Abstract
Systemic lupus erythematosus (SLE) is a chronic and potentially severe autoimmune disease that disproportionately affects women. Despite a known role for hormonal factors impacting autoimmunity and disease pathogenesis, the specific mechanisms of action remain poorly understood. Our laboratory previously backcrossed “estrogen receptor alpha knockout (ERαKO)” mice onto the NZM2410 lupus prone background to generate NZM/ERαKO mice. This original ERαKO mouse, developed in the mid-1990s and utilized in hundreds of published studies, is not in fact ERα null. They express an N-terminally truncated ERα, and are considered a functional KO. They have physiologic deficiencies including infertility due to disruption of a critical activation domain (AF-1) at the N terminus of ERα, required for most classic estrogen (E2) actions. We demonstrated that female NZM/ERαKO mice had significantly less renal disease and significantly prolonged survival compared to WT littermates despite similar serum levels of autoantibodies and glomerular immune complex deposition. Herein, we present results of experiments using a lupus prone true ERα−/− mice (deletional KO mice on the NZM2410 background), surprisingly finding that these animals were not protected if they were ovariectomized, even if E2-repleted, suggesting that another hormonal component confers protection, possibly testosterone, rather than the absence of the full-length ERα.
Keywords: Lupus, estrogen receptor alpha, testosterone, estrogen, autoantibody
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by production of autoantibodies and immune complex-mediated end-organ damage. Nine out of ten patients diagnosed with lupus are female, thus biologic sex is key in disease susceptibility. The mechanisms underlying the sex disparity in SLE are multifactorial, and likely involve the sex chromosomes, sex hormones and their receptors. There is strong epidemiologic data to support a role for estrogen impact on disease since the incidence of disease is highest during reproductive years when women are most hormonally active. This is in contrast to pre-menarche and post-menopause, when lupus incidence is lower and the female to male ratio is much less profound. Estrogens, mainly via ERα, may promote lupus by facilitating loss of immunologic tolerance and enhancing production of autoantibodies [1, 2]. In recent years, other immune effects of estrogen were identified, including modulation of Toll-like receptor (TLR) pathways and dendritic cell development that both play a significant role in lupus pathogenesis [3, 4]. The molecular pathways through which estrogens exert these effects are not fully defined.
In murine models of lupus, manipulation of sex hormones has significant impact on disease expression. NZB/NZW mice and some of the derived NZM strains (i.e. NZM2328) have significant female predominance of disease [5]. Both genders of MRL/lpr and NZM2410 mice develop severe lupus, however, there is a trend towards earlier and more severe disease in females [6]. In classic experiments by Roubinian et al., and later by Tarkowski et al., manipulation of sex hormones and castration led to significant effects on disease expression [7–12]. Specifically, castration of male NZB/NZW or MRL/lpr mice led to female-like disease, and ovariectomy with androgen replacement in female mice led to disease protection. Administration of pharmacologic doses of estrogen led to significant enhancement of disease in ovariectomized (OVX) females, castrated males, and un-manipulated females [12].
Estrogen has pleiotropic effects on many different cell types, including immune cells. Thus, estrogens do not act in the same manner in all inflammatory diseases. Even within a disease, estrogen may play both anti-inflammatory and pro-inflammatory roles depending on the disease state, the target organ, the type of immune stimulus, the length of exposure and the presence of other hormones and their receptors. This is consistent with the immense variability in immune responses necessary throughout a female’s reproductive life. Females are known to respond more vigorously to infection and vaccination, but are unfortunately more susceptible to autoimmunity. Testosterone (T2) also plays a role in sex-based disparity in immune responses. Multiple lines of study have demonstrated immune-suppressive effects of androgens on both adaptive and innate immune responses (Reviewed in [13]).
This study was undertaken to clarify unanswered questions from previous studies done using ERαKO animals. The original ERαKO mice express an N-terminally truncated ERα that includes a disruption of a critical activation domain (AF-1) resulting in, strictly speaking, a functional knockout of ERα. They are infertile and have physiologic deficiencies wherever classic estrogen action via AF-1 is required (ex. reproductive tissues) [14]. However, these animals still express an ERα protein with an intact DNA-binding domain (DBD), ligand binding domain (LBD) and AF-2, and may retain some non-classical functions [15]. Thus, they have the potential for impacting the murine lupus phenotype differently than a complete ERα knockout. Additionally, both the functional knockout (ERαKO) and complete knockout (ERα−/−) mice have hypergonadism (supraphysiologic serum levels of estrogen and testosterone, as a result of ERα dysfunction/deletion) which can be immunomodulatory. In this study, we compared intact/unmanipulated NZM ERα−/− mice to ovariectomized NZM ERα−/− mice (E2 and T2 depleted). We also included a group of ovariectomized NZM ERα−/− mice with repletion of E2 via pellet, to determine estrogen impact on the lupus phenotype in the setting of ERα−/− (since E2 may also have immune effects via ERβ, ERR or non-ligand bound functions).
The goal of this study was to assess whether complete deficiency of ERα is similarly protective in lupus disease expression as the functional knockout. We additionally determined the immuno-phenotype of these animals. Our results reveal that the complete ERα knockout is not directly protective since ovariectomized (OVX’d) ERα−/− mice are not protected. Like the NZM ERαKO mice, unmanipulated NZM ERα−/− mice are protected. With removal of sex hormones via OVX, there is no effect of ERα−/−, thus, this study suggests that elevated testosterone (in this case, secondary to hypergonadism) confers protection in the setting of ERα−/−, rather than deficiency of the full length receptor itself. Further study with T2 treatment of the OVX’d NZM ERα−/− group would be required to confirm that androgen treatment, as in historical studies, confers a protective phenotype. This report is of clinical significance since it corrects a previously held belief that ERα and E2 are generally pro-inflammatory in females and lupus murine models, when in fact there is evidence that E2 can have anti-inflammatory effects in specific settings. Regardless, improved understanding of sex hormones and nuclear hormone receptor action (ER and AR) may enable therapeutic targeting of specific functions, as opposed to general manipulation of hormones, to allow for separation of hormone effects on different tissues (immune cells versus reproductive tissue, for example).
2. Materials and Methods
2.1 Mice
Mice were maintained at the Ralph H. Johnson VAMC Animal Facility (Charleston, SC). Animal protocols followed the principles outlined in the Guide for the Care and Use of Laboratory Animals, and were approved by MUSC’s and the VA’s IACUC. The NZM2410 mice were acquired from Jackson Laboratory (Bar Harbor, ME, USA), the ERα−/− C57BL/6 mouse strain was a kind gift of Dr. Ken Korach. The two strains were backcrossed for >10 generations to create the ERα−/− NZM2410 mouse (NZM ERα −/−). They were maintained on a 12 hr. light/dark cycle with access to food and water ad libitum. All experimental mice (n= 51) were female and were littermates when possible. Two cohorts (NZM WT and NZM ERα −/−) were unmanipulated. All other mice were ovariectomized (OVX) at 4 weeks of age, before puberty, and 2 groups subsequently received 0.1 mg, 90-day sustained release 17β-estradiol pellet, implanted sub-dermally (Innovative Research of America, Sarasota, FL, USA). Mice were sacrificed by cervical dislocation following induction of anesthesia by isoflurane at 32 weeks of age or when they reached pre-determined sacrifice requirements (>10% loss of weight, >500mg urine protein as assessed by dipstick, or upon recommendation by the animal facility veterinarian).
2.2 Serum estradiol, serum testosterone, and serum anti-dsDNA
Serum was collected throughout the experiment at 2–4 week intervals and at time of sacrifice via submandibular bleed. Serum anti-dsDNA was measured by ELISA assay as previously described [6]. Estradiol levels were assessed via ELISA (Calbiotech, San Diego, CA, USA), with an assay sensitivity of 3 pg/ml; precision: 3.1% (intra-assay), 9.9% (inter-assay). Testosterone serum levels were assessed by radioimmunoassay (RIA) at the University of Virginia Center for Research and Reproduction Ligand Assay and Analysis core.
2.3 Urine protein excretion
Mice were housed in metabolic cages for 24 urine hour collection at 2–4 week intervals starting at 10 weeks of age until sacrifice. To prevent bacterial growth, antibiotics (ampicillin 25ug/mL, gentamicin 50 ug/mL, chloramphenicol 200 ug/mL) were added to the collection tube. After 24 hrs, urine quantity was determined and samples were frozen at −20° for future analysis via mouse albumin ELISA with known standards.
2.4 Kidney processing and renal pathology
One kidney was divided evenly for renal pathology and immunofluorescent analysis (IF). One half was snap frozen in liquid nitrogen and stored at −80°C for IF analysis, the other half was fixed with buffered formalin, embedded in paraffin, and then sectioned and stained with hematoxylin and eosin. Kidney sections were analyzed in a blinded fashion by Dr. Phillip Ruiz (Department of Pathology, University of Miami School of Medicine, Miami, FL) and graded on glomerular hyper-cellularity, segmental mesangial expansion, neutrophils/cell debris, crescent formation, and necrosis. These scores were combined for a total glomerular pathology score as previously described (17). Deposition of IgG and complement component C3 was assessed by immunofluorescence after incubating slides with rabbit anti-mouse IgG FITC (MP Biomedical) and sheep anti-mouse C3 FITC (MP Biomedical). IgG and C3 were graded 0–3 for intensity of staining as previously described (17). [A second kidney was processed for flow cytometry staining. The outer membrane of the kidney was removed before being sliced into pieces and digested with DNase I (Roche Life Sciences, Indianapolis, Indiana) and collagenase IV (Sigma Aldrich, St. Louis, MO) for 30min at 37C on a shaker. The kidney was then put through a 70um strainer and PBMCs were isolated using a Percoll gradient (Sigma Aldrich, St. Louis, MO). PBMCs were washed 2× with PBS before staining for flow cytometry.]
2.5. Spleen Flow Cytometry
Spleens were harvested and kept in complete RPMI media (10% fetal bovine serum, 1% L-glutamine, 1% penicillin-streptomycin) on ice during processing. The spleens were processed through 40um strainers and depleted of red blood cells with red blood cell lysis buffer (144 mM NH4Cl and 17 mM Tris, pH 7.6). Cells were washed twice with cold complete RPMI before being stained for flow cytometry analysis. Spleen cells (4×106 per sample) were resuspended in staining buffer (0.5% BSA and 0.02% sodium azide in 1×PBS). Viability was assessed using LIVE/DEAD Fixable Dead Cell stain (Life Technologies, Carlsbad, CA, USA) at a concentration of 50 µl/million cells. Cells were stained with Panel I: F4/80-Brillaint violet 421 (1:100), CD19-PerCP/Cy5.5 (1:100), CD3-Brilliant violet 605 (1:100), or Panel II: MHCII-APC (1:200), CD11c-Brilliant violet 605 (1:100), CD8a-Brilliant violet 421 (1:100), CD11b-PE (1:400). Cells were incubated with antibodies for 30 minutes on ice in the dark. All antibodies were purchased from Biolegend (San Diego, CA, USA). Cells were washed twice with staining buffer and resuspended in 0.3 mL of staining buffer for flow cytometry. Cells were acquired on an LSR Fortessa cell analyzer (BD Biosciences, San Jose, CA, USA) and analysis was performed using FlowJo software (FlowJo LLC, Ashland, Oregon, USA). Gating strategies are outlined in the text; briefly, gates were first serially set on Time, Debris dump, Singlets, and Live cells (using L/D in APC-Cy7 channel). Gates were then set by fluorescence minus one (FMO) and back-gating was done to ensure accuracy of populations.
2.6 Statistical analysis
Log rank analysis was used to compare trends in animal survival. For all other experiments, depending on whether data was non-parametric or parametric, either Kruskal-Wallis one-way analysis of variance with posthoc Dunn’s multiple comparison test, or ANOVA with post-hoc Tukey’s test were utilized to test for significance for comparing multiple groups. Standard error of the means was reported where applicable. p values ≤ 0.05 were considered significant.
3. Results
3.1 Survival effect of ERα deletion mutant (ERα−/−) in NZM2410 lupus prone mice
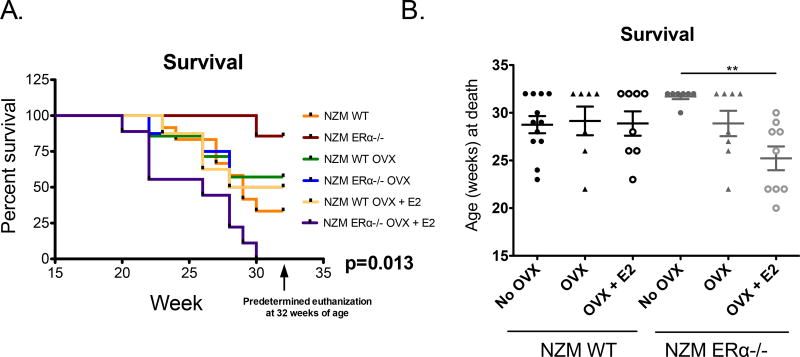
Ovariectomized (OVX) NZM WT mice had similar survival to the intact WT NZM (No OVX) mice regardless of whether they were E2-repleted (Figure 1A, 1B). Thus, OVX did not provide a protective effect in WT NZM2410 mice. In agreement with our prior study using ERα functional KO mice, NZM ERα−/− (ovaryintact) mice demonstrated protection from disease, with 6 of 7 animals surviving to the pre-determined euthanization age. The single animal that died early in that group did not have proteinuria or renal disease by pathology, and appeared to die of a cause likely unrelated to lupus (stomach obstruction). However, OVX of the NZM ERα−/− mice resulted in loss of protection (similar survival rates as NZM WT mice), indicating that the protection conferred by ERα deficiency was absent when sex hormones were removed. Repleting E2 in OVX’d NZM ERα−/− mice did not rescue them. In agreement with historical studies in murine mice, E2-treated mice had more severe disease. In fact, E2-treated ERα−/− mice had accelerated disease beyond that of NZM WT mice treated with E2. No animals survived beyond 30 weeks of age, suggesting that E2 can exacerbate lupus disease expression via a mechanism that is independent of ERα.
Figure 1. Survival of NZM2410 WT vs. NZM ERα−/− mice.
All mice were female. A subset underwent ovariectomy (OVX), and a subset of those were E2-repleted. A) Kaplan-Meier curve: 86% of intact NZM ERα−/− mice survived to the 32 week terminal point, whereas survival in other cohorts was 30–57% at 32 weeks. NZM ERα−/− mice that were both OVX’d and E2-repleted had exacerbated disease (0% survived to predetermined endpoint). Global p-value of differences among all 6 groups (using a log-rank Mantel-Cox test) was 0.013. B) Pair-wise comparisons of intact (No OVX) NZM ERα−/− mice to each of the other 5 groups (using a Dunn’s adjusted p-value), resulted in a significantly increased probability of survival in the No OVX NZM ERα −/− group in comparison with NZM ERα−/− mice that were OVX’d and E2-repleted (p< 0.01).
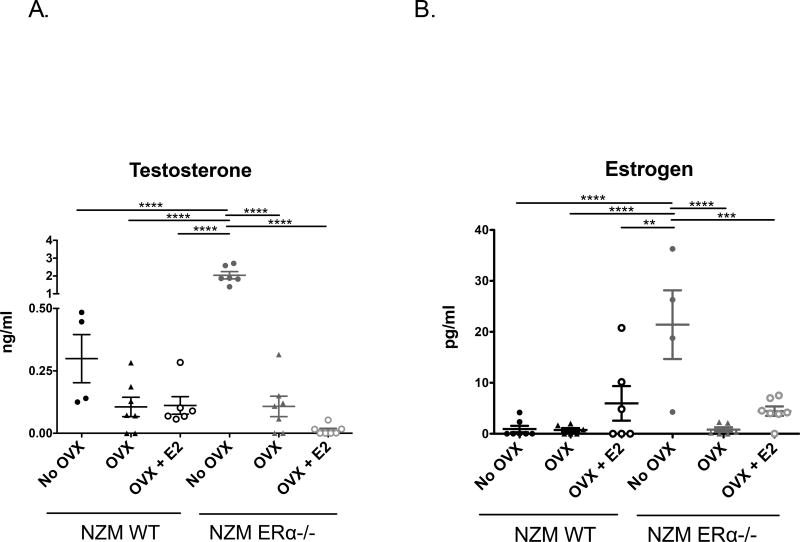
3.2 Testosterone levels in NZM ERα−/− mice correlate with survival
This study, in which NZM WT and NZM ERα−/− mice had E2 replaced via subcutaneous delayedrelease pellets (0.1 mg) following OVX, was designed to mitigate potential confounding effects of hypergonadism resulting in high endogenous testosterone levels in female NZM2410 mice without an intact ERα, as observed in our previous study. Serum testosterone (T2) levels were assayed at 2 different time points, 18 weeks and 32 weeks, with radioimmunoassay. Figure 2A shows testosterone levels from 32 weeks (or terminal endpoint). As expected, all OVX’d mice had low or undetectable T2 levels. Mice that were not OVX’d had higher levels, but significantly so for the NZM ERα−/− mice, which had levels approaching that of male mice. The significantly elevated T2 levels measured in the intact NZM ERα−/− mice may have contributed to the protected phenotype seen in this group that is not seen in the other groups where T2 levels are low. Specifically, NZM ERα−/− mice that were OVX’d had testosterone levels similar to that of NZM WT OVX, and their survival was remarkably similar. Thus, ERα deficiency alone did not directly protect from lupus disease expression, since the protective effect was lost following OVX. Also of note, E2 treatment was not protective in the WT or ERα deficient mice. Consistent with historical reports, E2 exacerbated disease, but only impacted survival in ERα null mice, not WT. Interestingly, this did not correlate with E2 levels (which were generally low or physiologic), but correlated with T2 levels. NZM ERα−/− OVX +E2 mice had the lowest T2 of all the groups and had significantly worse survival. These data suggest that in the setting of ERα deficiency, E2 treatment acted either directly or indirectly to lower T2 levels, and this may be either receptor-independent or via another receptor (ERβ, ERR). All together, this experiment demonstrated that complete knockout of ERα alone does not protect NZM lupus-prone mice from disease expression as had been previously concluded based on the unmanipulated NZM ERKO model. Most likely, testosterone, or lack thereof, plays a critical role in disease development in females in this model, perhaps more so than estrogen.
Figure 2. Hormone levels in NZM2410 WT and NZM ERα−/− mice +/− OVX and E2-repletion.
All mice were female. A) Serum testosterone (T2) levels where determined by radioimmunoassay and ELISA. Intact NZM ERα−/− mice had the highest levels of T2 due to known hypergonadism, and were significantly increased over all groups, including intact NZM WT mice. B) Estrogen (E2) levels in serum samples were assessed via ELISA. A subset of NZM WT and NZM ERα−/− mice had E2 replaced via subcutaneous 90d sustained-release pellet after OVX (implanted twice). Intact NZM ERα−/− mice had significantly higher levels of E2 (although not technically supraphysiologic), even more than those that were E2-repleted.
17β-estradiol levels in mice range from <5 pg/ml to >1000 pg/ml (sick/old/non-breeding mice on the low end vs. healthy/young/pregnant mice on the high end). In this study, serum 17β-estradiol levels for all treatment groups were assessed by estradiol ELISA at two time points, 18 weeks and 32 weeks. Results from the 32 week (or terminal) time point are displayed in Figure 2B. Intact NZM WT females had lower normal estrogen levels than expected, potentially due to a variety of factors including age, disease state, and hormonal cycle. Intact NZM ERα−/− mice had significantly higher estrogen levels compared to all other cohorts, consistent with hypergonadism (also seen in ERαKO mice).
3.3 Autoimmunity in NZM ERα−/− mice is increased, not decreased, compared with NZM WT mice
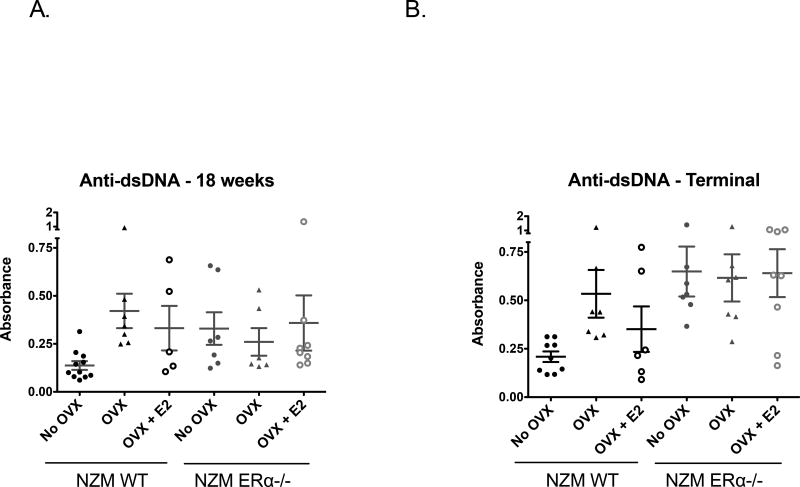
Anti-double stranded DNA (anti-dsDNA) levels were assessed via ELISA at 18 weeks (early-mid stage disease) and 32 weeks (terminal endpoint). While some individual animals had higher anti-dsDNA levels than others within a particular group, all cohorts had similar levels of anti-dsDNA antibodies at the 18 week time point with the exception of the intact NZM WT which trended towards lower levels (Figure 3A). This trend was further borne out at 32 weeks at which time all 3 groups of NZM ERα−/− mice had similar elevations in anti-dsDNA antibody levels regardless of OVX or E2 treatment (Figure 3B). Since levels of E2 and especially T2 are quite different amongst the ERα−/− groups, these data suggest that sex hormones alone do not significantly impact autoantibody production, consistent with our previous data. Also in agreement with our previous study, lack of ERα appears to increase, not decrease, autoantibody levels across all groups suggesting that the etiology of the protective disease phenotype and improved survival observed in intact NZM ERα−/− mice is not due to a difference in tolerance or autoantibody production.
Figure 3. Anti-double stranded DNA in NZM WT vs. NZM ERα−/− mice.
A) Anti-dsDNA levels as measured by ELISA were mildly elevated in WT NZM mice by 18 weeks, but moreso in all other groups. There was no significant difference, ANOVA=0.22 B) At the terminal endpoint (32 weeks or at sacrifice), serum anti-dsDNA antibody levels were high in all groups with no significant differences between individual groups after multiple comparisons, however trends suggested only groups that were deficient in ERα, E2, or both had the highest levels, ANOVA p=0.021.
There are also differences amongst the NZM WT mice, where OVX without E2 replacement resulted in higher anti-dsDNA levels. E2 treatment resulted in diminished autoantibody production in NZM2410 WT mice, in contrast to prior reports of E2 (also via ERα) inducing breaks in tolerance in other lupus models. Since E2 is an important driver of ERα expression, and OVX’d animals lack both E2 and T2, these data suggest that ERα plays a critical role in tolerance in vivo, and its complete deficiency may trigger or exacerbate autoimmunity.
3.4 Effects of ERα receptor knockout on lupus renal disease: proteinuria, C3 and IgG deposition, and histopathology
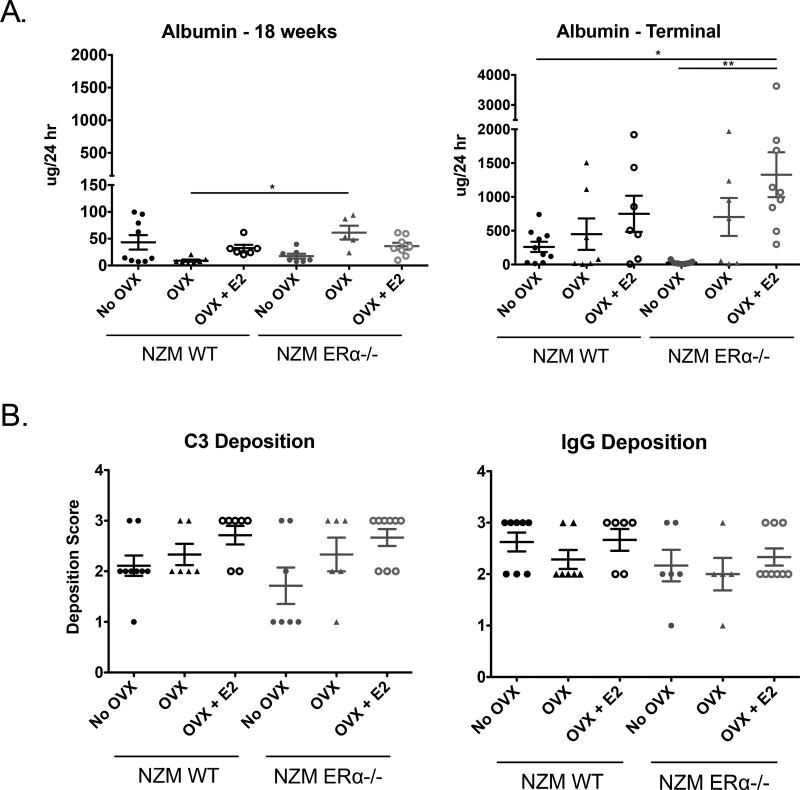
Urine protein levels (24h collection) were assessed using albumin ELISA at 18 and 32 weeks of age. All groups had mild proteinuria at 18 weeks except NZM WT OVX mice which appeared to be fully protected from early renal damage that was significantly different from NZM ERα−/− OVX (Figure 4A). By 32 weeks or the terminal endpoint, there were clearer differences among the cohorts, with only the intact NZM ERα−/− mice protected from progression to having pathologic proteinuria, consistent with their improved survival (Figure 4B). Intact NZM WT mice also showed a trend towards reduced albuminuria compared to the OVX’d groups, perhaps owing to their slightly higher T2 levels compared with OVX’d mice. Of note, intact NZM ERα−/− mice had the highest T2 levels. Also of note, E2-treated mice had exacerbated proteinuria, despite ERα knockout, suggesting that E2 may have renal-damaging effects that are ERα-independent. Together, these data definitively demonstrate that complete knockout of ERα is not directly protective. The data suggest that higher levels of serum T2 may be protective, and E2 may be detrimental in lupus nephritis even when ERα is absent.
Figure 4. 24h Proteinuria and C3 and IgG Deposition in NZM WT vs. NZM ERα−/− mice.
A) 24h proteinuria was assessed by albumin ELISA. At 18 weeks, mild proteinuria was observed in most groups although NZM WT OVX mice were protected from early proteinuria. B) By 32 weeks (or the terminal time point), most NZM mice had clinically significant proteinuria with the exception of intact NZM ERα−/− mice, which were protected. E2 exacerbated proteinuria, even in NZM ERα−/− mice which had significantly more proteinuria than intact NZM WT or NZM ERα−/− mice. C) C3 and IgG deposition were assessed via immunofluorescence after incubating slides with sheep anti-mouse C3 or rabbit anti-mouse IgG antibody. Slides were scored on a 0–3 scale by a blinded investigator. Almost all mice had moderate to high C3 and IgG deposition, and there were no significant differences in immune complex deposition among the groups, although NZM ERα−/− mice trended towards reduced C3 deposition.
Deposition of C3 and IgG, markers of kidney involvement in lupus diseases, was assessed via immunofluorescence. Samples were graded on a 0–3 scale by a blinded scorer. Similar to the albuminuria data, we observed that intact NZM ERα−/− mice had significantly lower C3 deposition (Figure 4C) compared to NZM WT mice or NZM ERα−/− OVX + E2 mice after accounting for multiple comparisons. It is worth noting, however, that all of the mice had at least some C3 deposition, and several animals had substantial deposition (grade 3) despite the fact that no animal in this group had significant proteinuria or died of lupus-related kidney disease. The immune complex deposition in this group could perhaps be expected given that serum autoantibody levels were still high in these mice. Consistent with our previous report in NZM ERαKO mice, there is a disconnect between effects of E2 and ERα on autoantibody production vs. renal pathology and mortality. This appears to also be the case with IgG deposition - all cohorts had similar moderate-severe IgG deposition scores, with most animals being scored at 2 or higher (Figure 4D) despite stark differences in other disease parameters. As in human disease, autoantibody production is necessary but clearly not sufficient for renal disease manifestation in this model.
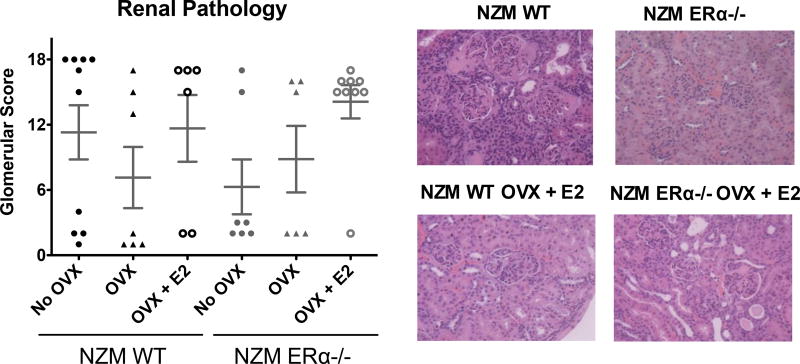
This is further supported by renal pathology results from these mice. H&E stained kidney samples were scored, range: 0–18, by a blinded pathologist who used multiple parameters to assess disease. The most common changes contributing to an elevated glomerular score in WT NZM mice were hypercellularity, mesangial expansion, membrane thickening, and focal segmental glomerular sclerosis. We observed a protected renal phenotype in intact NZM ERα−/− mice, whereas OVX’d NZM ERα−/− mice were not significantly different from NZM WT, and were in fact most damaged when ERα−/− mice were both OVX’d and E2-repleted (Figure 5). Thus NZM mice developed proliferative renal disease similar to WT NZM regardless of hormone receptor status, but impacted by hormones themselves. Nearly 90% of NZM ERα−/− OVX + E2 had glomerular scores >15. In parallel with the albuminuria results, renal pathology scores correlated with T2 in NZM ERα−/− mice. As is typically observed in this model, all cohorts had some animals that did, and some that did not, have high renal pathology scores, indicating that a subset of NZM mice are able to “escape” lupus kidney disease despite similar genetic backgrounds and exposures. There was also a slight trend towards renal protection in the OVX’d NZM WT mice without E2 repletion. As in human lupus, the renal scoring system outlined above was based on glomerular pathology, but in all cases chronic inflammation and fibrosis in the tubulointerstitium (TI) were also assessed. It is worth noting that NZM mice had concomitant disease in the TI that paralleled the severity of disease in the glomerulus in this model with less severity in the intact NZM ERα−/− mice compared to the other groups (data not shown). While several mice in this cohort had evidence of mild-moderate chronic inflammation in the TI, no intact NZM ERα−/− mouse had fibrosis.
Figure 5. Renal pathology scores in NZM WT vs. NZM ERα−/− mice.
Histopathology was scored by a blinded pathologist based on multiple parameters including glomerular hypercellularity, segmental mesangial expansion, membrane thickening, neutrophils/cell debris, crescent formation and focal segmental glomerular sclerosis. Scores in all treatment groups were varied, with animals having either high (≥15) or low scores (<5), indicating few animals with moderate disease. Although there were no significant differences we observed trends toward a protected renal phenotype in intact NZM ERα−/− mice, and both groups of OVX’d mice that were not E2-repleted. NZM ERα−/− OVX +E2 mice appeared to be the most renally progressed, with 89% of mice exhibiting severe pathology scores (all died), ANOVA p=0.21.
3.5 Effects of deleting ERα receptor on numbers of innate and adaptive immune cells in the spleen
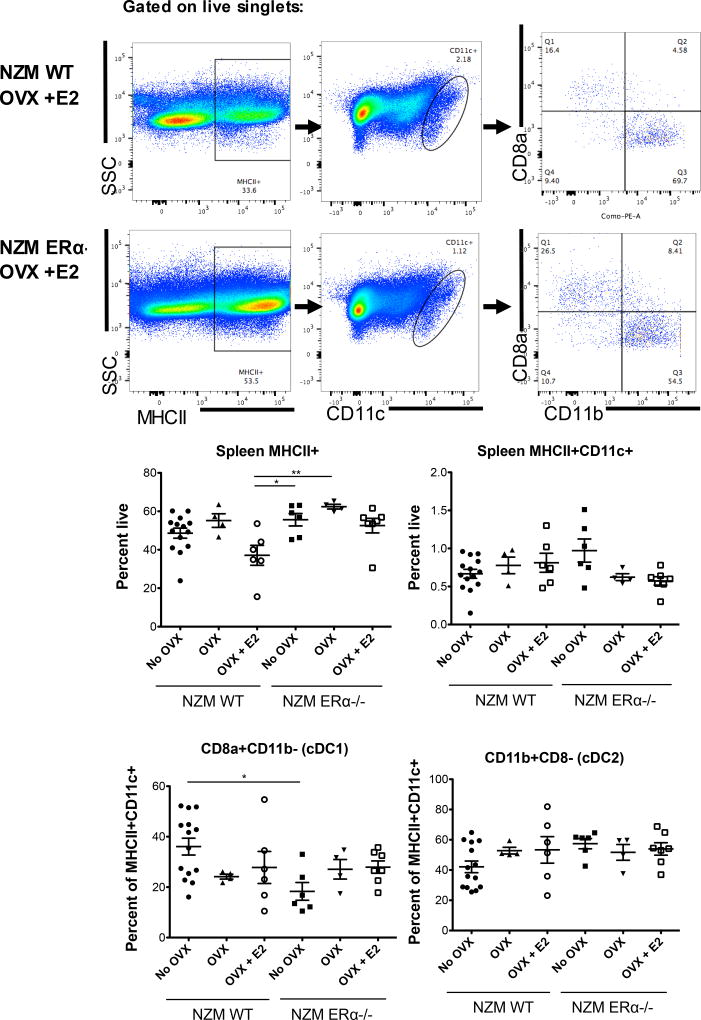
Spleen cells were isolated from all animals that were sacrificed, and stained for flow cytometry analysis. Based on our previous work in ERαKO mice, and work of others, we hypothesized that changes in E2 and ERα would significantly impact numbers and subsets of innate immune cells, specifically dendritic cells, that require E2 and ERα for normal development and differentiation [3, 16–18]. Spleen cells from NZM lupus prone mice were stained for MHCII, CD11c, CD11b, and CD8a to assess numbers of cDCs (classical/conventional dendritic cells) as well as cDC subsets: CD11b+ (DC2s) vs. CD8+ (DC1s). In a separate panel, PDCA1 was used to identify plasmacytoid dendritic cells. Spleen weights were not significantly different between groups and spleen counts were also not significantly different, although there was a trend toward an increased spleen cell count in untreated OVX’d NZM ERα−/− mice (Supplemental data). There was a decrease in percent of MHCII+ cells from spleens of NZM WT OVX +E2 mice versus other groups, which was significant compared with NZM ERα−/− OVX (+/− E2) (Figure 6); absolute numbers were not significantly different between the groups (data not shown). There were no significant differences in the percent of MHCII+CD11c+ spleen cells among groups, although there was a trend towards an increase in intact NZM ERα−/− mice. Surprisingly, absolute numbers of these cells were significantly increased in intact NZM ERα−/− mice versus NZM WT mice (Supplemental data). This data demonstrates that on the NZM background, ERα is not required for development of MHCII+CD11c+ cells, perhaps due to the elevated levels of Type 1 IFN that drive disease in this model (overriding a need for E2). Of the MHCII+CD11c+ spleen cells, DC1s (CD8+ DCs) were generally decreased compared with all other groups, and significantly decreased versus NZM WT mice, while DC2s (CD11b+ DCs) trended up in intact NZM ERα−/− mice. Percent of DC2s in spleens from intact NZM ERα−/− mice were also significantly increased versus intact NZM WT mice (supplemental data). With regard to pDCs (MHCII+CD11c+F4/80-PDCA1+ cells), there were small shifts in numbers, however there were no significant differences between groups (Figure 7). Together these data support the concept that sex hormones, including testosterone, play a role in modulating development of innate immune cells such as DCs. Perhaps more importantly, this work demonstrates that a full length ERα is not required for robust development of DCs on the NZM2410 background, since all three groups of ERα null mice were still able to produce DCs on par with that of WT animals. This further sheds light on previous work using ERαKO NZM mice in which DC development was negatively impacted [3]. Since AF-1 is missing in NZM ERαKO mice, our working model is that the AF-1 mutant of ERα actively suppresses early DC development, not seen in the ERα−/− mouse.
Figure 6. Flow cytometry of isolated spleen cells of NZM WT vs. NZM ERα−/− mice (DC panel).
Single cell suspensions of spleen cells were stained with MHCII, CD11c, CD11b, and CD8a and gates set by FMO. NZM WT OVX +E2 treated mice had decreased MHCII+ spleen cells compared to all groups, and were significantly reduced compared with intact ERα−/− and OVX’d ERα−/− mice, however this did not parallel MHCII+CD11c+ cells or subsets of DCs based on CD11b or CD8a staining. The only significant difference was seen in the DC1 subset which was reduced in intact NZM ERα−/− compared to intact NZM WT mice.
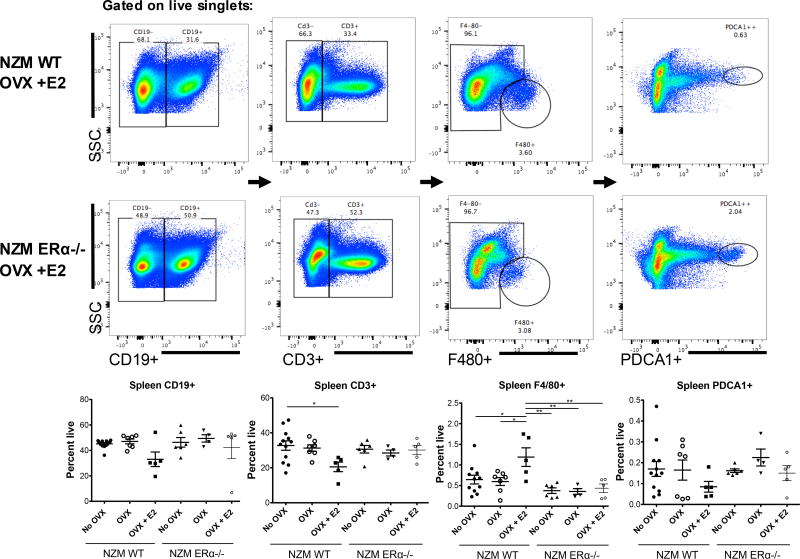
Figure 7. Flow cytometry of isolated spleen cells of NZM WT vs. NZM ERα−/− mice.
Single cell suspensions of spleen cells were stained with CD19, CD3, F4/80, and PDCA, with gates set by FMO. There were no significant differences in B cells among the groups although NZM WT OVX +E2 treated mice trended towards reduced numbers. These mice also had a significant reduction in total CD3+ T cells compared with intact NZM WT mice, and significantly increased percent of mature macrophage by F4/80 staining compared with all groups, suggesting ERα is required for this effect. Although there were slight differences in numbers of PDCA1+ cells (pDCs), there were no significant differences.
Panel II was designed to assess numbers of other spleen immune cells to look for any other possible differences in immune cells that might correlate with lupus phenotype differences. There were no significant differences in percent of CD19+ or CD3+ spleen cells except for a trend towards reduced numbers in WT OVX +E2 mice that was significantly different from intact WT mice (CD3+) (Figure 7); absolute numbers were not different between these groups, whereas there was a significant difference in absolute numbers (but not percent positive) of CD3+ and CD19+ in NZM ERα−/− OVX mice (data not shown), likely related to slightly increased spleen counts in these mice. These differences are unlikely to be of import, since there is no correlation with disease phenotype. Interestingly, although it also does not correlate with disease phenotype, there was a significant increase in both percent and absolute numbers of mature macrophage (F4/80+) in spleens of NZM WT OVX +E2 mice not seen in other groups, suggesting that this innate immune cell population is also impacted by E2 (higher in this group) and T2 (lower in this group) that does require ERα.
4. Discussion
Systemic lupus erythematosus and other autoimmune diseases have a significant female sex bias. Although this may in part be determined by the sex chromosomes, many studies have established a significant role for sex hormones and their receptors in triggering disease and modulating disease severity [19, 20]. Sex hormones, such as estrogens, are generally considered to be an environmental factor in disease pathogenesis, and thus may be considered a potentially modifiable risk, particularly if immune functions can be separated from reproductive functions. Extensive evidence indicates that E2, usually via ERα signaling, has significant immunomodulatory effects on most immune cell types, both developmentally and functionally. Testosterone and other sex hormones also have known immunomodulatory effects [21–24]. In murine lupus, manipulating sex hormones via classic ovariectomy and castration experiments demonstrated clear roles for E2 and T2 in exacerbating or ameliorating, respectively, lupus disease expression [25]. However, results from studies where ERα was knocked out are not as clear-cut. For example, ERα deficiency in wild-type B6/129 mice actually caused a lupus-like glomerulonephritis [26]. In contrast, targeted ERα disruption in NZM2410 and MRL/lpr female mice ameliorated glomerulonephritis, without decreasing autoantibody production, while in NZB/NZW F1 lupus-prone mice it resulted in attenuation of both autoantibody production and glomerulonephritis [6, 27].
The inconsistent results observed in these studies may at least be partially explained by two differences: 1) abnormal sex hormone profiles seen in ERαKOs due to loss of a negative feedback loop leading to hypergonadism, since most animals were not ovariectomized to control for this, and; 2) the structure/design of the knockout. One of the original ERαKO strains and the most widely used for several decades, expresses a truncated ERα (missing most of the AF-1 domain) (27), and is structurally quite similar to the AF-1 mutant mouse reported recently [18, 28, 29]. It is also similar in structure to an endogenous ERα splice variant, ERα46 [30–32]. This short ERα is unable to be classically activated by E2 in reproductive tissues or any tissue that requires E2-induced AF-1 transactivation (therefore resulting in infertility and other deficiencies).
To simultaneously determine the impact of sex hormones and complete ERα deletion on murine lupus, we investigated the effect of ovariectomy (OVX) +/− estradiol (E2) repletion on lupus disease phenotype in NZM2410 mice. This is the first report comparing NZM WT mice to a total body ERα deletion mutant on the NZM background (NZM ERα−/−). Similar to the protected phenotype seen in NZM ERαKO female mice, NZM ERα−/− mice were protected from lupus disease expression, but only if they had intact ovaries. OVX abrogated the protection, and OVX + E2 repletion exacerbated disease as in classic experiments. Intact NZM ERα−/− mice had significantly improved renal disease and significantly improved survival, despite similar or higher levels of autoantibodies, consistent with our earlier intact ERαKO studies. The fact that OVX’d NZM ERα−/− animals do the same or worse than NZM WT indicates that the hormonal milieu (ex. T2 levels) and not the estrogen receptor status accounts for the protective phenotype.
This study further suggests that hypergonadism and the resultant elevated testosterone is likely the etiology of the protective effect in intact NZM ERα−/− (and ERαKO) mice since T2 levels nicely parallel renal disease parameters and survival results. In this and previous studies, elevated E2 levels seen in intact NZM ERα−/− animals may also have been a confounder since high E2 levels could 1) act via the intact ERβ receptor to exert effects and/or 2) impact levels of other hormones such as prolactin which has immunomodulatory potential. Further experiments are necessary to definitely establish that T2 (vs. another impacted hormone such as E2, prolactin, progesterone, etc) results in the protective mechanism.
Flow cytometry of spleen cells to immunophenotype this new strain of mice (NZM ERα−/−) under various hormonal conditions did not suggest profound changes in numbers of any one immune cell type that might explain the significant differences in renal disease phenotype and survival. Based on the literature we hypothesized that there would be a decrease in total DCs, and perhaps also differences in DC subsets, in ERα null mice. Lack of estrogen after OVX in WT mice (without E2 repletion) was also expected to impact DC number. One possible explanation for the minimal impact of estrogen and ERα on DC endpoints is the highly inflammatory background (and late time point) of the NZM mice when analyzed, in comparison to normal B6 WT mice or young NZM mice that have been previously studied. NZM mice have Type I IFN-mediated lupus-like disease [33, 34] which may override some of the immune modulation of sex steroids that would be more impactful in an otherwise non-inflammatory setting. For example, Carreras et al. reported that ERα signaling promotes DC differentiation by inducing IRF4 expression [35], and the requirement for ERα can be circumvented by otherwise increasing IRF4 expression (which would be expected in this IFNα-induced model, likely via NfκB).
B cell and T cell spleen numbers (grossly identified with CD19 and CD3, respectively) were also not profoundly different between groups, but there were some trends that suggested hormonal modulation. ERα action was studied extensively in adaptive immune cells of multiple other models, however, one new finding herein was the increased anti-dsDNA production seen in all of the ERα−/− mice, regardless of T2 or E2 level. This autoantibody result, consistent with ERα deficiency in B6/129 mice [26], is of some interest and will require further experiments to determine the mechanism of increased autoimmunity seen in ERα−/− mice in this NZM model (not correlated with renal disease or survival).
To our knowledge, this study is the first report of complete ERα deficiency in murine lupus, correcting for the confounders of aberrant hormone levels, and demonstrates that complete ERα deficiency is not directly protective in murine lupus. We have also demonstrated that, consistent with classic reports, estrogen exacerbates lupus disease expression, but does so, interestingly, by an ERα-independent mechanism. We also provide evidence that deletion of ERα paradoxically increases autoantibody levels regardless of hormonal milieu. Pharmacological activation or inhibition of ERα already provides the foundation for therapeutic interventions in breast cancer and osteoporosis. Any future use of next generation selective estrogen receptor modulators (SERMs) in autoimmune diseases relies on our ability to understand the separate molecular actions of ERα in individual tissues and cell-types, and at appropriate times, so we may uncouple detrimental effects from beneficial ones.
In summary, despite myriad studies on the effects of estrogen on different cell types in lupus, our understanding of ERα molecular mechanisms of action in normal immunity and autoimmunity is still limited. Hormones clearly have variable effects on B, T, and dendritic cell functions [36]. Small clinical trials and case series have suggested benefits of androgens or known SERMs in lupus, but these are not well tolerated (ex. DHEA, fulvestrant) [37–39] or may even be associated with risks such as, in the case of anti-estrogens: clotting/strokes, bone loss and mood disorders, for which our patients are already at higher risk. Other trials confirmed the safety of estrogen-containing oral contraceptives, although hormone-replacement therapy induced mild flares in subsets of lupus patients [40, 41]. Overall, however, we have not sufficiently progressed our knowledge of sex hormone effects on lupus disease expression to benefit patients, as we have in other diseases where hormones play an important role. Contributing to the problem is a lack of detailed understanding of nuclear hormone receptor action and regulation in immune cells. The current study’s conclusions modify our previous working hypothesis that complete ERα deficiency is protective in murine lupus. Instead the study supports prior work that demonstrated a protective role for T2 and an exacerbating role for E2, although not via ERα. In contrast to the literature, mice with high T2 (in this case from ERα deficiency) are not protected from autoantibody development, but rather renal disease progression. In fact, ERα deficiency actually appears to worsen autoantibody development in this model, regardless of T2 or E2 levels, suggesting a role for ERα in the development of autoimmune B cells or individual cell autoantibody production. Additionally, E2 exacerbation of disease is much more evident in ERα−/− mice (no animals survived to endpoint), suggesting that E2 is pro-inflammatory either in a hormone receptor-independent manner, or via a different nuclear hormone receptor. Regardless, these data suggest that ERα is not the villainous receptor we once thought, and clearly has some protective functions in lupus as it does in other diseases such as experimental autoimmune encephalitis (EAE). Further work is necessary to determine more definitely the protective mechanisms of sex hormones and their receptors at particular time points and in particular tissues. Given that most autoimmune diseases are more prevalent in women, ongoing studies are needed to identify the mechanisms of hormone action that lead to increased female risk in autoimmunity.
Supplementary Material
Highlights.
NZM2410 ERα−/− mice are not protected from lupus disease expression if ovariectomized.
ERα disruption increases, not decreases, autoantibody production in the NZM2410 model.
E2 can exacerbate lupus disease expression via a mechanism that is independent of ERα.
DCs & their subsets (cDC1, cDC2) are not altered by E2 or ERα on the NZM background.
Acknowledgments
The authors wish to thank our long-time collaborator Gary Gilkeson at MUSC for comments on the manuscript and continued mentorship. We thank Ken Korach at NIEHS for both ERαKO strains of mice as well as his insight through the years. We would also like to acknowledge Erin Collins, Osama Naga, and Ivan Molano for help with experimental troubleshooting and ELISA assistance. We additionally express thanks to Adam Soloff in the Cell Evaluation & Therapy Shared Resource at the Hollings Cancer Center for help with flow cytometry set-up and helping us to produce high quality flow data for reproducibility. Lastly, we thank our funding sources: The South Carolina Clinical & Translational Research (SCTR) Institute/CTSA, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant number TR001452 and KL2 TR000060; and a VA Medical Research Service Merit Award 1I01BX000470-01, and NIH/NIAMS K08 Grant number AR068471. Supported in part by the Cell Evaluation & Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interest exists.
Author Contributions
JW, JS and MC conducted all studies with help from JE (breeding, genotyping, bleeding and urine collection), and PR (pathology). JS, JW and MC reviewed/interpreted and analyzed the data. MC designed the study and directed the work. MC, JW and JS prepared the manuscript and figures. All authors read and approved the final manuscript.
References
- 1.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–90. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Solal JF, Jeganathan V, Grimaldi CM, Peeva E, Diamond B. Sex hormones and SLE: influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham MA, Naga OS, Eudaly JG, Scott JL, Gilkeson GS. Estrogen receptor alpha modulates Toll -like receptor signaling in murine lupus. Clin Immunol. 2012;144:1–12. doi: 10.1016/j.clim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svenson J, Cunningham M, Dasgupta S, Gilkeson GS. Estrogen Receptor Alpha Modulates Mesangial Cell Responses to Toll-Like Receptor Ligands. The American journal of the medical sciences. 2014 doi: 10.1097/MAJ.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 5.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svenson JL, EuDaly J, Ruiz P, Korach KS, Gilkeson GS. Impact of estrogen receptor deficiency on disease expression in the NZM2410 lupus prone mouse. Clinical immunology. 2008;128:259–68. doi: 10.1016/j.clim.2008.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roubinian JR, Papoian R, Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977;59:1066–70. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talal N. Sex hormones and modulation of immune response in SLE. Clinics in rheumatic diseases. 1982;8:23–8. [PubMed] [Google Scholar]
- 9.Ansar Ahmed S, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. The American journal of pathology. 1985;121:531–51. [PMC free article] [PubMed] [Google Scholar]
- 10.Talal N, Ahmed SA, Dauphinee M. Hormonal approaches to immunotherapy of autoimmune disease. Ann N Y Acad Sci. 1986;475:320–8. doi: 10.1111/j.1749-6632.1986.tb20880.x. [DOI] [PubMed] [Google Scholar]
- 11.Talal N. Sex steroid hormones and systemic lupus erythematosus. Arthritis Rheum. 1981;24:1054–6. doi: 10.1002/art.1780240811. [DOI] [PubMed] [Google Scholar]
- 12.Carlsten H, Tarkowski A, Holmdahl R, Nilsson LA. Oestrogen is a potent disease accelerator in SLE-prone MRL lpr/lpr mice. Clin Exp Immunol. 1990;80:467–73. doi: 10.1111/j.1365-2249.1990.tb03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trigunaite A, Dimo J, Jorgensen TN. Suppressive effects of androgens on the immune system. Cellular immunology. 2015;294:87–94. doi: 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res. 1996;51:159–86. discussion 86-8. [PubMed] [Google Scholar]
- 15.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–21. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 16.Nalbandian G, Kovats S. Understanding sex biases in immunity: effects of estrogen on the differentiation and function of antigen-presenting cells. Immunologic research. 2005;31:91–106. doi: 10.1385/IR:31:2:091. [DOI] [PubMed] [Google Scholar]
- 17.Nalbandian G, Paharkova-Vatchkova V, Mao A, Nale S, Kovats S. The selective estrogen receptor modulators, tamoxifen and raloxifene, impair dendritic cell differentiation and activation. J Immunol. 2005;175:2666–75. doi: 10.4049/jimmunol.175.4.2666. [DOI] [PubMed] [Google Scholar]
- 18.Seillet C, Rouquie N, Foulon E, Douin-Echinard V, Krust A, Chambon P, Arnal JF, Guery JC, Laffont S. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor alpha. J Immunol. 2013;190:5459–70. doi: 10.4049/jimmunol.1203312. [DOI] [PubMed] [Google Scholar]
- 19.Lahita RG. The role of sex hormones in systemic lupus erythematosus. Curr Opin Rheumatol. 1999;11:352–6. doi: 10.1097/00002281-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17:412–5. doi: 10.1177/0961203308090026. [DOI] [PubMed] [Google Scholar]
- 21.Hughes GC. Progesterone and autoimmune disease. Autoimmun Rev. 2012;11:A502–14. doi: 10.1016/j.autrev.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, Straub RH. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–8. doi: 10.1191/0961203304lu1094oa. [DOI] [PubMed] [Google Scholar]
- 23.Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–61. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 24.Foo YZ, Nakagawa S, Rhodes G, Simmons LW. The effects of sex hormones on immune function: a meta-analysis. Biol Rev Camb Philos Soc. 2017;92:551–71. doi: 10.1111/brv.12243. [DOI] [PubMed] [Google Scholar]
- 25.Talal N. Natural history of murine lupus. Modulation by sex hormones. Arthritis Rheum. 1978;21:S58–63. [PubMed] [Google Scholar]
- 26.Shim GJ, Kis LL, Warner M, Gustafsson JA. Autoimmune glomerulonephritis with spontaneous formation of splenic germinal centers in mice lacking the estrogen receptor alpha gene. Proc Natl Acad Sci U S A. 2004;101:1720–4. doi: 10.1073/pnas.0307915100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bynote KK, Hackenberg JM, Korach KS, Lubahn DB, Lane PH, Gould KA. Estrogen receptor -alpha deficiency attenuates autoimmune disease in (NZB × NZW)F1 mice. Genes Immun. 2008;9:137–52. doi: 10.1038/sj.gene.6364458. [DOI] [PubMed] [Google Scholar]
- 28.Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Berges H, Laurell H, Gourdy P, Lenfant F, Arnal JF. The AF-1 activation function of estrogen receptor alpha is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154:2222–33. doi: 10.1210/en.2012-2059. [DOI] [PubMed] [Google Scholar]
- 29.Arnal JF, Fontaine C, Abot A, Valera MC, Laurell H, Gourdy P, Lenfant F. Lessons from the dissection of the activation functions (AF-1 and AF-2) of the estrogen receptor alpha in vivo. Steroids. 2013;78:576–82. doi: 10.1016/j.steroids.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Taylor SE, Martin-Hirsch PL, Martin FL. Oestrogen receptor splice variants in the pathogenesis of disease. Cancer Lett. 2010;288:133–48. doi: 10.1016/j.canlet.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. Identification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1. The EMBO journal. 2000;19:4688–700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irsik DL, Carmines PK, Lane PH. Classical estrogen receptors and ERalpha splice variants in the mouse. PLoS One. 2013;8:e70926. doi: 10.1371/journal.pone.0070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morel L, Wakeland EK. Lessons from the NZM2410 model and related strains. Int Rev Immunol. 2000;19:423–46. doi: 10.3109/08830180009055506. [DOI] [PubMed] [Google Scholar]
- 34.Agrawal H, Jacob N, Carreras E, Bajana S, Putterman C, Turner S, Neas B, Mathian A, Koss MN, Stohl W, Kovats S, Jacob CO. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J Immunol. 2009;183:6021–9. doi: 10.4049/jimmunol.0803872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carreras E, Turner S, Frank MB, Knowlton N, Osban J, Centola M, Park CG, Simmons A, Alberola-Ila J, Kovats S. Estrogen receptor signaling promotes dendritic cell differentiation by increasing expression of the transcription factor IRF4. Blood. 2010;115:238–46. doi: 10.1182/blood-2009-08-236935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40:66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 37.van Vollenhoven RF, Morabito LM, Engleman EG, McGuire JL. Treatment of systemic lupus erythematosus with dehydroepiandrosterone: 50 patients treated up to 12 months. J Rheumatol. 1998;25:285–9. [PubMed] [Google Scholar]
- 38.van Vollenhoven RF, Park JL, Genovese MC, West JP, McGuire JL. A double-blind, placebo-controlled, clinical trial of dehydroepiandrosterone in severe systemic lupus erythematosus. Lupus. 1999;8:181–7. doi: 10.1191/096120399678847588. [DOI] [PubMed] [Google Scholar]
- 39.Abdou NI, Rider V, Greenwell C, Li X, Kimler BF. Fulvestrant (Faslodex), an estrogen selective receptor downregulator, in therapy of women with systemic lupus erythematosus. clinical, serologic, bone density, and T cell activation marker studies: a double-blind placebo-controlled trial. J Rheumatol. 2008;35:797. [PubMed] [Google Scholar]
- 40.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, Merrill JT, Sammaritano L, Lockshin M, Alarcon GS, Manzi S, Belmont HM, Askanase AD, Sigler L, Dooley MA, Von Feldt J, McCune WJ, Friedman A, Wachs J, Cronin M, Hearth-Holmes M, Tan M, Licciardi F. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Annals of internal medicine. 2005;142:953–62. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 41.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM, Askanase AD, McCune WJ, Hearth-Holmes M, Dooley MA, Von Feldt J, Friedman A, Tan M, Davis J, Cronin M, Diamond B, Mackay M, Sigler L, Fillius M, Rupel A, Licciardi F, Buyon JP, Trial O-S. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–8. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.