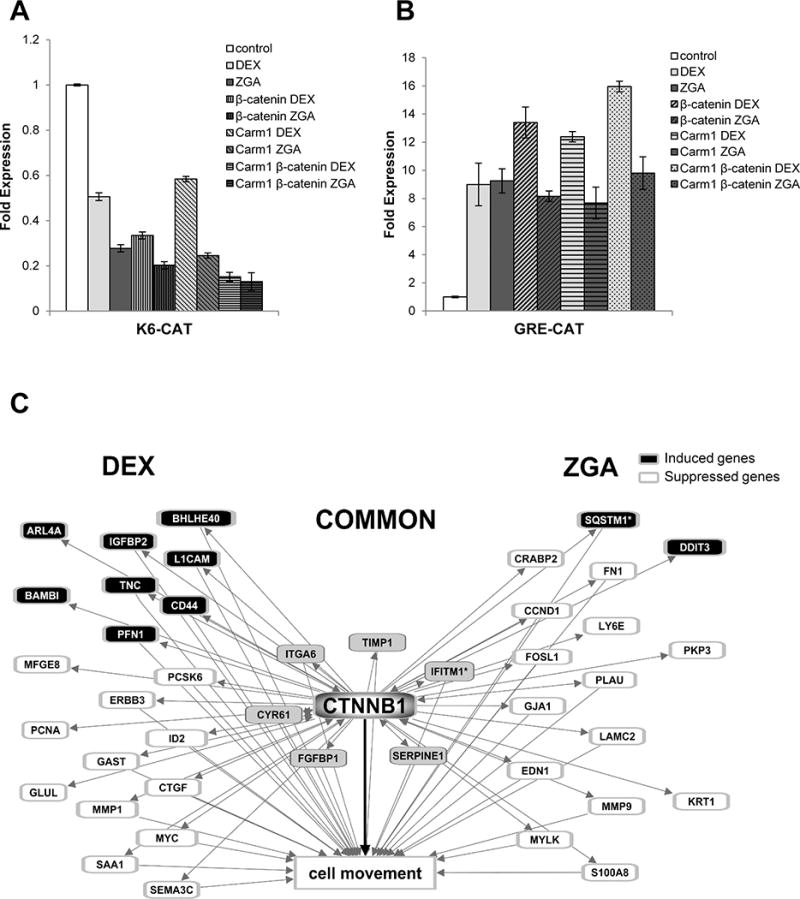
A. CAT assay after co-transfection of human keratinocytes with K6-CAT promoter. β-catenin enhances repression of K6 in the presence of both ZGA and DEX, acting as a co-repressor of GR. In the presence of accumulated FPP (ZGA), β-catenin-mediated K6 co-repression is further enhanced by CARM-1. B. Graphs represent quantitative CAT assay after co-transfection of human keratinocytes with GRE-CAT showing that unlike DEX, ZGA does not recruit CARM-1 or β-catenin to GRE. The data are presented as relative CAT activity, normalized to total protein. Values represent the mean ± S.D. from three independent experiments. C. Dex and ZGA regulate common and distinct β-catenin transcriptional targets which are predicted to collectively affect cellular movement. Black = upregulation, White = downregulation. 34 of the 42 ZGA and/or DEX-regulated β-catenin transcriptional targets are known to affect cell movement. Lines represent literature-supported findings in the Ingenuity Knowledge Base. Figure was generated using Ingenuity Pathway Analysis.
FPP, but not DEX, Suppresses the K6 Promoter through Recruitment of Grip1
To further test whether FPP as a ligand for GR changes the involvement of additional cofactors thus resulting in differential target gene expression, we performed co-transfection experiments with the nuclear receptor coactivator GRIP1 in the presence or absence of FPP and DEX (Fig. 7). Interestingly, in the context of K6, GRIP-1 acted as a co-repressor of the GR in the presence of FPP, but not in the presence of DEX (Fig. 7A). It is known that GRIP1 interacts with GR through the NR (nuclear receptor) Box II and III when DEX is the ligand (Chen et al., 1999; Koh et al., 2002). To determine if GRIP1 interacts with GR through similar binding sites when activated by FPP, we used GRIP1 nuclear receptor box mutant II/III. Mutation of both NR Box II and NR Box III completely eliminated functional and binding interactions with the GR (Fig. 7B). GRIP1 NR box mutant no longer showed co-repressor activity in the presence of FPP, indicating that these two sites are crucial for most of GRIP1’s binding activity to the FPP-activated GR (Ding et al., 1998). This result suggests that in the context of the K6 promoter, FPP-activated GR recruits GRIP1 as a co-repressor through NR box.
Figure 7. FPP targets the K6 promoter through co-repressor Grip1.
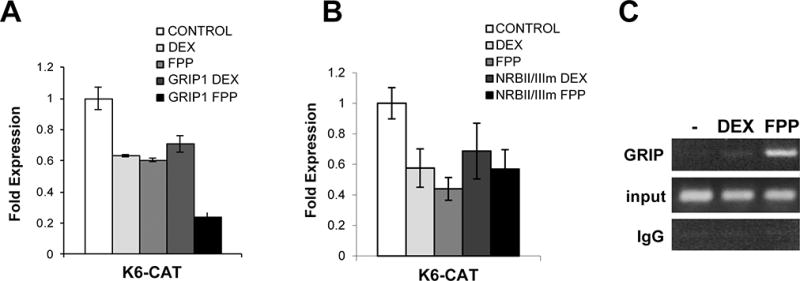
A. Transfection experiments of HEK with the K6-CAT reporters are shown. FPP treatment results in further suppression of K6 promoter in the presence of Grip1. B. GRIP1 DNA binding mutant – NRBmII/III abolishes K6 suppression mediated by FPP. Graphs represent quantitative CAT assay after co-transfection of human keratinocytes; values represent the mean ± S.D. from three independent experiments. C. ChIP with Grip1 antibody. HEK were either untreated (−) or treated with 1μM DEX or 10 μM FPP. Same amounts of genomic DNA (input) was used in each treatment. K6 promoter sequences were amplified by PCR after immunoprecipitation with anti-GRIP1 antibody or IgG.
To further confirm the binding of GRIP1 to GR in the context of K6 promoter, we carried out chromatin immunoprecipitation (ChIP) assays. We used polyclonal antibodies against GRIP1 (or IgG as a negative control) and primers amplifying a K6 promoter containing K6 response element (Lee et al., 2005). Cells were incubated in the presence or absence of FPP or DEX, and chromatin fragments containing identical amounts of total genomic DNA (input) were used for the immunoprecipitations (Fig. 7C). Treatment with FPP induced enrichment of GRIP1 at K6 promoter relative to untreated cells, while DEX treatment did not induce binding of GRIP1 (Fig. 7C). These results confirm our data from co-transfection experiments and show that DEX-activated GR does not recruit GRIP1 as a co-repressor to K6 promoter, whereas FPP does.
Discussion
In this paper we show that transcriptional changes caused by intracellularly accumulated FPP affect a wide range of processes in human keratinocytes, demonstrating the complexity of action of this cholesterol metabolite on epidermis. The predominant effect of FPP on gene transcription was suppression. Elevation of FPP inhibited epidermal differentiation, IFNγ signaling, and genes involved in IGF signaling pathway and cell migration (Fig 8). The observed downregulation of the gene expression can be, in part, explained by the ability of FPP to serve as a GR ligand (Das et al., 2007); (Vukelic et al., 2010), and, therefore, suppress GR target genes and downstream pathways. Indeed, FPP suppressed IFNγ signaling and genes involved in cell migration in a similar fashion as DEX (Stojadinovic et al., 2007). We have also shown that FPP can function as a ligand for nuclear receptors other than GR (Das et al., 2007), resulting in the FPP-specific gene expression signature. The effects of ZGA treatment may also be a consequence of the reduced levels of squalene, and based on the unique FPP-mediated suppression of differentiation may suggest importance of mevalonate pathway in undifferentiated basal keratinocytes or possibly, even stem cell compartment. The lack of mevalonate metabolites has been linked to induced cell differentiation of normal neurons and neuroblastoma cells (Paintlia et al., 2010). Moreover, inhibition of mevalonate pathway by statins has been shown to reduce the potential of mesenchymal stem cell to differentiate (Izadpanah et al., 2015).
Figure 8. Endogenous accumulation of farnesyl pyrophosphate (FPP) in epidermal keratinocytes results in global transcriptional changes, affecting major cellular processes: migration, differentiation, inflammation and oxidative stress response.
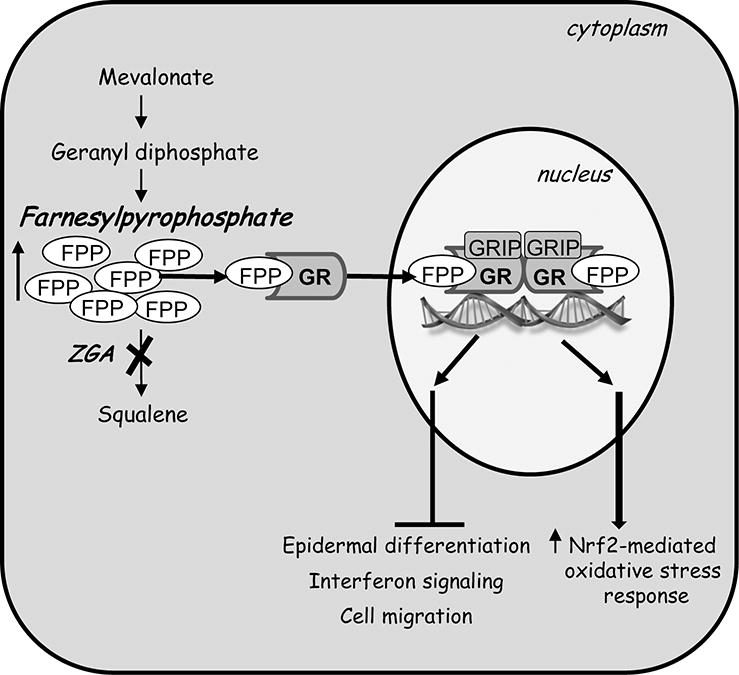
A diagram summarizing multiple effects of accumulated mevalonate metabolite-FPP upon treatment with zaragozic acid (ZGA) on epidermal keratinocytes. FPP acts as a glucocorticoid receptor (GR) ligand which upon binding to GR, recruits nuclear receptor co-factor GRIP1 to modulate epidermal gene expression. As a result, accumulated FPP inhibits keratinocyte migration, differentiation and interferon signaling and induces Nrf2-mediated oxidative stress response.
In contrast to overall transcriptional suppression, ZGA treatment resulted in upregulation of the cytoprotective transcription factor Nrf2 (Sykiotis and Bohmann, 2010), suggesting potential beneficial effects of elevated FPP levels at early time points during the treatment (Fig. 8). In addition, the ENC1 (Ectodermal-Neural Cortex 1) gene, a negative regulator of NRF2 (Wang and Zhang, 2009), was also found down-regulated by ZGA (Suppl. Material 1&2). The NRF2-mediated oxidative stress response pathway has been shown to play an important role in protecting cells against oxidative damage and promoting detoxification (Cordova et al., 2014). NRF2 has been shown to aid in the acute wound healing process by regulating inflammation and promoting survival of the keratinocytes under stress conditions (Braun et al., 2002). Induction of Nrf2 coupled with the overexpression of its target genes may therefore suggest enhanced protection of keratinocytes against environmental insults upon FPP treatment, underscoring some beneficial effects of epidermal cholesterol synthesis. A reduction in Nrf2 expression has been associated with increased oxidized proteins and high glucose-induced intracellular ROS in diabetic patients (Lee et al., 2015; Li et al., 2011) providing potential relationship between NRF2 and impaired wound healing in diabetic patients, thus suggesting that modulation of mevalonate pathway may offer new therapeutic approach.
The effects on FPP on epidermal differentiation and desmosomal and adherens junctions may suggest that elevated levels of FPP may compromise epidermal barrier overall. Decreased expression of desmosomal components (Dsg1 and Dsc1) along with downregulation of Vcl, an integral component of adherens junctions, may have structural impact on epidermal tissue by loosening its junctions and permitting entrance of microorganisms (Amagai et al., 2000). Therefore, on one end, cholesterol synthesis is essential component of lipid barrier (Feingold and Elias, 2014); Slominski et al., 2015a), and on the other when squalene synthesis does not progress, residual accumulated FPP compromises barrier by inhibiting keratinocyte differentiation and affecting tissue architecture.
In this study we further dissected molecular mechanism of the gene expression regulation by FPP. We have previously shown that FPP inhibits keratinocyte migration and wound closure through GR mediated inhibition of K6 (Vukelic et al., 2010). Our co-transfection data indicated that FPP as a GR agonist may change recruitment of co-repressors and co-activators and permit binding of more or different subset of regulatory proteins. In the situation where the ligand has a different chemical structure, such as FPP, a different conformational change of the receptor (GR) may be induced (Claessens et al., 2001; Flammer and Rogatsky, 2011; Kotovych et al., 1998; van Tilborg et al., 2000). In conjunction with specific receptor-DNA interactions such conformational change can either prevent or allow recruitment of co-regulators on specific promoters, resulting in distinct group of regulated genes. Taken together, our co-transfection and ChIP experiments showed that depending on the context (consensus GR element or K6 promoter) and type of co-regulator (ß-catenin, CARM, GRIP1), FPP could not only mimic the activity of GCs, but also expand the involvement of co-repressors or have no effect resulting in unique ZGA-mediated transcriptional changes. It is possible that FPP, upon binding to GR ligand binding domain, causes slightly different conformational change than GCs and acts as a partial agonist or mixed agonist-antagonist. Thus, FPP as a ligand makes GR more permissive for interactions with co-regulatory molecules and expands upon its transcriptional potency. Given that both FPP and cortisol are synthetized by keratinocytes and are two of the few GR agonists naturally present in the cell (Slominski et al., 2014; Vukelic et al., 2011), altering the levels of FPP in cells may have dual effect: direct, through targeting promoters and regulating expression, i.e. FPP -GR-agonist activity and indirect, by antagonizing activity of the endogenous GCs (Vukelic et al., 2011).
Our data suggest that FPP as an endogenous GR ligand may compete, to some degree, with the therapeutic effects of glucocorticoids by regulating keratinocyte functions. As an intermediate product of a mevalonate pathway, FPP levels can be further altered by commonly used drugs, statins (Stojadinovic O, 2010; Vukelic et al., 2010). Our findings that FPP acts as an agonist for GR could also aid better understanding of mechanism of action of statins and lead to additional applications and novel treatments for various skin disorders. Furthermore, the mechanisms by which FPP targets multiple major cellular process may have major implications to epidermal physiology in the context of elevated stress (Jozic et al., 2014) and metabolic disorders, such as diabetes. FPP intersects glucocorticoid signaling and oxidative stress, both of which are activated in the context of various types of stress conditions (Jozic et al., 2014; Slominski et al., 2014). In addition, as a cholesterol metabolite it integrates lipid metabolism into the framework of stress response, which may have major implications for skin physiology.
Supplementary Material
Supplementary Online Material 1. List of ZGA-regulated genes 48 h post-treatment are shown. 50 genes were upregulated and 95 genes were downregulated at 48 h. Fold change cutoff of 2. Fold changes reflect expression from ZGA treated cells vs. untreated control at 48 h; for each gene, paired t-test p<0.05.
Supplementary Online Material 2. List of ZGA-regulated genes 72 h post-treatment are shown. After 72 h of ZGA treatment, 58 genes were upregulated and 160 genes were downregulated. Fold change cutoff of 2. Fold changes reflect expression from ZGA treated cells vs. untreated control at 72 h; for each gene, paired t-test p<0.05.
Supplementary Online Material 3. A. Keratin 6 (KRT6B) expression, as represented by box-and-whisker plots of normalized microarray intensity values, decreases in ZGA-treated cells at both time point. *, p<0.001 by paired t-test. B. qPCR confirmation of regulated genes post ZGA treatment; Control=untreated keratinocytes. Data represent the mean ± S.D. (n=3) *p≤0.05; ** p≤0.001.
Acknowledgments
We thank Dr. Inez Rogatsky for the gift of the ChIP grade anti-GRIP antibody and Dr. Michael Stallcup for the gift of GRIP-1 and GRIP-1 NRBmII/III plasmids. We also thank Natalie Yin, Brian Lee and Elizabeth Nestor for their support and technical assistance and Dr. Antonio Barrientos for generously sharing laboratory resources and equipment. Our research is supported by National Institutes of Health AR060562 (MT-C), NR013881 (MT-C) and University of Miami SAC Award SAC 2013-19 (MT-C).
Footnotes
Conflict of Interest Disclosure: authors state no conflicts of interest.
LITERATURE CITED
- Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nature medicine. 2000;6(11):1275–1277. doi: 10.1038/81385. [DOI] [PubMed] [Google Scholar]
- Ando Y, Jensen PJ. Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. The Journal of investigative dermatology. 1993;100(5):633–639. doi: 10.1111/1523-1747.ep12472297. [DOI] [PubMed] [Google Scholar]
- Bouslimani A, Porto C, Rath CM, Wang M, Guo Y, Gonzalez A, Berg-Lyon D, Ackermann G, Moeller Christensen GJ, Nakatsuji T, Zhang L, Borkowski AW, Meehan MJ, Dorrestein K, Gallo RL, Bandeira N, Knight R, Alexandrov T, Dorrestein PC. Molecular cartography of the human skin surface in 3D. Proc Natl Acad Sci U S A. 2015;112(17):E2120–2129. doi: 10.1073/pnas.1424409112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Molecular and cellular biology. 2002;22(15):5492–5505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PG, Durham SK, Hayes JD, Suwanichkul A, Powell DR. Insulin-like growth factor-binding protein-3 binds fibrinogen and fibrin. J Biol Chem. 1999;274(42):30215–30221. doi: 10.1074/jbc.274.42.30215. [DOI] [PubMed] [Google Scholar]
- Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284(5423):2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- Collins CA, Watt FM. Dynamic regulation of retinoic acid-binding proteins in developing, adult and neoplastic skin reveals roles for beta-catenin and Notch signalling. Developmental biology. 2008;324(1):55–67. doi: 10.1016/j.ydbio.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Cordova EJ, Martinez-Hernandez A, Uribe-Figueroa L, Centeno F, Morales-Marin M, Koneru H, Coleman MA, Orozco L. The NRF2-KEAP1 pathway is an early responsive gene network in arsenic exposed lymphoblastoid cells. PloS one. 2014;9(2):e88069. doi: 10.1371/journal.pone.0088069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Schapira M, Tomic-Canic M, Goyanka R, Cardozo T, Samuels HH. Farnesyl pyrophosphate is a novel transcriptional activator for a subset of nuclear hormone receptors. Molecular endocrinology (Baltimore, Md) 2007;21(11):2672–2686. doi: 10.1210/me.2007-0080. [DOI] [PubMed] [Google Scholar]
- de Forges H, Bouissou A, Perez F. Interplay between microtubule dynamics and intracellular organization. The international journal of biochemistry & cell biology. 2012;44(2):266–274. doi: 10.1016/j.biocel.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Ding XF, Anderson CM, Ma H, Hong H, Uht RM, Kushner PJ, Stallcup MR. Nuclear receptor-binding sites of coactivators glucocorticoid receptor interacting protein 1 (GRIP1) and steroid receptor coactivator 1 (SRC-1): multiple motifs with different binding specificities. Mol Endocrinol. 1998;12(2):302–313. doi: 10.1210/mend.12.2.0065. [DOI] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson E, Campbell L, Davies FCJ, Ross NL, Ashcroft GS, Krust A, Chambon P, Hardman MJ. Insulin-like growth factor-1 promotes wound healing in estrogen-deprived mice: new insights into cutaneous IGF-1R/ERalpha cross talk. The Journal of investigative dermatology. 2012;132(12):2838–2848. doi: 10.1038/jid.2012.228. [DOI] [PubMed] [Google Scholar]
- Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res. 2009;50(Suppl):S417–422. doi: 10.1194/jlr.R800039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochimica et biophysica acta. 2014;1841(3):280–294. doi: 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Goyanka R, Das S, Samuels HH, Cardozo T. Nuclear receptor engineering based on novel structure activity relationships revealed by farnesyl pyrophosphate. Protein Eng Des Sel. 2010;23(11):809–815. doi: 10.1093/protein/gzq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci. 2003;116(Pt 15):3227–3238. doi: 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]
- Hamill KJ, Hiroyasu S, Colburn ZT, Ventrella RV, Hopkinson SB, Skalli O, Jones JC. Alpha actinin-1 regulates cell-matrix adhesion organization in keratinocytes: consequences for skin cell motility. The Journal of investigative dermatology. 2015;135(4):1043–1052. doi: 10.1038/jid.2014.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadpanah R, Schachtele DJ, Pfnur AB, Lin D, Slakey DP, Kadowitz PJ, Alt EU. The Impact of Statins on Biological Characteristics of Stem Cells Provides a Novel Explanation for Their Pleotropic Beneficial and Adverse Clinical Effects. Am J Physiol Cell Physiol. 2015 doi: 10.1152/ajpcell.00406.2014. ajpcell00406 02014. [DOI] [PubMed] [Google Scholar]
- Jozic I, Stojadinovic O, Kirsner RS, Tomic-Canic M. Stressing the steroids in skin: paradox or fine-tuning? The Journal of investigative dermatology. 2014;134(12):2869–2872. doi: 10.1038/jid.2014.363. [DOI] [PubMed] [Google Scholar]
- Jozic I, Stojadinovic O, Kirsner RS, Tomic-Canic M. Skin under the (Spot)-Light: Cross-Talk with the Central Hypothalamic-Pituitary-Adrenal (HPA) Axis. The Journal of investigative dermatology. 2015;135(6):1469–1471. doi: 10.1038/jid.2015.56. [DOI] [PubMed] [Google Scholar]
- Keller RK. Squalene synthase inhibition alters metabolism of nonsterols in rat liver. Biochimica et biophysica acta. 1996;1303(3):169–179. doi: 10.1016/0005-2760(96)00081-1. [DOI] [PubMed] [Google Scholar]
- Koh SS, Li H, Lee YH, Widelitz RB, Chuong CM, Stallcup MR. Synergistic coactivator function by coactivator-associated arginine methyltransferase (CARM) 1 and beta-catenin with two different classes of DNA-binding transcriptional activators. J Biol Chem. 2002;277(29):26031–26035. doi: 10.1074/jbc.M110865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Vouthounis C, Stojadinovic O, Brem H, Im M, Tomic-Canic M. From an enhanceosome to a repressosome: molecular antagonism between glucocorticoids and EGF leads to inhibition of wound healing. Journal of molecular biology. 2005;345(5):1083–1097. doi: 10.1016/j.jmb.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kwon SB, An JM, Kim CH, Lee SH, Choi CY, Nam DH, Park JW, Nam HS, Lee SH, Lee MW, Cho MK. Increased protein oxidation and decreased expression of nuclear factor E2-related factor 2 protein in skin tissue of patients with diabetes. Clinical and experimental dermatology. 2015;40(2):192–200. doi: 10.1111/ced.12487. [DOI] [PubMed] [Google Scholar]
- Li H, Wang F, Zhang L, Cao Y, Liu W, Hao J, Liu Q, Duan H. Modulation of Nrf2 expression alters high glucose-induced oxidative stress and antioxidant gene expression in mouse mesangial cells. Cellular signalling. 2011;23(10):1625–1632. doi: 10.1016/j.cellsig.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation; research in biological diversity. 2007;75(9):853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Paintlia AS, Paintlia MK, Singh AK, Orak JK, Singh I. Activation of PPAR-gamma and PTEN cascade participates in lovastatin-mediated accelerated differentiation of oligodendrocyte progenitor cells. Glia. 2010;58(14):1669–1685. doi: 10.1002/glia.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I, Stojadinovic O, Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surg Technol Int. 2008;17:105–112. [PubMed] [Google Scholar]
- Radoja N, Komine M, Jho SH, Blumenberg M, Tomic-Canic M. Novel mechanism of steroid action in skin through glucocorticoid receptor monomers. Molecular and cellular biology. 2000;20(12):4328–4339. doi: 10.1128/mcb.20.12.4328-4339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Zarember KA, Yamamoto KR. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. The EMBO journal. 2001;20(21):6071–6083. doi: 10.1093/emboj/20.21.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roupe KM, Veerla S, Olson J, Stone EL, Sorensen OE, Hedrick SM, Nizet V. Transcription factor binding site analysis identifies FOXO transcription factors as regulators of the cutaneous wound healing process. PloS one. 2014;9(2):e89274. doi: 10.1371/journal.pone.0089274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath H, Flowers MT, Liu X, Paton CM, Sullivan R, Chu K, Zhao M, Ntambi JM. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J Biol Chem. 2009;284(30):19961–19973. doi: 10.1074/jbc.M109.014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011;47(1):R1–10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. The Journal of steroid biochemistry and molecular biology. 2015a;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol. 2014;23(6):369–374. doi: 10.1111/exd.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Manna PR, Tuckey RC. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids. 2015b doi: 10.1016/j.steroids.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Syndecan-1 and Its Expanding List of Contacts. Adv Wound Care (New Rochelle) 2015;4(4):235–249. doi: 10.1089/wound.2014.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, Merchant A, Galiano RD, Tomic-Canic M. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167(1):59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic OLE, Pastar I, Kirsner I, Davis SC, Tomic-Canic M. Statins as Potential Therapeutic Agents for Healing Disorders. Expert Reviews Dermatology. 2010;5(6):689–698. [Google Scholar]
- Stojadinovic O, Lee B, Vouthounis C, Vukelic S, Pastar I, Blumenberg M, Brem H, Tomic-Canic M. Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-gamma pathway, and wound healing along with promotion of terminal differentiation. J Biol Chem. 2007;282(6):4021–4034. doi: 10.1074/jbc.M606262200. [DOI] [PubMed] [Google Scholar]
- Stojadinovic O, Tomic-Canic M. Human ex vivo wound healing model. Methods in molecular biology. 2013;1037:255–264. doi: 10.1007/978-1-62703-505-7_14. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Stress-activated cap‘n’collar transcription factors in aging and human disease. Science signaling. 2010;3(112):re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Rogatsky I, Schwarcz LE, Darimont BD. The glucocorticoid receptor represses cyclin D1 by targeting the Tcf-beta-catenin complex. The Journal of biological chemistry. 2006;281(26):17856–17863. doi: 10.1074/jbc.M602290200. [DOI] [PubMed] [Google Scholar]
- Tong H, Holstein SA, Hohl RJ. Simultaneous determination of farnesyl and geranylgeranyl pyrophosphate levels in cultured cells. Anal Biochem. 2005;336(1):51–59. doi: 10.1016/j.ab.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. The Journal of biological chemistry. 2011;286(12):10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukelic S, Stojadinovic O, Pastar I, Vouthounis C, Krzyzanowska A, Das S, Samuels HH, Tomic-Canic M. Farnesyl pyrophosphate inhibits epithelialization and wound healing through the glucocorticoid receptor. The Journal of biological chemistry. 2010;285(3):1980–1988. doi: 10.1074/jbc.M109.016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-J, Zhang DD. Ectodermal-neural cortex 1 down-regulates Nrf2 at the translational level. PloS one. 2009;4(5):e5492. doi: 10.1371/journal.pone.0005492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Online Material 1. List of ZGA-regulated genes 48 h post-treatment are shown. 50 genes were upregulated and 95 genes were downregulated at 48 h. Fold change cutoff of 2. Fold changes reflect expression from ZGA treated cells vs. untreated control at 48 h; for each gene, paired t-test p<0.05.
Supplementary Online Material 2. List of ZGA-regulated genes 72 h post-treatment are shown. After 72 h of ZGA treatment, 58 genes were upregulated and 160 genes were downregulated. Fold change cutoff of 2. Fold changes reflect expression from ZGA treated cells vs. untreated control at 72 h; for each gene, paired t-test p<0.05.
Supplementary Online Material 3. A. Keratin 6 (KRT6B) expression, as represented by box-and-whisker plots of normalized microarray intensity values, decreases in ZGA-treated cells at both time point. *, p<0.001 by paired t-test. B. qPCR confirmation of regulated genes post ZGA treatment; Control=untreated keratinocytes. Data represent the mean ± S.D. (n=3) *p≤0.05; ** p≤0.001.


