Abstract
Carbohydrates are an important class of biomolecules which are involved in a multitude of cellular functions. In the field of glycomics, the structure and function of various carbohydrates, oligosaccharides, glycans and their conjugates are constantly under investigation. In the continuing quest to understand the roles of carbohydrates in their interactions with proteins, immunogens, and other cell-surface carbohydrates, scientists have developed methods for observing the effects of specific saccharide sequences on various cellular components. Carbohydrate immobilization has allowed researchers to study the impact of specific sequences, leading to a deeper understanding of many cellular processes. The goal of this review is to highlight the chemical reactions and interactions that have been used for glycan immobilization.
Keywords: catalysis, carbohydrates, glycosylation, glycoconjugates, oligosaccharides
Graphical abstract
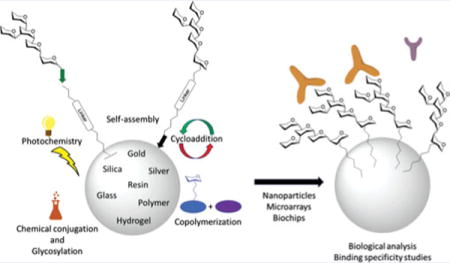
Introduction
Carbohydrates are an important class of biomolecules which are involved in many biological processes. Most people are familiar with the importance of carbohydrates in providing energy to the body, evidenced by the familiar “carb-loading” athletes, and “low carb” diets, etc. However, important scientific discoveries have revealed the involvement of carbohydrates in myriad cellular functions.[1,2] Carbohydrates are present in the cell primarily in conjugation with other biomolecules, for example, proteins, lipids and even other carbohydrates.[3–5] Their presence is essential for many common biological processes, and they are at least partially responsible for the toxicity of certain bacteria and biological poisons.
In the field of glycomics, sugars present in the cell are studied to gain a better understanding of their interactions with other biomolecules. Various carbohydrates, oligosaccharides, glycans and their conjugates are currently being investigated to determine the effects of specific monosaccharide structures and oligosaccharide sequences on cellular processes.[6,7] The explosive growth of glycosciences has increased our understanding of the roles of sugars as “molecules of death” due to their contribution to “every major disease.”[8] Studies which have helped to elucidate the roles of sugars in pathogenesis of cancer, AIDS, pneumonia, septicemia, diabetes, hepatitis, and malaria have stimulated many additional efforts in the field.[9–11] As scientists continue to investigate the roles of carbohydrates in their interactions with proteins, immunogens, and other cell-surface carbohydrates, new analytical methods continue to emerge. Carbohydrate immobilization has enabled the development of effective studies of carbohydrate interaction by allowing researchers to observe the impact of specific sequences in a format intended to mimic natural glycan presentation, leading to a deeper understanding of many cellular processes.
Carbohydrate-binding proteins, lectins, are known to bind specific saccharide sequences on the cell surface for a variety of purposes.[12–16] Given their specificity, they have been employed in science as the ideal tool for the study of oligosaccharide sequence structure and interaction. In solution, carbohydrate-lectin binding has been found to be weak. The strength of this binding is greatly enhanced when multiple copies of the saccharide sequence are presented on a protein or cell surface. This observation suggested that it might be beneficial to measure interactions on solid-phase.
There are multiple benefits of studying saccharide structures immobilized on solid surfaces. In the area of biological analysis alone, researchers have uncovered many aspects of carbohydrate-protein interactions through the use of solid-phase analytical tools such as carbohydrate microarrays.[17–19] Immobilization allows for multi-valent interactions between multiple copies of the saccharide ligand and multiple binding sites of single and multimeric proteins. The microarray format allows for high-throughput screening of multiple sequences, using very little material, which is important for both complicated synthetic sequences and low-abundance natural extracts.[18,20–22] Both synthetic and natural oligosaccharides have been immobilized at various surface densities to probe the importance of a given glycan in terms of its presentation and ligand density in interactions with carbohydrate binding proteins. Glycan-presenting nanostructures have the added potential of mimicking the cell surface in presentation and orientation of glycans. A particularly interesting carbohydrate immobilization format, three-dimensional microarrays are designed to incorporate many benefits for a more accurate study of carbohydrate functions.[23] Through these studies, many functions of carbohydrates have been discovered, such as their roles in fertilization, infection, and cell-cell communication.[24,25]
Chemical or enzymatic synthesis[26] can produce natural glycans that can be useful for studying their composition,[27] conformation,[28] interaction with other molecules,[15,29] and roles.[1] Although isolation from natural sources offers a viable means to obtain glycans,[30,31] only chemical synthesis can provide access to unnatural mimetics that attract rising interest due to their therapeutic[32,33] or diagnostic potential.[34–36] In spite of significant progress,[37–60] the chemical synthesis of glycans remains a lengthy and complicated process. Protecting groups for each individual saccharide must be thoughtfully chosen as they may affect the reactivity, stereoselectivity and chemoselectivity of the building block. Additionally, the hydroxyl for subsequent glycosylation needs to be liberated without causing the unintentional liberation of other functional groups. As a result, a synthetic sequence that entails protection, work-up, purification, characterization, glycosylation, work-up, purification, characterization, deprotection, etc. can be extremely tedious, even for a fairly simple target.
Glycan synthesis on solid-phase[61,62] has the potential to do for glycomics what solid-phase peptide synthesis has done for proteomics.[63–65] By eliminating or simplifying intermediate steps such as work-up and purification, solid-phase oligosaccharide synthesis streamlines access to pure quantities of specific oligosaccharide sequences.[66–70] Synthesis on solid phase has also opened the door to automation of oligosaccharide synthesis for the purpose of producing glycans of interest and making them readily accessible to researchers.[71–74] One such potential application of carbohydrate synthesis is in oncology. For example, the saccharide sequences presented on cancerous cells have been shown to be quite different from the saccharide sequences presented on their normal counterparts.[63–65] Chemical synthesis allows the production of these aberrant structures for the study of their interactions. Additionally, such synthetic structures could be applied to the development of carbohydrate-coated biomimetics for targeted drug delivery, diagnostics and immuno-prevention (vaccines and poison remedies). A broader application of the synthetic glycans is immobilization onto different substances or surfaces for subsequent study and application. The goal of this review is to highlight the chemical reactions, methods and interaction modes that have been used for glycan immobilization.
Reactions, methods, and interactions
Immobilization through non-covalent interactions
Non-covalent interactions may provide the simplest method for immobilizing carbohydrates on solid surfaces. The carbohydrate binding position can be non-specific, which may provide little control over glycan orientation. Methods for specific binding have also been reported, in which the carbohydrates have been modified, if necessary, at a specific point in their structure for interaction with the desired surface. A few examples are provided below.
The Wang group sought to demonstrate that microbial polysaccharides could be immobilized on a nitrocellulose-coated glass slide without chemical conjugation. Various fluorescein isothiocyanate-conjugated dextrans and inulin were printed on the slides and determined to be stable, though the immobilization efficiency was found to be better for higher molecular weight molecules.[75] The Pohl group immobilized synthetic monosaccharides through non-covalent fluorous interaction (fluorophilic attraction between per-fluorinated compounds). The monosaccharides were tagged with C8F17 chains which allowed them to be immobilized on fluorocarbon-coated glass slides.[76] An amine-modified galactofuranose derivative was converted to a Schiff base by reaction with 3,5-di-tert-butyl-2-hydroxybenzaldehyde (Figure 1) for immobilization through coordination with silver and gold nanoparticles.[77]
Figure 1.
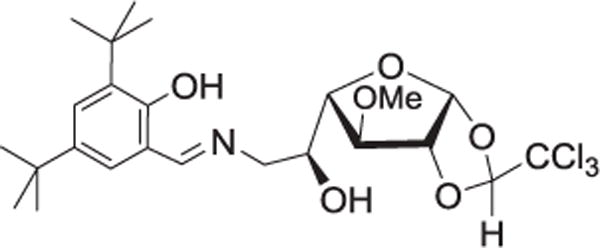
Structure of Schiff base ligand from aminochloralose derivative of galactose.
The high affinity biotin-avidin interaction has found use for the immobilization of many biological and synthetic molecules. In 1997, a group of researchers investigating saccharide-lectin interactions discovered a difference in results depending on which element was immobilized. In the lectin-immobilized system, the measured association constants were in agreement with reported values in solution. However, in the saccharide-immobilized system, the calculated constants varied greatly, with some over 10,000 times those reported for solution measurement. In investigating this effect, saccharides were conjugated with biotin through hydrazone formation with 4-(biotinamido) phenylacetylhydrazide. The biotinylated saccharides were immobilized onto avidin-functionalized biochips (gold film on glass).[78] Dubois and coworkers provide the first report of the immobilization of biotinylated-carbohydrate structures onto biotinylated polypyrrolic films through the formation of avidin bridges.[79] The Hernáiz group presented a general strategy for the synthesis of glycoconjugates functionalized with 2,6-diaminopyridine installed as a fluorescent tag for subsequent covalent immobilization or further functionalization with biotin for immobilization on an avidin-coated surface (Figure 2).[80]
Figure 2.
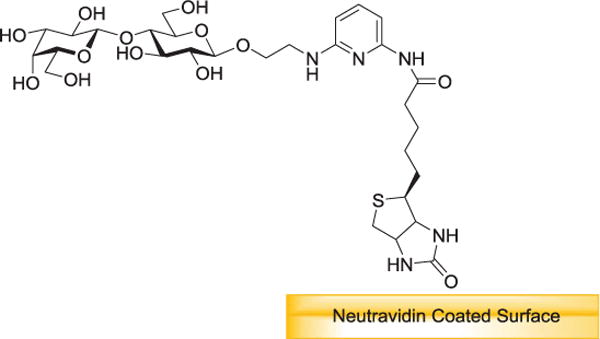
Immobilization of properly functionalized glycans. Biotinylated molecules are immobilized by its affinity to streptavidin/neutravidin.
Covalent methods of immobilization
Self-assembly
The most common method for immobilizing molecules on solid phase is the formation of self-assembled monolayers on gold. This method has been frequently used over the years for the facile immobilization of glycans. Saccharides can be functionalized with thiol and disulfide-bearing linkers for direct formation of self-assembled monolayers, or specially functionalized linkers can be similarly pre-assembled on the surface for subsequent immobilization of carbohydrates through reaction with the free linker terminus.
In situ nanoparticle preparation
Functionalized gold nanoparticles can be prepared by reduction of a gold salt in the presence of thiol-bearing ligands. The Kataoka group was able to prepare gold nanoparticles by reducing HAuCl4 in the presence of a polyethylene glycol chain with a thiol at one end for attaching to gold and a protected aldehyde at the other for reductive amination with p-aminophenyl β-D-lactopyranoside.[81] A similar procedure was used for the formation of carbohydrate encapsulated gold nanoparticles. Glycosyl bromides were glycosylated with 4-pentenol, which was further functionalized with the necessary thio-group by radical-initiated hydrolysis in the presence of thioacetic acid, which gives a free thiol following global deprotection.[82,83]
The Penadés group prepared conjugates of lactose and Lewisx antigen with thiol-terminated long chain alkyl linkers as glycosphingolipid mimics. Following trichloroacetimidate (TCA) glycosylation with protected thiol-terminated linkers, the glycoconjugates were used for the preparation of gold nanoparticles from tetrachloroauric acid in the presence of sodium borohydride. The resulting water-soluble 3D polyvalent glyconanoparticles were used to study carbohydrate-protein, as well as carbohydrate-carbohydrate self-recognition by Lex.[84,85] The same group later used a similar method for the formation of gold nanoclusters containing mixed monolayers of thiol-functionalized tumor-associated carbohydrate antigens and immunogenic peptides for investigation into their potential use as anticancer vaccines.[86]
Assembly on prepared nanoparticles
Huang reported the immobilization of 11-mercapto-3,6,9-trioxaundecyl-α-D-mannopyranoside onto gold nanoparticles prepared through reduction of HAuCl4·3H2O with tetrakis(hydroxymethyl) phosphonium chloride to provide improved·water-solubility over fluorescent polymers for bioassays.[87] Kim et al. modified amino-dextran through reaction with 2-iminothiolane to produce dextran-conjugated gold nanoparticles by self-assembly on prepared particles. These were used as proof of concept in the detection of protein glycosylation with lectin-coated quantum dots.[88] The Russell group functionalized simple sugars with a thiol moiety to form a stabilizing layer through self-assembly on gold nanoparticles.[89–93] The Jensen group modified unprotected carbohydrates with a thiol linker via formation of carbohydrate oximes for attachment of glycans to gold nanoparticles.[94] The Barboiu group also formed glyconanoparticles through self-assembly of thio-linked simple sugars.[95,96]
The Gibson group attached 2-amino sugars to the pentafluorophenyl ester terminus of an N-hydroxyethyl acrylamide polymer, which was subsequently used to coat the gold nanoparticle through the opposite thio-functionalized terminus. The resulting glyconanoparticles are reportedly stable at physiological salt concentration (Figure 3).[97,98] Witten and others synthesized glyconanoparticles via attachment to DNA ligands for control of lectin binding via temperature-induced DNA-duplex melting. DNA-saccharide conjugates were formed through reaction of respective thiol and amine terminated linkers with 4-(N-maleimidomethyl) cyclohexanecarboxylic acid N-hydroxysuccinimide ester before being conjugated with citrate-stabilized gold nanoparticles.[99]
Figure 3.
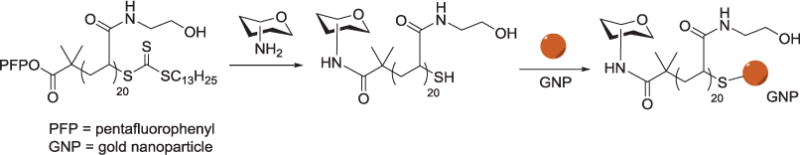
Synthetic route to glycosylated gold nanoparticles: polyhydroxyethyl acrylamide with amino-sugars and immobilization of carbohydrate terminal polymers onto preformed 60 nm gold nanoparticles.
Assembly on a surface
A group of salmonella disaccharide epitopes was immobilized onto thin gold films in an array format to probe antibody binding using surface plasmon resonance (SPR) imaging. The disaccharides were modified with an alkyl thiol linker for facile immobilization to gold.[100]
The reaction of thiols with maleimide is also frequently exploited for the immobilization of biomolecules on surfaces. In one example, the Seeberger group constructed carbohydrate microarrays through assembly of thiol-linked glycans on BSA-coated glass slides derivatized with maleimide.[101] Many more examples of immobilization using gold-sulfur interaction and thiol-maleimide connection exist. A few additional examples, included in the subsequent sections, are more closely focused on the chemistry used to covalently attach the sugar to linkers for immobilization.
Functional group modifications
A variety of chemical methods have been used for the immobilization of carbohydrates. Many require modification of, or reaction at the anomeric position to form specific, covalent linkages.
Amine
Amine functionalization, through both carbohydrate and surface modification, is a frequently used method for immobilization of carbohydrates on solid surfaces. Reaction of amines with molecules such as succinimides and carboxylic acids provides a convenient means of attachment. The Seeberger group created glycoprotein microarrays on the surface of amine-modified glass slides through use of the amino-reactive linker, ethylene glycol disuccinimide.[102] Blixt et al. constructed glycan microarrays by coupling amine-linked glycans to N-hydroxysuccinimide (NHS) activated glass slides using standard robotic printing technology.[103] In order to simplify the synthesis of free, reducing glycans with primary amines for the preparation of glycan microarrays, the Cummings group derivatized glycans with 2,6-diaminopyridine to generate fluorescently labeled glycans that contain a primary amine for further conjugation with NHS activated glass slides, among other surfaces.[104] For simplified synthesis and increased stability of glyco-quantum dots, nanoparticles made of semiconductor material, Earhart and colleagues developed a method for coating quantum dots with silica that provided a protective shell and an amenable surface for functionalization. An amine functionalized silica coating was prepared by hydrolysis of 2-aminoethyl-aminopropyltrimethoxysilane in the presence of the quantum dots, and conjugated with dextran which was pre-activated by coupling with disuccinimidyl carbonate (Figure 4).[105]
Figure 4.
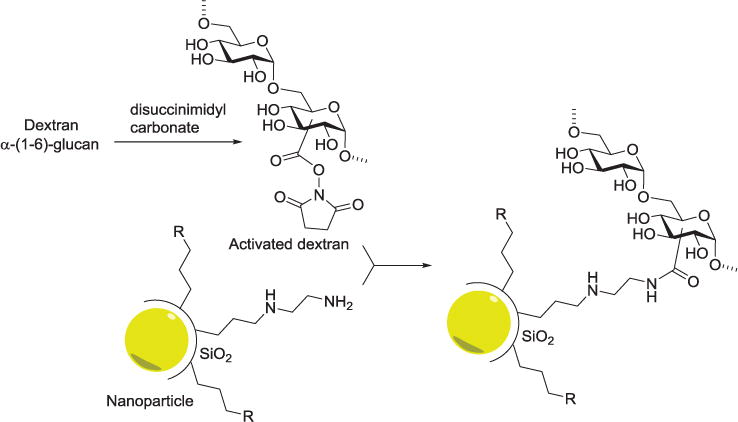
Disuccinimidyl carbonate-mediated dextran conjugation to amine-functionalized nanoparticles. R represents phosphonate or PEG functional group. The nanoparticles can be Ag, Fe3O4, or ZnS-CdSe. They are first coated with silica and then functionalized with dextran.
The first reported synthesis of silicon nanoparticles functionalized with carbohydrates was prepared by Ahire et al. The anomeric position of mannose was functionalized with a carboxylic acid linker for attachment to the amino-functionalized resin by amide formation. These silicon nanoparticles were shown to target cancerous cells through fluorescence imaging.[106] Chitin and cellulose nanocrystals were oxidized at C-6 of the glycopolymers to provide a point of attachment for amine-functionalized carbohydrate ligands and a quinolone fluorophore for fluorescent detection of lectin binding.[107,108] In medicine, immobilization of carbohydrates can be important for reasons other than diagnosis, detection and treatment. The Cai group immobilized mannose derivatives onto an inert silicon surface for the purpose of preventing infectious bacterial adhesion on devices such as catheters and implants. The silicon surface was initially activated with CO2 plasma for attachment of a poly(amidoamine) dendrimer which provided an amino terminated surface to which carboxy-terminated mannose derivatives were covalently attached. Benign Escherichia coli 83972 expressing mannose-binding type 1 fimbriae was reported to form a stable film and reduce adherence of undesirable bacteria.[109]
Shang and others functionalized a silica surface using an organophosphate self-assembled monolayer. 11-hydroxyundecylphosphonic acid was assembled on the silica surface and activated for coupling by conjugation with divinyl sulfone. The prepared amine-linked saccharides were then immobilized through reaction with the vinyl sulfone terminus (Figure 5).[110]
Figure 5.
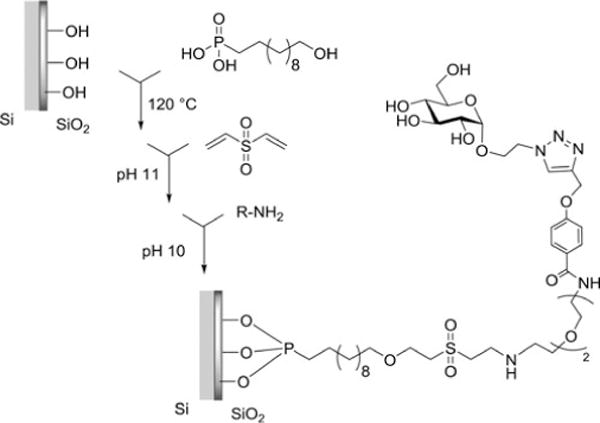
Functionalization of Silicon Substrate Using 11-Hydroxyundecylphosphonic Acid (UDPA) and Divinyl Sulfone (DVS) Linking Chemistry.
Reductive amination
Seo et al. developed a method for immobilizing carbohydrates on gold surface by reductive amination (open chain) of free oligosaccharides with aminophenyl disulfide, which can easily form a self-assembled monolayer on a gold surface.[111] Manimala and coworkers used an approach toward immobilizing carbohydrates through conjugations with bovine or human serum albumin by reductive amination. The glycoprotein conjugates were then printed on epoxide-functionalized glass slides for analysis of carbohydrate–protein interactions.[112] Synthetic, as well as naturally sourced oligosaccharides were tagged with fluorescent labels by reductive amination and immobilized on epoxide-activated gold affinity chips.[113]
Hydrazide and aminooxy
Hydrazide and aminooxy groups also provide a convenient, chemo-selective method for immobilizing carbohydrates on solid surfaces. For improvement in the immobilization of unmodified carbohydrates onto solid surfaces, the Shin group took advantage of the specificity of hydrazide (and aminooxy) groups in reaction with the reducing end of free carbohydrates. Glass slides were modified with these functional groups and simple mono- and disaccharides were attached. They also confirmed by NMR analysis that the products of the reactions of the carbohydrates with hydrazide groups gave predominantly cyclic β-anomeric products (Figure 6).[114,115] Zhi et al. prepared a hydrazide-derivatized self-assembled monolayer on gold surface by modifying various self-assembled mercapto-alkanoic acid linkers with adipic dihydrazide. Unmodified oligosaccharides, as well as synthetic glycans were attached at their reducing ends for protein interaction detection by a broader range of techniques than are available for substrates immobilized on glass, including surface plasmon resonance and quartz crystal microbalance.[116] The Cairo group reported the use of anomeric toluene sulfonylhydrazone to activate unprotected carbohydrates for immobilization onto a cross-linked agarose support.[117]
Figure 6.
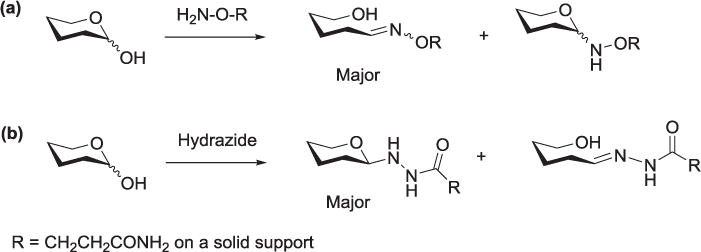
Adducts obtained from reactions of carbohydrates with (a) aminooxy and (b) hydrazide groups.
Cyanuric chloride
Cyanuric chloride (2,4,6-trichloro-1,3,5-triazine, CC), features three positions of attachment, spaced at 120° and allows free rotation around the newly formed bonds. The Chen group surmised that carbohydrates immobilized via this attachment might have the potential to adopt the most favorable spatial position for protein interaction. Intact and unmodified carbohydrates were immobilized on gold nanoparticles functionalized with cyanuric chloride in a one pot reaction at pH ∼9.0. CC was first linked to the acid termini of linkers which had been loaded on the gold surface through self-assembly. The degree of carbohydrate substitution was controlled by controlling the reaction temperature, as the reactivity of each chloride decreases with each subsequent substitution.[118,119] Various saccharides, reducing and non-reducing, mono-, oligo- and polysaccharides were also immobilized on hydroxy-terminated glass chips and gold film through covalent attachment to CC (Figure 7).[120]
Figure 7.
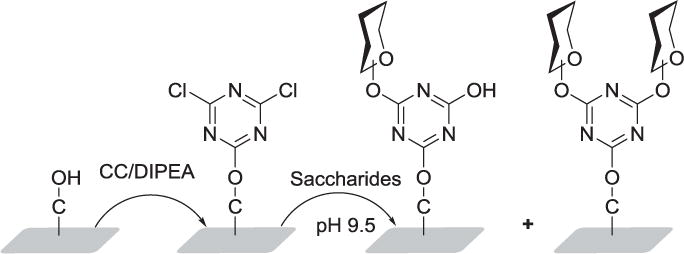
Immobilization of saccharides on rotatable cyanuric chloride-terminated solid surfaces to free their turning for better recognition position.
Boronic acids
Boronic acids tend to reversibly form covalent esters with diols and are frequently used as carbohydrate binders. It is therefore not surprising to find their use in the immobilization of carbohydrates. The Lin group chose to immobilize unmodified carbohydrates for microarray formation by creating stable boronic esters. Aldehyde-functionalized glass slides were coated with bovine serum albumin. The reactive amine groups of the protein were converted to acid by reaction with glutaric anhydride. Carbodiimide coupling was used to attach amino phenylboronic acid to the slide surface.[121]
Other
Zhai and colleagues reported the synthesis of water-soluble silicon nanocrystals which could be used as potential cancer imaging agents. Hydride-terminated silicon nanocrystals were activated by reaction with phosphorous pentachloride to provide a silicon chloride surface for direct conjugation with hydroxyl-linked peracetylated mannose.[122] Jayasundara and others described a mild method for immobilizing unprotected sugars on carbon and metal surfaces using diazonium chemistry. The method is reportedly mild, with no pre-modification of the surface required. p-Aminophenyl glycosides were treated with a solution of sodium nitrite and fluoroboric acid to produce the reactive species, which loses N2 and reductively adds to the solid substrate.[123] Barboiu and co-workers tested the utility of a complex anionic molybdenum oxide based structure, Müller’s spherical, anionic, molybdenum oxide based capsule (Mo132) for glycan immobilization and lectin detection. D-Mannose-ammonium chloride was immobilized by ion exchange with the ammonium counterions of the molybdenum compound to form glyconanocapsules.[124]
Photochemical methods
Carbohydrate immobilization by photochemical attachment provides a means for the direct use of unmodified carbohydrates, though the site of attachment to the saccharide can be non-specific. Carroll et al. reported a simple photo-immobilization approach where a molecule with a light-sensitive head-group is self-assembled onto glass plates. Upon irradiation in the presence of unmodified sugars, covalent linkage is formed through a radical mechanism. Specifically, phthalimide chromophores were presented on the surface that, upon exposure to light, react with unmodified carbohydrates by hydrogen abstraction followed by radical recombination. The carbohydrate density was controlled by the size of the illuminated section.[125] Sharma et al. presented a carbohydrate photoimmobilization scheme using1-fluoro-2-nitro-4-azidobenzene (FNAB) as a simple azido photo linker. A photoreactive-cellulose membrane was prepared in a thermochemical reaction with FNAB, onto which the saccharide was immobilized by photoirradiation.[126]
Ramström, Yan and coworkers have developed a versatile method for coupling underivatized carbohydrates to gold nanoparticles by a photochemical reaction with perfluorophenylazide (PFPA). After functionalization with a disulfide linker, the PFPA was assembled on gold nanoparticles. Carbohydrates were then immobilized by fast light activation via the C-H insertion reaction of the perfluorophenylnitrene generated by photoactivation of PFPA (Figure 8).[127–130] This group also produced a new glyconanoparticle microarray by first photocoupling the glycans to silica nanoparticles, then immobilizing the glyconanoparticles into array format onto PFPA functionalized poly(allylamine) film.[24] The group also used their photochemical method to produce glycans labeled with dye-doped silica nanoparticles for use in probing lectin microarrays.[131] The same researchers later leveraged PFPA-based photochemistry for carbohydrate immobilization by introducing the PFPA as a photo-reactive tag to the anomeric linker of various carbohydrates, instead of to the solid surface. This reversed method enabled rapid, covalent, and most importantly, nondestructive immobilization of carbohydrates onto solid supports.[132]
Figure 8.
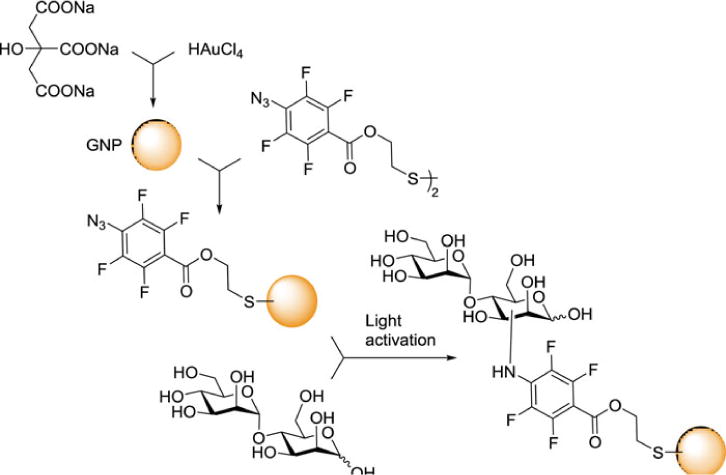
Synthesis of PFPA-gold nanoparticles and subsequent coupling with α-1,4-mannobiose.
In lieu of undertaking the synthetic effort required for pre-functionalizing di- and oligosaccharides for click nanodiamond formation, the Szunerits group produced diamond nanodots functionalized with PFPA for photochemical linkage for unmodified mono-, di- and polysaccharides, and compared lectin recognition results with those formed by click chemistry. Due to the non-specificity of the photo-coupling, lectins showed weaker affinity with monosaccharides, but comparable affinity for di- and oligosaccharides.[133] In an instance where specific attachment might be inconsequential, hyaluronan was grafted onto PFPA-functionalized polystyrene microspheres through photochemical reaction for investigation into its function in the pericellular coat.[134]
Cycloaddition
The Cu(I)-catalyzed heterocyclo-addition of azide to alkyne functional groups has emerged as a convenient reaction for the immobilization of carbohydrates on solid surfaces, particularly due to its amenability to aqueous conditions and compatibility with bioanalysis. Both copper-catalyzed and copper-free methods have been exploited in carbohydrate immobilization. The Diels-Alder, a concerted cycloaddition reaction, has also found use in functionalizing surfaces and in immobilization. The Mrksich group prepared carbohydrate arrays by using Diels-Alder-mediated immobilization of carbohydrates. The gold surface was activated by self-assembly of alkane-thiolates presenting benzoquinone at the surface. Carbohydrates functionalized with a linker containing cyclopentadiene were immobilized through cycloaddition with benzoquinone.[135] Sun and colleagues covalently immobilized carbohydrates onto a maleimidocaproyl-functionalized glass slide via Diels-Alder attachment of a cyclopentadiene-containing linker, which featured an alkyne terminus for convenient immobilization of azide-containing sugars.[136]
The Wong group found, in searching for methods for noncovalent carbohydrate immobilization, that saturated hydrocarbons could be non-covalently attached to polystyrene plates, and that thus displayed glycolipids were stable and functional in biological assays. However, when the synthesis of glycolipids presented challenges, the group looked for a more efficient way to immobilize carbohydrates in this manner. 1,3-dipolar cycloaddition between alkynes and azides provided a solution. Alkane moieties featuring terminal alkyne functional groups were immobilized, then reacted with sugars fitted with an azido-linker.[137] The Wong group went on to develop a more stable and reusable covalent array format through the use of a surface-tethered cross-linker with a terminal alkyne for conjugation to the azide-containing saccharides. The group employed cleavable cross-linkers for covalent attachment to both amine- and N-hydroxysuccinimide derivatized surfaces through isothiocyanate- and amine-functionalized termini, respectively (Figure 9).[138]
Figure 9.
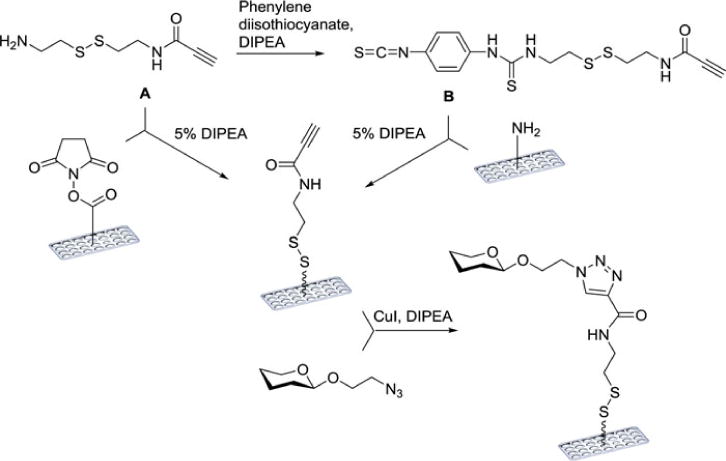
Cleavable linkers A and B can be attached to either NHS- or amine-coated surfaces to give the alkyne-functionalized surface.
Ramström, Yan and co-workers investigated the feasibility of using PFPA azide photoligation in conjunction with Cu-catalyzed azide-alkyne cycloaddition (CuAAC) for the immobilization of carbohydrates. An alkyne-functionalized polystyrene surface was formed by photoligation, onto which azide-linked sugars could easily be attached.[139] Later, they leveraged the multi-functionality of the perfluorophenyl azide reagent by immobilizing carbohydrates onto PFPA-functionalized silica nanoparticles via the CuAAC reaction with alkyne-linked monosaccharides. Ligand density and conjugation yields were observable by 19F NMR.[140]
The Wang group used a self-assembled monolayer terminated with alkyne groups to immobilize azido-polyethylene glycol-linked saccharides on gold surface for use in the analysis of carbohydrate-protein interactions.[141] Ravoo and coworkers were able to benefit from the ease of the click reaction by ink-jet printing alkyne-functionalized carbohydrates onto glass and silica surfaces modified with azide-functionalized monolayers. The surfaces were prepared by adsorption of an bromo-alkyltriethoxysilane, which was then substituted with azide.[142]
With the intent of adding electrochemical impedance spectroscopy to the methods used for detection of carbohydrate-protein interactions, Szunerits, and others built a sensor consisting of a boron-doped diamond electrode terminated with alkynyl surface groups for the immobilization of azide-bearing carbohydrates.[143] They later produced glyco-nanodiamonds using click coupling to both azide- and propargyl-functionalized nanodiamonds.[144,145] The group also used the click reaction to produce nanodiamonds functionalized with phenylboronic acid for the pH-triggered capture and release of man-nose, reporting its effects on lectin recognition.[146] The same group produced a graphene-tetrathiofulvene (TTF) nanohybrid material from graphene oxide sheets. The reduced graphene-TTF complex containing azide groups was conjugated to propargyl mannoside using click chemistry. The TTF-mannose units were releasable from the graphene either by oxidation, or by the formation of stronger host-guest complexes with cyclophane cyclobis(paraquat-p-phenylene) (Figure 10).[147]
Figure 10.
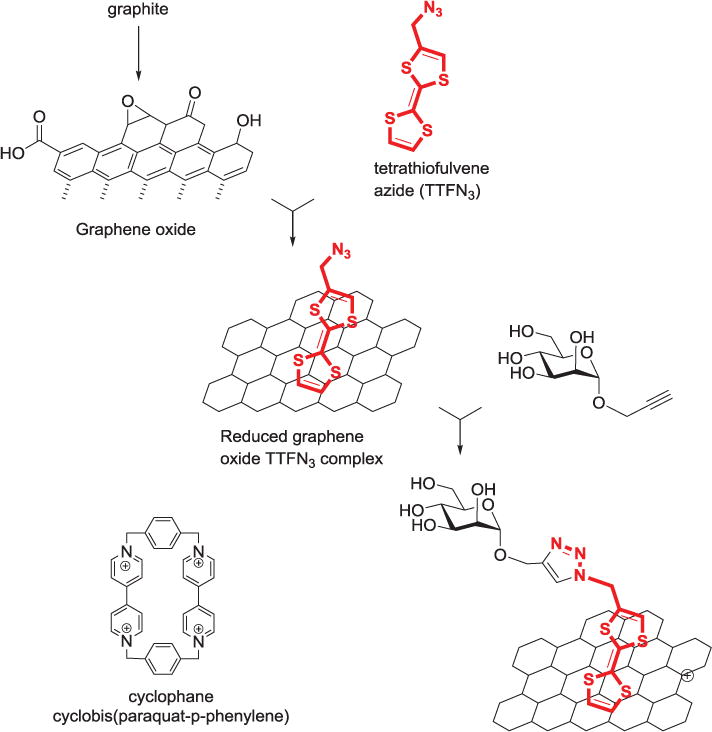
Schematic illustrations of the formation of graphene/TTF-N3 nanosheets and Cu(I)-catalyzed “click”chemistry with alkynyl-mannose.
A combination of reaction types was used to produce a carbohydrate-immobilized silicon platelet, with the click reaction featured as the final immobilization step. The silicon surface was first functionalized with undecylenic acid via hydrosilylation, and the terminal acid group was reacted with the amine terminus of an oligoethylene oxide chain which presented the azide function at the free end. Propargyl-functionalized glycans were then immobilized to the surface through copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition.[148] Aykaç and colleagues utilized click chemistry to produce disulfide-linker function-alized analogues of lactose and β-cyclodextrin for self-assembly onto gold nanopar-ticles to form potential site-specific delivery systems for anticancer drugs.[149] Self-assembled (aqueous) polymeric glyco-nanoparticles were prepared in both brush and linear form by Sun et al. The polymer bases were synthesized by atom transfer radical polymerization (ATRP), functionalized with azide groups, and conjugated with glycans using alkyne-linked sugars (Figure 11).[150,151]
Figure 11.
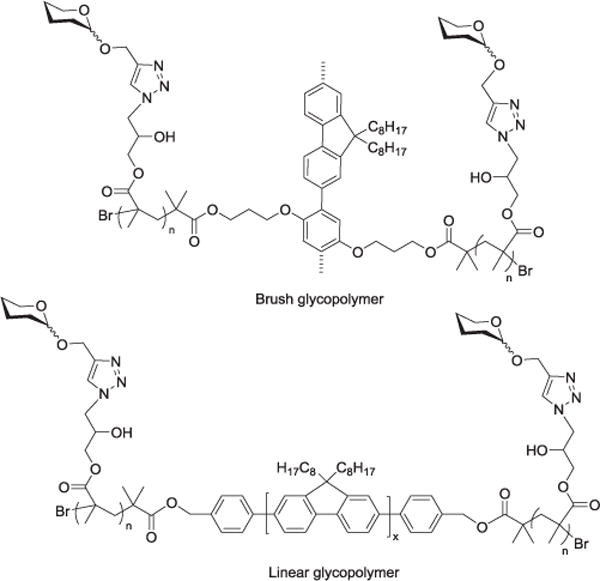
Structures of brush and linear glycopolymers investigated by Sun et al.
Copper-free click chemistry
In an alternative coupling strategy, a mannose-linked azide was covalently immobilized on a phosphane-derivatized glass slide to demonstrate the utility of using the copper-free Staudinger ligation for the formation of microarrays.[152] Strain-promoted azide-alkyne cycloaddition (i.e. copper-free click chemistry) was used to produce gold-glyconanoparticles. Azido-galactoside was reacted with a lipid-linked cyclooctyne group to produce a glycolipid which was subsequently self-assembled onto PEG-coated gold nanoparticles.[153]
Copolymerization
Glycan immobilization by direct polymer formation is a convenient way to form glycopolymers, such as hydrogels. Some of these polymers provide a hydrophilic, three-dimensional surface capable of mimicking cell-surface oligosaccharide presentation for more accurate carbohydrate-protein analysis. The Bovin group adapted an approach developed for grafting oligonucleotides and proteins into 3D polyacrylamide gel to form carbohydrate-immobilized 3D hydrogels. Glycans or glycoconjugates were fitted with amino-functionalized linkers or conjugated with an allyl-substituted polyacrylamide building block for polymerization with methacrylamide, to form the carbohydrate-immobilized gel.[17,154] This group later reported the use of 2-aminopyridine as a bifunctional fluorescent label for the immobilization of oligosaccharides. The reductive amination product was copolymerized directly into a polyacrylamide gel to form carbohydrate hydrogels. Additionally, this product could also be converted to the amino alditol form, and printed onto N-hydroxysuccinimide activated slides.[155] Liu and coworkers formed a 3D carbohydrate microarray on glutaraldehyde (GA) activated polyacrylamide hydrogel spots using 4-aminophenyl-modified monosaccharides.[156]
Beer and colleagues reported the use of polyethylene oxide-based star gel polymers functionalized with isocyanate for immobilizing amine-functionalized GlcNAc and LacNAc and poly-LacNAc onto microtiter plates. Later, amine-linked poly LacNAc was coupled to the isocyanate groups of the polymer as the first step in creating a layered biomimetic display of extracellular matrix components.[157,158]
Ethyl methacrylate-linked β-D-glucosamine was directly polymerized on a prepared silicon wafer to form glycopolymer brushes. The base glycans were functionally elongated by enzymatic glycosylation to form multivalent oligosaccharides.[159] Jans and co-workers recently reported the preparation of polyethylene glycol micro-gels carrying glycan motifs for lectin binding. The PEG-prepolymers (PEG poly-merized onto a sorbitol base) were terminated with acrylate functions which were polymerized in the presence of allyl-glycosylated glycans.[160]
Mannose was attached to an acrylamide-terminated anomeric linker and directly copolymerized with unmodified acrylamide to form a glycopolymer for analysis of interaction with concanavalin A.[161] Glisoni and Sosnik reported the formation of glucose-functionalized polymeric micelles which displayed lectin-promoted agglutination. Gluconolactone was attached to the polymer by microwave-assisted ring opening.[162]
Solid-phase synthesis
Solid-phase oligosaccharide synthesis (SPOS) is likely one of the most important developments for the promotion of glycomic research. It has the potential to provide access to a greater variety of oligosaccharide sequences and modifications, in quantities and purities not readily achievable through biological extraction. Solid-phase synthesis is generally far less time-intensive and potentially less expensive than traditional solution synthesis. As such, it may be one of the most important reasons for the immobilization of carbohydrates. Immobilization techniques for synthesis are required to be suitable for the range of solvents and conditions (acidic, basic) employed. While the purpose of SPOS is to generate free structures following cleavage from solid support, it also has the potential to provide immobilized sac-charide products. Fully developed techniques could lead to the facile production of immobilized carbohydrate sequences for direct use in many of the purposes mentioned above.
In an early report on the glycal assembly method, protected glycals were immo-bilized to a silyl chloride activated polystyrene resin through reaction at the primary hydroxyl position. The resin-bound glycals were activated for glycosylation via epoxidation using 3,3-dimethyldioxirane, and released from the resin using tetrabutylammonium fluoride.[163–165] The Mrksich group developed a method for direct synthesis of oligosaccharide sequences on glass biochips for the formation of carbohydrate microarrays. A mixed self-assembled monolayer of alkane thiolate included a terminal phenol group at a density of 10% to serve as the nucleophile for glycosylation with the first building block. The glycosylation conditions used were triflate activation of the glycosyl-trichloroacetimidate (TCA), with levulinoyl ester (Lev) as the selectively removable protecting group at the site of subsequent glycosylation.[166]
The Seeberger group used diisopropylcarbodiimide coupling to functionalize various resins with a stable, hydrogenolysis-labile linker for automated solid-phase oligosaccharide synthesis. Carbohydrate immobilization occurred by glycosylation with the primary hydroxy terminus of the linker after it was conjugated with the resin. Both TCA- and thio-activated donors were used.[167] A solid-phase carbohydrate library was prepared on TentaGel resin by Liang and coworkers using direct glycosylation with sulfoxide donors. The initial saccharide was immobilized by reacting its carboxylic acid terminated linker with free amine groups on the resin. Coupling conditions used were similar to those used for solid-phase peptide synthesis, including HOBT, HBTU and NMP.[168] Loening and others described the solid-phase synthesis of 13C-labeled oligosaccharides for on-resin NMR analysis. Phosphate activated monosaccharides were immobilized on Merrifield polystyrene resin modified with an octenediol linker by direct glycosylation.[169]
Toward the development of their surface-tethered iterative carbohydrate synthesis (STICS), Demchenko and Stine immobilized carbohydrates on a nanoporous gold (NPG) surface. NPG is a high-surface area, sponge-like nanomaterial, it can be utilized as a set of NPG plates (8 × 8 × 0.2 mm) assembled in a stacked Teflon mini reactor as shown in (Figure 12). Monosaccharide acceptors were fitted with sulfide-functionalized linkers for self-assembly onto the gold, and glycosylated using a variety of common glycosyl donors, including TCA, thioglycoside, and thioimidate donors.[170,171] The oligosaccharide assembly was accomplished via alternating glycosylation, washing, deprotection and drying steps.
Figure 12.
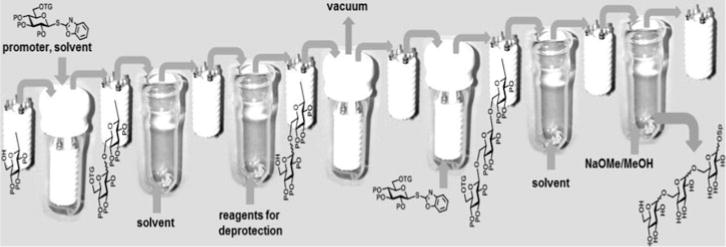
STICS: Surface-tethered iterative carbohydrate synthesis.
Summary and conclusions
Carbohydrate immobilization has proven to be an invaluable tool for research and development. Table 1 summarizes the key attributes of the various immobilization methods. For more specific information on the products of carbohydrate immobilization, several reviews have been published on topics including glyco-nanomaterials,[172–175] carbohydrate microarrays[19,25,176,177] and hydrogels.[23] Attachment of carbohydrates to surfaces has enabled the study of carbohydrate-biomolecule interactions through the development of research tools such as carbohydrate microarrays. Immobilization for solid-phase oligosaccharide synthesis has the potential to further expand interaction studies by providing access to a greater variety of oligosaccharide structures than available through biological extraction. Glyco-modified materials and nanoparticles have also been produced as a result of the development of immobilization chemistry.
Table 1.
The key attributes of various immobilization methods.
| Non-Covalent Immobilization | |||
|---|---|---|---|
|
| |||
| Glycan type | Advantages | Disadvantages | Examples |
| Un-modified | Minimal carbohydrate processing | Non-specific binding and orientation; Low MW glycans not stable |
High MW polysaccharides on nitrocellulose-coated glass |
| Linker-modified | Specific binding at linker terminus | Pre-modification required | Biotinylated glycans for avidin-modified surfaces |
| Covalent Immobilization | |||
| Self-assembly | |||
| Linker-modified | Facile immobilization process; Compatible with variety of bioanalytical methods |
Conjugation with linker required | Glycans fitted with thiol terminated linker for gold surfaces |
| Unmodified | Activated linker pre-assembled on surface | Potentially non-specific conjugation; May alter carbohydrate structure |
Reductive amination or oxime formation to connect immobilized linker |
| Functional group modifications | |||
| Modified with functional group for reaction with surface | Modification at specific carbohydrate position; Controlled presentation; Wide variety of surface materials and formats available |
Requires chemical modification of surface and/or sugar | Amine-functionalized sugar conjugated through amide bond formation |
| Unmodified | No pre-modification of glycan required | Potentially non-specific conjugation; May alter carbohydrate structure |
Hydrazine or aminooxy formation at anomeric position; Non-specific linkage to CC |
| Photochemical | |||
| Unmodified | No pre-modification of glycan required | Non-specific binding; Potentially destructive to monosaccharide structure |
Surfaces modified with PFPA |
| Linker-modified | Not destructive; Specific binding | Requires linker installation | Glycan modified with PFPA linker |
| Cycloaddition | |||
| Linker-modified | Amenable to aqueous, bioanalytical conditions; Inkjet compatible |
Requires linker installation and compatibly modified surface | CuAAC; Diels-Alder; Staudinger; Strain-promoted |
| Copolymerization | |||
| Natural/Synthetic; Amino-linked; Monomer or allyl-functionalized |
Potentially hydrophilic; 3-D cell surface mimic |
Requires linker installation; Pre-modification may alter monosaccharide structure |
Glycohydrogel; Glyconanopolymers; 3D microarray |
| Glycosylation | |||
| Protected monosaccharides | Synthesis of pure natural and uncommon glycans; Enzymatic synthesis compatible with bioanalytical formats |
Time intensive reaction sequences; Chemical synthetic conditions not readily compatible for bioanalysis |
Immobilized to activated resin by nucleophilic substitution |
When immobilizing carbohydrates, particularly for the purpose of observing binding interactions, there are many variables to consider. The format and chemistry used for immobilization will require compatible techniques for measuring carbohydrate-protein interactions, and potentially affect the data calculated in binding studies. For example, spectroscopic methods may be used to measure lectin-mediated glyconanoparticle aggregation in solution, while surface plasmon resonance and quartz crystal microbalance techniques are more appropriate for carbohydrate microarrays, and other surface-bound formats. A few instances of protein-carbohydrate interaction measurements using immobilized mannose with the lectin Con A illustrate the challenge with gathering consistent data across multiple platforms.
The three-dimensional carbohydrate hydrogel microarray reported by Liu et al., showed stronger binding between Con A and immobilized mannose (KD/nM 52.45) when compared with binding experiments performed on 2D (NHS-activated glass slide) microarray (KD/nM 66) by the Wong group.[156,178] The three-dimensional array presents a much higher binding capacity and supports “cluster configuration” in the presentation of carbohydrates, leading to the observed increase in lectin binding affinity. In another instance, binding affinity of mannose-bearing acrylamide glycopolymers tested using surface immobilized ConA by SPR provided different results (KD of 3.3 μM and 53 μM) depending on the ratio of glycosylated monomer (ligand density) used for the polymer synthesis.[161] In experiments with heterogeneous gold glyconanoparticles, researchers reported an apparent binding constant of 173.4 nM, using absorbance data for 2-amino-mannosylated particles attached through amide bond to an N-hydroxyethyl acrylamide polymer.[97] Watanabe and coworkers measured dissociation constants for the interaction of Con A with mannose immobilized on 50 nM gold nanoparticles (conjugated to carboxylic-acid functionalized, self-assembled thioglucose linkers), reporting KD values for high and low binding modes of 11.3 and 66.5 pM with data obtained by SPR. Also reported was an apparent KD for the interacting sites of 1.11 fM.[179]
The immobilization methods used to date can provide valuable information for future developments. Research studies have indicated the importance of such aspects as glycan orientation and ligand density in interactions with biomolecules. In the case of glyconanoparticles, linker length and carbohydrate ligand density can affect the specificity and affinity of lectin binding events.[97]
Different immobilization methods have been employed to achieve specific sac-charide binding (for favorable ligand presentation), as well as for the facile binding of unmodified substrates (desirable for biological extracts). Continued discovery of new methods for attaching unmodified carbohydrates to solid surfaces could lead to simpler, more accurate, and more precise analyses of carbohydrate interactions. The design of more bio-compatible glyconanoparticles and materials could lead to their regular use in the medical environment as in targeted therapeutics, imaging agents and improved medical devices.
Acknowledgments
Funding
This work was supported by grants from the National Institute of General Medical Sciences (GM111835 and GM120673).
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/lcar.
Author Contributions
The manuscript was written through contributions of all authors./All authors have given approval to the final version of the manuscript.
References
- 1.Varki A. Glycobiology. 1993;3:97. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varki A, Cummings RD, Esko JD, Freeze HH, Bertozzi CR, Stanley P, Hart GW, Etzler ME. Essentials of Glycobiology. 2nd. CSH Laboratory Press; New York: 2009. [PubMed] [Google Scholar]
- 3.BeMiller JN. In: Glycoscience: Chemistry and Chemical Biology. Fraser-Reid B, Tatsuta K, Thiem J, editors. Vol. 3. Springer; Berlin, Heidelberg, New York: 2001. p. 1865. [Google Scholar]
- 4.Holst O. In: Glycoscience: Chemistry and Chemical Biology. Fraser-Reid B, Tatsuta K, Thiem J, editors. Vol. 3. Springer; Berlin, Heidelberg, New York: 2001. p. 2083. [Google Scholar]
- 5.Wittmann V. In: Glycoscience: Chemistry and Chemical Biology. Fraser-Reid B, Tatsuta K, Thiem J, editors. Vol. 3. Springer; Berlin – Heidelberg – New York: 2001. p. 2253. [Google Scholar]
- 6.Cummings RD, Pierce JM. Chem Biol. 2014;21:1. doi: 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo L, Cipolla L. Chem Eur J. 2016;22:13380. doi: 10.1002/chem.201602156. [DOI] [PubMed] [Google Scholar]
- 8.Transforming Glycoscience: A Roadmap for the Future. 2012 http://dels.nas.edu/Report/Transforming-Glycoscience-Roadmap/13446. [PubMed]
- 9.Witczak ZJ. In: Carbohydrates in Drug Design. Witczak ZJ, Nieforth KA, editors. Marcel Dekker, Inc.; New York: 1997. p. 1. [Google Scholar]
- 10.Wong CH, editor. Carbohydrate-Based Drug Discovery. Wiley-VCH; Weinheim: 2003. [Google Scholar]
- 11.Klyosov AA, Witczak ZJ, Platt D, editors. Carbohydrate Drug Design. Vol. 932 ACS; Washington: 2006. [Google Scholar]
- 12.Sharon N, Lis H. Science. 1989;246:227. doi: 10.1126/science.2552581. [DOI] [PubMed] [Google Scholar]
- 13.Rosen SD, Bertozzi CR. Curr Opin Cell Biol. 1994;6:663. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 14.Dam TK, Brewer CF. Chem Rev. 2002;102:387. doi: 10.1021/cr000401x. [DOI] [PubMed] [Google Scholar]
- 15.Jin S, Cheng Y, Reid S, Li M, Wang B. Med Res Rev. 2010;30:171. doi: 10.1002/med.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghazarian H, Idoni B, Oppenheimer SB. Acta Histochem. 2011;113:236. doi: 10.1016/j.acthis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyukova VI, Shilova NV, Galanina OE, Rubina AY, Bovin NV. Biochim Biophys Acta. 2006;1760:603. doi: 10.1016/j.bbagen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Song X, Heimburg-Molinaro J, Smith DF, Cummings RD. Methods Mol Biol. 2012;800:163. doi: 10.1007/978-1-61779-349-3_11. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Gildersleeve JC, Blixt O, Shin I. Chem Soc Rev. 2013;42:4310. doi: 10.1039/c2cs35401b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazio F, Bryan MC, Blixt O, Paulson JC, Wong CH. J Am Chem Soc. 2002;124:14397. doi: 10.1021/ja020887u. [DOI] [PubMed] [Google Scholar]
- 21.Katrlík J, Švitel J, Gemeiner P, Kožár T, Tkac J. Med Res Rev. 2010;30:394. doi: 10.1002/med.20195. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Cummings RD, Smith DF, Huflejt M, Campbell CT, Gildersleeve JC, Gerlach JQ, Kilcoyne M, Joshi L, Serna S, Reichardt NC, Parera Pera N, Pieters RJ, Eng W, Mahal LK. Glycobiology. 2014;24:507. doi: 10.1093/glycob/cwu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubina AY, Kolchinsky A, Makarov AA, Zasedatelev AS. PROTEOMICS. 2008;8:817. doi: 10.1002/pmic.200700629. [DOI] [PubMed] [Google Scholar]
- 24.Tong Q, Wang X, Wang H, Kubo T, Yan M. Anal Chem. 2012;84:3049. doi: 10.1021/ac203455b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyelaran O, Gildersleeve JC. Curr Opin Chem Biol. 2009;13:406. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muthana S, Cao H, Chen X. Curr Opin Chem Biol. 2009;13:573. doi: 10.1016/j.cbpa.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duus JO, Gotfredsen CH, Bock K. Chem Rev. 2000;100:4589. doi: 10.1021/cr990302n. [DOI] [PubMed] [Google Scholar]
- 28.Wormald MR, Petrescu AJ, Pao YL, Glithero A, Elliott T, Dwek RA. Chem Rev. 2002;102:371. doi: 10.1021/cr990368i. [DOI] [PubMed] [Google Scholar]
- 29.Seah H, Basu A. In: Encyclopedia of Chemical Biology. Begley T, editor. John Wiley & Sons; 2008. [Google Scholar]
- 30.Song X, Smith DF, Cummings RD. Anal Biochem. 2012;429:82. doi: 10.1016/j.ab.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Song X, Ju H, Zhao C, Lasanajak Y. Bioconjug Chem. 2014;25:1881. doi: 10.1021/bc500366v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cipolla L, Arajo AC, Bini D, Gabrielli L, Russo L, Shaikh N. Expert Opin Drug Discovery. 2010;5:721. doi: 10.1517/17460441.2010.497811. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Y, Li M, Wang S, Peng H, Reid S, Ni N, Fang H, Xu W, Wang B. Sci China Chem. 2010;53:3. doi: 10.1007/s11426-010-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dube DH, Bertozzi CR. Nature Rev. 2005;4:477. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 35.Murrey HE, Hsieh-Wilson LC. Chem Rev. 2008;108:1708. doi: 10.1021/cr078215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaubard JL, Krishnamurthy C, Yi W, Smith DF, Hsieh-Wilson LC. J Am Chem Soc. 2012;134:4489. doi: 10.1021/ja211312u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemieux RU. Adv Carbohydr Chem Biochem. 1954;9:1. [PubMed] [Google Scholar]
- 38.Capon B. Chem Rev. 1969;69:407. [Google Scholar]
- 39.Gervay J, Nguyen TN, Hadd MJ. Carbohydr Res. 1997;300:119. [Google Scholar]
- 40.Nukada T, Berces A, Zgierski MZ, Whitfield DM. J Am Chem Soc. 1998;120:13291. [Google Scholar]
- 41.Nguyen HM, Chen YN, Duron SG, Gin DY. J Am Chem Soc. 2001;123:8766. doi: 10.1021/ja015968p. [DOI] [PubMed] [Google Scholar]
- 42.Ayala L, Lucero CG, Romero JAC, Tabacco SA, Woerpel KA. J Am Chem Soc. 2003;125:15521. doi: 10.1021/ja037935a. [DOI] [PubMed] [Google Scholar]
- 43.Crich D, Chandrasekera NS. Angew Chem Int Ed. 2004;43:5386. doi: 10.1002/anie.200453688. [DOI] [PubMed] [Google Scholar]
- 44.Boebel TA, Gin DY. J Org Chem. 2005;70:5818. doi: 10.1021/jo050294c. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Gildersleeve J. J Am Chem Soc. 2006;128:11612. doi: 10.1021/ja063247q. [DOI] [PubMed] [Google Scholar]
- 46.Jensen HH, Bols M. Acc Chem Res. 2006;39:259. doi: 10.1021/ar050189p. [DOI] [PubMed] [Google Scholar]
- 47.Whitfield DM. Adv Carbohydr Chem Biochem. 2009;62:83. doi: 10.1016/S0065-2318(09)00004-3. [DOI] [PubMed] [Google Scholar]
- 48.Mydock LK, Demchenko AV. Org Biomol Chem. 2010;8:497. doi: 10.1039/b916088d. [DOI] [PubMed] [Google Scholar]
- 49.Beaver MG, Woerpel KA. J Org Chem. 2010;75:1107. doi: 10.1021/jo902222a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crich D. Acc Chem Res. 2010;43:1144. doi: 10.1021/ar100035r. [DOI] [PubMed] [Google Scholar]
- 51.Pedersen CM, Marinescu LG, Bols M. C R Chimie. 2010;14:17. [Google Scholar]
- 52.Nokami T, Shibuya A, Manabe S, Ito Y, Yoshida J. Chem Eur J. 2009;15:2252. doi: 10.1002/chem.200802293. [DOI] [PubMed] [Google Scholar]
- 53.Huang M, Retailleau P, Bohe L, Crich D. J Am Chem Soc. 2012;134:14746. doi: 10.1021/ja307266n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang M, Garrett GE, Birlirakis N, Bohe L, Pratt DA, Crich D. Nature: Chemistry. 2012;4:663. doi: 10.1038/nchem.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crich D. J Org Chem. 2011;76:9193. doi: 10.1021/jo2017026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaeothip S, Yasomanee JP, Demchenko AV. J Org Chem. 2012;77:291. doi: 10.1021/jo2019174. [DOI] [PubMed] [Google Scholar]
- 57.Kononov LO, Malysheva NN, Orlova AV, Zinin AI, Laptinskaya TV, Kononova EG, Kolotyrkina NG. Eur J Org Chem. 2012:1926. [Google Scholar]
- 58.Whitfield DM. Carbohydr Res. 2012;356:180. doi: 10.1016/j.carres.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 59.Whitfield DM. Carbohydr Res. 2012;356:191. doi: 10.1016/j.carres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Ranade SC, Demchenko AV. J Carbohydr Chem. 2013;32:1. [Google Scholar]
- 61.Fruchtel JS, Jung G. Angew Chem Int Ed Engl. 1996;35:17. [Google Scholar]
- 62.Winter M. In: Combinatorial peptide and nonpeptide libraries: a handbook. Jung G, editor. VCH; Wienheim, New York, Basel, Cambridge, Tokyo: 1996. p. 465. [Google Scholar]
- 63.Adamczyk B, Tharmalingam T, Rudd PM. Biochim Biophys Acta. 2012;1280:1347. doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Yin Z, Huang X. J Carbohydr Chem. 2012;31:143. doi: 10.1080/07328303.2012.659364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stowell SR, Ju T, Cummings RD. Annu Rev Pathol. 2015;10:473. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frechet JM, Schuerch C. J Am Chem Soc. 1971;93:492. [Google Scholar]
- 67.Schmidt RR, Jonke S, Liu K. In: ACS Symp Ser (Frontiers in Modern Carbohydrate Chemistry) Demchenko AV, editor. Vol. 960. Oxford Univ. Press; 2007. p. 209. [Google Scholar]
- 68.Seeberger PH. J Carbohydr Chem. 2002;21:613. [Google Scholar]
- 69.Seeberger PH, Haase WC. Chem Rev. 2000;100:4349. doi: 10.1021/cr9903104. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka K, Fukase K. In: Solid-Phase Organic Synthesis. Toy PH, Lam Y, editors. John Wiley & Sons, Inc.; Hoboken: 2012. p. 489. [Google Scholar]
- 71.Plante OJ, Palmacci ER, Seeberger PH. Science. 2001;291:1523. doi: 10.1126/science.1057324. [DOI] [PubMed] [Google Scholar]
- 72.Seeberger PH. Chem Soc Rev. 2008;37:19. doi: 10.1039/b511197h. [DOI] [PubMed] [Google Scholar]
- 73.Plante OJ, Palmacci ER, Seeberger PH. Adv Carbohydr Chem Biochem. 2003;58:35. doi: 10.1016/s0065-2318(03)58002-7. [DOI] [PubMed] [Google Scholar]
- 74.Seeberger PH. Acc Chem Res. 2015;48:1450. doi: 10.1021/ar5004362. [DOI] [PubMed] [Google Scholar]
- 75.Wang D, Liu S, Trummer BJ, Deng C, Wang A. Nat Biotech. 2002;20:275. doi: 10.1038/nbt0302-275. [DOI] [PubMed] [Google Scholar]
- 76.Jaipuri FA, Collet BYM, Pohl NL. Angew Chem Int Ed. 2008;47:1707. doi: 10.1002/anie.200704262. [DOI] [PubMed] [Google Scholar]
- 77.Telli FC, Demir B, Barlas FB, Guler E, Timur S, Salman Y. RSC Advances. 2016;6:105806. [Google Scholar]
- 78.Shinohara Y, Hasegawa Y, Kaku H, Shibuya N. Glycobiology. 1997;7:1201. doi: 10.1093/glycob/7.8.1201. [DOI] [PubMed] [Google Scholar]
- 79.Dubois M-P, Gondran C, Renaudet O, Dumy P, Driguez H, Fort S, Cosnier S. Chem Commun. 2005:4318. doi: 10.1039/b506699a. [DOI] [PubMed] [Google Scholar]
- 80.Muñoz FJ, Rumbero Á, Sinisterra JV, Santos JI, André S, Gabius H-J, Jiménez-Barbero J, Hernáiz MJ. Glycoconjugate J. 2008;25:633. doi: 10.1007/s10719-008-9115-y. [DOI] [PubMed] [Google Scholar]
- 81.Otsuka H, Akiyama Y, Nagasaki Y, Kataoka K. J Am Chem Soc. 2001;123:8226. doi: 10.1021/ja010437m. [DOI] [PubMed] [Google Scholar]
- 82.Lin C-C, Yeh Y-C, Yang C-Y, Chen C-L, Chen G-F, Chen C-C, Wu Y-C. J Am Chem Soc. 2002;124:3508. doi: 10.1021/ja0200903. [DOI] [PubMed] [Google Scholar]
- 83.Lin C-C, Yeh Y-C, Yang C-Y, Chen G-F, Chen Y-C, Wu Y-C, Chen C-C. Chem Commun. 2003:2920. doi: 10.1039/b308995a. [DOI] [PubMed] [Google Scholar]
- 84.de la Fuente JM, Barrientos AG, Rojas TC, Rojo J, Cañada J, Fernández A, Penadés S. Angew Chem Int Ed. 2001;40:2257. doi: 10.1002/1521-3773(20010618)40:12<2257::AID-ANIE2257>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 85.Hernáiz MJ, de la Fuente JM, Barrientos ÁG, Penadés S. Angew Chem Int Ed. 2002;41:1554. doi: 10.1002/1521-3773(20020503)41:9<1554::aid-anie1554>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 86.Ojeda R, de Paz JL, Barrientos AG, Martín-Lomas M, Penadés S. Carbohydr Res. 2007;342:448. doi: 10.1016/j.carres.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 87.Huang C-C, Chen C-T, Shiang Y-C, Lin Z-H, Chang H-T. Anal Chem. 2009;81:875. doi: 10.1021/ac8010654. [DOI] [PubMed] [Google Scholar]
- 88.Kim Y-P, Park S, Oh E, Oh Y-H, Kim H-S. Biosensors and Bioelectronics. 2009;24:1189. doi: 10.1016/j.bios.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 89.Hone DC, Haines AH, Russell DA. Langmuir. 2003;19:7141. [Google Scholar]
- 90.Karamanska R, Mukhopadhyay B, Russell DA, Field RA. Chem Commun. 2005:3334. doi: 10.1039/b503843j. [DOI] [PubMed] [Google Scholar]
- 91.Reynolds AJ, Haines AH, Russell DA. Langmuir. 2006;22:1156. doi: 10.1021/la052261y. [DOI] [PubMed] [Google Scholar]
- 92.Schofield CL, Field RA, Russell DA. Anal Chem. 2007;79:1356. doi: 10.1021/ac061462j. [DOI] [PubMed] [Google Scholar]
- 93.Schofield CL, Haines AH, Field RA, Russell DA. Langmuir. 2006;22:6707. doi: 10.1021/la060288r. [DOI] [PubMed] [Google Scholar]
- 94.Thygesen MB, Sauer J, Jensen KJ. Chem Eur J. 2009;15:1649. doi: 10.1002/chem.200801521. [DOI] [PubMed] [Google Scholar]
- 95.Mahon E, Aastrup T, Barboiu M. Chem Commun. 2010;46:5491. doi: 10.1039/c002652b. [DOI] [PubMed] [Google Scholar]
- 96.Mahon E, Mouline Z, Silion M, Gilles A, Pinteala M, Barboiu M. Chem Commun. 2013;49:3004. doi: 10.1039/c3cc41074a. [DOI] [PubMed] [Google Scholar]
- 97.Otten L, Vlachou D, Richards S-J, Gibson MI. The Analyst. 2016;141:4305. doi: 10.1039/c6an00549g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richards S-J, Gibson MI. ACS Macro Letters. 2014;3:1004. doi: 10.1021/mz5004882. [DOI] [PubMed] [Google Scholar]
- 99.Witten KG, Rech C, Eckert T, Charrak S, Richtering W, Elling L, Simon U. Small. 2011;7:1954. doi: 10.1002/smll.201100492. [DOI] [PubMed] [Google Scholar]
- 100.Grant CF, Kanda V, Yu H, Bundle DR, McDermott MT. Langmuir. 2008;24:14125. doi: 10.1021/la8026489. [DOI] [PubMed] [Google Scholar]
- 101.Ratner DM, Adams EW, Su J, O’Keefe BR, Mrksich M, Seeberger PH. Chem-biochem: a European journal of chemical biology. 2004;5:379. doi: 10.1002/cbic.200300804. [DOI] [PubMed] [Google Scholar]
- 102.Adams EW, Ratner DM, Bokesch HR, McMahon JB, O’Keefe BR, Seeberger PH. Chemistry & Biology. 2004;11:875. doi: 10.1016/j.chembiol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 103.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong C-H, Paulson JC. PNAS. 2004;101:17033. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xia B, Kawar ZS, Ju T, Alvarez RA, Sachdev GP, Cummings RD. Nat Meth. 2005;2:845. doi: 10.1038/nmeth808. [DOI] [PubMed] [Google Scholar]
- 105.Earhart C, Jana NR, Erathodiyil N, Ying JY. Langmuir. 2008;24:6215. doi: 10.1021/la800066g. [DOI] [PubMed] [Google Scholar]
- 106.Ahire JH, Chambrier I, Mueller A, Bao Y, Chao Y. ACS Applied Materials & Interfaces. 2013;5:7384. doi: 10.1021/am4017126. [DOI] [PubMed] [Google Scholar]
- 107.Zhou J, Butchosa N, Jayawardena HSN, Zhou Q, Yan M, Ramström O. Bioconj Chem. 2014;25:640. doi: 10.1021/bc500004c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou J, Butchosa N, Jayawardena HSN, Park J, Zhou Q, Yan M, Ramström O. Biomacromolecules. 2015;16:1426. doi: 10.1021/acs.biomac.5b00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lopez AI, Kumar A, Planas MR, Li Y, Nguyen TV, Cai C. Biomaterials. 2011;32:4336. doi: 10.1016/j.biomaterials.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shang J, Cheng F, Dubey M, Kaplan JM, Rawal M, Jiang X, Newburg DS, Sullivan PA, Andrade RB, Ratner DM. Langmuir. 2012;28:3338. doi: 10.1021/la2043153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seo JH, Adachi K, Lee BK, Kang DG, Kim YK, Kim KR, Lee HY, Kawai T, Cha HJ. Bioconj Chem. 2007;18:2197. doi: 10.1021/bc700288z. [DOI] [PubMed] [Google Scholar]
- 112.Manimala JC, Roach TA, Li Z, Gildersleeve JC. Angew Chem Int Ed. 2006;45:3607. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 113.de Boer AR, Hokke CH, Deelder AM, Wuhrer M. Glycoconjugate J. 2008;25:75. doi: 10.1007/s10719-007-9100-x. [DOI] [PubMed] [Google Scholar]
- 114.Lee M-R, Shin I. Org Lett. 2005;7:4269. doi: 10.1021/ol051753z. [DOI] [PubMed] [Google Scholar]
- 115.Park S, Lee M-R, Shin I. Bioconj Chem. 2009;20:155. doi: 10.1021/bc800442z. [DOI] [PubMed] [Google Scholar]
- 116.Zhi Z-l, Powell AK, Turnbull JE. Anal Chem. 2006;78:4786. doi: 10.1021/ac060084f. [DOI] [PubMed] [Google Scholar]
- 117.Hernandez Armada D, Santos JT, Richards MR, Cairo CW. Carbohydr Res. 2015;417:109. doi: 10.1016/j.carres.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 118.Chen Y, Liu C, Wang X. Fabrication of Bio-function-Preserved Saccharide Microarray Chips with Cyanuric Chloride as a Rotatable Linker. Vol. 1518. Springer Science + Business Media; New York: 2017. [DOI] [PubMed] [Google Scholar]
- 119.Wang X, Xu J, Wang Y, Wang F, Chen Y. RSC Advances. 2016;6:85333. [Google Scholar]
- 120.Liang K, Chen Y. Bioconj Chem. 2012;23:1300. doi: 10.1021/bc300142s. [DOI] [PubMed] [Google Scholar]
- 121.Hsiao H-Y, Chen M-L, Wu H-T, Huang L-D, Chien W-T, Yu C-C, Jan F-D, Sahabuddin S, Chang T-C, Lin C-C. Chem Commun. 2011;47:1187. doi: 10.1039/c0cc03816d. [DOI] [PubMed] [Google Scholar]
- 122.Zhai Y, Dasog M, Snitynsky RB, Purkait TK, Aghajamali M, Hahn AH, Sturdy CB, Lowary TL, Veinot JGC. J, Mater Chem B. 2014;2:8427. doi: 10.1039/c4tb01161a. [DOI] [PubMed] [Google Scholar]
- 123.Jayasundara DR, Duff T, Angione MD, Bourke J, Murphy DM, Scanlan EM, Colavita PE. Chem Mater. 2013;25:4122. [Google Scholar]
- 124.Barboiu M, Mouline Z, Silion M, Licsandru E, Simionescu BC, Mahon E, Pinteala M. Chem Eur J. 2014;20:6678. doi: 10.1002/chem.201402187. [DOI] [PubMed] [Google Scholar]
- 125.Carroll GT, Wang D, Turro NJ, Koberstein JT. Langmuir. 2006;22:2899. doi: 10.1021/la0531042. [DOI] [PubMed] [Google Scholar]
- 126.Sharma P, Basir SF, Nahar P. J Colloid Interface Sci. 2010;342:202. doi: 10.1016/j.jcis.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 127.Wang X, Matei E, Deng L, Ramstrom O, Gronenborn AM, Yan M. Chem Commun. 2011;47:8620. doi: 10.1039/c1cc12981c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang X, Ramstroem O, Yan M. J Mater Chem. 2009;19:8944. doi: 10.1039/B917900C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X, Ramström O, Yan M. Anal Chem. 2010;82:9082. doi: 10.1021/ac102114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang X, Ramström O, Yan M. Adv Mater. 2010;22:1946. doi: 10.1002/adma.200903908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang X, Matei E, Deng L, Koharudin L, Gronenborn AM, Ramström O, Yan M. Biosensors and Bioelectronics. 2013;47:258. doi: 10.1016/j.bios.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deng L, Norberg O, Uppalapati S, Yan M, Ramstrom O. Org Biomol Chem. 2011;9:3188. doi: 10.1039/c1ob05040k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Turcheniuk V, Turcheniuk K, Bouckaert J, Barras A, Dumych T, Bilyy R, Zaitsev V, Siriwardena A, Wang Q, Boukherroub R, Szunerits S. ChemNanoMat. 2016;2:307. [Google Scholar]
- 134.Joester D, Klein E, Geiger B, Addadi L. J Am Chem Soc. 2006;128:1119. doi: 10.1021/ja0537474. [DOI] [PubMed] [Google Scholar]
- 135.Houseman BT, Mrksich M. Chem Biol. 2002;9:443. doi: 10.1016/s1074-5521(02)00124-2. [DOI] [PubMed] [Google Scholar]
- 136.Sun X-L, Stabler CL, Cazalis CS, Chaikof EL. Bioconj Chem. 2006;17:52. doi: 10.1021/bc0502311. [DOI] [PubMed] [Google Scholar]
- 137.Fazio F, Bryan MC, Blixt O, Paulson JC, Wong C-H. J Am Chem Soc. 2002;124:14397. doi: 10.1021/ja020887u. [DOI] [PubMed] [Google Scholar]
- 138.Bryan MC, Fazio F, Lee H-K, Huang C-Y, Chang A, Best MD, Calarese DA, Blixt O, Paulson JC, Burton D, Wilson IA, Wong C-H. J Am Chem Soc. 2004;126:8640. doi: 10.1021/ja048433f. [DOI] [PubMed] [Google Scholar]
- 139.Norberg O, Deng L, Yan M, Ramström O. Bioconj Chem. 2009;20:2364. doi: 10.1021/bc9003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kong N, Zhou J, Park J, Xie S, Ramström O, Yan M. Anal Chem. 2015;87:9451. doi: 10.1021/acs.analchem.5b02507. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Y, Luo S, Tang Y, Yu L, Hou K-Y, Cheng J-P, Zeng X, Wang PG. Anal Chem. 2006;78:2001. doi: 10.1021/ac051919+. [DOI] [PubMed] [Google Scholar]
- 142.Michel O, Ravoo BJ. Langmuir. 2008;24:12116. doi: 10.1021/la802304w. [DOI] [PubMed] [Google Scholar]
- 143.Szunerits S, Niedziolka-Jonsson J, Boukherroub R, Woisel P, Baumann J-S, Siriwar-dena A. Anal Chem. 2010;82:8203. doi: 10.1021/ac1016387. [DOI] [PubMed] [Google Scholar]
- 144.Barras A, Martin FA, Bande O, Baumann J-S, Ghigo J-M, Boukherroub R, Beloin C, Siriwardena A, Szunerits S. Nanoscale. 2013;5:2307. doi: 10.1039/c3nr33826f. [DOI] [PubMed] [Google Scholar]
- 145.Khanal M, Turcheniuk V, Barras A, Rosay E, Bande O, Siriwardena A, Zaitsev V, Pan G-H, Boukherroub R, Szunerits S. Langmuir. 2015;31:3926. doi: 10.1021/acs.langmuir.5b00643. [DOI] [PubMed] [Google Scholar]
- 146.Khanal M, Barras A, Siriwardena A, Boukherroub R, Szunerits S. Phys Status Solidi A. 2016;213:2124. [Google Scholar]
- 147.Kaminska I, Barras A, Coffinier Y, Lisowski W, Roy S, Niedziolka-Jonsson J, Woisel P, Lyskawa J, Opallo M, Siriwardena A, Boukherroub R, Szunerits S. ACS Applied Materials & Interfaces. 2012;4:5386. doi: 10.1021/am3013196. [DOI] [PubMed] [Google Scholar]
- 148.Gouget-Laemmel AC, Yang J, Lodhi MA, Siriwardena A, Aureau D, Boukherroub R, Chazalviel JN, Ozanam F, Szunerits S. J Phys Chem C. 2013;117:368. [Google Scholar]
- 149.Aykaç A, Martos-Maldonado MC, Casas-Solvas JM, Quesada-Soriano I, García-Maroto F, García-Fuentes L, Vargas-Berenguel A. Langmuir. 2014;30:234. doi: 10.1021/la403454p. [DOI] [PubMed] [Google Scholar]
- 150.Sun P, He Y, Lin M, Zhao Y, Ding Y, Chen G, Jiang M. ACS Macro Letters. 2014;3:96. doi: 10.1021/mz400577p. [DOI] [PubMed] [Google Scholar]
- 151.Sun P, Lin M, Zhao Y, Chen G, Jiang M. Colloids and Surfaces B: Biointerfaces. 2015;133:12. doi: 10.1016/j.colsurfb.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 152.Köhn M, Wacker R, Peters C, Schröder H, Soulère L, Breinbauer R, Niemeyer CM, Waldmann H. Angew Chem Int Ed. 2003;42:5830. doi: 10.1002/anie.200352877. [DOI] [PubMed] [Google Scholar]
- 153.Hu X-L, Jin H-Y, He X-P, James TD, Chen G-R, Long Y-T. ACS Applied Materials & Interfaces. 2015;7:1874. doi: 10.1021/am5076293. [DOI] [PubMed] [Google Scholar]
- 154.Dyukova VI, Dementieva EI, Zubtsov DA, Galanina OE, Bovin NV, Rubina AY. Anal Biochem. 2005;347:94. doi: 10.1016/j.ab.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 155.Shilova NV, Galanina OE, Rubina AY, Butvilovskaya VI, Huflejt ME, Chambers J, Roucoux A, Bovin NV. Glycoconjugate J. 2008;25:11. doi: 10.1007/s10719-007-9050-3. [DOI] [PubMed] [Google Scholar]
- 156.Liu X, Lei Z, Liu F, Liu D, Wang Z. Biosensors and Bioelectronics. 2014;58:92. doi: 10.1016/j.bios.2014.02.056. [DOI] [PubMed] [Google Scholar]
- 157.Beer MV, Rech C, Diederichs S, Hahn K, Bruellhoff K, Möller M, Elling L, Groll J. Anal Bioanal Chem. 2012;403:517. doi: 10.1007/s00216-012-5850-9. [DOI] [PubMed] [Google Scholar]
- 158.Beer MV, Rech C, Gasteier P, Sauerzapfe B, Salber J, Ewald A, Möller M, Elling L, Groll J. Advanced Healthcare Materials. 2013;2:306. doi: 10.1002/adhm.201200080. [DOI] [PubMed] [Google Scholar]
- 159.Park H, Rosencrantz RR, Elling L, Böker A. Macromol Rapid Commun. 2015;36:45. doi: 10.1002/marc.201400453. [DOI] [PubMed] [Google Scholar]
- 160.Jans A, Rosencrantz RR, Mandić AD, Anwar N, Boesveld S, Trautwein C, Moeller M, Sellge G, Elling L, Kuehne AJC. Biomacromol. 2017;18:1460. doi: 10.1021/acs.biomac.6b01754. [DOI] [PubMed] [Google Scholar]
- 161.Deepti D, Kohei S, Toshihiro T, Bin C, Hidenao A, Tetsuo K, Ken H, Koji M. Molecules. 2017;22:1. [Google Scholar]
- 162.Glisoni RJ, Sosnik A. Macromol Biosci. 2014;14:1639. doi: 10.1002/mabi.201400235. [DOI] [PubMed] [Google Scholar]
- 163.Seeberger PH, Bilodeau MT, Danishefsky SJ. Aldrichim Acta. 1997;30:75. [Google Scholar]
- 164.Danishefsky S, McClure K, Randolph J, Ruggeri R. Science. 1993;260:1307. doi: 10.1126/science.8493573. [DOI] [PubMed] [Google Scholar]
- 165.Francisco CG, Leon EI, Martin A, Moreno P, Rodriguez MS, Suarez E. J Org Chem. 2001;66:6967. doi: 10.1021/jo0156565. [DOI] [PubMed] [Google Scholar]
- 166.Ban L, Mrksich M. Angew Chem, Int Ed. 2008;47:3396. doi: 10.1002/anie.200704998. [DOI] [PubMed] [Google Scholar]
- 167.Collot M, Eller S, Weishaupt M, Seeberger PH. Beilstein J Org Chem. 2013;9:97. doi: 10.3762/bjoc.9.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liang R, Yan L, Loebach J, Ge M, Uozumi Y, Sekanina K, Horan N, Gildersleeve J, Thompson C, Smith A, Biswas K, Still WC, Kahne D. Science. 1996;274:1520. doi: 10.1126/science.274.5292.1520. [DOI] [PubMed] [Google Scholar]
- 169.Loening NM, Kanemitsu T, Seeberger PH, Griffin RG. Magnetic Resonance in Chemistry. 2004;42:453. doi: 10.1002/mrc.1364. [DOI] [PubMed] [Google Scholar]
- 170.Vijaya Ganesh N, Fujikawa K, Tan YH, Nigudkar SS, Stine KJ, Demchenko AV. J Org Chem. 2013;78:6849. doi: 10.1021/jo400095u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pornsuriyasak P, Ranade SC, Li A, Parlato MC, Sims CR, Shulga OV, Stine KJ, Demchenko AV. Chem Commun. 2009:1834. doi: 10.1039/b817684a. [DOI] [PubMed] [Google Scholar]
- 172.Chen X, Ramström O, Yan M. Nano Res. 2014;7:1381. doi: 10.1007/s12274-014-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hao N, Neranon K, Ramström O, Yan M. Biosensors and Bioelectronics. 2016;76:113. doi: 10.1016/j.bios.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marin MJ, Schofield CL, Field RA, Russell DA. The Analyst. 2015;140:59. doi: 10.1039/c4an01466a. [DOI] [PubMed] [Google Scholar]
- 175.Santoyo-Gonzalez F, Hernandez-Mateo F. Chem Soc Rev. 2009;38:3449. doi: 10.1039/b909363j. [DOI] [PubMed] [Google Scholar]
- 176.Shin I, Park S, Lee M-R. Chem Eur J. 2005;11:2894. doi: 10.1002/chem.200401030. [DOI] [PubMed] [Google Scholar]
- 177.Monzo A, Guttman A. QSAR Comb Sci. 2006;25:1033. [Google Scholar]
- 178.Liang C-H, Wang C-C, Lin Y-C, Chen C-H, Wong C-H, Wu C-Y. Anal Chem. 2009;81:7750. doi: 10.1021/ac9012286. [DOI] [PubMed] [Google Scholar]
- 179.Watanabe S, Yoshida K, Shinkawa K, Kumagawa D, Seguchi H. Colloids and Surfaces B: Biointerfaces. 2010;81:570. doi: 10.1016/j.colsurfb.2010.07.061. [DOI] [PubMed] [Google Scholar]


