Abstract
From basic studies in understanding the role of signaling pathways to therapeutic applications in engineering new cellular functions, efficient and safe techniques to monitor and modulate molecular targets from cells to organs have been extensively developed. The developmental advancement of engineering devices such as microscope and ultrasonic transducers allows us to investigate biological processes at different scales. Synthetic biology has further emerged recently as a powerful platform for the development of new diagnostic and therapeutic molecular tools. The synergetic amalgamation between engineering tools and synthetic biology has rapidly become a new front in the field of bioengineering and biotechnology. In this review, ultrasound and its generated mechanical perturbation are introduced to serve as a non-invasive engineering approach and, integrated with synthetic biology, to remotely control signaling and genetic activities for the guidance of cellular functions deep inside tissue with high spatiotemporal resolutions. This ultrasound-based approach together with synthetic biology has been applied in immunotherapy, neuroscience, and gene delivery, paving the way for the development of next-generation therapeutic tools.
Keywords: Ultrasound, mechanogenetics, synthetic biology, bioengineering, biotechnology
Graphic abstract
Introduction
Ultrasound has been used for more than 50 years as a diagnosis and therapeutic tool. Ultrasound imaging using a single element, or an array transducer has long history and is widely used at research laboratories and clinics [1, 2]. An effort to use ultrasound as therapeutic tools has been surged due to non-invasive, remote, and safe characteristics that can be used to target biological objects ranging from cells to tissues [3-5].
Current methods to control genetic activities rely mainly on chemicals, radio waves, magnetics, or light [6, 7]. Chemical inducers, radio waves, and magnetic field typically lack spatial resolution, with radio and magnetic waves requiring additional signal amplification and transmission to activate genetically engineered cells. Although light or optogenetics can control genetic regulations with high precision in space and time, it cannot reach deep tissues [8]. In contrast, ultrasound waves can be safely and non-invasively focused into small volumes of tissue deep inside the body [9].
In this review, we first introduce basic definitions and general concepts as well as the mechanical effects of ultrasound. We then introduce techniques that modulate functions of biological targets from cells to organs. Applications to immunotherapy and the intracellular delivery of macromolecules using ultrasound are also presented. Lastly, future perspectives are discussed.
General principles of ultrasound and interaction with biological target
History
In the early 1940s, Karl Theodore Dussik first developed an idea to visualize cerebral ventricle using transmission ultrasound [10] while ultrasonic surgery was proposed after recognizing the benefits of noninvasiveness using thermal ablation [11, 12]. Ultrasound hence showed a potential as a versatile source for both diagnosis and therapy. In the 1950s, Edler and Hertz developed echocardiography, with the invention of array transducers further advancing the ultrasound medical imaging field. On the therapeutic direction, thermal effect by ultrasound was the main mechanism before the cavitation effect was observed. Indeed, the cavitation was studied intensively in the 1980s with cavitation of microbubble proposed for diagnostic and therapeutic purposes [13, 14]. Since then, studies on the effects of ultrasound to biological targets from cells to animals have been conducted and have shown that ultrasound has the capability to modulate function and fate of biological targets. Low frequency ultrasound conjugated with microbubbles as an amplifier and high frequency ultrasound directly have demonstrated that noninvasive and specific control of biological targets can advance in the field of immunotherapy and drug delivery. The scale of biological targets and the types of applications can be broadly extended by tuning the properties of the ultrasound.
Sound waves
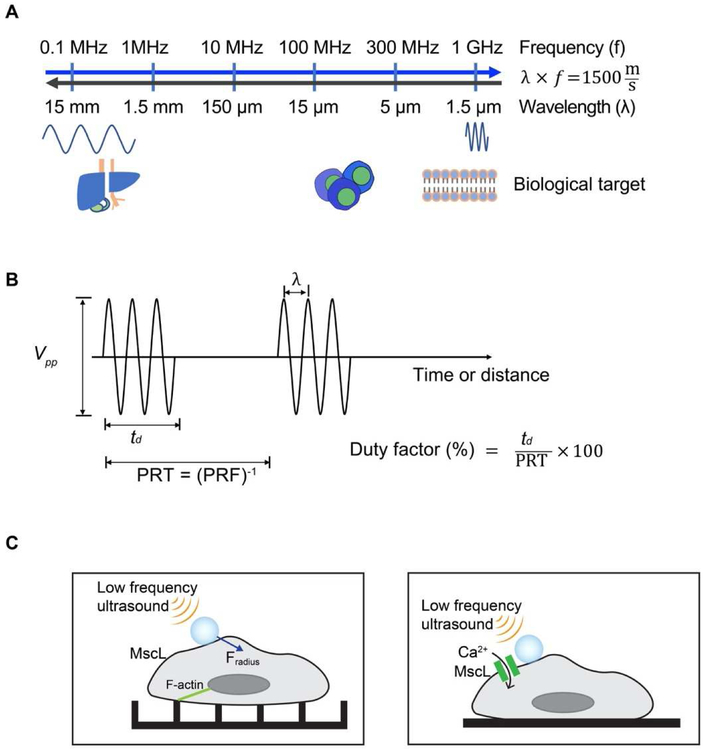
Sound waves can cause a disturbance of molecules in medium that propagates from one point to another in finite time without requiring gross movement of medium. The spectrum of sound can vary from several hertz (Hz) to several hundred mega hertz (MHz). The audible sound spectrum for human beings is generally from 20 to 20,000 Hz. Ultrasound is usually considered as the frequency ranges over 20 kHz. Medical ultrasound usually uses frequency ranges between 1 and 20 MHz for imaging purposes and below 2 MHz for therapeutic purposes as shown in Figure 1A. In research laboratories, frequency ranges over 50 MHz are often used to investigate cellular response and develop high resolution imaging device because of technical advancement of fabrication process for high frequency ultrasonic transducers [15, 16].
Figure 1. Sound waves and ultrasound parameters.
(A) In ultrasound field, frequency and wavelength are related to speed of sound of water or biological targets. Therapeutic ultrasound usually use frequency below 1 MHz and medical imaging applications uses 1 to 20 MHz frequency ranges. Recent development of ultra-high frequency ultrasonic transducers opens a new area for high frequency ultrasound research applications such as cellular manipulation using ultrasound. (B) Depending on applications, appropriate pulse parameters are required. PRT (PRF) represents pulse repetition time (frequency). (C) Schematics showing low frequency ultrasound excitation on microbubbles (MB) attached via RGD-integrin binding to the cell membrane to study actin cytoskeleton signaling (left); or to activate exogenously expressed mechanosensitive channel MscL opening (right).
Ultrasound design parameters
Depending on the size of biological targets and engineering purposes, users must select the appropriate center frequency of ultrasound waves, bandwidth, intensity (power), spatial resolution. The center frequency of ultrasonic waves and bandwidth are first determined depending on engineering purposes. If the biological target is made of very lossy (high attenuation) material, low frequency ultrasound is preferred. An appropriate center frequency is required to resonate the specific size of the microbubbles. High center frequency and large bandwidth are required to obtain fine images with improved lateral and axial resolutions, respectively. Narrow bandwidth is desired to interrogate specific target regions within biological tissues. Ultrasound medical imaging does not need high intensity but needs frequent samplings with high pulse repetition frequency (PRF). Therapeutic ultrasound requires high intensity at the focal region to ablate abnormalities or break kidney stones which can be achieved by increasing the duty factor or peak-to-peak voltages (Vpp). Most properties are easily adjusted by using a waveform generator or a function generator (Figure 1B).
Thermal interaction
The amplitude of ultrasound waves decreases when the sound waves propagate in a lossy medium such as biological tissue. Attenuation is the main cause of the dissipation and it can be categorized by absorption and scattering. Naturally, the energy of ultrasound waves may turn into heat as the waves propagate, and ultrasound waves may change its propagation direction after the interaction with structures in a medium. As long as the rate at which heat is converted is greater than the rate at which the heat is leaving, temperature may increase due to absorption. Studies have established mathematical equations to explain thermal interactions between ultrasound waves and biological tissues, and the temperature increase can be predicted by simple equations to provide a rough estimation within a short duration of time [17, 18]. Photoacoustics can be explained by thermal and stress confinements, which are mainly due to the interaction of light with biological tissues. After thermal expansion, ultrasound waves with wide frequency spectrum propagate, which are then used to reconstruct images for photoacoustic imaging modality [19].
Non-thermal interactions
The most representative non-thermal interaction between ultrasound waves and biological tissue is the cavitation or acoustically generated cavitation [20]. US Food and Drug Administration (FDA) regulates the exposure of ultrasound energy within certain levels depending on organs. One of the regulations is Mechanical Index (MI), which explains the mechanical impact of ultrasound energy to biological tissue. The relationship between acoustic pressure and the generation of cavitation is presented previously [21]. Besides serving as ultrasound contrast agents to improve echogenicity and image contrast, microbubbles are routinely applied herein to amplify ultrasound energy for therapeutic purposes. Oscillatory motion of microbubbles under sonication may break blood-brain-barrier (BBB) or loosen connective tissues for easier drug delivery [22]. By conjugating moieties with microbubbles, targeted delivery to specific sites such as tumors and safe activation by ultrasound sonication remotely can be achieved [23]. Another therapeutic application using microbubbles and low frequency ultrasound is to enhance cell permeability. Upon exposure to ultrasound, stable or unstable inertial cavitation affects the permeability of cells, named sonoporation. This membrane alteration can be transient or sustained to cause permanent membrane disruption [24]. Many studies have shown the success of sonoporation-related intracellular delivery, although quite some of these methods suffer from high cell cytotoxicity and unreliable control of microbubbles [25, 26].
Overview of acoustic and mechanical effects on the biological system and medical applications
Tissue ablation
Tissue ablation is broadly and frequently used in clinical surgeries, especially in cancer therapy. Traditional tumor ablations, including laser ablation, radiofrequency ablation and ultrasound ablation, have been widely used in clinical treatments [27]. Among them, ultrasound ablation can achieve non-invasiveness with high spatial precision. High-intensity focused ultrasound (HIFU) can be focused at the region of interest and the absorption of the ultrasound energy by the tissue can rapidly increase the tissue temperature and cause cell necrosis. Starting from 1990s, the thermal effect of ultrasound has been used in the clinical treatment of prostate tumor, breast tumor, uterine fibroids and many other tumors [28]. However, thermal effects usually have limited spatial resolution due to tissue inhomogeneity. Considering this, more effort has been focused on the pure mechanical ablation of tissues. In the last two decades, two approaches that use shock waves to provide non-thermal tissue ablation (called histotripsy) have been proposed [29, 30]. The first approach uses short (10-20μs) and intense ultrasound pulses to rapidly generate the ‘cloud bubbles’ only at the local focus in the tissue and the implosion of the bubbles can disintegrate the cells. The second approach is called the boiling histotripsy. Relatively longer and intense acoustic pluses are used to initiate ‘boiling bubbles’ within milliseconds, and the interaction of the boiling bubbles with the tissue can fragment cells into debris. Although these two methods use different principles to generate the pure mechanical effects in tissue, both methods produce lesions in the tissue[31, 32].
Ultrasound stimulation of brain activities
Ultrasound has also been applied to stimulate brain activities. Non-thermal stimulation of neuron cells in a brain circuit was first demonstrated using low intensity ultrasound by activating voltage-gated ion channels [33]. Since then, pulsed low frequency and low intensity ultrasound has been applied to modulate deep-brain function and human brain activity by stimulating brain and peripheral nerve circuits, verifying the mechanical effect of pulsed ultrasound beam on activation of neuronal membranes and mechanotransduction channels [5]. Non-invasive stimulation of cortical and hippocampal circuits, as well as the peripheral nerve circuits in human subjects, were also achieved without undesired heating effects [34-36].
Ultrasound application at molecular/cellular levels
Cells are constantly exposed to physical cues in the forms of shear stress, compression, and stretching [37]. Early applications of acoustic mechanical stimulations were mainly applied at the tissue level with innate mechanosensitivity. With the development of synthetic biology and cellular/molecular imaging tools, recent studies applied ultrasound to induce mechanical perturbation for the control of molecular signaling pathways. For example, ultrasound was used to actuate functionalized lipid microbubbles covalently attached to single mechanosensitive live cells to study cytoskeleton and RohA/ROCK signaling [38] (Figure 1C). In another study, by exogenously expressing an E. coli-derived mechanosensitive ion channel MscL in mammalian cells coupled with microbubbles, low frequency ultrasound was demonstrated to induce the opening of engineered mechanochannels for the calcium influx in vitro [39] (Figure 1C). In worm Caenorhabditis elegans, the low-pressure ultrasound was coupled with microbubbles to sensitize specific neurons that endogenously express the mechanosensitive channel TRP-4 and induce neuronal behavior changes [4].
The application of ultrasound to cellular engineering
Ultrasound application for mechanogenetics
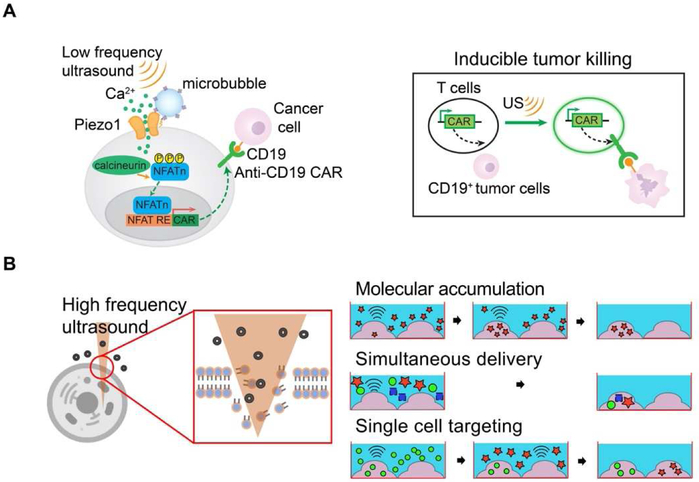
One promising therapeutic application of acoustic mechanogenetics is immunotherapy. With the use of central memory T cells capable of suppressing cancer relapse for several years [40]. CAR T cell immunotherapy is transforming cancer therapy. However, major challenges remain for CAR-based immunotherapy against solid tumors. For instance, the on-target, off-tumor toxicity due to non-specific targeting of the CAR-T cells against normal/non-malignant tissues can be life-threatening [41]. There is hence an urgent need for the control of CAR-T cell activation with high spatiotemporal precision. Ultrasound could serve as a promising inducer for gene activation in clinical settings because it can be safely and non-invasively delivered into small volumes of tissue deep inside the body with high spatiotemporal resolutions [9]. Accordingly, A modular method has been developed to engineer cells with synthetic genetic circuits that can sense ultrasound stimulation. Ultrasound can hence be applied remotely and non-invasively to these engineered cells to control genetics and CAR protein expression for recognizing antigens to kill the target tumor cells [23]. This method utilizes Piezo1 ion channel as a mechanical sensor (Figure 2A), which has been identified as a component of mechanically activated (MA) cation channels [42]. With microbubbles conjugated on HEK293T cells or primary T cells, the ultrasound stimulation of Piezo1 and the consequent calcium influx can activate calcium-sensitive pathways to drive the expression of designed target genes. While continuous evolution and optimization will be required for in vivo and clinical applications, this study laid the basis for developing remote and non-invasive modular system for controllable cancer immunotherapy.
Figure 2. Cell engineering applications.
(A) Schematic showing low frequency ultrasound excitation on microbubbles (MB) attached to the mechanosensitive ion channel Piezo1 expressing cell membrane to activate anti-CD19 CAR gene expression. The subsequent calcium entry triggers the downstream pathways, including calcineurin activation, NFAT dephoshorylation and translocation into the nucleus. The nucleus-translocated NFAT can bind to upstream response elements to initiate gene expression (left). Ultrasound induced T cells can specifically kill antigen expressing tumor cells (right). (B) 150 MHz high frequency ultrasound beam directly disturbs cell membrane for intracellular delivery of macromolecules, known as acoustic-transfection technique. Three prominent capabilities of acoustic-transfection are molecular accumulation by repeated acoustic-transfection, simultaneous delivery of multiple molecules, and single cell level targeting to deliver multiple molecules into target cells.
Intracellular delivery of macromolecules in vitro
We have developed an ultrasound technique to deliver macromolecules into target cells in vitro [43, 44]. Ultra-high frequency ultrasound beam can mechanically disrupt cell membrane to increase permeability of cells. Direct membrane disruption can only be achieved by ultra-high frequency ultrasound because it has a very confined focal area of smaller than the size of a single cell and enough focusing gain (Figure 2B). 150 MHz ultra-high frequency ultrasonic transducer was specifically developed for this application with a lithium niobate (LibNO3) single crystal. For the purposes of diagnosis and therapeutics, the function of cells can be modulated by the introduction of such exogenous molecules as DNA plasmids and recombinant proteins. Direct intracellular delivery of macromolecules using 150 MHz high frequency ultrasound was named as acoustic-transfection. Acoustic-transfection has three prominent benefits: 1) simultaneous intracellular delivery of various types of molecules ranging from nucleic acids to recombinant proteins, 2) the accumulation of molecules at target cells, and 3) single cell level selectivity (Figure 2B). These studies lay the groundwork for developing future devices for therapies involving patient-specific induced pluripotent stem cells and for deciphering intratumor heterogeneity and tumor evolution through CTC analysis.
Future Perspectives
Fluorescent proteins (FPs) and their derived biosensors, such as those based on fluorescence resonance energy transfer (FRET), have allowed the visualization of dynamic molecular activities at subcellular levels in live cells [45]. Optogenetics integrating optical and genetic methods has enabled the control of specific molecular events in living systems with high spatiotemporal resolutions [46]. The ultrasound- controllable mechanogenetics can non-invasively manipulate cells deep in the body for the control of physiological and therapeutic outcomes, hence bringing the full power of remote control of gene and cell activation to the general scientific and clinical community, similar to how fluorescent proteins and optogenetics have revolutionized live biological sensing and actuating.
By combining the orthogonal mechanogenetic and optogenetic controls, fully controllable live cells can be developed, activated or deactivated at any given time for cellular studies and therapeutic approaches. In addition, the combination of ultrasound-controllable mechano-genetics with genetic regulation tools, e.g. CRISPR and Cas9, can allow dynamic genome editing in vivo at any location deep in living organisms[47]. Integrated with epigenetic modulators and nuclease-deficient Cas9 (dCas9) targeting specific genome locus, this ultrasound-controllable mechano-genetics can be applied to remotely and non-invasively control the locus-specific epigenetic landscapes and endogenous gene expression profiles (Figure 3A) [48]. It is expected that each component of this ultrasound-controllable mechano-genetics, i.e. ultrasound controllers and transducers, genetic/epigenetic transducing modules, and mechano-sensors, will continue to be evolved for greater precision. The leverage of technological advancements of these different fields into this ultrasound-controllable mechano-genetics should in turn drive the development of these individual fields to open up new avenues.
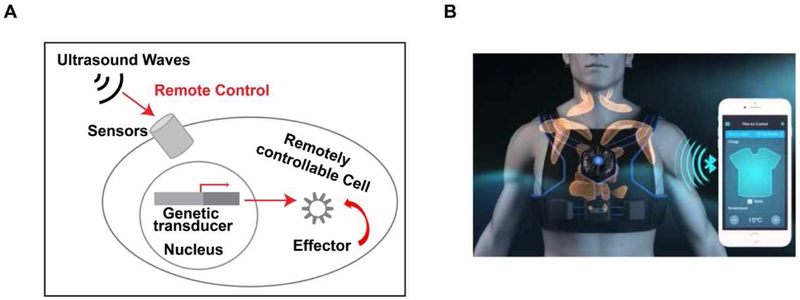
Figure 3. A diagram depicting the future application of ultrasound-controllable mechanogenetics.
(A) Ultrasound can be applied to remotely activate molecular sensors and genetic transducer for the production of effector in controlling the target cell. (B) A cartoon depicting the remotely controlled telemedicine. Wearable patches containing ultrasound transducers can be distally activated by mobile phones via internet and wireless network to control engineered cells for therapeutics.
We envision that wearable patches carrying ultrasound transducers will be developed in the near future to be controllable by mobile phones and/or control devices via wireless and Internet connection. In fact, stretchable electronic circuits have been developed to produce wearable patches and ultrasound transducer arrays [49]. As such, this ultrasound-controllable mechano-genetics technology will lead to a paradigm shift in translational medicine and pave the way for future telemedicine approaches, providing programmable health care at a remote distance for individual patients transplanted with ultrasound-controllable cells (Figure 3B). In summary, the ultrasound-controllable cell activation will usher in a new era in life science and technology, and highlight the translational power by integrating fundamental science and engineering principles for biomedical and clinical applications.
Acknowledgements
This work is supported by grants from NIH CA204704, CA209629, GM125379, HL121365, and NSF CBET1360341. This work is also supported by NIH under grant No. K99-GM120493.
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Ritter TA, Shrout TR, Tutwiler R, and Shung KK: ‘A 30-MHz piezo- composite ultrasound array for medical imaging applications’, IEEE Trans Ultrason Ferroelectr Freq Control, 2002, 49, (2), pp. 217–230 [DOI] [PubMed] [Google Scholar]
- 2.Shung KK: ‘General engineering principles in diagnostic ultrasound’, IEEE Eng Med Biol Mag, 1987, 6, (4), pp. 7–13 [DOI] [PubMed] [Google Scholar]
- 3.Bourdeau RW, Lee-Gosselin A, Lakshmanan A, Farhadi A, Kumar SR, Nety SP, and Shapiro MG: ‘Acoustic reporter genes for noninvasive imaging of microorganisms in mammalian hosts’, Nature, 2018, 553, (7686), pp. 86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibsen S, Tong A, Schutt C, Esener S, and Chalasani SH: ‘Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans’, Nat Commun, 2015, 6, pp. 8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyler WJ: ‘The mechanobiology of brain function’, Nat Rev Neurosci, 2012, 13, (12), pp. 867–878 [DOI] [PubMed] [Google Scholar]
- 6.Seo D, Southard KM, Kim JW, Lee HJ, Farlow J, Lee JU, Litt DB, Haas T, Alivisatos AP, Cheon J, Gartner ZJ, and Jun YW: ‘A Mechanogenetic Toolkit for Interrogating Cell Signaling in Space and Time’, Cell, 2016, 165, (6), pp. 1507–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, Sauer J, Dyke JP, Dordick JS, and Friedman JM: ‘Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism’, Nature, 2016, 531, (7596), pp. 647–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Chen X, and Yang Y: ‘Spatiotemporal control of gene expression by a light-switchable transgene system’, Nat Methods, 2012, 9, (3), pp. 266–269 [DOI] [PubMed] [Google Scholar]
- 9.Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, DeSalles A, Min BK, and Yoo SS: ‘A review of low-intensity focused ultrasound pulsation’, Brain Stimul, 2011, 4, (3), pp. 125–136 [DOI] [PubMed] [Google Scholar]
- 10.Shampo MA, and Kyle RA: ‘Karl Theodore Dussik--pioneer in ultrasound’, Mayo Clin Proc, 1995, 70, (12), pp. 1136. [DOI] [PubMed] [Google Scholar]
- 11.Lynn JG, Zwemer RL, Chick AJ, and Miller AE: ‘A NEW METHOD FOR THE GENERATION AND USE OF FOCUSED ULTRASOUND IN EXPERIMENTAL BIOLOGY’, The Journal of General Physiology, 1942, 26, (2), pp. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynn JG, and Putnam TJ: ‘Histology of Cerebral Lesions Produced by Focused Ultrasound’, Am J Pathol, 1944, 20, (3), pp. 637–649 [PMC free article] [PubMed] [Google Scholar]
- 13.Apfel RE: ‘Acoustic cavitation prediction’, The Journal of the Acoustical Society of America, 1981, 69, (6), pp. 1624–1633 [Google Scholar]
- 14.Flynn HG: ‘Generation of transient cavities in liquids by microsecond pulses of ultrasound’, The Journal of the Acoustical Society of America, 1982, 72, (6), pp. 1926–1932 [Google Scholar]
- 15.Lam KH, Hsu HS, Li Y, Lee C, Lin A, Zhou Q, Kim ES, and Shung KK: ‘Ultrahigh frequency lensless ultrasonic transducers for acoustic tweezers application’, Biotechnol Bioeng, 2013, 110, (3), pp. 881–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon S, Kim MG, Williams JA, Yoon C, Kang BJ, Cabrera-Munoz N, Shung KK, and Kim HH: ‘Dual-element needle transducer for intravascular ultrasound imaging’, J Med Imaging (Bellingham), 2015, 2, (2), pp. 027001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubbers J, Hekkenberg RT, and Bezemer RA: ‘Time to threshold (TT), a safety parameter for heating by diagnostic ultrasound’, Ultrasound Med Biol, 2003, 29, (5), pp. 755–764 [DOI] [PubMed] [Google Scholar]
- 18.Nyborg WL: ‘Solutions of the bio-heat transfer equation’, Phys Med Biol, 1988, 33, (7), pp. 785–792 [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Yantsen E, Larson T, Karpiouk AB, Sethuraman S, Su JL, Sokolov K, and Emelianov SY: ‘Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques’, Nano Lett, 2009, 9, (6), pp. 2212–2217 [DOI] [PubMed] [Google Scholar]
- 20.Leighton TG: ‘The acoustic bubble’ (Academic Press, 1994. 1994) [Google Scholar]
- 21.Holland CK, and Apfel RE: ‘An improved theory for the prediction of microcavitation thresholds’, IEEE Trans Ultrason Ferroelectr Freq Control, 1989, 36, (2), pp. 204–208 [DOI] [PubMed] [Google Scholar]
- 22.Downs ME, Buch A, Karakatsani ME, Konofagou EE, and Ferrera VP: ‘Blood-Brain Barrier Opening in Behaving Non-Human Primates via Focused Ultrasound with Systemically Administered Microbubbles’, Sci Rep, 2015, 5, pp. 15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Pan Y, Yoon S, Sun J, Huang Z, Lee C, Allen M, Wu Y, Chang YJ, Sadelain M, Shung KK, Chien S, and Wang Y: ‘Mechanogenetics for the remote and noninvasive control of cancer immunotherapy’, Proc Natl Acad Sci U S A, 2018, 115, (5), pp. 992–997 [DOI] [PMC free article] [PubMed] [Google Scholar]; **This paper demonstrated for the first time that ultrasound can remotely induce desired gene activities by integrating a mechanosensitive Piezo 1 ion channel and engineered genetic circuits in mammalian cells.
- 24.McNeil PL: ‘Chapter 10 Incorporation of Macromolecules into Living Cells’, in Wang Y-L, Taylor DL, and Jeon KW (Eds.): ‘Methods in Cell Biology’ (Academic Press, 1988), pp. 153–173 [DOI] [PubMed] [Google Scholar]
- 25.Guzman HR, Nguyen DX, McNamara AJ, and Prausnitz MR: ‘Equilibrium loading of cells with macromolecules by ultrasound: effects of molecular size and acoustic energy’, J Pharm Sci, 2002, 91, (7), pp. 1693–1701 [DOI] [PubMed] [Google Scholar]
- 26.Bao S, Thrall BD, and Miller DL: ‘Transfection of a reporter plasmid into cultured cells by sonoporation in vitro’, Ultrasound Med Biol, 1997, 23, (6), pp. 953–959 [DOI] [PubMed] [Google Scholar]
- 27.Knavel EM, and Brace CL: ‘Tumor ablation: common modalities and general practices’, Tech Vasc Interv Radiol, 2013, 16, (4), pp. 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou YF: ‘High intensity focused ultrasound in clinical tumor ablation’, World J Clin Oncol, 2011, 2, (1), pp. 8–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z, Ludomirsky A, Eun LY, Hall TL, Tran BC, Fowlkes JB, and Cain CA: ‘Controlled ultrasound tissue erosion’, IEEE Trans Ultrason Ferroelectr Freq Control, 2004, 51, (6), pp. 726–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canney MS, Khokhlova TD, Khokhlova VA, Bailey MR, Hwang JH, and Crum LA: ‘Tissue Erosion Using Shock Wave Heating and Millisecond Boiling in HIFU Fields’, Aip Conf Proc, 2010, 1215, pp. 36–39 [Google Scholar]
- 31*.Khokhlova VA, Fowlkes JB, Roberts WW, Schade GR, Xu Z, Khokhlova TD, Hall TL, Maxwell AD, Wang YN, and Cain CA: ‘Histotripsy methods in mechanical disintegration of tissue: towards clinical applications’, Int J Hyperthermia, 2015, 31, (2), pp. 145–162 [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper presented two histotripsy approaches and their clinical applications utilizing mechanical perturbation.
- 32.MAXWELL A, SAPOZHNIKOV O, BAILEY M, CRUM L, ZHEN X, FOWLKES B, CAIN C, and KHOKHLOVA V: ‘DISINTEGRATION OF TISSUE USING HIGH INTENSITY FOCUSED ULTRASOUND: TWO APPROACHES THAT UTILIZE SHOCK WAVES’, Acoustics today, 2012, 8, (4), pp. 24–37 [Google Scholar]
- 33*.Tyler WJ, Tufail Y, Finsterwald M, Tauchmann ML, Olson EJ, and Majestic C: ‘Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound’, PLoS One, 2008, 3, (10), pp. e3511. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper demonstrated that low intensity and pulsed ultrasound can stimulate neuron cells and brain circuits with non-thermal mechanism.
- 34.Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SI, and Tyler WJ: ‘Transcranial pulsed ultrasound stimulates intact brain circuits’, Neuron, 2010, 66, (5), pp. 681–694 [DOI] [PubMed] [Google Scholar]
- 35.Tufail Y, Yoshihiro A, Pati S, Li MM, and Tyler WJ: ‘Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound’, Nat Protoc, 2011, 6, (9), pp. 1453–1470 [DOI] [PubMed] [Google Scholar]
- 36.Legon W, Rowlands A, Opitz A, Sato TF, and Tyler WJ: ‘Pulsed ultrasound differentially stimulates somatosensory circuits in humans as indicated by EEG and FMRI’, PLoS One, 2012, 7, (12), pp. e51177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DuFort CC, Paszek MJ, and Weaver VM: ‘Balancing forces: architectural control of mechanotransduction’, Nat Rev Mol Cell Biol, 2011, 12, (5), pp. 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Z, Sun Y, Di C, Tay D, Chen W, Deng CX, and Fu J: ‘Acoustic tweezing cytometry for live-cell subcellular modulation of intracellular cytoskeleton contractility’, Scientific reports, 2013, 3, pp. 2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Heureaux J, Chen D, Murray VL, Deng CX, and Liu AP: ‘Activation of a bacterial mechanosensitive channel in mammalian cells by cytoskeletal stress’, Cell Mol Bioeng, 2014, 7, (3), pp. 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper demonstrated that ultrasound can stimulate the opening of a heterogeneously expressed mechanosensitive channel MscL in mammalian cells.
- 40.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, and June CH: ‘Chimeric antigen receptor-modified T cells for acute lymphoid leukemia’, The New England journal of medicine, 2013, 368, (16), pp. 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, and Milone MC: ‘CAR T cell immunotherapy for human cancer’, Science, 2018, 359, (6382), pp. 1361–1365 [DOI] [PubMed] [Google Scholar]
- 42.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, and Patapoutian A: ‘Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels’, Science, 2010, 330, (6000), pp. 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Yoon S, Wang P, Peng Q, Wang Y, and Shung KK: ‘Acoustic-transfection for genomic manipulation of single-cells using high frequency ultrasound’, Sci Rep, 2017, 7, (1), pp. 5275. [DOI] [PMC free article] [PubMed] [Google Scholar]; **High frequency ultrasound over 150 MHz was applied for the first time to directly deliver various types of macromolecules into cells.
- 44.Yoon S, Kim MG, Chiu CT, Hwang JY, Kim HH, Wang Y, and Shung KK: ‘Direct and sustained intracellular delivery of exogenous molecules using acoustic-transfection with high frequency ultrasound’, Sci Rep, 2016, 6, pp. 20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Shyy JY, and Chien S: ‘Fluorescence proteins, live-cell imaging, and mechanobiology: seeing is believing’, Annu Rev Biomed Eng, 2008, 10, pp. 1–38 [DOI] [PubMed] [Google Scholar]
- 46.Deisseroth K: ‘Optogenetics: 10 years of microbial opsins in neuroscience’, Nature neuroscience, 2015, 18, (9), pp. 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA, Church GM, and Zhang F: ‘Optical control of mammalian endogenous transcription and epigenetic states’, Nature, 2013, 500, (7463), pp. 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singhal N, Graumann J, Wu G, Arauzo-Bravo MJ, Han DW, Greber B, Gentile L, Mann M, and Scholer HR: ‘Chromatin-Remodeling Components of the BAF Complex Facilitate Reprogramming’, Cell, 2010, 141, (6), pp. 943–955 [DOI] [PubMed] [Google Scholar]
- 49.Rogers JA, Someya T, and Huang Y: ‘Materials and mechanics for stretchable electronics’, Science, 2010, 327, (5973), pp. 1603–1607 [DOI] [PubMed] [Google Scholar]