Abstract
How the principal functions of the Golgi apparatus - protein processing, lipid synthesis, and sorting of macromolecules - are integrated to constitute cargo-specific trafficking pathways originating from the trans Golgi network (TGN) is unknown. Here we show that the activity of the Golgi localized SPCA1 calcium pump couples sorting and export of secreted proteins to synthesis of new lipid in the TGN membrane. A secreted Ca2+-binding protein, Cab45, constitutes the core component of a Ca2+-dependent, oligomerization-driven sorting mechanism whereby secreted proteins bound to Cab45 are packaged into a TGN-derived vesicular carrier whose membrane is enriched in sphingomyelin, a lipid implicated in TGN-to-cell surface transport. SPCA1 activity is controlled by the sphingomyelin content of the TGN membrane, such that local sphingomyelin synthesis promotes Ca2+ flux into the lumen of the TGN, which drives secretory protein sorting and export, thereby establishing a protein-and lipid-specific secretion pathway.
Keywords: sphingomyelin, Golgi apparatus, secretion, Ca2+, Cab45, SPCA1
eTOC
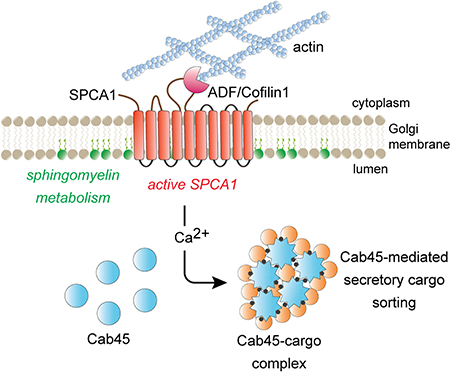
In the trans Golgi network, newly synthesized proteins and lipids are sorted into distinct vesicular carriers that mediate inter-organelle transport and secretion. Deng et al. delineate a Ca2+-dependent, oligomerization-driven sorting mechanism whereby Golgi membrane sphingomyelin stimulates SPCA1 calcium pump activity to induce Cab45 oligomerization-dependent client protein packaging into vesicles.
Graphical Abstract
Introduction
After protein and lipid secretory cargo traverses the Golgi cisternae, it is sorted into distinct vesicular transport carriers that bud from the trans Golgi network (TGN) and mediate inter organelle transport and secretion. Elucidating the mechanisms by which proteins and lipids are selected to be incorporated into Golgi-derived carriers is fundamental for understanding organelle biogenesis pathways. At present, these sorting mechanisms are poorly understood (Anitei and Hoflack, 2011; De Matteis and Luini, 2008; Kienzle and Blume, 2014).
Many integral membrane proteins, such as recycling sorting receptors, present sorting signals to the cytoplasm that are recognized by cytosolic coat proteins that concentrate cargo and elicit budding of a transport carrier (Ang and Fölsch, 2012; Bonifacino, 2014; Fölsch et al., 2003). However, most Golgi-derived transport vesicles, such as those that mediate secretion, lack cytoplasmic coats and the mechanisms that coordinate cargo selection, carrier budding and fission are poorly understood (Kienzle and Blume, 2014; Pakdel and Blume, 2018). An especially vexing question regards how soluble secreted proteins for which no integral membrane sorting receptors have been identified are packaged into Golgi-derived secretory carriers. Existing models posit that such proteins are captured non-selectively as components of the aqueous bulk (Pfeffer and Rothman, 1987) yet some proteins, such as insulin and other peptide hormones, are concentrated in essentially pure form in secretory vesicles (Huttner and Tooze, 1989; Molinete et al., 2000; Tooze et al., 1991; Tooze and Huttner, 1990). A secreted Ca2+ binding protein, Cab45, binds to soluble secreted proteins and is proposed to concentrate bound proteins from the bulk milieu prior to export from the TGN (Blume et al., 2012; Crevenna et al., 2016; Scherer et al., 1996).
In addition to its role in protein sorting, the Golgi apparatus is also considered a “lipid-based sorting station” (van Meer et al., 2008). How the lipid content of Golgi-derived carriers is determined, and if the membranes of different carriers contain the same, or different, proportions of lipids, is unknown. Synthesis of complex sphingolipids, such as sphingomyelin (SM) and gangliosides, occurs in the Golgi apparatus from where they are trafficked to other organelles, principally the plasma membrane, which contains a high proportion of cellular sphingolipid (van Meer et al., 2008). Interfering with sphingolipid synthesis in the Golgi apparatus impairs glycosylation of secretory cargo and slows the rate of secretion from the cell (Duran et al., 2012; Subathra et al., 2011; Tafesse et al., 2013; van Galen et al., 2014; Wakana et al., 2015), suggesting that glycoprotein processing, sphingolipid synthesis, and anterograde trafficking are coupled (Campelo et al., 2017; Capasso et al., 2017). A potential mechanism to couple sphingolipid synthesis and vesicle formation is suggested by the finding that production of diacylglycerol (DAG), a lipid that is produced during sphingomyelin synthesis and which promotes negative membrane curvature, is necessary for fission of at least one class of transport vesicle from the TGN membrane (Baron and Malhotra, 2002; Campelo and Malhotra, 2012; Litvak et al., 2005; Sarri et al., 2011).
The most abundant sphingolipid, sphingomyelin, is synthesized in the lumenal leaflets of Golgi membranes (Barenholz and Thompson, 1980) and then transported to the PM. To visualize SM trafficking in cells, we engineered a natural SM-binding protein, equinatoxin II, produced by the marine organism, Actinia equina, into a non-toxic SM reporter protein, termed “EQ-SM” (Deng et al., 2016). When addressed to the secretory pathway as a fusion to a fluorescent protein, EQ-SM is observed to be exported from the TGN in pleiomorphic carriers and to be released from the cell via exocytosis, where it remains bound to SM in the PM (Deng et al., 2016). The population of putative secretory carriers observed to bud from the TGN containing EQ-SM are also enriched with a secreted glycosylphosphatidylinositol (GPI)anchored fluorescent reporter protein, to a similar extent as EQ-SM containing exocytic carriers observed to fuse with the PM (Deng et al., 2016). These observations suggest that SM and GPI-anchored proteins are sorted in the Golgi into a distinct arm of the secretory pathway that we refer to as the “sphingomyelin secretion (SMS) pathway” to suggest its role in trafficking SM from the TGN to the PM. To date, no native proteins have been identified that rely on the SMS pathway for secretion.
Here we identify native protein cargo of the SMS pathway using unbiased proteomics analyses of Golgi-derived vesicles that contain EQ-SM. One identified protein, Cab45, was previously implicated in calcium-dependent sorting of a subset of secreted proteins, hereafter referred to as Cab45 ‘clients’ (Blume et al., 2012; Crevenna et al., 2016). We show that Cab45 and its clients are secreted via the SMS pathway and that sorting into the SMS pathway requires active SPCA1, a TGN-localized calcium pump. Cab45- and Ca2+-dependent sorting of soluble secretory cargo in the TGN fails when SM synthesis in the TGN is disrupted due to deficient Ca2+ pumping activity by SPCA1, which resides in sphingolipid-rich membrane. Our findings reveal an unanticipated functional coupling between SM synthesis in the TGN membrane and Ca2+-dependent sorting of soluble secretory cargo within the lumen of the TGN.
Results
Identification of native protein cargos of the SMS pathway
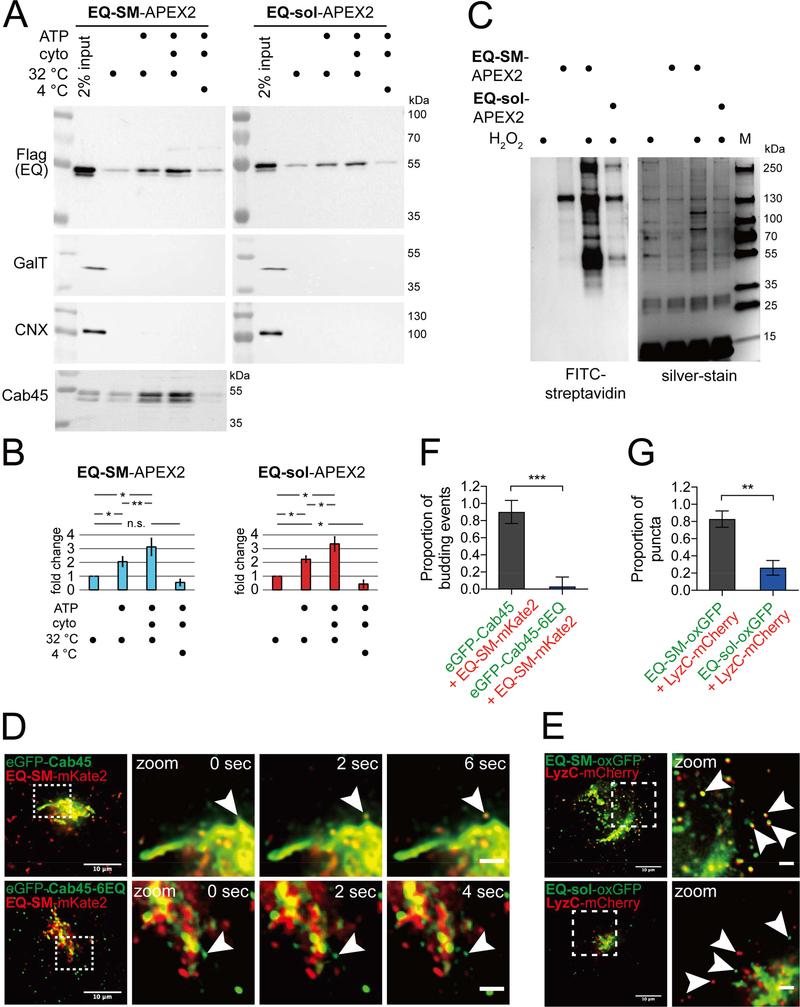
We have previously shown that EQ-SM is exported from the Golgi in a distinct secretory transport carrier (Deng et al., 2016). A comparative proximity biotinylation proteomics approach was implemented to identify candidate native proteins that are secreted with EQ-SM via the SMS pathway. The general strategy entailed collecting vesicle-enriched fractions produced by permeabilized HeLa cells that express EQ-SM, or a control protein that does not bind SM termed ‘EQ-sol’, as fusions to engineered ascorbate peroxidase 2 (APEX2) (Hung et al., 2016) followed by APEX2-mediated biotinylation and comparative proteomic analyses of proteins purified on streptavidin matrix. HeLa cell lines stably expressing EQ-SM-APEX2 or EQ-sol-APEX2 were constructed and confirmed to elicit biotinylation of Golgi-localized proteins by in situ FITC-streptavidin detection (data not shown), confirming that they localize similarly to previously characterized GFP-tagged forms of EQ-SM and EQ-sol (Deng et al., 2016). As a source of Golgi-derived vesicles, we applied a previously published protocol to generate a crude vesicle fraction from permeabilized cells (Wakana et al., 2012). The plasma membranes of cells expressing EQ-SM-APEX2 or control EQ-sol-APEX2 were permeabilized, the cytosol was washed out, and conditions were established to generate Golgi-derived vesicles containing EQ-SM-APEX2 or EQ-sol-APEX2, but not galactose transferase T1 (GalT), a resident of the trans-Golgi, or calnexin, a resident of the ER (Fig. 1A, B). The appearance of EQ-SM-APEX2 or EQsol-APEX2 in vesicle fractions was promoted approximately 3-fold by incubation of permeabilized cells at physiological temperature (32°C) with exogenous cytosol and an ATP regeneration system.
Figure 1. Comparative proteomics of Golgi-derived vesicles identifies Cab45 as a cargo of the sphingomyelin secretion pathway.
(A) Preparation of Golgi-derived vesicles. Cell lines expressing EQ-SM-APEX2 or EQ-sol-APEX2 were permeabilized and then incubated with rat liver cytosol, ATP generating reaction components, at 32ºC or on ice for 45 minutes, as indicated. Released vesicular material was collected by centrifugation and the relative proportions of EQ-SM-APEX2 or EQ-sol-APEX2, β1,4-galactosyltransferase (GalT; a resident of late Golgi compartments), calnexin (CNX; an ER resident), and Cab45, in each fraction were determined by immunoblotting. Note that EQ-SM-APEX2, EQ-sol-APEX2, and Cab45 are detected in the budded vesicle fractions, but GalT (a Golgi resident) and CNX (an ER resident) are not. An example experiment for EQ-SM-APEX2 and EQ-sol-APEX2 is shown. Proteomic analyses were performed for two independent vesicle preparations. See also Table S1. (B) Proportions of EQ-SM-APEX2 and EQ-sol-APEX2 in vesicle fractions. The fold increases in the amounts of EQ-SM-APEX2 and EQ-sol-APEX2 in the vesicle fractions (mean ± s.d.; 3 independent experiments) are plotted. (C) Purification of biotinylated proteins from vesicle fractions. Vesicle fractions from EQ-SM-APEX2 or EQ-sol-APEX2 expressing or control (no EQ probe) cells were incubated with APEX-mediated biotinylation reagents, unless otherwise noted. Detergent (1% TritonX-100 and 0.1% SDS) was added to solubilize vesicle-associated proteins and biotinylated proteins were purified using immobilized streptavidin. An aliquot of each purified fraction was separated by SDS-PAGE and proteins were visualized by silver stain. Biotinylated proteins in each fraction were identified by blotting with fluorescent streptavidin. The migration of protein molecular mass standards is indicated to the right of each gel. (D) Example micrographs of cells expressing eGFP-Cab45 and EQ-SM-mKate2. Each micrograph shows the Golgi and surrounding cytoplasm of cells expressing the indicated fluorescent proteins. Cab45–6EQ is an engineered variant of Cab45 that does not bind Ca2+. To quantify the proportion of Golgi-derived vesicles containing both proteins, time-lapse movies were acquired and examined to identify vesicular/tubular profiles that underwent budding and fission from the Golgi apparatus. The arrowheads indicate a budding vesicle. Data was collected from 3 independent experiments. Merge bars, 10 μm. Zoom bars, 2 μm. (E) LyzC is exported from the Golgi in vesicles marked by EQ-SM, but not EQ-sol. Images of the Golgi region of cells expressing mCherry-tagged LyzC and EQ-SM-oxGFP or EQ-sol-oxGFP are shown. Arrowheads point to post-Golgi cytoplasmic vesicles. Merge bars, 10 μm. Zoom bars, 2 μm. (F) The mean (± s.d.) proportion of 26 budding events from 4 cells expressing eGFP-Cab45 and EQ-SM as well as 32 budding events from 9 cells expressing eGFP-Cab45–6EQ and EQ-SM are plotted. Data was collected from 2 independent experiments. (G) The mean (± s.d.) proportion of detected postGolgi LyzC vesicles that were either EQ-SM (n=185 vesicles from 6 cells) or EQ-sol (n=146 vesicles from 5 cells) positive was quantified. Data was collected from 2 independent experiments.
To identify native proteins that are specifically co-packaged with EQ-SM-APEX2 or EQ-sol-APEX2 into budded vesicles, vesicle fractions from permeabilized cells were incubated with APEX biotinylation reagents to elicit labeling of vicinal proteins. Lysis buffer containing detergent was then used to solubilize vesicle content, biotin-labeled proteins were purified on a solid streptavidin matrix (Fig. 1C), and mass spectrometry was applied to identify proteins in each vesicle fraction. We defined candidate cargo proteins of the SMS pathway as those proteins containing a signal sequence, a transmembrane domain, or a GPI anchor, that were identified in EQ-SM-APEX2 vesicle fractions, but not in EQ-sol-APEX2 vesicle fractions, in each of two independent vesicle preparations.
One protein that met these criteria is Cab45 (Table S1), a soluble Ca2+-binding protein that has been previously characterized as a Golgi-localized protein that facilitates sorting and secretion of certain proteins, including lysozyme C (LyzC) and cartilage oligomeric matrix protein (COMP) (Blume et al., 2012; Crevenna et al., 2016). The presence of Cab45 in the EQ-SM-APEX2 budded vesicle fraction was also validated by immunoblotting of permeabilized cell vesicle fractions (Fig. 1A). These data suggest that Cab45 exits the Golgi in vesicles enriched in SM.
Next, we investigated if Cab45 and EQ-SM exit the Golgi in the same or distinct carriers using time lapse imaging of fluorescently tagged Cab45 (eGFP-Cab45) and EQ-SM (EQ-SM-mKate2) in live cells (Fig. 1D). Tubular and vesicular structures containing Cab45 were observed to bud and fission from the TGN and the proportion of these putative secretory carriers that also contained EQ-SM was determined. This analysis showed that nearly all Cab45 containing vesicles (23/26 budding events) also contained EQ-SM (Fig. 1F). Importantly, a Ca2+ binding-defective mutant form of Cab45 (“Cab45–6EQ”) and EQ-SM are exported from the TGN via a different carrier(s) (2/32 budding events), indicating a physiologic requirement for Ca2+ binding for co-sorting of Cab45 and EQ-SM upon exit from the Golgi.
Ca2+-dependent sorting of a Cab45 client into the SMS pathway
Ca2+ binding by Cab45 drives its oligomerization, recognition of secretory clients, and efficient client secretion (Blume et al., 2012; Crevenna et al., 2016). Therefore, we tested whether EQ-SM is also enriched in cytoplasmic vesicles that contain a Cab45 dependent cargo, LyzC, using live-cell fluorescence microscopy of cells expressing LyzC-mCherry and either EQ-SM or EQ-sol expressed with a C-terminal fusion to oxGFP, a form of GFP optimized for use within the secretory pathway (Costantini et al., 2015) (Fig. 1E). We observed that 83 ± 10% LyzC-mCherry puncta are also labelled by EQ-SM-oxGFP, but EQ-sol, which does not recognize SM, was detected in only 26 ± 9% of the Lyz-mCherry puncta (Fig. 1G). These data indicate that a Cab45 client, LyzC, is sorted into TGN-derived vesicles that contain EQ-SM.
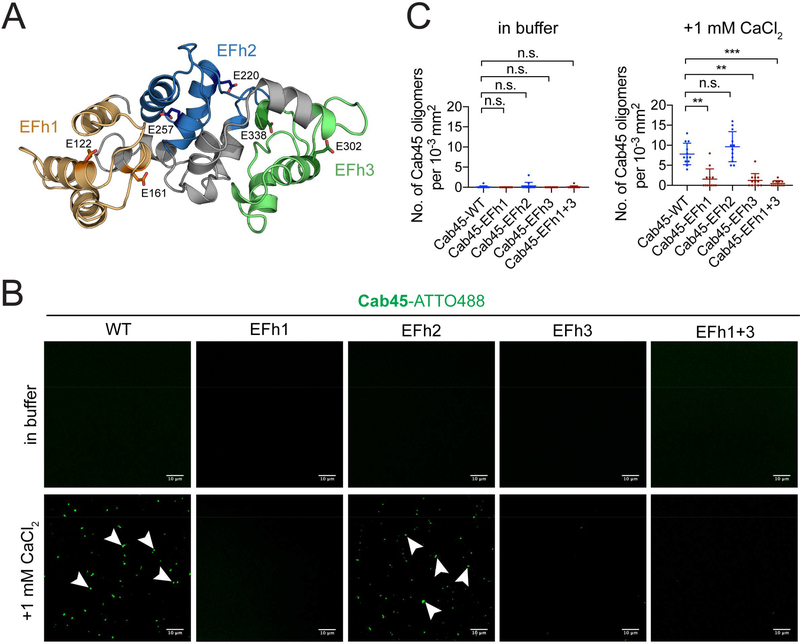
We next addressed Cab45 structural requirements for LyzC sorting. Structural modeling of Cab45 (Blank and Blume, 2017; Crevenna et al., 2016) suggests that pairs of contiguous EF hand domains form three structurally independent modules that we term EFh1 (consisting of EF hands 1 and 2), EFh2 (EF hands 3 and 4), and EFh3 (EF hands 5 and 6) (Fig. 2A). To determine which EF hand modules are required for Cab45 oligomerization, we first visualized Ca2+ dependent oligomerization by using confocal microscopy of ATTO488-labeled recombinant Cab45 compared to Cab45-EFh mutants in which the Ca2+ binding sites of each functional EFh pair was mutated (Fig. 2B). The addition of 1 mM Ca2+ promoted the formation of fluorescent Cab45 oligomers of WT and EFh2-mut but not EFh1-mut, EFh3-mut and EFh1+3-mut (Fig. 2B, C). In line with this observation, circular dichroism (CD) measurements showed a change in the secondary structure upon addition of Ca2+ of Cab45-WT and EFh2-mut, but not EFh1-mut, EFh3-mut and EFh1+3-mut (Fig. S1A). Confocal microscopy of HeLa cells expressing Cab45-EFh mutants showed that EFh1-mut, EFh3-mut and EFh1+3-mut co-localize with p230, a TGN marker, and also to cytoplasmic vesicles, similar to Cab45–6EQ, while EFh2-mut showed reduced cytoplasmic vesicular localization (Fig. S1B, C). These results indicate that EFh1 and EFh3 are critical for Ca2+-induced change in secondary structure, oligomerization of Cab45, and correct localization in the TGN.
Figure 2. Cab45 oligomerization and client binding requires EF hand modules 1 and 3.
(A) Model of Cab45 EF hand modules. Structural modeling predicts that contiguous pairs of EF hands function as independent Ca2+-binding modules (Crevenna et al., 2016); EF hand module 1 (consisting of EF hands 1 and 2) is orange, EF hand module 2 (consisting of EF hands 3 and 4) is blue, and EF hand module 3 (consisting of EF hands 5 and 6) is green. The positions and amino acid side chains of calcium binding glutamic acid residues from each module are indicated. These residues were replaced by Glutamine in Cab45 EFh mutants to disable Ca2+ binding. (B) In vitro oligomerization assay analyzed by confocal microscopy. Recombinant Cab45-WT and the Cab45-EFh1, Cab45-EFh2, Cab45-EFh3 and Cab45-EFh1+3 mutants labeled with ATTO488, were incubated in Ca2+-free buffer or with 1 mM CaCl2. Bars, 10 μm. Arrowheads indicate fluorescent puncta of Cab45 oligomers. (C) Quantification of in vitro oligomerization assay. Data are presented as mean number of Cab45 puncta per 10−3 mm2 area from 12 regions of interest (± s.d.) from 2 independent experiments. See also Figure S1.
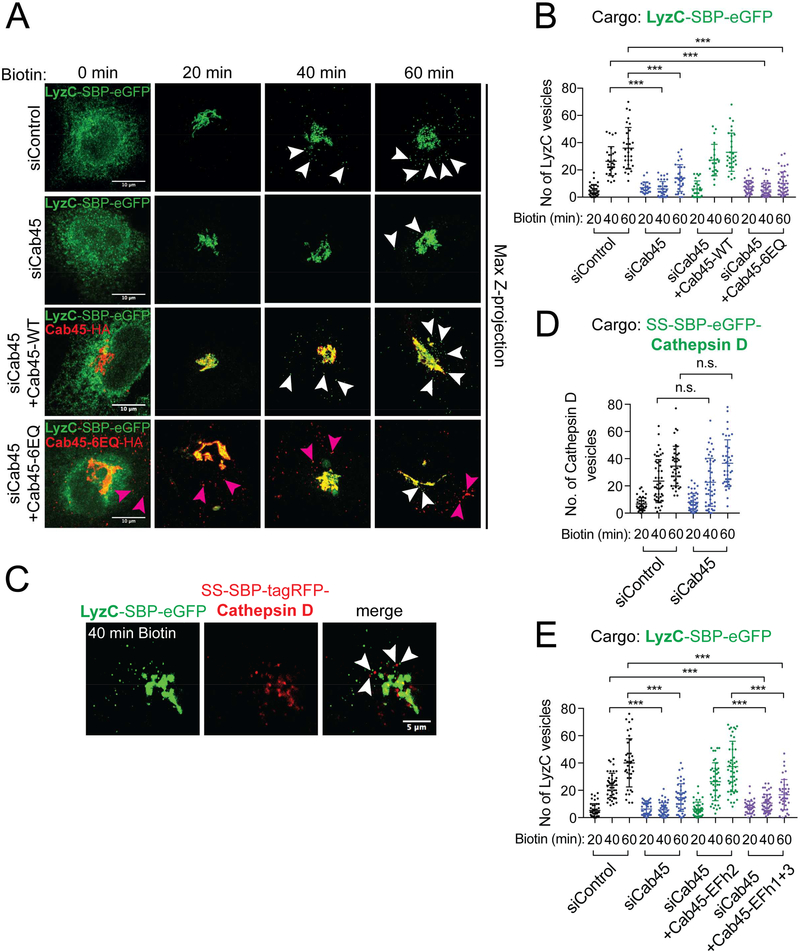
To correlate these results to sorting of a Cab45 client into the SMS pathway, we first monitored transit of GFP-tagged LyzC through the secretory pathway in cells expressing mutant forms of Cab45. A gene replacement strategy was employed where native Cab45 was depleted by Cab45 siRNAs and then siRNA resistant WT or mutant forms of Cab45 were expressed. To monitor trafficking of a single cohort of LyzC, the “retention using selective hooks” (RUSH) system (Boncompain et al., 2012) was used to synchronously release LyzC-eGFP from the ER by addition of biotin to the culture medium. Accordingly, the streptavidin binding peptide (SBP) sequence was appended to the C-terminus of LyzC, followed by enhanced GFP (LyzC-SBP-eGFP). Whereas in the absence of added biotin, LyzC-SBP-eGFP is restricted to the ER (Fig. 3A), twenty minutes after biotin addition to the culture medium, nearly all LyzC-SBP-eGFP localized to the Golgi apparatus. At 40 and 60 minutes after biotin addition, vesicles containing LyzC-SBP-eGFP are observed in the cytoplasm (mean number of vesicles/cell: 26 ± 11 and 36 ± 15, respectively) (Fig. 3A, B). In contrast, in cells depleted of Cab45, LyzC-SBP-eGFP is largely retained within the Golgi apparatus; with just 6 ± 5 and 14 ± 10 vesicles per cell observed 40 and 60 minutes after ER release, respectively (Fig 3A, B, Fig. S2A, B). Expression of siRNA-resistant, HA epitope-tagged Cab45-WT restored export of LyzC-SBP-eGFP from the Golgi (27 ± 12 vesicles per cell and 33 ± 14 vesicles per cell, respectively), however, a Ca2+ binding defective mutant protein form of Cab45 (Cab45–6EQ-HA) did not (5 ± 5 vesicles per cell and 10 ± 8 vesicles per cell, respectively) (Fig 3A, B), even though both proteins were expressed at similar levels (data not shown). Importantly, Cathepsin D, which is not a Cab45 client (Crevenna et al., 2016), does not colocalize within the same post-Golgi vesicles and showed no export defect in Cab45 depleted cells, indicating that it is exported from the Golgi by a distinct carrier (Fig. 3C, D). Next, we examined requirements for the EFh Ca2+ binding modules of Cab45 in promoting export of LyzC-SBP-eGFP from the Golgi apparatus. Expression of siRNA resistant Cab45-EFh2-mut rescued LyzC-SBP-eGFP to WT levels (26 ± 14 and 37 ± 18 at 40 and 60 minutes post-release, respectively) (Fig. 3E). Importantly, expression of Cab45-EFh1+3-mut (11 ± 6 and 17 ± 11, respectively) failed to rescue export of LyzC-SBPeGFP from the Golgi (Fig. 3E). These results demonstrate that Ca2+ binding by Cab45 EFh1 and EFh3 is required for efficient export of LyzC from the Golgi apparatus.
Figure 3. Ca2+ binding by Cab45 is required for sorting of a Cab45 client, lysozyme C (LyzC), into Golgi-derived vesicles.
(A) HeLa cells were transfected with Cab45 siRNA and a plasmid that directs expression of siRNA-insensitive HA-epitope tagged Cab45-WT, or the Ca2+ binding defective Cab45–6EQ mutant variant. All cells were co-transfected with plasmids that direct expression of a LyzC – streptavidin binding peptide (SBP) – eGFP fusion protein (LyzC-SBP-eGFP), and streptavidin-KDEL ‘anchor’ that confers ER retention of SBP-containing proteins. Biotin was added to the culture medium to elicit release of LyzC-SBP-eGFP from the ER (0 min). Micrographs were captured after fixing cells 20, 40, and 60 minutes after biotin addition and the number of cytoplasmic vesicles was determined at each time point. Arrowheads point to cytoplasmic vesicles. Note that Golgi derived vesicles containing LyzC-SBP-eGFP are abundant in the cytoplasm of cells that express native, but not Ca2+ binding defective, Cab45. Magenta arrowheads point to Cab45–6EQ cytoplasmic vesicles that localize to distinct vesicles from LyzC-SBP-eGFP. See also Fig S1. Vesicle counts from at least 22 cells per condition are plotted in (B). The means of 3 independent experiments (± s.d.) are plotted. (C) Cathepsin D and lysozyme C fusion proteins are exported from the Golgi in different vesicles. HeLa cells were transfected with plasmids that direct expression of LyzC-SBP-eGFP or SS-SBP-tagRFP-Cathepsin D fusion proteins. Proteins were released from the ER by the addition of biotin and the cargo loads of Golgi derived cytoplasmic vesicles was determined as described in the legend to panel A. Micrographs show representative cells 40 minutes after release of cargo from the ER. Bars, 5 μm. (D) Export of Cathepsin D from the Golgi is unaffected by Cab45 gene silencing. The SS-SBP-eGFP-Cathepsin D fusion protein was released from the ER of Cab45 siRNA-silenced, or control, cells by addition of biotin. The mean number of post-Golgi vesicles per cell (>39 cells per condition, ± s.d.) are plotted from 3 independent experiments as a function of time. Vesicle counts for Cab45 siRNA and control siRNA cell populations are not statistically significant. (E) Ca2+ binding by EFh modules 1 and 3 is required for efficient export of LyzC from the Golgi apparatus. Cells were depleted of endogenous Cab45 by siRNA and siRNA-insensitive Cab45 cDNAs with mutations in each of the EF hand modules were expressed. The number of cytoplasmic vesicles containing LyzC-SBP-eGFP per cell was determined after release of LyzC-SBP-eGFP from the ER. Only the data for EFh2 and EFh1+3 are shown in this figure; additional data is shown in Fig. S1. The mean vesicle counts from at least 38 cells per condition (± s.d.) are plotted.
EQ-SM, Cab45 and LyzC are exocytosed via the same carrier
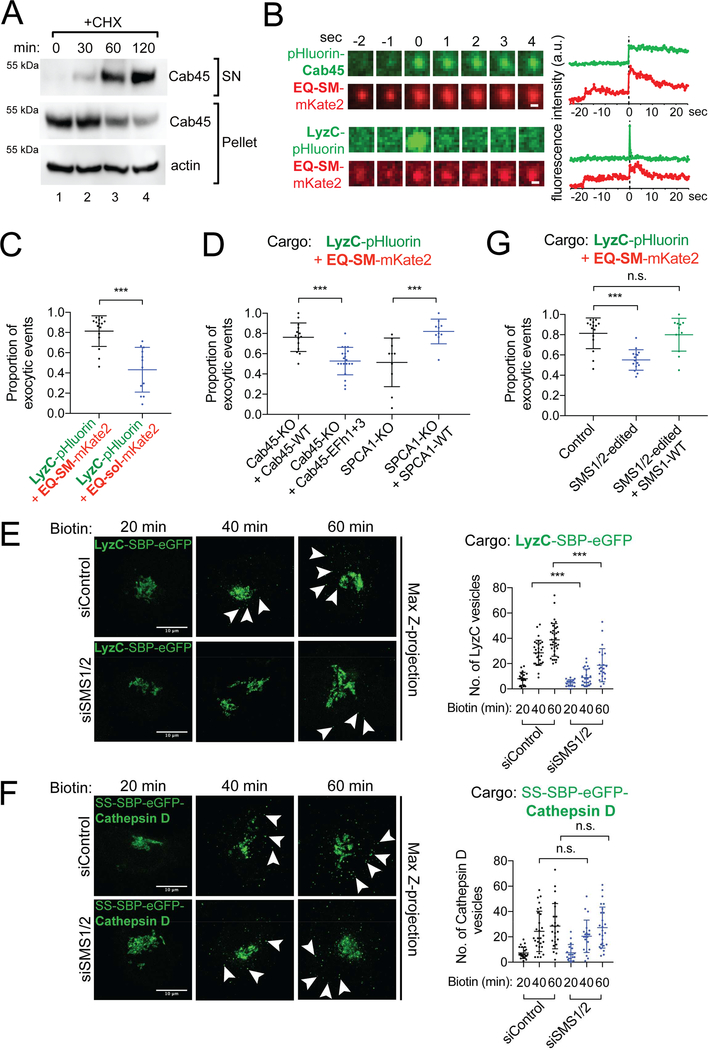
The results establish that EQ-SM, Cab45 and LyzC exit the Golgi in the same carrier, and we next sought to determine if these proteins are also secreted from the cell via the same carrier. Secretion of endogenous Cab45 and the requirement for Cab45 for efficient secretion of LyzC were confirmed by probing the cell medium of cycloheximide (CHX) treated HeLa cells with anti-Cab45 antiserum (Fig. 4A, Fig. S2A, B). To determine if Cab45 and LyzC are exocytosed in vesicles of the SMS pathway, we used time-lapse total internal reflection fluorescence microscopy (TIRFM) of live cells co-expressing EQ-SM-mKate2 or EQ-sol-mKate2 and pHlourin-Cab45 or LyzC-pHluorin (Fig. 4). Exocytic events were identified in movies by the flash of fluorescence emitted by pHluorin upon encountering the neutral pH of the culture medium, and then the presence of EQ-SM-mKate2 or EQ-sol-mKate2 was scored (Figure 4B). We observed that 98 ± 5% (40/42 exocytic events in 8 cells) of the pHlourin-Cab45 containing vesicles also contained EQ-SM-mKate2, but just 46 ± 37% of pHlourin-Cab45 containing vesicles contained EQ-sol-mKate2 (14/30 exocytic events in 8 cells). We next examined exocytosis of EQ reporters with LyzC and observed that 81 ± 15% (n=15 cells) of LyzC-pHluorin exocytosed vesicles also contained EQ-SM-mKate2, but just 43 ± 22 (n=12 cells) of LyzC-pHluorin vesicles contained EQ-sol-mKate2 (Fig. 4C). These data, considered together with the results of the Golgi export assays, confirm that Cab45 and the Cab45 client, LyzC, are sorted into the SMS pathway. We note that substantially fewer Cab45 exocytic events were observed compared to LyzC events, possibly indicating that Cab45 is retrieved from a post-Golgi compartment while the Cab45 client continues on the anterograde pathway.
Figure 4. Cab45, LyzC, and EQ-SM are exocytosed via the same vesicles in a sphingomyelin synthesis dependent manner.
(A) Cell culture supernatants and whole cell lysates of HeLa cells were collected after 0, 30, 60 and 120 min incubation with cycloheximide (CHX) and probed for Cab45 by immunoblotting. (B) Time-lapse gallery of Cab45, LyzC, and EQ-SM exocytosis. Galleries show example exocytic events of pHluorin-Cab45 or LyzC, and EQ-SM-mKate2 captured by TIRFM. The corresponding graphs show the summed fluorescence intensities for each frame in each channel over time. (C) LyzC is co-sorted with EQ-SM, but not EQ-sol, into exocytic vesicles. The mean proportions of exocytic events observed in 3 independent experiments (± s.d.) where LyzC-pHluorin containing vesicles also released mKate2-tagged EQ-SM or EQ-sol are indicated (362 events/15 cells for LyzC+EQ-SM and 268 events/12 cells for LyzC+EQ-sol). (D) Cab45 EFh1 and EFh3 and SPCA1 are required for cosorting of LyzC and EQ-SM. Genome edited Cab45 null cells expressed Cab45-WT or Cab45EFh1+3-mut by transfection or genome edited SPCA1 null cells that expressed or did not express SPCA1-WT by transfection. The cargo loads of exocytic vesicles were determined as described for (B). The means (± s.d.) are shown for n=239 events/14 cells for Cab45-WT, n=128 events/10 cells for Cab45-EFh1+3, n=163 events/10 cells for SPCA1-KO, n=157 events/10 cells for SPCA1-WT. (E) Depletion of sphingomyelin synthases (SMS1 and SMS2) delays export of LyzC-SBP-eGFP from the Golgi apparatus. HeLa cells were transfected with siRNAs targeting SMS1 and SMS2 or non-targeted control for two days prior to transfection with a plasmid that directs expression of LyzC-SBP-eGFP and the Streptavidin-KDEL ‘anchor’. The number of cytoplasmic vesicles per cell was determined at the indicated time points after release of LyzC-SBP-eGFP from the ER by addition of biotin. Arrowheads point to cytoplasmic vesicles. Bars, 10 μm. Vesicle counts (mean ± s.d.) from at least 18 cells per condition in 3 independent experiments are plotted in the graph on the right. (F) Depletion of SMS1 and SMS2 does not delay export of SS-SBP-eGFP-Cathepsin D vesicles from the Golgi apparatus. Arrowheads point to cytoplasmic vesicles. Assays were conducted as in (A). Bars, 10 μm. Vesicle counts from at least 18 cells per condition in 3 independent experiments are plotted (mean ± s.d.). (G) TIRF microscopy-based sorting assays were used to determine the proportion of LyzC-pHluorin exocytic vesicles that also contained EQ-SM-mKate2 (mean ± s.d.) in genome edited HeLa cells (SMS1/2-edited) that express SMS1 and SMS2 at reduced levels. See also Figure S3.
To address Cab45 structural requirements for co-sorting of LyzC and EQ-SM we conducted TIRFM-based exocytosis assays in genome edited Cab45 null cells (Crevenna et al., 2016) that re-express Cab45 or the Cab45-EFh1+3 mutant by transfection (Fig. 4D). In control cells that re-express Cab45-WT, 76 ± 14% (n=14 cells) of exocytic vesicles contained both LyzC-pHluorin and EQ-SM-mKate2, a value that is similar to that of LyzC-pHluorin and EQ-SM-mKate2 in unmodified cells (Fig. 4D). In contrast, in cells expressing EFh1+3-mut, only 53 ± 14% (n=20 cells) of LyzC-pHluorin containing exocytic vesicles also contained EQ-SM-mKate2 (Fig. 4D). Importantly, disruption of TGN Ca2+ homeostasis by deletion of SPCA1 reduced the number of exocytic vesicles containing both LyzC-pHluorin and EQ-SM-mKate2 to only 51 ± 24% (n=10 cells) (Fig. 4D) and this effect was rescued to 82 ± 12% (n=10 cells) by re-expression of SPCA1-WT (Fig. 4D). Furthermore, by using RUSH assays, we observed that cytoplasmic LyzC vesicle counts were significantly reduced after forty and sixty minutes of Biotin addition in SPCA1 null cells while this effect was rescued by re-expression of SPCA1-WT (Fig. S2C). In addition, SPCA1 depletion slowed the rate of LyzC secretion into the culture medium (Fig. S2A, B) (Blume et al., 2012). These data demonstrate that Ca2+ binding by Cab45 promotes sorting of LyzC into secretory vesicles of the SMS pathway in an SPCA1-dependent manner.
Depletion of SM synthases causes mis-sorting of LyzC
Having established roles for Cab45 and SPCA1 in Ca2+-dependent sorting of LyzC into Golgi-derived exocytic vesicles of the SMS pathway, we next sought to determine if SM synthesis is required for Cab45-dependent sorting. Using RUSH-based sorting assays, we examined export of LyzC and Cathepsin D from the Golgi of cells depleted of SM synthases by siRNA (Fig. S3A). Both SMS1, which localizes to the Golgi apparatus, and SMS2, which localizes to the PM, were depleted because published work shows that toxicity of exogenously added short chain ceramide are ameliorated only when both enzymes are depleted (Duran et al., 2012; van Galen et al., 2014). Whereas forty minutes after release of LyzC-SBP-eGFP from the ER, control siRNA cells contained 29 ± 10 vesicles per cell, SMS1/2 depleted cells contained 9 ± 7 vesicles per cell (Fig. 4E). Notably, SMS1 and SMS2 depletion did not affect the number of post-Golgi vesicles containing SS-SBP-eGFP-Cathepsin D (25 ± 16 versus 21 ± 13 vesicles per cell cytoplasm) (Fig. 4F). These data indicate that depletion of SMS1/2 selectively decrease the rate of export of LyzC, but not Cathepsin D, from the Golgi.
TIRFM-based exocytosis assays revealed that SM synthesis is required for co-sorting of LyzC-pHluorin and EQ-SM into exocytic vesicles (Fig. 4G). For these experiments, we used a fortuitously obtained genome edited HeLa cell line that expresses reduced amounts of SMS1 and SMS2 (‘SMS1/2-edited’). Quantitative mass spectrometry analysis of sphingolipids in this cell line shows that the sum total of all SM species is reduced by 31 ± 6.4% (s.e.m.) (Fig. S3B), a level that is below the detection limit for EQ-SM (Fig. S3B-E). Upon fusion of EQ-SM containing vesicles with the plasma membrane of SMS1/2-edited cells, EQ-SM rapidly diffuses from the site of exocytosis, in contrast to parental cells where EQ-SM remains at the site of fusion (Fig. S3C, D), indicating that these secretory vesicles are depleted of SM. Whereas in unmodified parental cells 81 ± 15% (n=15 cells) of LyzC-pHluorin containing exocytic vesicles contained EQ-SM-mKate2, in SMS1/2 depleted cells only 55 ± 10% (n=15 cells) contained both of these proteins (Fig. 4G). Depletion of SMS1/2 was also observed to slow secretion of LyzC into the medium (Fig. S2A, B). Importantly, re-expression solely of SMS1 in SMS1/2-edited cells restored co-sorting of LyzC-pHluorin and EQ-SM-mKate2 to control levels (Fig. 4G). We note that the magnitude of the effect of SMS1/2 depletion on co-sorting of LyzC and EQ-SM is similar to that observed for co-sorting of these proteins in Cab45-EFh1+3 mutant cells (53%; Fig. 4D), and that of LyzC and EQ-sol in unmodified parental cells (43%; Fig. 4C). The results establish that Cab45 mediates Ca2+-dependent sorting of LyzC into the SMS pathway and that SM synthesis is necessary for sorting.
SPCA1 associates with sphingolipid in TGN membrane
The Ca2+ pump activity of SPCA1 maintains Ca2+ in the lumen of the TGN (Lissandron et al., 2010). Curiously, SPCA1 is reported to purify with detergent resistant membrane (Baron et al., 2010) and its optimal activity in reconstituted liposomes requires the presence of SM (Chen et al., 2017), leading us to hypothesize that SPCA1 may provide a mechanistic link between the SM biosynthetic pathway and Cab45-mediated sorting and secretion. Accordingly, we asked if SPCA1 resides in a Golgi compartment where SM is produced. Two observations indicate that this is the case. First, endogenous SMS1 and SPCA1 partially co-localize in the TGN (Fig. 5A). This was determined by comparing the localization of an endogenously expressed SNAP-tagged SMS1 fusion protein generated by genome editing to that of endogenous SPCA1, which was detected by indirect immunofluorescence. Quantitation of colocalization with an endogenous TGN protein, p230, indicates that both SMS1 localizes predominantly to the TGN, while SPCA1 localizes to multiple Golgi compartments (Fig. 5A, Table S2).
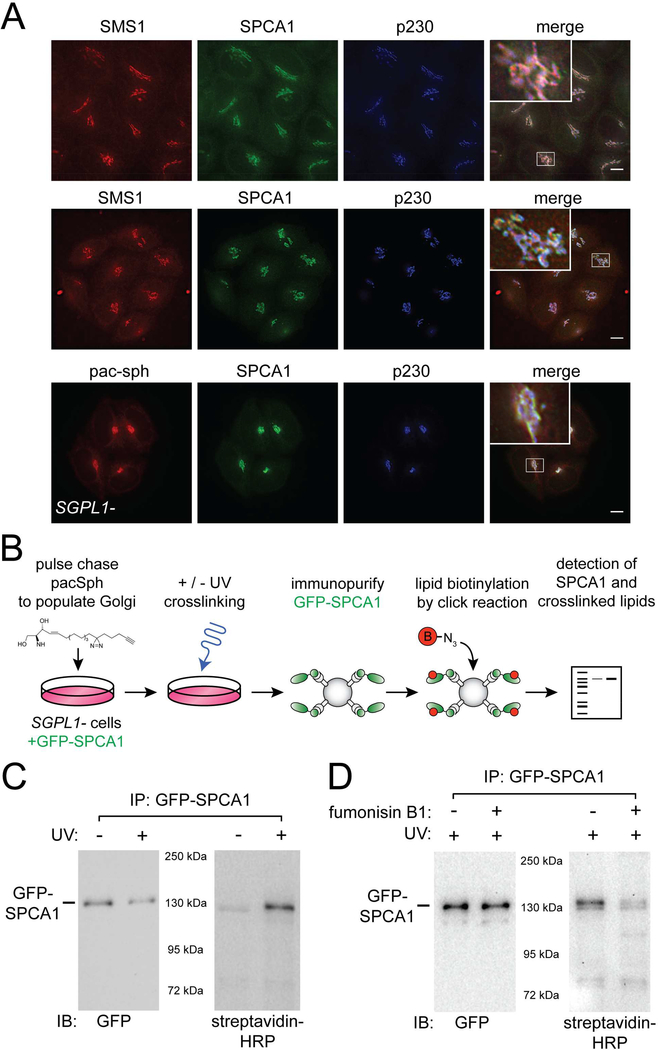
Figure 5. SPCA1 associates with sphingolipid in Golgi membrane.
(A) SMS1, SPCA1, and pacSph localize to the TGN. Antisera to SPCA1, p230 (TGN), or GM130 (cis Golgi) were used to detect each protein by immunofluorescence microscopy in gene edited HeLa cells. To detect endogenous SMS1, a SMS1-SNAP tag fusion protein (constructed by genome editing) was labeled with SNAP-Cell 647-SiR. To visualize sphingolipids in situ, sphingosine-1-phosphate lyase deficient (SGPL1-) HeLa cells were pulse labeled with 0.6 μM pacSph for 30 minutes, followed by a chase period of 1 hour. Fixed, permeabilized cells were incubated with click chemistry reagents to covalently attach Alexa647 fluorophore to pacSph. Cells were visualized by deconvolution fluorescence microscopy. Quantitative evaluation of co-localization was accomplished by determining Pearson’s correlation coefficients (Table S2). Scale bars, 10 μm. Insets in the merged images show a higher magnification view of the Golgi region. (B) Schematic diagram of protocol used to test for UV-induced crosslinking of SPCA1 and pacSph. (C) SPCA1 and pacSph can be crosslinked. The left panel is an anti-GFP immunoblot showing GFP-SPCA1 that was immunopurified from the UV treated and untreated samples. In the right hand blot, the same samples were probed with streptavidin-HRP to detect pacSph crosslinked to SPCA1. (D) An inhibitor of ceramide synthase, fumonisin B1, prevents crosslinking of SPCA1 and pacSph. Cells were incubated with fumonisin B1 (50 μM) for 24 hours prior to initiating the pulse labeling with pacSph. The left and right hand blots were processed as in panel C. See also Figure S4 and Table S2.
Co-localization of SMS1 and SPCA1 implies that SPCA1 resides in SM-rich membrane. We tested this first by directly visualizing SPCA1 and sphingolipids (Fig. 5A). To visualize sphingolipids in situ, we utilized a modified sphingolipid metabolic precursor, pac-sphingosine (pacSph), that contains a ‘clickable’ chemical bond that can be derivatized with a fluorescent moiety (Haberkant et al., 2016). A gene edited HeLa cell line lacking sphingosine 1-phosphate lyase (encoded by SGPL1) was used for these experiments to restrict metabolism of pacSph to the sphingolipid pathway (Gerl et al., 2016; Haberkant et al., 2016). In pilot experiments, a pulse-chase protocol was established which results in prominent localization of pacSph to the Golgi apparatus (Fig. S4A). In the time frame of these experiments (30 minute pulse, 60 minute chase), pacSph is incorporated into ceramide and SM, but not other sphingolipids (Gerl et al., 2016; Haberkant et al., 2016). Importantly, Golgi localization is prevented by inhibition of ceramide synthase with fumonisin B1, indicating that incorporation of pacSph into Golgi membrane requires its conversion to ceramide (Fig. S4B). These results demonstrate that SPCA1 and pacSph-labeled sphingolipids co-localize at the TGN (Fig. 5A).
To directly examine if SPCA1 and sphingolipids are associated in the Golgi, we took advantage of a diazirine ring incorporated into the fatty acyl chain of pacSph that can mediate lipid-protein crosslinking upon exposure to ultraviolet light (Gerl et al., 2016; Haberkant et al., 2016). After pulse labeling the Golgi of oxGFP-SPCA1 expressing cells with pacSph, the cells were exposed to ultraviolet (UV) light, solubilized with detergent, and oxGFP-SPCA1 was immunopurified (Fig. 5B, C). To determine if pacSph crosslinked to SPCA1, the purified material was subjected to click-mediated biotinylation of pacSph and crosslinked lipid was detected using streptavidin. The results show that UV exposure elicits crosslinking of pacSph to SPCA1. Importantly, inhibition of ceramide synthase with fumonisin B1, substantially eliminates crosslinking (Fig. 5D), indicating that SPCA1 is intimately associated with a complex sphingolipid, likely SM.
Golgi Ca2+ homeostasis is perturbed by SM depletion
Published results indicate that optimal activity of recombinant SPCA1 requires SM in its resident membrane (Chen et al., 2017). To determine if in vivo SPCA1 activity is influenced by a reduction in SM in the TGN membrane, we monitored Ca2+ influx into Golgi compartments of control and SM depleted (i.e., SMS1/2 siRNA) cells using an established TGN-localized fluorescence resonance energy transfer (FRET)–based Ca2+ sensor (Go-D1cpv) (Blume et al., 2011; Kienzle et al., 2014; Lissandron et al., 2010). Cells were depleted of Ca2+ using ionomycin and then Ca2+ was added and Go-D1cpv FRET signals were recorded. Fluorescence signals reflecting TGN [Ca2+] were normalized to ΔR/R0 where ΔR is the change in the ratio of YFP/CFP emission intensity at any time and R0 is the value obtained before addition of 2.2 mM Ca2+ at the first frame (Fig. 6A, B). Upon addition of Ca2+ (2.2 mM CaCl2) to the culture medium, control cells showed a 47.19 ± 7.34% increase in FRET signal. Depletion of SPCA1 by siRNA resulted in a reduced rate of calcium influx and a reduction of the maximal FRET value to 31.93 ± 5.38% (Fig. 6B, C). In SMS1/2 siRNA depleted cells, the rate of Ca2+ influx and maximal FRET value was reduced to 36.99 ± 5.44%. Re-expression of Golgi localized SM synthase 1 in SMS1/2 depleted cells not only restored the rate of Ca2+ influx, but resulted in a modest (7.74% FRET signal), reproducible increase in maximal FRET value to 54.93 ± 10.62%. In addition, ectopic overexpression of SPCA1 in SMS1/2 depleted cells restored Ca2+ influx to control levels (Fig. 6B, C), though it did not alter Ca2+ influx in control cells (Fig. S5A, B). The results show that depletion of SM impairs the TGN Ca2+ uptake mediated by SPCA1.
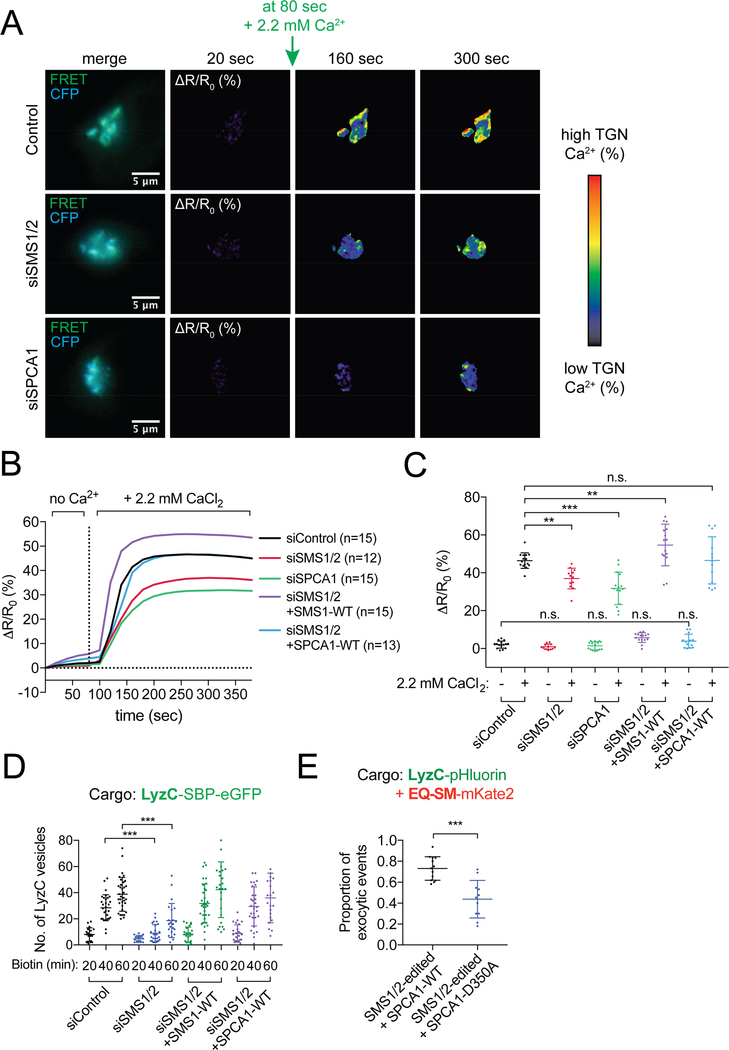
Figure 6. SM depletion inhibits Ca2+ influx into the TGN.
(A) Example time-lapse images of Golgi Ca2+ influx assays in control, siRNA SMS1/2 or SPCA1 depleted HeLa cells expressing the Go-D1-cpv Golgi Ca2+ sensor. Fluorescence micrographs of the FRET sensor in the TGN are shown in the left column. Cells were incubated with ionomycin to deplete Ca2+ from the lumen of the TGN and then live-cell ratiometric FRET microscopy was used to monitor Ca2+ influx by measuring the ΔR/R0 FRET ratio of YFP/CFP channels over time after addition of 2.2 mM CaCl2 to the cell medium, where R0 is the FRET ratio value obtained before addition of 2.2 mM CaCl2 (20 second time point). The color-coded ΔR/R0 heat map scale is shown on the right. Images are shown for representative cells 160 and 300 seconds after addition of CaCl2 at 80 sec. Scale bars, 5 μm. (B) Quantification of FRET images shown in A, as well as that of siRNA-treated cells expressing siRNA-insensitive SMS1 or SPCA1 cDNAs. Fluorescence signals reflecting TGN [Ca2+] are presented as ΔR/R0. Data are plotted as the mean Ca2+ influx over time. Data was acquired for at least 12 cells per condition in two independent experiments. (C) Data shown in B are plotted as the mean ± s.d Ca2+ influx before Ca2+ addition (at 80 sec) or after Ca2+ addition (at 300 sec). Data was acquired for at least 12 cells per condition in two independent experiments. (D) The number of LyzC vesicles was quantified in HeLa control or SMS1/2 siRNA depleted cells expressing either LyzC-SBP-eGFP alone, or co-transfected with SMS1-WT or SPCA1-WT. Vesicle counts from at least 18 cells per condition in 3 independent experiments are plotted. (E) TIRF microscopy-based sorting assays were used to determine the proportion of LyzC-pHluorin exocytic vesicles that also contained EQ-SM-mKate2 in SMS1/2edited cells that express either SPCA1-WT or SPCA1-D350A, a mutant that has no Ca2+ influx activity (means ± s.d. in 3 independent experiments; total of 201 events/13 cells for SPCA1-WT; total of 144 events/11 cells for SPCA1-D350A). See also Figure S5.
Rescue of Ca2+ influx in the Golgi of SMS1/2-depleted cells by overexpression of SPCA1 led us to determine if overexpression of SPCA1 impacts the deficiencies in cargo sorting and secretion of SM-depleted cells. We first monitored the number of Golgi-derived LyzC-containing vesicles in SMS1/2 depleted cells expressing either SPCA1 or as control, SMS1, using RUSH assays (Fig. 6D). Whereas 40 and 60 minutes after release of LyzC-SBP-eGFP from the ER, cytoplasmic vesicles counts were significantly decreased in SMS1/2-depleted compared to control siRNA cells, they were rescued to control level by overexpression of SPCA1 (Fig. 6D). Importantly, expression of siRNA-resistant SMS1 also rescues Golgi export of LyzC in these cells, demonstrating that synthesis of SM in the TGN sustains secretory cargo export from the TGN. TIRFM-based exocytosis assays (Fig. 6E) to evaluate co-sorting of LyzC-pHluorin and EQ-SM-mKate2 into exocytic vesicles in SMS1/2-edited cells showed that overexpression of SPCA1, but not SPCA1-D350A, a mutant defective in Ca2+ transport (Dode et al., 2005; Sorin et al., 1997) rescued cargo sorting into secretory vesicles. These results demonstrate that depletion of SM selectively impairs LyzC cargo sorting through reduced activity of SPCA1, impacting TGN Ca2+ homeostasis.
Discussion
Mechanisms for sorting and packaging soluble cargo proteins into transport vesicles within the secretory pathway typically involve transmembrane sorting receptors that contain a luminal domain that recognizes the cargo protein and a cytoplasmic domain capable of eliciting the recruitment of coat proteins that effect vesicle budding. At the TGN, this is best exemplified by the cation-independent mannose phosphate receptor that recognizes soluble lysosomal pro-enzymes decorated with mannose 6-phosphate and elicits their packaging into clathrin-coated vesicles that mediate TGN-to-endosome trafficking (Kornfeld and Mellman, 1989). Sorting of soluble secreted proteins, however, is far less well understood, especially in recognition of their structural diversity and lack of identified sorting motifs that might be recognized by an integral membrane sorting receptor.
One class of soluble secreted proteins relies on Cab45 for efficient secretion (Blume et al., 2012; Kienzle et al., 2014) and data presented here indicate that secretion of Cab45, and Cab45 clients, is mediated by a Golgi-derived carrier whose membrane is enriched in SM. In support of this conclusion, a subset of vesicles that emerge from the TGN contain Cab45 and the SM-binding protein, EQ-SM. Importantly, a similar proportion of vesicles contain LyzC and EQ-SM, confirming that these proteins are co-sorted in the Golgi into a common secretory carrier of the SMS pathway. Depletion or deletion of Cab45, SPCA1, or SMS1/2 all result in an accumulation of LyzC in the Golgi, though it is eventually exported from the TGN and secreted via vesicles that are not enriched with EQ-SM. Under these conditions, LyzC is secreted via vesicles that also contain a bulk secretion marker, EQ-sol, leading us to speculate that secretion by this alternative pathway is via non-specific, bulk flow secretion. Under control of SPCA1, Cab45 mediates a concentrative sorting step that increases the rate of secretion of its clients above that of bulk flow.
Prior work has shown that SPCA1-mediated influx of Ca2+ into the TGN is stimulated by recruitment of F-actin via cofilin1 to the P-domain of SPCA1 (Kienzle et al., 2014) and this study demonstrates that SM potentiates SPCA1-mediated Ca2+ influx (Fig. 6). Binding of calcium causes Cab45 to undergo Ca2+-dependent oligomerization and secretory client recognition (Crevenna et al., 2016). We find that SPCA1, SMS1, and sphingolipids populate the TGN and that SPCA1 Ca2+ pumping activity is promoted by maintenance a physiologic level of a physiologic level of SM in the TGN. At present, the mechanism by which SM-rich membrane facilitates SPCA1-mediated calcium pumping is unknown. Specific interactions between SM and the paddle domain of plasma membrane voltage-gated K+ channels are critical for voltage sensing (Combs et al., 2013; Milescu et al., 2009; Ramu et al., 2006; Xu et al., 2008) and it may be that SM acts as an agonist by binding to a site(s) on SPCA1 to activate Ca2+ pumping.
Consistent with this, a systematic survey of SPCA1 activity in reconstituted proteoliposomes of differing of lipid composition showed that SPCA1 activity is highest in vesicles containing SM (Chen et al., 2017). Local hotspots of SPCA1 activity, linked to synthesis and local enrichment of SM within the TGN membrane, will define TGN sorting domains and cargo exit sites.
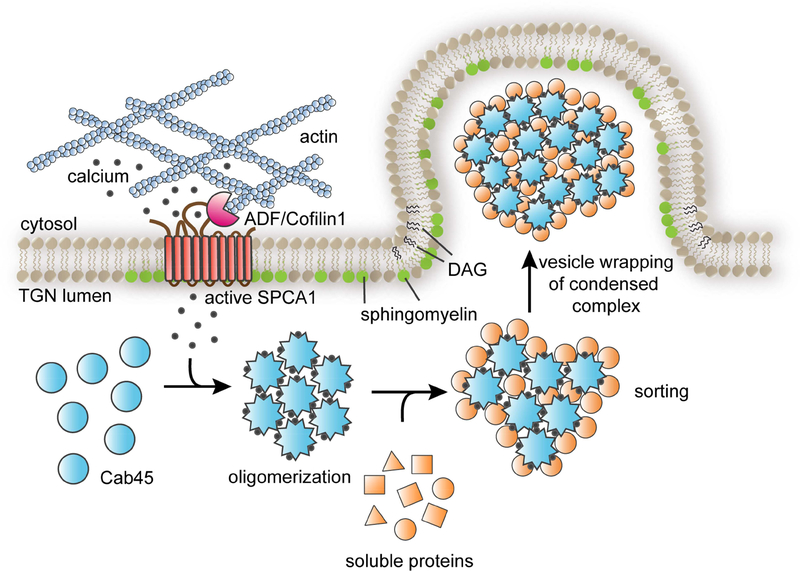
How is Cab45-mediated client sorting linked to the formation of a secretory carrier? Our findings suggest that a local increase in Ca2+ concentration within the lumen of the TGN drives Cab45 oligomerization and client capture through Cab45-client condensation from the bulk milieu (Fig. 7). Within the lumen of the TGN, Cab45 is concentrated within discrete regions that we speculate are sites of Cab45-mediated sorting and carrier formation (Crevenna et al., 2016). Surprisingly, we observed that sorting of LyzC into the SMS pathway is largely ablated by a modest (31%) decrease in cellular SM. This leads us to suggest that carrier formation is coupled to local SM synthesis by SMS1 which maintains a supply of membrane to sustain carrier formation at, and budding from, the TGN. Synthesis of SM produces an equivalent amount of diacylglycerol (DAG), a lipid that promotes negative membrane curvature of a bilayer, shown to be necessary for the formation and fission of a subset of secretory carriers from the TGN (Baron and Malhotra, 2002; Campelo and Malhotra, 2012; Litvak et al., 2005; Sarri et al., 2011). We speculate that chemical and physical coupling of SM synthesis, DAG production, Ca2+ influx, and capture of secretory cargo by Cab45 promotes engulfment of oligomeric Cab45-client complexes by TGN membrane, leading to vesicle budding (Fig. 7). As no integral membrane receptor(s) for Cab45 clients has been identified, we suggest the formation of secretory carriers of the SMS pathway carrying Cab45-client complexes resembles that of zymogen granule formation in pancreatic acinar cells, where the enzymes are proposed to form a “submembrane matrix” that deforms the membrane to induce budding of a secretory granule from the TGN (Dartsch et al., 1998; Schmidt et al., 2000). Thus, a common mechanism may be employed for the formation of secretory carriers containing different pools of proteins destined for secretion.
Figure 7. SPCA1 links sphingomyelin synthesis to Ca2+ dependent secretory protein and lipid sorting in the TGN.
The model depicts the events leading to the sorting and export of secretory cargo from the TGN, where the convergence of sphingomyelin synthesis and Ca2+ import into the lumen of the TGN drives the formation of a secretory carrier enriched in Cab45client complexes. Secretory cargo sorting is initiated by SPCA1-mediated Ca2+ influx, which is triggered by binding of ADF/cofilin1 to SPCA1 in the cytoplasm, where F-actin is associated with the TGN membrane. Synthesis of sphingomyelin in the TGN membrane potentiates SPCA1 mediated Ca2+ pumping in a region of the TGN membrane enriched in sphingomyelin. The local elevation of lumenal Ca2+ drives oligomerization of Cab45, which binds to soluble secretory protein clients, condensing them from the bulk milieu. The second product of SM synthesis, diacylglycerol (DAG), promotes engulfment of Cab45-client complexes by generating negative membrane curvature, leading to the formation of a secretory carrier enriched in oligomeric Cab45-client complexes.
New questions regarding spatiotemporal coupling of the events that lead to cargo capture and sorting in the SMS pathway are raised by the findings presented here. A critical issue regards how secretory load is sensed and coupled to SPCA1-mediated Ca2+ influx, which is stimulated by ADF/Cofilin and F-actin in the cytoplasm. Related, synthesis of SM in the Golgi is promoted by non-vesicular transport of ceramide and cholesterol (Ngo and Ridgway, 2009; Parmar and Duncan, 2016; Wakana et al., 2015) at ER-TGN contact sites, so it is of interest to determine the if the formation of carriers of the SMS pathway are chemically and physically coupled to the non-vesicular ceramide transport machinery. Intriguingly, siRNA-mediated depletion of components of the non-vesicular ER-to-TGN transport machinery perturbs glycosylation of secretory cargo in the Golgi and slows the rate of secretion (Wakana et al., 2015). Finally, the mechanism by which, and location where, Cab45-client complexes are dissociated, and if Cab45 is then retrieved to the TGN for re-use, will be essential to elucidate. The conceptual framework that emerges from our findings, along with new methods developed, present new opportunities for understanding the core function of the Golgi apparatus as a macromolecular trafficking hub within the cell.
STAR methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by Lead Contact, Julia von Blume (vonblume@biochem.mpg.de).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
HeLa cells were maintained in 5% CO2 at 37°C in DMEM supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, United States). T-REx™-HeLa Cells (Thermo Fisher Scientific) were grown in 15 ug/ml blasticidin and 200 ug/ml zeocin. After transfecting with pcDNA5-EQ-SM/sol-APEX2, zeocin was replaced with 200 ug/ml hygromycin.
METHOD DETAILS
DNA manipulations
APEX2 plasmid was acquired from Addgene (pcDNA3 APEX2-NES). APEX2 was PCR amplified and linked with EQ-SM/sol-Flag and inserted into pcDNA™5/FRT Vector (Invitrogen™).
LyzC-pHluorin was cloned by inserting LyzC into N1-pHluorin. SMS1-SNAP-tag repair template was generated by PCR amplified SMS1 left arm and right arm (around stop codon) from HeLa genomic DNA, linked with Flag-SNAP-tag and inserted into vector pBluescript II SK (+). LyzC-mCherry was cloned by amplifying LyzC-3xFlag and inserted N-terminally by EcoRI and BamHI sites into a pLPCX-mCherry vector.
Generation of plasmids that direct expression of WT and mutant Cab45 proteins and SPCA1-HA in pLPCX is described in (Blume et al., 2012).
For expression of His-SUMO-tagged Cab45 in SF9 cells, Cab45 cDNA was amplified from pLPCX-Cab45-WT or EFh mutant plasmid and inserted into pI-Insect Secretory SUMOstar Vector (3106; Life Sensors) as described previously (Crevenna et al., 2016).
The RUSH vector pIRESneo3 Str-KDEL_ST-SBP-eGFP was a gift from Franck Perez (Addgene plasmid # 65264). The ST region was replaced by amplifying LyzC-Flag (Blume et al., 2011) and insertion into the pIRESneo3 backbone by AscI and EcoRI cutting sites. To generate the SS-SBP-eGFP-Cathepsin D construct, first the Str-KDEL_SS-SBP-eGFP construct was generated by amplifying human Growth Hormone (hGH) signal peptide to insert it into the pIRESneo3 backbone by AscI and EcoRI cutting sites. In the second step, HA-Cathepsin D was C-terminally fused to SS-SBP-eGFP by overlap and extension PCR and inserted by AscI and XhoI into the pIRESneo3 vector. The SS-SBP-tagRFP-Cathepsin D construct was cloned by amplifying SS-SBP, tagRFP, Cathepsin D and the fragments were inserted by Gibson Assembly (Gibson, 2009) into the pIRESneo3 RUSH vector that was linearized with AscI and XhoI. The Go-D1cpv (Lissandron et al., 2010) construct was cloned into pLPCX by restriction digestion with HindIII and EcoRI. eGFP-Cab45 and eGFP-Cab45–6EQ were cloned by amplifying eGFP and inserted after the signal sequence by using HindIII and EcoRI sites into either pLPCX-SS-Cab45 or pLPCX-SS-Cab45–6EQ mutant vector.
A siRNA resistant SMS1-HA variant was generated by mutating the siRNA binding site 5’ – gacggcagcttcagcatcaagatta - 3’ to 5’ –gaTggAagTttTagTatAaaAatCa - 3’. For CRISPR/Cas9 targeting guide RNA oligos and their complementary sequences were cloned with suitable overhangs into a pX459 2.0 mammalian expression vector (Addgene plasmid # 62988) as described in (Ran et al., 2013). DNA oligonucleotides encoding CRISPR/Cas9 gRNA sequences were synthesized by Yale Keck Oligonucleotide Synthesis facility or by metabion international AG (Planegg, Germany). Oligonucleotides were annealed and cloned into pX459 2.0. The sequences of plasmids were confirmed by DNA sequencing.
Cab45-gRNA1: TTCTGATGGACGCGTCTGCA
Cab45-gRNA2: TTGATGAGGACGCGGAGCCG
SMS1-gRNA1: GAGGAGACTCGGCACAAGGG
SMS1-gRNA2: GGACTTGATCAACCTAACCC
SMS2-gRNA1: CGTCTTGACAACCGTCATGA
SMS2-gRNA2: AAAAGTACCCGGACTATATC
SMS1-tagging-gRNA: CATAACAGCTGTACAAAGTG
SGPL1-gRNA1: TGTGAAAGCTTTACCCTCCC
SGPL1-gRNA2: CTTAAGGAGTACAGCTCTAT
Antisera
Anti-FLAG (Sigma, F1804), anti-HA (rat monoclonal, 11867423001, Roche), anti-GalT (Abcam, ab178406), anti-Calnexin (Millipore, clone C8.B6), anti-SPCA1 (mouse monoclonal, H00027032-M01, Abnova), anti-p230 (mouse monoclonal, 611281, BD Biosciences), antiTGN46 (sheep polyclonal, AHP500G, AbD Serotec), anti-β-actin (mouse monoclonal, A5441, Sigma-Aldrich), anti-FLAG M2-Peroxidase (mouse monoclonal, A8592, Sigma-Aldrich). The anti-SPCA1 (rabbit polyclonal) antibody was a gift from Peter Vangheluwe, Department of Cellular and Molecular Medicine, University of Leuven, Belgium. Alexa Fluor Cross-Adsorbed secondary antibodies for immunofluorescence were purchased from Thermo Fisher Scientific (A-11034, A-11035, A-11029, A-11030, A-21209, A-21208, A-21203). Secondary anti-rabbit HRP conjugated antibodies were purchased from Thermo Fisher Scientific (32260), m-IgGκ BPHRP Antibody was from Santa Cruz Biotechnology (sc-516102).
For Cab45 antibody generation was performed by the immunization service by the animal facility of the Max Planck Institute of Biochemistry. Recombinant Cab45 full length protein (His Sumo tagged) was prepared in TiterMax Gold Adjuvant liquid (Sigma-Aldrich) according to the manufacturer’s protocol. Rabbits were injected and boosted three times before collecting serums Sera were stirred while incubated at room temperature for one hour and then at 4°C overnight. After centrifugation for 30 min at 5000 x g, collected supernatants were stored at – 20°C.
Cell culture and engineering
HeLa cells were maintained in 5% CO2 at 37°C in DMEM supplemented with 10% fetal bovine serum (Gbco, Grand Island, NY, United States). Cells were transfected with FuGENE HD (Promega), Lipofectamine 2000 (Thermo Fisher Scientific), 1.25 mg/ml Polyethylenimine (PEI) linear (Alfa Aesar) or Lipofectamine LTX PLUS (Thermo Fisher Scientific). To generate cell lines stably expressing Cab45-WT and Cab45–6EQ mutant VSV-G pseudotyped retroviral vectors were produced by transient transfection of HEK293T (human embryonic kidney) cells. To harvest virus particles, cell culture supernatants were filtered through 0.45-μm filters. The virus particles were then concentrated from cell culture supernatants by spinning at 68.000 x g for 2h, followed by a second spin at 59.000 x g for 2.5h at RT. The pellet was resuspended in 200 μl of HBSS (Pfeifer et al., 2000) and used for infection.
Stable cell lines expressing EQ-SM-APEX2 or EQ-sol-APEX2 were engineered as follows: HeLa T-Rex (Thermo Fisher Scientific) cells were transfected with pcDNA5/FRT-EQ-SM/solFlag-APEX2 and the recombinase pOG44. After two days, 200 ug/ml hygromycin and 15 ug/ml blasticidin was added to the medium and the cells were cultured for an additional for 10 days and then expanded.
For gene editing cell lines using CRISPR/Cas9, HeLa cells were transfected with two gRNA against the same gene. For SMS1-SNAP-tag CRISPR cells, HeLa cells were transfected with one gRNA and a repair template that has Flag and SNAP-tag. For selection, 24 h post-transfection cells were selected for 48 h in 2 μg/ml puromycin. 100 cells were then seeded in 15 cm culture dishes and cultured until single cell colonies were big enough to manually scrap them off the dish and transfer them to 96-well plates. Single clones were expanded and screened by PCR and Western Blotting. PCR amplification from the genomic DNA and gel electrophoresis were used to assess proper cleavage or tagging of the target sequences. The PCR products were confirmed by sequencing. For Cab45 KO and SMS1-SNAP-tag, protein levels were confirmed by Western Blotting.
siRNA silencing
For siRNA gene silencing following oligos were used: Cab45: 5’ –
GAGCCAGGACCUCACUUCCUCCUCU - 3’; SMS1: 5’ –
GACGGCAGCUUCAGCAUCAAGAUUA - 3’; SMS2: 5’- UCAAUAGUGGGACGCAGAUUCUGUU - 3’; SPCA1: 5’–
CAUCGAGAAGUAACAUUGCCUUUAU - 3’ and a scrambled negative control from Invitrogen.
The siRNA transfection mix was prepared by adding 20 nM siRNA and 12 μl HiPerFect
Transfection Reagent (Qiagen) to 100 μl Opti-MEM reduced serum medium (Gibco by Life Technologies) and incubated 15 min at RT. The transfection mix was added to HeLa cells seeded on glass slides in either 6-wells or live cell μ-dishes (μ-Dish 35 mm, high Glass Bottom from ibidi). Cells were processed for further analysis after 48h for Cab45 and 72h for SMS1/2 silencing.
Vesicle budding and APEX2-mediated biotinylation
To monitor budding of vesicles containing EQ-SM-APEX2 or EQ-sol-APEX2 in permeabilized cells, cells lines that stably express two 10-cm dishes of cell lines that stably express EQ-SM-APEX2 or EQ-sol-APEX2 were treated with 2 ug/ml tetracycline overnight to induce expression of EQ-SM/sol-APEX2 proteins. The next day, cells were trypsinized and harvested by centrifugation at 1000 g for 3 min. The cell pellet was washed with buffer A (20 mM Hepes, pH 7.4, 250 mM D-sorbitol, and150 mM potassium acetate) and digitonin (40 μg/ml) was added and incubated for 5 minutes on ice. Cells were washed one time with buffer A, resuspended in buffer A, and the mixture was then aliquoted into four tubes. The cells were incubated either with or without ATP regenerating system (1 mM ATP, 40 mM creatine phosphate, 0.2 mg/ml creatine phosphokinase and 0.1 mM GTP), with or without rat liver cytosol (1 mg/ml; Thermo Fisher Scientific), or incubated at 4°C or 32°C for 45 min. The reactions were centrifuged at 10,000 g for 10 minutes and the supernatant was collected and centrifuged at 100,000 g for 1 hour to collect vesicular material. The pelleted material was dissolved in SDSPAGE loading dye and analyzed by SDS-PAGE. Streptavidin-HRP (Thermo Scientific Pierce, PI-21130) was used to detect biotinylated proteins.
For proteomic analyses of vesicle fractions, EQ-SM/sol-APEX2 stable cells from ten 15 cm dishes were processed as described above, except that biotinylation reations were carried on S10 fractions. To do so, biotin-phenol (500 μM) was added to the reactions and incubated for 20 min at 32°C. Next, H 2O2 (1 mM) was added and the reactions were tumbled for 1 minute at room temperature. The reaction was stopped by adding Trolox (1 mM) and sodium ascorbate (10 mM). The reactions were centrifuged at 10,000 g for 5 min and the supernatant was removed and then centrifuged at 100,000 g for 1 hour to collect vesicular material. The vesicle pellets were washed once with buffer A containing Trolox (1 mM) and sodium ascorbate (10 mM) to remove the biotin and recentrifuged at 100,000 for 30 min.
Purification of biotinylated proteins was accomplished by affinity selection using NeutrAvidin beads. Pelleted vesicular material was solubilized in 1 ml RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 1% Triton X-100 PH 7.5, 0.5% sodium deoxycholate, 10 mM sodium ascorbate, 1 mM Trolox, 2Xprotease inhibitor) and sonicated at 40W for 2 min. The solution was centrifuged at 13,000 g for 15 min and the supernatant was recovered and then incubated with 100 μl NeutrAvidin beads for 6 hours at 4°C. The beads were washed with RIPA lysis buffer twice, once with 0.5 mL of 1 M KCl, once with 0.5 mL of 0.1 M Na2CO3, once with 0.5 mL of 2 M urea in 10 mM Tris-HCl pH 8.0, and three times with RIPA buffer. Purified proteins were eluted by boiling the beads in 100 μl 2× protein loading buffer for 5 min. Purified material was separated by SDS-PAGE on a 4–20% Mini-PROTEAN® TGX™ Precast Protein Gel (Bio-Rad). After staining with silver (Pierce™ Silver Stain Kit (24612), each lane was cut into five equal sized pieces and mass spectrometry was used to identify proteins in each gel piece. Mass spectrometry was done by the Yale Keck Biotechnology Resources Laboratory.
Protein detection by immunoblotting, silver staining or immunofluorescence
For immunoblotting, proteins were transferred to nitrocellulose membrane and then blocked in PBS-T with 5% milk or in TBS-T with 5% BSA. After immunoblotting, proteins were detected by chemiluminescence. Images of immunoblots were acquired by ChemiDoc Imaging System (Bio-Rad).
For immunostaining, cells were cultured on glass slides, fixed for 10 min with 4% paraformaldehyde, washed with PBS and subsequently permeabilized for 5 min in 0.2% Triton-X 100 and 0.5% SDS in 4% BSA solution. After washing with PBS and blocking of slides for 1 h in 4% BSA, cells were incubated with primary and secondary antibody for 1 h at room temperature in blocking buffer in the dark. Glass slides were mounted with ProLong Gold (Thermo Scientific). For immunofluorescence after pac-sphingosine labeled cells, saponin was used to permeabilize cells.
Lipidomics
One day after plating, cells from one 10 cm dish were collected by scraping, and washed twice with PBS. An aliquot was removed for protein quantification and the remainder was sent to the Virginia Commonwealth University Lipidomics/Metabolomics Core for mass spectrometry based sphingolipid analysis. Lipid levels were normalized to protein content.
Fluorescence microscopy and image analysis
For live cell deconvolution fluorescence microscopy, cells were washed twice with PBS, and the medium was replaced with live cell imaging solution (Molecular Probes) supplemented with 10 mM glucose. 3D image stacks were collected at 0.3 μm z increments on a DeltaVision Elite workstation (Applied Precision) based on an inverted microscope (IX-70; Olympus) using a 60×, 1.4 NA oil immersion lens. Images were captured with a sCMOS camera (CoolSnap HQ; Photometrics) and deconvolved with softWoRx (v.6.0) software using the iterative-constrained algorithm and the measured point spread function. Golgi budding experiments of living cells were performed at the widefield of the Imaging Facility of the Max Planck Institute of Biochemistry (MPIB-IF) on a GE Healthcare DeltaVision Elite system based on an OLYMPUS IX-71 inverted microscope, an OLYMPUS 60x/1.42 PLAPON oil objective and a PCO sCMOS 5.5 camera. Budding events were scored manually from time lapse series. Quantitative co-localization analyses were done using softWoRx (v.6.0) software.
Analysis of vesicle numbers was carried out by a custom-made ImageJ macro. The macro uses ImageJ’s rolling ball background subtraction algorithm, the enhance contrast function and a maximum z-projection of the RUSH reporter channel to cover all vesicles of the cell volume in a 2D image. After using a median filter, suitable cells were selected by drawing a polygon selection. A binary image was generated by the Threshold function. The threshold algorithm “Yen” was used by default while for low intensity images the threshold required manual correction. The vesicle objects in the binary images were compared and controlled by visual inspection with the original image. In the binary image, vesicle objects with sizes ranging from 420 pixels were then quantified by Analyze Particles function.
Confocal fluorescence microscopy of fixed cells was performed using a confocal laser-scanning microscope (LSM 780; Carl Zeiss) with a 40x/1.4 Plan-Apochromat oil or 100x/1.46 oil α-Plan-Apochromat oil objective lens. For detection of Alexa Fluor, the 488 nm laser line was used. Pictures were acquired using Leica software (ZEN 2010) and adjusted in ImageJ (version 1.51u).
Total internal reflection microscopy was done using a microscope (IX-70; Olympus) equipped with argon (488 nm) and argon/krypton (568 nm) laser lines, a TIRFM condenser (Olympus or custom condenser), a 60× 1.45 NA oil immersion objective lens (Olympus), and an EMCCD camera (iXon887; 0.18 μm per pixel, 16 bits; Andor Technology). The TIRFM system was controlled by iQ software (Andor Technology). HeLa cells were grown in MatTek dishes and imaged 16–20 h after transfection. All experiments were done at 37°C in live cell imaging solution (Molecular Probes) containing 10 mM glucose, pH 7.4. Cells were imaged in one channel at 5 Hz or two channels by sequential excitation at 2 Hz.
Analysis of TIRF images was done as described in Deng et al (Deng et al., 2016). Briefly, each image stack was manually reviewed to identify putative vesicle fusion events that released pHluorin. The coordinates of the fusion events were labeled and a small region of interest around each exocytic event in each channel was used for further analysis. Circular regions with diameters of 4 pixels were used to calculate the intensity of a single vesicle. Colocalization of proteins in the same vesicle was determined manually based on the coincident appearance and release of fluorescence signals by each fusion protein. No nonlinear adjustments were made to alter fluorescent signals.
RUSH cargo sorting assay using confocal microscopy
HeLa cells were cultured on sterile glass slides in 6-wells and gene silenced as described above. Cells were transfected using either pIRESneo3-LyzC-SBP-eGFP or pIRESneo3-SSSBP-eGFP-Cathepsin D for 16h. Cells were incubated with 40 μM d-Biotin (SUPELCO) in DMEM for 20, 40 and 60 min and as a control without d-Biotin to confirm the retention of the reporter. Cells were then washed once in 1x PBS and fixed in 4% PFA in PBS for 10 min and further processed for immunofluorescence microscopy as described above. Samples were acquired using a confocal laser-scanning microscope (LSM 780; Carl Zeiss). Only cells were processed that showed proper transport of the reporter to the Golgi after Biotin addition while cells showing ER signal after Biotin addition were discarded from the analysis. To cover the whole volume of the cells, typically 8–16 z-stacks with a step-size of 0.35 μm were acquired of each field of view.
RUSH cargo sorting assay using western blot analysis
HeLa cells were cultured in 6-wells and gene silenced as described above. Cells were transfected with pIRESneo3-LyzC-SBP-eGFP for 16h. Cells were washed 3x in PBS followed by 3x with DMEM w/o FCS. Cells were incubated with or without 1 mL 40 μM d-Biotin for 60 min in DMEM w/o FCS. Cell culture supernatants were collected and centrifuged for 5 min at 1,000 x g to pellet residual cells. Supernatants were concentrated by centrifugation at 16,000 x g for 8 min using Centrifugal Filters (Amicon Ultra, Ultracel 10K). Cell pellets were washed 3 times with PBS, trypsinized and transferred to tubes. Cells were then washed PBS and lysed with 1% TritonX-100 in 1 time with PBS. Concentrated supernatants and lysed cell pellets were further processed for SDS-PAGE and Western Blot analysis. Western Blot quantification from three independent experiments was performed by using Image Lab Version 5.2.1 build 11 (Bio-Rad) software. Supernatant signals were normalized to each corresponding actin signal of the cell pellet to correct for protein levels. Rates of secretion for each condition were determined by calculating the ratio of 60 min to 0 min LyzC-SBP-eGFP supernatant signals and were normalized to 100% of control cells and plotted as a bar graph.
In vivo Golgi vesicle budding assays
HeLa cells seeded in live cell μ-dishes were transfected with EQ-SM-mKate2 together with either eGFP-Cab45 or eGFP-Cab45–6EQ for 16h. Cells were acquired by live-cell widefield microscopy and focused at the Golgi region. Dual-channel acquisition for eGFP and mCherry was performed at 1 sec intervals for 100 frames. The time-lapse movies were analyzed by identifying each eGFP-Cab45 or GFP-Cab45–6EQ budding event from the Golgi. Cab45 budding events were then scored whether or not EQ-SM-mKate2 was also present. At least 26 budding events from 4 cells for eGFP-Cab45 and 32 budding events from 13 cells for eGFP-Cab45–6EQ were analyzed. The ratios of Cab45 budding with or without EQ-SM were calculated to total budding events. The average and SD of the ratios were plotted as a bar graph.
Pac-sphingosine labeling
SGPL1 null HeLa cells were incubated with 0.5 μM pacSph (Avanti, 900600) in DMEM/delipidated FBS (pre-warmed to 37°C) for 30 mi nutes at 37°C. the medium was then removed and the cells were three times with DMEM/delipidated FBS. Cells were then incubated in DMEM/FBS for 1 hour at 37°C. Cells were washed with PBS three times and the medium was replaced with 4% paraformaldehyde in PBS and incubated for 20 minutes. Cells were washed with PBS three times and then incubated with permeabilization and blocking Buffer (0.1% saponin with 5% serum in PBS) for 1 hour. For immunofluorescence detection of proteins in these cells, primary and secondary antibodies were added. Cells were then washed with PBS three times. A Click-iT™ Cell Reaction Buffer kit was used to click label pacSph according to the manufacture’s instructions (Thermo Fisher Scientific, C10269). After the reaction, cells were washed 5 times with PBS and then visualized by fluorescence microscopy.
Pac-sphingosine crosslinking
SGPL1 null HeLa cells were transfected with 25 μg oxGFP-SPCA1 and lipofectamine for 18 hours prior to labeling with pacSph (0.5 μM) for 30min and then chased in DMEM/FBS for 1 hour. Two 10-cm dishes cells were subjected to 365 nm UV (8W) (or kept in the dark, as control) for 10 min. The cells were collected and washed with PBS. The cell pellets were resuspended in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton, protein inhibitor) for 1 hour and then sonicated (with tip sonicator) for 30 seconds. The lysate was centrifuged for 30 min at 13,000 x g. The supernatant was collected and incubated with 15 μl GFP-Trap beads (gtma-20, ChromoTek GmbH) for 1 hour. The beads were washed once with wash buffer (20 mM Tris, pH 7.5, 150 mM NaCl) and then incubated with Click reagents and azide-biotin (Thermo Fisher Scientific) for 1 hour. The beads were washed twice with wash buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Triton). 30 μl SDS loading buffer was used to elute from the beads. 25 μM Fumonisin B1 (Sigma) was added to the cells when transfected and in the pulse- chase experiments.
Ca2+ influx assay
Ca2+ entry into the TGN was measured as described previously (Lissandron et al., 2010). Measurements of [Ca2+] in the TGN were performed using a fluorescent Ca2+ sensor (Go-D1-cpv) expressed as a fusion to sialyltransferase which targets to the TGN. Changes in Ca2+ concentration are observed as changes in the efficiency of FRET between CFP and YFP fluorescent proteins linked by a modified calmodulin and calmodulin-binding domain. HeLa cells were transfected with control, SPCA1, or SMS1/2 siRNAs for 72 h. Then Go-D1-cpv was transfected alone, or with siRNA resistant SMS1-HA, or SPCA1-HA. Ca2+ entry into the TGN was measured in Ca2+-depleted cells after 1 h of incubation at 4°C in H BSS (20 mM Hepes, Ca2+/Mg2+-free HBSS [Gibco by Life Technologies], 2 gl−1 glucose, 490 μM MgCl2, and 450 μM MgSO4; 300 mOsmol/liter, pH 7.4) with 1 μM ionomycin (Abcam) and 0.5 mM EGTA (von Blume 2011). Then cells were washed twice in HBSS + 0.5 mM EGTA followed by three washes in HBSS only. Image acquisition was performed on a GE DeltaVision Elite as described above. To generate the images, the excitation filter (430/24), dual-band Sedat CFP/YFP beam splitter (Chroma Technology Corp.), and the emission filters (535/25 for FRET and 470/24 for CFP) were rapidly changed using an external filter wheel controlled by a motorized unit. Fluorescent signals reflecting TGN [Ca2+] were presented as ΔR/R0, where R0 is the value obtained before addition of 2.2 mM CaCl2 to the cell’s bathing solution. Images were acquired using softWoRx 5.5 software (GE Healthcare). Image analysis was carried out by a custom-made ImageJ macro and is based on ratiometric FRET analysis described previously (Kardash et al., 2011; Kienzle et al., 2014). The macro uses ImageJ’s rolling ball background subtraction algorithm followed by a mean filter to smooth the edges of the objects. A binary image was generated by the Auto Threshold function using the “Moments” algorithm. The images of the FRET and CFP channel were multiplied by the “ImageCalculator” plug-in with their respective binary images resulting in images that show 0 intensities outside of the threshold Golgi region while retaining intensities within the Golgi. Next, a ratio image of FRET/CFP was generated by using the “Ratio Plus” plug-in. The Golgi objects were detected by using the “Find Maxima” function and added to the ROI manager. The mean intensities of each ROI were then measured in the ratio image for each frame. The ratio values of each frame were subtracted to the first frame. These values were normalized to the first frame and presented as percentage ΔR/R0 to obtain normalized ratio values before the addition of CaCl2. A pool of ~35% of SMS1/2 knock down cells that did not show a Ca2+ influx defect due to knock down variability of two independent genes, were discarded. Samples that showed over 15% signal reduction of the maximum amplitude value compared to the last frame were discarded due to high photo bleaching.
Quantitative real-time PCR
HeLa cells seeded in 6-wells were transfected with control or with SMS1/2 siRNA as described above. RNA was isolated from cells using the RNeasy Mini Kit (Qiagen). 1 μg RNA was used for the reverse transcription reaction using the iScript cDNA Synthesis kit (Bio-Rad). Quantitative PCR reaction were performed with the LightCycler 480 II (Roche) using iQ SYBR green Supermix (Bio-Rad) with following primers that were described previously (van Galen et al., 2014): SMS1: 5’ – ACTGTGAGCCTCTGGAGCAT - 3’ and 5’ –TGCTCCATTTTCAGGGTTTC - 3’; SMS2: 5’ – CAATTCCTTGCTGCTTCTCC - 3’ and 5’ – CCTTTGTTTTGCTCCTCAG - 3’; GAPDH: 5’ – TGCACCACCAACTGCTTAGC - 3’ and 5’ – GGCATGGACTGTGGTCATGAG - 3’. The Ct values of control and siRNA transfected samples of the experimental genes SMS1 and SMS2 were subtracted from the housekeeping gene GAPDH to obtain the ΔCt values. The ΔCt value of the siRNA transfected sample was then subtracted from the control value to obtain the ΔΔCt values. Next, by calculating 2-ΔΔCt, the relative expression fold change was determined for each replicate and plotted at as a bar graph.
Purification of recombinant proteins and labeling
His-SUMO tagged Cab45 was expressed in SF9 cells with pI-secSUMOstar plasmid and purified from cell supernatants with nickel-based affinity chromatography using a NaP pH 8.0, 500 mM NaCl buffer and cOmplete His-tag Purification Resin (Roche). Following elution by 250 mM imidazole, proteins were dialyzed to 20 mM PIPES, pH 6.8, 500 mM NaCl, 10% Glycerol for storage.
Recombinant Cab45 labeling with NHS-Atto488 (Sigma Aldrich) was performed according to manufacturer’s instructions.
Cab45 oligomerization assay
For oligomerization assays recombinant Cab45 proteins and EFh mutants were thawed on ice and centrifuged 15 min at 13.200 rpm at 4 °C to remove aggregates. Protein concentrations were adjusted to 2.5 μM in PBS pH 7.4. For measurements proteins were diluted 1:100 in a total volume of 100 μl in PBS pH 7.4 and analyzed in Lab-Tek 8 Chamber #1.0 borosilicate coverglass system (Nunc, Rochester, USA) with a LSM780 confocal microscope as described above under the indicated conditions.
Circular dichroism spectroscopy
Circular dichroism spectroscopy (CD) measurements were performed in a 1-mm (path length) cuvette at 10 °C on a JASCO J-715 spectrome ter. Protein samples (0.2 mg/ml) were dissolved in CD buffer (20 mM PIPES pH 6.8, 50 mM NaCl) and the indicated amounts of Ca2+ were added before spectra were recorded. An average of 10 (± Ca2+ analysis) independent spectra (from 195 to 250 nm with 0.1 nm spacing) were documented. Data was normalized to molecular elipticity of protein and FFT filter was applied.
QUANTIFICATION AND STATISTICAL ANALYSIS
For statistical evaluation GraphPad Prism version 7.0b for Mac OS X (GraphPad Software, La Jolla California USA) was used. RUSH cargo sorting assays were analyzed by using a non-parametric Kruskal-Wallis test followed by Dunn’s multiple comparisons test. TIRF exocytosis assays were analyzed using Student’s unpaired t-test. In vitro vesicle budding data were analyzed using a ratio paired t-test. The following P-value style was used: ≤ 0.05 (*), ≤ 0.01 (**), ≤ 0.001 (***).
Supplementary Material
Table S1. Identification of native protein cargos of the SMS pathway, Related to Figure 1. Mass spectrometry proteomics analysis list containing candidate cargo proteins of the SMS pathway that were defined as proteins containing a signal sequence, a transmembrane domain, or a GPI anchor, that were identified in EQ-SM-APEX2 vesicle fractions, but not in EQ-sol-APEX2 vesicle fractions, in each of two independent vesicle preparations.
Table S3. List of oligonucleotides used for genomic validation, cloning and siRNA targeting, Related to STAR Methods.
Highlights.
Golgi membrane sphingomyelin stimulates SPCA1 Ca2+ ATPase
Ca2+ binding by Cab45 causes it to oligomerize in the lumen of the TGN
Oligomeric Cab45 condenses secreted protein clients within the luminal milieu • Cab45 clients are secreted via vesicles enriched in sphingomyelin
Acknowledgements
We are grateful to Andreas Ernst for advice with pacSph labeling, Michael Caplan and Zhe Lu for assistance interpreting the Ca2+ influx assays, Peter Vangheluwe for SPCA1 antiserum, and Franck Perez for RUSH plasmids. Research reported in this publication was supported by the following sources: the National Institute of General Medical Sciences of the United States National Institutes of Health under award numbers GM095766 and GM060221 to C.G.B., award number T32GM007223 to E.S., the Perspective Program (Boehringer Ingelheim Foundation), CRC914 (TP A09), and a project grant (BL 1186/4-1) from the Deutsche Forschungsgemeinschaft (DFG) to J.v.B., and by the Max Planck Institute of Biochemistry and by the department led by Reinhard Fässler. Proteomic analyses were provided by the W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University. Lipidomic analyses were provided by the Virginia Commonwealth University (VCU) Lipidomics/Metabolomics Core, which is supported by the NIH-NCI Cancer Center Support Grant P30 CA016059 to the VCU Massey Cancer Center, as well as a shared resource grant (S10RR031535) from the US
National Institutes of Health.
Abbreviations List:
- PM
plasma membrane
- SM
sphingomyelin
- ER
endoplasmic reticulum
- SPCA1
Secretory Pathway Ca2+ ATPase 1
- TGN
trans Golgi network
- GPI
glycosylphosphatidylinisotol
- EQ-SM
non-toxic sphingomyelin reporter protein
- EQ-sol
nontoxic sphingomyelin reporter mutant that can no longer bind to sphingomyelin
- APEX2
engineered ascorbate peroxidase 2
- FITC
Fluorescein isothiocyanate
- GalT
galactose transferase T1
- ATP
adenosine triphosphate
- LyzC
Lysozyme C
- COMP
cartilage oligomeric matrix protein
- eGFP
enhanced green fluorescent protein
- Cab45
45 kDa Ca2+-binding protein
- mut
mutant
- RUSH
retention using selective hooks
- SBP
Streptavidin binding peptide
- TIRF
total internal reflection fluorescence
- SMS
sphingomyelin synthase
- pacSph
pac-sphingosine
- SGPL1
sphingosine 1-phosphate lyase
- FRET
Förster resonance energy transfer
- SR
sarcoplasmic reticulum
- SERCA
sarcoendoplasmic reticulum Ca2+ transport ATPase
- WT
wild-type
- DAG
diacylglycerol
Footnotes
Declaration of Interest:
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ang SF, Fölsch H, 2012. The role of secretory and endocytic pathways in the maintenance of cell polarity. Essays Biochem. 53, 29–39. doi:10.1042/bse0530029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitei M, Hoflack B, 2011. Exit from the trans-Golgi network: from molecules to mechanisms. Curr. Opin. Cell Biol 23, 443–451. doi:10.1016/j.ceb.2011.03.013 [DOI] [PubMed] [Google Scholar]
- Barenholz Y, Thompson TE, 1980. Sphingomyelins in bilayers and biological membranes. Biochim Biophys Acta 604, 129–158. [DOI] [PubMed] [Google Scholar]
- Baron CL, Malhotra V, 2002. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295, 325–328. doi:10.1126/science.1066759 [DOI] [PubMed] [Google Scholar]
- Baron S, Vangheluwe P, Sepúlveda MR, Wuytack F, Raeymaekers L, Vanoevelen J, 2010. The secretory pathway Ca(2+)-ATPase 1 is associated with cholesterol-rich microdomains of human colon adenocarcinoma cells. Biochim Biophys Acta 1798, 1512–1521. doi:10.1016/j.bbamem.2010.03.023 [DOI] [PubMed] [Google Scholar]
- Blank B, Blume, von J, 2017. Cab45-Unraveling key features of a novel secretory cargo sorter at the trans-Golgi network. Eur. J. Cell Biol doi:10.1016/j.ejcb.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Blume, von J, Alleaume A-M, Cantero-Recasens G, Curwin A, Carreras-Sureda A, Zimmermann T, van Galen J, Wakana Y, Valverde MA, Malhotra V, 2011. ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev. Cell 20, 652–662. doi:10.1016/j.devcel.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Blume, von J, Alleaume A-M, Kienzle C, Carreras-Sureda A, Valverde M, Malhotra V, 2012. Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network. J. Cell Biol 199, 1057–1066. doi:10.1083/jcb.201207180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncompain G, Divoux S, Gareil N, de Forges H, Lescure A, Latreche L, Mercanti V, Jollivet F, Raposo G, Perez F, 2012. Synchronization of secretory protein traffic in populations of cells. Nat. Methods 9, 493–498. doi:10.1038/nmeth.1928 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, 2014. Adaptor proteins involved in polarized sorting. J. Cell Biol 204, 7–17. doi:10.1083/jcb.201310021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo F, Malhotra V, 2012. Membrane fission: the biogenesis of transport carriers. Annu. Rev. Biochem 81, 407–427. doi:10.1146/annurev-biochem-051710-094912 [DOI] [PubMed] [Google Scholar]
- Campelo F, van Galen J, Turacchio G, Parashuraman S, Kozlov MM, García-Parajo MF, Malhotra V, 2017. Sphingomyelin metabolism controls the shape and function of the Golgi cisternae. Elife 6, 158104. doi:10.7554/eLife.24603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso S, Sticco L, Rizzo R, Pirozzi M, Russo D, Dathan NA, Campelo F, van Galen J, Hölttä-Vuori M, Turacchio G, Hausser A, Malhotra V, Riezman I, Riezman H, Ikonen E, Luberto C, Parashuraman S, Luini A, D’Angelo G, 2017. Sphingolipid metabolic flow controls phosphoinositide turnover at the trans-Golgi network. EMBO J. 36, 1736–1754. doi:10.15252/embj.201696048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, De Raeymaecker J, Hovgaard JB, Smaardijk S, Vandecaetsbeek I, Wuytack F, Møller JV, Eggermont J, De Maeyer M, Christensen SB, Vangheluwe P, 2017. Structure/activity relationship of thapsigargin inhibition on the purified Golgi/secretory pathway Ca(2+)/Mn(2+)-transport ATPase (SPCA1a). J. Biol. Chem 292, 6938–6951. doi:10.1074/jbc.M117.778431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs DJ, Shin H-G, Xu Y, Ramu Y, Lu Z, 2013. Tuning voltage-gated channel activity and cellular excitability with a sphingomyelinase. J. Gen. Physiol 142, 367–380. doi:10.1085/jgp.201310986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini LM, Baloban M, Markwardt ML, Rizzo M, Guo F, Verkhusha VV, Snapp EL, 2015. A palette of fluorescent proteins optimized for diverse cellular environments. Nat Commun 6, 7670. doi:10.1038/ncomms8670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevenna AH, Blank B, Maiser A, Emin D, Prescher J, Beck G, Kienzle C, Bartnik K, Habermann B, Pakdel M, Leonhardt H, Lamb DC, Blume, von J, 2016. Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J. Cell Biol 213, 305–314. doi:10.1083/jcb.201601089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartsch H, Kleene R, Kern HF, 1998. In vitro condensation-sorting of enzyme proteins isolated from rat pancreatic acinar cells. Eur. J. Cell Biol 75, 211–222. doi:10.1016/S01719335(98)80115-5 [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Luini A, 2008. Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol 9, 273–284. doi:10.1038/nrm2378 [DOI] [PubMed] [Google Scholar]
- Deng Y, Rivera-Molina FE, Toomre DK, Burd CG, 2016. Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc. Natl. Acad. Sci. U.S.A 113, 6677–6682. doi:10.1073/pnas.1602875113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dode L, Andersen JP, Raeymaekers L, Missiaen L, Vilsen B, Wuytack F, 2005. Functional comparison between secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and sarcoplasmic reticulum Ca2+-ATPase (SERCA) 1 isoforms by steady-state and transient kinetic analyses. J. Biol. Chem 280, 39124–39134. doi:10.1074/jbc.M506181200 [DOI] [PubMed] [Google Scholar]
- Duran JM, Campelo F, van Galen J, Sachsenheimer T, Sot J, Egorov MV, Rentero C, Enrich C, Polishchuk RS, Goñi FM, Brügger B, Wieland F, Malhotra V, 2012. Sphingomyelin organization is required for vesicle biogenesis at the Golgi complex. EMBO J. 31, 4535–4546. doi:10.1038/emboj.2012.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H, Pypaert M, Maday S, Pelletier L, Mellman I, 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol 163, 351–362. doi:10.1083/jcb.200309020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerl MJ, Bittl V, Kirchner S, Sachsenheimer T, Brunner HL, Lüchtenborg C, Özbalci C, Wiedemann H, Wegehingel S, Nickel W, Haberkant P, Schultz C, Krüger M, Brügger B, 2016. Sphingosine-1-Phosphate Lyase Deficient Cells as a Tool to Study Protein Lipid Interactions. PLoS ONE 11, e0153009. doi:10.1371/journal.pone.0153009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, 2009. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucleic Acids Res. 37, 6984–6990. doi:10.1093/nar/gkp687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkant P, Stein F, Höglinger D, Gerl MJ, Brügger B, Van Veldhoven PP, Krijgsveld J, Gavin A-C, Schultz C, 2016. Bifunctional Sphingosine for Cell-Based Analysis of Protein-Sphingolipid Interactions. ACS Chem. Biol 11, 222–230. doi:10.1021/acschembio.5b00810 [DOI] [PubMed] [Google Scholar]
- Hung V, Udeshi ND, Lam SS, Loh KH, Cox KJ, Pedram K, Carr SA, Ting AY, 2016. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat Protoc 11, 456–475. doi:10.1038/nprot.2016.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Tooze SA, 1989. Biosynthetic protein transport in the secretory pathway. Curr. Opin. Cell Biol 1, 648–654. [DOI] [PubMed] [Google Scholar]
- Kardash E, Bandemer J, Raz E, 2011. Imaging protein activity in live embryos using fluorescence resonance energy transfer biosensors. Nat Protoc 6, 1835–1846. doi:10.1038/nprot.2011.395 [DOI] [PubMed] [Google Scholar]
- Kienzle C, Basnet N, Crevenna AH, Beck G, Habermann B, Mizuno N, Blume, von J, 2014. Cofilin recruits F-actin to SPCA1 and promotes Ca2+-mediated secretory cargo sorting. J. Cell Biol 206, 635–654. doi:10.1083/jcb.201311052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzle C, Blume, von J, 2014. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. doi:10.1016/j.tcb.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I, 1989. The biogenesis of lysosomes. Annu. Rev. Cell Biol 5, 483–525. doi:10.1146/annurev.cb.05.110189.002411 [DOI] [PubMed] [Google Scholar]
- Lissandron V, Podini P, Pizzo P, Pozzan T, 2010. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc. Natl. Acad. Sci. U.S.A 107, 9198–9203. doi:10.1073/pnas.1004702107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S, 2005. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat. Cell Biol 7, 225–234. doi:10.1038/ncb1221 [DOI] [PubMed] [Google Scholar]
- Milescu M, Bosmans F, Lee S, Alabi AA, Kim JI, Swartz KJ, 2009. Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nat. Struct. Mol. Biol 16, 1080–1085. doi:10.1038/nsmb.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinete M, Irminger JC, Tooze SA, Halban PA, 2000. Trafficking/sorting and granule biogenesis in the beta-cell. Semin Cell Dev Biol 11, 243–251. doi:10.1006/scdb.2000.0173 [DOI] [PubMed] [Google Scholar]
- Ngo M, Ridgway ND, 2009. Oxysterol binding protein-related Protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function. Mol. Biol. Cell 20, 1388–1399. doi:10.1091/mbc.E08-09-0905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakdel M, Blume, von J, 2018. Exploring new routes for secretory protein export from the trans-Golgi network. Mol. Biol. Cell 29, 235–240. doi:10.1091/mbc.E17-02-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar HB, Duncan R, 2016. A novel tribasic Golgi export signal directs cargo protein interaction with activated Rab11 and AP-1-dependent Golgi-plasma membrane trafficking. Mol. Biol. Cell 27, 1320–1331. doi:10.1091/mbc.E15-12-0845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR, Rothman JE, 1987. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu. Rev. Biochem 56, 829–852. doi:10.1146/annurev.bi.56.070187.004145 [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Kessler T, Silletti S, Cheresh DA, Verma IM, 2000. Suppression of angiogenesis by lentiviral delivery of PEX, a noncatalytic fragment of matrix metalloproteinase 2. Proc. Natl. Acad. Sci. U.S.A 97, 12227–12232. doi:10.1073/pnas.220399597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu Y, Xu Y, Lu Z, 2006. Enzymatic activation of voltage-gated potassium channels. Nature 442, 696–699. doi:10.1038/nature04880 [DOI] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F, 2013. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8, 2281–2308. doi:10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarri E, Sicart A, Lázaro-Diéguez F, Egea G, 2011. Phospholipid synthesis participates in the regulation of diacylglycerol required for membrane trafficking at the Golgi complex. J. Biol. Chem 286, 28632–28643. doi:10.1074/jbc.M111.267534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Lederkremer GZ, Williams S, Fogliano M, Baldini G, Lodish HF, 1996. Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J. Cell Biol 133, 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Dartsch H, Linder D, Kern HF, Kleene R, 2000. A submembranous matrix of proteoglycans on zymogen granule membranes is involved in granule formation in rat pancreatic acinar cells. J. Cell. Sci 113 ( Pt 12), 2233–2242. [DOI] [PubMed] [Google Scholar]
- Sorin A, Rosas G, Rao R, 1997. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem 272, 9895–9901. [DOI] [PubMed] [Google Scholar]
- Subathra M, Qureshi A, Luberto C, 2011. Sphingomyelin synthases regulate protein trafficking and secretion. PLoS ONE 6, e23644. doi:10.1371/journal.pone.0023644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafesse FG, Sanyal S, Ashour J, Guimaraes CP, Hermansson M, Somerharju P, Ploegh HL, 2013. Intact sphingomyelin biosynthetic pathway is essential for intracellular transport of influenza virus glycoproteins. Proc. Natl. Acad. Sci. U.S.A 110, 6406–6411. doi:10.1073/pnas.1219909110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Flatmark T, Tooze J, Huttner WB, 1991. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J. Cell Biol 115, 1491–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Huttner WB, 1990. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell 60, 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Galen J, Campelo F, Martínez-Alonso E, Scarpa M, Martínez-Menárguez JÁ, Malhotra V, 2014. Sphingomyelin homeostasis is required to form functional enzymatic domains at the trans-Golgi network. J. Cell Biol 206, 609–618. doi:10.1083/jcb.201405009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW, 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol 9, 112–124. doi:10.1038/nrm2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana Y, Kotake R, Oyama N, Murate M, Kobayashi T, Arasaki K, Inoue H, Tagaya M, 2015. CARTS biogenesis requires VAP-lipid transfer protein complexes functioning at the endoplasmic reticulum-Golgi interface. Mol. Biol. Cell 26, 4686–4699. doi:10.1091/mbc.E15-08-0599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana Y, van Galen J, Meissner F, Scarpa M, Polishchuk RS, Mann M, Malhotra V, 2012. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. EMBO J. 31, 3976–3990. doi:10.1038/emboj.2012.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ramu Y, Lu Z, 2008. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature 451, 826–829. doi:10.1038/nature06618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Identification of native protein cargos of the SMS pathway, Related to Figure 1. Mass spectrometry proteomics analysis list containing candidate cargo proteins of the SMS pathway that were defined as proteins containing a signal sequence, a transmembrane domain, or a GPI anchor, that were identified in EQ-SM-APEX2 vesicle fractions, but not in EQ-sol-APEX2 vesicle fractions, in each of two independent vesicle preparations.
Table S3. List of oligonucleotides used for genomic validation, cloning and siRNA targeting, Related to STAR Methods.